Abstract
Technological procedures for immobilizing agrochemical pollutants and activating soil enzymes that break down contaminants are still lacking due to industry’s ever-increasing number of new products to enhance agricultural production systems. Using animal manure as organic fertilizers in plant production is an affordable way to alleviate the production cost of inorganic fertilizers and improve crop yield and quality at an affordable price to limited-resource farmers. Microorganisms in animal manure secrete various extracellular hydrolyzing enzymes capable of breaking down organic matter and releasing C, N, and P for plant uptake. A field experiment was conducted to investigate the impact of combining biochar with animal manure on the activity of three enzymes involved in the N, C, and P cycles as a promising strategy for promoting soil health. The results have revealed variability among animal manure and biochar amendments in the activities of the three hydrolyzing enzymes. Biochar decreased the activity of urease and invertase in soil, indicating that some analytes in biochar act as enzyme inhibitors. The results also indicate that not all soil amendments promote soil enzymes activity, and this might be due to the various characteristics and composition of each animal manure.
1. Introduction
There is an increasing concern about human and environmental health effects resulting from the manufacturing, use, and dumping of several organic and inorganic compounds that enhance agriculture, industry, and domestic detergents. The use of fertilizers and pesticides is necessary in agriculture to meet the increasing need for food production worldwide, which ultimately contributes to the increased entry of pollutants into the environment. Therefore, an important environmental concern of the 21st century is preserving the integrity of crops, soil, and water resources. A high rate of food production necessitates better plant and soil management techniques that reduce reliance on the expensive agrochemicals used in the production processes. It will be beneficial for farmers with limited resources to increase crop output and fruit quality at a reasonable cost when using animal manures and other soil amendments in agricultural production systems as alternatives to the overpriced synthetic elemental fertilizers [1,2]. Heavy metals, synthetic pesticides, inorganic fertilizers, and other pollutants are the cause of environmental contamination, which has become a national concern.
The discharge of environmental pollutants from inadequate soil management practice, the disposal of industrial waste into rivers and streams, mining operations, toxic metal contaminants, the use of N and P fertilizers (that cause the eutrophication of natural water resources), and the disposal of pharmaceuticals and their metabolites, antibiotics, and hormones that contaminate soil and natural water resources, all require imminent and emerging solutions.
The biomass conversions of wood, manure, and plant leaves are operated under high temperatures to produce biochar, an inexpensive organic adsorbent product. The conversion of biomass to biochar lowers the atmospheric concentration of CO2 and produces sustainable energy in the form of synthetic gas and bio-oil. Biochar’s high surface area, functional groups, and hygroscopic and porous structure (Figure 1) hold water molecules and water-soluble nutrients, making this material a home for many beneficial microorganisms. Biochar production for agricultural applications is a viable technique for carbon sequestration and improving soil productivity, as it improves nutrient availability to growing plants, soil physical, chemical and biological qualities, and environmental quality [3]. When added to soil, biochar acts as a long-term carbon storage solution by improving soil characteristics [4]. Biochar decreases soil acidity via ion exchange, which reduces nutrient leaching from agricultural soils. Biochar’s large surface area absorbs contaminants from soil while also benefiting soil biota [5,6] by hosting and sheltering algae, protozoa, fungi, and bacteria. According to the literature review, when compared to other soil amendments, the enhanced structure, nutrient usage efficacy, aeration, permeability, and water-holding capacity of soil containing biochar is all likely to increase crop yield [7]. Biochar can restore degraded soil, and play a significant role in preventing water molecules and nutrient minerals in the upper soil surface from evaporating following rainfall or rainfall and irrigation events, as it contains numerous tiny tunnels that retain water and minerals in the soil layers, increasing water availability for plants. This boosts crop cost-savings and decreases the need for inorganic elemental fertilizers. The minerals (C, H, N, S, O, etc.) that make up biomolecules like cellulose, hemicelluloses, and lignin give biochar its characteristics. Biochar’s application to soil increases soil fertility by improving nutrient retention and uptake by developing plants. Additionally, biochar adsorbs nutrients on its surfaces, reducing the amounts of nutrients (NO3−–N, NH4+–N, and PO43−–P) that leach after fertilizer application [8]. Inorganic-N (NO3−–N, NH4+–N) is adsorbed on the surface of biochar and released gradually in the soil solution, thus functioning as a slow-release fertilizer [9]. This suggests that the surfaces of biochar exhibit a higher affinity for the positive NH4+–N ions than for NO3− ions.
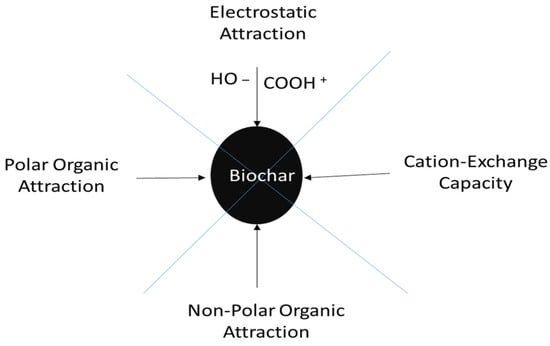
Figure 1.
Schematic structure of biochar surface functional groups (ether C-O-R, carboxylic COOH, hydroxyl -OH, aromatic hydrocarbon groups) on its extensive surface area that bind a broad spectrum of toxic organic and inorganic pollutants from contaminated environments by cation exchange, complexation, and precipitation capacity.
