Evaluation of the Effectiveness of Plant-Protein-Based Cleaning Agents in the Production of Industrial-Filtered Clarified Apple Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple and Apple Juice
2.2. Clarification Agents
2.3. Spectrophotometer and Chemicals
2.4. Methods
2.4.1. Volume of Sediment
2.4.2. Water-Soluble Solids (Brix°)
2.4.3. Transmittance (T%)
2.4.4. Color (ΔE*)
2.4.5. Turbidity (NTU = Nephelometric Turbidity Unit)
2.4.6. Antioxidant Capacity (FRAP)
2.4.7. Total Polyphenol Content (TPC)
2.4.8. Statistical Analysis
3. Results and Discussion
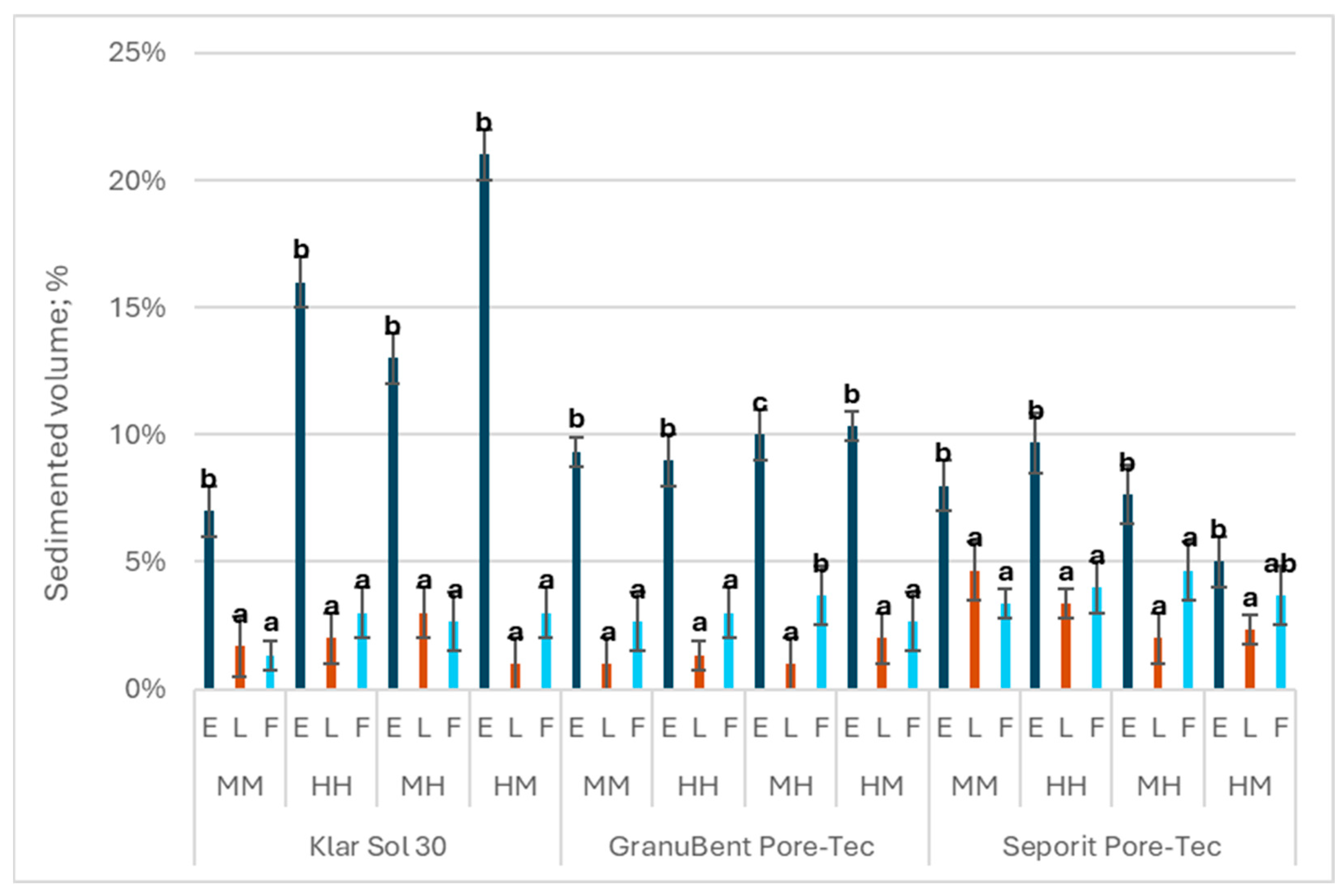
3.1. Volume of Sediment
3.2. Water-Soluble Solids (BRIX°)
3.3. Transmittance (T%)
3.4. Color
3.5. Turbidity (NTU)
3.6. Antioxidant Capacity (FRAP)
3.7. Total Polyphenol Content (TPC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apple Juice. Available online: https://www.statista.com/outlook/cmo/non-alcoholic-drinks/juices/apple-juice/worldwide (accessed on 7 February 2025).
