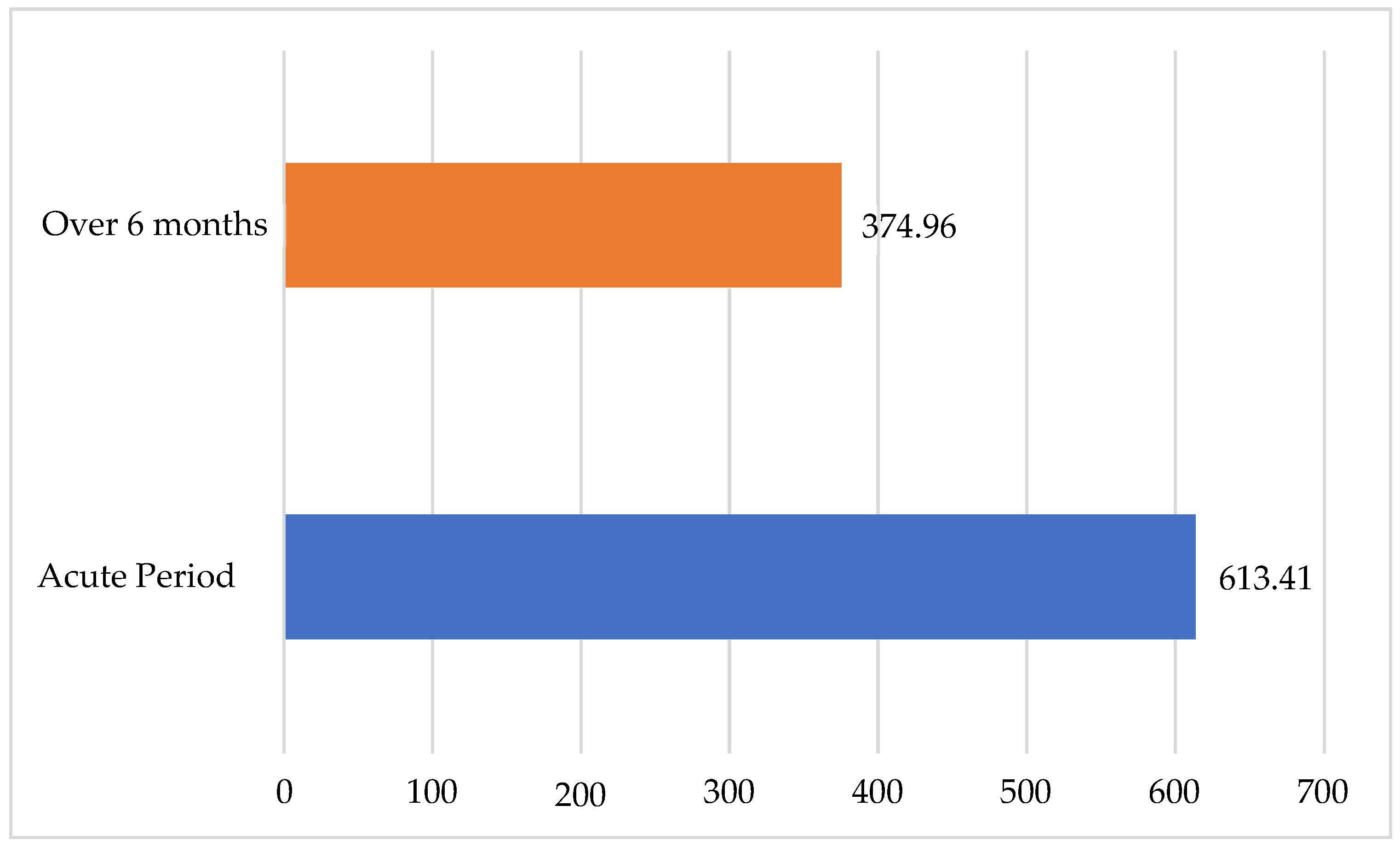1. Introduction
Stroke is a rare condition in children and adolescents, often underestimated, but it has a significant impact on morbidity and mortality. The incidence of pediatric stroke is 2–13/100,000 for ischemic stroke (IS), 1–5/100,000 for hemorrhagic stroke, and 0.67/100,000 for cerebral venous thrombosis (CVT) [
1]. IS most commonly occurs in the prenatal period and within the first 28 days after birth, with a frequency of 1 in 4000 live births [
1]. Fetal IS occurs between the 14th week of gestation and birth. This condition is caused by an ischemic lesion that can occur from 20 weeks of gestation to 28 days postnatal, with an occurrence rate of 1 in 2300–5000 live births and an estimated annual mortality rate of 3.49/100,000 [
1]. Neonatal IS, a subcategory of pediatric IS, leads to significant morbidity and long-term neurological and cognitive deficits, including cerebral palsy, epilepsy, neurodevelopmental disabilities, behavioral disorders, and vision and language impairment. In some children, neonatal IS is recognized retrospectively when hemiparesis or seizures occur [
2].
The study of the epidemiology, determinants, and predictive aspects of pediatric Disability-Adjusted Life Years (DALYs) is a current issue in contemporary neurology, both locally and globally. The incidence of neonatal IS is increasing in the Republic of Moldova, highlighting the need for research on the predictive and therapeutic aspects of IS in children during early developmental stages to optimize the medical, psychological, and educational resources.
As defined by the World Health Organization (WHO), stroke is a vascular disease characterized by the rapid-onset focal or global impairment of brain activity, which can lead to death, with symptoms lasting at least 24 h. This definition also includes arterial occlusion or venous thrombosis leading to IS [
3].
According to the WHO classification, stroke can be categorized into several types.
Transient ischemic stroke (TIS): Ischemia in a localized area of the brain with a motor deficit lasting up to 24 h (minutes to hours), without neurological sequelae [
4]. Involutional stroke: Acute cerebral ischemia with a motor deficit lasting more than 24 h, with complete resolution within 21 days [
4]. Lacunar IS: A type of stroke in which the blood flow is blocked in a group of very small arteries within the brain, primarily those supplying deep brain areas. Ischemic brain lesions in these cases range in diameter from 2 to 20 mm [
4]. Evolving ischemic stroke: A stroke with a slow, progressive course, lasting from a few hours to several days. Clinical symptoms persist for more than three weeks, with motor deficits evolving or resolving in about 30 days. If it progresses, it may lead to constitutive IS, resulting from neuronal destruction in the affected vascular territory and causing the clinical presentation of a definite motor deficit [
4].
According to several investigators, stroke is defined as a focal neurologic deficit lasting more than 24 h, with neuroimaging evidence of cerebral infarction [
4]. In cases where the neurologic deficit resolves within 24 h and there are no neuroradiologic changes, the event is classified as a transient ischemic attack. However, if clinical manifestations last less than 24 h but neuroimaging reveals signs of infarction, the event is considered an ischemic stroke.
Rosa M. et al. define pediatric stroke as a sudden focal infarction of brain tissue, diagnosed either through neuroimaging or post mortem examination, which may result in arterial stroke or venous infarction. A stroke occurs when there is an abrupt occlusion of one or more cerebral arteries. In children, arterial stroke is the most common subtype, accounting for just over half of all strokes [
4].
Freundlich C.L. et al. describe pediatric stroke as a cerebrovascular event occurring during fetal or neonatal life, up to 28 days after birth, with radiologic evidence of a focal arterial infarction of the brain. This type of stroke is 17 times more frequent in the perinatal period than later in infancy [
5].
In recent years, significant attention has been given to serum biomarkers associated with inflammation and hypercoagulability in pediatric IS [
6]. According to Coelho Junior H.J., C-reactive protein (CRP) levels are often elevated during acute stroke episodes. A Canadian study documented elevated levels of stroke markers in adults, including IL-6, IL-10, GM-CSF, and IL-1Ra [
6]. Another study on four immune mediators in children—CRP, serum amyloid A (SAA), myeloperoxidase, and tumor necrosis factor (TNF) α—revealed that CRP and myeloperoxidase levels were elevated in the cardioembolic stroke group compared to the idiopathic group. Both cardioembolic and arteriopathic groups exhibited increased serum amyloid A levels compared to the idiopathic group, while elevated CRP and SAA levels in the arteriopathic group were predictive of recurrent arterial stroke [
7].
The biomolecular school scientific literature emphasizes the critical role of biomarkers in diagnosing pediatric stroke, assessing the neurologic prognosis, understanding stroke pathogenesis, and supporting recovery. In addition to traditional inflammatory markers like IL-6, TNF-α, and IL-1β, other molecules and biological factors—including cytokines (e.g., myokines, adipokines), growth factors, hormones, and microRNAs—have been identified. The peripheral levels of immunosoluble factors and immune cells provide valuable insights into stroke onset, pathogenesis, and recovery [
7].
Research also suggests that neurotrophins, including VEGF and erythropoietin (EPO), activate intracellular signaling pathways and exert neuroprotective effects [
7]. Novel therapeutic strategies, such as neurotrophic factor treatments and mesenchymal stem cell therapy, hold promise for stimulating nerve cell regeneration and improving the quality of life in infants affected by pediatric IS (PIS) [
8,
9].
Aim: To study the enzyme immunoassay parameters in IS in children, with a focus on understanding the disease pathogenesis, improving early diagnosis, and identifying predictive factors.
2. Materials and Methods
Between 2017 and 2019, a clinical study was conducted in the Republic of Moldova as part of the state program “Systemogenesis of Risk Factors, Optimization of Healthcare Service, Sustainable Evaluation, and Mathematical Modeling of Stroke”. This project included a dedicated pediatric component: the “Evaluation of Incidence, Prevalence, Risk Factors, and Research of Clinical, Neuroimaging, Neurophysiological, and Neurotrophic Management of Strokes in Children”.
Based on this project, a prospective study was conducted on a sample of 53 children diagnosed with IS who had been examined and consulted at the Institute of Mother and Child. In the 53 patients with IS (study sample, SS), during the acute period of the disease, the serum levels of some enzyme immunoassay markers were assessed by ELISA, including CD105 (endoglin), antiphospholipid antibodies (APAs) and interleukin-6 (IL-6). As reference values, the serum levels of the above-mentioned markers in a sample of 53 “practically healthy” children (control sample, CS) were considered. Initially, clinical symptoms of IS were registered, followed by the imaging findings, and venous blood sampling was performed with centrifugation and the separation of the serum for storage at (−20 °C). An enzyme immunoassay was carried out using the ELISA method and the SYNERGY-H1 analyzer (Seattle, WA, USA, BioTek). The results were analyzed using standard statistical methods of determining frequencies, the confidence interval, and averages with the standard deviation and standard error. The Pearson correlation, chi-square, and Mann–Whitney tests were used as statistical tests where appropriate.
The study we conducted included retrospective and prospective analyses. It was a non-randomized cohort case–control study conducted between 1 January 2010 and 1 January 2020 at the Pediatric Neurology Clinic, Pediatrics Department of the Nicolae Testemițanu State University of Medicine and Pharmacy (PI SUMP), with the respective departments located in the Institute of Mother and Child and the MHC No. 1 institution. This served as the basis for the study design. The study focused on analyzing cases of acute ischemic stroke (AIS) and acute ischemic pediatric stroke (AIPS), including research on epidemiological aspects, risk factors, clinical–paraclinical features, and immunoenzymatic changes.
This study had two components: (1) the retrospective analysis of 402 medical records of newborn children (291 children, 57.1%; 95% CI [54.91–59.29]) and children aged 28 days to 18 years (111 children, 21.8%; 95% CI [19.97–23.63]) treated with a confirmed diagnosis of AIS or AIPS between 2010 and 2016 in the neurology departments of the IMC and MHC No. 1, and (2) a prospective study conducted between 2017 and 2020 at the same medical institutions, involving a sample of 108 children aged 0 to 18 years, divided into the age categories of newborns—71 (65.7%; 95% CI [61.13–70.27])—and children aged 28 days to 18 years (meeting the criteria for AIS)—37 (28.7%; 95% CI [24.35–33.05])—with the same diagnosis (AIS). In the prospective study, the serum levels of immunoenzymatic parameters, such as VEGF, the S-100B protein, CNTF, IL-6, APAs, and CD105 (endoglin), were assessed using the ELISA method in 53 children diagnosed with AIS. Additionally, similar immunological analyses were performed on 53 children in the control group.
To analyze the epidemiological aspects, clinical manifestations, and neurological outcomes in the retrospective and prospective studies, data from 510 medical records of cases diagnosed with AIS in childhood were reviewed, categorized by age: newborn—362 (71%, 95% CI [68.99–73.01]); child—148 (29%, 95% CI [26.99–31.01]). The analysis of imaging, neurofunctional, and immunological manifestations was based on the results obtained from the 108 AIS patients in the prospective study.
This study allowed for the elucidation of epidemiological features and the stratification of the clinical–paraclinical manifestations of AIS in children of different ages, as well as the identification of predictive methods for this disease in newborns and children.
