Characterization and Selection of Lactobacillus Strains with Potential Probiotic Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
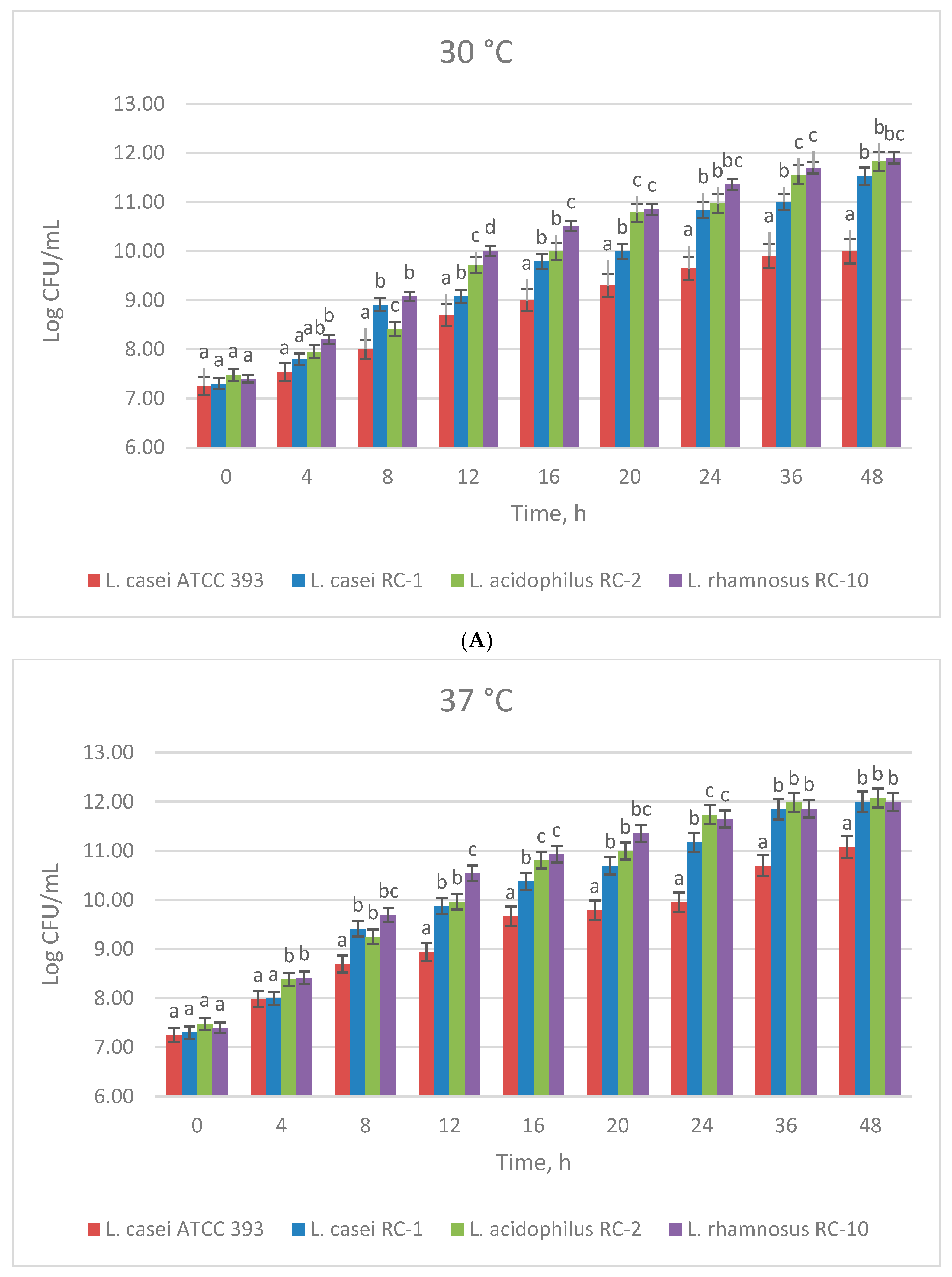
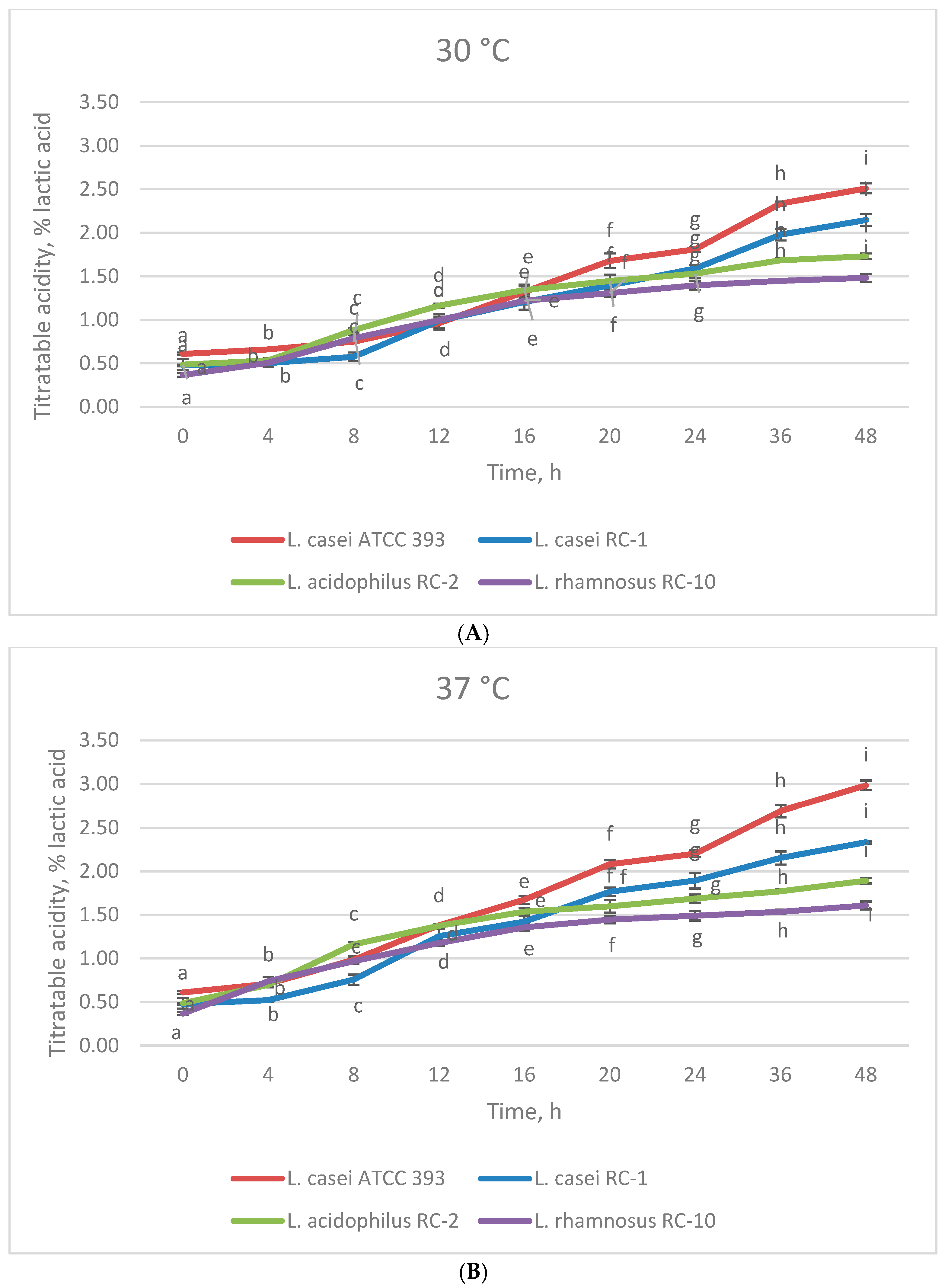
3.1. The Effect of Temperature on the Growth of Lactobacillus Strains
3.2. Resistance of Lactobacillus Strains to Simulated Upper Gastrointestinal Tract Conditions
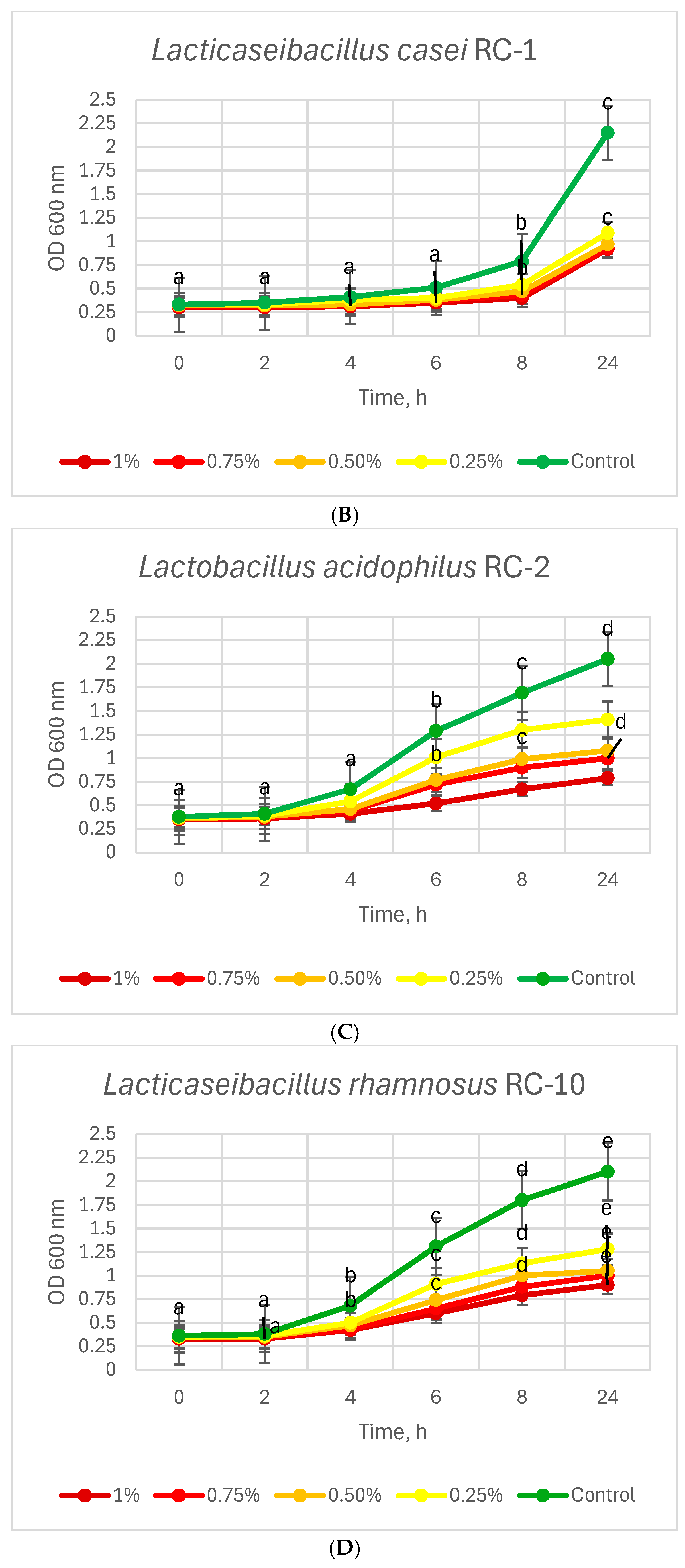
3.3. Resistance of Lactobacillus Strains to Bile Salts
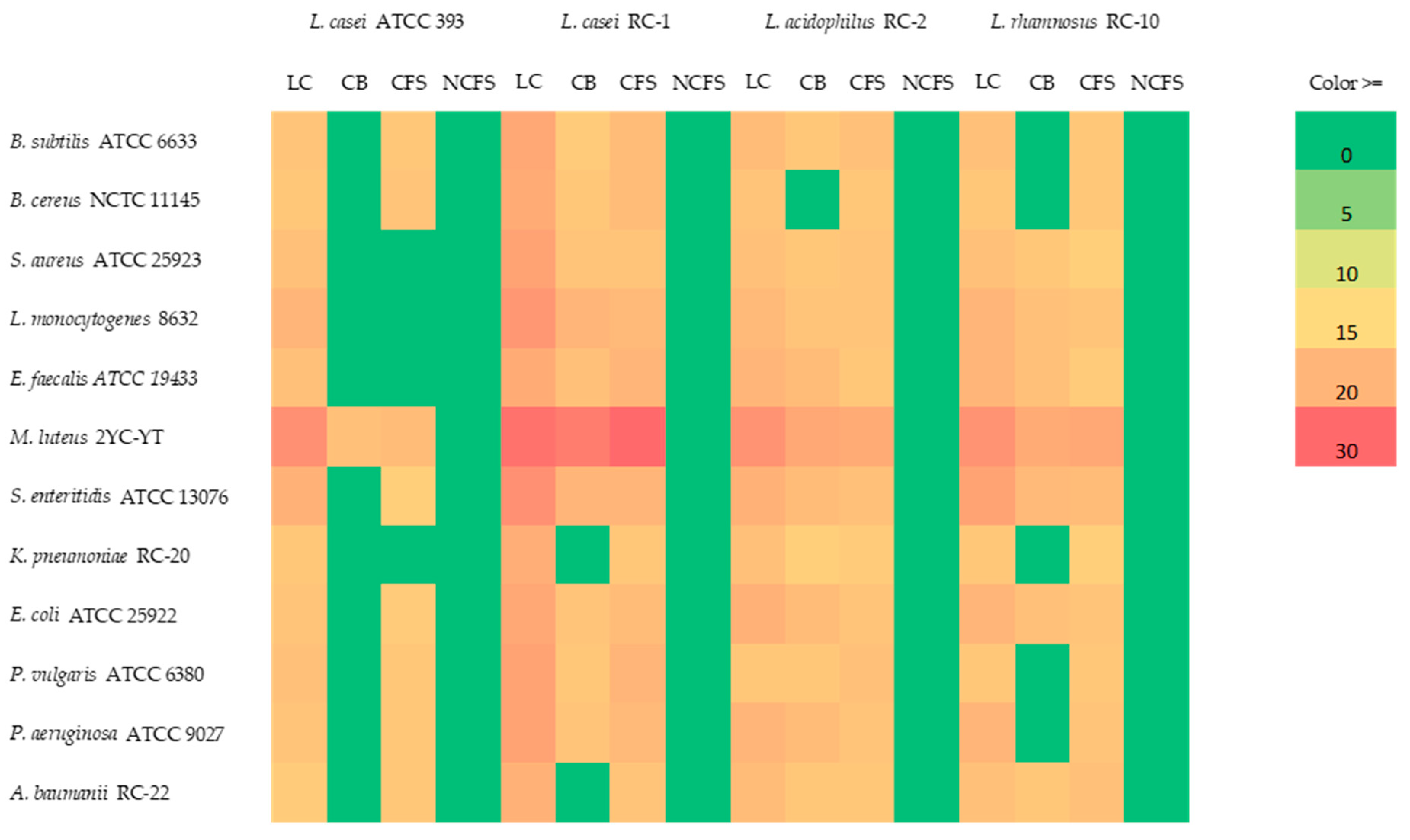
3.4. Antimicrobial Activity of Lactobacillus Strains
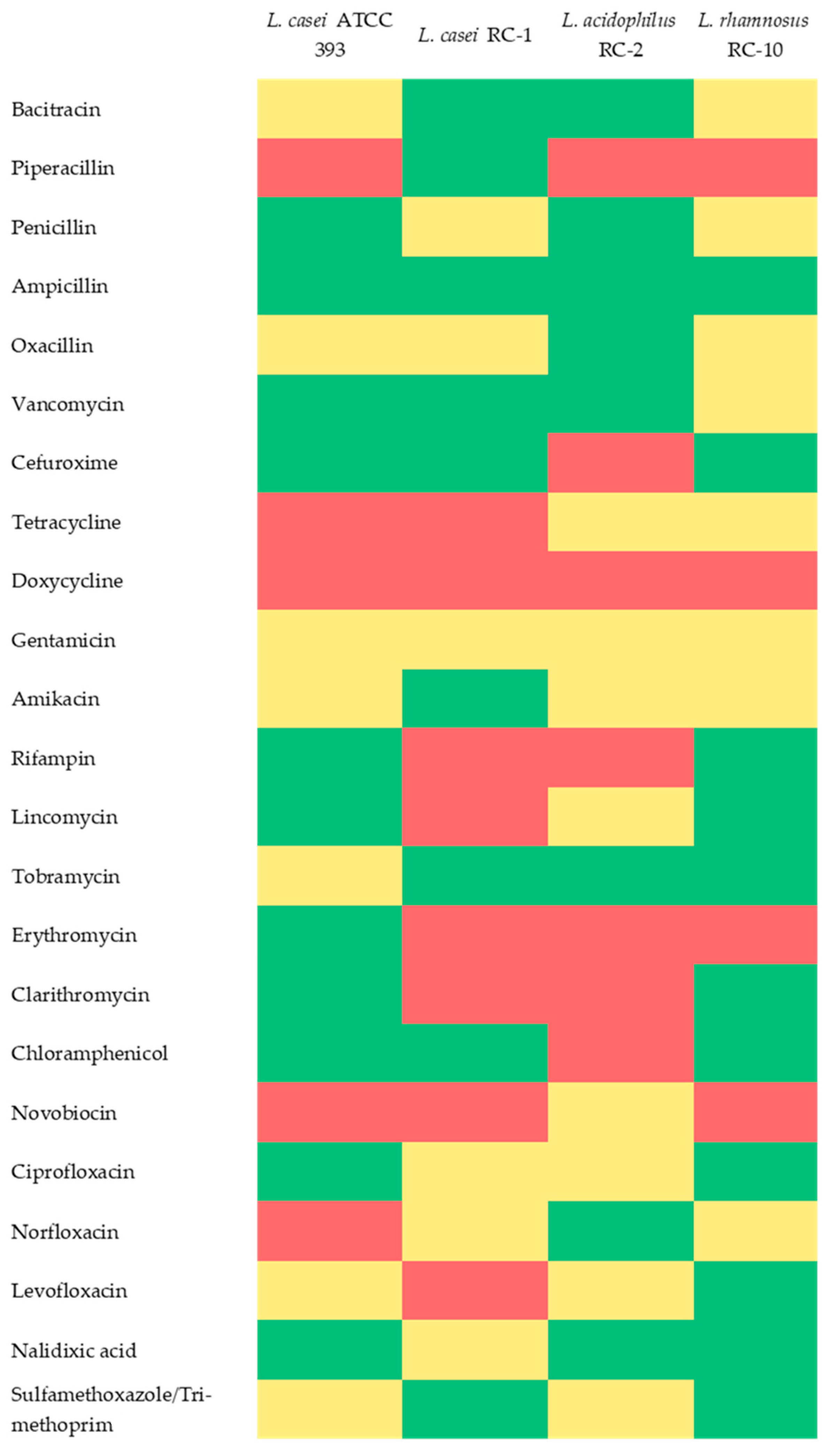
3.5. Antibiotic Susceptibility of Lactobacillus Strains
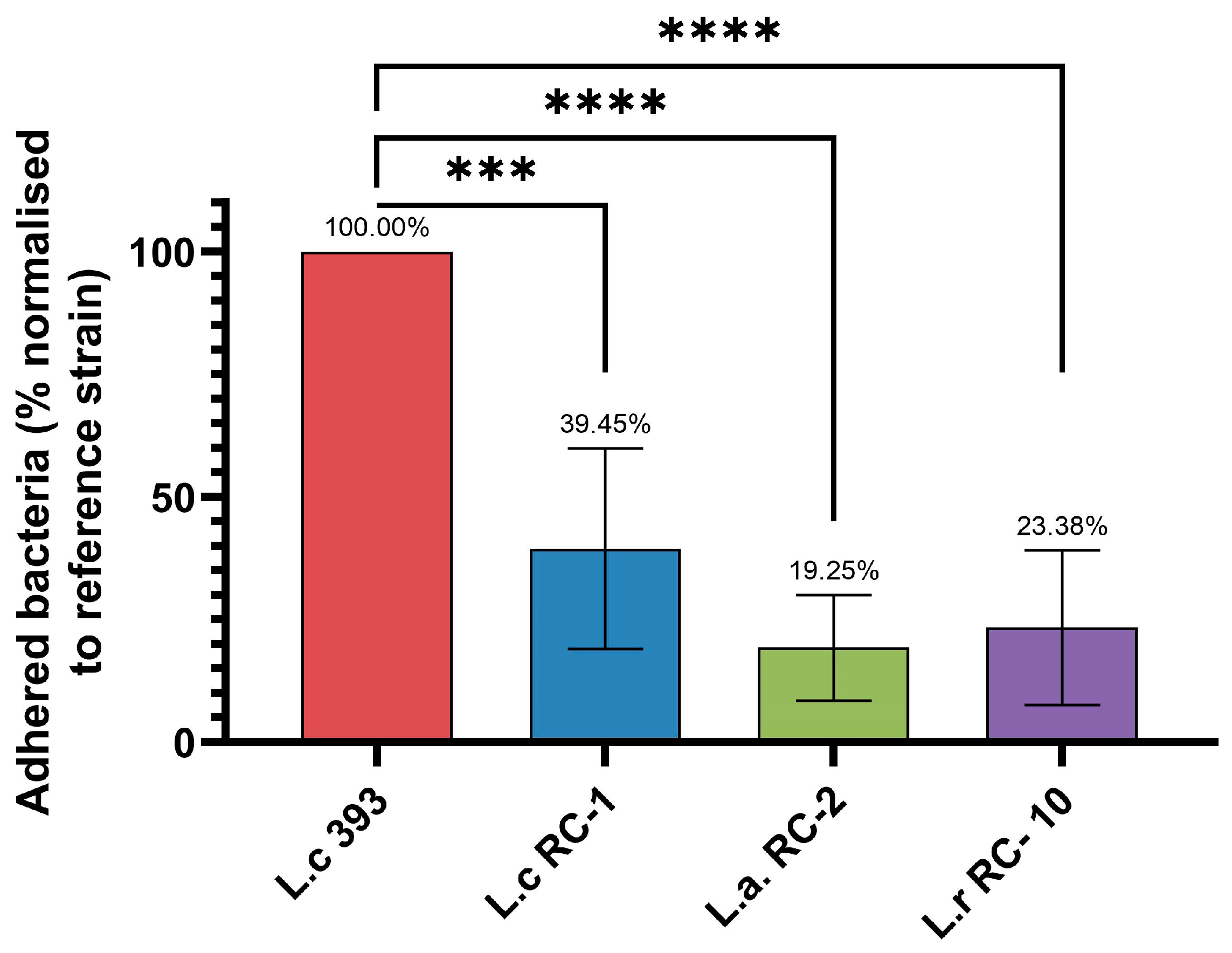
3.6. Adhesion Ability of Lactobacillus Strains to Intestinal Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food; WHO: Geneva, Switzerland, 2002; pp. 1–11. [Google Scholar]
- Zielinska, D.; Kolohyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. BioMed. Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef] [PubMed]
- Caggia, C.; De Angelis, M.; Pitino, I.; Pino, A.; Randazzo, C.L. Probiotic features of Lactobacillus strains isolated from Ragusano and Pecorino Siciliano cheeses. Food Microbiol. 2015, 50, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 2017, 30, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Zhao, M.; Zhong, H.; Luo, W.; Feng, F. In vitro and in vivo investigations of probiotic properties of lactic acid bacteria isolated from Chinese traditional sourdough. Appl. Microbiol. Biotechnol. 2019, 103, 1893–1903. [Google Scholar] [CrossRef]
- Shehata, M.G.; El Sohaimy, S.A.; El-Sahn, M.A.; Youssef, M.M. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016, 61, 65–75. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D. Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe 2015, 33, 117–123. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Chaudhary, A.; Saharan, B.S. Probiotic Properties of Lactobacillus plantarum. J. Pure Appl. Microbiol. 2019, 13, 933–948. [Google Scholar] [CrossRef]
