Abstract
Various advanced oxidation processes have been used to degrade perfluorooctane sulfonate (PFOS), one of the persistent organic pollutants that dissolves in aquatic ecosystems, but these processes suffer from inherent limitations. This study proposes aeration-assisted cold plasma (CP) technology as an alternative. PFOS removal via CP treatment reached 62.5% after 1 h of exposure, with a degradation rate constant of 3.1 h−1. The detection of sulfate (SO42−) in the solution provides evidence of effective PFOS degradation. The close agreement between the measured and estimated fluoride concentrations further confirms mass balance after degradation. Acute toxicity tests indicate that PFOS degradation may initially increase the acute toxicity, possibly due to the formation of degradation by-products. However, this increased toxicity can be mitigated through additional exposure to the reactive species generated by CP. Furthermore, investigations into the energy per order of CP and the quantification of hydroxyl radicals support its operational effectiveness. This study confirms that aeration-assisted CP has the potential to serve as a viable treatment option for mitigating the environmental threats posed by PFOS.
1. Introduction
Per- and polyfluoroalkyl substances (PFASs) are carbon-chain-based chemical compounds containing C-F bonds with high bond dissociation energy (536 kJ/mol) [1]. PFASs have a number of unique physical and chemical properties, including heat resistance and hydrophobicity [2,3]. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) are among the most commonly occurring PFASs, and both have a wide range of commercial, industrial, and public uses, including in firefighting foams, cleaning products, paints, waxes, water-repellent fabrics, and so on [4,5].
In particular, PFOS has been found not only in surface water and sediment downstream of production facilities but also in wastewater treatment plant effluent, sewage sludge, and landfill leachate [6,7,8], and it comes as no surprise that many people have been exposed to it through the consumption of normal water or by exposure to contaminated soil and sediment [9,10]. Although the amount of PFOS detected in many studies is very small, the environmental persistence, tendency to bioaccumulate, and potential toxicity of PFOS have generated great concern [11]. Due to its high biological resistance, PFOS is not naturally biodegradable, leading to its bioaccumulation and persistence in organisms [6,12,13,14,15]. The estimated half-life of PFOS in the environment is 41–97 years, but in adult human bodies it decreases to 4–5 years due to excretion [16,17]. These characteristics have led to the discontinuation of PFOS production in North America and Europe [18]. Moreover, PFOS has been added to Annex B of the Stockholm Convention on persistent organic pollutants (POPs) [19].
Several studies have been conducted to identify the most effective means of destroying PFOS in water to ensure the safety of water, humans, and ecosystems. Activated carbon and ion exchanges have been the primary means used to remove PFOS dissolved in water [1,20,21,22]. However, the generation of by-products that can only be cleaned with adsorbents and the concentrated brine solution used to regenerate the ion exchange resin limit the usage of these means, respectively [23].
Various advanced oxidation processes (AOPs) have been proposed as alternatives [24], primarily because hydroxyl radicals, key agents in the degradation of organic pollutants when using AOPs, can oxidize PFOS [25]. The literature indicates that electrochemical oxidation [26], photocatalysis [27], electrocatalytic oxidation [28], sonolysis [29], and electron beam irradiation [30] may be applicable. These technologies, however, are economically inefficient, as they require additional chemicals and electrical energy [24]. Also, after these treatments by-products still remain, and residual halogen compounds may be harmful to humans [31,32].
To overcome the limitations of conventional AOPs in PFOS treatment, aeration-assisted cold plasma (CP) technology has been applied instead. CP is one of the AOPs that has been examined as being potentially beneficial in wastewater treatment [33,34]. Because the electrical discharge in plasma generation apparatus produces reactive oxygen and nitrogen species (ROS/RNS) such as ozone (O3), hydroxyl radical (·OH), hydrogen peroxide (H2O2), superoxide (O2·−), nitric oxide (·NO), peroxynitrite (ONOO−), and so on, by using air as a carrier gas, tests of CP report a high removal efficiency of non-biodegradable substances [35]. The formation and recombination of radicals are mainly due to their dissociation and ionization during electron collisions [36]. Collisions between electrons and the contents of normal air with humidity can cause many kinds of reactions, as shown in Equations (1)–(3) [37]. The production of hydrogen peroxide through the recombination of hydroxyl radicals can occur in the gas phase or at an interface (Equation (4)) [38,39]. The reaction in Equation (5) is well known to generate ozone through the collision of oxygen molecules [40].
e− + H2O → ·OH + ·H + e−
e− + H2O → H2 + O+ + 2 e−
e− + H2O → H+ + ·H + ·O + 2 e−
·OH + ·OH → H2O2
O + O2 → O3
Produced ROS/RNS move to the liquid phase, accompanying recombination, and subsequently react with target pollutants in the liquid phase; thus, CP requires no additive chemicals or auxiliary equipment due to its in situ generation of ROS/RON [33,41,42]. Despite such advantages, however, little research has focused on the removal of residual PFOS in the water phase via aeration-assisted CP.
This study determines how rapidly PFOS removal occurs from a kinetics standpoint. Additionally, the mass balance upon PFOS degradation was verified by the analytical determination of SO42- and F- in the suspension. We further demonstrate how PFOS degradation is associated with variations in acute toxicity based on definitive evidence. We also confirm the potential of aeration-assisted CP through the comparison of its electrical energy per order (EE/O) and hydroxyl radical production with that of other AOPs.
2. Materials and Methods
2.1. Experimental Set-Up
Figure 1 illustrates a CP device equipped with an aeration pump (2546C-10, WELCH, Skokie, IL, USA).
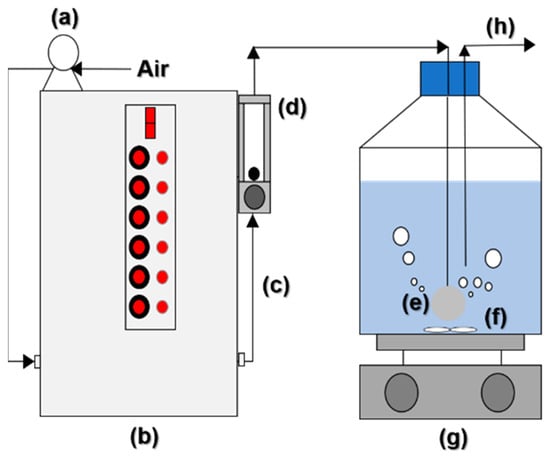
Figure 1.
