Olfactory Capacity and Obesity: A Narrative Review of the Literature
Abstract
1. Introduction
Smell and Diet
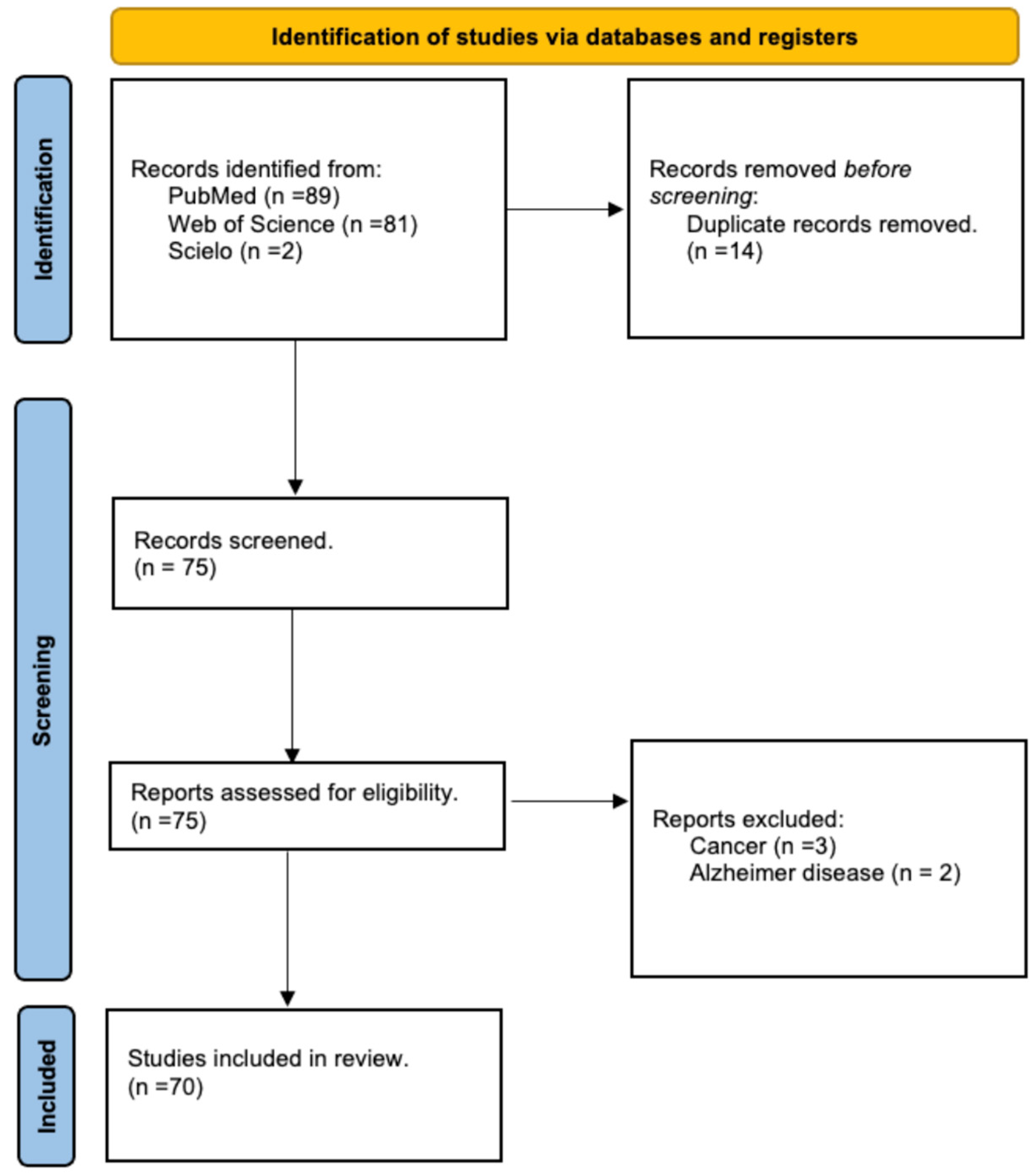
2. Materials and Methods
3. Results
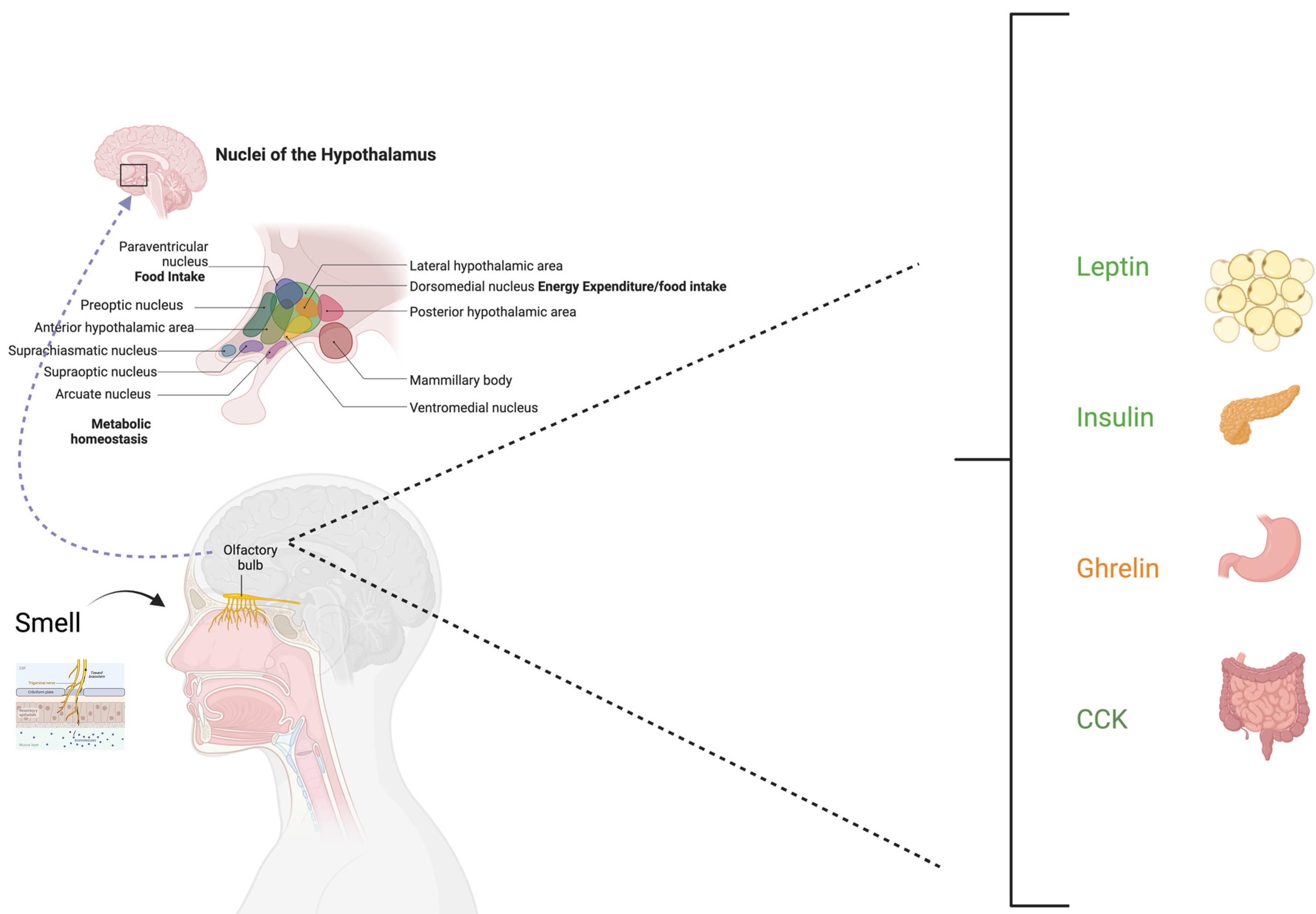
3.1. Smell and Energy Regulation
3.2. Studies on Animals and Insects
3.3. Human Studies
3.4. Studies in Children
3.5. Studies in Adults
3.6. Eating Behavior Disorders
3.7. Bariatric Surgery
3.8. Olfactory Dysfunction, BMI, and COVID-19
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [PubMed]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 28 January 2025).