One excellent source of plant nutrients is animal manure. It increases soil biological activity and improves soil health when added to agricultural soils [10,11,12]. For farmers with limited resources, the availability of animal manures offers a stabilized form of nutrients that support the development and yield of a wide range of crops. An important source of soil nitrogen is animal manures, such as sewage sludge, chicken manure, horse manure, etc. Soil bioremediation can be improved by using sewage sludge as a soil conditioner to improve the physical, chemical, and microbiological characteristics of the soil. Chicken manure improves the proliferation of many kinds of microorganisms, including bacteria, fungi, and actinomycetes, as well as the biological activity and fertility of the soil. The nutrients that are most frequently utilized in inorganic fertilizers, such as N and K, are found in abundance in animal manures, along with other minerals [13,14].
N is the main nutrient required to increase plant growth and crop yield [15]. N is required for plants and animals due to its role in the formation of several energetic proteins and amino acids. Amiri et al. [16] published information on the role of N in eggplant production, and confirmed that eggplants depend on N to supply up to 120 kg of N ha−1, whereas Trani [17] suggested using up to 200 kg of N ha−1 for farming. De Souza et al. [14] also found that the maximum number of fruits per plant−1 was obtained when N concentration ranged between 14.0 and 17.0 g of N per plant−1 (145–177 kg of N ha−1). On the contrary, N rates greater than 15.03 g per plant−1 minimized crop yields.
The application of animal manure in agricultural production systems reduces dependence on synthetic inorganic fertilizers. In addition, animal manure application in agricultural systems provides a useful means of waste disposal. However, animal dung and biochar are some of the pathways by which heavy metals enter the ecosystem and accumulate in the soil, and these then discharge the heavy metals into natural water resources captivated by the growing crops, and they are consumed by humans, posing a threat to anthropological health [18,19]. Therefore, keeping such metal concentrations below legal limits is challenging for farmers and health authorities. Heavy metals influence the microbial population that secretes soil enzymes. Heavy metals can reduce bacterial species abundance and lessen bacterial communities’ biomass and diversity in polluted soils [20]. Karaca et al. [21] reported that Cd is more harmful to hydrolyzing enzymes than Pb. Additionally, Cu inhibits the activity of β-glucosidase more notably than cellulase due to Cu’s higher mobility and lower affinity for soil colloids. In contrast, high levels of Pb in the soil significantly reduce the activities of urease, invertase, catalase, and acid phosphatase.
Soil enzymes play a vital role in numerous essential soil functions. They are deeply involved in nutrient recycling, enhancing fertilizer efficiency, reflecting soil microbiological activity, and as indicators of soil transformations. Bioindicators, such as soil enzymes in animal manures, are tools for monitoring soil health [10,22,23]. Enzymes are susceptible to conservational stress caused by elevated levels of heavy metals [24,25], soil hormones, and antibiotics [26] in animal manures, which affect soil biological activity.
Urease (urea amidohydrolase, EC 3.5.1.5) converts urea fertilizers into NH3 and CO2, which raises soil pH [27] and causes N loss to the troposphere because of NH3 volatilization. Soil urease is created from plants and microorganisms [28]. Soil microorganisms, plants, and animals secrete the enzyme invertase under acid and alkaline conditions [29]. These enzymes discharge the C and N needed for the development and growth of soil microorganisms. Phosphatases in the soil exchange organic phosphate esters to orthophosphate ions, which represent an important connection between biologically unavailable and bioavailable phosphorus (P) for plant uptake.
Microorganisms respond to low amounts of inorganic phosphates in soil by producing phosphatases. Only a small portion of the soil’s P content, roughly 1% to 5%, is accessible to plants [30]. Non-mammalian species such as bacteria, fungi, parasites, and plants contain acid phosphatase (EC 3.1.3.2) that is structurally identical to mammalian acid phosphatase. Every year, 1.3 billion tons of garbage are produced worldwide from animal waste. Global biosolids production could reach 2.2 billion tons annually [31]. Recycling animal excrement and biosolids for use as organic fertilizers would lessen the need for synthetic fertilizers and the issue of waste disposal space. It would also provide low-cost means for farmers with limited resources to employ to improve nutrient status and soil structure. Microbiological activity is increased when organic resources, such as animal manure or municipal sewage sludge compost, are added to soil. The possibility that soil biological characteristics could serve as sensitive and early markers of soil ecological stress and restoration is being more widely supported by several investigators [30]. Only a few studies have addressed the impact of animal manure mixed with biochar on soil enzyme activity. In attempts to fill the gaps in biochar technology and the mechanisms of microbial activity and enzyme secretion, this chapter aims to investigate the impact of animal manure mixed with biochar on the activity of the three enzymes involved in the nitrogen, carbon, and phosphorus cycles (urease, invertase, and phosphatase, respectively).
2. Materials and Methods
2.1. Methods of Application of Animal Manure
Forty-eight field plots measuring 1.2 × 3 m2 each were established at the University of Kentucky Research Farm using a randomized complete block design (RCBD). The study comprised three replicates across eight treatments. Eight organic soil treatments and a control were set up, including the following: (1) horse manure (HM); (2) sewage sludge SS; (3) vermicompost, worm castings; (4) chicken manure (CM); (5) inorganic fertilizer (Inorg) from Southern State NPK 19:19:19; (6) organic (Org) fertilizer (Nature Safe 10:2:8); (7) biochar 10% (w/w) purchased from Wakefield Agricultural Carbon (Columbia, MO, USA), and (8) a control no-amendment (NA) untreated soil. The remaining 24 treatments (8 treatments × 3 replicates) included similar soil treatments as described above mixed with 10% biochar 10% (w/w) to investigate the impacts of biochar on soil enzyme activity. Green peppers (Capsicum annuum L.) were planted in the experimental area after spraying the soil with a dimethenamid herbicide to control pre-emergent annual grasses and broadleaf weeds at a single application of 1.46 L ha−1 [32]. Weeding and other agricultural tasks were routinely performed as needed throughout the growing season.
