Abstract
Herein, a recently developed UAV/Drone approach as a new vector for the collection of airborne particulate matter is reported. In this study, airborne particle emissions from plumes generated in a holiday fireworks display were collected. A platform fabricated using a 3D printer was mounted on the drone, which allowed for particulate capture using double-sided carbon tape attached to aluminum disks. The drone platform was used to trap airborne samples from two types of plumes: high-altitude sampling (HAS), which relates to professional fireworks, and low-altitude sampling (LAS), associated with personal fireworks. Collected samples were studied using a Scanning Electron Microscope alongside Electron Dispersal X-ray Spectroscopy (EDX) for elemental composition analysis. The overall findings regarding the physical morphology reveal several key observations. Firstly, particles from professional fireworks are significantly larger and more spheroidal than those from personal fireworks. Secondly, both types of fireworks show a consistent trend in which some of the larger particles have finer particulates deposited on their surfaces. Lastly, the plumes produced by both types contain spheres that are either solid, hollow or exhibit a core–shell structure. EDX analysis revealed the presence of various types of metals within the samples. EDX analysis shows that the samples collected from the HAS and LAS contain particulates with common elements. However, the samples from the plume of professional fireworks appear to have Ba, Mg, and Fe compared to the samples from personal fireworks. These elements are known to be used in powerful fireworks to create colored displays. A proposed mechanism for particulate growth in fireworks is proposed and discussed.
1. Introduction
On a global scale, the deployment of pyrotechnics constitutes a distinctive and integral aspect for the commemoration of significant events and festivals. Pyrotechnics encompass a range of devices, including fireworks and firecrackers, constructed of a diverse array of metallic elements designed to produce both explosive sounds and colorful captivating displays or sparkling effects. The discharge of these pyrotechnic devices can lead to the emission of plumes loaded with elevated concentrations of gaseous and particular matter (PM) pollutants. The particulate matter pollutants comprise organic and inorganic metal-based, posing potential threats to both the environment and human health. Moreover, the composition and design of fireworks and firecrackers are influenced by regional traditions and cultural preferences, resulting in notable variations across different regions. For example, fireworks are a key part of celebrating Diwali in India [1,2], the Lunar New Year (also known as Chinese New Year in China, Taiwan, Vietnam, and Korea) and Lantern Festival (in several East and Southeast Asian countries) [3,4], Guy Fawkes Night in the United Kingdom [5,6], Bastille Day in France, New Year’s celebrations worldwide, and National Day or Independence Day in various countries around the world [7,8]. For instance, fireworks are used in both large and small towns across the United States to celebrate Independence Day on 4 July.
Given the widespread use of fireworks across various countries and cultures, and the significant role they play in major celebrations, numerous studies have been conducted to investigate the pollutants emitted. These studies include real-time air quality monitoring of plumes and the physical collection of bulk samples using various aerosol sampling methods. For instance, to assess the specific pollutants researchers employ air quality monitors to measure particulate matter (PM), gaseous pollutants (such as nitrogen oxides, carbon monoxide, and sulfur dioxide), and volatile organic compounds (VOCs) during firework displays [9,10,11]. Instruments like optical particle counters or gravimetric samplers have been used for real-time data collection [12]. Other researchers have utilized aerosol filter-based sampling (e.g., Teflon or quartz filters) to capture airborne particulate matter for analysis [3,13,14,15,16,17,18,19]. For instance, Li et al. used a real-time method to monitor PM2.5 emissions from intensive firecracker and firework displays during the Chinese New Year (CNY) [20]. The collected samples included before, during, and after the fireworks using a pump system with quartz filters to trap particulates emitted during the festival for a comparative analysis. Additionally, an Aethalometer was employed to measure black carbon concentrations during the festival. The study reported that the daily average PM2.5 concentration reached 183 μg/m3 during CNY, which was six times higher than the levels before and after the festival. Similarly, black carbon concentrations were elevated throughout the CNY. Interestingly, the study showed that intensive fireworks can lead to the formation of haze, which can be suspended in the region for several days after an event. Moreover, emissions from the fireworks were found to undergo significant transformations, such as the conversion of SO2 to sulfate. Yan et al. [21] reported using quartz filters to trap particulate matter on the intake side of a pump system. An ion chromatography system alongside chemical reaction analyses was employed to analyze the trapped samples. The study identified five major subgroups of organic compounds, including four common types: carbon-, hydrogen-, and oxygen-only containing CHO compounds; nitrogen-containing CHON compounds; sulfur-containing CHOS compounds; and sulfur- and nitrogen-containing CHONS compounds. Particularly, a subgroup of halogen (e.g., chlorine, bromine, and iodine)-containing compounds (HCCs) was also identified, including CHOSI, CHOSCl, CHONSCl, CHONI, CHONCl, CHONBr, CHOClBr, CHOClI, CHOI, CHOCl, and CHOBr. Saha et al. used an Aethalometer during the Diwali Festival to demonstrate the level of pollution caused by fireworks plumes by measuring the concentration of black carbon particles released into the environment during large-scale firework displays [22]. In addition to particulate matter (PM), several chemical species were also reported, including NO2, SO2, PM10, BC (black carbon), CO, and O3. These were compared for three periods: pre-fireworks (normal day), during fireworks, and post-fireworks. The study found that PM10 was the most hazardous pollutant, with concentrations of approximately 90 µg/m3 on normal days, rising to 1660 µg/m3 after the fireworks. NO2 and CO concentrations exceeded the National Ambient Air Quality Standard (NAAQS) limits during the three-day festival, whereas they remained well below these limits on normal days. NO2 levels were three times higher than on normal days, and SO2 was 10 times greater. Alarmingly, PM10 levels were found to be 12 times the normal day levels, which is about eight times above the NAAQS limit. BC and surface O3 concentrations were also elevated during the Diwali period compared to normal days. Additionally, their investigation had a broader scope, examining various meteorological parameters that can affect emissions during the festival. The aforementioned methods are used for bulk sample analysis of pollutant emissions. Researchers have also developed computational models to predict or represent how fine particulate matter (PM2.5) from fireworks spreads into the atmosphere. These models can help estimate exposure levels at different distances from the fireworks display and predict the plume trajectory (pollutants) movement based on weather conditions, wind speed, and terrain [23,24].
