Can Anaerobically Digested Food Effluent Support Arthrospira platensis Cultivation in Open Ponds?
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Culture
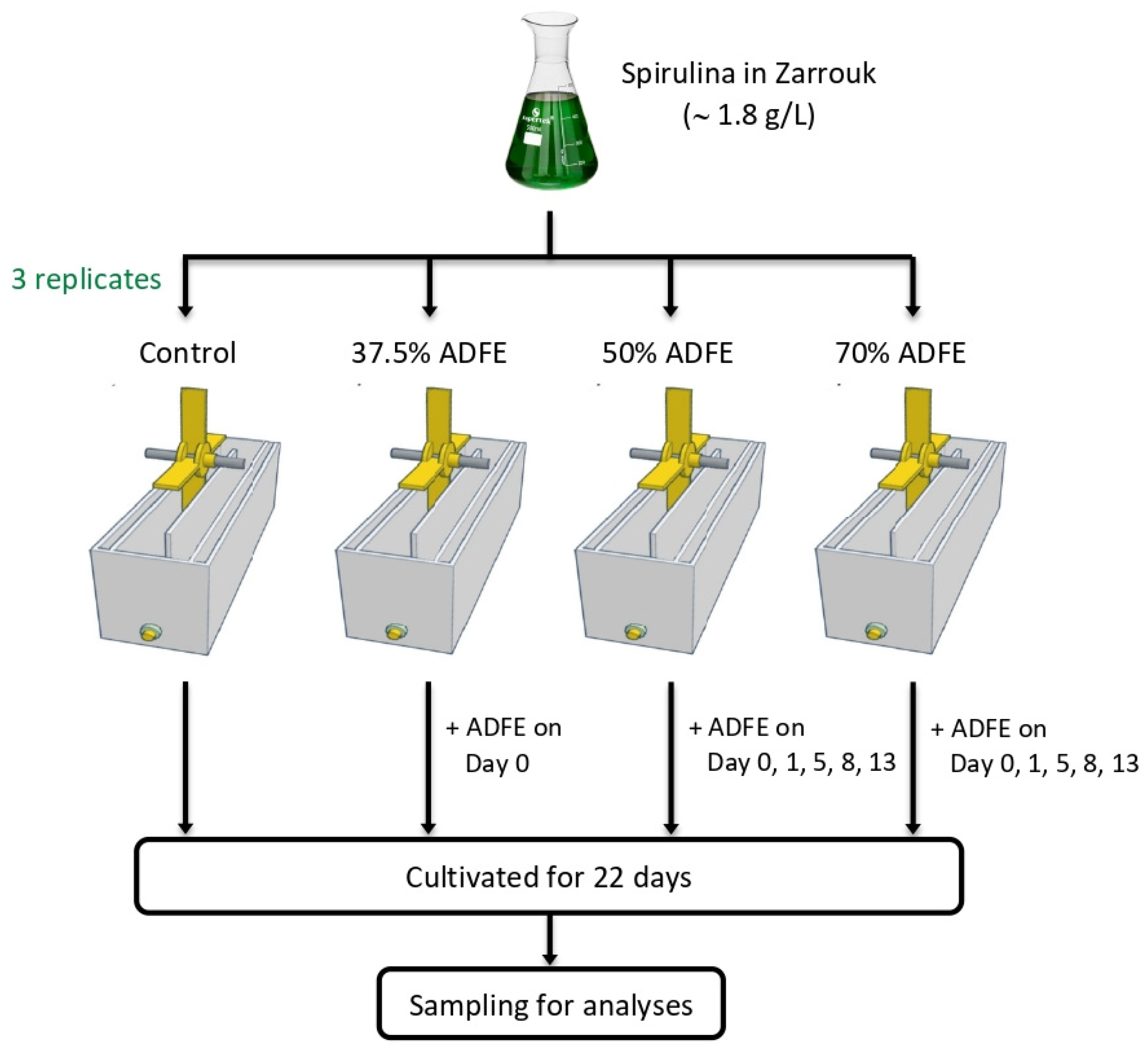
2.2. Experimental Setup and Cultivation Conditions
2.3. Biomass and Chlorophyll a Determination
2.4. Analytical Methods
2.5. Public Data Sources
2.6. Statistical Analysis
3. Results
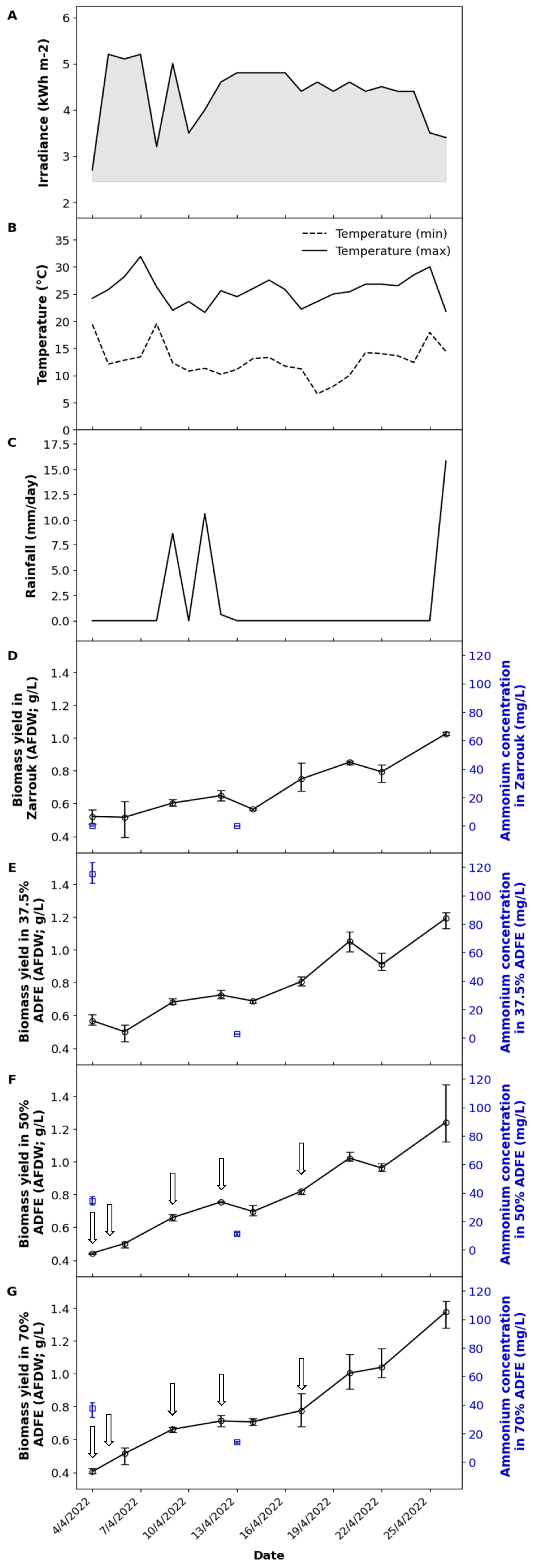
3.1. Culture Conditions
3.2. Physicochemical Parameters and Ammonium Content
3.3. Chlorophyll a Content of Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, E.R.; Van Vliet, M.T.H.; Qadir, M.; Bierkens, M.F.P. Country-Level and Gridded Estimates of Wastewater Production, Collection, Treatment and Reuse. Earth Syst. Sci. Data 2021, 13, 237–254. [Google Scholar] [CrossRef]
- Ahuja, S. Water Quality Worldwide. In Handbook of Water Purity and Quality; Academic Press: Cambridge, MA, USA, 2021; pp. 19–33. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Natasha; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Analysing Global Food Waste Problem: Pinpointing the Facts and Estimating the Energy Content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- He, L.; Lin, Z.; Zhu, K.; Wang, Y.; He, X.; Zhou, J. Mesophilic Condition Favors Simultaneous Partial Nitrification and Denitrification (SPND) and Anammox for Carbon and Nitrogen Removal from Anaerobic Digestate Food Waste Effluent. Sci. Total Environ. 2022, 816, 151498. [Google Scholar] [CrossRef]
- Li, G.; Bai, X.; Li, H.; Lu, Z.; Zhou, Y.; Wang, Y.; Cao, J.; Huang, Z. Nutrients Removal and Biomass Production from Anaerobic Digested Effluent by Microalgae: A Review. Int. J. Agric. Biol. Eng. 2019, 12, 8–13. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent Developments in Physical, Biological, Chemical, and Hybrid Treatment Techniques for Removing Emerging Contaminants from Wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Mesa, A.P.; Grattz, P.A.C.; Vargas, J.J.V.; Ríos, L.A.; Echeverri, D.O.; Parra, A.M.M. Feasibility of Nitrogen and Phosphorus Removal from Treated Wastewater Using Microalgae and Potential Microalgae Use as Biofertilizer. J. Water Process Eng. 2025, 70, 107023. [Google Scholar] [CrossRef]
