Abstract
This study investigates the essential oil (EO) isolated from the seeds and cones of Canadian hemlock (Tsuga canadensis), highlighting notable differences in their chemical composition and biological activities. The seed EO was uniquely dominated by oxygenated derivatives of monoterpene hydrocarbons, particularly bornyl acetate (40%), whereas the cone EO exhibited higher levels of monoterpene hydrocarbons such as α-pinene (23%), β-pinene (20%), and myrcene (23%). A significant finding was the strong cytotoxic activity of cone EO against melanoma cell lines, with IC50 values as low as 0.104 ± 0.015 μL/mL, compared to the minimal effects of seed EO. Additionally, cone EO demonstrated stronger antimicrobial activity, with lower minimum inhibitory concentrations (MICs) against Gram-positive and Gram-negative bacteria, further highlighting its therapeutic potential. Lipophilic extracts from seeds were characterized by unsaturated fatty acids (linoleic, oleic, and sciadonic acids—specific to conifers) and bioactive molecules with high antioxidant and nutritional potential, such as β-tocopherol, β-sitosterol, and campestrol. These findings underscore the unique chemical composition of T. canadensis seed EO and its lipophilic extract, along with the potent cytotoxic and antimicrobial properties of cone EO, offering insights into their potential applications in natural products for pharmaceutical and therapeutic uses.
1. Introduction
Canadian hemlock, also known as eastern hemlock (Tsuga canadensis L.), is an evergreen tree native to eastern North America; however, as an ornamental tree, it occurs in the northern part of our globe. It has been recognized as one of the keystone species responsible for the stability of forest ecosystems [1]. This tree is important from a scientific point of view since it may be used for medicinal and industrial purposes [2,3]. Chemical research conducted so far has focused on determining the composition of the essential oil from the tree’s needles and the fatty acid composition of the T. canadensis seeds. It has been reported that T. canadensis needle’s essential oils (EOs) from collection sites in northern Alabama and northwestern Georgia were dominated by monoterpene hydrocarbons; among them, α-pinene, camphene, and limonene were the majority. Also, the oxygenated derivatives of monoterpene hydrocarbons were huge, among which isobornyl acetate and piperitone were the most quantitatively important [4]. This study also demonstrated the analgesic and anti-inflammatory activities of needle EOs constituents, which support the traditional uses of this plant in the treatment of rheumatism [5].
In recent research, the antioxidant and antibacterial activities of green cone extracts from T. canadensis were evaluated. Kaempferol glycosides were identified as the most effective antioxidants in these extracts. The extracts showed clear inhibition against Staphylococcus aureus but exhibited limited antimicrobial activity against Escherichia coli [6]. In another study, T. canadensis bark was used for pharmaceutical purposes in the early 20th century. However, no recent studies have been made available on the composition of T. canadensis bark [1,6]. A study on the antioxidant properties of extracts of conifer cones from six species, including T. canadensis, revealed that cones of this plant, particularly green and mature ones, had the highest total polyphenol content and antioxidant capacity, as measured by ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays [7]. However, while these findings provide a strong foundation, little attention has been paid to the essential oils of T. canadensis seeds and cones or seed extract, which may harbor unique bioactive compounds and expand the plant’s utility in natural product research.
The present study aims to address this knowledge gap by investigating, for the first time, the composition and bioactive properties of essential oils derived from the seeds and cones of T. canadensis. This research will build on findings by identifying and characterizing the volatile constituents and assessing their capacity using advanced biochemical assays. By exploring the therapeutic potential of these essential oils, this study could contribute to developing novel antioxidant-rich natural products and reinforce the significance of T. canadensis as a valuable resource for medicinal and industrial applications. Antioxidants reduce the amount of reactive oxygen forms, which are the main factors of oxidative stress. Reduced oxidative stress can improve cell survival, which is why this paper focuses on the analysis of essential oils isolated from seeds and cones as well as lipophilic seed extracts of Canadian hemlock.