Schematic of the aeration-assisted CP system: (a) aeration pump; (b) CP generator; (c) air with reactive oxidizing species; (d) flowrate meter; (e) ceramic sparger; (f) magnetic bar; (g) magnetic stirrer; and (h) sampling port.
The plasma generator, manufactured by G-Company in Jeonju, Korea, operates by creating high-voltage pulses that induce a glow discharge, thereby generating plasma. [35]. In the CP device, high-energy electrons collide with air coming from the air flow meter (RMA-22-SSV, DwyerOmega, Michigan City, IN, USA). After passing through the CP, the carrier gas is introduced into the liquid phase. Produced ROS/RNS in the carrier gas contribute to the oxidation or recombination of the reactive species with proton, hydroxyl ion, water molecule, and other substances, including PFOS.
For each test, the reactor was filled with 3.0 L of an 8 mg/L PFOS solution. This solution was then treated for reaction times of 1, 3, 6, and 10 h. Table 1 summarizes the operating conditions of the CP reactor.

Table 1.
Experimental set-up of the CP reactor.
2.2. Sample Preparation and Characterization
PFOS with 95% purity was purchased from Sigma-Aldrich (St. Louis, MO, USA). Its molecular formula is C8HF17O3S, and its molecular weight is 500.13 g/mole. After CP test sampling, the samples were stored in a refrigerator at 4 °C. PFOS was identified and quantified by UPLC/MS using the Waters Acquity UPLC system in multiple-reaction-monitoring (MRM) mode according to the standard curve (R2 > 0.98). Its quantification was based on a linear four-point calibration curve (0.3–961 µg/L), and each CP-treated sample was diluted due to the high concentration of PFOS they contained. Standard PFOS samples were diluted in MeOH to prepare stock solutions of 250 µg/L. PFOS was mixed and diluted with MeOH to make a 7.5 µg/L of internal standard (IS) working solution. PFOS separation was achieved using the Acquity UPLC BEH C18 column (2.1 × 50 mm, Waters, Milford, MA, USA), with mobile phase A consisting of 2 mM ammonium acetate in water/MeOH (95:5) and mobile phase B consisting of 2 mM ammonium acetate in MeOH.
The concentration of total organic carbon (TOC) was analyzed using a TOC analyzer (TOC-L, Shimadzu, Kyoto, Japan). Fluoride (F−) and SO42− concentrations were determined using an ion chromatograph (Dionex Aquion IC system, Thermo Fisher Scientific Inc., Seoul, Korea) equipped with an IonPac AS14 (4 mm i.d.) column and a conductivity detector. The eluent solution contained sodium carbonate (Na2CO3, 3.5 mM) and sodium bicarbonate (NaHCO3, 1mM), with a constant flow rate of 1.2 mL/min.
2.3. Statistical Analyses and Regressions
To estimate the PFOS removal rate constant, k (h−1), SigmaPlot 12 (Systat Software, Inc., San Jose, CA, USA) was used for the regression analysis, based on the assumption of a first-order exponential decay of the PFOS concentration over the duration of aeration-assisted CP operation.
2.4. Acute Toxicity Test
An acute toxicity test was conducted to determine the toxicity unit (TU) of effluent that causes an adverse effect on a group of test organisms during short-term exposure, following prescribed procedures [43,44]. Daphnia magna (Crustacea), which were used as standard organisms, were obtained from the National Institute of Environmental Research, South Korea.
The Daphnia magna were separated by age, with young individuals born within 24 h separated from endemic animals older than 14 days [45]. The initial concentration of PFOS was set at 8 mg/L and diluted according to the dilution ratio used in the experiment. Four replicates were performed for each dilution of the PFOS sample. Five young Daphnia magna were placed in each beaker within 24 h of their birth and the number of deaths and the extent of swimming inhibition seen after 24 h were recorded. Swimming inhibition was recorded if (1) the Daphnia magna did not move for more than 15 s, even when the test beaker was moved slowly, and (2) if some of their organs showed activity, including tactile movements, but the organism did not swim [44].
To obtain a TU (=100/EC50) and the half-effect concentration (EC50) of the diluted sample (%), this study followed the Probit and Trimmed Spearman–Karber (TSK) statistical methods provided by the US Environmental Protection Agency. TU is a measure of toxicity in an effluent as determined by its acute toxicity units (TUa) [43]. Table 2 presents the experimental conditions of the acute toxicity test using Daphnia magna.

Table 2.
Experimental set-up of acute toxicity test using Daphnia magna.
2.5. Calculation of Electrical Energy per Order (EE/O)
Economics is a crucial factor in selecting a wastewater treatment technology [46]. To compare the energetic efficiency of CP with other AOPs, its electrical energy per order (EE/O) was computed as described in Equation (6) [47,48]. EE/O represents the energy, measured in kilowatt-hours (kWh), required to degrade a contaminant by an order of magnitude in a unit volume (1 m3).
where P, t, V, C0, and Ct represent the power of the equipment (kW), the reaction time (h), the solution volume (L), the concentration of initial PFOS (mg/L), and the measured concentration of PFOS at time t (mg/L), respectively.
2.6. Quantification of Hydroxyl Radical
In numerous studies, various methods have been proposed to quantify the hydroxyl radical, one of the most reactive radicals. In this study, a quantitative method employing dimethyl sulfoxide (DMSO) was selected [49]. DMSO with a purity of 99.7% was purchased from Sigma-Aldrich (St. Louis, MO, USA). The initial concentration of DMSO was 10 mg/L, and the reactor’s working volume was 3 L. The air flow rate was adjusted to 5 L/min using an air flow pump, and the plasma reaction was conducted.
The literature indicates that DMSO reacts with hydroxyl radicals, resulting in the formation of methyl radicals and methane sulfonic acid as primary intermediate products [50]. The methyl radical is formed by the reaction of DMSO, and the hydroxyl radical reacts with oxygen to form the methyl peroxy radical, as shown in Equation (8). Equation (9) indicates that 2 moles of methyl peroxy radical yield 1 mole of formaldehyde. In summary, it is possible to quantify hydroxyl radicals using reaction stoichiometry and experimental evidence because 2 moles of hydroxyl radical yields 1 mole of formaldehyde according to Reactions (7)–(9).
