1. Introduction
The bottarga, also called
avgotaracho in Greece or
karasumi in Japan, is a product created during the process of drying and salting ovaries from different fish species [
1]. It is produced worldwide with different manufacturing methods; Sardinia’s bottarga is the end-product of several treatments of female gonads exclusively from mullet (
Mugil cephalus L.) that occur in specific humidity (53%) and temperature (25 °C) conditions [
2]. The total production of bottarga in Sardinia is equal to 400 tons/year [
3]. Considering the high economic value of the product (200 EUR/kg), the production of bottarga represents an important economic sector for the region. Moreover, it has been recognized as a traditional product of Sardinia (National list of traditional products, Ministerial Decree 18/07/2000).
As a fish product, bottarga has an interesting chemical composition and fatty acid profile with a high nutritional value due to the high concentration of some long-chain polyunsaturated fatty acids (LC-PUFA) from the omega-3 family, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [
4]. Approximately, EPA and DHA amounted to 22% of total fatty acids of bottarga [
2]. These fatty acids (FAs) play an important role in the prevention of cardiovascular diseases, inflammatory processes, and metabolic syndrome [
5,
6]. Also, Rosa et al. [
2] evidenced a high content of free fatty acids due to the hydrolysis that occurs during the manufacturing and storage conditions.
In terms of microbiological properties, due to the low level of water activity, which can drop as low as 0.77 [
4], bottarga is not an ideal substrate for the growth of microorganisms [
7]. However, previous studies [
8,
9] evidenced a contamination of bottarga and similar products with some pathogens. The authors found a variable number of spoilage/pathogen bacteria in the samples, such as
Micrococcus spp.,
Clostridium perfringens, and
Staphylococcus aureus.
S. aureus is a halophilic bacteria capable of growing with a W
a value < 0.872.
S. aureus could be normally found in the human skin, and if good hygienic practices during the manual work steps were not followed, contamination of the final product may occur [
10]. Several steps of bottarga manufacturing involve manual work, and for this reason, biological contamination could occur in some phases of the process [
10]. The data about the microbiological analysis of bottarga is quite limited; however, this type of analysis is important for the determination of the safety of the final product.
Consumer acceptance has received attention in recent years [
11] and sensory analysis plays an important role in understanding the palatability of food products. Several factors, such as manufacturing, processing, or preparation, may influence the characteristics (e.g., color, freshness, and humidity) of fresh products and their acceptability. Despite the fact that bottarga is largely appreciated for its properties, there is scant literature about the sensory analysis of this seafood. Only Rosa et al. [
12] have investigated the sensory evaluation of different bottarga samples and shown the relationship between lipid content and sensory properties (taste and odor) of the product.
The mullet from which the Sardinian bottarga comes from is a schooling species that lives in the coastal waters of tropical, subtropical, and temperate zones; therefore, Sardinia represents a natural habitat for the rearing of this species. The production of bottarga, traditionally associated with the consumption of mullet [
13], is born in the natural habitat favorable to the survival of this fish species, in particular in the ponds of Cabras (Oristano), Tortolì (Ogliastra), San Teodoro (Nuoro), and in the lagoons of the San Pietro Island and Sant’Antioco (Cagliari).
Each lagoon has peculiar features strongly related to freshwater inputs, tidal fluctuation, and human activity. For this reason, the lagoons are considered a heterogeneous environment that can strongly differ between them in terms of flora and found endemic species [
14]. The Cabras lagoon, the largest lagoon in Sardinia, is characterized by two main tributaries that give the basin a low salinity (<10 psu) during rainfall period [
15]. The Tortolì lagoon is considered a medium-size basin and, due to its physiographic features, is strongly influenced by the sea. The Tortolì lagoon is considered a peculiar ecosystem, characterized by a high level of biodiversity and by a marked degree of marinization [
16].
There is a great variability in the physico-chemical characteristics among roes from different geographical origins [
3], depending on both the manufacturing processes and the different environmental conditions. However, there is scant information on the differences in the bottarga produced in Sardinia from different local fish.
Due to the different characteristics of the lagoons and the bottarga manufacturing process, the bottarga produced in the east (Tortolì) and west coasts (Cabras) of Sardinia may have different physico-chemical characteristics, quality, and sensory properties. For this reason, the nutritional and microbiological qualities of the bottarga from Sardinia were investigated. Furthermore, sensory analyses were carried out to assess consumer acceptance of the product.