2. Materials and Methods
2.1. Plant Material
Cones with ripe seeds were hand-picked from Tsuga canadensis trees growing in Arboretum—Rogów Forestry Experimental Station, Warsaw University of Life Sciences, Poland. The plant material was classified in terms of species by the head of Rogów Arboretum. Cones and seeds of T. canadensis were collected during early autumn in 2015, ensuring consistency in sampling to account for potential seasonal variations in secondary metabolite production. The samples were stored in tight plastic bags in a freezer (−24 °C) until needed. The seeds were separated from the cones and ground in a mill (Bosch MKM6000UC) just before hydrodistillation or extraction. The voucher specimen (No: I-28/Tca/N/15 and I-28/Tca/S/15) of seeds and cone scales was deposited in the Institute of Natural Products and Cosmetic, Łódź, Poland.
2.2. Hydrodistillation
Volatile terpenes were isolated during hydrodistillation in Clevenger-type apparatus for 4 h. Mass of 30–100 g of cone scales or milled seeds were used for the isolation. The distillation was carried out in three replications. The EOs content was expressed in mL/100 g plant material.
2.3. Extraction
The lipophilic extractives were separated in a Soxhlet apparatus using 20 g of conifer seeds in 300 mL of n-hexane (Poland, POCH) for 8 h. The solvent was removed using rotatory evaporator (Heidolph HeiVap Value with G3 Vertical Condenser), and the residue (extract amount) was determined gravimetrically. The results was expressed in the percentage of seed weight.
2.4. Fatty Acids Methyl Esters (FAMEs) Determination
To determine fatty acids (FAs) in hemlock seeds, they were converted to their methyl esters. Transesterification process was performed as follows: to 0.1 g of extract 5 mL of tert-butylmethyl ether (Witko-CHS, Łódź, Poland) and 100 μL of the standard solution of nonadecanoic acid (Merck, Poznań, Poland) were added. The sample was stirred; next, silylation was conducted. To the above solution of 100 μL, 50 μL of trimethylsulfonium hydroxide (Sigma-Aldrich, Poznań, Poland) was added and gently heated for 30 min at a higher temperature. The samples were analyzed using GC-MS-FID (see below). The content of fatty acids was calculated as % w/w of dry extract.
2.5. Methodology for Tocopherol Detection and Quantification
A sample of 100 mg of seed extract was subjected to alkaline hydrolysis using 5 mL of a 2% sodium hydroxide solution in ethanol. Subsequently, a standard cholesterol solution (Sigma-Aldrich, Poznań, Poland) was added into the mixture. Following an hour of reflux heating, the resultant mixture was allowed to cool, after which 6 mL of a 2% hydrochloric acid solution in methanol was introduced. The sample was stirred, and then 4 mL of hexane along with 2 mL of distilled water was added. The hexane phase was collected into a screw-capped tube and subjected to centrifugation. To 1 mL of the resulting solution, 200 µL of the silylation reagent N,O-bis-(trimethylsilyl) trifluoroacetamide combined with 1-trimethylchlorosilane (Sigma-Aldrich, Poznań, Poland) was added. The samples were analyzed via gas chromatography–mass spectrometry with flame ionization detection (GC-FID-MS). The quantitative analysis of tocopherols was conducted independently, utilizing external calibration curves. The peak areas corresponding to individual tocopherol isomers were adjusted by their previously established response factors. The content of β-tocopherol was expressed as a percentage (w/w) of the dry extract.
2.6. Methodology for Phytosterol Detection and Quantification
Phytosterols were converted into trimethylsilyl ethers through the application of a reagent mixture comprising N,O-bis(trimethylsilyl)trifluoroacetamide and 1-trimethylchlorosilane (Sigma-Aldrich, Poznań, Poland) and according to the literature [8]. Cholesterol served as the internal standard to facilitate quantification.