(CH3)2SO3 + ·OH → CH3SOOH + ·CH3
·CH3 + O2 → ·CH3OO
2 ·CH3OO → HCHO + CH3OH + O2
The formaldehyde was quantified using the Korean standard methods for the examination of water and wastewater (ES 04605.2) [51]. Table 3 lists the analytical conditions of the GC-ECD for the detection of formaldehyde.

Table 3.
Analytical conditions of GC-ECD.
3. Results and Discussion
3.1. PFOS Removal by CP
Figure 2a illustrates the removal of PFOS over time during duplicate experiments. The initial concentration of PFOS (8.0 mg/L) sharply decreased to approximately 3.0 mg/L within 1 h (about a 62.5% removal efficiency). Subsequently, the rapid degradation of PFOS suddenly ceased, and its concentration very slowly decreased to around 2.5 mg/L (a 68.8% removal efficiency) on average, even after 10 h.
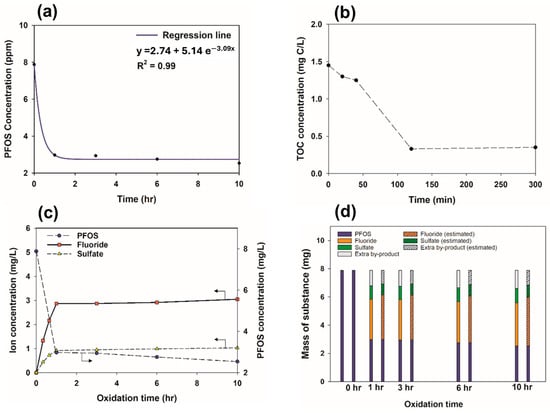
Figure 2.
Overview of PFOS degradation and its associated dynamics in the aeration-assisted CP system. (a) PFOS concentration dynamics and regressed degradation line over time. (b) Variation in the TOC concentration in CP over time. (c) Detection of fluoride and sulfate produced during PFOS degradation in the system. (d) Mass balance of fluoride and sulfate, comparing experimental data with theoretical calculations, during PFOS degradation.
The rapid removal efficiency of PFOS over time is likely associated with the generation of ROS/RNS by CP, as these strong oxidizing species can readily and quickly attack PFOS’s sulfonyl group (-SO3H). When comparing CP’s PFOS-degrading ability with other AOPs, Yamamoto et al. reported that applying UV radiation to a PFOS sample with an initial concentration of 40 µmol over a 24 h period resulted in a removal efficiency of 27.2% [52]. According to Trautmann [53], the electrochemical application of boron-doped diamond (BDD) to PFOS at an initial concentration of 4.6 µmol resulted in a 98% removal efficiency after 43 h. Zhuo [54] determined that the electrochemical application of a lead dioxide anode for 180 min led to a 41.6% removal efficiency for PFOS with an initial concentration of 0.0929 mmol/L. Kim [55] found that applying an electron beam (900 kGy) to PFOS with an initial concentration of 100 mg/L resulted in a removal efficiency of 61.0%. Additionally, Lewis [56] reported that PFOS treated for 1 h by applying gliding arc plasma achieved a removal efficiency of 93.1%. Singh [57] reported that an initial concentration of 8.3 mg/L of PFOS was treated for 1 h with a high-voltage (HV) electrode plasma reactor, achieving a removal efficiency of over 90%.
Indeed, CP demonstrates a stable PFOS removal efficiency in a shorter timeframe compared to alternative AOPs. Furthermore, CP is relatively economical in its power usage (0.15 kW) and does not require additives such as ozone, hydrogen peroxide, or iron. As a result, CP represents a viable and cost-effective method for removing PFOS.
Previous studies investigating the efficacy of AOPs in removing PFOS have examined their effects on the by-products of PFOS’s degradation, including PFOA, perfluorobutanoic acid (PFBA), perfluorobutanesulfonate (PFBS), perfluoroheptanoic acid (PFHpA), perfluorohexanesulfonate (PFHxS), perfluorohexanoic acid (PFHxA), perfluoropentanoic acid (PFPeA), and others. This area represents a promising avenue for future research on the CP method. Figure 2b show the TOC analysis conducted during PFOS’s degradation by CP. The initial TOC value was observed to be 1.45 mg C/L, with a removal efficiency of approximately 77% within 2 h, a trend that continued beyond that point. Therefore, the TOC analysis results suggest the potential for the effective removal of the organic carbon in PFOS by CP.
3.2. Degradation Kinetics of PFOS
The regression of the PFOS concentration was generated under the assumption that PFOS’s degradation by CP followed the kinetics of a first-order exponential decay reaction. Subsequent regression analysis revealed that the degradation kinetic constant of PFOS was 3.09 h−1 (kPFOS). These results are reflected in Table 4, which compares the first-order degradation rate constants of AOPs when treating non-biodegradable substances and PFOS, as determined in prior research.

Table 4.
Comparison of first-order kinetic constant of non-biodegradable contaminants and PFOS for various AOPs.
A literature review reveals that antibiotics degrade faster than PFOS. This is attributed to the greater oxidation power required to break the strong C-F chain of PFOS compared to antibiotics. Although the rate of PFOS degradation has been consistent across prior studies, it was lower than the rate observed in this study, highlighting CP’s superior oxidizing abilities. However, our regression analysis shows that the CP application achieved a 62.5% removal efficiency within 1 h, with subsequent results indicating only incremental PFOS removal. This was hypothesized to be the result of the by-products produced by PFOS acting as radical scavengers. To verify this theory, an acute toxicity test was performed to assess the effect of these by-products according to the PFOS degradation time.
3.3. Detection of Sulfate in the Suspension
Several studies have reported F− and SO42− to be the final by-products of PFOS degradation. An IC analysis was performed to quantitatively determine the concentrations of F− and SO42− generated as PFOS degraded. Figure 2c illustrates the concentrations of F− and SO42− during CP-induced PFOS degradation. After 10 h of PFOS’s exposure to CP, a concentration of SO42− was detected that corresponded to 120% of the theoretical SO42− that could be produced from the oxidation of the sulfur present in 8.0 mg/L (the initial concentration) of PFOS. This indicates a significant mineralization of PFOS and its by-product compounds. Along with a rapid decrease in PFOS concentration, there was a correspondingly rapid increase in F- and SO42− concentrations within 1 h of CP exposure. Over the next eight hours, the PFOS concentration slowly decreased as the F− and SO42− concentrations slowly increased. Clearly, F− and SO42− are produced as by-products of the degradation process.