- Das, V.; Dunford, N.; Deka, D. Waste Utilization and Biodiesel Production from Desmodesmus Maximus Grown in Swine Wastewater Using Waste Eggshells as a Catalyst. Aquac. Eng. 2022, 99, 102293. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Moheimani, N.R.; Ubi, B.E.; Ogbonna, J.C.; Vadiveloo, A.; Pluske, J.R.; Huisman, J.M. Macroalgae Culture to Treat Anaerobic Digestion Piggery Effluent (ADPE). Bioresour. Technol. 2017, 227, 15–23. [Google Scholar] [CrossRef]
- Chuka-ogwude, D.; Ogbonna, J.; Moheimani, N.R. A Review on Microalgal Culture to Treat Anaerobic Digestate Food Waste Effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of Microalgae on Undiluted Anaerobic Digestate of Piggery Effluent with High Ammonium Concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient Recovery from Wastewater Streams by Microalgae: Status and Prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging Applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Hossain, M.S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A Review on Spirulina: Alternative Media for Cultivation and Nutritive Value as an Aquafeed. Rev. Aquac. 2020, 12, 2371–2395. [Google Scholar] [CrossRef]
- Chalermthai, B.; Charoensuppanimit, P.; Nootong, K.; Olsen, B.D.; Assabumrungrat, S. Techno-Economic Assessment of Co-Production of Edible Bioplastic and Food Supplements from Spirulina. Sci. Rep. 2023, 13, 10190. [Google Scholar] [CrossRef]
- El-Sayed, A.E.-K.; El-Sheekh, M. Outdoor Cultivation of Spirulina Platensis for Mass Production. Not. Sci. Biol. 2018, 10, 38–44. [Google Scholar] [CrossRef]
- Cheunbarn, S.; Peerapornpisal, Y. Cultivation of Spirulina Platensis Using Anaerobically Swine Wastewater Treatment Effluent. Int. J. Agric. Biol. 2015, 12, 586–590. [Google Scholar]
- Pumas, P.; Pumas, C. Cultivation of Arthrospira (Spirulina) platensis Using Low Cost Medium Supplemented with Lac Wastewater. Chiang Mai. J. Sci. 2016, 43, 1037–1047. [Google Scholar]
- Lucakova, S.; Branyikova, I.; Branyik, T.; Matoulkova, D.; Krausova, G. Wastewater from the Demineralization of Cheese Whey for Cost-Efficient Cultivation of Spirulina. J. Appl. Phycol. 2022, 34, 89–99. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.; Noh, W.; Yeom, D.H.; Kim, S.; Kim, D.W.; Kim, J. Regeneration of Nitrate and Phosphate from Toilet Wastewater Using Waste Alumina Adsorbent for Cultivation of Spirulina Platensis. Environ. Eng. Res. 2020, 25, 393–399. [Google Scholar] [CrossRef]