Each soil amendment was added to the native soil at 5% nitrogen (N) on a dry weight basis. To achieve the rate of 5% addition, a total of 10,189.6 kg per hectare−1 of chicken manure (1.1% N) and 2241.7 kg per hectare−1 of sewage sludge, which naturally contains 5% N, purchased from Metropolitan Sewer District in Louisville, Kentucky, USA, were mixed with native soil. Biochar was used at 15,567.4 kg per hectare−1, after being acquired from Wakefield Agricultural Carbon Company (Columbia, MO, USA). To guarantee the even dispersion of each soil amendment, each soil amendment was combined with native soil and roto-tilled to a depth of 15 cm (~0.5 ft.) topsoil before pepper planting (Figure 2).

Figure 2.
Flowchart representing methods of application of animal manure used at 5% N in the preparation of soil for growing pepper.
2.2. Collection of Soil Samples
Soil samples representing the various field treatments (a total of 48 treatments) were collected from the rhizosphere of pepper plants grown in animal manure (Figure 3), amended soil and animal manure amended with biochar treatments during the study period (from 1 h to 35 days) to a depth of 15 cm, which is typically where microbial and enzyme activity is greatest. The samples were carefully transported to the laboratory on ice. A core sampler (Clements Associates, Newton, IA, USA) fitted with a plastic liner tube of 2.5 cm in inside diameter was used to collect and preserve the integrity of the samples. The collected soil samples were air-dried at room temperature, sifted through a 2 mm non-metal sieve, and stored at 4 °C for no more than 24 h before processing.


Figure 3.
Soil amendments (biochar (A), vermicompost (B), sewage sludge (C), chicken manure (D), horse manure (E), inorganic fertilizer (F), and organic mineral fertilizer (G)) used for growing bell pepper, Capsicum annuum L., at the University of Kentucky Research Farm.
2.3. Measurement of Soil Enzymes Activity
The assessment of the activity of soil urease, the enzyme that breaks down urea to form NH4 and CO2, was conducted following the procedure outlined by Tabatabi and Bremner [33], with modifications introduced by Antonious and Turley [34], where the concentrations of NH4+ ions released in the soil solutions were measured using a selective electrode. Urease activity was represented as µg NH4-N released per g of dry soil over the incubation period. The activity of invertase, the enzyme that breaks down sucrose to form glucose and fructose in the soil, was measured using the technique detailed by Balasubramanian et al. [35]. The method created by Tabatabai and Bremner [36] was used to measure the activity of acid and alkaline phosphatase. To determine soil urease activity, 5 g of soil was collected from each treatment, and 10 mL measures of 0.1 M phosphate buffer (pH 6.7) in 50 mL volumetric flasks were kept in an incubator at 37 °C for 24 h [34]. The procedure was then completed as described by Tabatabi and Bremner [33]. The method was elaborated by measuring the concentration of NH4+ ions released in the soil solutions by the selective electrode method. A series of standard solutions of NH4Cl covering the concentrations of 0.1–100 µg NH4-N mL−1 of water were calibrated. For invertase activity, 5 g of sieved soil was taken in 50 mL flasks, and 2.5 mL toluene was added. After 15 min, 20 mL of 0.1 acetate phosphate buffer (pH 5.5) and 20 mL of 5% sucrose solution were added. A standard calibration curve was obtained for each group of samples, using analytical grade glucose in the range of 10–50 µg mL−1 glucose. Then, invertase activity in the soil was determined as described by Balasubramanian et al. [35]. Acid and alkaline phosphatase activities were determined using the method developed by Tabatabai and Bremner [36].
Phosphatases catalyze the removal of a phosphate group from the substrate molecule by breaking down the phosphomonoester bond. The method of determination measures the amount of p-nitro phenol released during soil incubation using the p-nitro phenol phosphate sodium test for acid phosphatase at pH 6.7 and pH 11 for alkaline phosphatase. A colorimetric technique that involves hydrolyzing p-nitro phenyl phosphate disodium hexahydrate (p-NPP) to p-nitro phenol (PNP) and measuring the absorbance at 520 nm was used to track extracellular acid and alkaline phosphatase activities.