Figure 2d shows the mass balances based on the measured and estimated amounts of F− and SO42− from PFOS degradation. Estimated values were calculated from the molecular weights of F- and SO42−. The difference between the measured and estimated values of F− and SO42− was approximately 10% and 20%, respectively. These results imply that the mass balance of F− and SO42− with the degradation of PFOS over time is correct.
Produced ions such as F− and SO42− can still adversely affect human health when present in drinking water [65]. Therefore, in practical applications, post-treatment techniques such as electrodialysis, adsorption, and biological conversion are required [66,67,68,69].
3.4. Evaluation of the Acute Toxicity of the PFOS
Figure 3 presents the variations in the acute toxicity of PFOS over time.
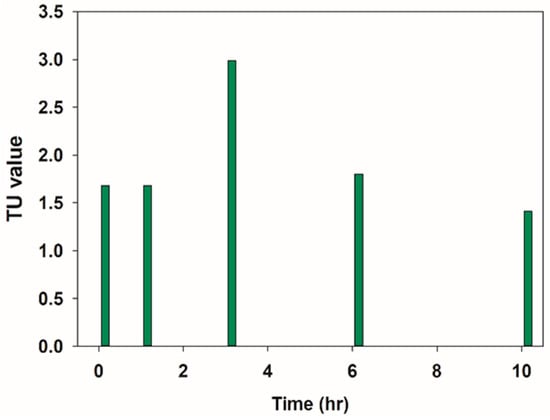
Figure 3.
Variations in the acute toxicity of PFOS and its degradation intermediates in the aeration-assisted CP system.
The TU value (95% confidence interval) of the PFOS sample at its initial concentration of 8 mg/L is recorded as 1.68 (with an EC50 of 59.46%). After applying CP for 24 h, the TU value is 1.41 (with an EC50 of 70.71%).
There was no change in acute toxicity within the first hour of CP exposure. However, by hour three, the TU value had sharply increased to 2.99 (with an EC50 of 33.48%). After that, acute toxicity gradually decreased, until the EC50 reached 11.25%. This result suggests that PFOS’s degradation intermediates may be more toxic than the original PFOS, although the toxicity continuously decreases with additional exposure to CP.
In general, some AOPs may increase toxicity due to the chemicals added as part of the process [70]. However, CP does not use chemicals, meaning that any increases in toxicity must be caused by PFOS’s degradation intermediates. These findings suggest that PFOS’s intermediates acted as scavengers, resulting in the incomplete degradation of PFOS.
3.5. Comparison of Energy Cost of CP Versus Other AOPs
We have demonstrated that CP can effectively treat PFOS in a short period of time due to its high oxidation ability. However, considerations of its feasibility must also include electrical costs. The electric power consumption of a CP device with an aeration pump is 0.15 kWh.
We calculated the EE/O value and energy cost for the CP process to be 117 kWh/m3 and 10.6 USD/m3, respectively. The energy cost was determined by multiplying the obtained EE/O by the average US electricity rate (0.09 USD/kWh) obtained from SiteWiseTM Version 3.1 [71]. The EE/O values and energy costs for the CP and other AOPs are summarized in Table 5.

Table 5.
Comparison of energy cost of CP and AOPs used to treat PFOS.
Generally, an EE/O value under 2.5 kWh/m3 is considered practical and economical for water treatment [76]. However, due to the strong C-F bond in PFOS, higher power consumption and longer reaction times are required to achieve a high removal efficiency, leading to higher EE/O values for the AOPs. Therefore, for the cost-effective treatment of PFOS, CP could be considered a viable alternative.
3.6. Comparison of Hydroxyl Radical Formation Rates of Various AOPs
Figure 4 shows the concentration of formaldehyde produced by the reaction of DMSO and hydroxyl radical.
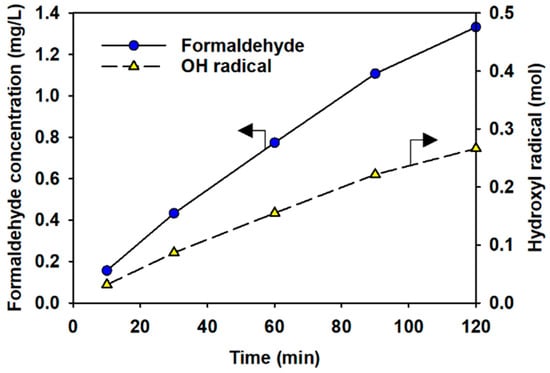
Figure 4.
Detection of formaldehyde and quantification of hydroxyl radicals. The left arrow denotes formaldehyde concentration, and the right arrow indicates hydroxyl radicals.
Additionally, the quantitative results for the hydroxyl radicals, calculated through stoichiometry and using the concentration of formaldehyde detected, are presented. GC-ECD analysis was performed to quantitatively determine the presence of formaldehyde. Ten minutes after the experiment started, the concentration of formaldehyde detected was 0.16 mg/L, while after 2 h 1.33 mg/L of formaldehyde was detected. This indicates that 0.03 mol and 0.27 mol of hydroxyl radicals were produced after 10 min and after 2 h, respectively. The detected hydroxyl radicals might have originated from the recombination of plasma-induced reactive chemicals, as the half-life of the hydroxyl radical is very short (~10−9 s) [77].
Figure 5 presents the results of calculating, from the amount of hydroxyl radical produced by CP, the instantaneous formation rates of hydroxyl radicals at the measured time intervals.
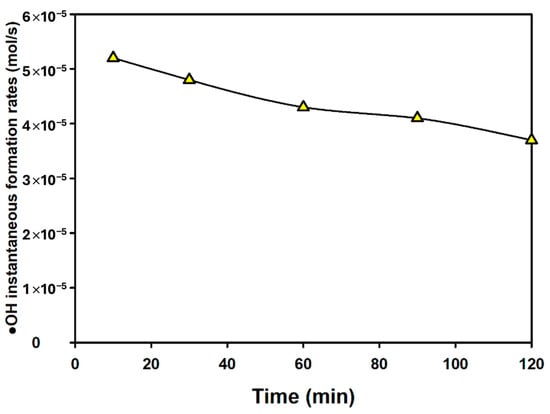
Figure 5.