- Vallée Marcotte, B.; Verheyde, M.; Pomerleau, S.; Doyen, A.; Couillard, C. Health benefits of apple juice consumption: A review of interventional trials on humans. Nutrients 2022, 14, 821. [Google Scholar] [CrossRef]
- Tsoupras, A.; Gkika, D.A.; Markopoulos, T.; Curran, R.; Scallon, C.; Karali, M.; Kyzas, G.Z. Apple products (apple juice and cider) and by-products (apple pomace): Bioactive compounds and biological properties. In Natural Products in Beverages: Botany, Phytochemistry, Pharmacology and Processing; Mérillon, J.-M., Rivière, C., Lefèvre, G., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–42. [Google Scholar] [CrossRef]
- Sharma, H.P.; Patel, H.; Sharma, S. Enzymatic extraction and clarification of juice from various fruits—A review. Trends Post Harvest Technol. 2014, 2, 1–14. [Google Scholar]
- Codex Alimentarius Commission. General Standard for Fruit Juices and Nectars; FAO/WHO: Geneva, Switzerland, 2005; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/jp/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2S47-2005%252FCXS_247e.pdf (accessed on 16 September 2024).
- Sharma, H.P.; Patel, H.; Sugandha. Enzymatic added extraction and clarification of fruit juices—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1215–1227. [Google Scholar]
- Oszmiański, J.; Wojdyło, A. Effects of various clarification treatments on phenolic compounds and color of apple juice. Eur. Food Res. Technol. 2007, 224, 755–762. [Google Scholar]
- Sarbatly, R.; Sariau, J.; Krishnaiah, D. Recent developments of membrane technology in the clarification and concentration of fruit juices. Food Eng. Rev. 2023, 15, 420–437. [Google Scholar]
- Vitolo, M. Enzymes in the production of juices and beverages. World J. Pharm. Pharm. Sci. 2020, 9, 504–517. [Google Scholar]
- Wang, F.; Xu, H.; Wang, M.; Yu, X.; Cui, Y.; Xu, L.; Qian, J. Application of immobilized enzymes in juice clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef]
- Horváth-Kerkai, E.; Stéger-Máté, M. Manufacturing fruit beverages and concentrates. In Handbook Fruits and Fruit Processing, 2nd ed.; Nirmal, K.S., Jiwan, S., Barta, J., Wu, B., Eds.; Wiley-Blavkwell: Ames, IA, USA, 2012; pp. 215–228. [Google Scholar]
- Wongmaneepratip, W.; Tongkhao, K.; Vangnai, K. Effect of clarifying agent type and dose on the reduction of pyrethroid residues in apple juice. Food Control 2023, 153, 109909. [Google Scholar]
- Vegan Food Market Size, Share & Trends Analysis Report by Product (Meat & Seafood, Creamer, Ice Cream & Frozen Novelties, Yogurt, Cheese, Butter, Meals, Protein Bars, Others), by Distribution Channel, by Region, and Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/vegan-food-market (accessed on 22 November 2024).