2. Materials and Methods
2.1. Technological Process
As described in
Figure 1, the technological process of bottarga starts with the fishing of the mullet that is performed with a particular fishing basin called a “
lavoriero”. This fishing technique takes advantage of the reproductive migration of the fish toward the sea. The
lavoriero is a traditional enclosure placed at the mouth of the lagoon, which makes it possible to fish only the mullet that have completed their natural cycle and that have ovaries ready for bottarga production. The mullets are carefully caught with nets. After fishing, the mullets are selected based on their size and the integrity of the animals. The evisceration is performed manually with knives. The weight of the ovaries ranged between 200 and 500 g. To remove the ovaries, the fish is desquamated in the ventral part, and several incisions are made to take them off perfectly intact. The roes are then washed, and the blood is removed. The roes are salted with marine salt to reduce moisture (4% weight loss [
7]). After two hours, the roes are rinsed with water to remove the excess salt. Then, the drying process starts, and the roes are kept in ventilated, humidity- (about 35%) and temperature-controlled rooms (at 10–15 °C) for about 2 weeks. A specialized operator determines the end of the drying period by applying manual pressure on the product.
2.2. Sampling and Analysis
Eighteen samples of bottarga were collected from two fishing companies, namely BEC and BWC, located along the east coast of Sardinia at Tortolì (39°56′36′′ N, 9°40′13′′ E) and along the west cost of Sardinia at Cabras (39°54′16′′ N, 8°27′14′′ E), respectively (
Figure 2).
For each manufacturer, three replicates (one sample from three different trays) and two repetitions of each analysis were carried out.
2.3. Physico-Chemical and Microbiological Analyses
For the determination of the physico-chemical analysis, the Bottarga samples were finely ground and analyzed for water activity (W
a) using an electric hygrometer (Thermoconstanter model, Novasina, Germany). pH was measured by a pH meter (Thermo Fisher Scientific 0250A0, Milan, Italy, pH/mV/relative mV/temperature meter, model 250A). The AOAC methods [
17] were used for the analysis of carbohydrates (method 974.06), total fats (method 948.15), and proteins (method 981.10). For the determination of sodium, calcium potassium, and phosphorus (method 985.01), the samples underwent a digestion process with a microwave system [
18].
Finally, cholesterol was determined according to Shen et al. [
19] using an enzymatic kit (Boehringer Mannheim/R-Biopharm, Milan, Italy).
For the microbiological analysis, 25 g of bottarga sample was homogenized in a 225 mL sterile Ringer’s solution for 3 min in a Stomacher Lab Blender 80 (PBI, Milan, Italy). Aliquots (1 mL) were 10-fold diluted in Ringer’s solution and plated/inoculated on specific media used to quantify different microbial groups. Both mesophilic (MMC) and psychrophilic (PMC) microbial counts were enumerated on plate count agar (Oxoid, Milan, Italy) at 30 °C and 5 °C for 72 h, respectively; yeasts and molds were counted on YPDA that consisted of 1% w/v yeast extract, 2% w/v dextrose, 2% w/v peptone, 1.5% w/v agar (all ingredients were purchased from Oxoid), and with 100 mg mL−1 of chloramphenicol (Merk, Darmstadt, Germany) at a pH of 4.5 after incubating at 25 °C for 48–96 h; enterobacteria in VRBGA (Oxoid) and subsequent incubation at 37 °C for 48 h; fecal coliforms were enumerated in brilliant-green bile broth (Oxoid) with Durham bell after 48 h of incubation at 44 °C; and presumptive E. coli was counted by indole testing: a subculture (0.1 mL) of fecal coliforms with positive BGBB tubes was transferred to tryptone water to perform the indole production test with Kovac’s reagent (Sigma-Aldrich, Darmstadt, Germany) after 37 °C for 24 h (MPN method).
Staphylococci in Baird–Parker agar (Oxoid) supplemented with egg yolk tellurite emulsion (Oxoid) were incubated at 37 °C for 48 h; presumptive colonies of coagulase-positive staphylococci were assayed for coagulase activity using the staphylase test (Oxoid, Italy); spores of sulphite-reducing clostridia (MPN method) were placed in DRCM broth (Oxoid) after heat treatment (80 °C for 10 min) of the samples and incubated at 37 °C for 48 h in anaerobic conditions.