2.7. Analytical Techniques of Bioactive Compounds
Chromatographic methodologies combined with mass spectrometry were used for identification and quantification (FID-detector) of EOs constituents, fatty acid methyl esters (FAMEs), and silylated derivatives of tocopherols and phytosterols. The analyses of bioactive compounds were conducted on a Trace GC Ultra coupled with a DSQ II mass spectrometer (Thermo Electron, Waltham, MA, USA). The GC-FID and MS analysis were performed simultaneously using an MS-FID splitter (SGE Analytical Science, Milton Keynes, UK). The mass range was 33–750 amu; ion source-heating, 200 °C; and ionization energy, 70 eV. A capillary column was used, and its working conditions were as follows: Rtx-1 MS (60 m × 0.25 mm i.d., film thickness 0.25 μm); temp. program, 50 (3 min) to 300 °C (30 min) at 4 °C min−1. The injector and detector temp. were 280 °C and 300 °C, respectively. The carrier gas was constant pressure, 300 kPa. The above methodology was used in previously published material [9]. For FAMEs, we used a polar capillary column TG-WAX MS (30 m × 0.25 mm i.d., film thickness 0.25 μm); its condition was as follows: temperature program, 50 (3 min) to 240 °C (30 min) at 4 °C min−1. The injector and detector temperatures were 250 °C and 260 °C, respectively. While the operating conditions for silylated derivatives of phytosterols were as follows: semi-polar capillary column BPX-5 (30 m × 0.25 mm i.d., film thickness 0.25 μm); temp. program, 100 °C (0.5 min, 4 °C min−1) to 250 °C (15 min, 4 °C min−1) to 310 °C (30 min). The temperature of injector and detector were 150–310 °C and 200 °C, respectively. The GC-MS analyses of the silylated derivatives of tocopherols were performed using a Pegasus 4D (LECO Corp., St. Joseph, MI, USA) apparatus with a chromatograph of semi-polar BPX5 capillary column (30 m long, inside diameter 0.25 mm, film thickness 0.25 μm). The injector temperature was 320 °C in a splitless mode. The temperature program was 100 °C (for 0.5 min) to 310 °C (for 15 min) at 4 °C min−1 and then to 330 °C (for 2 min) at 4 °C/min; carrier gas: helium, flow 1 mL/min. Detector: TOF-MS, ion source temp. 200 °C, ionization energy 70 eV, scan range 33–800 amu at 30 spectra/s. An extracted ion chromatogram was used for the quantification (the sum of ions at m/z = 368, 468, 474, 482, 488, 496, and 502). The solution of tocopherols (tocotrienol and tocopherol Mixed Solution Standard; ChromaDex, Irvine, CA, USA) and the response factors were determined. The above methodology was used in a previously published material [10].
2.8. Identification of Bioactive Compounds
Fatty acids, following alkaline hydrolysis, were quantified as fatty acid methyl esters (FAMEs), while phytosterols and tocopherols were evaluated as trimethylsilyl (TMS) derivatives. The constituents of EOs were analyzed, alongside their mass spectra, which were compared with those from mass spectral libraries, specifically the NIST 2012 and the Wiley Registry of Mass Spectral Data (10th edition), as well as relative retention indices (RIs) of volatile compounds. The retention times (RTs) of both FAMEs and tocopherols were matched against standard mixtures: a FAME standard solution obtained from Sigma–Aldrich and a mixed solution of tocotrienols and tocopherols sourced from ChromaDex, respectively.
2.9. Cell Culture
Normal human skin fibroblasts CCD25Sk (ATCC, Manassas, VA, USA), keratinocytes HaCaT (AddexBio, San Diego, CA, USA), melanoma cells A375 (Sigma-Aldrich, St. Louis, MO, USA), and melanoma cells C32 (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (all purchased from Gibco, Waltham, MA, USA) in a 5% CO2 incubator at 37 °C.
2.10. Cell Viability Assay
Cell viability was determined using an MTT assay. The cells were seeded at a density of 1 × 104 cells per well in 96-well plates and allowed to adhere for 24 h. Tsuga oseed and Tsuga cone were solubilized in dimethyl sulfoxide (DMSO, Loba Chemie, Mumbai, India) just prior to the experiment. The cells were treated with essential oils at concentrations of 0.012, 0.025, 0.05, 0.1, and 0.2 μL/mL for 24 h. The highest final concentration of DMSO in the culture medium was 0.1%. Control cells were treated with 0.1% DMSO. After incubation, a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution was added to each well, and the cells were incubated at 37 °C for 4 h in the dark. Then, the medium was removed, and formazan crystals were solubilized in 100 μL of DMSO and 12.5 μL of Sorensen’s glycine buffer on a plate shaker. Optical density was read at 570 nm using a microplate reader. The half-maximal inhibitory concentration (IC50) was calculated using GraphPad Prism 7 software (version 7.04).