- Song, M.; Yun, B.; Moon, J.-H.; Park, D.-J.; Lim, K.; Oh, S. Characterization of Selected Lactobacillus Strains for Use as Probiotics. Korean J. Food Sci. Anim. Resour. 2015, 35, 551–556. [Google Scholar] [CrossRef]
- Xu, S.; Liu, T.; Radji, C.A.I.; Yang, J.; Chen, L. Isolation, Identification, and Evaluation of New Lactic Acid Bacteria Strains with Both Cellular Antioxidant and Bile Salt Hydrolase Activities In Vitro. J. Food Prot. 2016, 79, 1919–1928. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Physiological Characteristics of Lactobacillus casei Strains and Their Alleviation Effects against Inflammatory Bowel Disease. J. Microbiol. Biotechnol. 2021, 31, 92–103. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Todorova, M.; Topuzova, M.; Georgieva, P.; Ganeva, Z.; Mihov, R.; Yanakieva, V. Antifungal Activity of Carboxymethyl Cellulose Edible Films Enriched with Propolis Extracts and Their Role in Improvement of the Storage Life of Kashkaval Cheese. Curr. Res. Nutr. Food Sci. 2021, 9, 487–499. [Google Scholar] [CrossRef]
- García-Hernández, Y.; Pérez-Sánchez, T.; Boucourt, R.; Balcázar, J.L.; Nicoli, J.R.; Moreira-Silva, J.; Rodríguez, Z.; Fuertes, H.; Nuñez, O.; Albelo, N.; et al. Isolation, characterization and evaluation of probiotic lactic acid bacteria for potential use in animal production. Res. Vet. Sci. 2016, 108, 125–132. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Yanakieva, V.; Denkova, R.; Denkova, Z. Isolation, Identification and Comparison of Some Properties of Lactobacillus delbrueckii subsp. bulgaricus Strains from Traditional Bulgarian and Italian Yogurts. Carpathian J. Food Sci. Technol. 2021, 13, 38–54. [Google Scholar] [CrossRef]