The results of microbiological analysis were expressed as CFU/g or MPN/g. Three replicates (one sample from three different trays) and two repetitions of each analysis were carried out.
The Listeria spp. was determined, involving two pre-enrichment phases. Briefly, 25 g of sample was first diluted in 225 mL of Fraser Broth (Oxoid) and enriched with Fraser Supplement SR166M and placed to incubate at 30 °C for 21–24 (1st enrichment). Subsequently, 1 mL of the primary enrichment was transferred into 10 mL of buffered listeria enrichment broth base (BLEBB) enriched with the SR141E supplement and incubated at 30 °C for 21–24 min (2nd enrichment).
In case of blackening of the broth culture, a smear is carried out on Palcam Agar (Microbiol, Cagliari, Italy) differential and selective media after incubation at 30 °C for 48–72 h. The results for presumptive Listeria spp. were expressed as present (+)/absent (−) in 25 g.
2.4. Sensory Analysis: Descriptive Analysis by Expert Panelists
All panel members agreed to participate and received a nuisance allowance for their participation. The sensory evaluation of the bottarga was conducted following a modified quantitative descriptive analysis methodology [
20]. The sensory profiles of the bottarga were determined using twelve trained panelists (8 males, at the age of 45–60). The panelists employed in the bottarga descriptive analysis were habitual consumers of bottarga and had, on average, 10 years of experience in sensory analysis. Samples were stored at 8 ± 2 °C until evaluation. The samples were presented in plastic containers (petri dishes), taken out of the refrigerator three hours beforehand, and labeled with three-digit random codes. Each sample was represented by 3 mm thick slices, served at a controlled temperature of 18 ± 2 °C in a testing room with a combination of natural light.
The order of presentation was randomized across the panelists and sessions; to quantify the intensity of the baffa attributes, the panel used a nine-point horizontally oriented scale anchored as “not perceived at all” and “extremely intense” at the left and right ends, respectively.
The assessors were selected and trained in 9 sessions according to ISO 13299:2016 [
21] during which they agreed on a list of 12 attributes describing the appearance of the
baffa (uniformity of the color of the
baffa and the slice), the texture (friability, stickiness, and greasiness), and the sea odor, flavor, and taste (acid, bitter, and salty).
The panel’s performance was monitored during the training, with the purpose of examining the reproducibility and discriminatory ability of the panel individually and as a group. The performance of the panel was evaluated using PanelCheck (V1.4.2), according to the workflow suggested by Tomic et al. [
22].
The panel was trained with orientation sessions, after which it agreed to evaluate the following attributes, the reference standards they have been prepared and used in the training sessions. The judges used water (Smeraldina SpA, Sardinia, Italy) and unsalted crackers (Mulino Bianco SpA, Barilla, Italy) to cleanse the palate and minimize sample entrainment. All samples and reference solutions were prepared in a food-safe environment. All subjects gave their written informed consent prior to the beginning of the study, and they were instructed to refrain from smoking, eating, and drinking (except water) in the hour before tasting.
2.5. Consumer Test
The hedonic survey of consumer acceptability was conducted in the Food Science Laboratory Sensory (Porto Conte Ricerche, Alghero, Italy). The evaluation was carried out using an acceptance test and a 9-point hedonic scale. The consumers gave scores of 1–9 to the samples, ranging from “extremely dislike” to “extremely like” [
23]. A total of 64 subjects were included in the consumer study: 48 males and 16 females aged 32–60 years. They were asked to fill out an anonymous questionnaire with their demographic data (age, gender, education level, and specific food frequency questions). The samples were presented in plastic containers (petri dishes), taken out of the refrigerator three hours beforehand, and labeled with three-digit random codes. Each sample was represented by 3 mm thick slices, served at a controlled temperature of 18 ± 2 °C in a testing room with a combination of natural light. All consumers were screened before participating. Participation was voluntary. Furthermore, all participants provided written or verbal consent prior to the sensory evaluation. The final products tested in this study were confirmed safe for consumption, and participants were given the option to withdraw from the study at any time without justifications. Consumers received non-monetary compensation for their participation.