2.11. Statistical Analysis
Data were analyzed in GraphPad Prism software (version 7.04) using a one-way ANOVA followed by Tukey’s test and reported as mean ± standard deviation. Statistically significant values of p < 0.05 were considered for further calculations. Statistical data analyses were conducted utilizing GraphPad Prism software (version 7.04), employing a one-way ANOVA followed by a Tukey’s post hoc test. Results are expressed as mean ± standard deviation, with significance defined at a p-value threshold of less than 0.05, which was utilized in subsequent analyses.
2.12. Bacterial Strains and Culture Conditions
The following reference strains were used in this study: Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 8739. Bacteria were inoculated on Columbia agar medium with 5% sheep blood (bioMeriéux, Warsaw, Poland) and then incubated at 37 °C for 18 h under aerobic conditions.
2.13. Determination of the Effectiveness of the EOs Against the Tested Bacteria
The minimum inhibitory concentration (MIC) of EOs isolated from seeds and cones, along with positive controls, such as thymol (Sigma-Aldrich, Darmstadt, Germany) and gentamicin sulfate (Krka, Novo Mesto, Slovenia), was established against the above bacterial strains according to the serial dilution method in a Mueller–Hinton broth (MHB, Sigma-Aldrich, Darmstadt, Germany), according to the Clinical and Laboratory Standards Institute recommendations [11]. A stock solution of the tested essential oils was prepared with the incorporation of Tween 80 (1.0%, v/v) (Sigma-Aldrich, Darmstadt, Germany), resulting in a range of concentrations from 250 to 0.03 µL/mL. Subsequently, 50 µL of each EO concentration was dispensed into a 96-well microplate, followed by the addition of 50 µL of a bacterial suspension with a concentration of 106 CFU/mL. After an incubation period of 18 h at 37 °C, the MIC was determined by adding 20 µL of 0.02% a resazurin solution (Sigma-Aldrich, Darmstadt, Germany) to the wells. The presence of bacteria was indicated by a color change from navy blue to pink after incubation with resazurin at 37 °C for an additional 3 h. The MIC value was regarded as the first well where the navy blue color persisted. Additionally, a positive control was conducted utilizing gentamicin (Krka, Slovenia) at a concentration of 40 mg/mL, and the test was replicated for each substance in triplicate. The minimum bactericidal concentration (MBC), defined as the lowest oil concentration that results in the death of 99.9% of the bacterial population, was identified by transferring 20 µL of bacterial cultures from wells with concentrations exceeding the MIC into fresh MHB-filled wells. This incubation also occurred for 18 h at 37 °C, and the MBC was established as the concentration at which no bacterial growth was detectable in the respective well [12]. To check the efficacy of the substances, the ratio of MBC to MIC was calculated, where a ratio of MBC/MIC ≤ 4 indicated a bactericidal effect, and a ratio of MBC/MIC > 4 denoted a bacteriostatic effect.
3. Results and Discussion
3.1. Composition of Seed and Cone EOs
The EOs isolated from hemlock seeds and cones were characterized by a yellowish color and an intense forest-like scent. The seed EO exhibited a fresher and greener odor due to its higher bornyl acetate content. The yield of EOs was 0.1% for both seeds and cones, with the seed EO yield being lower compared to previous studies on Abies or Picea seed EOs [9,13,14]. There is no literature data on EO yield of Canadian hemlock seeds or cones. The observed EO yield of 0.1% for T. canadensis is lower compared to other coniferous species belonging to A. alba and P. orientalis, which is irrevocably attributed to plant genera related to shape of cones and seeds, geographical origin, seasonal variations, and EO isolation conditions. Environmental factors such as soil composition, altitude, and climate at the collection site likely impact EO production, while seasonal fluctuations in secondary metabolism could explain yield differences in many plant genera. Additionally, the use of hydrodistillation may not fully optimize yield, as conditions like temperature and duration can affect EO recovery and thermolabile compound preservation.