- Grzymajło, K.; Dutkiewicz, A.; Czajkowska, J.; Carolak, E.; Aleksandrowicz, A.; Waszczuk, W. Salmonella adhesion is decreased by hypoxia due to adhesion and motility structure crosstalk. Vet. Res. 2023, 54, 99. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth Kinetics of Probiotic Lactobacillus Strains in the Alternative, Cost-Efficient Semi-Solid Fermentation Medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.-Q.; Shah, N.P. Characterization of new probiotics from dairy and nondairy products—Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- Das, P.; Khowala, S.; Biswas, S. In vitro probiotic characterization of Lactobacillus casei isolated from marine samples. LWT—Food Sci. Technol. 2016, 73, 383–390. [Google Scholar] [CrossRef]
- Nagyzbekkyzy, E.; Abitayeva, G.; Anuarbekova, S.; Shaikhina, D.; Li, K.; Shaikhin, S.; Almagambetov, K.; Abzhalelov, A.; Saduakhassova, S.; Kushugulova, A.; et al. Investigation of acid and bile tolerance, antimicrobial activity and antibiotic resistance of Lactobacillus strains isolated from kazakh dairy foods. Asian J. Appl. Sci. 2016, 9, 143–158. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT—Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT—Food Sci. Technol. 2019, 116, 108501. [Google Scholar] [CrossRef]
- Farid, W.; Masud, T.; Sohail, A.; Ahmad, N.; Saqlan Naqvi, S.M.; Khan, S.; Ali, A.; Khalifa, S.A.; Hussain, A.; Ali, A.; et al. Gastrointestinal transit tolerance, cell surface hydrophobicity, and functional attributes of Lactobacillus acidophilus strains isolated from Indigenous Dahi. Food Sci. Nutr. 2021, 9, 5092–5102. [Google Scholar] [CrossRef]
- Han, Q.; Kong, B.; Chen, Q.; Sun, F.; Zhang, H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods 2017, 32, 391–400. [Google Scholar] [CrossRef]
- Altamirano-Rios, A.V.; Guadarrama-Lezama, A.Y.; Arroyo-Maya, I.J.; Hernandez-Alvarez, A.-J.; Orozco-Villafuerte, J. Effect of encapsulation methods and materials on the survival and viability of Lactobacillus acidophilus: A review. Int. J. Food Sci. Technol. 2022, 57, 4027–4040. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, Y.; Zhong, J.; Zhang, D. Assessing the Safety and Probiotic Characteristics of Lacticaseibacillus rhamnosus X253 via Complete Genome and Phenotype Analysis. Microorganisms 2023, 11, 140. [Google Scholar] [CrossRef]