CP-enabled instantaneous formation rates of hydroxyl radical.
After 10 min of the plasma reaction, the hydroxyl radical instantaneous formation rate was calculated to be 5.2 × 10−5 mol/s, but it was subsequently confirmed that this rate gradually decreased to 3.7 × 10−5 mol/s at 2 h. This decrease is estimated to be due to the fact that even the produced formaldehyde acts as a scavenger for plasma reactions over time and that some of the formaldehyde was oxidized.
Many studies have demonstrated that formic acid is formed as an intermediate due to the oxidation reaction of plasma with formaldehyde [78]. Additionally, the reactions related to formaldehyde decomposition are shown in Equations ((10)–(13)) [79,80,81]. The degradation of formaldehyde due to its plasma reaction depends on two mechanisms, including (a) the direct degradation caused by the collision of electrons with formaldehyde and (b) the reaction between formaldehyde molecules and various oxidizing species [80]. Finally, formic acid degrades to end products including H2O, CO2, etc. [79]
HCHO + e− → ·H + CHO−
HCHO + O3 → H2O + CO2 + ·O
HCHO + · OH → H2O + ·CHO
HCHO + ·OH → HCOOH + ·H
HCOOH + ·OH → H2O + ·H + CO2
Using the 10 min measurement time, the hydroxyl radical instantaneous formation rates of CP and other AOPs are compared and summarized, as shown in Table 6.

Table 6.
Instantaneous formation rates of various AOPs.
It was confirmed that CP generated more hydroxyl radicals in a shorter time period compared to other AOPs. These rapidly generated hydroxyl radicals are able to efficiently decompose organic pollutants. This is why CP was able to efficiently degrade PFOS within a short period of time.
4. Conclusions
This study verifies the utility of CP as a PFOS degradation system and demonstrates the rapidity with which CP removes unwanted PFOS. After 1 h of exposure, PFOS’s removal efficiency was 62.5%, which is similar to the removal efficiency seen after 10 h of CP exposure. This similarity is hypothesized to be due to the presence of PFOS degradation intermediates. The kinetic constant obtained by regression was 3.09 h−1, while CP’s superiority over alternative AOPs was further demonstrated by their degradation rate constants. CP-induced PFOS degradation was confirmed by measuring the sulfate and fluoride concentrations in the solution. Acute toxicity testing with Daphnia magna illustrated the relationship between toxicity and PFOS degradation, showing that the degradation intermediates may have a higher toxicity than PFOS itself. This supports the theory that PFOS intermediates act as scavengers, causing incomplete PFOS degradation. Notably, although aeration-assisted cold plasma is known to generate reactive species such as NOx, H2O2, and O3, our results revealed that the levels of NO3− and NO2− remained unchanged during CP exposure, indicating that the observed toxicity variations are primarily attributed to the degradation intermediates rather than these reactive species.
Additionally, when considering the electrical cost of PFOS degradation, CP was confirmed to be a cost-effective option. The rapid decomposition of PFOS by CP was attributed to its rapid formation of hydroxyl radicals. Ultimately, these results suggest that CP technology is a viable physico-chemical option for mineralizing non-biodegradable and ecotoxic PFOS.
This study illustrates the superiority of CP as a remediation method compared to alternative AOPs, based on their degradation rate constants. Acute toxicity testing reveals how PFOS’s toxicity is altered by CP, and the relationship between this and CP’s PFOS removal efficiency is discussed. The results suggest that CP technology may be a viable physico-chemical option for mineralizing non-biodegradable, problematic, and ecotoxic PFOS.
Author Contributions
S.O.: Data curation, Formal analysis, Writing—original draft; J.-Y.N.: Conceptualization, Data curation, Formal analysis, Methodology, Writing—original draft; Y.H.: Investigation, Resources, Validation, Visualization; T.-H.L.: Data curation, Resources, Software, Validation, Visualization; H.-W.K.: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing—review and editing; J.-C.L.: Conceptualization, Investigation, Supervision, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by research funds from Jeonbuk National University in 2023. This work was also supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. 2022R1F1A1073198) and a research grant from the project BK21 FOUR. The authors are grateful for the financial support from the 2023 Research Development Program at the Jeonbuk Green Environment Center (grant no. 23-04-50-52-09) and the R&D grant given by the Jeollabukdo business agency. This study was also supported by the Korean Ministry of Environment (MOE) as a Waste-to-Energy Recycling Human Resource Development Project (grant no. YL-WE-23-001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors, Youngpyo Hong and Tae-Hun Lee, were employed by the company Groon, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-Chain per- and Polyfluoroalkyl Substances in Aquatic Systems: Occurrence, Impacts and Treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, W.; Wang, W.; Lin, Y.; Ruan, T.; Jiang, G. Occurrence and Degradation Potential of Fluoroalkylsilane Substances as Precursors of Perfluoroalkyl Carboxylic Acids. Environ. Sci. Technol. 2019, 53, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Daniels, K.D.; Wu, S.; Ziska, A.D.; Snyder, S.A. Magnetic Ion-Exchange (MIEX) Resin for Perfluorinated Alkylsubstance (PFAS) Removal in Groundwater: Roles of Atomic Charges for Adsorption. Water Res. 2020, 181, 115897. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J.-J.; Rodenburg, L.A.; Cai, M.; Wu, Z.; Ke, H.; Chitsaz, M. Perfluoroalkyl Substances in Sediments from the Bering Sea to the Western Arctic: Source and Pathway Analysis. Environ. Int. 2020, 139, 105699. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Jian, J.-M.; Chen, D.; Han, F.-J.; Guo, Y.; Zeng, L.; Lu, X.; Wang, F. A Short Review on Human Exposure to and Tissue Distribution of Per- and Polyfluoroalkyl Substances (PFASs). Sci. Total Environ. 2018, 636, 1058–1069. [Google Scholar] [CrossRef]