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018; p. 2018. [Google Scholar]
- Lara-Castor, L.; Micha, R.; Cudhea, F.; Miller, V.; Shi, P.; Zhang, J.; Sharib, J.R.; Erndt-Marino, J.; Cash, S.B.; Mozaffarian, D.; et al. Sugar-sweetened beverage intakes among adults between 1990 and 2018 in 185 countries. Nat. Commun. 2023, 14, 5957. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Webb, P.; Cudhea, F.; Shi, P.; Zhang, J.; Reedy, J.; Erndt-Marino, J.; Coates, J.; Mozaffarian, D.; Global Dietary Database. Global dietary quality in 185 countries from 1990 to 2018 show wide differences by nation, age, education, and urbanicity. Nat. Food 2022, 3, 694–702. [Google Scholar]
- Spence, C. Multisensory Flavor Perception. Cell 2015, 161, 24–35. [Google Scholar]
- Micarelli, A.; Malacrida, S.; Strapazzon, G.; Mrakic-Sposta, S.; Micarelli, B.; Alessandrini, N.; Carbini, V.; Caputo, S.; Falla, M.; Alessandrini, M. Impact of Nutritional Intervention on Taste Perception-A Scoping Review. Foods 2021, 10, 2747. [Google Scholar] [CrossRef]
- McCrickerd, K.; Forde, C.G. Sensory influences on food intake control: Moving beyond palatability. Obes. Rev. 2016, 17, 18–29. [Google Scholar] [CrossRef]
- Yeomans, M.R. Olfactory influences on appetite and satiety in humans. Physiol. Behav. 2006, 89, 10–14. [Google Scholar]
- Reisenman, C.E.; Scott, K. Food-derived volatiles enhance consumption in Drosophila melanogaster. J. Exp. Biol. 2019, 222, jeb202762. [Google Scholar]
- Manesse, C.; Ferdenzi, C.; Sabri, M.; Bessy, M.; Rouby, C.; Faure, F.; Bellil, D.; Jomain, S.; Landis, B.N.; Hugentobler, M.; et al. Dysosmia-Associated Changes in Eating Behavior. Chemosens. Perception 2017, 10, 104–113. [Google Scholar]
- Roxbury, C.R.; Bernstein, I.A.; Lin, S.Y.; Rowan, N.R. Association Between Chemosensory Dysfunction and Diet Quality in United States Adults. Am. J. Rhinol. Allergy 2022, 36, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Touhara, K.; Vosshall, L.B. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef]
- Murphy, C. Nutrition and chemosensory perception in the elderly. Crit. Rev. Food Sci. Nutr. 1993, 33, 3–15. [Google Scholar] [PubMed]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [PubMed]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar]
- Jovanovic, P.; Riera, C.E. Olfactory system and energy metabolism: A two-way street. Trends Endocrinol. Metab. 2022, 33, 281–291. [Google Scholar]
- Petrovich, G.D.; Setlow, B.; Holland, P.C.; Gallagher, M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J. Neurosci. 2002, 22, 8748–8753. [Google Scholar] [CrossRef]
- Su, Z.; Alhadeff, A.L.; Betley, J.N. Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 2017, 21, 2724–2736. [Google Scholar]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Farr, O.M.; Li, C.-S.R.; Mantzoros, C.S. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism 2016, 65, 699–713. [Google Scholar] [CrossRef]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC neuronal heterogeneity in energy balance and beyond: An integrated view. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [PubMed]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar]