- Vougiouklaki, D.; Tsironi, T.; Tsantes, A.G.; Tsakali, E.; Van Impe, J.F.M.; Houhoula, D. Probiotic Properties and Antioxidant Activity In Vitro of Lactic Acid Bacteria. Microorganisms 2023, 11, 1264. [Google Scholar] [CrossRef]
- Sieladie, D.V.; Zambou, N.F.; Kaktcham, P.M.; Cresci, A.; Fonteh, F. Probiotic properties of Lactobacilli strains isolated from raw cow milk in the western highlands of Cameroon. Innov. Rom. Food Biotechnol. 2011, 9, 12–28. [Google Scholar]
- Thaweesang, S.; Leenanon, B. Survival of Lactobacillus acidophilus TISTR1338 in bile salt stress conditions. Asian J. Microbiol. Biotech. Env. Sci. 2016, 18, 569–579. [Google Scholar]
- Tarrah, A.; da Silva Duarte, V.; de Castilhos, J.; Pakroo, S.; Lemos Junior, W.J.F.; Luchese, R.H.; Fioravante Guerra, A.; Rossi, R.C.; Righetto Ziegler, D.; Corich, V.; et al. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 2019, 54, 489–497. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Masalam, M.S.B.; Bahieldin, A.; Alharbi, M.G.; Al-Masaudi, S.; Al-Jaouni, S.K.; Harakeh, S.M.; Al-Hindi, R.R. Isolation, Molecular Characterization and Probiotic Potential of Lactic Acid Bacteria in Saudi Raw and Fermented Milk. Evid. Based Complement. Alternat. Med. 2018, 2018, 7970463. [Google Scholar] [CrossRef]
- Nawaz, Z.; Zahoor, M.K.; Shafique, M.; Athar, R.; Yasmin, A.; Zahoor, M.A. In vitro assessment of probiotic properties of lactic acid bacteria isolated from camel milk: Enhancing sustainable foods. Front. Sustain. Food Syst. 2024, 8, 1437201. [Google Scholar] [CrossRef]
- El-Sayed, A.I.M.; El-Borai, A.M.; Akl, S.H.; EL-Aassar, S.A.; Abdel-Latif, M.S. Identification of Lactobacillus strains from human mother milk and cottage cheese revealed potential probiotic properties with enzymatic activity. Sci Rep. 2022, 12, 22522. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef]