- Gao, L.; Liu, J.; Bao, K.; Chen, N.; Meng, B. Multicompartment Occurrence and Partitioning of Alternative and Legacy Per- and Polyfluoroalkyl Substances in an Impacted River in China. Sci. Total Environ. 2020, 729, 138753. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced Photocatalytic Degradation of Perfluorooctanoic Acid Using Carbon-Modified Bismuth Phosphate Composite: Effectiveness, Material Synergy and Roles of Carbon. Chem. Eng. J. 2020, 395, 124991. [Google Scholar] [CrossRef]
- Domingo, J.L. Health Risks of Dietary Exposure to Perfluorinated Compounds. Environ. Int. 2012, 40, 187–195. [Google Scholar] [CrossRef]
- Kim, S.; Stroski, K.M.; Killeen, G.; Smitherman, C.; Simcik, M.F.; Brooks, B.W. 8:8 Perfluoroalkyl Phosphinic Acid Affects Neurobehavioral Development, Thyroid Disruption, and DNA Methylation in Developing Zebrafish. Sci. Total Environ. 2020, 736, 139600. [Google Scholar] [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Pelch, K.E.; Reade, A.; Wolffe, T.A.M.; Kwiatkowski, C.F. PFAS Health Effects Database: Protocol for a Systematic Evidence Map. Environ. Int. 2019, 130, 104851. [Google Scholar] [CrossRef]
- Page, D.; Vanderzalm, J.; Kumar, A.; Cheng, K.Y.; Kaksonen, A.H.; Simpson, S. Risks of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) for Sustainable Water Recycling via Aquifers. Water 2019, 11, 1737. [Google Scholar] [CrossRef]
- Li, P.; Xiao, Z.; Sun, J.; Oyang, X.; Xie, X.; Li, Z.; Tian, X.; Li, J. Metabolic Regulations in Lettuce Root under Combined Exposure to Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Hydroponic Media. Sci. Total Environ. 2020, 726, 138382. [Google Scholar] [CrossRef]
- Jian, J.-M.; Guo, Y.; Zeng, L.; Liang-Ying, L.; Lu, X.; Wang, F.; Zeng, E.Y. Global Distribution of Perfluorochemicals (PFCs) in Potential Human Exposure Source–A Review. Environ. Int. 2017, 108, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Mair, D.C.; Church, T.R.; Ellefson, M.E.; Reagen, W.K.; Boyd, T.M.; Herron, R.M.; Medhdizadehkashi, Z.; Nobiletti, J.B.; Rios, J.A.; et al. Decline in Perfluorooctanesulfonate and Other Polyfluoroalkyl Chemicals in American Red Cross Adult Blood Donors, 2000−2006. Environ. Sci. Technol. 2008, 42, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Shane, H.L.; Baur, R.; Lukomska, E.; Weatherly, L.; Anderson, S.E. Immunotoxicity and Allergenic Potential Induced by Topical Application of Perfluorooctanoic Acid (PFOA) in a Murine Model. Food Chem. Toxicol. 2020, 136, 111114. [Google Scholar] [CrossRef]
- Schultes, L.; Vestergren, R.; Volkova, K.; Westberg, E.; Jacobson, T.; Benskin, J.P. Per- and Polyfluoroalkyl Substances and Fluorine Mass Balance in Cosmetic Products from the Swedish Market: Implications for Environmental Emissions and Human Exposure. Environ. Sci. Process Impacts 2018, 20, 1680–1690. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Yeung, L.W.Y.; Wei, S.; Dai, J. Analysis of Emerging Per- and Polyfluoroalkyl Substances: Progress and Current Issues. TrAC Trends Anal. Chem. 2020, 124, 115481. [Google Scholar] [CrossRef]
- Woodard, S.; Berry, J.; Newman, B. Ion Exchange Resin for PFAS Removal and Pilot Test Comparison to GAC. Remediat. J. 2017, 27, 19–27. [Google Scholar] [CrossRef]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of Per- and Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in Drinking Water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- Rodowa, A.E.; Knappe, D.R.U.; Chiang, S.-Y.D.; Pohlmann, D.; Varley, C.; Bodour, A.; Field, J.A. Pilot Scale Removal of Per- and Polyfluoroalkyl Substances and Precursors from AFFF-Impacted Groundwater by Granular Activated Carbon. Environ. Sci. Water Res. Technol. 2020, 6, 1083–1094. [Google Scholar] [CrossRef]
- Horst, J.; McDonough, J.; Ross, I.; Houtz, E. Understanding and Managing the Potential By-Products of PFAS Destruction. Groundw. Monit. Rem 2020, 40, 17–27. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Alam, M.M.; Zhou, J.L.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Rahman, M.S.; Hossen, J.; Hasan, A.T.M.K.; Moni, M.A. Advanced Treatment Technologies Efficacies and Mechanism of Per- and Poly-Fluoroalkyl Substances Removal from Water. Process Saf. Environ. Prot. 2020, 136, 1–14. [Google Scholar] [CrossRef]
- Dai, X.; Xie, Z.; Dorian, B.; Gray, S.; Zhang, J. Comparative Study of PFAS Treatment by UV, UV/Ozone, and Fractionations with Air and Ozonated Air. Environ. Sci. Water Res. Technol. 2019, 5, 1897–1907. [Google Scholar] [CrossRef]
- Fang, C.; Sobhani, Z.; Niu, J.; Naidu, R. Removal of PFAS from Aqueous Solution Using PbO2 from Lead-Acid Battery. Chemosphere 2019, 219, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic Degradation of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Water: A Critical Review. Chem. Eng. J. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Zhuo, Q.; Wang, J.; Niu, J.; Yang, B.; Yang, Y. Electrochemical Oxidation of Perfluorooctane Sulfonate (PFOS) Substitute by Modified Boron Doped Diamond (BDD) Anodes. Chem. Eng. J. 2020, 379, 122280. [Google Scholar] [CrossRef]