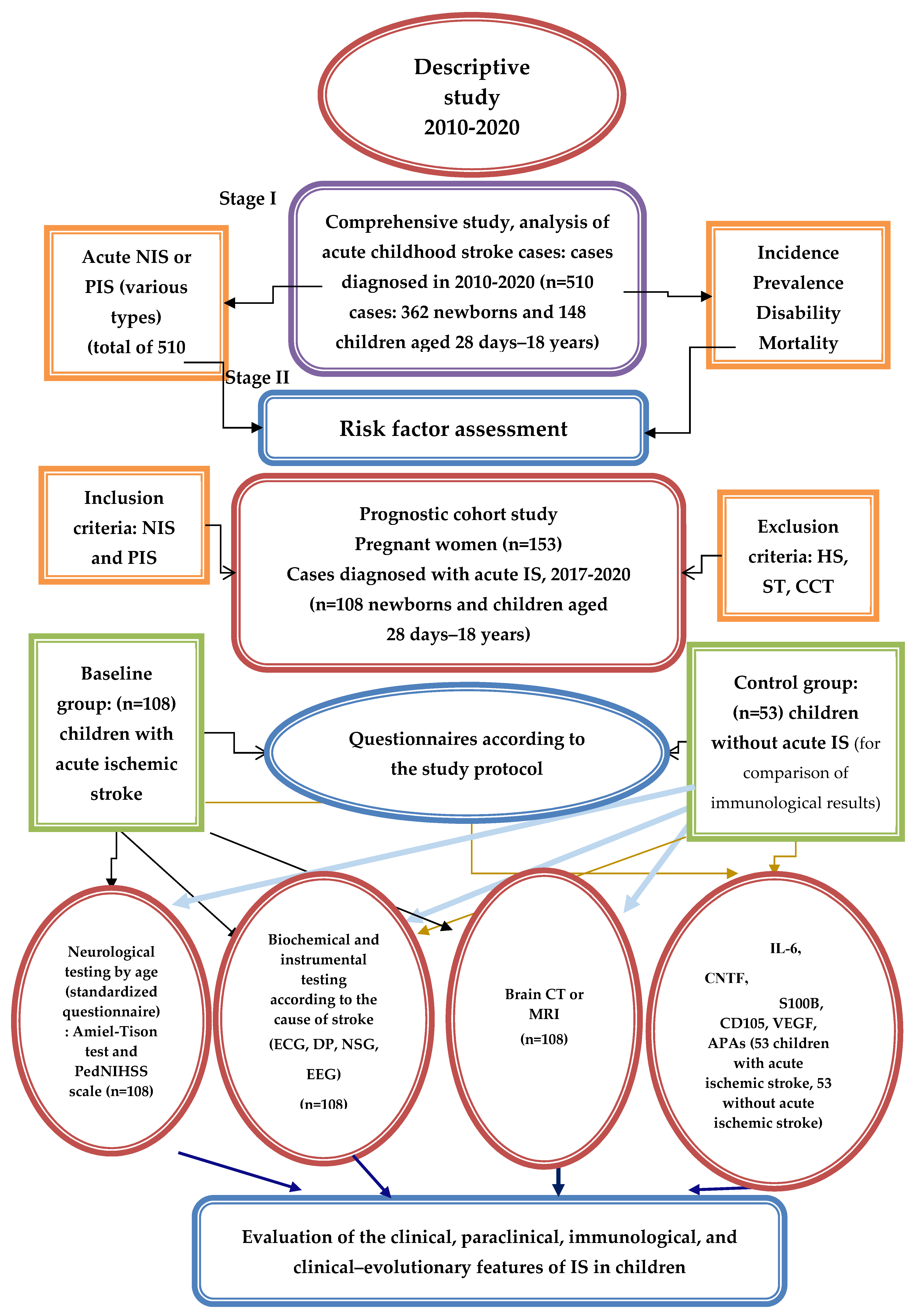
The study consisted of two complementary pilot studies. In both, the aim was to find new predictors for IS. In the first study, the aim was to find paraclinical predictors for primary IS diagnosis in children, while in the second, it was to find immunoenzymatic ones. According to the study design (
Figure 1), the study was conducted in four stages. The pilot study researching immunoenzymatic predictors was realized in stage two. To ensure a better overview of the whole study, in this article the methods and results of the first pilot study on paraclinical predictors are also described; more detailed information about this pilot study can be obtained in the paper published prior by Wessel N. et al. [
10].
Both pilot studies encompassed the following:
A comprehensive descriptive study to analyze the incidence, prevalence, disabilities, and mortality in children who experienced AIS or AIPS between 2010 and 2020.
A cohort prognostic study, n = (Z)2 P(1 − P)/e2 (1), where P is the pediatric stroke incidence, estimated to be between 2 and 13/100,000, with an average P = 0.0075; Z = 1.96 for a 95% confidence interval; e = 0.03 is the accepted error; n = 0.0075 × 0.9925 (1.96/0.03)2 = 31.74; and n × design effect (1.5) = 47.6. There was a 10.0% non-response rate, and the representative sample included 53 children with AIS.
The research was conducted in several stages. The first stage involved a comprehensive descriptive study (2010–2020) analyzing the evolutionary aspects and characteristics of neonatal and pediatric AIS in the Republic of Moldova (2010–2020), based on an observational analytical study (of the incidence, morbidity, and mortality) conducted retro- and prospectively at the Pediatric Neurology Clinic of the Pediatrics Department of IP USMF Nicolae Testemițanu, with the respective departments at the IMC and MHC No. 1; the distribution of AIS cases across the territory of Moldova (in various regions) was considered, along with the distribution of patients by age and sex. The analysis also included the determinants involved in triggering AIS in the pediatric population in Moldova according to the age category, as well as estimating the diagnosed cases of neonatal and pediatric AIS between 2010 and 2020 (510 children) based on the clinical and paraclinical manifestations.
The second stage involved a prognostic study, carried out from two perspectives: (1) We analyzed the results of examinations of pregnant women at risk of giving birth to children with AIS (153 pregnant women) performed using non-invasive prenatal diagnostic methods (fetal ultrasonography and biochemical screening: double/triple test) and invasive methods (amniocentesis with fetal karyotype study) at the Reproductive Health and Medical Genetics Center, in accordance with the national program for evaluating pregnant women and preventing hereditary pathologies. The results of 153 pregnant women were evaluated in the medical–genetic consultation, providing genetic counseling to prevent and predict cerebral pathology in children. (2) We conducted a study on 108 children who experienced AIS between 2017 and 2020. This study highlighted and specified the clinical–paraclinical manifestations of AIS and AIPS depending on the involved determinants (perinatal and postnatal) and the child’s age.
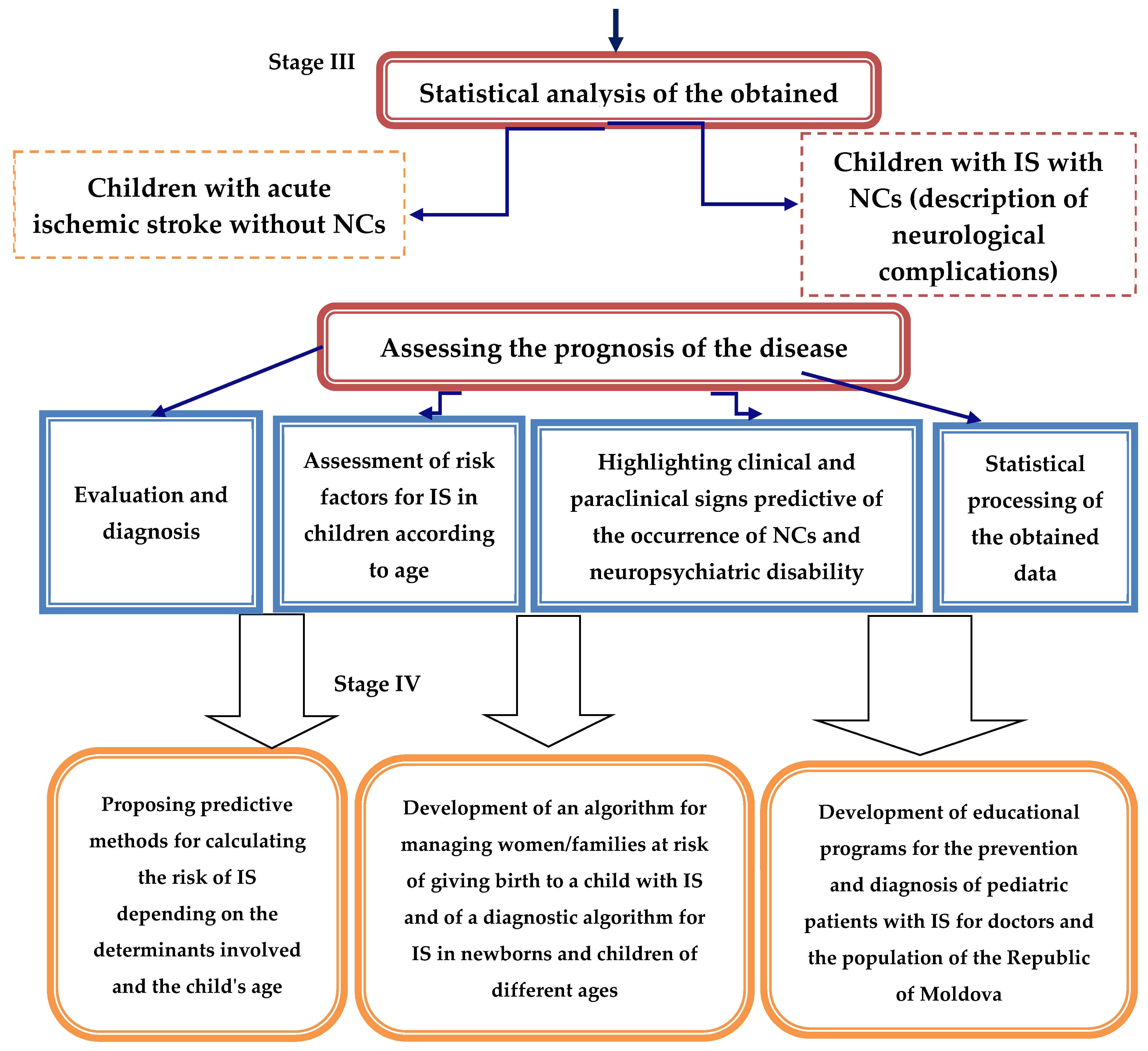
In the third stage, the neurological complication prognosis of AIS patients was assessed. Mathematical analysis methods were used to evaluate the relationship between the determinants involved in AIS, the child’s age, the clinical features at the time of diagnosis, immunoenzymatic changes, and the risk of neurological complications. Predictive methods for the prophylaxis of AIS and neurological complications in children were proposed.
The fourth stage involved developing an algorithm for managing women/families at risk of giving birth to a child with AIS and an algorithm for diagnosing and managing newborns and children with AIS. A National Clinical Protocol for Pediatric Stroke was also developed for the prophylaxis, early diagnosis, and definitive treatment of stroke in pediatric patients for doctors and the population of the Republic of Moldova.
In the context of this study, the following inclusion/exclusion criteria served as conceptual and methodological benchmarks for patient selection.
The inclusion criteria for patients in the study were as follows:
Children with acute ischemic stroke of various types.
Newborn children (0–28 days old).
Children aged between 28 days and 18 years.
Pregnant women.
Informed consent from the parents or caregivers.
The exclusion criteria for the study were as follows:
Children with hemorrhagic stroke.
Venous sinus thrombosis.
Children older than 18 years.
Children with acute traumatic brain injury.
Premature children.
A lack of informed consent from the parents or caregivers.
A retrospective study involving the review and analysis of 402 medical records of children diagnosed with acute ischemic stroke (AIS) between 2010 and 2016 and a prospective, non-randomized study of 108 children who experienced AIS between 2017 and 2020 were carried out. In the prospective study, all 108 children with confirmed AIS were evaluated according to Amiel-Tison and Gosselin neurological examination (designed for children of various age groups). Additionally, children older than three months in the study group were prospectively evaluated using the pedNIHSS scale, adapted for children. Out of the 108 children examined, 82 (75.9%; 95% CI [71.79–80.01]) exhibited motor deficits, 45 (41.7%; 95% CI [36.96–46.44]) had seizures, and 19 (17.6%; 95% CI [13.94–21.26]) experienced consciousness disturbances. Furthermore, 87 (80.6%; 95% CI [76.79–84.41]) presented with unilateral AIS, and 77 (71.3%; 95% CI [66.95–75.65]) were found to have signs of damage in the territory of the middle and anterior cerebral artery circulation.
Additionally, in this stage of the study, we evaluated a group of pregnant women, considering that developing predictive methods for AIS in children could only be feasible within the context of a medical–genetic consultation. The medical–genetic consultations for the pregnant women in this study were conducted in the Medical Genetics Department of the MHCI IMC and in the Consultative Department of MHCI HCM nr. 1 in Chisinau. Currently, according to several authors, medical–genetic consultation is one of the most widespread and effective methods for diagnosing and preventing hereditary diseases, including fetal pathologies occurring during the perinatal period. Medical–genetic consultation is considered the cornerstone that connects several aspects of medicine, genetics, psychology, education, and social work.
During the medical–genetic consultations of families at high risk for cerebral pathology, we gathered information regarding their family history, which allowed us to construct a pedigree for each family in the risk group. These data, including the disease history, family history, socio-economic conditions, demographics, and consanguinity (the degree of kinship between parents), were collected during conversations with the parents, relatives, and caregivers of the sick children. The methods used in the medical–genetic consultations included gathering anamnesis data, constructing the family pedigree of families with sick children, the psychoneurological examination of subjects based on clinical syndromes, prenatal diagnostics, laboratory methods (cytogenetic, molecular–genetic, biochemical), screening, and ultrasound (USG). Prenatal diagnostics play an essential role in preventing the birth of children with fetal pathologies, as they enabled the forecasting of a child’s health in high-genetic-risk groups for perinatal neurological pathologies.
The second study (clinical–immunoenzymatic), a non-randomized prospective prognostic study, was conducted at the Pediatric Neurology Clinic of the Department of Pediatrics of USMF Nicolae Testemiţanu, in the psychoneurology departments of the MHCI IMC and MHCI HCM nr. 1, between 2017 and 2020. The study included the immunoenzymatic testing of biomarkers such as IL-6, the S100B protein, VEGF, CD105 (endoglin), CNTF, and APAs during the acute phase of the disease, as well as the testing of VEGF and S100B protein levels six months after AIS. To achieve this goal, we selected 69 children, 8 of whom did not meet the inclusion criteria: 5 refused to participate, and 3 had other reasons. The study ultimately included 53 children with AIS.