- Clem, J.; Barthel, B. A look at plant-based diets. Mo. Med. 2021, 118, 233. [Google Scholar]
- Gelatin Was Yesterday—Plant Protein Is Today. Available online: www.eaton.com (accessed on 16 September 2024).
- Ramezani, M.; Ferrentino, G.; Morozova, K.; Kamrul, S.H.; Scampicchio, M. Clarification of apple juices with vegetable proteins monitored by multiple light scattering. J. Food Sci. 2020, 85, 316–323. [Google Scholar]
- Ahamad, S.; Choupdar, G.K.; Kumar, R.; Kumar, A.; Bihari, C.; Singh, S.; Wamiq, M. Enhancing Clarity and Quality: The Role of Clarifying Agents in Horticulture Foods and Formulations. Int. J. Environ. Clim. Change 2023, 13, 549–559. [Google Scholar]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine fining with plant proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef]
- Jafari, S.; Shiekh, K.A.; Mishra, D.K.; Kijpatanasilp, I.; Assatarakul, K. Combined Effects of Clarifying Agents Improve Physicochemical, Microbial and Sensorial Qualities of Fresh Indian Gooseberry (Phyllanthus emblica L.) Juice during Refrigerated Storage. Foods 2024, 13, 290. [Google Scholar] [CrossRef]
- Tobolka, A.; Škorpilová, T.; Beňo, F.; Podskalská, T.; Rajchl, A. Effect of Various Carbohydrates in Aqueous Solutions on Color Stability and Degradation Kinetics of Selected Anthocyanins During Storage. Foods 2024, 13, 3628. [Google Scholar] [CrossRef]
- Bryant, C.J. We can’t keep meating like this: Attitudes towards vegetarian and vegan diets in the United Kingdom. Sustainability 2019, 11, 6844. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, P.R.; Hu, F.; Zhu, W.; Wei, Z.J. Updates on plant-based protein products as an alternative to animal protein: Technology, properties, and their health benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef]
- Aziz, M.B.; Mouls, L.; Fulcrand, H.; Douieb, H.; Hajjaj, H. Phenolic compounds of Moroccan red press wines: Influence of fining agents and micro-oxygenation treatments. LWT 2017, 78, 143–150. [Google Scholar]
- Ridge, M.; Sommer, S.; Dycus, D.A. Addressing enzymatic clarification challenges of Muscat grape juice. Fermentation 2021, 7, 198. [Google Scholar] [CrossRef]
- Marchal, R.; Lallement, A.; Jeandet, P.; Establet, G. Clarification of Muscat musts using wheat proteins and the flotation technique. J. Agric. Food Chem. 2003, 51, 2040–2048. [Google Scholar]
- Gaspar, L.M.; Machado, A.; Coutinho, R.; Sousa, S.; Santos, R.; Xavier, A.; Simões, J. Development of potential yeast protein extracts for red wine clarification and stabilization. Front. Microbiol. 2019, 10, 2310. [Google Scholar]
- Gazzola, D.; Vincenzi, S.; Marangon, M.; Pasini, G.; Curioni, A. Grape seed extract: The first protein-based fining agent endogenous to grapes. Aust. J. Grape Wine Res. 2017, 23, 215–225. [Google Scholar]
- Gambuti, A.; Rinaldi, A.; Romano, R.; Manzo, N.; Moio, L. Performance of a protein extracted from potatoes for fining of white musts. Food Chem. 2016, 190, 237–243. [Google Scholar]
- Kang, W.; Niimi, J.; Bastian, S.E.P. Reduction of red wine astringency perception using vegetable protein fining agents. Am. J. Enol. Vitic. 2018, 69, 22–31. [Google Scholar]
- Dedehou, E.S.; Dossou, J.; Ahohuendo, B.; Saidou, A.; Ahanchede, A.; Soumanou, M.M. Optimization of cashew (Anacardium occidentale L.) apple juice’s clarification process by using cassava and rice starch. J. Appl. Biosci. 2015, 95, 8989–9002. [Google Scholar]
- Nehmé, L.; El Tekle, M.; Barakat, N.; El Khoury, A.; Azzi-Achkouty, S.; El Rayess, Y. Alternative processes for apple juice stabilization and clarification: A bibliometric and comprehensive review. Processes 2024, 12, 296. [Google Scholar] [CrossRef]
- Available online: https://erbsloeh.com/produkt-kategorie/all-products/ (accessed on 8 September 2024).