- Vennemann, M.M.; Hummel, T.; Berger, K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets? Diabetes Metab. J. 2020, 44, 509–528. [Google Scholar]
- Poessel, M.; Breuer, N.; Joshi, A.; Pampel, A.; Villringer, A.; Hummel, T.; Horstmann, A. Reduced Olfactory Bulb Volume in Obesity and Its Relation to Metabolic Health Status. Front. Hum Neurosci. 2020, 14, 586998. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Schreder, T.; Kleemann, A.M.; Schöpf, V.; Kopietz, R.; Anzinger, A.; Demmel, M.; Linn, J.; Kettenmann, B.; Wiesmann, M. Olfactory detection thresholds and pleasantness of a food-related and a non-food odour in hunger and satiety. Rhinology 2009, 47, 160–165. [Google Scholar]
- Soria-Gómez, E.; Bellocchio, L.; Reguero, L.; Lepousez, G.; Martin, C.; Bendahmane, M.; Ruehle, S.; Remmers, F.; Desprez, T.; Matias, I.; et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014, 17, 407–415. [Google Scholar]
- Root, C.M.; Ko, K.I.; Jafari, A.; Wang, J.W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 2011, 145, 133–144. [Google Scholar]
- Savigner, A.; Duchamp-Viret, P.; Grosmaitre, X.; Chaput, M.; Garcia, S.; Ma, M.; Palouzier-Paulignan, B. Modulation of spontaneous and odorant-evoked activity of rat olfactory sensory neurons by two anorectic peptides, insulin and leptin. J. Neurophysiol. 2009, 101, 2898–2906. [Google Scholar] [CrossRef]
- Velluzzi, F.; Deledda, A.; Onida, M.; Loviselli, A.; Crnjar, R.; Sollai, G. Relationship between Olfactory Function and BMI in Normal Weight Healthy Subjects and Patients with Overweight or Obesity. Nutrients 2022, 14, 1262. [Google Scholar] [CrossRef]
- Palouzier-Paulignan, B.; Lacroix, M.-C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Micarelli, A.; Vezzoli, A.; Malacrida, S.; Micarelli, B.; Misici, I.; Carbini, V.; Iennaco, I.; Caputo, S.; Mrakic-Sposta, S.; Alessandrini, M. Taste Function in Adult Humans from Lean Condition to Stage II Obesity: Interactions with Biochemical Regulators, Dietary Habits, and Clinical Aspects. Nutrients 2023, 15, 1114. [Google Scholar] [CrossRef]
- Wesson, D.W.; Wilson, D.A. Sniffing out the contributions of the olfactory tubercle to the sense of smell: Hedonics, sensory integration, and more? Neurosci. Biobehav. Rev. 2011, 35, 655–668. [Google Scholar] [CrossRef]
- Weismann, M.; Yousry, I.; Heuberger, E.; Nolte, A.; Ilmberger, J.; Kobal, G.; Yousry, T.A.; Kettenmann, B.; Naidich, T.P. Functional magnetic resonance imaging of human olfaction. Neuroimaging Clin. N. Am. 2001, 11, 237–250. [Google Scholar] [CrossRef]
- Wang, J.; Eslinger, P.J.; Smith, M.B.; Yang, Q.X. Functional magnetic resonance imaging study of human olfaction and normal aging. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Micarelli, A.; Mrakic-Sposta, S.; Micarelli, B.; Malacrida, S.; Misici, I.; Carbini, V.; Lennaco, I.; Caputo, S.; Vezzoli, A.; Alessandrini, M. Smell Impairment in Stage I-II Obesity: Correlation with Biochemical Regulators and Clinical Aspects. Laryngoscope 2022, 132, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.A.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab. 2017, 26, 198–211.e5. [Google Scholar] [CrossRef]
- He, J.; Tuo, W.; Zhang, X.; Dai, Y.; Fang, M.; Zhou, T.; Xiu, M.; Liu, Y. Olfactory Senses Modulate Food Consumption and Physiology in Drosophila melanogaster. Front. Behav. Neurosci. 2022, 16, 788633. [Google Scholar] [CrossRef]