We estimated the following core components: correlations between the serum levels of neuroinflammatory biomarkers (BN) (IL-6, S100B protein, VEGF, CD105 endoglin, CNTF, and APA), as well as correlations with scores on scales evaluating the degree of patient suffering, imaging and electrophysiological results, and neurological outcomes in children post-AIS. The BN research was made possible by the project “Evaluation of the incidence, prevalence, risk factors, clinical aspects, neuroimaging, neurophysiological aspects, and neurotrophic recovery of cerebrovascular accidents in children”, part of the state program “Systemogenesis of risk factors, optimization of healthcare services, sustainable evaluation, and mathematical modeling of stroke”, taking into account the costs and methodological difficulties of the method (rarely used for scientific purposes). The project was carried out in the Department of Pediatrics at USMF Nicolae Testemiţanu.
The parents of the children included in the study were informed about the scientific purpose of the research, and informed consent was obtained for their participation. All relevant parties, including the heads of clinics, heads of neurology departments, and pediatric neurologists, were informed about the necessary examinations. We collaborated with the Biochemical Diagnostic Laboratory at USMF Nicolae Testemiţanu to perform immunological tests while maintaining the confidentiality of the data. The data on the total number and characteristics of the patients included in the study, according to the age criteria, are presented in detail and systematically in
Table 1.
In the current research, a questionnaire developed for this study was applied to determine and analyze the risk factors, life history, disease history, and the clinical and paraclinical profile of IS in children. A total of 510 children with acute IS were included in the study, of which 402 were examined retrospectively and 108 children prospectively.
The gender characteristics of the children included in this study showed a predominance of boys—317 (62.2%; 95% CI [57.9–66.4])—compared to girls—193 (37.8%; 95% CI [33.6–42.1])—with no statistically significant differences between the groups, both being represented by a higher proportion of boys (χ2 = 0.038; df = 1; p > 0.05). Among the children with neonatal IS, 242 (66.9%; 95% CI [64.43–69.37]) were boys, and 120 (33.1%; 95% CI [30.63–35.57]) were girls. In the prospective analysis study, among a group of 108 children, 72 (66.7%; 95% CI [62.16–71.24]) were boys and 36 (33.3%; 95% CI [28.76–37.84]) were girls with neonatal or pediatric IS.
Of the total of 510 cases of acute IS in children, according to the study results, 362 (71%, 95% CI [68.99–73.01]) occurred in the neonatal period and 148 (29%, 95% CI [26.99–31.01]) in early childhood and adolescence. Among the neonatal IS cases, 242 (66.9%; 95% CI [64.43–69.37]) were boys, and 120 (33.1%; 95% CI [30.63–35.57]) were girls. It was found that, in newborns, the anterior and middle circulation were predominantly affected—228 (63.0%; 95% CI [60.46–65.54])—as well as the left cerebral hemisphere—245 (67.7%; 95% CI [65.24–70.16]). Multifocal brain lesions were found in about one-third of the children—119 (32.9%; 95% CI [30.43–35.37]).
For monitoring, children with IS were selected, and their perinatal history as well as the factors of high risk for IS development were evaluated. In the approach to acute IS in children, the following aspects were monitored: the degree of central nervous system (CNS) impairment at birth and later in children who had suffered acute IS, the evolution of neurological manifestations during the neonatal and childhood periods, and the development of clinical manifestations in the first months after IS (paroxysmal manifestations and motor, cognitive, and behavioral performance: communication, memory, intelligence, and social adaptability). Additionally, the following were evaluated: (1) immunoenzymatic changes in VEGF, CNTF, IL-6, endoglin, APAa, and the S100B protein, as well as the VEGF and S100B protein levels, assessed during the acute phase and six months after IS; (2) EEG patterns; and (3) CT and MRI brain imaging characteristics.
Patients were examined in neurological departments, intensive care units, and neonatal neurology. The periodic observation of patients was conducted repeatedly, depending on the established diagnoses and the child’s age. All data were included in the questionnaire (Annex 1), comprising interviews including data on risk factors, determinants, and clinical outcomes after the Amiel-Tison test (for neonates and children up to 3 months) and the PedNIHSS scale (after 3 months). To assess the functional and structural changes in the brain, additional exams such as CT, MRI, and EEG were used. Investigations were conducted in a stationary setting. EEG was performed based on existing indications. Imaging examinations were performed on 108 children with acute neonatal or pediatric IS in the prospective study group. The results of the additional investigations confirmed the diagnosis of acute IS in the children, assessed the dimensions of the cerebral ischemic focus, and evaluated the prognosis of the disease.
The prospective clinico-immunoenzymatic study was conducted on a group of 53 children with neonatal or pediatric IS selected from the general research group, enrolled during the acute phase of the disease, and 53 children from the control group, who were considered healthy and without any health issues. Correlations between the serum levels of IS markers and the severity of IS, as assessed by the Amiel-Tison test and the PedNIHSS scale, as well as imaging and electroencephalographic manifestations, were evaluated. Thus, the modified serum levels of immunoenzymatic markers correlated with selected comparison variables for IS in children.
In general, the study was initiated to evaluate the clinical–neurological characteristics of children with acute IS, assess symptoms suggestive of IS, select methods of immunological, functional, and imaging exploration, and perform the statistical processing of the obtained results. The synthesis of the clinical–paraclinical results allowed for an approach to diagnosis based on age, the identification of predictive factors for IS, the elucidation of the disease prognosis, the assessment of risk factors for neuropsychological disability, and support for neuroprotective therapies.
The approval of ethical aspects and the obtaining of consent to participate in the study was carried out following protocol No. 69 dated 21 March 2017, which was approved by the Research Ethics Board of the State University of Medicine and Pharmacy ”Nicolae Testemitsanu”.
3. Results
Biochemical markers have recently gained significance in identifying brain lesions. In this context, our study aimed to assess the expression of immune parameters and biological factors involved in childhood stroke. We analyzed their relationship with the disease severity, infarct size, and long-term clinical outcomes. Additionally, we investigated the correlation between these parameters and their potential early prognostic role in pediatric ischemic stroke (IS). Given the limited data on the role of these markers in the mechanisms of acute childhood stroke—which remains poorly studied—we focused on assessing the expression of interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF), the S-100B protein, CD105 (endoglin), and antiphospholipid antibodies (APAs) in peripheral blood. The aim was to understand their role in inflammation at disease onset and in angiogenesis during the onset and progression of the disease.
Following the research objectives, a prospective study was conducted during the years 2017–2020 on a sample of 53 children who suffered neonatal or pediatric IS. Also, an equal number of children were included in the control group. Concomitantly with the evaluation of children with acute IS using the PedNIHSS scale, the serum concentrations of IL-6, VEGF, CNTF, S100B, endoglin (CD105), and APAs in patients with acute IS and children in the control group were determined. In addition, the serum VEGF and S100B protein levels were assessed during the acute period of the disease and more than six months after the acute stroke. We considered certain practically determined and scientifically substantiated normal concentration limit levels. The obtained results were characterized by statistically significant variations in the serum concentrations of these biomarkers.
Concerning endoglin (ENG) or CD105, it is known to be a membrane glycoprotein that can bind the β1 and β3 isoforms of transforming growth factor beta (TGFβ). Endoglin is a TGFβ-associated receptor and is required for both vasculogenesis (the formation of an embryonic vascular system) and angiogenesis (the growth of new vessels in an existing vascular system), as the process of new blood vessel formation in an organ or tissue, during which the reorganization of the primary capillary network results in it being reduced to a simpler and more distinct system of capillaries, arteries and veins, is known to be continuous. Normally, angiogenesis processes in the body continue with moderate intensity and are activated only during the regeneration of injured tissues, drainage of blood clots, elimination of inflammatory outbreaks, scar formation, and similar processes of recovery, as well as during the growth and development of the body. However, the impact of ENG on recovery after stroke and brain surgery is unclear. In this study, the serum CD105 concentration was determined to be lower in children with acute stroke, and endoglin deficiency impairs recovery processes after stroke [
11]. Thus, ENG-CD105 deficiency may impair recovery after ischemic brain injury.
Endoglin deficiency was associated with impaired recovery processes post-stroke, suggesting that ENG-CD105 deficiency might hinder angiogenesis, alter macrophage adhesion, and delay the resolution of inflammation [
11,
12,
13,
14]. Experimental studies have indicated that CD105 deficiency exacerbates ischemic brain injury and delays neurobehavioral recovery processes.
According to the literature, “hypoxia-induced CD105” expression is regulated by hypoxia-inducible factor 1 (HIF-1), which directly binds to the hypoxia response element in the CD105 promoter. HIF-1, an essential factor for cellular adaptation to low oxygen levels, is predominantly found in the brain, heart, and lungs. The activation of HIF in response to hypoxia may facilitate improved recovery from IS in children by improving therapeutic responses. Hypoxia is a complicated biological process, and hypoxia-regulated CD105 expression (in peripheral blood) suggests its possible involvement in several neurophysiological pathways.
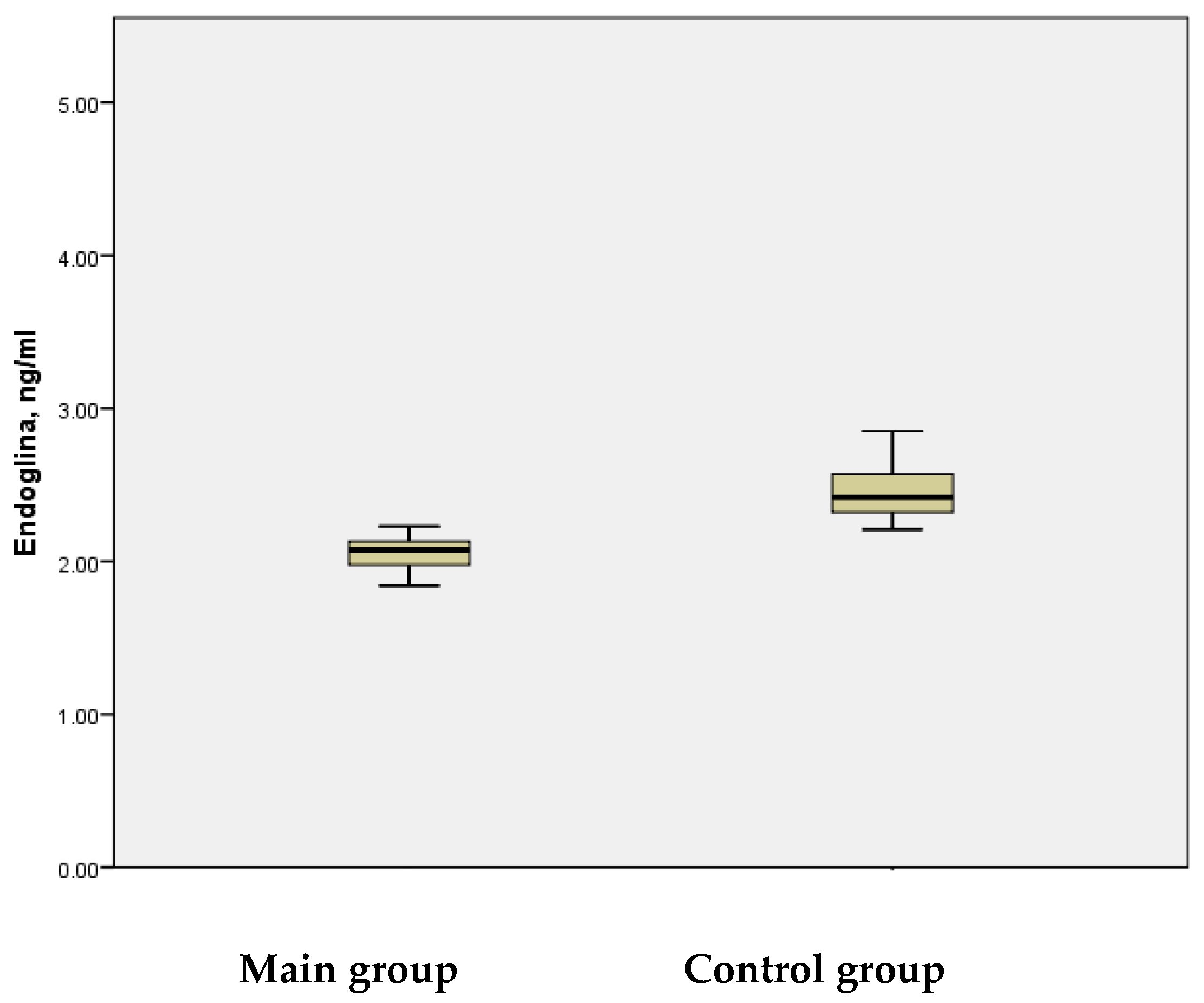
In the main group, the mean serum endoglin level was measured at 2.06 ± 0.012 ng/mL, with a maximum value of 2.23 ng/mL, whereas in the control group, the mean level was 2.51 ± 0.071 ng/mL, reaching a maximum of 4.02 ng/mL (
Figure 2). The analysis revealed that the mean serum endoglin levels were statistically significantly lower in the study group compared to the control group (
p < 0.001). These results indicate that the reduced concentration of serum endoglin CD105 during the acute phase of stroke in children correlated with the degree of hypoxia experienced by these patients. A deficiency of CD105 could impair recovery processes following ischemic brain injury by reducing angiogenesis, a critical component in tissue repair and neuroregeneration.
Also, the role of another marker of ischemic stroke, the S100B protein, was investigated. It represents a member of the S100 protein family. S100B is a glial-specific, multifunctional protein selectively synthesized by certain astroglial cells (astrocytes) in the brain. Specifically, it is produced by a subtype of mature astrocytes lining blood vessels, which play a role in nerve cell differentiation, neural growth during development, and nerve repair after injury, serving a protective function at physiological levels.