2.4. Soil Metal and Nutrient Analysis
An inductively coupled plasma–mass spectrometer (ICP-OES, Perkin Elmer, Shelton, CT, USA) was employed to analyze the concentrations of Cd, Cr, Ni, Pb, Zn, Cu, and Mo following the U.S. EPA method 6020a [37]. Spike recovery rates ranged from 85% to 100%, with a relative percentage difference of less than 3% for repeated analyses. The m/z values for quantification were 52 for Cr, 60 for Ni, 65 for Cu, 66 for Zn, 98 for Mo, 112 for Cd, and 208 for Pb. The native soil utilized in this study consisted of approximately 56% silt, 38% clay, and 6% sand, with a pH of 6.2, a CEC of 14.7 meq100 g−1, and an OM content of 2.2%. Nutrient levels included total N at 0.18%, N-NO3 at 20.7 ppm, N-NH4 at 5.7 ppm, P at 95.8 ppm, K at 336.2 ppm, and C at 1091 ppm. A Fisher brand XL500 Benchtop Meter (Thermo Fisher, Waltham, MA, USA) equipped with Orion High-Performance ammonia and nitrate electrodes was used to measure NH4 and NO3, adhering to APHA guidelines [38]. The pH value was determined using a distilled water slurry of 1:5 (w/v), EC was measured using an electrical conductivity meter (Multi 3430 WTW unit, Weilheim, Germany) and C was measured using an ICP-OES spectrometer. One-way ANOVA was applied to analyze heavy metals, NH4, and NO3 in soil and animal manure amendments [39], complemented by Duncan’s multiple range test to compare means.
3. Results and Discussion
3.1. Composition of Soil Amendments
Table 1 indicates that the soil pH (6.2) was greater in chicken manure compared to other animal dung. NH4–N was greater (66.7 ppm) in chicken manure, whereas NO3–N concentration was higher (93 ppm) in biochar with P, Cd, Cr, Cu, Zn, Pb, As, and Ni concentrations among all the animal manures. K (1024 ppm) and C (50,623 ppm) were greater in inorganic and biochar treatments compared to other manures. Soil urease activity in vermicompost was also greater (1106 µg−1 dry soil), whereas invertase activity was greater in all manures compared to the native soil (control). Acid phosphatase was greater in vermicompost and sewage sludge compared to chicken and horse manures. Alkaline phosphatase was greater (524 µg g−1 dry soil) in chicken manure compared to the other manures (Table 1).

Table 1.
Properties and chemical composition in µg g−1 of soil amendments and native soil used in agricultural management practices for growing pepper, Capsicum annuum L. and other vegetables grown at the University of Kentucky Research Farm.
3.2. Soil Enzyme Activity
Regardless of soil treatments, Figure 4A illustrates the activities of soil urease and invertase collected at sampling dates during the green pepper growing season. As described earlier, urease is the enzyme that breaks down urea N fertilizers and produces CO2 and NH4+ in soil. Following the application of soil amendments, in early June, the increased activity of urease was followed by an increase in NH4+ concentration in the soil. Since NH4+ is a weak base, one of the effects of this hydrolysis is raising the soil pH (the hydrogenionic potential). Accordingly, the increase in urease activity after manure application was followed by a decrease in invertase activity, as shown by the trend line (the dashed line in Figure 4A). Investigators [40] stated that the optimal invertase activity in soil was witnessed at pH 5.0. Figure 4A also reveals that, at a higher urease activity, the activity of invertase tends to decrease, indicating that the pH value plays a significant role in the activity of soil invertase. A high pH can alter the invertase structure and reduce its efficiency, and consequently, the rate of invertase hydrolysis can decrease, impacting the availability of simple sugars for microbial and plant uptake [41].
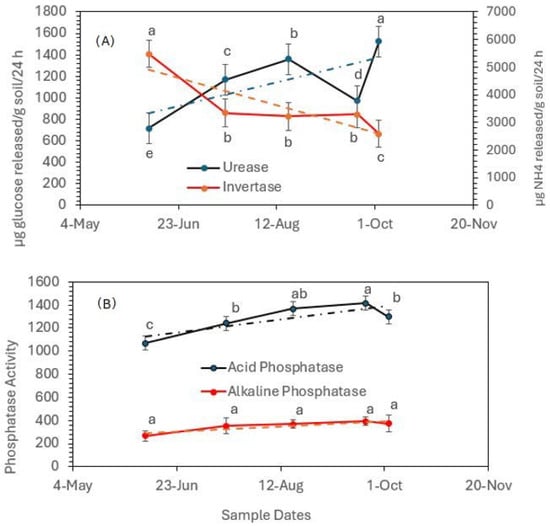
Figure 4.
Overall soil urease and invertase activity (A) and acid and alkaline phosphatase activity (B) at different sampling dates during the green pepper growing season, regardless of soil amendments. Statistical analysis was carried out among the dates of sampling for each enzyme using analysis of variance (ANOVA). Values accompanied by different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple test for mean comparisons. The dotted lines indicate trend lines.
Figure 4B presents the activity of phosphatase in soil, regardless of soil treatments at different time intervals during the pepper growing season. Soil acid phosphatase activity was much greater compared to alkaline phosphatase activity. Soil pH is not the only parameter that controls phosphatase activity. Several factors can also impact the activity of phosphatases. The reduced activity (inhibition) of alkaline phosphatase shown in Figure 4B could be due to the presence of Cd2+ in the soil, which reduces the enzyme’s affinity to its substrates and reduces reaction velocity [42]. In addition, according to Zho et al. [43], Al3+, Fe2+, and Ca2+ ions (not determined in this study) can inhibit alkaline phosphatase activities, and excess S2− ions can also reduce alkaline phosphatase activity by removing Zn2+ from the active sites of the enzyme [43].