As part of ongoing studies on pollutants from fireworks, recent advancements in electron microscopy techniques, such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM), and energy disperse X-ray (EDX) have enabled researchers to focus on the physical and chemical morphology of the particulates formed during fireworks displays. Table 1 lists illustrative works related to the use of electron microscopy in pyrotechnics including as-received (confiscated fireworks), form of collection, chemical analysis, and representative physical morphology. Kosanke et al. (in 2001) reported that SEM coupled with EDX is the most reliable method for detecting gunpowder residue (Table 1) [25]. Additionally, it was shown in that study that SEM/EDX can be successfully employed to study various pyrotechnic fireworks. Their study served as a proof of concept for using SEM and EDX to identify particulates in pyrotechnic residue. They were able to successfully differentiate between sample origins based on the chemical composition and physical structure of the particulates, confirming that the applied methodology works effectively and can be used on a variety of samples. The SEM micrographs revealed that the pyrotechnic reaction residue (PRR) is composed of spheroidal-shaped particulates with fine condensed particles on their surfaces. The spheroidal particles exhibit a polydisperse diameter, ranging from approximately 10 to 20 microns. Some of the spheroidal structures are smooth, while others appear to have a very coarse surface. The surfaces of these larger irregular particles are covered with smaller aggregates formed of finer shapeless particles. The SEM micrographs also revealed that some of the structures had fractured surfaces and, to some extent, collapsed inward, with the shell appearing compromised and less spheroidal. Micrographs of large structures with irregular shapes were also reported. In the work of Castro et al., it was reported the application of various techniques including Raman spectroscopy, SEM, EDX, and Fourier-Transfer-Infrared-Spectroscopy (FTIR) to determine the physical and chemical analysis of several type of unused confiscated pyrotechnic powders and artifacts (Table 1) [26]. The combination of these various techniques can result in a complementary performance as one technique may not be very suitable to fully characterize or detect certain compounds. The SEM micrographs of this work in one of the analyzed powders showed the presence of irregular-shaped structures referred as “grains”. Through the application of SEM/EDX mapping and Raman spectroscopy on one the formulations/samples the presence of two layers of materials or “grains” intermingled was revealed. A base layer or “grains”, without physical symmetry, is composed of nitrite compounds with deposited smaller carbon-containing agglomerates on top. The inorganic “grains”, that are mostly large, have different shapes that are jagged with uneven edges or surfaces. Li et al. collected various aerosol particulates directly on Cu TEM grids using a single-stage impactor with a 0.5 mm diameter jet nozzle operating under an air flowrate of 1.0 L/min during the Chinese New Year festival [20]. The TEM grids were analyzed using a transmission electron microscope coupled with EDX to determine the elemental composition of the particles. The EDX analysis showed that sulfate- and organic-rich particles were dominant in the atmosphere before and after the CNY. In contrast, K-rich sulfates and other metal-rich particles (e.g., Ba, Al, Mg, and Fe) were much more abundant than ammonia-reacted sulfate particles during the CNY. The study also presented a physical view of the morphology of the structures formed in the fireworks. A close inspection of the TEM images showed that the particulates resulting from the fireworks were large in size and irregular in shape. Some of the surfaces of these large particles frequently have condensed or deposited finer structures. TEM images of particles collected from firecracker samples revealed that they were much smaller, more spherical in shape, and aggregated (Table 1). A study by Grima et al. focused on using SEM/EDX to identify and compare the morphology of particles at the microscale—both physically and chemically—that can be generated from gunshot residue (GSR) and those from fireworks (Table 1) [27]. The PM generated from fireworks was collected by placing 12 mm SEM double-sided carbon tapes in a glass Petri dish for SEM/EDX analysis. The sample holders were positioned randomly near the firework discharge to collect fallout particulates during the festivities on the Maltese Islands. Electron microscopy of the fireworks samples revealed large, regular spheroidal-shaped structures, spheroidal structures with smaller condensed particulates, and hollowed spheroidal shapes, depending on the location of the firework discharge. The study concluded that SEM and EDX revealed striking similarities in the physical and chemical characteristics of firework residue and GSR particle formation. Mishra et al. conducted SEM/EDX studies in which aerosol samples were collected by trapping them using pump system filters and an Anderson sampler during the Diwali festival in New Delhi [28]. Samples were gathered before, during, and after the event to assess potential changes in the elemental composition of the suspended particulates generated by the fireworks. The SEM/EDX comparative analysis showed that, overall, the particles collected before the event were mostly composed of carbon-rich (organic) material, frequently accompanied by particles rich in Al and Ca (mineral dust). However, the SEM/EDX analysis revealed that the samples collected during and after the fireworks event contained both organic and inorganic particles, including several metallic base elements. Their work identified numerous metallic compounds, such as TiO2, MnO, and CuO, that were introduced into the environment as a result of the celebrations. These compounds were not present in the samples taken before the festival, indicating that a significant amount of metal oxide compounds were released due to the fireworks. A brief inspection of the SEM images from this study reveals that large spheroidal-shaped structures were absent from the SEM micrographs. The comparative SEM analysis shows that the particles collected after the fireworks were of various shapes: some were irregular and lacked symmetry, others appeared rectangular, while some consisted of smaller spheroidal-like and polygonal-like structures forming large clusters. A noteworthy observation is that the particles collected after the fireworks were relatively larger in size (super micron) compared to those collected during the fireworks.

Table 1.
Illustrative microscopy studies of particulate emissions from fireworks bursting and non-bursting.
In this work, the novel application of a drone coupled with a customized particulate collector is used to capture samples of the particulates released from two different types of plumes generated from fireworks in their airborne state rather than using ground-based samplers that rely on particulates settling onto their filters. This approach contrasts with traditional ground-based samplers that rely on particulates settling onto collectors, such as double-sided carbon tape, which are left in the atmosphere for days to weeks. It also differs from techniques using suction systems with filters. This study presents a detailed analysis of the chemical and physical morphology of individual particles. The focus of this study is on collecting particulate samples from two types of plumes: one generated by professional fireworks and the other by personal fireworks. The physical and chemical morphology of the particulates in the collected samples was obtained using scanning electron microscopy (SEM) and SEM energy-dispersive X-rays (SEM-EDX). A proposed mechanism for particulate growth in fireworks is proposed and discussed. The investigation was centered around a municipal firework display conducted in Norman, Oklahoma, USA as a part of Independence Day celebrations. The city of Norman is located approximately at 35°11′ N, 97°26′ W within the state of Oklahoma in the south-central United States.
2. Materials and Methods
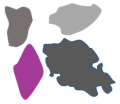
To collect samples from the firework plumes, a DJI (Shenzhen, China) Mavic Pro Platinum drone and a customized mount for the drone with a particle sampling mechanism were used. The full platform is mounted on the back of the drone. This drone mount was designed using CAD software (SolidWorks 2022: Waltham, MA, USA). The particulate capture method consisted of a 3D Printed mount capable of holding 1.5-inch diameter aluminum disks. These disks were coated with electrically conductive double-sided carbon tape on the outside, and just before flight, the covering of this tape was removed, allowing the airborne particulates in a plume to stick to the carbon tape for later study. The full platform also included a counterweight on the opposite side of the drone to ensure the whole system remained in balance. The isometric and top views of the sampling drone mount can be seen in Figure 1a. The exploded view with detailed components for particulate collection, including the sampling cone, is shown in Figure 1b. Figure 1c shows a photograph of the drone with the drone mount installed. Figure 1d shows the drone/sampler hovering at a low altitude, while Figure 1e depicts it during high-altitude sampling. At both altitudes, the drone/sampler remained stable.
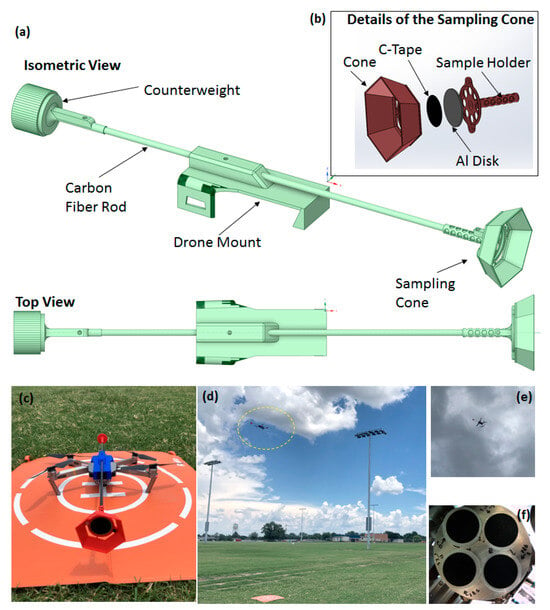
Figure 1.
Various aspects of the experimental setup. (a) Isometric and top view of the sampling drone mount; (b) exploded view with detailed components of the sampling cone assembly; (c) a photograph of the actual drone and sampling platform; (d) drone with mounted platform hovering at low altitude; (e) drone with mounted platform hovering at high altitude; (f) example sample disk for SEM analysis with 4 samples.
Samples were collected from two different types of plumes. The plume generated by professional fireworks is higher above the ground, at an altitude at or above 25 m, and is referred to as high-altitude sampling (HAS). The plume from personal fireworks is closer to the ground, at or below an altitude of 10 m, and is referred to as low-altitude sampling (LAS). The LAS plumes were formed mainly of smaller personal fireworks used by the general public in the park including sparking devices, firecrackers, and fountains while the HAS samples were primarily the larger-scale fireworks used as a part of the municipal display hosted by the city of Norman. Given the distinct nature of these two sources, one of the objectives of this study is to understand the type of particulates present in the two types of plumes emitted from these fireworks. In order to ensure particulate capture while remaining at a safe distance from the firework launch site, the drone was flown in a position downwind from the launch site, as seen in Figure 2. The plume generated by professional fireworks is higher above the ground (HAS) compared to the plume from personal fireworks that is closer to the ground (LAS). Therefore, the materials and methods required testing of the drone/collector before the firework event at various altitudes.
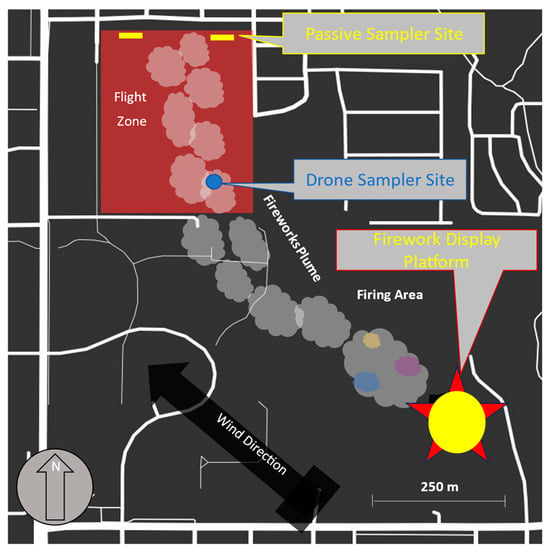
Figure 2.