Table 1 presents the detailed composition of the EOs. Using the GC-FID-MS method it was possible to identified nearly 100 volatiles in examined EOs. In the seed oil, the major components were bornyl acetate (41.4%), camphene (11.7%), and α-pinene (10.2%). In contrast, the cone oil was primarily composed of myrcene (23.1%), α-pinene (22.9%), and β-pinene (19.6%). Notably, bornyl acetate, a significant component of the seed oil, was present in much lower amounts in the cone oil (7.1%). The identified compounds accounted for 99.1% and 99.7% of the total composition in the seed and cone oils, respectively.

Table 1.
Composition of Tsuga canadensis seed and cone essential oils.
While the seed EO was dominated by oxygenated derivatives of monoterpene hydrocarbons—primarily bornyl acetate (over 40%)—the cone EO contained over 90% monoterpene hydrocarbons, including α-pinene, β-pinene, and myrcene. Other oxygenated monoterpenoids in the cone EO were present in small quantities, such as α-terpineol (2.2%), trans-pinocarveol (1.5%), borneol (0.7%), and terpinen-4-ol (0.6%). Understanding the components of essential oils highlights the strong biological properties of oxygenated derivatives of monoterpene hydrocarbons [15].
Sesquiterpenes were less abundantly represented in the hemlock EOs. Surprisingly, the seed oil was composed of 15% of sesquiterpenes, while the total content in the cone oil, also considering oxygenated derivatives of sesquiterpenes, was 0.4%. The compounds from this group that appeared in both oils were α-humulene, humulene oxide II, τ-muurolol, and α-cadinol, while the seed EO was dominated by germacrene D (3%), (E)-β-caryophellene (2%), and α-humulene (2%). Diterpene hydrocarbons and their oxygenated derivatives constituted a small percentage of the analyzed seed (2%) and cone (1%) EOs, although quite numerous. Pimaradiene and dehydroabietal were the majority in the above group. The differences in EOs composition are shown in Figure 1.
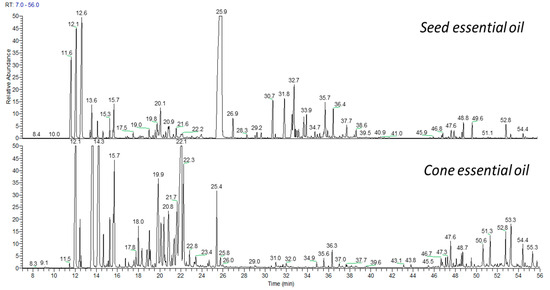
Figure 1.
The GC chromatograms of seed and cone essential oils of Canadian hemlock.
In a similar research, needle EOs were obtained using hydrodistillation and analyzed via GC-FID and GC-MS of a wild variety of T. canadensis in north Alabama and northwest Georgia, revealing the same major constituents of α-pinene, camphene, and bornyl acetate [16]. However, the relative proportions were different, due to different plant parts and geographical location. Furthermore, in another study, the major constituents of EOs from needles of T. canadensis were bornyl acetate (26.8%), α-pinene (23.7%), and camphene (11.93%) [17]. Results of both studies indicate a common chemical profile for T. canadensis, but content levels could deviate. There is no analysis of the composition of oils from the seeds or cones of Canadian hemlock, so the composition will be compared with the composition of essential oils from the seeds and cones of other related conifer species. EOs from Californian fir seeds and cones show notable differences compared to those from T. canadensis. Abies concolor seed oil is dominated by monoterpene hydrocarbons, particularly limonene (47.2%) and α-pinene (40.2%), while cone oil contains higher levels of α-pinene (58.3%) and sabinene (10.7%). Oxygenated monoterpenes occurred in lower content than monoterpene hydrocarbons in fir EOs [9], in contrast with T. canadensis seed oil, which is rich in bornyl acetate (41.4%)—a volatile with a balsamic type odor and a camphorous type flavor. Notably, bornyl acetate occurred in significantly lower concentrations in its cone oil (7.1%); thus, the scent of the seed oil is fresher compared to the heavy resinous scent of the cone EO. The high content of bornyl acetate (41.4%) in T. canadensis seed EOs underscores its potential for cosmetic and therapeutic applications, particularly due to its fragrance, anti-inflammatory, and antimicrobial properties. However, practical challenges, such as formulation stability, compatibility with other ingredients, and effective delivery systems, need to be addressed. The chemical profiles of EOs are dependent upon the plant part used to obtain the essential oil, whether seeds, cones, or needles, and it may also be associated with the geographical origin [16]. The chemical profiles of T. canadensis seed and cone EOs were compared with those of Abies alba and Picea orientalis using a bar chart (Supplementary Figure S1).