2.6. Statistical Analysis
The statistical analysis was performed using XLSTAT analysis software (version 2018.01) (Addinsoft, New York, NY, USA). The mixed three-way ANOVA model (sample, replica, and panelist) was conducted on all sensory attributes, and the corresponding interactions between the factors were used to determine the effects of the panel’s performance. Tukey’s honest significant difference (HSD) test was applied to evaluate the significant differences between samples for each attribute.
3. Results and Discussion
3.1. Physico-Chemical Parameters
The results of the physico-chemical analysis were shown in
Table 1. Bottarga reported a pH value of 5.3 and 5.45 without significant difference between BWC and BEC samples. Also, water activity (W
a) did not differ between the samples from the east and west coasts. The values of Aw were found to be 0.90 ± 0.46 and 0.91 ± 0.62 (mean ± SD) for the samples of the west coast and east coast, respectively. There is no information in the literature about the Aw regarding bottarga samples; however, the growth of the bacteria is limited when this value is <0.91 [
24]. The concentration of protein was 40.5% ± 1.73 and 38% ± 0.46 for the bottarga from the west and east coasts, without any difference between the two areas. The fat concentration was, on average, 18.6%. The bottarga also had a good concentration of calcium, equal to 455 and 413 mg/kg for the west and east coasts, respectively. The concentration of sodium in the samples, equal to 0.70%, was lower than reported by other authors (4.3% of dried weight) [
25]. The cholesterol found in the samples was 417 and 389 mg/100 g of the edible part for bottarga from the west and east coasts, respectively. These values were lower than that found by Rosa et al. [
25], who reported a concentration of cholesterol of 730 mg/100 g of the edible part.
3.2. Microbiological Quality Parameters
The results of the microbiological analysis were shown in
Table 2. Both mesophilic (MMC) and psychrophilic microbial count (PMC) were approximately 4 log CFU/g in all bottarga samples analyzed. Slightly higher microbial concentrations were reported in previous research conducted on Italian/Sardinian Bottarga [
10]; on the contrary, a much lower count (1.23 log CFU/g) has been determined in similar products from Caspian fish [
26].
Spoilage microorganisms indicative of environmental contamination, such as clostridia, yeasts, and molds, were not found in the samples. This result suggested that the significant number of the microbial population could be related to the intrinsic characteristics of the roe, such as pH and Aw, as well as to the technology used to make the product. In fact, the pH and the Wa of the bottarga and the manufacturing technologies, which involve the use of the hands, make the product favorable to microbial growth.
Moreover, the presence of coagulase-negative staphylococci (CNS) in all the samples analyzed suggested some improper applications of hygienic procedures. The potential pathogens coagulase positive staphylococci (CPS) were not determined in the east coast samples and in very low concentrations in the west coast samples, meeting the safety criteria [
27]. In fact, current food law requires the determination of staphylococcal enterotoxins when the presence of CP staphylococci exceeds 10
5 CFU/gr. In addition, presumptive pathogens, such as
Listeria and
E. coli, were found to be absent in all the samples analyzed. Other researchers found the presence of bacteria, albeit at low levels, in grey mullet roe from Greece [
8]. Brandas et al. [
10] also found contamination of bottarga samples with
S. aureus and other pathogens during the manufacturing processes. Both studies suggested that the contamination occurred mainly during the manufacturing process due to human manipulation.