Map of sampling location in relation to observed firework origin courtesy of Google Maps via atlist.com.
Once the samples were collected, they were kept in a desiccator and consolidated onto a larger platform for Scanning Electron Microscopy analysis, as shown in Figure 1f.
The samples underwent microscopy analysis with a Thermo-Fisher Scientific (Waltham, MA, USA) Quattro Scanning Electron Microscope (FE-ESEM) and focused primarily on conventional Everhart-Thornley (ETD, also known as Secondary Electron Imaging) and Concentric Back Scatter (CBS) methods for obtaining the physical characteristics of the sampled particulates. Prior to analysis, the samples were treated with iridium sputter-coating (Emitech K-575D, Ashford, UK) to allow for electrons to dissipate through the sample and avoid any charging artifacts that can occur in SEM samples that are not properly grounded. The microscopy analyses also included Electron Dispersal X-ray Spectroscopy (EDX) to obtain the chemical compositions of the particulates.
3. Results and Discussion
During the Independence Day firework celebration, the drone platform was successfully implemented to capture airborne particulate matter for later analysis. Samples were also taken on the day before the celebration in order to ensure there were no false conclusions made as a result of the analysis included in this work. SEM imaging and EDX analysis were used as a means of studying the captured particulate matter and a potential formation mechanism for the pollution in question has been developed and is presented herein.
3.1. Control Sampling
To establish a baseline for comparison, airborne particles were sampled the day before the festival in the same park that hosted the fireworks. Figure 3 shows SEM images of airborne particles “as-trapped” using the drone carbon tape system developed for this study. This baseline allowed for distinguishing firework-generated particles from other pollutants. Figure 3a is an SEM image of typical capture structure, which has a non-spherical shape with a “rocky-like” overall appearance. Figure 3(a2) shows a higher-magnification image of a selected area on the surface of the structure in Figure 3a, revealing both smooth and textured regions that appear porous in nature. The EDX point analysis conducted on a selected area of the particle in Figure 1a shows X-ray signatures for O, Na, Al, and Si (Figure 3(a3)). The peaks for Si and O are much more prominent than those for Na and Al, indicating that the analyzed region is predominantly composed of Si and O. The presence of a Na peak suggests some form of salt in the particle, while the Al peak could indicate environmental background pollution. Aluminum is commonly found in trace amounts in pollutants generated by automobile engines, which could explain its presence, as sampling was conducted near a large university campus with major roads nearby [29]. Figure 3b shows a SEM image of another group of non-spherical particles with two “rocky-like” appearance. The elemental analysis of this particle, shown in Figure 3(b2), reveals two clear peaks corresponding to O and Si. Figure 3c displays another type of “rocky-like” appearance, but it has a more elliptical shape and lacks the sharp edges or corners seen in the other trapped particles. The elemental analysis of this particle, seen in the spectrum in Figure 3(c2), reveals three peaks, with major peaks for O and Si, alongside a minor peak for Al. All three samples contained a majority of Si and O, suggesting the presence of SiO2 in these particles. This finding is supported by literature, as silicon dioxide is one of the most common minerals on Earth’s surface and a major component of various soil compositions worldwide [30]. The particles and elements in the control sample suggest that any detectable particulate matter in the environment on the sampling day is likely to be dust or dirt, swept up by wind.
Figure 3.
Assorted SEM images of representative particulates collected from airborne control samples: (a) single non-spherical particulate; (a2) high-resolution image of a selected area on the surface of the particulate in (a), corresponding to the white dashed arrow, revealing a porous region; (a3) EDX spectrum associated with the selected area (PRE1 Point), as indicated by the solid white arrow in (a); (b) SEM image of a pair of non-spherical particles; (b2) EDX spectrum associated with the particle in (b) (PRE2 Point), as indicated by the solid white arrow; (c) SEM image of another representative non-spherical particle collected in the control sample; (c2) EDX spectrum associated with a selected point (PRE3) in (c), as indicated by the solid white arrow in the corresponding SEM image.
3.2. Particulate Sizing
3.2.1. Low-Altitude Samples
Figure 4 shows SEM images of the airborne “as-trapped” particulates collected on disks at the low altitude sampling. It appears that the smoke plume resulted from personal fireworks (or samples from LAS) is composed of a variety of particulates (e.g., shape and size). Samples taken at the low altitude often featured particles with a “rocky” type construction as seen in Figure 4a,b,e. Figure 4a shows a large irregularly shaped structure with multiple sharp edges and a coarse surface texture. A higher resolution SEM image of the boxed area in Figure 4a shows that smaller shapeless particles are deposited on the surface of this larger structure (Figure 4(a2)). Figure 4b shows a relatively large cohesive particle with multiple facets and a smooth surface surrounded by smaller shapeless particles. This can be seen best in Figure 4c,d, especially within Figure 4c as many of the particulates imaged appear to be on the smaller end of the micro-scale and well below the threshold for PM2.5. The LAS group also had a larger number of sampled particulates, which has two potential causes.
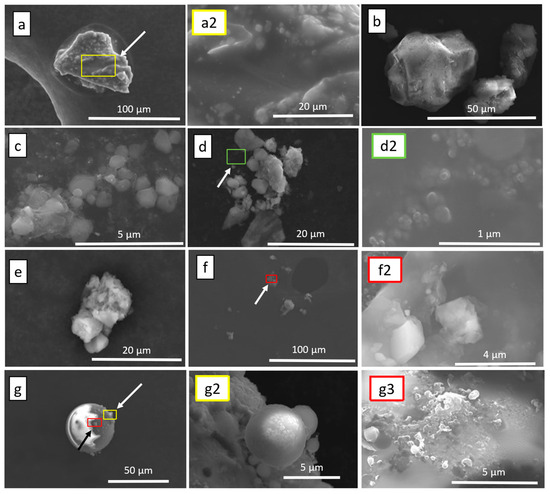
Figure 4.
Assorted SEM images of low-altitude sampled particulates; (a) single particulate with surface deposition; (a2) high-resolution image of particulate A deposited material corresponding with the white arrow of particle A; (b) particulate cluster; (c) large particulate cluster with many particles on a nano-scale; (d) particulate cluster; (d2) high-resolution image of particulate D with emphasis on splatter-formation of particulate matter corresponding with the white arrow of particulate D; (e) single particulate; (f) extended particulate cluster; (f2) high-resolution image of frame F showing smaller length scale corresponding with the white arrow of F; (g) spherical composite particulate; (g2) high resolution of smaller spherical particulates attached to frame G corresponding with the white arrow of particulate G; (g3) high resolution of non-spherical deposits on frame G corresponding with the black arrow.
The LAS samples were taken at a lower altitude closer to the source, meaning it was significantly easier to ensure that the drone platform remained fully within the plumes originating from the various fireworks sources. This trend was also supported by how LAS samples were taken earlier in the evening, as the smaller personal fireworks used by the general public were greater in number and much closer than their professional counterparts. The samples taken at this lower altitude also displayed a more diverse array of physical compositions as seen in Figure 4 with a diverse array of both sizes and shapes within the particulates collected. Many of these imaging results are similar in size and shape to particulate residue found in gunshot residue by Argente-García et al. in 2019 [31]. Their work was focused on differences between swabs of non-shooters and shooters in order to detect certain chemicals in the residue [31]. In their included SEM imaging of detected particulates in gunshot residue, many similar structures are seen which is reasonable considering that in both cases the propellant is the same compound—gunpowder [31].
In the LAS samples, it is observed that the morphology of the finer structures differs greatly between the samples collected. Figure 4a,g display larger particulates with smaller deposits of material, as indicated by the arrows in each Frame with higher resolution images focused on surface deposits seen in Figure 4(a2,g2,g3). Figure 4c,d display clusters composed of very fine particulate distribution, in a “splatter” formation where the particulates appear randomly distributed in a given area with little organization, including particulates that appear stacked on one another as seen in Figure 4c. Many of the particulates in Figure 4c,d appear to be of the same construction with many being hexagonal in shape, indicating that this could be a common particulate type being formed during the firework displays. It is theorized that these structures are indicative of sodium salts that are commonly used in sparklers and fountains, as seen later in the EDX maps of similar structures.