Additionally, S100B is synthesized by cells expressing NG2 (neuroglial antigen 2), a chondroitin sulfate proteoglycan encoded by the CSPG4 gene in humans. NG2-related actions include the stimulation of Ca2+ influx, the inhibition of phosphorylation mediated by protein kinase C (PKC), and astrocytosis. PKC, a family of enzymes involved in phosphorylating proteins, plays a role in various cell signaling cascades, including those regulated by increased diacylglycerol or Ca2+ ions. However, in adults, these processes can lead to nervous system damage, making NG2 and related proteins potential clinical and prognostic markers.
S100B is released from injured brain cells into the extracellular space or bloodstream and can be detected in peripheral circulation following brain injury. Elevated extracellular concentrations of S100B have been associated with cell damage, possibly contributing to the pathophysiology of neurodegenerative processes [
15,
16]. Consequently, a normal serum concentration of the S100B protein typically excludes the likelihood of severe central nervous system (CNS) pathology. The rapid release of S100B into biological fluids, combined with its short half-life, makes it a crucial marker for acute brain tissue injury, such as traumatic brain injury or ischemic/hemorrhagic stroke.
Furthermore, chromosomal rearrangements and an altered expression of the S100B gene have been linked to various neurologic, neoplastic, and other diseases, such as Alzheimer’s disease, Down syndrome, epilepsy, amyotrophic lateral sclerosis, schwannoma, melanoma, and type I diabetes mellitus [
16]. It has been suggested that the regulation of S100B by melittin has potential for the treatment of epilepsy [
17].
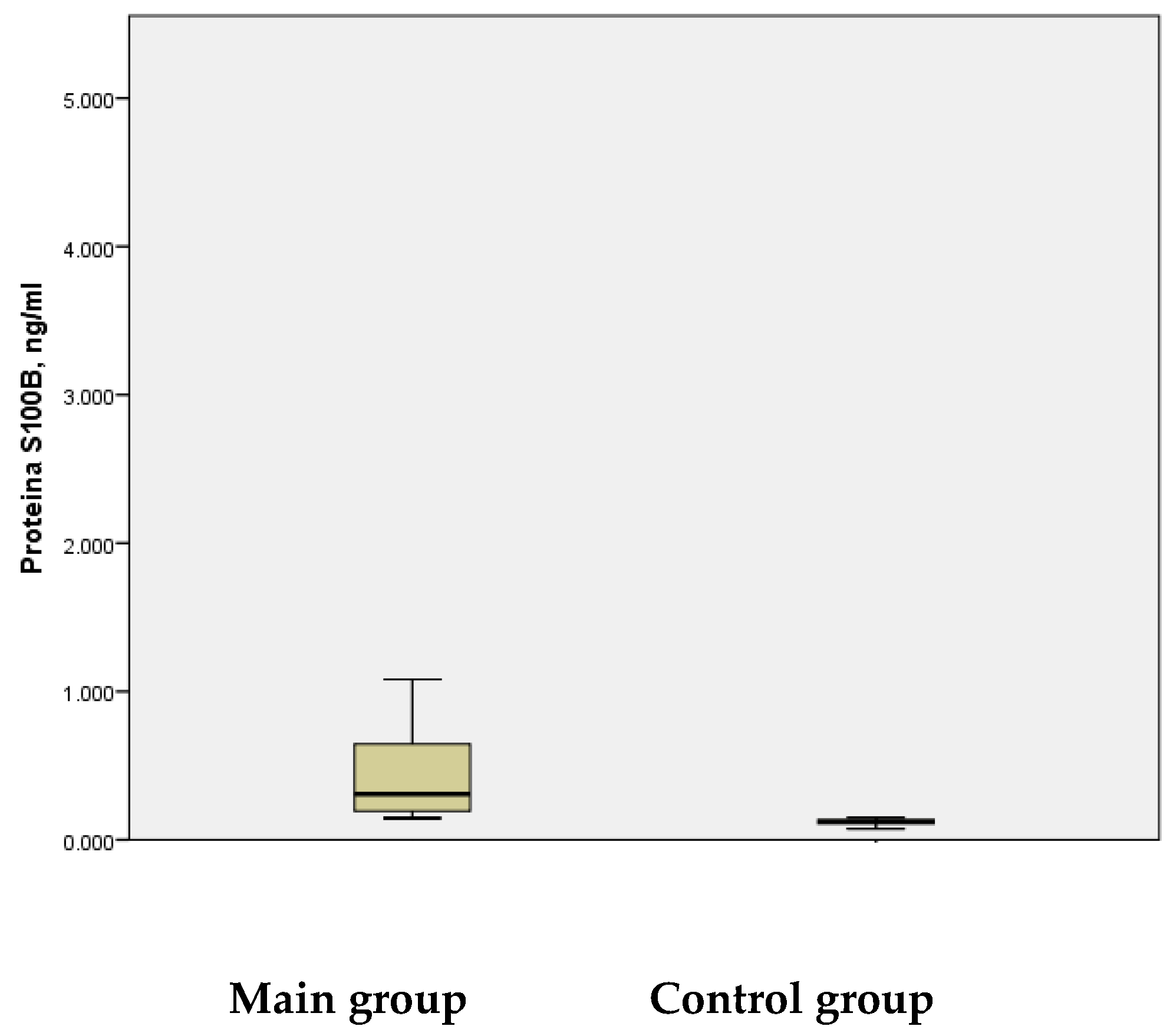
In this study, it was determined that the serum levels of the S100B protein were significantly elevated in patients during the acute phase of stroke-induced brain injury. Unlike endoglin, S100B protein levels increased during the acute phase in the main group compared to the control group. Specifically, in children with ischemic stroke, the mean S100B protein level was 0.524 ± 0.0850 ng/mL, with a maximum value of 4.390 ng/mL. Conversely, in the control group without IS, the mean S100B level was 0.120 ± 0.0038 ng/mL, with a maximum value of 0.149 ng/mL. The difference between these groups was statistically significant (
p < 0.01) (
Figure 3).
There was no statistically significant correlation observed between endoglin and S100B protein levels in either the main group or the control group (p < 0.1).
In this context, based on elevated values of 0.6–1.4 pg/mL, the astroglial-derived serum protein S100B was considered not only a potential clinical marker for assessing the degree of IS manifestation in children, but also a screening marker for evaluating the prognosis of long-term neurological sequelae.
Next, markers from the vascular endothelial growth factor (VEGF) family were evaluated due to their known role in regulating vascularization and stimulating blood vessel formation. In the brain, VEGFs are crucial for regulating vasculo- and angiogenesis, neuroprotection, and neurogenesis. VEGF may enhance collateral ischemic remodeling and has recently been recognized as a biomarker for stroke.
VEGF is a signaling protein produced by cells to stimulate vasculogenesis (the formation of an embryonic vascular system) and angiogenesis (the growth of new blood vessels within an existing vascular system).
Several factors within the VEGF family (a subclass of a larger class of growth factors) have been identified. VEGF proteins are part of the system that restores oxygen supply to tissues during periods of inadequate blood circulation, such as during a stroke.
The main functions of VEGF include the formation of new blood vessels during embryonic development and following various diseases, as well as facilitating collateral circulation to form new vessels when existing ones are blocked. Increased VEGF activity is associated with various diseases, including solid cancers, and its overexpression is linked to vascular diseases and epilepsy. Drugs designed to inhibit VEGF, such as bevacizumab, have recently been developed.
Controlling or slowing the progression of such diseases remains a critical goal. The current research indicates that VEGF proteins are not the sole activators of angiogenesis. During the acute phase of ischemic stroke (IS), elevated VEGF levels increase the blood–brain barrier permeability and vessel injuries, leading to disruptions in homeostasis, peripheral immune cell infiltration, and localized edema. While VEGF has adverse effects on vascular integrity in the acute phase, these effects are transient. Beyond the acute phase, VEGF exhibits neuroprotective properties when maintained at subthreshold levels.
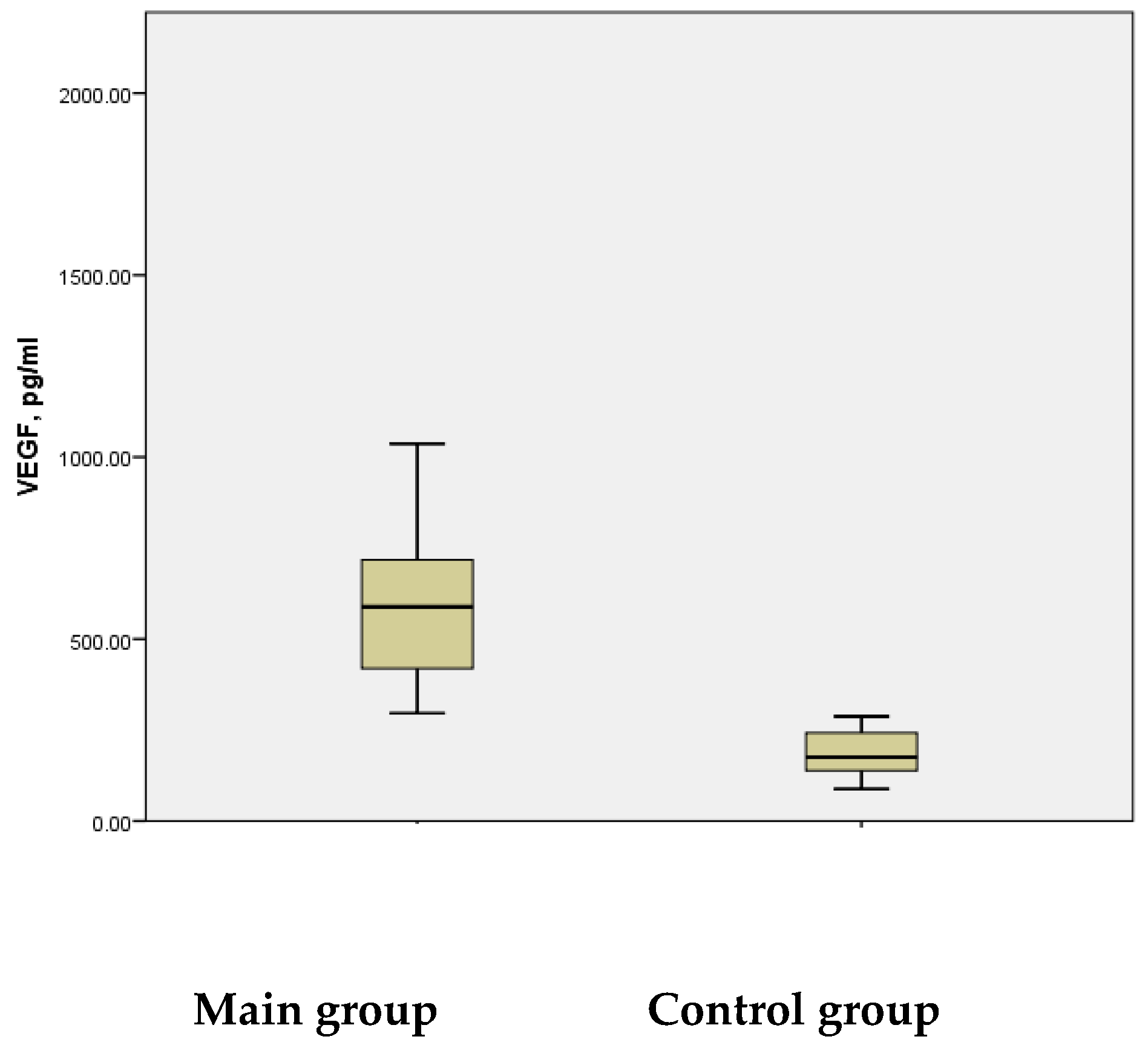
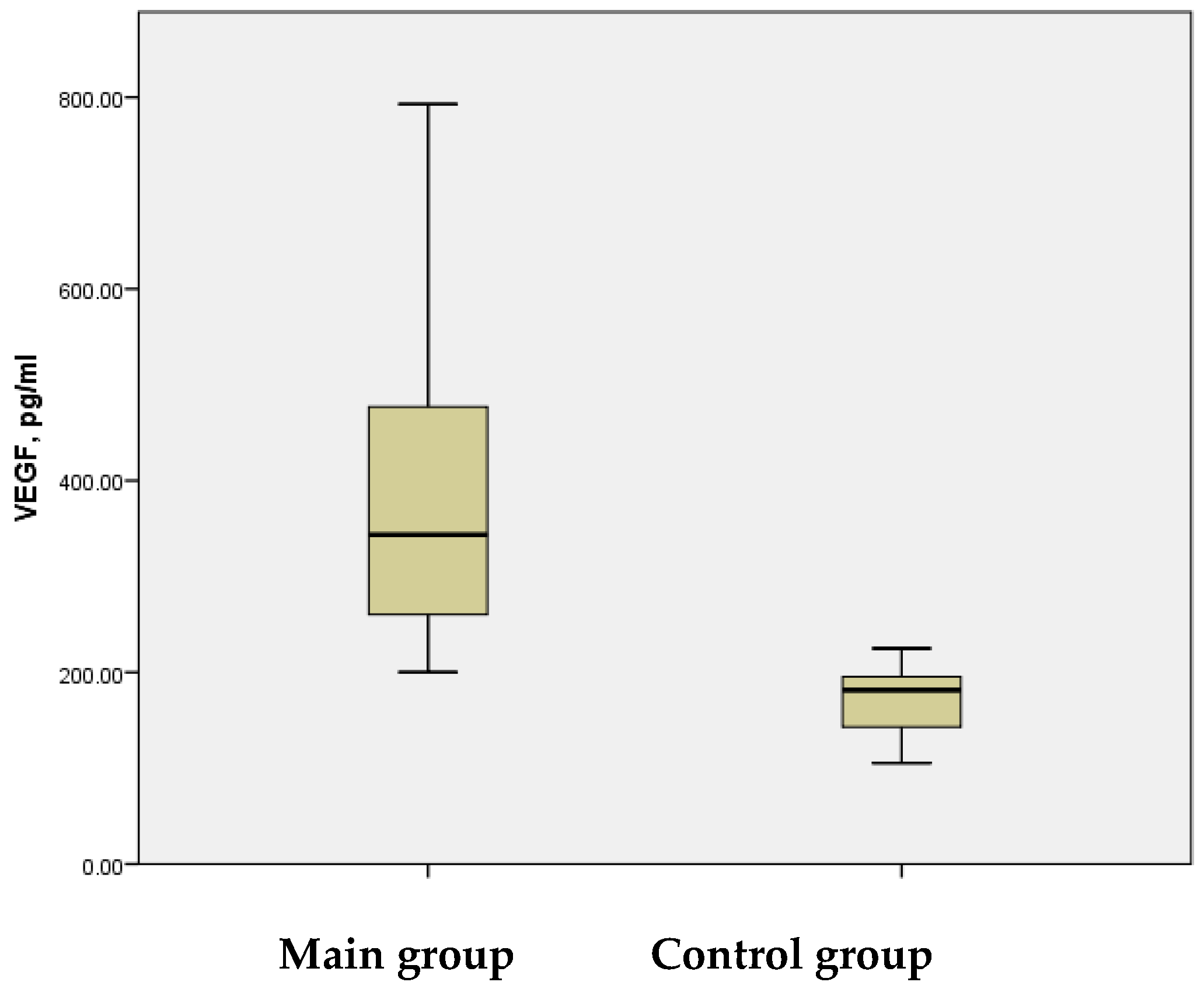
Literature reports consistently indicate elevated serum VEGF levels during the acute phase of IS. In our study, significantly increased mean serum VEGF levels were observed in children during Disability-Adjusted Life Years (DALYs) associated with the acute phase of the disease. The study group demonstrated mean VEGF levels of 613.41 ± 39.299 pg/mL, with a range from 296.23 pg/mL to 1705.81 pg/mL. In contrast, the control group showed a mean VEGF level of 185.50 ± 12.039 pg/mL, with a maximum of 287.44 pg/mL—significantly lower than the minimum VEGF levels observed in the study group. The difference between the groups was statistically significant (
p < 0.001) (
Figure 4).