Bornyl acetate, showed highly active whitening and antioxidant activities, which was confirmed in experiments based on bornyl acetate fractionated from C. japonica essential oil. The authors of the experiments concluded that this ingredient may be a potential raw material in cosmetics. Globally, bornyl acetate is widely found in EOs from Ledum palustre, Siberian fir needles, cinnamon twigs (Cinnamomum osmophloeum), Pseudotsuga menziesii, and Chrysanthemum indicum. This volatile is relatively stable under normal conditions but can hydrolyze into borneol and acetic acid under acidic or alkaline conditions. Due to its safety, it is widely used as a popular raw chemical or as an organic synthesis intermediate. We can find this volatile in the daily chemical, food, and cosmetic industries [18].
3.2. Composition of Seed Lipophilic Extract
In the next part of our research, we focused on lipophilic seed extract, which is still a little-explored “piece of knowledge”. The yield of the non-polar extract of Canadian hemlock seeds was 35%. This is a high value compared to the yield of extracts obtained from the seeds of other conifer species and published by us earlier: Abies alba had the highest total lipophilic extract content (41.1%), followed by the A. cephalonica (32.0%), Picea pungens (21.5%), Abies concolor (19.8%), Picea abies (16.8%), Abies koreana (16.3%), and Picea orientalis (9.8%) [10]. Our analysis of the T. canadensis seed extract revealed a diverse composition of fatty acids, tocopherols, and phytosterols (Table 2). The most abundant fatty acid (FA) in the extract was linoleic acid (C18:2; 9, 12; n-6), constituting 0.408% w/w of the identified fatty acids, followed sciedonic (C20:3; 5,11,14; n-6; cis) at 0.541% w/w, and oleic acid (C18:1; n-9) at 0.089% w/w. These results are in agreement with our previous study focused on fatty acids (FAs) content in seeds of chosen conifers [10]. As was found in the literature, linoleic acid is almost always, except in a few cases, the most prominent of total fatty acids, ranging from 40 to 60%. The second habitual is oleic acid, which accounts for from 12 to 30% [19,20]. In our study, the content of this acid is lower. Polyunsaturated fats play crucial roles in cellular functions and have been associated with antioxidant and anti-inflammatory activities [21]. The presence of these fatty acids in the plant extracts suggests the antioxidant potential of this plant.

Table 2.
Fatty acids, tocopherols, and phytosterols of T. canadensis seed extract (% w/w of dry extract).
Interestingly, the fatty acid composition of the seeds of coniferous trees of the Abies, Tsuga, Pseudotsuga, Larix, Picea, Pinus, and Cedrus species are rich in unusual unsaturated fatty acids with the first site of unsaturation at the fifth carbon atom (Δ5-FAs) and cis (Z) configuration, such as: cis-5,9-octadecadienoic (taxoleic), cis-5,9,12-octadecatrienoic (pinolenic), cis-5,11-octadecadienoic (ephedrenic), cis-5,11-eicosadienoic (keteleeronic), cis-5,11,14-eicosatrienoic (sciadonic), cis-5,9,12,15-eicosatetraenoic (coniferonic), and cis-5,11,14,17-eicosatetraenoic (juniperonic) acids. The proportions of these acids have been documented in a few studies, based on the seeds of popular coniferous species [19,20]. The highest level of total Δ5-FAs was 30–31% of all FAs for P. sylvestris. Our GC-MS analysis of lipophilic seed extract from the Tsuga canadensis allowed us to identify pinolenic and sciadonic acid from the Δ5-FAs group (1.56 mg/g). The proportions of acids, including Δ5-FAs, differ within species. They are probably a chemotaxonomic determinant. According to literature data, the acid composition of the Tsuga species is more similar to Picea Pinus than to Abies [19]. From our observations, the shape and size of Tsuga seeds are also closer to spruces than to firs. Fatty acids constitute a large proportion of the lipophilic extract from Tsuga canadensis seeds, with monounsaturated and polyunsaturated fatty acids being the dominant ones. In recent decades, intensive research has been conducted on the properties of unsaturated fatty acids and their action in the human body. Fatty acid micelles scavenged superoxide in an unsaturation-dependent manner, where long-chain polyunsaturated acids, especially those of n-3 series, have the strongest antioxidant properties [22,23].