- He, Y.; Ji, Z.; Li, S. Effective clarification of apple juice using membrane filtration without enzyme and pasteurization pretreatment. Sep. Purif. Technol. 2007, 57, 366–373. [Google Scholar] [CrossRef]
- Lukács, G. Színmérés; Műszaki Könyvkiadó: Budapest, Hungary, 1982; pp. 125–167. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Tulek, Y.; Yilmaz, S. Use of clarifying agents and ultra filter to decrease fumaric acid, HMF and increase clarity of apple juice. J. Food Qual. 2006, 29, 216–228. [Google Scholar]
- Araya-Farias, M.; Mondor, M.; Lamarche, F.; Tajchakavit, S.; és Makhlouf, J. Clarification of apple juice by electroflotation. Innov. Food Sci. Emerg. Technol. 2008, 9, 320–327. [Google Scholar]
- Mirzaaghaei, M.; Goli, S.A.H.; Fathi, M. Clarification of apple juice using activated sepiolite as a new fining clay. Clay Miner. 2017, 52, 497–508. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Kolniak, J. Effect of pectinase treatment on extraction of antioxidant phenols from pomace, for the production of puree-enriched cloudy apple juices. Food Chem. 2011, 127, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Erkan-Koç, B.; Türkyılmaz, M.; Yemiş, O.; Özkan, M. Effects of various protein-and polysaccharide-based clarification agents on antioxidative compounds and colour of pomegranate juice. Food Chem. 2015, 184, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, A.; Mirzaaghaei, M.; Goli, S.A.H. Application of natural fining agents to clarify fruit juices. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4190–4216. [Google Scholar] [CrossRef]



| Code | Name of Agent | Properties | Surface Charge | Form | Recommended Dosage | Medium Dosage; mL/100 L (M) | High Dosage; mL/100 L (H) |
|---|---|---|---|---|---|---|---|
| K | KlarSol 30 | alkaline, silica sol | − | Liquid | 20–250 mL/100 L | 135 | 250 |
| G | GranuBent Pore-Tec | sodium bentonite, granules | − | Granules | 35–75 g/100 L | 55 | 75 |
| S | Seporit Pore-Tec | calcium bentonite granules | − | Granules | 100–200 g/100 L | 150 | 200 |
| E | ErbiGel Liquid | gelatine | + | Liquid | 20–50 mL/100 L | 35 | 50 |
| L | LittoFresh Liquid | vegetable protein | + | Liquid | 50–200 mL/100 L | 125 | 200 |
| F | FloaClair | pea protein isolate | + | Powder | 20–60 g/100 L | 40 | 60 |
| E | L | F | |
|---|---|---|---|
| Klar Sol 30 | |||
| MM | 12.4 ± 0.1 a | 12.2 ± 0.1 a | 12.3 ± 0.0 a |
| HH | 12.2 ± 0.0 a | 12.2 ± 0.1 a | 12.2 ± 0.1 a |
| MH | 12.2 ± 0.0 a | 12.2 ± 0.0 a | 12.2 ± 0.0 a |
| HM | 12.3 ± 0.1 a | 12.3 ± 0.0 a | 12.3 ± 0.1 a |
| GranuBent Pore-Tec | |||