A close examination of the SEM images shows that the samples from personal fireworks consist of structures with various shapes and sizes. Particularly, the size distribution of the spheroidal structures is quite diverse. The spheroid depicted in Figure 4g has a diameter of about 40 μm. High-resolution SEM images of its surface reveal smaller microspheres deposited on the larger spheroid (Figure 4(g2)). The particle in Figure 4(g2) measures approximately 6 μm and is surrounded by spheres that are only a few micrometers in diameter (indicated by arrows in Figure 4(g2)). A proposed underlying growth mechanism of the structures is presented.
3.2.2. High-Altitude Samples
Figure 5 shows the SEM images of the airborne “as-trapped” particulates collected on disks at the HAS. It should be noted that, as seen in Figure 5(a2,a3,b2), these larger particles often had depositions of other particulates below these thresholds.
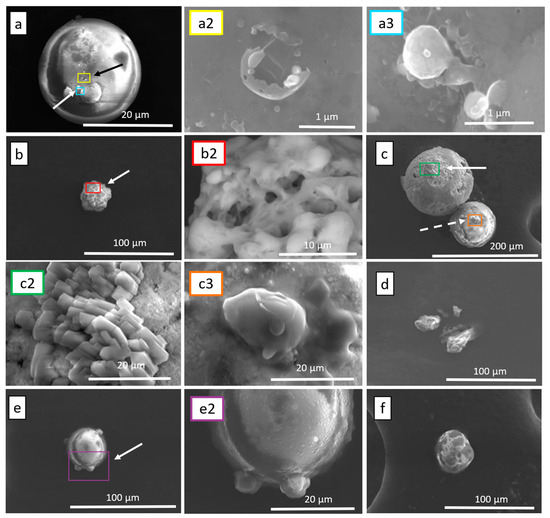
Figure 5.
Assorted SEM images of high altitude sampled particulates (a) compound spherical particulate with various surface deposits; (a2) hollow spherical surface deposit of frame A corresponding with black arrow; (a3) spherical deposits on the surface of frame A corresponding with the white arrow; (b) single semi-spherical particulate; (b2) high-resolution image of frame B showing hollow semi-spherical composition corresponding with white arrow; (c) double spherical particulate; (c2) crystalline surface deposits on frame C corresponding with white arrow; (c3) amorphous surface deposits on frame C corresponding with dashed white arrow; (d) particulate cluster; (e) single compound particulate; (e2) image focused on spherical deposits of frame E corresponding with white arrow; (f) single semi-spherical particulate.
A comparison between the two sampling altitudes (SEM images in Figure 4 and Figure 5), reveals some distinct aspects of the particulates. One notable difference is the difference in size. That is, the particulates from HAS are much larger in size. A second observation is that the particulates from HAS appear to have a more uniform shape. That is, the particles from this type of plumes (professional fireworks) have a spherical or near-spherical shape. The particulates sometimes were still compound, featuring smaller particulates deposited onto the larger surface as seen through the various arrows indicating regions of interest with higher magnification imaging of these regions (Figure 5a–c,e). These deposited particulates were also often spherical in shape but did not exclusively maintain this shape profile. For instance, Figure 5a shows a particulate that is approximately 20 μm in diameter with a smooth surface. Higher resolution SEM imaging on a selected area, the boxed area in Figure 5a, shows surface depositions that appear to be spherical in nature (Figure 5(a2,a3)). Other particulates from the HAS appear to be roughened (Figure 5(b,b2)). Figure 5c shows two large particles from HAS that are bonded (Figure 5c). The larger particle is approximately 150 μm in diameter while the smaller is approximately 85 μm in diameter. High-resolution SEM imaging, the selected area on the larger particle, shows that the deposits have a platelet-like structure (Figure 5(c2)). Meanwhile, the deposits on the smaller particles are more spherical (Figure 5(c3)). Other large particles in the HAS are coarse and have prominent irregularities or larger protrusions on its surface (Figure 5(e,e2)).
3.3. Elemental Analysis
3.3.1. High-Altitude Samples
Through the EDX maps, it is observed that many of the particulates appear to be well mixed chemically and contain regions with somewhat even distributions of different elements. This is true for the particle seen in Figure 6(a1) and the corresponding maps of oxygen, magnesium, barium, and aluminum of the particle.

Figure 6.
Energy dispersive X-ray (EDX) maps and EDX spectra collected on selected airborne particulate matter of high-altitude fireworks. (a1) Secondary electron SEM image of a porous-like particulate morphology; (a2–a5) EDX maps of O-K, Mg-K, Ba-L, and Al-K on the porous structure; (a6) associated EDX spectra; (b1) secondary electron SEM image of a spheroidal-like structure; (b2–b5) EDX maps of Mg-K, Ba-L, Al-K, and O-K; (b6) associated EDX spectra; (c1) secondary electron SEM image of large spheroidal with a smaller particle diffused to its surface; (c2–c6) EDX maps of elements present including Sr-K, O-K, Mg-K, Fe-K, Cu-K; (c7) overlaid EDX map showing the distinct elemental regions within the particle; (c8) is the spectra of the large spheroidal and diffused particle.
The same trend is seen in the particle imaged in Figure 6(b1), as its corresponding maps between Figure 6(b2–b5) show a nearly uniform distribution of the mapped elements within the nearly perfectly spherical particle. This observed uniform distribution is much more interesting, however, for the particle imaged in Figure 6(c1). In the corresponding maps of this particle, it is observed that there are regions of uniformity that contain certain specific elements while other regions feature other elements almost exclusively. Figure 6(c7) features an overlay of different maps with the observed regions outlined. These observed patterns in chemical composition, alongside the proposed formation mechanism of multistage particulate formation, could indicate that the particulate conglomeration process is not necessarily uniform within a given region.
As seen in the EDX analysis, various alkaline and transition metals including Al, Ba, Sr, Cu, and Fe were detected within the samples using both point analysis and elemental mapping as seen above in Figure 6(a2–a5,b2–b5,c2–c7). The presence of these elements is expected, as previously noted due to the role these elements serve in creating certain colors and effects within fireworks. It should be noted that many of the spectra produced during EDX analysis indicated the presence of iridium. This is a result of using iridium sputter-coating to reduce the effect of charging in the sample and enhance the quality of the imaging. Figure 6(a2–a5) indicate that the imaged particulate is a compound particulate with the signature of oxygen, magnesium, barium, and aluminum which suggests that these elements potentially came from a common origin and were mixed in a gaseous state prior to forming a particulate. A similar observation is made for the particulate featured in Figure 6(b2–b5) with the same elements being present in a different physical state suggesting entrapment by the airborne method either in a different state of formation or distinct conditions during particulate formation. The well-mixed nature of the various present elements indicates that the firework shells of origin were of distinct color and effect, as is to be expected in a large display of this nature. The theorized mechanism by which the particulates form is discussed in Section 3.4. Many of these particulates were simple in composition, consisting of two or three different metallic elements, while others, as seen in Figure 6(c2–c7), were far more complex, containing specific regions that were associated with certain elements. Figure 6(c1–c8) shows a secondary electron image of the particulate and comparing this against other images, specifically Figure 6(c4,c6), illustrating how the particulate’s magnesium and copper appear to occupy distinct regions of the particulate. The elemental peaks observed in the EDX spectra of Figure 6(a6,b6,c8) display prominent oxygen peaks with respect to the other present metals, indicating that there are likely metal oxides present in the observed particulates, though this cannot be fully verified without further analysis using Electron Energy Loss Spectroscopy (EELS). It should be noted that the spectra also feature a very prominent carbon peak while the elemental maps do not include carbon, and this is a result of the carbon tape used to trap the particulates rather than a reflection of the particulates themselves.
Exposure to certain heavy metals has been specifically highlighted in published medical literature due to the especially large risk from those particular elements [32]. Several metals identified through EDX analysis have been included as presenting risks for human health when present in elevated concentrations, specifically Fe and Al. Increased exposure to Fe, despite its crucial role within the human body, has been known to cause the presence of extra radicals that damage biomolecules, cells, and tissue within the body. Aluminum exposure presents a risk mainly for those with preexisting conditions like kidney disease, but extended exposure has also been tied to other conditions like aluminum-induced adynamic bone disease and aluminum-induced osteomalacia. For the majority of the population, however, brief exposure to aluminum can cause nausea, ulcers, and vomiting. These symptoms are reported to be brief and mild, unlike the more potent risks of extended exposure.