3.3. Panel Performance
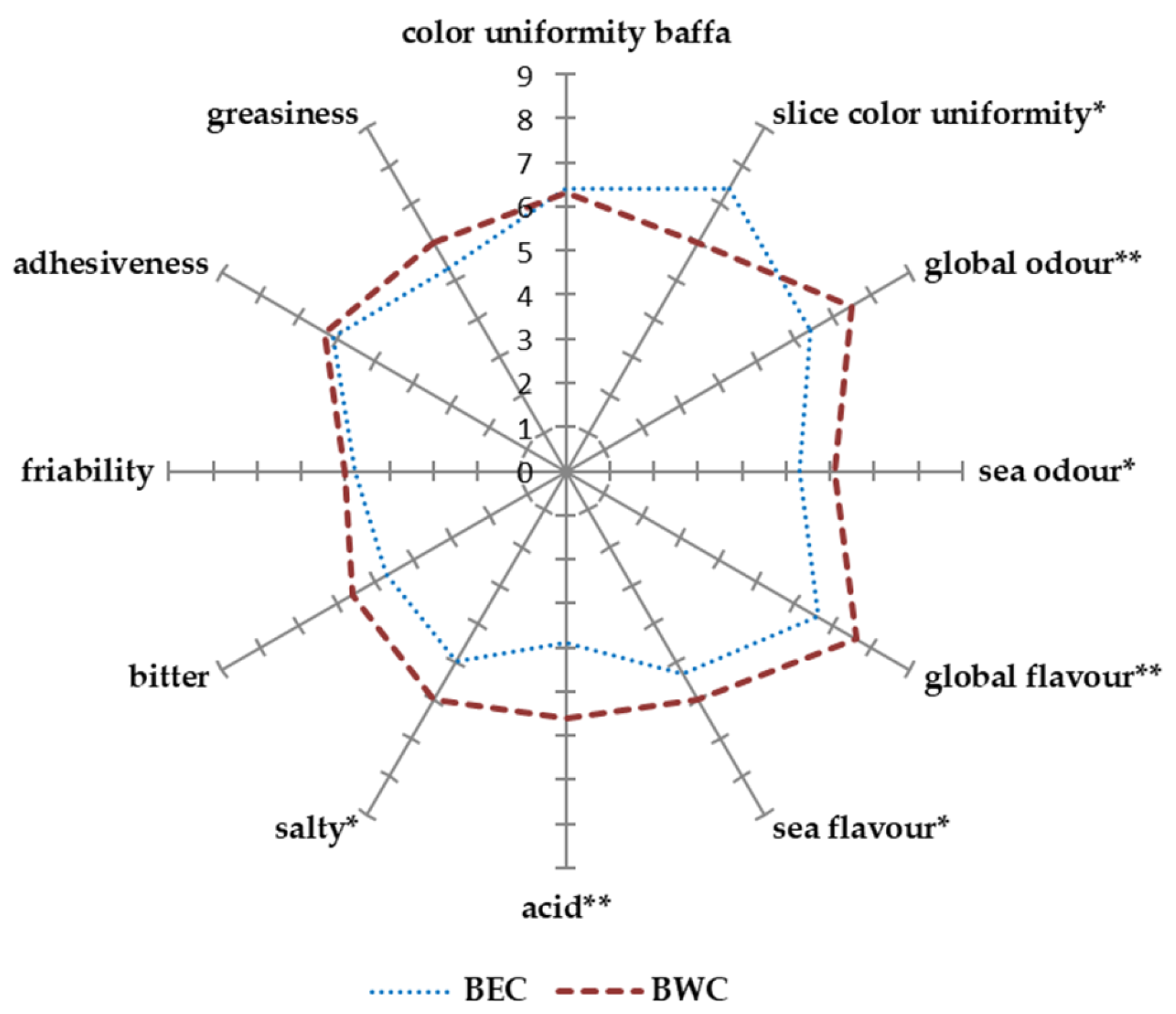
Based on the panel test, for two of eleven attributes (bitter and acid), the interaction between sample and panelist was significant (
p < 0.05), indicating that for those attributes the panelists differed in use of the scale. The factor “panelist” had a significant effect (
p < 0.05) for all the attributes. Furthermore, for all attributes, there were no significant effects of the replicate (
p > 0.05). Overall, 7 out of 12 attributes (
p < 0.05) were found to discriminate the samples (
Figure 3). Several factors could affect the color of a commercial grated bottarga sample: the quality of the raw material, the procedures (salting, drying, grinding, and storage), and the conditions (time, temperature, and light exposure) of production.
The BEC samples exhibited significantly higher scores than BWC, indicating a more homogeneous appearance of slice color uniformity. This could be attributed to differences in the salting or drying methods, which may affect the distribution of pigments in the final product.
No significant differences were observed in bottarga color uniformity, suggesting a homogeneity in the raw material quality or preliminary processing phases, such as ovary selection and handling. The texture attributes (greasiness, adhesiveness, and friability) did not show significant differences between the two samples. This could reflect similar drying techniques and the fat content levels, which can influence texture-related perceptions. The global odor and the sea odor were higher for the west coast bottarga, suggesting a more pronounced aromatic profile. This could be due to variations in the diet of the fish or to environmental conditions that affect the raw material, such as water salinity or water temperature.
The west coast sample (BWC) also produced a significantly higher score for global flavor and sea flavor, probably due to the natural biochemical composition of the roe due to differences in mullet environmental conditions; however, differences related to specific handling practices during processing should be considered.