- Hu, P.-L.; Yuan, Y.-H.; Yue, T.-L.; Guo, C.-F. A new method for the in vitro determination of the bile tolerance of potentially probiotic lactobacilli. Appl. Microbiol. Biotechnol. 2018, 102, 1903–1910. [Google Scholar] [CrossRef]
- Malilay, J.K.T.; Oliveros, M.C.R.; Bautista, J.A.N.; Castillo-Israel, K.A.T. Functional, safety and technological properties of Lactobacillus acidophilus BIOTECH 1900. Philipp. J. Vet. Anim. Sci. 2019, 45, 11–21. [Google Scholar]
- Barzegar, H.; Behbahani, B.A.; Falah, F. Safety, probiotic properties, antimicrobial activity, and technological performance of Lactobacillus strains isolated from Iranian raw milk cheeses. Food Sci Nutr. 2021, 9, 4094–4107. [Google Scholar] [CrossRef] [PubMed]
- George-Okafor, U.; Ozoani, U.; Tasie, F.; Mba-Omeje, K. The efficacy of cell-free supernatants from Lactobacillus plantarum Cs and Lactobacillus acidophilus ATCC 314 for the preservation of home-processed tomato-paste. Sci. Afr. 2020, 8, e00395. [Google Scholar] [CrossRef]
- Ali, F.S.; Zayed, G.; Saad, O.A.O.; Salwa, A.; Gharib, H. Antimicrobial activity and Probiotic Properties of Lactic Acid Bacteria Isolated From Traditional Fermented Dairy Products. J. Mod. Res. 2020, 2, 40–48. [Google Scholar] [CrossRef]
- Bohora, A.A.; Kokate, S.R.; Khedkar, S.; Vankudre, A. Antimicrobial activity of probiotics against endodontic pathogens:- A preliminary study. Indian J. Med. Microbiol. 2019, 37, 5–11. [Google Scholar] [CrossRef]
- Soltani, N.; Abbasi, S.; Baghaeifar, S.; Taheri, E.; Jadid, M.F.S.; Emami, P.; Abolhasani, K.; Aslanshirzadeh, F. Antibacterial and antibiofilm activity of Lactobacillus strains secretome and extraction against Escherichia coli isolated from urinary tract infection. Biotechnol. Rep. 2022, 36, e00760. [Google Scholar] [CrossRef]