- Nogueira, S.M.S.; Junior, J.S.; Maia, H.D.; Saboya, J.P.S.; Farias, W.R.L. Use of Spirulina Platensis in Treatment of Fish Farming Wastewater. Rev. Cienc. Agron. 2018, 49, 599–606. [Google Scholar] [CrossRef]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual Productivity of Spirulina (Arthrospira) and Nutrient Removal in a Pig Wastewater Recycling Process under Tropical Conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Matos, Â.P.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Anaerobic Digestate Abattoir Effluent (ADAE), a Suitable Source of Nutrients for Arthrospira Platensis Cultivation. Algal. Res. 2021, 54, 102216. [Google Scholar] [CrossRef]
- Nematollahi, M.A.; Laird, D.W.; Hughes, L.J.; Raeisossadati, M.; Moheimani, N.R. Effect of Organic Carbon Source and Nutrient Depletion on the Simultaneous Production of a High Value Bioplastic and a Specialty Pigment by Arthrospira platensis. Algal Res. 2020, 47, 101844. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, b, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Und Physiol. Der Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae-Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Raeisossadati, M.; Vadiveloo, A.; Bahri, P.A.; Parlevliet, D.; Moheimani, N.R. Treating Anaerobically Digested Piggery Effluent (ADPE) Using Microalgae in Thin Layer Reactor and Raceway Pond. J. Appl. Phycol. 2019, 31, 2321. [Google Scholar] [CrossRef]
- Chuka-ogwude, D.; Ogbonna, J.; Borowitzka, M.A.; Moheimani, N.R. Screening, Acclimation and Ammonia Tolerance of Microalgae Grown in Food Waste Digestate. J. Appl. Phycol. 2020, 32, 3775–3785. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of Liquid Digestate Treatment on Sustainable Microalgae Biomass Production. Bioenergy Res. 2021, 15, 357–370. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.P. Anaerobic Digestate as Substrate for Microalgae Culture: The Role of Ammonium Concentration on the Microalgae Productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S. Effect of Ammonia Concentrations on Growth of Chlorella vulgaris and Nitrogen Removal from Media. Bioresour. Technol. 1996, 57, 45–50. [Google Scholar] [CrossRef]
- Magro, F.G.; Margarites, A.C.; Reinehr, C.O.; Gonçalves, G.C.; Rodigheri, G.; Costa, J.A.V.; Colla, L.M. Spirulina Platensis Biomass Composition Is Influenced by the Light Availability and Harvest Phase in Raceway Ponds. Environ. Technol. 2018, 39, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Belay, A. Mass Culture of Spirulina Outdoors-the Earthrise Farms Experience. In Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology; Vonshak, A., Ed.; Taylor and Francis Group, CRC Press: London, UK, 1997; pp. 131–158. [Google Scholar]