The effects of adding biochar to soil on urease and invertase activities are shown in Figure 5A,B, respectively. Overall, the addition of 10% w/w biochar slightly decreased the activities of urease, as shown by the trend line in Figure 5A. Biochar, a carbon-rich substance created during the pyrolysis and thermochemical breakdown of biomass, has total organic carbon 88%, total inorganic carbon 0.34%, a surface area of 366 m2 g−1 dry, and 54% moisture. The organic content in biomass breaks down during the pyrolysis process, leaving behind biochar’s undegradable heavy metals. According to a 2024 study [19], biochar significantly affects soil-hydrolzing enzymes and their secretions. This could be due to the influences of heavy metals in biochar that impact urease, invertase, and phosphatase, which have been determined to be positive [44] or negative [45], and this might be also due to the different compositions of heavy metals in biochar and variations in the levels of absorbing and retaining water molecules that impact soil microbial secretions and soil enzymes’ activity. However, Figure 5A,B does not show significant differences between biochar added to soil and soil not amended with biochar. Accordingly, there is no evidence of urease and invertase inhibition in response to heavy metals stress.
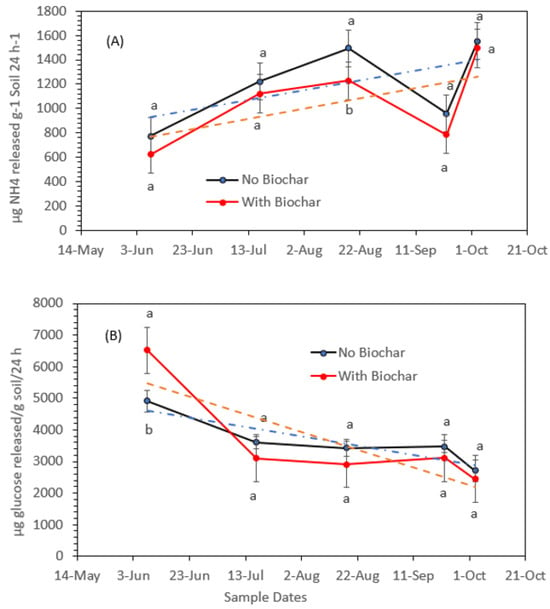
Figure 5.
Overall urease activity in soil amended with biochar and soil with no biochar (A) and invertase activity in soil amended with biochar and soil with no biochar (B) at different sampling dates during the green pepper growing season. Statistical analysis was carried out among biochar and no-biochar treatments at each sampling date using analysis of variance (ANOVA). Values accompanied by different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple test for mean comparisons. The dotted lines indicate trend lines.
However, the results indicate a reduction in alkaline phosphatase activity compared to acid phosphatase (Figure 4B). Alkaline phosphatase has three active sites for binding metals; two of them are occupied with Zn+2 ions, and the third Cd site is occupied with Mg+2 ions [46]. Accordingly, these active sites can be occupied by mineral ions in biochar and/or animal manure, causing enzyme inhibition. Tan et al. [47] reported that enzyme inhibition can also be accomplished by Cd ions that reduce the enzyme’s affinity to its substrate.
Figure 6A,B represent the impacts of biochar added to soil on the activity of alkaline- and acid phosphatase, expressed as µg p-nitrophenol released per g−1 soil. Overall, acid phosphatase activity before and after biochar addition tends to be similar, whereas the activity of alkaline phosphatase was slightly reduced due to the addition of biochar, as indicated by the trend line. This might be due to the impacts of Cd [42], Al3+, Fe2+, and Ca2+ ions [43] on alkaline phosphatase activity.
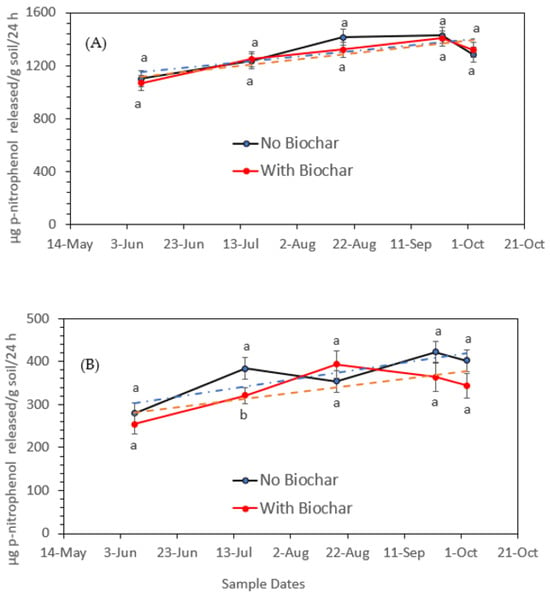
Figure 6.
Overall acid phosphatase activity in soil amended with biochar and soil with no biochar (A) and alkaline phosphatase activity in soil amended with biochar and soil with no biochar (B) at different sampling dates during the green pepper growing season. Statistical analysis was carried out among biochar and no-biochar treatments at each date using analysis of variance (ANOVA). Values accompanied by different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple test for mean comparisons. The dotted lines indicate trend lines.
Figure 7A reveals that biochar added to vermicompost, horse manure, and the control treatments did not impact soil urease activity. Biochar added to inorganic mineral fertilizer, sewage sludge, and chicken manure reduced the activity of urease. Biochar added to the organic mineral fertilizer significantly increased urease activity. Figure 7B also reveals that biochar added to horse manure, sewage sludge, chicken manure, and the control treatments did not impact invertase activity, whereas biochar added to inorganic mineral and organic elemental fertilizers reduced invertase activity. On the contrary, vermicompost amended with biochar showed significantly increased invertase activity. These results are consistent with those of Wu et al. [48], who reported that biochar and vermicompost significantly increased the diversity of bacterial and fungal communities, and the abundance of Actinomycetes, Acidobacteria, Ascomycetes, and Aspergillus, in soil under greenhouse conditions.