- Stivala, A.; Carota, G.; Fuochi, V.; Furneri, P.M. Lactobacillus rhamnosus AD3 as a Promising Alternative for Probiotic Products. Biomolecules 2021, 11, 94. [Google Scholar] [CrossRef]
- Anil, A.; Raj, A.; Azhar, M.M.; Kuriakose, N.; Murali, R.; Kumar, H.K.S. Evaluation of antimicrobial activity of Lactobacillus acidophilus and Lactobacillus rhamnosus against clinically isolated Klebsiella pneumoniae: An in vitro study. Int. J. Res. Med. Sci. 2024, 12, 1140–1146. [Google Scholar] [CrossRef]
- Darsanaki, R.K.; Aliabadi, M.A.; Chakoosari, M.M.D. Antibiotic resistance of lactic acid bacteria. Sci. J. Microbiol. 2013, 2, 201–206. [Google Scholar]
- Anisimova, E.A.; Yarullina, D.R. Antibiotic Resistance of LACTOBACILLUS Strains. Curr Microbiol. 2019, 76, 1407–1416. [Google Scholar] [CrossRef]
- De Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum Strains Isolated from Mozzarella Cheese: Probiotic Potential, Safety, Acidifying Kinetic Parameters and Viability under Gastrointestinal Tract Conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef]
- Selvin, J.; Maity, D.; Sajayan, A.; Kiran, G.S. Revealing antibiotic resistance in therapeutic and dietary probiotic supplements. J. Glob. Antimicrob. Resist. 2020, 22, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhang, W.; Guo, H.; Pan, L.; Zhang, H.; Sun, T. Comparative studies on antibiotic resistance in Lactobacillus casei and Lactobacillus plantarum. Food Control 2015, 50, 250–258. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Svensson, B. Dietary Nutrients, Proteomes, and Adhesion of Probiotic Lactobacilli to Mucin and Host Epithelial Cells. Microorganisms 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Romeih, E.; Gamba, R.R.; Nagai, E.; Suzuki, T.; Koyanagi, T.; Enomoto, T. The biological activity of fermented milk produced by Lactobacillus casei ATCC 393 during cold storage. Int. Dairy J. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Esmail, G.A.; Alzeer, A.F.; Arasu, M.V.; Vijayaraghavan, P.; Choi, K.C.; Al-Dhabi, N.A. Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J. Biol. Sci. 2020, 27, 3505–3513. [Google Scholar] [CrossRef]