- Kaushik, R.; Prasanna, R.; Joshi, H.C. Utilization of Anaerobically Digested Distillery Effluent for the Production of Spirulina platensis (ARM 730). J. Sci. Ind. Res. 2006, 65, 521–525. [Google Scholar]
- Ajijah, N.; Tjandra, B.C.; Hamidah, U.; Widyarani; Sintawardani, N. Utilization of Tofu Wastewater as a Cultivation Medium for Chlorella Vulgaris and Arthrospira Platensis. In Proceedings of the 4th International Symposium on Green Technology for Value Chains, Tangerang, Indonesia, 23–24 October 2019; IOP Conference Series: Earth and Environmental Science. Institute of Physics Publishing: Bristol, UK, 2020; Volume 483. [Google Scholar]
- Krishnamoorthy, S.; Manickam, P.; Muthukaruppan, V. Evaluation of Distillery Wastewater Treatability in a Customized Photobioreactor Using Blue-Green Microalgae—Laboratory and Outdoor Study. J. Environ. Manag. 2019, 234, 412–423. [Google Scholar] [CrossRef]
- Matos, Â.P.; da Silva, T.; Sant’Anna, E.S. The Feasibility of Using Inland Desalination Concentrate (DC) as an Alternative Substrate for Spirulina platensis Mass Cultivation. Waste Biomass Valorization 2021, 12, 3193–3203. [Google Scholar] [CrossRef]
- McDowell, D.; Dick, J.T.; Eagling, L.; Julius, M.; Sheldrake, G.N.; Theodoridou, K.; Walsh, P.J. Recycling Nutrients from Anaerobic Digestates for the Cultivation of Phaeodactylum Tricornutum: A Feasibility Study. Algal. Res. 2020, 48, 101893. [Google Scholar] [CrossRef]
- Sánchez-Quintero, Á.; Leca, M.A.; Bennici, S.; Limousy, L.; Monlau, F.; Beigbeder, J.B. Treatment and Valorization of Agro-Industrial Anaerobic Digestate Using Activated Carbon Followed by Spirulina Platensis Cultivation. Sustainability 2023, 15, 4571. [Google Scholar] [CrossRef]
- Borges, J.A.; Rosa, G.M.; Meza, L.H.R.; Henrard, A.A.; Souza, M.R.A.Z.; Costa, J.A.V. Spirulina Sp. LEB-18 Culture Using Effluent from the Anaerobic Digestion. Braz. J. Chem. Eng. 2013, 30, 277–287. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Ammonia (mg N-NH3 L−1) | 2800 |
| Nitrate (mg N-NO3− L−1) | <1 |
| NOx-N (mg L−1) | <1 |
| Total nitrogen (mg L−1) | 5300 |
| Total phosphorus (mg L−1) | 520 |
| Total iron (mg L−1) | 16 |
| Total calcium (mg L−1) | 170 |
| Magnesium (mg L−1) | 24 |
| Chemical oxygen demand, COD (mg L−1) | 50,000 |
| Potassium (mg L−1) | 120 |
| Treatment Group | Zarrouk (%) | ADFE (%) | Description |
|---|---|---|---|
| Zarrouk | 100 | 0 | No ADFE added |