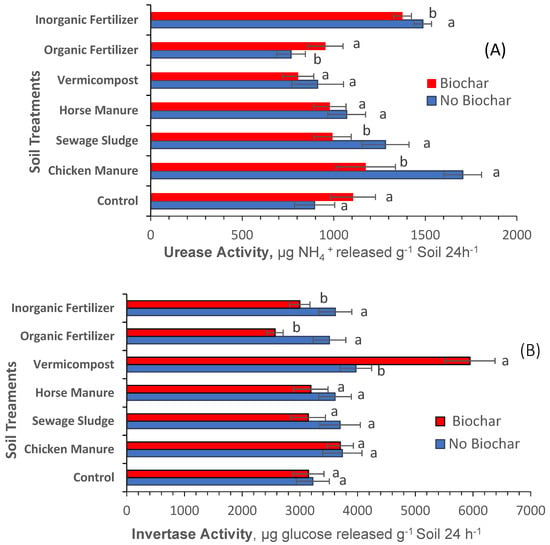
Figure 7.
Impact of soil treatments amended with biochar and treatments not amended with biochar at harvest time on the activity of urease (A) and invertase (B). Each bar is an average of three replicates ± standard error. Statistical comparisons were made between soil treatments amended with biochar and soil treatments not amended with biochar using the ANOVA procedure (SAS Institute 2016). Values accompanied by different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple test for mean comparisons.
Figure 8A reveals that inorganic elemental fertilizer, sewage sludge, and the control treatments amended with biochar did not impact soil acid phosphatase activity, whereas organic mineral fertilizer and vermicompost significantly increased acid phosphatase activity. On the contrary, horse manure and chicken manure amended with biochar at 10% reduced the activity of acid phosphatase. Figure 8B indicates that biochar added to inorganic fertilizer and chicken manure significantly increased alkaline phosphatase activity, whereas biochar added to organic mineral fertilizer and vermicompost did not impact the activity of alkaline phosphatase.
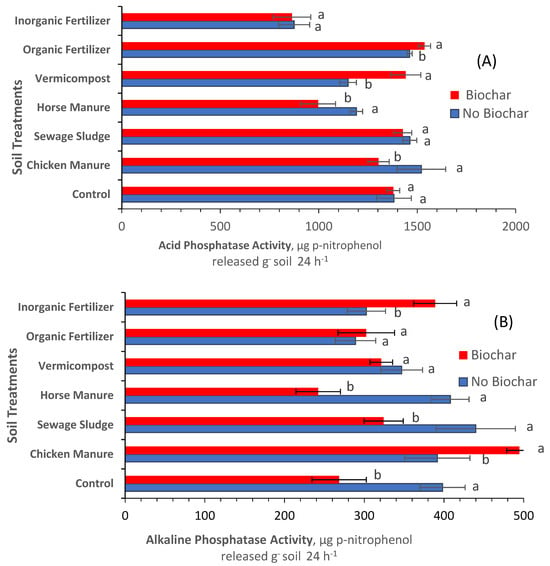
Figure 8.
Impacts of soil treatments on the activity of acid phosphatase (A) and alkaline phosphatase (B). Each bar is an average of three replicates ± standard error. Statistical comparisons were made between soil treatments amended with biochar and soil treatments not amended with biochar using the ANOVA procedure (SAS Institute 2016). Values accompanied by different letter(s) are significantly different (p ≤ 0.05) using Duncan’s multiple test for mean comparisons.
Biochar added to the horse manure, sewage sludge, and control treatments significantly reduced the alkaline phosphatase activity. Again, several factors could be responsible for the variability in and duality of the effects of biochar on soil enzyme activity. For example, they could be due to the presence of Cd at high concentrations in soil [42], and/or Al3+, Fe2+, and Ca2+ ions [43] in the soil solution (these elements were not among the elements quantified in the current study). The active sites on soil-hydrolyzing enzymes can be occupied by mineral ions in biochar and/or animal manure, causing enzyme inhibition due to a reduction in the enzyme’s affinity in binding to its substrate. Accordingly, not all soil amendments promote soil enzymes’ activity due to the various characteristics of each animal manure. Some animal manures contribute to the introduction of antibiotics into the soil, and there are few studies on the introduction of antibiotics into agricultural soil or its impacts. Enzyme inhibition can also occur due to the differences in the concentrations of dissolved humic substances among animal manures [49].
4. Conclusions
Soil microbial activity, impacted by soil nutrient recycling, is controlled by N, C and P cycles, and their impacts on the activities of urease, invertase, acid and alkaline phosphatase, respectively, along with other soil-hydrolyzing enzymes. Animal manure is a source of nutrients required for growing vegetables and fruits, and has the potential to replace the expensive inorganic mineral fertilizers. However, animal manures, such as sewage sludge, horse manure, chicken manure, and vermicompost, can contain various heavy metals that may affect soil enzyme activity. Additionally, the application of biochar, produced through the incineration of biomass, as a soil amendment is suggested to enhance plant nutrient availability, increase soil electrical conductivity, and improve soil organic matter. Biochar is also considered a potential solution for mitigating climate change and retaining soil water content. Nonetheless, the heavy metals present in both biochar and animal manures can act as enzyme inhibitors. Using enzyme activity as a bioindicator to assess soil health is an effective method for monitoring the impacts of heavy metals and animal manures on soil-hydrolyzing enzymes’ activities when evaluating the effectiveness of soil remediation techniques.