Recently, vascular endothelial growth factor (VEGF) has been linked with ciliary neurotrophic factor (CNTF) as an important biomarker in stroke. CNTF, a pleiotropic cytokine from the neurokinin family, is expressed primarily in gray matter, including the cortex, hippocampus, and astrocytes. Its biological effects are mediated through CNTFR receptors. CNTF plays a vital role in maintaining nerve cell survival in neurodegenerative diseases and promoting axonal growth. It significantly contributes to the development and maintenance of the nervous system, as well as the health of cardiomyocytes, osteoblasts, immune cells, adipocytes, and skeletal muscle cells.
CNTF exerts multiple beneficial effects on the central nervous system (CNS) and peripheral nervous system, offering potent neuroprotective and neurotrophic actions across various neuron classes, particularly motor neurons. It is known to protect against neuronal death, as confirmed by animal model studies. Additionally, CNTF is upregulated following spinal cord injury and IS. In animal models, an astroglial upregulation of CNTF in the brain and CNTF expression in the hippocampus were observed for two weeks post-stroke. CNTF is recognized as a significant biomarker in various neurological diseases, including childhood IS. It directly impacts the nervous system by promoting neuron and oligodendrocyte survival, mediating neurogenesis, and exerting anti-inflammatory effects.
Ciliary neurotrophic factor (CNTF) is reported to have a safe biological range, beyond which it may pose a risk of neurotoxicity. However, the dosing range appears sufficiently broad to minimize such risks. Improved functional outcomes have been observed following the administration of combined CNTF preparations via both oral and topical routes.
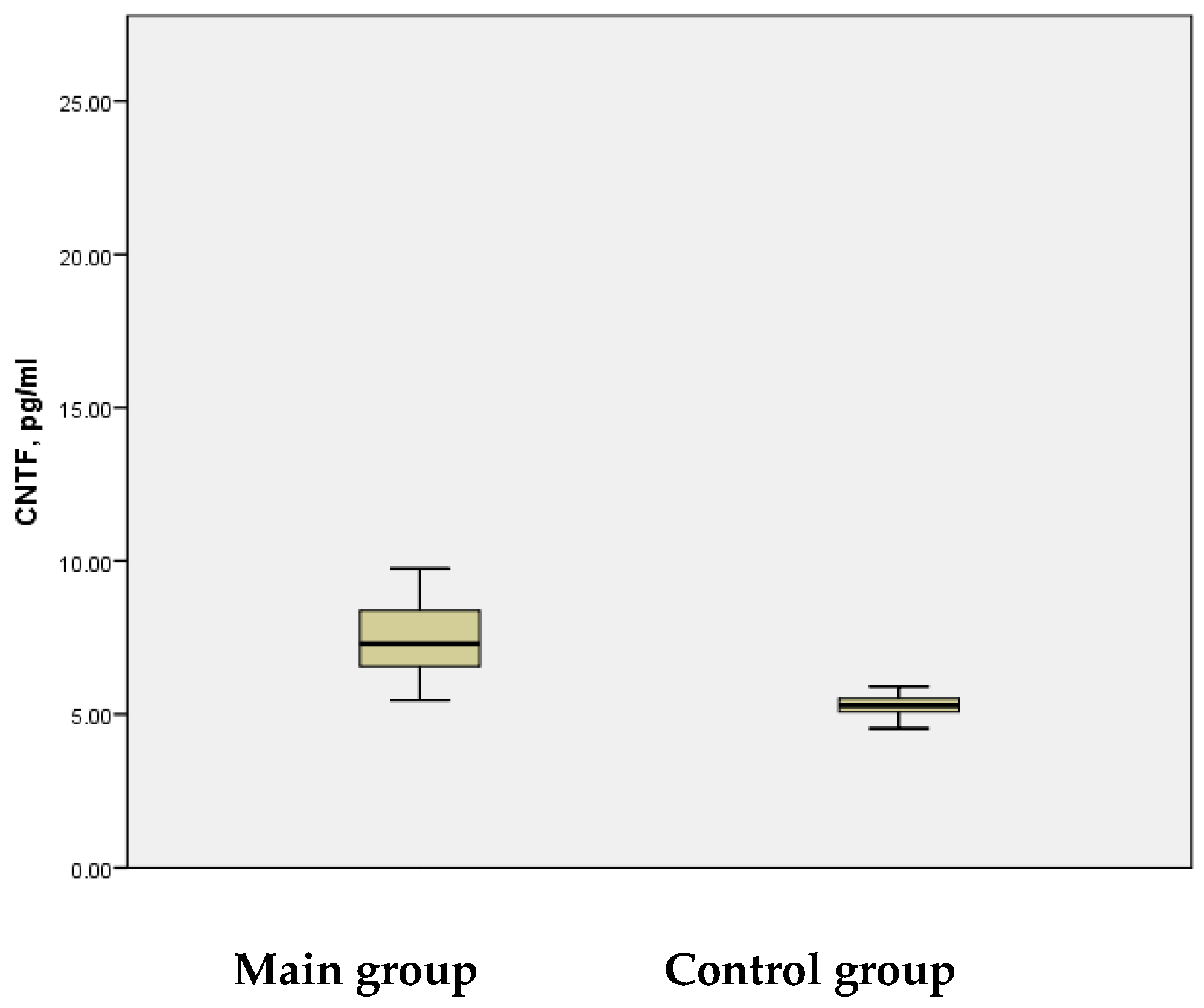
In this study, the CNTF levels in the serum of patients with acute ischemic stroke were assessed, revealing a mean value of 7.84 ± 0.322 pg/mL, with a wide range of variation (5.46 pg/mL to 20.26 pg/mL). In contrast, the control group showed a considerably narrower range of CNTF levels (4.94 pg/mL to 5.90 pg/mL) and a mean value of 5.29 ± 0.067 pg/mL. This difference between the groups was statistically significant (
p < 0.001) (
Figure 5).
A significant positive correlation was observed between the CNTF values in the main group (r = 0.72), compared to the control group (r = 0.208). The elevated CNTF levels in peripheral blood may reflect the concentration of this protein in the brain during IS, suggesting its potential role as a key biomarker of the disease. Notably, elevated CNTF levels are associated with significant neurotoxicity. However, during the progression of IS, CNTF levels below physiological thresholds may act as a neuroprotective factor, mitigating the pathological process in ischemic areas and preventing neuronal death, particularly in motor and sensory cells.
The findings of the given study show that in this case, when CNTF levels exceeded the threshold for neurotoxicity, then neuroinflammatory and neurodestructive processes intensified. Early in the course of IS, reducing elevated CNTF levels through the administration of combined drugs—targeting pathways such as the CNTF-STAT3-IL-6 axis—may help to modulate the inflammatory cascade. The therapeutic regulation of CNTF levels in such cases represents a promising approach for preventing neurological complications, particularly in pediatric IS. Improved functional outcomes following the use of recombinant CNTF preparations have been widely documented in animal models.
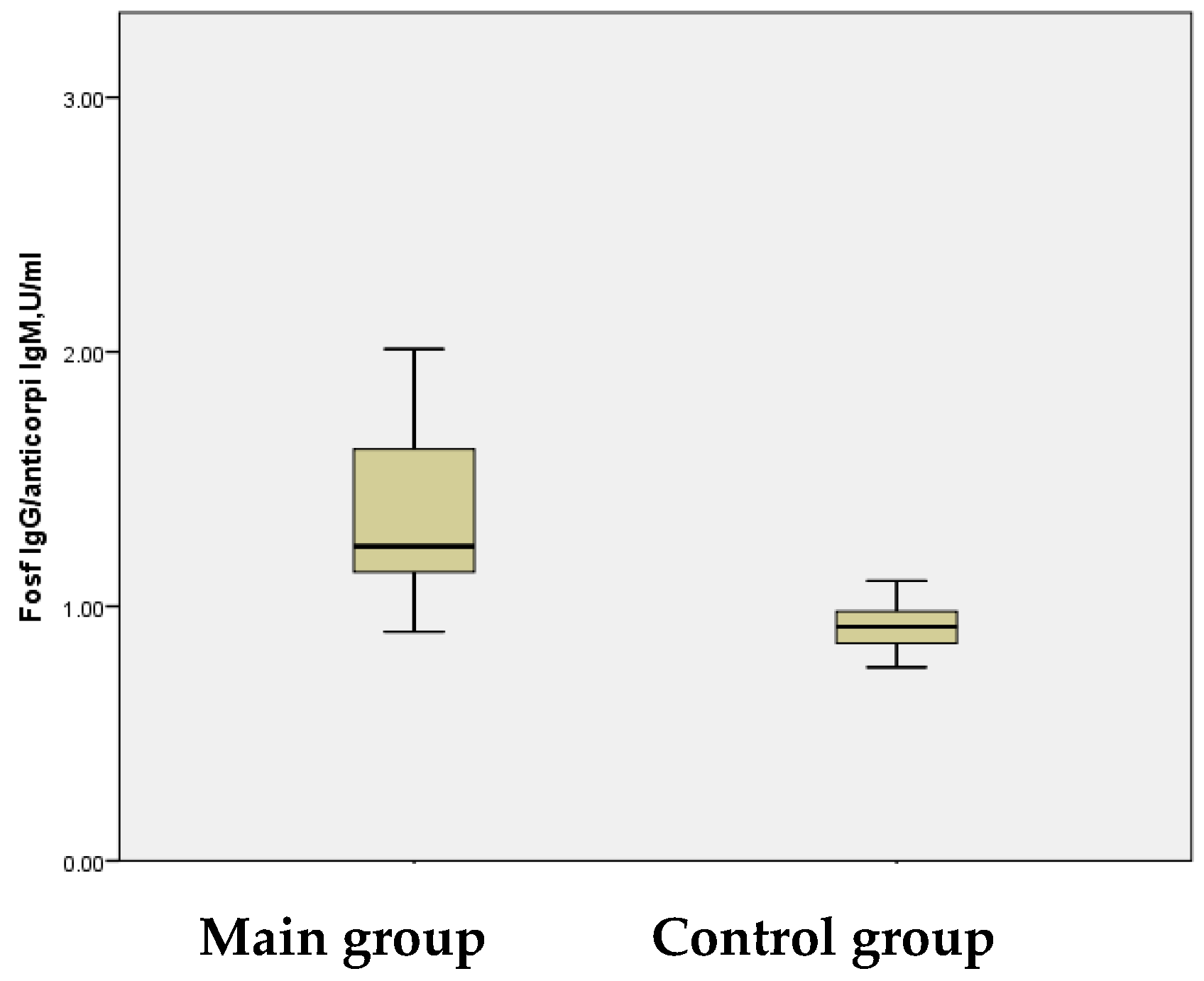
Additionally, this study analyzed other pro-inflammatory cytokines, including antiphospholipid antibodies (APAs), which act as vascular components and inflammatory markers. Elevated APA levels are associated with a hypercoagulable state, contributing to IS and other ischemic events. APAs, a heterogeneous group of autoantibodies, play a role in thrombosis through mechanisms such as the activation of coagulation cascades, ultimately leading to cerebral ischemia. The study group exhibited a mean APA level of 1.37 ± 0.046 U/mL, significantly higher than the control group’s mean level of 0.92 ± 0.021 U/mL, activating coagulation cascades, causing cerebral ischemia.
The figures reflect the APA values. Although the difference between the two groups may appear modest, a statistically significant disparity was observed (
p < 0.001) (
Figure 6).
Molecular markers of inflammation are valuable tools for managing patients in the acute phase of ischemic stroke (IS), assessing their prognosis, and preventing neurologic complications. Interleukin-6 (IL-6) is a key inflammatory mediator, with elevated levels commonly observed in children with IS. While inflammation plays a significant role in the pathophysiology of IS, correlating the serum IL-6 levels with C-reactive protein (CRP) and other inflammatory markers is essential for evaluating the infarct volume and predicting clinical outcomes.