| MM | 12.0 ± 0.0 b | 11.8 ± 0.1 a | 11.9 ± 0.1ab |
| HH | 11.9 ± 0.0 a | 11.8 ± 0.1 a | 11.8 ± 0.1 a |
| MH | 11.9 ± 0.1 a | 11.9 ± 0.1 a | 11.9 ± 0.1 a |
| HM | 11.9 ± 0.0 a | 11.9 ± 0.1 a | 11.8 ± 0.1 a |
| Seporit Pore-Tec | |||
| MM | 11.9 ± 0.1 ab | 11.8 ± 0.1 a | 12.1 ± 0.1 b |
| HH | 11.8 ± 0.1 a | 11.9 ± 0.1 ab | 12.0 ± 0.1 b |
| MH | 12.0 ± 0.1 a | 11.9 ± 0.1 a | 12.1 ± 0.1 a |
| HM | 11.9 ± 0.1 a | 11.9 ± 0.1 a | 12.0 ± 0.1 a |
| E | L | F | |
|---|---|---|---|
| Klar Sol 30 | |||
| MM | 7.6 ± 0.6 a | 9.6 ± 0.6 b | 11.2 ± 0.6 c |
| HH | 7.9 ± 0.6 a | 6.9 ± 1.0 a | 8.9 ± 0.0 b |
| MH | 10.1 ± 0.6 c | 7.5 ± 0.6 b | 5.7 ± 0.6 a |
| HM | 6.8 ± 1.0 a | 6.4 ± 1.0 a | 11.4 ± 0.6 b |
| GranuBent Pore-Tec | |||
| MM | 29.9 ± 0.6 c | 9.2 ± 0.0 a | 24.5 ± 0.6 b |
| HH | 21.5 ± 0.6 b | 25.5 ± 0.6 c | 7.9 ± 0.6 a |
| MH | 18.4 ± 0.6 b | 13.5 ± 0.6 a | 13.7 ± 0.0 a |
| HM | 16.8 ± 0.6 b | 23.6 ± 0.0 c | 9.3 ± 0.6 a |
| Seporit Pore-Tec | |||
| MM | 16.5 ± 0.6 c | 6.7 ± 0.0 a | 8.9 ± 0.0 b |
| HH | 14.4 ± 0.0 a | 11.6 ± 0.0 a | 12.6 ± 0.6 a |
| MH | 14.9 ± 0.6 c | 12.0 ± 1.0 b | 9.0 ± 1.0 a |
| HM | 13.3 ± 1.0 a | 16.5 ± 1.0 b | 11.7 ± 1.0 a |
| Klar Sol 30 | GranuBent Pore-Tec | Seporit Pore-Tec | ||||
|---|---|---|---|---|---|---|
| L | F | L | F | L | F | |
| MM | 2.95 | 3.11 | 9.63 | 9.11 | 9.28 | 8.57 |
| HH | 6.77 | 5.85 | 11.05 | 9.74 | 6.05 | 3.51 |
| MH | 11.38 | 10.11 | 10.95 | 7.46 | 10.08 | 7.41 |
| HM | 4.52 | 3.73 | 8.71 | 6.78 | 5.96 | 3.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kereszturi, J.; Béres, C.; Dalmadi, I.; Máté, M. Evaluation of the Effectiveness of Plant-Protein-Based Cleaning Agents in the Production of Industrial-Filtered Clarified Apple Juice. Appl. Sci. 2025, 15, 3415. https://doi.org/10.3390/app15063415
Kereszturi J, Béres C, Dalmadi I, Máté M. Evaluation of the Effectiveness of Plant-Protein-Based Cleaning Agents in the Production of Industrial-Filtered Clarified Apple Juice. Applied Sciences. 2025; 15(6):3415. https://doi.org/10.3390/app15063415
Chicago/Turabian StyleKereszturi, Julianna, Csenge Béres, István Dalmadi, and Mónika Máté. 2025. "Evaluation of the Effectiveness of Plant-Protein-Based Cleaning Agents in the Production of Industrial-Filtered Clarified Apple Juice" Applied Sciences 15, no. 6: 3415. https://doi.org/10.3390/app15063415
APA StyleKereszturi, J., Béres, C., Dalmadi, I., & Máté, M. (2025). Evaluation of the Effectiveness of Plant-Protein-Based Cleaning Agents in the Production of Industrial-Filtered Clarified Apple Juice. Applied Sciences, 15(6), 3415. https://doi.org/10.3390/app15063415