The results of this investigation reveal that biochar added to vermicompost, or horse manure treatments did not influence soil urease activity, whereas biochar added to inorganic fertilizer, sewage sludge or chicken manure reduced the activity of urease. Adding biochar to organic mineral fertilizer significantly increased urease activity. In addition, the results also reveal that adding biochar to horse manure, municipal sewage sludge or chicken manure treatments did not affect invertase activity, whereas the biochar added to inorganic mineral fertilizer and organic elemental fertilizer reduced invertase activity, and this might be because of the effects of the heavy metals in biochar on the soil microbial activity. On the contrary, vermicompost amended with biochar showed significantly increased invertase activity, indicating that the biochar and vermicompost formulations significantly increased the diversity of bacterial and fungal communities in soil. Accordingly, this chapter highlights the importance of combining biochar and vermicompost as organic amendments for promoting the physicochemical properties of soil. However, some analytes in biochar and animal manure, such as heavy metals, may act as enzyme inhibitors. The use of enzyme activity as a bioindicator for testing soil health and the potential impacts of heavy metals and animal manure on soil enzymes is a simple and safe way to monitor soil health and the efficiency of remediation techniques.
Funding
This project is funded by a grant from the United States Department of Agriculture, National Institute of Food and Agriculture (USDA/NIFA), given to the Kentucky State University under the agreement # KYX-10-23-80P Accession 7005611 to Kentucky State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author (george.antonious@kysu.edu).
Acknowledgments
I thank Anjan Nepal, Eric Turley, Basanta Neupane, and the farm crew at Kentucky State University Research Farm for their support in undertaking farm work.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Antonious, G.F.; Dawood, M.H.; Turley, E.T.; Paxton, R.B. Biochar and animal manures increased yield of three varieties of turnips. Int. J. Appl. Agric. Sci. 2022, 8, 50–56. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Gyawali, R.B.; Freeman, A.C. Influence of biochar and animal manures application on ammonia and nitrate concentrations in the root and shoot of three varieties of turnips. Agriculture 2023, 13, 137. [Google Scholar] [CrossRef]
- Antonious, G.F. The Impact of Organic, Inorganic Fertilizers, and Biochar on Phytochemicals Content of Three Brassicaceae Vegetables. Appl. Sci. 2023, 13, 8801. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Benefits and Limitations of Biochar Amendment in Agricultural Soils: A Review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Huang, X.; Li, Z.; Tan, X.; Zeng, G.; Zhou, L. Potential Benefits of Biochar in Agricultural Soils: A Review. Pedosphere 2017, 27, 645–661. [Google Scholar] [CrossRef]
- Shaaban, M.; Van Zwieten, L.; Bashir, S.; Younas, A.; Núñez-Delgado, A.; Chhajro, M.A.; Kubar, K.A.; Ali, U.; Rana, M.S.; Mehmood, M.A.; et al. A Concise Review of Biochar Application to Agricultural Soils to Improve Soil Conditions and Fight Pollution. J. Environ. Manag. 2018, 228, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications. Fuel 2021, 292, 20243. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Antonious, G.F. Soil amendments for agricultural production. In Organic Fertilizers: From Basic Concepts to Applied Outcomes; Larramendy, M.L., Soloneski, S., Eds.; Intech: Rijeka, Croatia, 2016; pp. 157–187. [Google Scholar]
- Antonious, G.F.; Turley, E.T.; Dawood, M. Monitoring soil enzymes activity before and after animal manure application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Antonious, G.F.; Dawood, M.H.; Turley, E.T.; Trivette, T.G. Soil amendments enhanced summer squash yield, fruit composition, quality, and soil enzyme activity. Agric. Sci. 2022, 13, 684–701. [Google Scholar] [CrossRef]
- Lopes, L.N.; Souza, C.F.; Santoro, B.L. Utilização da TDR para monitoramento da solução de nitrato de potássioem Latossolo Vermelho-Amarelo. Eng. Agric. 2010, 30, 932–947. [Google Scholar]
- De Souza, A.H.C.; Rezende, R.; Lorenzoni, M.Z.; Seron, C.C.; Hachmann, T.L.; Lozano, C.S. Response of eggplant crop fertigated with doses of nitrogen and potassium. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 1. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Aroiee, H.; Fatemi, H.; Ameri, A.; Karimpour, S. Responses of eggplant (Solanum melongena L.) to different rates of nitrogen under field conditions. J. Cent. Eur. Agric. 2010, 11, 453–458. [Google Scholar] [CrossRef]
- Amiri, E.; Gohari, A.A.; Esmailian, Y. Effect of irrigation and nitrogen on yield, yield components and water use efficiency of eggplant. Afr. J. Biotechnol. 2012, 11, 3070–3079. [Google Scholar]
- Trani, P.E. Calagem e Adubação para Hortaliças sob Cultivo Protegido; Instituto Agronômico: Campinas, Brazil, 2014; p. 25. [Google Scholar]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Antonious, G.F. Mobility of Nitrates and Phosphates from Animal Manure-Amended Soil to Runoff and Seepage Water from a Sweet Potato Field. Water 2024, 16, 204. [Google Scholar] [CrossRef]
- Chen, L.; Guo, H.; Luo, S.; Xiao, X.; Xi, Q.; Wei, W.; He, Y. Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresour. Technol. 2010, 101, 8599–8605. [Google Scholar]
- Karaca, A.; Cetin, S.C.; Turgay, Q.C.; Kizilkaya, R. Effects of Heavy Metals on Soil Enzyme Activities. In Soil Enzyme Activities; Springer: Berlin/Heidelberg, Germany, 2010; pp. 195–218. [Google Scholar] [CrossRef]
- Antonious, G.F. Biochar and animal manure impact on soil, crop yield and quality. In Agricultural Waste; Aladjadjiyan, A., Ed.; National Biomass Association, Bulgaria & IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Antonious, G.F. Animal Manure Improved Antioxidants in two Brassicaceae Plants: Arugula and Mustard. EMS Environ. Sci. 2018, 1, 003. Available online: https://emspublishers.org/article.php?articleId=1026 (accessed on 24 December 2024).