- Sophatha, B.; Piwat, S.; Teanpaisan, R. Adhesion, anti-adhesion and aggregation properties relating to surface charges of selected Lactobacillus strains: Study in Caco-2 and H357 cells. Arch. Microbiol. 2020, 202, 1349–1357. [Google Scholar] [CrossRef]
- Feng, Y.; Qiao, L.; Liu, R.; Yao, H.; Gao, C. Potential probiotic properties of lactic acid bacteria isolated from the intestinal mucosa of healthy piglets. Ann. Microbiol. 2017, 67, 239–253. [Google Scholar] [CrossRef]
- Stojanov, S.; Plavec, T.V.; Zupančič, Š.; Berlec, A. Modified vaginal lactobacilli expressing fluorescent and luminescent proteins for more effective monitoring of their release from nanofibers, safety and cell adhesion. Microb. Cell Fact. 2024, 23, 333. [Google Scholar] [CrossRef]



| Strain | Conditions | Cell Recovery, % | |
|---|---|---|---|
| 6 h | 24 h | ||
| L. casei ATCC 393 | pH 2.5 + pepsin | 65.45 ± 0.02 aA | 75.35 ± 0.06 aB |
| pH 7.4 + pancreatin | 80.91 ± 0.03 bA | 93.95 ± 0.07 bB | |
| L. casei RC-1 | pH 2.5 + pepsin | 60.25 ± 0.01 aA | 74.24 ± 0.03 aB |
| pH 7.4 + pancreatin | 86.96 ± 0.05 bA | 96.07 ± 0.03 bB | |
| L. acidophilus RC-2 | pH 2.5 + pepsin | 66.67 ± 0.01 aA | 87.50 ± 0.15 aB |
| pH 7.4 + pancreatin | 87.10 ± 0.02 bA | 98.15 ± 0.02 bB | |
| L. rhamnosus RC-10 | pH 2.5 + pepsin | 70.33 ± 0.12 aA | 87.30 ± 0.06 aB |
| pH 7.4 + pancreatin | 81.32 ± 0.07 bA | 96.83 ± 0.02 bB | |
| Strain | Bile Salts Concentration | Survival Rate, % | ||
|---|---|---|---|---|
| 4 h | 8 h | 24 h | ||
L. casei ATCC 393 | 1% | 82.50 ± 0.02 aA | 60.00 ± 0.02 aB | 36.72 ± 0.06 aC |
| 0.75% | 87.50 ± 0.01 bA | 61.67 ± 0.02 bB | 38.98 ± 0.04 bC | |
| 0.5% | 90.00 ± 0.02 cA | 65.00 ± 0.04 cB | 41.81 ± 0.06 cC | |
| 0.25% | 92.50 ± 0.03 dA | 68.33 ± 0.02 dB | 50.85 ± 0.08 dC | |