- James Wood, R.; Sidnell, T.; Ross, I.; McDonough, J.; Lee, J.; Bussemaker, M.J. Ultrasonic Degradation of Perfluorooctane Sulfonic Acid (PFOS) Correlated with Sonochemical and Sonoluminescence Characterisation. Ultrason. Sonochemistry 2020, 68, 105196. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, S.-H.; Kim, H.Y.; Doudrick, K.; Yu, S.; Kim, S.D. Decomposition of Perfluorooctane Sulfonate (PFOS) Using a Hybrid Process with Electron Beam and Chemical Oxidants. Chem. Eng. J. 2019, 361, 1363–1370. [Google Scholar] [CrossRef]
- Yang, Y. Recent Advances in the Electrochemical Oxidation Water Treatment: Spotlight on Byproduct Control. Front. Environ. Sci. Eng. 2020, 14, 85. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.-C.; Nam, J.-Y.; Kim, H.-W. Degradation of Sulfonamide Antibiotics and Their Intermediates Toxicity in an Aeration-Assisted Non-Thermal Plasma While Treating Strong Wastewater. Chemosphere 2018, 209, 901–907. [Google Scholar] [CrossRef]
- Park, R.; Kim, J.-G.; Kim, H.-W. Prediction of Varying Microcystins during Non-Thermal Plasma Oxidation of Harvested Microalgal Biomass. J. Hazard. Mater. 2021, 403, 123596. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Nam, G.-S.; Jang, J.-S.; Won, C.-H.; Kim, H.-W. Cold Plasma Treatment for Efficient Control over Algal Bloom Products in Surface Water. Water 2019, 11, 1513. [Google Scholar] [CrossRef]
- Locke, B.R.; Thagard, S.M. Analysis and Review of Chemical Reactions and Transport Processes in Pulsed Electrical Discharge Plasma Formed Directly in Liquid Water. Plasma Chem Plasma Process 2012, 32, 875–917. [Google Scholar] [CrossRef]
- Dolan, T.J. Electron and Ion Collisions with Water Vapour. J. Phys. D Appl. Phys. 1993, 26, 4–8. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–Liquid Interactions: A Review and Roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Lee, J.K.; Walker, K.L.; Han, H.S.; Kang, J.; Prinz, F.B.; Waymouth, R.M.; Nam, H.G.; Zare, R.N. Spontaneous Generation of Hydrogen Peroxide from Aqueous Microdroplets. Proc. Natl. Acad. Sci. USA 2019, 116, 19294–19298. [Google Scholar] [CrossRef]
- Hong, D.; Rabat, H.; Bauchire, J.M.; Chang, M.B. Measurement of Ozone Production in Non-Thermal Plasma Actuator Using Surface Dielectric Barrier Discharge. Plasma Chem Plasma Process 2014, 34, 887–897. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Lee, J.-C.; Park, R.; Kim, H.-W. Integration of Submerged Microfiltration and Cold Plasma for High-Strength Livestock Excreta. J. Hazard. Mater. 2021, 401, 123280. [Google Scholar] [CrossRef]
- US EPA. Understanding and Accounting for Method Variability in Whole Effluent Toxicity Applications under the NPDES Program; US EPA: Washington, DC, USA, 2000.
- OECD. Daphnia Sp. Acute Immobilisation Test. In OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2004. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E.B., Eds.; American Public Health Association: Washington, DC, USA, 2023; ISBN 0-87553-299-3. [Google Scholar]
- Behnajady, M.A.; Vahid, B.; Modirshahla, N.; Shokri, M. Evaluation of Electrical Energy per Order (EEO) with Kinetic Modeling on the Removal of Malachite Green by US/UV/H2O2 Process. Desalination 2009, 249, 99–103. [Google Scholar] [CrossRef]
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Woodard, S.; Nickelsen, M.; Mededovic Thagard, S.; Holsen, T.M. Removal of Poly- and Per-Fluorinated Compounds from Ion Exchange Regenerant Still Bottom Samples in a Plasma Reactor. Environ. Sci. Technol. 2020, 54, 13973–13980. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Sahni, M.; Locke, B.R. Quantification of Hydroxyl Radicals Produced in Aqueous Phase Pulsed Electrical Discharge Reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Eberhardt, M.K.; Colina, R. The Reaction of OH Radicals with Dimethyl Sulfoxide. A Comparative Study of Fenton’s Reagent and the Radiolysis of Aqueous Dimethyl Sulfoxide Solutions. J. Org. Chem. 1988, 53, 1071–1074. [Google Scholar] [CrossRef]
- ES 04605.2; Official Test Standards for Environmental Pollution. Ministry of Environment: Sejong-si, Republic of Korea, 2014; (Written in Korean).
- Yamamoto, T.; Noma, Y.; Sakai, S.; Shibata, Y. Photodegradation of Perfluorooctane Sulfonate by UV Irradiation in Water and Alkaline 2-Propanol. Environ. Sci. Technol. 2007, 41, 5660–5665. [Google Scholar] [CrossRef]
- Trautmann, A.M.; Schell, H.; Schmidt, K.R.; Mangold, K.-M.; Tiehm, A. Electrochemical Degradation of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Groundwater. Water Sci. Technol. 2015, 71, 1569–1575. [Google Scholar] [CrossRef]
- Zhuo, Q.; Luo, M.; Guo, Q.; Yu, G.; Deng, S.; Xu, Z.; Yang, B.; Liang, X. Electrochemical Oxidation of Environmentally Persistent Perfluorooctane Sulfonate by a Novel Lead Dioxide Anode. Electrochim. Acta 2016, 213, 358–367. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yu, S.; Choi, Y.; Jeong, T.-Y.; Kim, S.D. Profiling the Decomposition Products of Perfluorooctane Sulfonate (PFOS) Irradiated Using an Electron Beam. Sci. Total Environ. 2018, 631–632, 1295–1303. [Google Scholar] [CrossRef]
- Lewis, A.J.; Joyce, T.; Hadaya, M.; Ebrahimi, F.; Dragiev, I.; Giardetti, N.; Yang, J.; Fridman, G.; Rabinovich, A.; Fridman, A.A.; et al. Rapid Degradation of PFAS in Aqueous Solutions by Reverse Vortex Flow Gliding Arc Plasma. Environ. Sci. Water Res. Technol. 2020, 6, 1044–1057. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown Products from Perfluorinated Alkyl Substances (PFAS) Degradation in a Plasma-Based Water Treatment Process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.B.; Vitale, P.; Barreto, G.P.; Aparicio, F.; de los Ángeles Dublan, M.; Eyler, G.N. Treatment of Real Non-Biodegradable Wastewater: Feasibility Analysis of a Zero-Valent Iron/H2O2 Process. J. Environ. Chem. Eng. 2020, 8, 103954. [Google Scholar] [CrossRef]