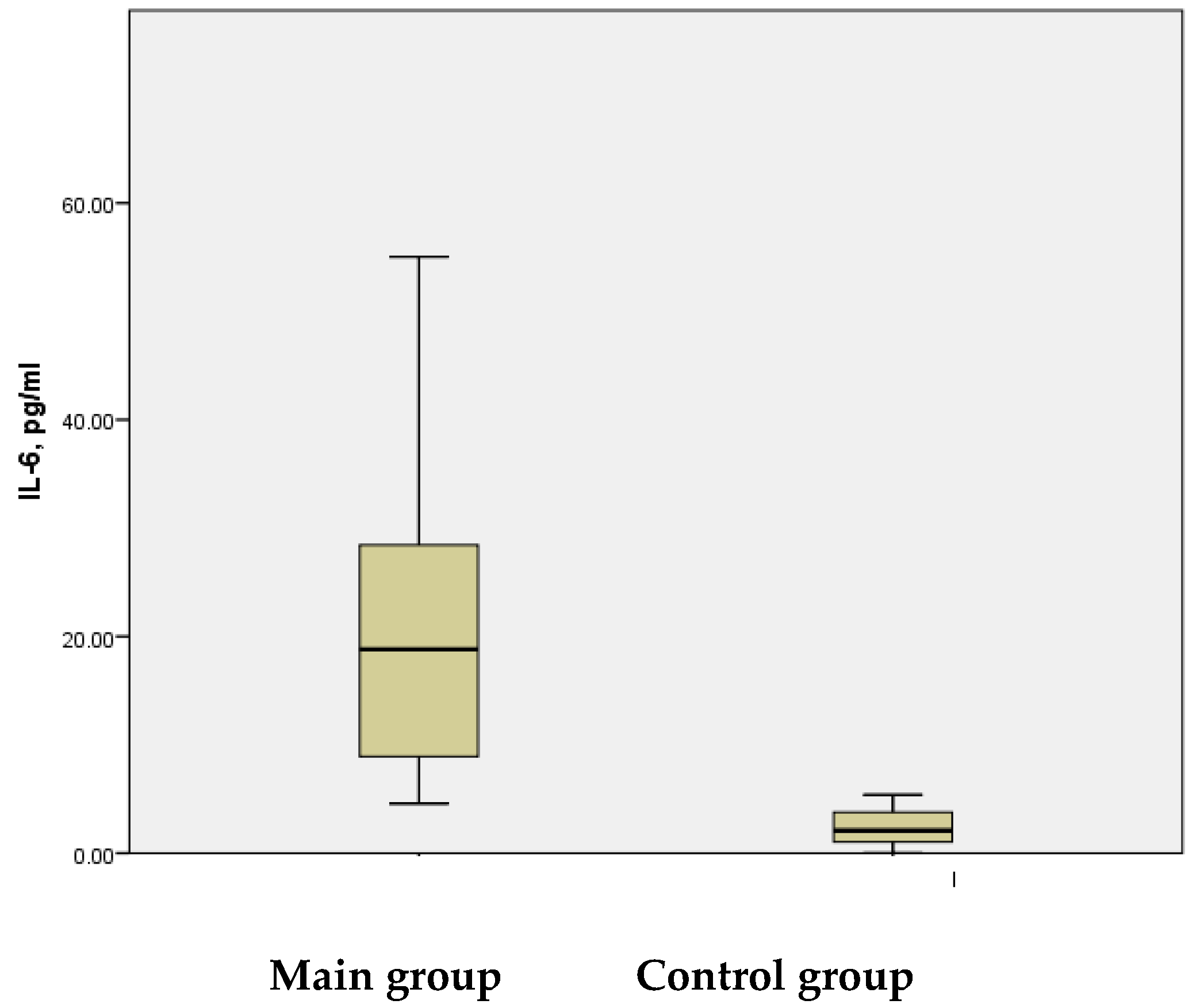
In this study, high serum IL-6 levels were detected in the study group, with a mean value of 22.02 ± 2.143 pg/mL, ranging from 4.58 pg/mL to 65.71 pg/mL. In contrast, the control group exhibited IL-6 levels that were approximately ten times lower, with a mean value of 2.38 ± 0.302 pg/mL, ranging from 0.01 pg/mL to 5.38 pg/mL. This difference was statistically significant (
p < 0.001) (
Figure 7).
The results of this study reveal that elevated serum IL-6 levels during the acute phase of IS, compared with those of healthy controls (p < 0.001), were strongly associated with the infarct volume (p < 0.004) and a less favorable prognosis during the six months post-stroke, as indicated by the severity of neurological sequelae (p < 0.01).
The assessment of serum IL-6 levels in children during the acute phase of ischemic stroke (IS) provides valuable insights into the severity of brain injury by reflecting the extent of neuroinflammation. It also serves as a predictor of the long-term neurological outcomes and overall prognosis in IS. A positive correlation was also observed between the CNTF and IL-6 levels in the main group (r = 0.580), highlighting their combined role in promoting and sustaining neuroinflammation, as well as in assessing the extent of brain injury resulting in serious sequelae in pediatric IS. In this study, there were no significant correlations found between other pro-inflammatory cytokines and immune mediators.
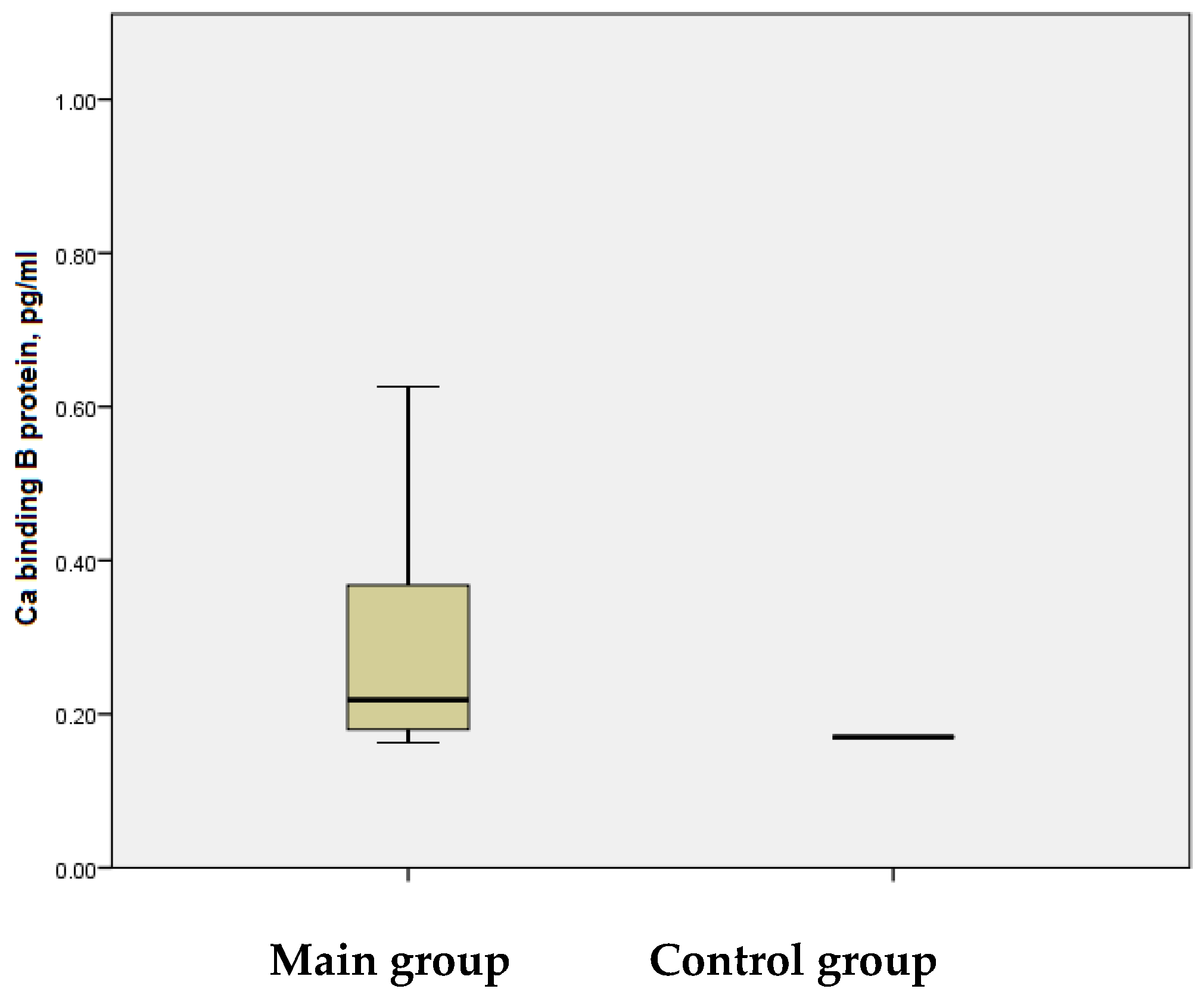
Additionally, the S100B protein levels, measured six months after the onset of IS, were significantly higher in the main group, with a mean value of *0.30 ± 0.022 ng/mL* and a range from *0.16 ng/mL to 0.81 ng/mL. In contrast, the control group exhibited relatively stable S100B levels (0.17 ± 0.001 ng/mL) with minimal variability (0.16–0.18 ng/mL). This difference between the groups was statistically significant (
p < 0.001) (
Figure 8).
An analysis of the S100B protein levels six months after in terms of disease-adjusted life years (DALYs) revealed a significant decrease in their mean values, confirmed by a statistically significant difference (*t = 2.702,
p < 0.01) (
Figure 9). Over this period, the S100B levels declined from **0.524 ± 0.0850 ng/mL* to *0.30 ± 0.022 ng/mL*, normalizing in most patients. This trend suggests the involvement of the S100B protein in recovery mechanisms following ischemic stroke.
However, persistently elevated S100B levels in some patients, particularly those with severe brain injury, were noted six months post-stroke (e.g., from *4.390 ng/mL* at disease onset to *0.81 ng/mL* after six months). This persistence highlights the risk of recurrent stroke and underscores S100B’s potential as a biomarker for both the brain injury severity and the extent of ongoing neurological impairment.
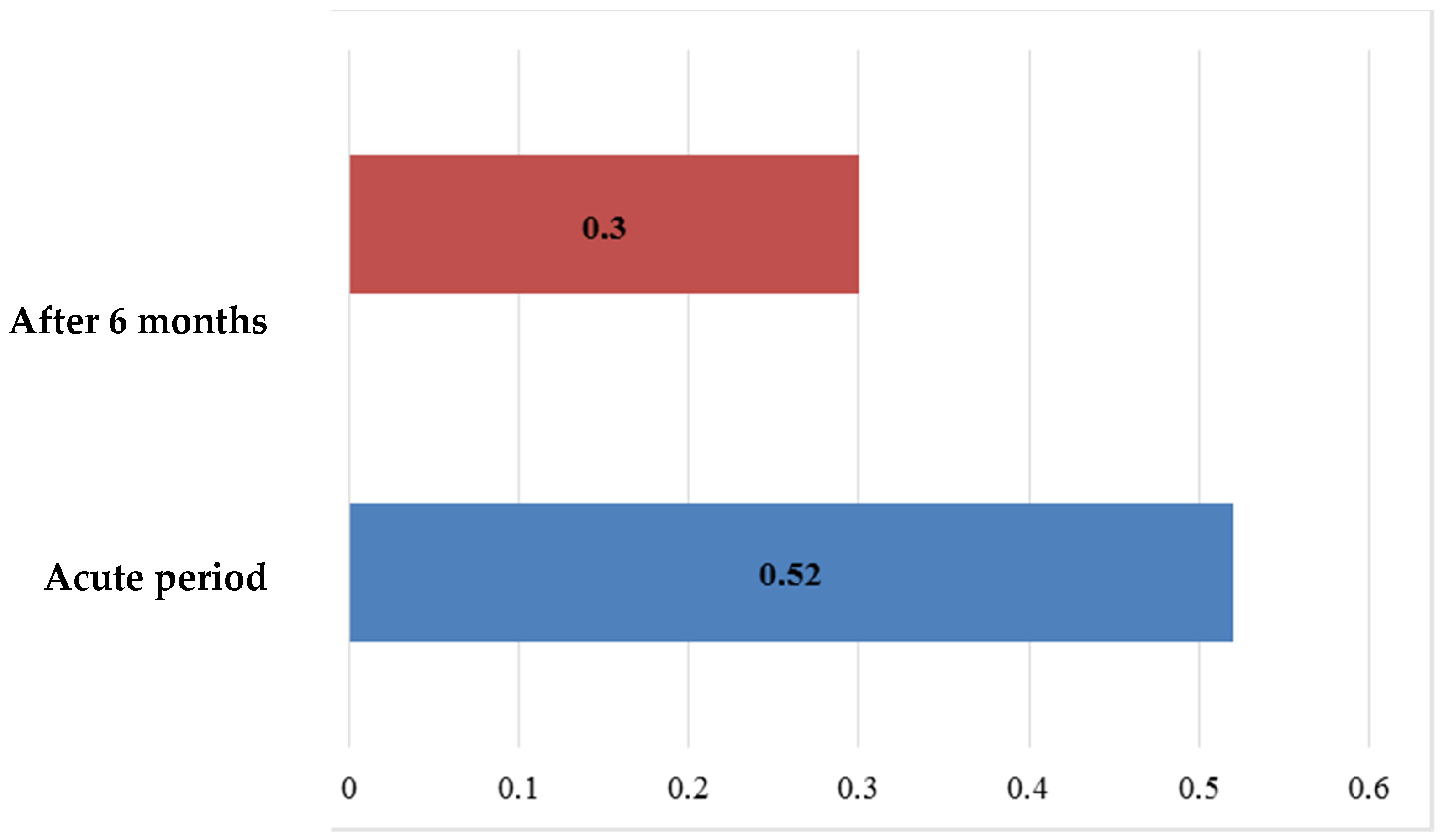
Similarly to that of the S100B protein, the mean VEGF level, when assessed six months after ischemic stroke (
Figure 10), had decreased by nearly half in the main group, from 613.41 ± 39.299 pg/mL to 374.96 ± 19.506 pg/mL; the evident dynamics were also confirmed statistically (
p < 0.01). To visualize the change in the VEGF values over time, it is represented in a flow-chart diagram. This change demonstrated a statistically significant difference over time (
Figure 11), comparing the dynamics of the values in the acute period of IS and after six months.