L. casei RC-1 | 1% | 75.61 ± 0.08 aA | 50.63 ± 0.08 aB | 42.79 ± 0.08 aC |
| 0.75% | 78.05 ± 0.06 bA | 54.43 ± 0.08 bB | 43.26 ± 0.12 bC | |
| 0.5% | 80.49 ± 0.04 cA | 59.49 ± 0.06 cB | 45.12 ± 0.09 cC | |
| 0.25% | 92.68 ± 0.02 dA | 68.35 ± 0.03 dB | 50.70 ± 0.03 dC | |
L. acidophilus RC-2 | 1% | 61.19 ± 0.01 aA | 40.24 ± 0.04 aB | 38.54 ± 0.08 aC |
| 0.75% | 65.67 ± 0.01 bA | 53.25 ± 0.09 bB | 48.78 ± 0.04 bC | |
| 0.5% | 68.66 ± 0.03 cA | 58.58 ± 0.06 cB | 52.68 ± 0.10 cC | |
| 0.25% | 80.60 ± 0.09 dA | 76.92 ± 0.19 dB | 68.78 ± 0.04 dC | |
L. rhamnosus RC-10 | 1% | 61.76 ± 0.01 aA | 43.89 ± 0.10 aB | 42.86 ± 0.06 aC |
| 0.75% | 63.24 ± 0.08 bA | 48.89 ± 0.11 bB | 47.62 ± 0.05 bC | |
| 0.5% | 69.12 ± 0.03 cA | 55.56 ± 0.12 cB | 50.00 ± 0.12 cC | |
| 0.25% | 73.53 ± 0.14 dA | 62.78 ± 0.11 dB | 60.95 ± 0.10 dC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumbarski, Y.; Peykova-Shapkova, I.; Ivanova, M.; Cholakov, R.; Dutkiewicz, A.; Grzymajło, K. Characterization and Selection of Lactobacillus Strains with Potential Probiotic Applications. Appl. Sci. 2025, 15, 2902. https://doi.org/10.3390/app15062902
Tumbarski Y, Peykova-Shapkova I, Ivanova M, Cholakov R, Dutkiewicz A, Grzymajło K. Characterization and Selection of Lactobacillus Strains with Potential Probiotic Applications. Applied Sciences. 2025; 15(6):2902. https://doi.org/10.3390/app15062902
Chicago/Turabian StyleTumbarski, Yulian, Ivelina Peykova-Shapkova, Mihaela Ivanova, Remzi Cholakov, Agata Dutkiewicz, and Krzysztof Grzymajło. 2025. "Characterization and Selection of Lactobacillus Strains with Potential Probiotic Applications" Applied Sciences 15, no. 6: 2902. https://doi.org/10.3390/app15062902
APA StyleTumbarski, Y., Peykova-Shapkova, I., Ivanova, M., Cholakov, R., Dutkiewicz, A., & Grzymajło, K. (2025). Characterization and Selection of Lactobacillus Strains with Potential Probiotic Applications. Applied Sciences, 15(6), 2902. https://doi.org/10.3390/app15062902