3.3.2. Low-Altitude Samples
Through the above EDX maps, it is observed that the particulates sampled at high altitudes appear much less chemically diverse than the low-altitude samples. Low-altitude samples were also observed to have a significant amount of potassium within the particulates, which is consistent with these particles coming from the gunpowder used in lift charges for smaller mortar-type fireworks or the use of potassium as oxidizers in sparkler fireworks [33].
Figure 7(a4,c4) give EDX maps for potassium in these samples, and it is theorized that considering the closer proximity to the origin, these samples were able to capture these potassium particles much more effectively, as potassium is commonly utilized in the propellant for larger fireworks, as well as a fuel source for smaller handheld firework options. Figure 7(a1–a5) indicate that the mapped particulates are very well mixed chemically, with aluminum, oxygen, silicon, and potassium being the materials making up the particulates. This suggests that the low-altitude samples contain a mixture of elements used in various aspects of these fireworks, specifically elements tied to smaller sparklers or fountains, which is consistent with observed differences between higher and lower-altitude fireworks on the day of the celebration. The EDX spectrum seen in Figure 7(a6) has strong peaks for the elements included in the elemental maps, especially oxygen and silicon, indicating much of the imaged particulate can be silicon dioxide combined with other compounds in the particulate. The EDX maps also feature a strong correlation between sodium and chlorine in Figure 6(b2,b3), which suggests that sodium chloride was used in some of the lower fireworks that were used. This conclusion is supported by the EDX spectrum seen in Figure 6(b4), where both sodium and chlorine are the largest peaks aside from the background peaks of carbon and oxygen.
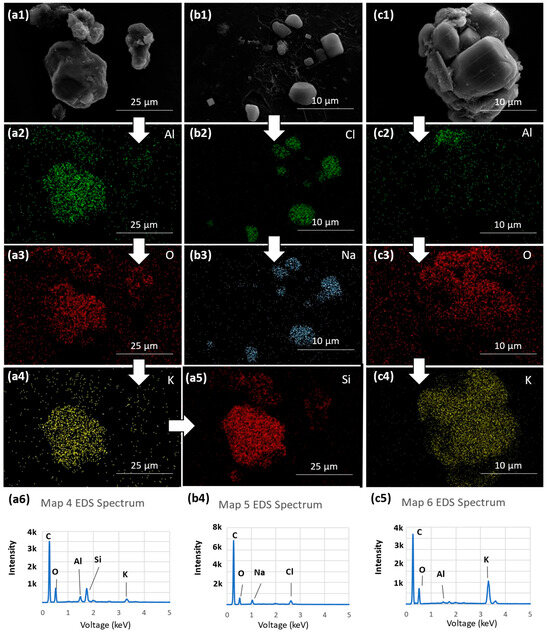
Figure 7.
Energy dispersive X-ray (EDX) maps and EDX spectra collected on selected airborne particulate matter of low-altitude fireworks. (a1) Secondary electron SEM image of a region with 5 grouped particles; (a2–a5) EDX maps of Al-K, O-K, K-K, Si-K in the grouped structure; (a6) associated EDX spectra; (b1) secondary electron SEM image of a grouped “splatter” region (b2,b3) EDX maps of Cl-K and Na-K; (b4) associated EDX spectra; (c1) secondary electron SEM image of compound granular particle; (c2–c4) EDX maps of elements present including Al-K, O-K, K-K; (c5) associated EDX Spectra.
This is also supported by the known literature, as certain sodium salts including sodium chloride are often used to create yellow effects, as would commonly be seen in fountain-style fireworks [34]. Across all samples, the presence of eight different metals are confirmed that there are either components in the fireworks’ propellant or a metal used for its light properties as a part of the display. These detected elements were potassium, sodium, barium, calcium, magnesium, iron, strontium, and titanium. Each of these detected metals has a role in creating certain colors or effects in firework displays and, as a result, all are expected to be found within the collected samples [34]. Figure 7(c1–c4) indicate a different process is taking place, with a shared region of potassium and oxygen (Figure 7(c3,c4)) while there is a distinct region for aluminum as seen in Figure 7(c2). This particulate region displays a very prominent peak for potassium alongside a smaller aluminum peak which likely indicates that it originates from a gunpowder compound used in one of the low-altitude fireworks with smaller depositions (Al) on the larger existing particulate. This is more consistent with the observed patterns of Figure 4, with distinct elemental depositions on existent particulates. This theorized formation mechanism is described in the following section.
3.4. Proposed Growth Mechanism
A possible growth mechanism for the formation of particulates from fireworks is proposed in this study. It can be hypothesized that all structures (e.g., microspheres, irregular, and finer particles) are formed instantaneously during the reaction zone or burning of the fireworks, regardless of whether they are generated from professional or personal fireworks. Although the temperature and chemical species present in professional fireworks are generally much stronger due to the potent and energetic chemical compounds, they can contribute to higher combustion temperatures and more intensive reactions. A comparison of the SEM analysis conducted on collected samples from professional fireworks (HAS) and personal fireworks (LAS) shows that, overall, (i) particles from the professional fireworks are much larger in size and more spherical compared to those from the personal fireworks (Table 2); (ii) both types of fireworks exhibit a common trend where some of the larger particles have finer particulates deposited on their surfaces; and (iii) there is evidence that the plumes in both types of fireworks contain spheres that are hollow or core–shell, resembling bubble dynamics. Figure 8(a1–a18,b,c1–d2) illustrate the schematics of the two types of fireworks: HAS and LAS. Specifically, Figure 8(a1–a4) depict the firing process of professional fireworks, where mortar-style mechanisms are frequently used. Figure 8(a4) shows a series of images that such fireworks result from explosions. Figure 8(a5) is an illustration of the applied airborne collection in this study. The SEM analysis of the airborne samples indicates that the formation of the microspheres may occur via the Ostwald Ripening effect through contact recrystallization [35]. During the firework explosion, the intense heat can cause various metal compounds to vaporize or sublimate and then condense as they cool. Additionally, solid particulates undergoing a heating process can become hollow core–shell structures [36]. In materials science, it is postulated that large particles grow at the expense of smaller particles of the same solid species [37]. The SEM analysis of the airborne samples from professional fireworks reveals that most of the trapped particles are large and spherical in shape. Figure 8(a6) shows a sphere approximately 20 μm in diameter with several smaller spherical particles deposited on its surface. Figure 8(a7) represents a higher-resolution SEM image of the boxed area in Figure 8(a6), revealing morphological details of the smaller spheres. It is evident that the microsphere has several smaller spheres attached to its surface (Figure 8(a7)). The variation in the diameter of the smaller particles may be because, during the growth process, the smaller particles continuously shrink as they are consumed by the larger particles. The smaller particles have different diameters, with the largest approximately two micrometers in size. Backscattering SEM imaging analysis reveals that both large and smaller particles have the same contrast, indicating a uniform material composition (Figure 8(a8)). This strongly suggests that the growth of the larger particles resulted from the coalescence of smaller particles into larger ones (Figure 8b). Another feature observed in the samples from HAS is the appearance of microspheres with core–shell morphology. This is evident from the openings on the surfaces of the microspheres (Figure 8(a9–a12)). Some microspheres have various openings (Figure 8(a10–a12)). Large, smooth, and perfectly spherical structures without openings and condensate materials on their surfaces are frequently encountered in analysis of the airborne samples (Figure 8(a13)). Figure 8(a14) is an SEM image of a fractured microsphere. Close inspection of the fracture region indicates that this microsphere is solid. However, scanning its surface shows initial openings (Figure 8(a15)). This indicates that the core–shell morphology of the microspheres follows after the coalescence process. Pyrotechnics are highly energetic compounds, and during the explosion, these materials burn to generate volatile gasses that expand and create pressure within the particles. Temperature, phase transformation, and heat transfer in and around a particle are crucial factors influencing the growth process. Lebel et al. (2013) were able to successfully measure the temperatures within an explosive fireball based on the light emissions from the fireball itself [38]. It was reported that the temperatures inside the fireball are in the range of 1600 K to 1900 K with a peak of 1850 K occurring 12 milliseconds after the explosion, with a uniform temperature distribution throughout the fireball. Additionally, Grima et al. (2012) demonstrated that the physical (spheroidal, solid, and hollow) and chemical morphology of firework residue is similar to that found in gunshot residue [27]. It is reasonable to suggest that during the explosion of the fireworks, as nucleation and growth occur (depending on the explosion’s location), there is a uniform particle volume temperature. Nucleation and growth begin at the surface of the sphere. However, the temperature at the center can be significantly higher than at the surface, which is continuously cooled by convection and radiation. As the spheroidal particles are carried away from the reaction zone by the plume toward cooler regions, volatile gasses become trapped, resulting in the formation of hollow or core–shell morphology.