- Geng, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yuan, C.; Du, J.; Wang, C. Energy-Saving Photo-Degradation of Three Fluoroquinolone Antibiotics under VUV/UV Irradiation: Kinetics, Mechanism, and Antibacterial Activity Reduction. Chem. Eng. J. 2020, 383, 123145. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, D.M.; Ávila-Torres, Y.; Serna-Galvis, E.A.; Torres-Palma, R.A. Data on Treatment of Nafcillin and Ampicillin Antibiotics in Water by Sonochemistry. Data Brief 2020, 29, 105361. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential Removal of Pesticides from Water by Molecular Imprinting on TiO2 Photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bartosiewicz, I.; Bojanowska-Czajka, A.; Szreder, T.; Bobrowski, K.; Nałęcz-Jawecki, G.; Męczyńska-Wielgosz, S.; Nichipor, H. Application of Ionizing Radiation in Decomposition of Perfluorooctane Sulfonate (PFOS) in Aqueous Solutions. Chem. Eng. J. 2020, 379, 122303. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, C.; Xing, L.; Zhou, Q.; Dong, W.; Hoffmann, M.R. UV/Nitrilotriacetic Acid Process as a Novel Strategy for Efficient Photoreductive Degradation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2018, 52, 2953–2962. [Google Scholar] [CrossRef]
- Campbell, T.; Hoffmann, M.R. Sonochemical Degradation of Perfluorinated Surfactants: Power and Multiple Frequency Effects. Sep. Purif. Technol. 2015, 156, 1019–1027. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 92-4-004506-6. [Google Scholar]
- Arar, O.; Yavuz, E.; Yuksel, U.; Kabay, N. Separation of Low Concentration of Fluoride from Water by Electrodialysis (ED) in the Presence of Chloride and Sulfate Ions. Sep. Sci. Technol. 2009, 44, 1562–1573. [Google Scholar] [CrossRef]
- Velazquez-Peña, G.C.; Solache-Ríos, M.; Martínez-Miranda, V. Competing Effects of Chloride, Nitrate, and Sulfate Ions on the Removal of Fluoride by a Modified Zeolitic Tuff. Water Air Soil Pollut. 2015, 226, 2236. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A Zirconium-Based Nanoparticle: Essential Factors for Sustainable Application in Treatment of Fluoride Containing Water. J. Colloid Interface Sci. 2014, 416, 227–234. [Google Scholar] [CrossRef]
- Hao, T.; Xiang, P.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.; Van Loosdrecht, M.C.M.; Chen, G.-H. A Review of Biological Sulfate Conversions in Wastewater Treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Oturan, N.; Trajkovska, S.; Oturan, M.A.; Couderchet, M.; Aaron, J.-J. Study of the Toxicity of Diuron and Its Metabolites Formed in Aqueous Medium during Application of the Electrochemical Advanced Oxidation Process “Electro-Fenton. ” Chemosphere 2008, 73, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-Chemical Processes for the Treatment of per-and Polyfluoroalkyl Substances (PFAS): A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.R.; Higgins, C.P. Electrochemical Treatment of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) in Groundwater Impacted by Aqueous Film Forming Foams (AFFFs). J. Hazard. Mater. 2015, 295, 170–175. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, P.; Shao, T.; Zhao, S. Ferric Ion Mediated Photodecomposition of Aqueous Perfluorooctane Sulfonate (PFOS) under UV Irradiation and Its Mechanism. J. Hazard. Mater. 2014, 271, 9–15. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Kinetics and Mechanism of the Sonolytic Conversion of the Aqueous Perfluorinated Surfactants, Perfluorooctanoate (PFOA), and Perfluorooctane Sulfonate (PFOS) into Inorganic Products. J. Phys. Chem. A 2008, 112, 4261–4270. [Google Scholar] [CrossRef]
- Tai, C.; Peng, J.-F.; Liu, J.-F.; Jiang, G.-B.; Zou, H. Determination of Hydroxyl Radicals in Advanced Oxidation Processes with Dimethyl Sulfoxide Trapping and Liquid Chromatography. Anal. Chim. Acta 2004, 527, 73–80. [Google Scholar] [CrossRef]
- Park, J.-A.; Yang, B.; Park, C.; Choi, J.-W.; Van Genuchten, C.M.; Lee, S.-H. Oxidation of Microcystin-LR by the Fenton Process: Kinetics, Degradation Intermediates, Water Quality and Toxicity Assessment. Chem. Eng. J. 2017, 309, 339–348. [Google Scholar] [CrossRef]
- Sies, H. Strategies of Antioxidant Defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, T.; Sun, Y.; Yan, X. The Roles of Various Plasma Species in the Plasma and Plasma-Catalytic Removal of Low-Concentration Formaldehyde in Air. J. Hazard. Mater. 2011, 196, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-J.; Li, J.; Li, J.-X.; Zhu, T.; Jin, Y.-Q. Formaldehyde Removal from Gas Streams by Means of NaNO2 Dielectric Barrier Discharge Plasma. J. Hazard. Mater. 2010, 175, 1090–1095. [Google Scholar] [CrossRef]
- Chang, M.B.; Lee, C.C. Destruction of Formaldehyde with Dielectric Barrier Discharge Plasmas. Environ. Sci. Technol. 1995, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Storch, D.G.; Kushner, M.J. Destruction Mechanisms for Formaldehyde in Atmospheric Pressure Low Temperature Plasmas. J. Appl. Phys. 1993, 73, 51–55. [Google Scholar] [CrossRef]
- Zhang, J.; Grzelczak, M.; Hou, Y.; Maeda, K.; Domen, K.; Fu, X.; Antonietti, M.; Wang, X. Photocatalytic Oxidation of Water by Polymeric Carbon Nitride Nanohybrids Made of Sustainable Elements. Chem. Sci. 2012, 3, 443–446. [Google Scholar] [CrossRef]
- Kwan, W.P.; Voelker, B.M. Rates of Hydroxyl Radical Generation and Organic Compound Oxidation in Mineral-Catalyzed Fenton-like Systems. Environ. Sci. Technol. 2003, 37, 1150–1158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).