In most patients, their VEGF levels reached normal values within six months. However, some children continued to exhibit elevated levels of VEGF, equal to 1705.81 pg/mL during the acute phase of the disease and 792.94 pg/mL in the recovery phase. Persistently high VEGF levels six months post-IS suggest ongoing remodeling and the growth of new vessels in the ischemic focus, indicating active angiogenesis and improved collateral circulation. These processes were particularly intense in children with extensive brain lesions, as evidenced by significantly elevated VEGF levels. These findings underscore the need for pharmacological interventions to regulate these phenomena.
No significant correlations were found between the S100B protein and VEGF levels at six months post-IS, either in the main group (r = −0.088) or the control group (r = 0.195). However, IL-6, VEGF, CNTF, the S100B protein, endoglin (CD105), and APAs play critical roles in promoting mechanisms associated with pediatric IS, including neuroinflammation and blood–brain barrier disruption, which contribute to dysregulated angiogenesis in ischemic areas. The serum levels of IL-6, VEGF, CNTF, S100B, CD105, and APAs are essential biomarkers for pediatric IS. These markers, detectable in peripheral blood, provide insights into the extent of brain tissue damage and long-term neurological prognosis.
The study of such reliable biomarkers for IS in children has a vital role in Moldova, as well as worldwide. Over recent decades, there has been an alarming trend in the neuropsychological health of children globally and in the Republic of Moldova. The pediatric morbidity, mortality, and rates of neuropsychological disabilities remain high [
9]. DALYs (disease-adjusted life years) in children, frequently accompanied by psychoneurological developmental disorders, are also of significant concern. In children under three years old in the Republic of Moldova, neurological conditions account for 65–70% of morbidity [
9].
While the incidence of pediatric stroke is lower than in adults, it has been rising due to improved recognition of the disease, from 2.5 to 2.7 per 100,000 children per year in the 1990s to 10.7 per 100,000 per year by 2002. Stroke is now among the top 10 causes of childhood mortality. However, the issue of DALYs in children remains understudied. Its causes are complex and multifactorial, and the disease often progresses rapidly, rendering earlier research outdated [
16]. Ethical and logistical challenges continue to impede further research into the etiology and manifestations of pediatric IS.
Moral dilemmas often arise in diagnosing diseases in their early stages. The etiopathogenetic factors contributing to DALYs (Disability-Adjusted Life Years) are diverse; in some cases, significant risk factors may be absent from the diagnostic framework [
16].
Over the past century, IS emerged as a critical global concern, with imaging modalities becoming indispensable for diagnosis. Pediatric stroke is now recognized as a major neurological emergency and a leading cause of morbidity and mortality. In the neonatal period, stroke occurs in approximately 1 in 2500 to 4000 live-born infants, while in infants older than one month, the incidence is 1.2 to 8 per 100,000 [
17]. Unlike in adults, the clinical manifestations of stroke in children are age-dependent, often subtle, and exhibit variable clinical presentations. Stroke may even occur during pregnancy or immediately postpartum without prominent symptoms [
18]. The mortality rate for pediatric stroke ranges between 5% and 10%, with over half of survivors experiencing long-term neurological sequelae. Additionally, 10–20% of survivors face stroke recurrence. These statistics underscore the need for specialized emergency care and rehabilitation centers, a multidisciplinary approach, and highly specialized diagnostic and therapeutic strategies. These efforts place significant demands on healthcare systems, families, and communities. Therefore, studying the predictive and therapeutic aspects of pediatric stroke in early developmental stages is essential to optimize medical, psychological, and educational resources.
Clinical, Functional, Imaging, and Immunologic Correlates of Ischemic Stroke in Children
An analysis of the relationship between laboratory markers and the severity of clinical manifestations, as assessed by the PedNIHSS score, revealed a positive linear correlation between most markers and the size of the ischemic focus based on imaging results and electrophysiological abnormalities. However, an inverse linear correlation was observed for endoglin, and the S100B protein levels showed a distinct relationship with NSG (neuronal-specific gamma-enolase) and CT/MRI brain imaging findings in children (
Table 2).
A correlation analysis of the data obtained more than six months post-stroke, including laboratory markers, clinical evaluations, and imaging results, demonstrated a strong direct relationship with most of the studied markers, with the exception of the S100B protein (
Table 3). These findings suggest that the selected factors used to assess the health status of children who had experienced a stroke were both demonstrative and clinically relevant.
An analysis of the variables assessed in ischemic stroke in children revealed significant correlations between changes in immunoenzymatic markers and clinical–paraclinical parameters.
Markers such as IL-6, VEGF, CNTF, APAs, ENG, and S-100B demonstrated correlations of varying intensity with the PedNIHSS score, imaging results, and electrophysiological findings. These correlations underscore the selective role of these enzymes in determining the disease severity, the size of the ischemic focus, and the prognosis for neurological sequelae.
Notably, the peak VEGF levels were observed during the acute phase of the disease. A strong positive correlation was identified between the serum VEGF levels and disease severity, as assessed by the PedNIHSS score (r = 0.800). High correlations were also observed between the VEGF levels and infarct size, as determined by imaging (CT: r = 0.761; MRI: r = 0.801). These findings indicate that larger infarct volumes were associated with higher serum VEGF levels.
The analysis of electrophysiological manifestations (r = 0.744) demonstrated a high risk of seizure occurrence in children with ischemic stroke. A strong direct correlation of VEGF (r = 0.678) with patients’ functional outcomes six months post-stroke was also observed, alongside correlations with the size of the pathological focus (r = 0.671) and electrophysiological abnormalities (r = 0.644). The elevated levels of VEGF detected in peripheral circulation during acute pediatric stroke and their correlation with the disease severity and infarct size highlight VEGF as a potential peripheral marker for acute stroke in children. Furthermore, the strong correlation of VEGF with the long-term functional outcomes supports its role as a prognostic marker for post-stroke neurological sequelae.
VEGF is thus regarded as a clinical marker for assessing the disease severity and volume of the affected area and a reliable prognostic marker for long-term neurological outcomes. The detection of elevated VEGF levels during acute stroke and their strong correlation with functional manifestations underscore its utility as a sufficient peripheral biomarker for both acute stroke assessment and prognostic purposes.
The study revealed several significant reciprocal correlations, including the following:
- (1)
IL-6: A very strong direct correlation with the disease severity (r = 0.901) and the size of the ischemia area (r = 0.881) and a strong correlation with electrophysiological manifestations (r = 0.686).
- (2)
CNTF: A moderate direct correlation with the disease severity (r = 0.532) and the size of the ischemia area (r = 0.543), and a weak correlation with electrophysiological manifestations (r = 0.368).
- (3)
APAs: A moderate direct correlation with the disease severity (r = 0.553) and the size of the ischemia area (r = 0.543), and a weak correlation with electrophysiological manifestations (r = 0.368).
- (4)
S100B protein: A weak direct correlation with the disease severity (r = 0.311), the size of the ischemia area (r = 0.326), and electrophysiological manifestations (r = 0.350).
- (5)
Endoglin (ENG/CD105): A moderate inverse correlation with the disease severity (r = −0.434) and the size of the ischemia area (r = −0.444), and a weak inverse correlation with electrophysiological manifestations (r = −0.293).
4. Discussion
The results highlighted very strong correlations of the serum IL-6 and VEGF levels with the disease severity and strong correlations with the infarct size and functional outcomes six months post-stroke. For markers such as CNTF, APAs, endoglin, and the S100B protein, the correlations ranged from moderate to weak. Nonetheless, a statistically significant but non-linear relationship was identified between the S100B protein levels and disease severity (p < 0.023), ischemic focus size (p < 0.017), and six-month functional outcomes (p < 0.052).
Additionally, significant correlations were observed between the ENG levels and variables such as the disease severity, infarct size, functional outcomes, APAs, IL-6, VEGF, and CNTF (p < 0.0001 to p < 0.001). These findings support VEGF being a strong peripheral marker for acute pediatric IS and IL-6 being a strong marker of inflammation in this context. CNTF, APAs, endoglin (CD105), and S100B were identified as supplementary markers, ranked in descending order of relevance.
However, the role of ENG/CD105 in recovery after stroke and brain injury remains unclear. Our findings revealed that lower serum CD105 concentrations in children with acute stroke impaired recovery processes. A deficiency of ENG/CD105 may hinder recovery from ischemic brain injuries. Although the literature on the role of ENG is inconclusive, evidence suggests its involvement in stroke recovery processes.
Endoglin (ENG) is recognized as a receptor associated with transforming growth factor β (TGFβ) and plays a critical role in vasculogenesis and angiogenesis. Angiogenesis is vital for the development of cerebral vasculature and the pathogenesis of cerebrovascular diseases, including pediatric ischemic stroke. Ischemia triggers a significant increase in the microvascular density, indicative of angiogenesis, within the penumbra of cerebral infarctions. Enhanced angiogenesis has been linked to improved functional outcomes in both animal models and human stroke patients. Research by Choi E.J., Walker E.J., and others has shown a high expression of ENG in the penumbra of human stroke lesions, correlating with increased angiogenesis [
11,
18]. These findings are consistent with the present study, which identified that ENG deficiency impairs recovery in cerebral ischemic lesions by reducing angiogenesis, delaying inflammation resolution, and exacerbating ischemic damage. These impairments contribute to hypoxia and delayed neurobehavioral recovery.
The researchers Coelho Junior H.J. and Gambassi B.B. et al. demonstrated that cytokine levels increase following a stroke due to the heightened production of inflammatory cells, glial cells, and neurons [
6]. Key cytokines involved include IL-1, IL-6, IL-10, tumor necrosis factor alpha, and TGFβ. This aligns with the current findings, which underscore the role of VEGF as a strong prognostic marker in pediatric IS. The detection of elevated serum VEGF levels at disease onset and their dynamic presence over six months showed a significant correlation with patients’ functional status at six months post-stroke. These observations establish VEGF as a reliable predictor of the prognosis in children with IS.
Another significant finding was that comorbidities in children with acute IS did not influence the levels of the investigated biomarkers in peripheral circulation. This suggests that some biomarkers are specifically indicative of brain lesions in children with IS. Elevated VEGF levels in peripheral circulation were strongly associated with childhood disabilities, such as paresis, paralysis, communication disorders, and epilepsy. Consequently, VEGF can be considered a sufficient marker for predicting the IS prognosis in children and for reflecting the presence of brain injury detectable in peripheral blood during IS events.
Understanding the involvement of immunoenzymatic factors in ischemic brain injury may offer new therapeutic opportunities to improve outcomes in children. Data from the biomolecular scientific literature further highlight the essential role of biomarkers in diagnosis, evaluating the neurological prognosis, understanding pathogenesis, and promoting recovery in pediatric IS. Inflammatory markers, such as pro-inflammatory cytokines IL-6 and IL-1β, as well as other biological factors like VEGF, ciliary neurotrophic factor (CNTF), the S100B protein, ENG (CD105), and antiphospholipid antibodies, have been identified as critical contributors.
Among these, the S100B protein is the most extensively studied biomarker in IS. It has intracellular and extracellular functions, including regulating calcium homeostasis and signaling as a secondary messenger. In experimental settings, S100B promotes cell differentiation, cell cycle progression, and the inhibition of apoptosis. Extracellularly, it supports neurogenesis and neuronal plasticity and enhances learning and memory processes in both normal and traumatic conditions.
The role of VEGF in promoting angiogenesis, the role of S100B in neurogenesis, and the findings on ENG deficiency’s impact on recovery processes emphasize the importance of further exploring these biomarkers. Collectively, these insights offer critical avenues for understanding and improving interventions in pediatric ischemic stroke.
This study highlights the role of interleukin-6 (IL-6) in promoting and sustaining neuroinflammation in pediatric ischemic stroke (IS), with an emphasis on evaluating the extent of brain lesions and subsequent sequelae [
18]. The findings suggest that IL-6 serves as a neuroinflammatory biomarker in IS. Based on the confirmed inflammatory role of IL-6 in stroke progression, this study suggests incorporating anti-inflammatory therapies to complement standard treatments, offering a novel approach for enhancing recovery following ischemic stroke.
Choi E. et al. have identified vascular endothelial growth factor (VEGF) and ciliary neurotrophic factor (CNTF) as key biomarkers in stroke. In animal models, CNTF, which is endogenously upregulated during IS onset, mediates neurogenesis and exerts anti-inflammatory effects. The present study corroborated these findings, emphasizing the importance of CNTF as a biomarker. Specifically, CNTF is involved in increased neurotoxicity during the early stages of IS and the progression of neuroinflammatory and neurodegenerative processes and has a protective role in brain tissue during disease progression. CNTF appears to limit the pathological spread in ischemic areas and prevent neuronal death, particularly safeguarding motor and sensory neurons.
VEGF is implicated in processes such as atherosclerosis, arteriogenesis, cerebral edema, neuroprotection, neurogenesis, angiogenesis, and post-ischemic brain repair [
18]. Additionally, VEGF contributes to the effects of transplanted stem cells in experimental IS research. Research by Mackenzie F. et al. demonstrated that vascular growth factors, including VEGF-A, VEGF-B, and placental growth factor (PlGF), play pivotal roles in the development and function of the circulatory and nervous systems, further supporting their involvement in stroke through the interaction of these systems [
19]. Both the literature and the current findings indicate that VEGF is a potent peripheral biomarker in acute pediatric IS. The strong correlation between VEGF levels and the functional outcomes six months post-stroke reinforces its role as a marker for long-term neurological sequelae. VEGF is thus considered a clinical marker for assessing distress, the infarct volume, and the prognosis in pediatric IS.