| 37.5% ADFE | 62.5 | 37.5 | ADFE added on day 0 |
| 50% ADFE-addition | 50 | 50 | ADFE added incrementally on days 0, 1, 5, 8, 13 |
| 70% ADFE-addition | 30 | 70 | ADFE added incrementally on days 0, 1, 5, 8, 13 |
| Nutrients | Zarrouk | 37.5% ADFE | 50% ADFE | 70% ADFE | Chemical Detail |
|---|---|---|---|---|---|
| NaHCO3 (g) | 336 | 210 | 168 | 100.8 | Merck KGaA, Darmstadt, Germany |
| N-NO3−1 (g) * | 8.3 | 5.19 | 4.15 | 2.49 | Merck KGaA, Darmstadt, Germany |
| NaCl (g) | 20 | 12.5 | 10 | 6 | Merck KGaA, Darmstadt, Germany |
| K2SO4 (g) | 20 | 12.5 | 10 | 6 | Sigma-Aldrich, St. Louis, MO, USA |
| K2HPO4 (g) | 10 | 6.25 | 5 | 3 | Sigma-Aldrich, St. Louis, MO, USA |
| Solution A (mL) ** | 20 | 12.5 | 10 | 6 | Composition specified in the method |
| Solution B (mL) ** | 20 | 12.5 | 10 | 6 | |
| Solution Fe (mL) ** | 20 | 12.5 | 10 | 6 | |
| N-NH3 (g) * | 0 | 3.17 | 4.15 | 5.81 | Richgro, Garden Product, Jandakot, WA, Australia |
| Treatment | Overall Volumetric Productivity (mg L−1 d−1) | Overall Areal Productivity (g m−2 d−1) | Maximum Specific Growth Rate (μmax) | Chlorophyll a (mg/g Biomass) |
|---|---|---|---|---|
| Zarrouk | 22.9 ± 1.76 a | 4.58 ± 0.35 a | 0.093 ± 0.03 a | 6.95 ± 0.76 a |
| 37.5% ADFE | 28.3 ± 1.71 b | 5.66 ± 0.34 b | 0.13 ± 0.03 a | 6.98 ± 0.15 a |
| 50% ADFE-addition | 41.0 ± 0.61 c | 8.20 ± 0.12 c | 0.11 ± 0.01 a | 7.17 ± 0.98 a |
| 70% ADFE-addition | 44.2 ± 3.48 c | 8.83 ± 0.69 c | 0.13 ± 0.01 a | 7.09 ± 0.80 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raeisossadati, M.; Bumandalai, O.; Moheimani, N.R. Can Anaerobically Digested Food Effluent Support Arthrospira platensis Cultivation in Open Ponds? Appl. Sci. 2025, 15, 3115. https://doi.org/10.3390/app15063115
Raeisossadati M, Bumandalai O, Moheimani NR. Can Anaerobically Digested Food Effluent Support Arthrospira platensis Cultivation in Open Ponds? Applied Sciences. 2025; 15(6):3115. https://doi.org/10.3390/app15063115
Chicago/Turabian StyleRaeisossadati, Mohammadjavad, Odgerel Bumandalai, and Navid Reza Moheimani. 2025. "Can Anaerobically Digested Food Effluent Support Arthrospira platensis Cultivation in Open Ponds?" Applied Sciences 15, no. 6: 3115. https://doi.org/10.3390/app15063115
APA StyleRaeisossadati, M., Bumandalai, O., & Moheimani, N. R. (2025). Can Anaerobically Digested Food Effluent Support Arthrospira platensis Cultivation in Open Ponds? Applied Sciences, 15(6), 3115. https://doi.org/10.3390/app15063115