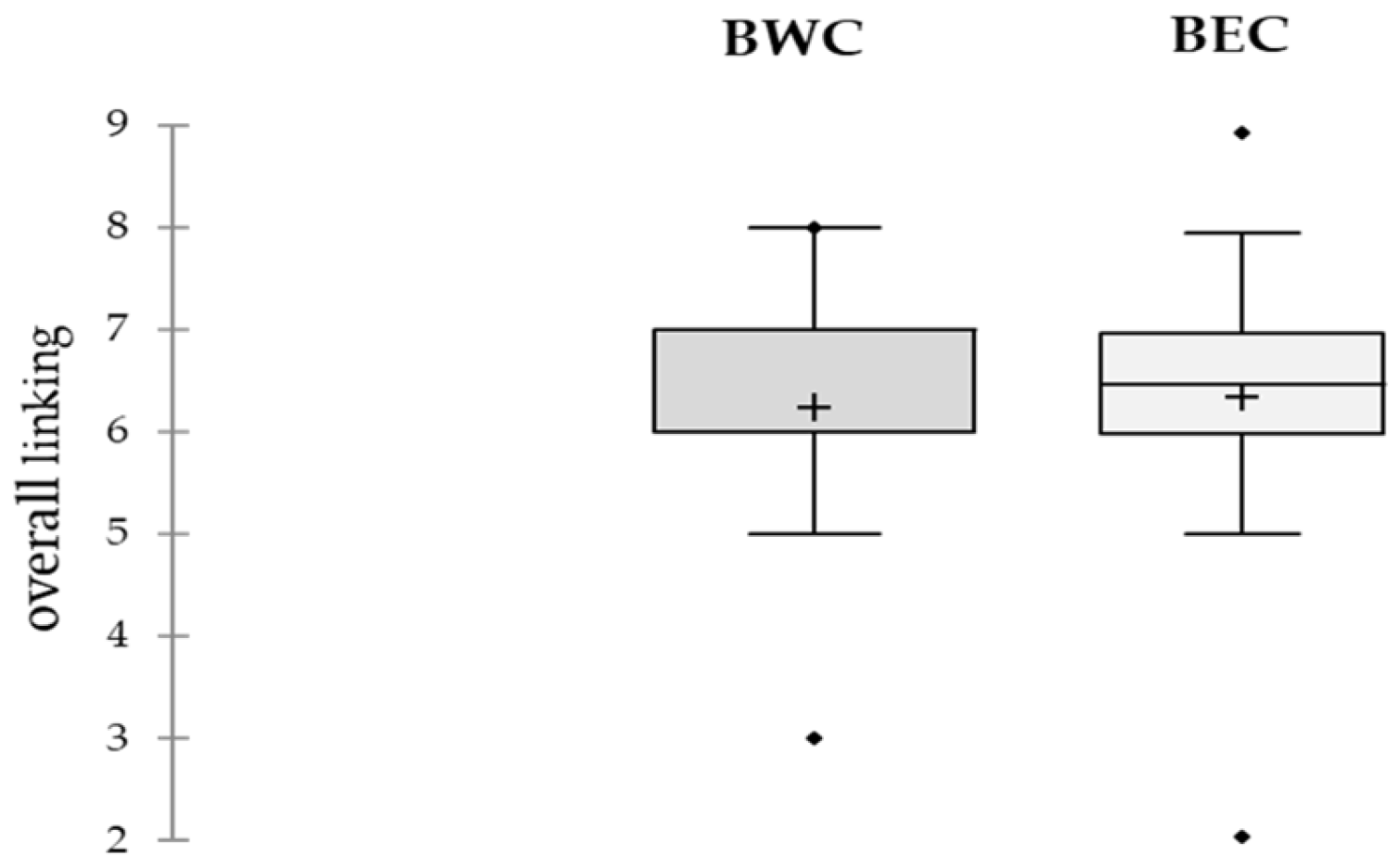
The acid and salty attributes showed a significantly higher score for the BWC bottarga compared to BEC, reflecting a sharper and more balanced taste. This could indicate differences in salting methods during drying, which can have a greater impact on flavor than on other sensory traits. No differences in bitterness were found between the samples, suggesting that this attribute is not affected by origin or processing procedures. Furthermore, overall consumer acceptance (
Figure 4) also showed no significant differences between BEC and BWC.