Table 2.
LAS and HAS sizing comparison.
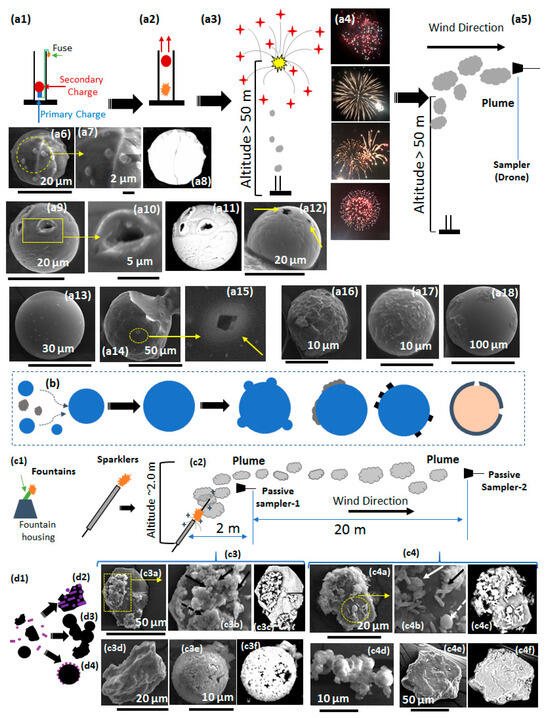
Figure 8.
Various firework schematics (a1–a4) mortar-style firework function process; (a5) various effects produced in mortar-style fireworks; (b) schematic illustrating the growth of microspheres resulted from coalesce; (a6–a18) SEM images of the various morphologies of the microspheres supporting the growth mechanism; (c1,c2) sparkler and fountain function process; (d1–d4) schematic illustrating the growth process; (c3,c4) SEM images of the various morphologies of the structures at 2 m and 20 m from the reaction zone, respectively.
Other surrounding vapors can act as the source material for the finer particles. In the airborne samples, crystallization of these finer particles on the surface of the microspheres is evident (Figure 8(a16–a18)). The larger surface areas of these structures provide ideal sites for the recrystallization of “leftover” vapors from the explosion as they condense. A similar mechanism has been proposed for the formation of finer materials on the surfaces of larger structures found in ashes formed during volcanic explosions (Moran et al., 2023) [39]. Oderji, H et al. (2016) conducted laser-induced explosions on the surface of transition metal specimens revealing that vapors or sources of material for recrystallization of particulates were formed through sublimation [40]. To further support the proposed mechanism for the formation of structures in the HAS, LAS (near ground level) was conducted using passive samplers installed at two different locations relative to the reaction zone, in the direction of the wind. The plumes released from the personal fireworks are very close to the ground, making them ideal for this study. Figure 8(c2) depicts the firing process of personal fireworks (mostly sparklers and fountains) and the collection process. One passive sampler was located about 2 m from the reaction zone (at a height of ~1 m), while the other was about 20 m away (at a height of ~2 m) (Figure 8(c2)). The smoke plume from the LAS passive samplers appears to be composed of a greater variety of particulates compared to the HAS. SEM analysis of the passive sampler located 1 m from the reaction zone reveals the presence of a heterogeneous microstructure, including spheroidal, irregular or shapeless, and fine materials. The set of images in Figure 8(c3a–c3f) displays SEM images of particulates collected at a distance of approximately 2 m and a height of 1 m from the reaction zone. Figure 8(c3a) shows a hexagonal platelet-like structure (~50 μm along the axis and 40 μm across) with a high deposition of condensate particulates on its upper surface. A high-resolution SEM image of the boxed area in Figure 8(c3a) shows that the condensate particles consist of finer spherical shapes and irregular or shapeless structures (Figure 8(c3b)). The samples collected from this close location to the reaction zone also show the presence of very large irregular structures (e.g., those with sharp and pointy edges) without finer materials deposited on their surfaces (Figure 8(c3d)). The size of this irregular structure is approximately 25 μm along the axis and about 18 μm across (Figure 8(c3d)). The presence of spherical-shaped particulates with condensate or crystallized fine particles on their surfaces is evident (Figure 8(c3e,c3f)).
The set of images of Figure 8(c4a–c4f) represents SEM images of particulates collected at approximately 20 m and a height of 2 m from the reaction zone. Higher-resolution SEM imaging on the condensed structures show that they are composed of spheroidal (white dotted arrow), slender platelets (black solid arrow), and shapeless particles (white solid arrow) (Figure 8(c4b)). Large irregular structures with sharp and pointy edges and minimal condensate particles are also present (Figure 8(c4e,c4f)). At this distance, agglomerated particles forming clusters are evident (Figure 8(c4d)). The cluster in Figure 8(c4d) is mainly composed of spheroidal shapes, each only a few microns in diameter.
Analysis of passive samplers placed at 22 m from the reaction zone indicates that their physical morphology is very similar to those collected and analyzed at 2 m (set of images of Figure 8(c3) and 8(c4), respectively). Specifically, the presence of platelet-like structures and finer condensate formations is a typical characteristic observed in the samples. It is theorized that these clusters may form from the collision of particulates as they are carried within the plume. Professional fireworks contain more energetic compounds, making them more explosive and resulting in higher temperatures. These results strongly support the presented hypothesis of a two-step formation process: the first step involves the formation of large structures, and the second step is the condensation of volatile gasses on the surfaces of the larger structures, leading to the nucleation of finer particles. The spherical particles in personal fireworks are smaller due to the lack of a coalescence process, which is limited by the nature of personal fireworks (e.g., being less explosive).
The HAS samples, however, did not exhibit these characteristics and were instead formed into large spheres that were less concentrated than in the LAS samples. This finding is supported by the same line of logic, considering that the origin of the HAS particulates was an explosive reaction intended to disperse combustible transition metals to create the glow effect that fireworks of this style are famous for [41]. Considering these two vastly different genesis processes for the particulates, the observed discrepancy in average particulate sizing between the two sample groups is a reasonable expectation and finding.
The detected presence of particulates significantly varying in size is consistent with the previous literature regarding firework smoke including the article regarding Diwali Celebrations in Kolkata by Saha [22]. As shown in the Supplemental Figure S1, there was an increase in the concentrations of both PM10 and PM2.5 around the time of the fireworks display. The data in the supplementary plots were measured optically using a Teledyne (Thousand Oaks, CA, USA) Model T640 PM mass monitor. For example, at 21:00 (9:00 PM) on 4 July, the concentration of PM2.5 raised to 25 µg m−3, up from around 8 µg m−3 at 18:00 (6:00 PM), with an annual average of 10.5 µg m−3. However, for PM10, several peaks were observed at different times. Specifically, at 21:00 (9:00 PM) on 4 July, the concentration of PM10 increased to 34 µg m−3, up from about 15 µg m−3 (Figure S1). The annual average for PM10 is 26 µg m−3. Particles larger than 10 μm (shapeless) are typically fugitive dust carried by winds from roadways, fields, and construction sites, and they correlate well with the morphology of particulates in the control samples. Analysis of control samples revealed airborne particulate matter on the PM10 scale but not the PM2.5 scale, while many particles captured during the fireworks display were on the PM2.5 scale, which is consistent with studies from Gonzalez and Saporito [42,43]. The measured concentrations for each type of particulate matter are consistent with the observed patterns in particulate size from the day of the festival, supporting the conclusion that fireworks displays specifically increase the concentration of PM2.5 in the surrounding environment. Additionally, most of the particles collected from high-altitude sampling of the fireworks have a spheroidal shape and unique chemical composition.
It should be noted, however, that the PM data presented in the plot were collected approximately 10 miles away in the same metropolitan area as the firework display rather than its immediate vicinity. Previous studies of particulate matter dispersion have determined that for low wind speeds (at or below 4 km per hour), very limited particulate dispersion occurs, and pollution is likely to remain localized [44,45]. As seen in Figure S2, the wind speed was under 1 m per second (3.6 km h−1) at the time of sampling, meaning extended dispersion of particulate matter was unlikely to occur. As a result, the PM concentrations near the firework display were likely much higher than the reported values due to the limited wind speeds.