VEGF exhibits direct trophic and protective effects on neurons, making its vascular and neuronal roles highly relevant to IS. Several studies [
20,
21] highlight the effects of cerebral ischemia on VEGF expression and its receptors, particularly in the ischemic penumbra (the transitional zone between the infarct core and the surrounding region). This penumbra remains partially perfused, and its fate significantly influences the clinical outcomes. Kimura R. et al. identified VEGF-A as the principal mediator of cerebral angiogenesis, with increased levels observed after stroke in both rodent models and humans. Angiogenesis in the brain is a tightly regulated process governed by neuroectodermal-derived growth factors that activate tyrosine kinase receptors on endothelial cells.
Studies by Bouvier D. et al. and Calcagnile O. et al. validated the S100B protein as a biomarker for brain injury [
22]. The current study confirmed its significance in IS, demonstrating that higher S100B levels are associated with more severe brain injuries and longer recovery times. These findings align with previous research, underscoring S100B’s role in promoting inflammation.
A notable clinical and fundamental contribution of this study is the dysregulation of angiogenesis mechanisms in pediatric IS, evidenced by low endoglin levels, alongside disrupted thrombotic mechanisms indicated by increased antiphospholipid antibody (APA) levels. Elevated APA levels reflect the activation of coagulation cascades, contributing to thrombogenesis. Additionally, the study assessed and described the activation of ischemic remodeling processes, marking a significant advancement in understanding pediatric IS pathophysiology.
Increased levels of VEGF, CNTF, and S100B proteins contribute to promoting neuronal plasticity and stimulating angiogenesis in the context of inflammation. The practical implications of these novel pathophysiological mechanisms include the potential to predict ischemic stroke in children by evaluating inflammatory biomarkers such as IL-6, S100B, and CNTF. Irregularities in the serum concentrations of these markers reveal common features of the acute phase of pediatric IS, including inflammation and toxicity characterized by significant elevations in IL-6, S100B, and CNTF. These changes contribute to early neurological depression and infarct expansion.
Another key practical application of the study’s findings is the ability to assess the severity of IS in children by analyzing disruptions in homeostasis, as evidenced by the elevated serum levels of VEGF, CNTF, and S100B. The clinical utility of S100B lies in its role as a marker for brain injury and its predictive value for neurorecovery processes. Higher levels of S100B are associated with more severe recovery trajectories and increased neurological complications. This biomarker can guide the selection of appropriate neurorehabilitation strategies on a case-by-case basis.
So, as the results of the given study showed, IS in children is a progressing problem worldwide, and the literature analysis on this subject also confirms that. Addressing this challenge requires the integration of computing algorithms supported by AI technologies into routine clinical practice. Such modern diagnostic approaches have the potential to significantly enhance human decision-making for the timely recognition of stroke-related warning signs [
15,
23,
24,
25]. Establishing efficient pediatric diagnosis and monitoring systems necessitates reliable and recent data sets. This pilot study aimed to identify critical immunoenzymatic parameters that can serve as early predictors of pediatric stroke, facilitating the creation of a meaningful database for machine learning applications and artificial neural network development [
15,
26,
27,
28,
29,
30]. Examples of such a successful approach that includes the integration of medical data sets and machine learning for prediction construction were shown in predictive cardiology, when step-by-step data set development—from the discovery of new fundamental cardiovascular mechanisms [
28] to their recognition in biosignal registrations and testing under different psychoemotional conditions [
29,
30]— was transformed into predictive construction models that provided the clinical decision-makers with essential information. So, the important results of the present study show that IL-6, VEGF, CNTF, the S100B protein, CD105 (endoglin), and APAs play a significant role in promoting mechanisms that characterize pediatric ischemic stroke. They are among the triggers of neuroinflammation and contribute to the disruption of cerebral circulation, ultimately leading to the deregulation of angiogenesis in the cerebral ischemic focus. IL-6, VEGF, CNTF, S100B, CD105, and APAs are important markers of pediatric ischemic stroke that can be detected in peripheral blood, reflecting the extent of brain tissue damage and the long-term neurological prognosis. These diagnostic parameters will aid specialists in pediatric monitoring and facilitate early stroke diagnosis in children.
The findings of this study emphasize the significant role of VEGF and IL-6 as key biomarkers in pediatric ischemic stroke (PIS). The strong correlations observed between the serum IL-6 and VEGF levels and the disease severity, infarct size, and functional outcomes at six months post-stroke reinforce their prognostic value. Additionally, while CNTF, APAs, endoglin (CD105), and the S100B protein showed moderate to weak correlations, their statistical significance underscores their potential supplementary role in evaluating pediatric IS.
This study underscores the complex interplay between angiogenesis and neuroinflammation in stroke recovery. VEGF emerges as a potent peripheral marker for acute pediatric IS, whereas IL-6 serves as a strong inflammatory marker in this context. Further research into supplementary markers, including CNTF, APAs, ENG/CD105, and S100B, is necessary to refine their clinical applications.
A key aspect of this research was the role of ENG/CD105 in stroke recovery. The observed lower serum concentrations of ENG/CD105 in children with acute stroke suggest that its deficiency may impair angiogenesis, delay inflammatory resolution, and exacerbate ischemic damage. While the literature on ENG/CD105 remains inconclusive, previous studies have indicated its involvement in stroke recovery. Kirton, A et al. demonstrated an increased expression of ENG in human stroke penumbra regions, correlating with enhanced angiogenesis [
31,
32]. Similarly, research by Deveber, G. supports the role of cytokines such as IL-6, IL-1β, and VEGF in stroke pathology and recovery [
33,
34]. Moreover, this study establishes VEGF as a predictor of the neurological prognosis in children with IS, with elevated VEGF levels in peripheral circulation strongly linked to childhood disabilities, including paresis, paralysis, and communication disorders. These findings are consistent with prior biomolecular studies highlighting the importance of inflammatory and angiogenic biomarkers in pediatric stroke [
35]. The results suggest that targeted therapeutic approaches focusing on modulating the IL-6 and VEGF pathways could improve recovery outcomes. The incorporation of anti-inflammatory strategies alongside standard stroke management holds promise for enhancing functional recovery in pediatric IS [
36]. Future research should aim to expand the data set to improve generalizability. A larger cohort obtained through multicenter collaboration and extended study periods would enhance the robustness of these findings. Additionally, longitudinal studies could further elucidate the dynamic roles of these biomarkers throughout stroke progression and recovery. VEGF-A is a key mediator of cerebral angiogenesis, with increased levels observed after IS in rodents and humans [
37]. Brain angiogenesis is a well-regulated process controlled by neuroectoderm-derived growth factors that bind to tyrosine kinase receptors expressed on endothelial cells [
2]. In the rat brain, angiogenesis is completed around postnatal day 20. However, in the adult brain, under pathological conditions such as hypoxia/ischemia and brain tumor growth, endothelial cells can proliferate. Current evidence suggests that physiological angiogenesis in the brain is regulated by mechanisms similar to those of pathological angiogenesis induced by tumors or hypoxia/ischemia [
38]. Hypoxia-inducible endothelial mitogen, vascular permeability factor, and VEGF appear to play essential roles in most of these processes. VEGF is expressed when angiogenesis is upregulated, as seen in the embryonic neuroectoderm, glioblastomas, and around infarcts, whereas it is expressed at low levels in the absence of angiogenesis, such as in the adult neuroectoderm. Additionally, the induction of angiogenesis through growth factors (pro-angiogenesis) could be a rational therapy for IS patients [
38]. VEGF is important in branched morphogenesis. Data presented by Mcglennan, C. et al. support the idea that the collateral density and vascular branching are closely linked [
39]. Few studies have analyzed whether endogenous VEGF mediates collateral growth. It is suggested that VEGF acting through VEGFR-1 mediates collateral remodeling. This contrasts with the role of VEGFR-1 as a VEGF inhibitor during development [
39,
40,
41]. Other research indicates that measuring VEGFR-1 signaling is challenging due to its weak kinase activity [
40]. However, it has been found that VEGF expression determines the collateral density in secondary tissues, and high VEGF levels contribute to collateral formation in the embryo. In our clinical study on children with IS, we found that the VEGF levels in the acute phase of the disease were significantly higher in the study group than in the control group, which is consistent with the findings of other experimental and clinical studies.
Acute ischemic stroke (AIS) is a heterogeneous disease, and the elevated levels of the studied biomarkers showed varied results correlated with the severity of the ischemic lesion [
40]. The ischemic focus can be identified using brain MRI, while the assessment of certain immunological biomarkers can provide insights into the mechanisms and severity of AIS, a finding strongly supported by the results of our study. Thus, patients with severe AIS, characterized by pronounced neurological symptoms, exhibited significantly elevated levels of S100B, VEGF, CNTF, IL-6, and APAs, along with decreased CD105 levels. In contrast, in mild to moderate AIS cases, these values fluctuated, showing slight to moderate abnormal increases. Although the primary goal of studying AIS biomarkers is to assess their correlation with the disease severity, as well as the size and topography of the ischemic lesion detected on MRI scans, further research is necessary to evaluate their role in optimizing treatment strategies for improving both early and long-term neurological outcomes. The application of tests for endoglin (CD105), S100B, APAs, IL-6, CNTF, and VEGF significantly enhances diagnostic accuracy, helps determine the disease severity, and predicts the long-term neurological outcomes. The likelihood of detecting neurological issues increases with an excessive rise in certain biomarkers and a decline in others, underscoring the need for early therapeutic intervention in all patients suspected of having AIS.
Prediction also regards certain bioethics issues that should be taken in account, especially in the case of prediction in child care [
42]. Artificial intelligence (AI) tools, such as chatbots (ChatGPT, DeepSeek, Claude, Meta AI, Zapier Agents), search engines (Perplexity, Google AI Overviews, Arc Search), content creation (Jasper, Anyword, Writer), video creation and editing (Runway, Descript, Wondershare Filmora), image generation (DALL·E 3, Midjourney, Ideogram), knowledge management and AI grounding (Mem, Notion AI Q&A, Personal AI), task and project management (Asana, Any.do, BeeDone), etc., have been asserting themselves, in recent years, as elements in pediatric diagnostics that can change traditional emphases. Thus, AI is currently associated with a series of important challenges, with new perspectives and obstacles but also involving new possibilities and new opportunities. Since its inception, AI has been thought of and developed more as a substitute and replacement for human expertise in all spheres of life, but also to make the decision-making and approval process more rational. The last decades demonstrate that AI is becoming more than a tool for improving and qualitatively amplifying the medical decision-making process in diagnosis and treatment. This tool, AI, significantly influences the reassessment of traditional ethical principles and values in medicine in general and in pediatric diagnostics, in particular. Therefore, AI challenges the universality and validity of traditional ethical values and principles in medicine; on the other hand, AI generates new realities that require new attitudes, based on strengthened beliefs about Good and Evil—the most important moral and ethical categories. For example, in pediatric diagnostics, AI amplifies ethical issues by disregarding the vulnerable status of the child who is subject to the diagnosis of the disease and its treatment. Thus, the minor patient is not only a recipient of synthesized knowledge, but also a source of new data and information for future patients, data that will not be able to meet future confidentiality requirements and standards, resulting from the simple fact that the current data protection mechanisms have been imperfect from the start in the context of their permanent improvement. The vulnerability status of the minor patient, in the context of the use of various AI tools, is very often disregarded and relativized. We will advocate, in this context, that future research using various AI tools should consequently focus not only on obtaining comprehensive data to ensure the generalizability of research results, but also be more strictly coordinated with the new guidelines and requirements of medical and research ethics, which will be developed by competent collegial forums.
The next step is to adopt these biomarker data sets for training a fuzzy classifier-based diagnostic model and to develop machine learning algorithms [
24,
25,
26,
27,
42,
43] for prompt, time-saving stroke recognition, contributing to more efficient pediatric monitoring, diagnosis, and prediction.
5. Conclusions
- -
Elevated serum concentrations of S100B, VEGF, CNTF, IL-6, APAs, and decreased END levels are predictive immunoenzymatic biomarkers in pediatric IS.
- -
The S100B protein is a marker for brain injury and neurorecovery prediction. Higher S100B levels indicate more severe recovery processes and increased neurological complications. It is recommended that S100B be assessed in all children with IS to determine the severity and progression of IS and the effectiveness of neurorecovery strategies.
- -
VEGF serves as a clinical biomarker for infarct volume assessment and as a prognostic marker for the long-term neurological outcomes post-IS.
- -
The identification of serum concentration imbalances in the studied markers allowed for the determination of common characteristics in the acute phase of IS in children: (1) the presence of inflammation and toxicity, mediated by significantly increased IL-6 and CNTF levels, contributing to early neurological depression and infarct expansion; (2) homeostasis disruption, confirmed by elevated serum VEGF and CNTF levels, indicating the disease severity; (3) the dysregulation of angiogenesis mechanisms, as evidenced by low CD105 (endoglin) levels, which influence the disease prognosis; (4) the disturbance of thrombotic mechanisms due to increased APA levels, reflecting coagulation cascade activation; and (5) the activation of ischemic remodeling processes, indicated by increased levels of VEGF and CNTF, which promote neuronal plasticity and stimulate blood vessel formation under inflammatory conditions.
- -
A six-month follow-up VEGF assessment in IS patients revealed that persistently elevated serum levels correlated with ongoing pro-inflammatory processes, delayed recovery, and a higher likelihood of epileptogenesis. VEGF serves as a peripheral prognostic marker for predicting long-term neurological complications and sequelae.