- Kizilkaya, R.; Askin, T.; Bayraki, B.; Sağlam, M. Microbial characteristics of soil contaminated with heavy metals. Eur. J. Soil Biol. 2004, 40, 95–102. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Carreira, J.A.; Rodriguez-Moroto, J.M.; Garcia-Ruiz, R. Effects of pyrite sludge pollution on soil enzyme activities: Ecological dose-response model. Sci. Total Environ. 2008, 396, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Blakeley, R.L.; Zerner, B. Urease: A Ni (II) metalloenzyme. In The Bioinorganic Chemistry of Nickel; Lancaster, J.R., Ed.; VCH: New York, NY, USA, 1989; pp. 141–166. [Google Scholar]
- Mobley, H.L.T.; Hausinger, R.P. Microbial urease: Significance, regulation and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef]
- Spalding, B.P. Effect of divalent metal cations respiration and extractable enzyme activities of Douglas-fir needle litter. J. Environ. Qual. 1979, 8, 105–109. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Ocampo, J.A.; Garcia, R.I. Enzymes in the arbuscular mycorrhizal symbiosis. In Enzymes in the Environment: Activity, Ecology, and Applications; Burns, R.G., Dick, R.P., Eds.; Marcel Dekker, Inc.: Basel, Switzerland, 2002; Chapter 5. [Google Scholar]
- Moya, D.; Aldás, C.; López, G.; Kaparaju, P. Municipal solid waste as a valuable renewable energy resource: A worldwide opportunity of energy recovery by using waste-to-energy technologies. Energy Procedia 2017, 134, 286–295. [Google Scholar] [CrossRef]
- Kentucky Production Guide (2022–2024). The University of Kentucky, College of Agriculture, Food and Environment, Cooperative Extension Service, ID-36. Available online: https://kentuckypestnews.wordpress.com/2021/10/26/vegetable-production-guide-for-commercial-growers-2022-2023-id-36/ (accessed on 24 December 2024).
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T. Trace elements composition and enzymes activity of soil amended with municipal sewage sludge at three locations in Kentucky. Int. J. Appl. Agric. Sci. 2020, 6, 89–95. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Bagyaraj, D.J.; Rangaswami, G. Studies on the influence of foliar application of chemicals on the microflora and certain enzyme activities in the rhizosphere of Eleusine coracana Gaertn. Plant Soil 1970, 32, 198–206. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Method 6020A: Inductively Coupled Plasma-Mass Spectrometry; USEPA: Washington, DC, USA, 1998. Available online: https://19january2017snapshot.epa.gov/sites/production/files/2015-07/documents/epa-6020a.pdf (accessed on 24 December 2024).
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Eds.; Port City Press: Baltimore, MD, USA, 2005. [Google Scholar]
- SAS Institute Inc. SAS/STAT Guide; Version 9.1.3; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Frankenberger, W.T.; Johanson, J.B. Factors affecting invertase activity in soils. Plant Soil 1983, 3, 313–323. [Google Scholar] [CrossRef]
- Pabbisetty, S.K.; Shrivastava, A.; Satyanarayana, T. Effects of pH on the activity and stability of invertase from Thermotoga maritima. J. Enzym. Microb. Technol. 2010, 46, 98–103. [Google Scholar]
- Tan, X.; Liu, Y.; Yan, K.; Wang, Z.; Lu, G.; He, Y.; He, W. Differences in the response of soil dehydrogenase activity to Cd contamination are determined by the different substrates used for its determination. Chemosphere 2017, 169, 324–332. [Google Scholar] [CrossRef]
- Zhao, G.; Sheng, Y.; Li, C.; Liu, Q. Effects of macro metals on alkaline phosphatase activity under conditions of sulfide accumulation. Sci. Total Environ. 2019, 697, 134151. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Baran, A. Effect of wheat and Miscanthus straw biochars on soil enzymatic activity, ecotoxicity, and plant yield. Int. Agrophys. 2017, 31, 367–375. [Google Scholar] [CrossRef]
- Ameloot, N.; Neve, S.D.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Tan, X.; Machmuller, M.B.; Wang, Z.; Li, X.; He, W.; Cotrufo, M.F.; Shen, W. Temperature enhances the affinity of soil alkaline phosphatase to Cd. Chemosphere 2018, 196, 214–222. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Liu, X.; Chang, T.; Wang, Q.; Shaghaleh, H.; Hamoud, Y.A. Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front. Environ. Sci. 2023, 10, 1060277. [Google Scholar] [CrossRef]
- Boavida, M.J.; Wetzel, R.G. Inhibition of phosphatase activity by dissolved humic substances and hydrolytic reactivation by natural ultraviolet light. Freshw. Biol. 1998, 40, 285–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).