4. Conclusions
This study presents a newly developed and unique approach to the analysis of airborne particulate pollution resulting from the large-scale use of fireworks in a holiday setting. The novel use of drones as a method of particulate capture offers a new perspective on these pollutants. The presented system of particulate capture on carbon tape also facilitates direct analysis of captured particulates in an SEM without visual interference from fibers located on the filter as would be present in a pumping system. EDX analysis indicates the presence of various metallic elements including Sr, Ba, Al, Mg, Fe, and Cu which are all known to be ingredients in fireworks as they are used for certain colors and effects. This work’s findings confirm that these elements are making their way into the environment of the region in which they are used.
The novel approach also allowed for the development of a proposed formation mechanism by which the pollution from fireworks forms after their use. By sampling pollutants from various distances relative to the launch site as well as different heights, this work has created a new understanding of the processes that govern the formation of these particulates. This new area of unexplored investigation will allow for further research into how particulates form in explosive environments. Without the use of a drone-based approach to sampling, the insights presented here would not have been possible. This study was, however, limited in certain respects. Lacking measurements for various meteorological parameters limited the scope of this study. There was also a limitation because of the use of a single drone rather than multiple drones for sample collection. Due to the fact that the firework display was limited in time, using more than one drone would greatly increase the sampling capacity of this study. The aluminum disks upon which samples were collected needed to be changed during the display, greatly decreasing the effectiveness of the platform. Future investigations of firework particulate pollution would benefit from addressing these limitations and optimizing the sampling procedure to maximize the effectiveness of the drone platform.
In summary, this study provides an exploration of particulate pollution from firework use via a newly created sampling method using drones for airborne particulate capture. A proposed formation mechanism for this type of pollution to offer further insight into the underlying processes at work is also presented.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15063151/s1, Figure S1. Hourly Particulate Matter measurements taken near the sampling site of this study courtesy of the Oklahoma Department of Environmental Quality’s Air Quality Division. Figure S2. Hourly wind speed and direction vectors in Norman, OK near the sampling site of this study (courtesy of ambientweather.net) with integrated PM2.5 concentration values.
Author Contributions
Conceptualization, J.-T.M., M.L., F.S.S., D.P.A. and W.M.-M.; methodology, J.-T.M., M.L., F.S.S., D.P.A. and W.M.-M.; software J.-T.M., M.L. and W.M.-M.; validation, J.-T.M., M.L. and W.M.-M.; formal analysis, J.-T.M., M.L., F.S.S., D.P.A. and W.M.-M.; investigation, J.-T.M., M.L., F.S.S., D.P.A. and W.M.-M.; resources W.M.-M.; data curation, J.-T.M., M.L., F.S.S., D.P.A. and W.M.-M.; writing—original draft preparation, J.-T.M., M.L. and W.M.-M.; writing—review and editing, J.-T.M., M.L. and W.M.-M.; visualization, J.-T.M., M.L. and W.M.-M.; electron microscopy, J.-T.M., F.S.S., D.P.A.; supervision, W.M.-M.; project administration, W.M.-M.; funding acquisition, W.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was supported by the National Science Foundation REU-Site: Unmanned Aerial Systems with Real-World Applications in Oklahoma. Award Number 2150365.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article may be made available by the authors on request.
Acknowledgments
We would like to thank Kenneth Carson, who oversees the operation of unmanned aerial systems (UAS) on University-owned or University-managed property, for helping secure the drone flying permits for trapping particulates from the fireworks. We would also like to express appreciation to Preston Larson from the Samuel Roberts Noble Electron Microscopy Laboratory at the University of Oklahoma for his assistance in operating the Thermo-Fischer Scientific Quattro SEM, expert guidance in electron microscopy techniques, expertise in materials science, and continued support with microscopy. We would like to thank Daniel Moran-Zuloaga from Johannes Gutenberg University of Mainz and the Escuela Superior Politécnica del Litoral (ESPOL) for his assistance and for sharing his experience in real-time continuous monitoring of PM and wind speeds/direction. W.M.-M., J.-T.M., M.L., F.S.S., D.P.A., gratefully acknowledges the support for this work from the National Science Foundation REU-Site Award Number: 2150365.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| UAV | Unmanned Aerial Vehicle |
| SEM | Scanning Electron Microscopy |
| EDX/EDS | Energy Dispersive X-Ray Spectroscopy |
| TEM | Transmission Electron Microscopy |
| LAS | Low-Altitude Sample |
| HAS | High-Altitude Sample |
| PM | Particulate Matter |
| VOCs | Volatile Organic Compounds |
| XRF | X-Ray Fluorescence Spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
References
- Tiwari, S.; Chate, D.; Srivastava, M.; Safai, P.; Srivastava, A.; Bisht, D.; Padmanabhamurty, B. Statistical evaluation of PM 10 and distribution of PM 1, PM 2.5, and PM 10 in ambient air due to extreme fireworks episodes (Diwali festivals) in megacity Delhi. Nat. Hazards 2012, 61, 521–531. [Google Scholar] [CrossRef]
- Nasir, U.P.; Brahmaiah, D. Impact of fireworks on ambient air quality: A case study. Int. J. Environ. Sci. Technol. 2015, 12, 1379–1386. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Chen, J.; Mellouki, A.; Jiang, P.; Gao, Y.; Li, Y.; Yang, Y.; Wang, W. Influence of fireworks displays on the chemical characteristics of PM2.5 in rural and suburban areas in Central and East China. Sci. Total Environ. 2017, 578, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Huang, K.-L.; Chen, H.-L.; Tsai, J.-H.; Chiu, Y.-P.; Lee, J.-T.; Chen, S.-J. Influences of Beehive Firework Displays on Ambient Fine Particles during the Lantern Festival in the YanShuei Area of Southern Taiwan. Aerosol Air Qual. Res. 2014, 14, 1998–2009. [Google Scholar] [CrossRef]
- Godri, K.; Green, D.; Fuller, G.; Dall’Osto, M.; Beddows, D.; Kelly, F.; Harrison, R.; Mudway, I. Particulate oxidative burden associated with Firework activity. Environ. Sci. Technol. 2010, 44, 8295–8301. [Google Scholar] [CrossRef]
- Hamad, S.; Green, D.; Heo, J. Evaluation of health risk associated with fireworks activity at Central London. Air Qual. Atmos. Health 2016, 9, 735–741. [Google Scholar] [CrossRef]
- Plimpton, G. Fireworks; Doubleday & Co.: New York, NY, USA, 1984; p. 286. [Google Scholar]
- Tanda, S.; Ličbinský, R.; Hegrová, J.; Goessler, W. Impact of New Year’s Eve fireworks on the size resolved element distributions in airborne particles. Environ. Int. 2019, 128, 371–378. [Google Scholar] [CrossRef]
- Retama, A.; Neria-Hernandez, A.; Jaimes-Palomera, M.; Rivera-Hernandez, O.; Sanchez-Rodriguez, M.; Lopez-Medina, A.; Velasco, E. Fireworks: A major source of inorganic and organic aerosols during Christmas and New Year in Mexico City. Atmos. Environ. X 2019, 2, 100013. [Google Scholar] [CrossRef]
- Seidel, D.; Birnbaum, A. Effects of Independence Day fireworks on atmospheric concentrations of fine particulate matter in the United States. Atmos. Environ. 2015, 115, 192–198. [Google Scholar] [CrossRef]