- Guild, A.A. Olfactory acuity in normal and obese human subjects: Diurnal variations and the effect of d-amphetamine sulphate. J. Laryngol. Otol. 1956, 70, 408–414. [Google Scholar] [CrossRef]
- Thompson, D.A.; Moskowitz, H.R.; Campbell, R.G. Taste and olfaction in human obesity. Physiol. Behav. 1977, 19, 335–337. [Google Scholar] [PubMed]
- Hubert, H.B.; Fabsitz, R.R.; Feinleib, M.; Brown, K.S. Olfactory sensitivity in humans: Genetic versus environmental control. Science 1980, 208, 607–609. [Google Scholar] [CrossRef]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.-J.F. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur. J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [PubMed]
- Llewellyn, C.; Wardle, J. Behavioral Susceptibility to Obesity: Gene-Environment Interplay in the Development of Weight. Physiol. Behav. 2015, 152, 494–501. [Google Scholar] [CrossRef]
- Cecchetto, C.; Dal Bò, E.; Aiello, M.; Fischmeister, F.P.S.; Gentili, C.; Osimo, S.A. Alexithymia Modulates the Attitudes towards Odors but Not the Olfactory Abilities or the Affective Reactions to Odors. PLoS ONE 2023, 18, e0278496. [Google Scholar]
- Zoon, H.F.A.; de Graaf, C.; Boesveldt, S. Food Odours Direct Specific Appetite. Foods 2016, 5, 12. [Google Scholar] [CrossRef]
- Purdy, F.; Luo, Z.; Gardiner, J.C.; Pinto, J.M.; Shiroma, E.J.; Simonsick, E.M.; Harris, T.B.; Chen, H. Olfaction and Changes in Body Composition in a Large Cohort of Older U.S. Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2434–2440. [Google Scholar]
- Yuan, Y.; Li, C.; Luo, Z.; Simonsick, E.M.; Shiroma, E.J.; Chen, H. Olfaction and Physical Functioning in Older Adults: A Longitudinal Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1612–1619. [Google Scholar] [PubMed]
- Obrebowski, A.; Obrebowska-Karsznia, Z.; Gawliński, M. Smell and taste in children with simple obesity. Int. J. Pediatr. Otorhinolaryngol. 2000, 55, 191–196. [Google Scholar]
- Herz, R.S.; Van Reen, E.; Gredvig-Ardito, C.A.; Carskadon, M.A. Insights into smell and taste sensitivity in normal weight and overweight-obese adolescents. Physiol. Behav. 2020, 221, 112897. [Google Scholar] [CrossRef]
- Marty, L.; Bentivegna, H.; Nicklaus, S.; Monnery-Patris, S.; Chambaron, S. Non-Conscious Effect of Food Odors on Children’s Food Choices Varies by Weight Status. Front. Nutr. 2017, 4, 16. [Google Scholar]
- Stafford, L.D.; Welbeck, K. High hunger state increases olfactory sensitivity to neutral but not food odors. Chem. Senses 2011, 36, 189–198. [Google Scholar] [CrossRef]
- Stafford, L.D.; Whittle, A. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef]
- Micarelli, A.; Malacrida, S.; Vezzoli, A.; Micarelli, B.; Misici, I.; Carbini, V.; Caputo, S.; Mrakic-Sposta, S.; Alessandrini, M. Smell, taste and food habits changes along body mass index increase: An observational study. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 5595–5606. [Google Scholar]
- Richardson, B.E.; Vander Woude, E.A.; Sudan, R.; Thompson, J.S.; Leopold, D.A. Altered olfactory acuity in the morbidly obese. Obes. Surg. 2004, 14, 967–969. [Google Scholar] [CrossRef]