- Hoyos, C.; Herrera-Mejia, L.; Roldan-Henao, N.; Isaza, A. Effects of fireworks on particulate matter concentration in a narrow valley: The case of the Medell´ın metropolitan area. Environ. Monit. Assess. 2020, 192, 6. [Google Scholar] [CrossRef]
- Berger, B. Parameters Influencing the Pyrotechnic Reaction. Propellants Explos. Pyrotech. 2005, 30, 27–35. [Google Scholar] [CrossRef]
- Chhabra, A.; Turakhia, T.; Sharma, S.; Saha, S.; Iyer, R.; Chauhan, P. Environmental impacts of fireworks on aerosol characteristics and radiative properties over a mega city, India. City Environ. Interact. 2020, 7, 100049. [Google Scholar] [CrossRef]
- Ambade, B. The air pollution during Diwali festival by the burning of fireworks in Jamshedpur city, India. Urban Clim. 2018, 26, 149–160. [Google Scholar] [CrossRef]
- Kong, S.; Li, X.; Li, L.; Yin, Y.; Chen, K.; Yuan, L.; Zhang, Y.; Shan, Y.; Ji, Y. Variation of polycyclic aromatic hydrocarbons in atmospheric PM2.5 during winter haze period around 2014 Chinese Spring Festival at Nanjing: Insights of source changes, air mass direction and firework particle injection. Sci. Total Environ. 2015, 520, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Moreno, T.; Querol, X.; Alastuey, A.; Minguillon, M.-C.; Pey, J.; Rodriguez, S.; Miro, J.-V.; Felis, C.; Gibbons, W. Recreational atmospheric pollution episodes: Inhalable metalliferous particles from firework displays. Atmos. Environ. 2007, 41, 913–922. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Chien, L.-H.; Yuan, C.-S.; Lin, Y.-C.; Jen, Y.-H.; Ie, I.-R. Influences of fireworks on chemical characteristics of atmospheric fine and coarse particles during Taiwan’s Lantern Festival. Atmos. Environ. 2012, 62, 256–264. [Google Scholar] [CrossRef]
- Feng, J.; Yua, H.; Su, X.; Liu, S.; Li, Y.; Pan, Y.; Sun, J.-H. Chemical composition and source apportionment of PM2.5 during Chinese Spring Festival at Xinxiang, a heavily polluted city in North China: Fireworks and health risks. Atmos. Res. 2016, 182, 176–188. [Google Scholar] [CrossRef]
- Singh, D.-P.; Gadi, R.; Mandal, T.-K.; Dixit, C.-K.; Singh, K.; Saud, T.; Singh, N.; Gupta, P. Study of temporal variation in ambient air quality during Diwali festival in India. Environ. Monit. Assess. 2010, 169, 1–13. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Yan, L.; Dong, C.; Wang, W. Individual Metal-Bearing Particles in a Regional Haze Caused by Firecracker and Firework Emissions. Sci. Total Environ. 2013, 443, 464–469. [Google Scholar] [CrossRef]
- Yan, C.; Chen, H.; Xu, Q.; Zhou, Y.; Zhou, R.; Li, R.; Zheng, M.; Xie, M.; Jiang, B.; Zhang, Z.; et al. New insights into the influences of firework combustion on molecular composition and formation of sulfur- and halogen-containing organic compounds. Sci. Total Environ. 2024, 932, 172929. [Google Scholar] [CrossRef]
- Saha, U.; Talukdar, S.; Jana, S.; Maitra, A. Effects of Air Pollution on Meteorological Parameters During Diwali Festival Over an Indian Urban Metropolis. Sci. Total Environ. 2014, 98, 530–539. [Google Scholar]
- Parra, R.; Saud, C.; Espinoza, C. Simulating PM2.5. Concentrations duing New Year in Cuenca, Ecuador: Effects of Advancing the Time of Burning Activities. Toxics 2022, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kamp, D.; McKendry, I.; Wong, M.; Stull, R. Lidar ceilometer observations and modeling of a fireworks plume in Vancouver, British Columbia. Atmos. Environ. 2008, 42, 7174–7178. [Google Scholar] [CrossRef]
- Kosanke, K.L.; Kosanke, B.J.; Dujay, R.C. Pyrotechnic Reaction Residue Particle Identification by SEM/EDS. J. Pyrotech. 2001, 13, 40–53. [Google Scholar]
- Castro, K.; de Vallejuelo, S.; Astondoa, I.; Gonic, F.; Madariaga, J.-M. Analysis of confiscated fireworks using Raman spectroscopy assisted with SEM-EDS and FTIR. J. Raman Spectrosc. 2011, 42, 2000–2005. [Google Scholar] [CrossRef]
- Grima, M.; Butler, M.; Hanson, R.; Mohameden, A. Firework displays as sources of particles similar to gunshot residue. Sci. Justice 2012, 52, 49–57. [Google Scholar] [CrossRef]
- Mishra, S.; Khosla, D.; Arora, M.; Sharma, C.; Prasad, M.; Aggarwal, S.; Gupta, B.; Radhakrishnan, S.; Guleria, R.; Kotnala, R. SEM-EDS and FTIR Characterization of Aerosols during Diwali and Post Diwali Festival over Delhi: Implications to Human Health. J. Environ. Nanotechnol 2016, 5, 12–26. [Google Scholar]
- Anwar, K.; Ejaz, S.; Ashraf, M.; Ahmad, N.; Javeed, A. Monitoring trace elements generated by automobiles: Air pollutants with possible health impacts. Environ. Sci. Pollut. Res. 2013, 20, 4574–4586. [Google Scholar] [CrossRef]
- Gu, J.; Cai, X.; Wang, Y.; Guo, D.; Zeng, W. Evaluating the Effect of Nano-SiO2 on Different Types of Soils: A Multi-Scale Study. Int. J. Environ. Sci. Technol. 2022, 19, 16805. [Google Scholar] [CrossRef]
- Argente-García, A.; Hakobyan, L.; Guillem, C.; Campíns-Falcó, P. Estimating Diphenylamine in Gunshot Residues from a New Tool for Identifying both Inorganic and Organic Residues in the Same Sample. Separations 2019, 6, 16. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.; Beeregowda, K. Toxicity, mechanism, and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Arumugachamy, A.; Russel, N.; Rajamanickam, A. A preliminary study of combustion flame by digital image processing and residue for fireworks flashpowder. ISA Trans. 2024, 153, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, H.; Berger, B. Pyrotechnics in Fireworks. CHIMIA 2004, 58, 369. [Google Scholar] [CrossRef]
- Geng, L.; Liu, Q.; Chen, J.; Jia, P.; Ye, H.; Yan, J.; Zhang, L.; Tang, Y.; Huang, J. In situ observation of electrochemical Ostwald ripening during sodium deposition. Nano Res. 2022, 15, 2650–2654. [Google Scholar] [CrossRef]
- Fedraza, F.; Podor, R. Influence of annealing conditions on the formation of hollow Al2O3 microspheres studied by in situ ESEM. Mater. Charact. 2016, 113, 198–206. [Google Scholar] [CrossRef]
- Sugimoto, T. Chapter 4-Recrystallization. In Monodispersed Particles, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 167–179. [Google Scholar]
- Lebel, L.; Brousseau, P.; Erhardt, L.; Andrews, W. Measurements of the Temperature Inside an Explosive Fireball. J. Appl. Mech. 2013, 80, 031702. [Google Scholar] [CrossRef]
- Moran-Zuloaga, D.; Merchan-Merchan, W.; Rodriguez-Caballero, E.; Mulas, M.; Hernick, P. Long-range transport and microscopy analysis of Sangay volcanic ashes in Ecuador. Air Qual. Atmos. Health 2023, 17, 155–175. [Google Scholar] [CrossRef]
- Oderji, H.; Farid, N.; Sun, L.; Fu, C.; Ding, H. Evaluation of explosive sublimation as the mechanism of nanosecond laser ablation of tungsten under vacuum conditions. Spectrochim. Acta Part B At. Spectrosc. 2016, 122, 1–8. [Google Scholar] [CrossRef]
- Martín-Alberca, C.; García-Ruiz, C. Analytical Techniques for the Analysis of Consumer Fireworks. TrAC 2014, 56, 27–36. [Google Scholar] [CrossRef]
- Gonzalez, A.; Boies, A.; Swanson, J.; Kittelson, D. Measuring the effect of fireworks on air quality in Minneapolis, Minnesota. SN Appl. Sci. 2022, 4, 142. [Google Scholar] [CrossRef]
- Saporito, A.; Gordon, T.; Kim, B.; Huynh, T.; Khan, R.; Raja, A.; Terez, K.; Camacho-Rivera, N.; Gordon, R.; Gardella, J.; et al. Skyrocketing pollution: Assessing the environmental fate of July 4th fireworks in New York City. J. Expo. Sci. Environ. Epidemiol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hilker, N.; Wang, J.M.; Jeong, C.-H.; Healy, R.M.; Sofowote, U.; Debosz, J.; Su, Y.; Noble, M.; Munoz, A.; Doerksen, G.; et al. Traffic-related air pollution near roadways: Discerning local impacts from background. Atmos. Meas. Tech. 2019, 12, 5247–5261. [Google Scholar] [CrossRef]
- Coccia, M. The effects of atmospheric stability with low wind speed and of air pollution on the accelerated transmission dynamics of COVID-19. Int. J. Environ. Stud. 2020, 78, 1–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).