- Melis, M.; Mastinu, M.; Sollai, G. Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups. Nutrients 2024, 16, 821. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J. Am. Diet Assoc. 1995, 95, 876–879. [Google Scholar]
- Postma, E.M.; De Graaf, C.; Boesveldt, S. Food preferences and intake in a population of Dutch individuals with self-reported smell loss: An online survey. Food Qual. Prefer. 2020, 79, 103771. [Google Scholar]
- Brondel, L.; Romer, M.; Van Wymelbeke, V.; Walla, P.; Jiang, T.; Deecke, L.; Rigaud, D. Sensory-specific satiety with simple foods in humans: No influence of BMI? Int. J. Obes. 2007, 31, 987–995. [Google Scholar]
- Uygun, B.; Kiyici, S.; Ozmen, S.; Gul, Z.; Sigirli, D.; Cavun, S. The Association Between Olfaction and Taste Functions with Serum Ghrelin and Leptin Levels in Obese Women. Metab. Syndr. Relat. Disord. 2019, 17, 452–457. [Google Scholar] [CrossRef]
- Fernández-Aranda, F.; Agüera, Z.; Fernández-García, J.C.; Garrido-Sanchez, L.; Alcaide-Torres, J.; Tinahones, F.J.; Giner-Bartolomé, C.; Baños, R.M.; Botella, C.; Cebolla, A.; et al. Smell-taste dysfunctions in extreme weight/eating conditions: Analysis of hormonal and psychological interactions. Endocrine 2016, 51, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, F.; De Nitto, S.; Zambetti, G.; Loriedo, C.; Ciofalo, A. Alterations of the olfactory-gustatory functions in patients with eating disorders. Eur. Eat. Disord. Rev. 2013, 21, 382–385. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Scholze, N.; Joraschky, P.; Hummel, T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J. Psychiatr. Res. 2008, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Schreder, T.; Albrecht, J.; Kleemann, A.M.; Schöpf, V.; Kopietz, R.; Anzinger, A.; Demmel, M.; Linn, J.; Pollatos, O.; Wiesmann, M. Olfactory performance of patients with anorexia nervosa and healthy subjects in hunger and satiety. Rhinology 2008, 46, 175–183. [Google Scholar]
- Rapps, N.; Giel, K.E.; Söhngen, E.; Salini, A.; Enck, P.; Bischoff, S.C.; Zipfel, S. Olfactory deficits in patients with anorexia nervosa. Eur. Eat. Disord. Rev. 2010, 18, 385–389. [Google Scholar] [CrossRef]
- Richardson, B.E.; Vanderwoude, E.A.; Sudan, R.; Leopold, D.A.; Thompson, J.S. Gastric bypass does not influence olfactory function in obese patients. Obes. Surg. 2012, 22, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Matiashova, L.; Hoogkamer, A.L.; Timper, K. The Role of the Olfactory System in Obesity and Metabolism in Humans: A Systematic Review and Meta-Analysis. Metabolites 2023, 14, 16. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Islam, M.A.; Abdullah, B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. Laryngoscope 2021, 131, 865–878. [Google Scholar] [CrossRef]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Gary, J.B.; Gallagher, L.; Joseph, P.V.; Reed, D.; Gudis, D.A.; Overdevest, J.B. Qualitative Olfactory Dysfunction and COVID-19: An Evidence-Based Review with Recommendations for the Clinician. Am. J. Rhinol. Allergy 2023, 37, 95–101. [Google Scholar] [CrossRef]
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926. [Google Scholar] [PubMed]
- Chen, B.; Masala, C.; Oleszkiewicz, A.; Englmaier, V.; Gunder, N.; Menzel, S.; Haehner, A.; Hummel, T. Nonlinear association between chemosensory dysfunction and body mass index. J. Sens. Studies 2022, 37, e12715. [Google Scholar]
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging-A review. Age 2015, 37, 9821. [Google Scholar] [CrossRef] [PubMed]
- Burges Watson, D.L.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered smell and taste: Anosmia, parosmia and the impact of long COVID-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef]
- Algahtani, S.N.; Alzarroug, A.F.; Alghamdi, H.K.; Algahtani, H.K.; Alsywina, N.B.; Bin Abdulrahman, K.A. Investigation on the Factors Associated with the Persistence of Anosmia and Ageusia in Saudi COVID-19 Patients. Int. J. Environ. Res. Public Health 2022, 19, 1047. [Google Scholar] [CrossRef]
- Perna, S.; Abdulsattar, S.; Alalwan, T.A.; Zahid, M.N.; Gasparri, C.; Peroni, G.; Faragli, A.; La Porta, E.; Ali Redha, A.; Janahi, E.M.; et al. A cross-sectional analysis of post-acute COVID-19 symptoms. Ann. Ig. Med. Prev. E Comunita. 2022, 34, 478–489. [Google Scholar]
- Taş, B.M.; Alpaydın, T.; Akçalı, S.; Kaygusuz, S.; Özlük Erol, Ö.; Şencan, Z.; Cömert, E.; Bayar Muluk, N.; Özel, G. Evaluation of Clinical Features and Olfactory Functions in COVID-19: A Multicentre Study. Cureus 2023, 15, e40027. [Google Scholar]
- Bhutani, S.; Coppin, G.; Veldhuizen, M.G.; Parma, V.; Joseph, P.V. COVID-19 related chemosensory changes in individuals with self-reported obesity. Rhinology 2022, 60, 128–138. [Google Scholar]
- Valladares Vega, M.; Obregón Rivas, A.M. Association of olfactory sensitivity with energy intake: Role in development of obesity. Nutr. Hosp. 2015, 32, 2385–2389. [Google Scholar]
- Ferrulli, A.; Senesi, P.; Terruzzi, I.; Luzi, L. Eating Habits and Body Weight Changes Induced by Variation in Smell and Taste in Patients with Previous SARS-CoV-2 Infection. Nutrients 2022, 14, 5068. [Google Scholar] [CrossRef]
- Vilarello, B.J.; Jacobson, P.T.; Tervo, J.P.; Gallagher, L.W.; Caruana, F.F.; Gary, J.B.; Saak, T.M.; Gudis, D.A.; Joseph, P.V.; Goldberg, T.E.; et al. BMI Increases in Individuals with COVID-19-Associated Olfactory Dysfunction. Nutrients 2023, 15, 4538. [Google Scholar] [CrossRef] [PubMed]
- Liem, D.G.; Russell, C.G. The Influence of Taste Liking on the Consumption of Nutrient Rich and Nutrient Poor Foods. Front. Nutr. 2019, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, N.; Høier, A.T.Z.B.; Andersen, B.V. A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease. Foods 2021, 10, 892. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Agüero, S.; Obregón-Rivas, A.M. Olfactory Capacity and Obesity: A Narrative Review of the Literature. Appl. Sci. 2025, 15, 3590. https://doi.org/10.3390/app15073590
Durán-Agüero S, Obregón-Rivas AM. Olfactory Capacity and Obesity: A Narrative Review of the Literature. Applied Sciences. 2025; 15(7):3590. https://doi.org/10.3390/app15073590
Chicago/Turabian StyleDurán-Agüero, Samuel, and Ana María Obregón-Rivas. 2025. "Olfactory Capacity and Obesity: A Narrative Review of the Literature" Applied Sciences 15, no. 7: 3590. https://doi.org/10.3390/app15073590
APA StyleDurán-Agüero, S., & Obregón-Rivas, A. M. (2025). Olfactory Capacity and Obesity: A Narrative Review of the Literature. Applied Sciences, 15(7), 3590. https://doi.org/10.3390/app15073590