Tocopherols are common lipophilic antioxidants abundant in many plant oils and nuts, but their presence in Canadian hemlock seeds provides vitamin E activity and antioxidant potential as well. A study based on antioxidant potential was conducted on Norway spruce cones and eastern hemlock cones found that these cones possess high antioxidant properties [5]. The findings of this study align with the present study findings and further support the antioxidant potential of T. canadensis seed extract. It is known that vitamin E, which acts as the main lipophilic compound with antioxidant properties, is composed of tocopherol and tocotrienol isomers. In the series of antioxidant activities, the α isomer has the strongest antioxidant effect, while the δ isomer is the weakest [24]. Almost all isomers were identified in our previous studies in the seeds of the selected Alba and Picea species, but only Abies koreana, Pieca abies, and P. orientalis seeds contained β-isomer [10]. The β isomer ranks second in the antioxidant activity of tocopherols and trienols. It exhibits antioxidant activity by virtue of the phenolic hydrogen on the 2H-1-benzopyran-6-ol nucleus. The presence of the β form of tocopherol in T. canadensis seeds (0.0291% w/w) probably affects the preservation of the stability of unsaturated fatty acids or other components sensitive to oxygen or light [25]. It is well documented that the extracts or cold-pressed oils from drupes are highly unsaturated, but they contain enough vitamin E to keep the oxidative stability of unsaturated fatty acids present in the oil. Furthermore, tocopherols and tocotrienols possessing activity of vitamin E are exogenous antioxidant molecules with ROS-scavenging properties that are considered useful in preventing oxidative stress in mammalian cells and organisms [24,25,26].
Another biologically active group of compounds identified in seed extract were phytosterols (Table 2). β-Sitosterol was the major sterol. It constituted 0.1532% w/w (1.5 mg/g) of the lipophilic extract. Campesterol was also detected but in a much smaller quantity (0.0192 mg/g). These characteristics of hemlock seeds suggest possible roles in human nutritional products. Sum of detected phytosterols was 0.1724% w/w (1.7 mg/g). Among the conifer species tested for the presence of phytosterols, the result for T. canadensis is the closest to the phytosterol content in the extract from Abies concolor seeds. The record-holder in this case is Picea orientalis seed (1% w/w) [10].
3.3. Cell Cytotoxicity Activity of T. canadensis Seed and Cone EOS
In order to determine the viability of skin fibroblasts, keratinocytes, and melanoma cells (A375 and C32) after 24 h of treatment with five concentrations of essential oils (0.012, 0.025, 0.05, 0.1, and 0.2 μL/mL), the MTT assay was used. As shown in Figure 2, Tsuga seed is less cytotoxic than Tsuga cone, and its IC50 values are higher than 0.2 μL/mL in all tested cell lines. Our previous studies on essential oils from seeds of various species of the Abies genus belonging, like Tsuga canadensis, to the Pinaceae family showed higher cytotoxicity in fibroblasts (IC50 values about 0.11 μL/mL) than Tsuga seed [1,2]. Similar to a previous study based on some Picea EOs, where cone EO exhibited stronger antimicrobial and cytotoxic effects compared to seed EO [14], the cone EO of T. canadensis demonstrated significant cytotoxicity, particularly against keratinocytes and melanoma cell lines (A375 and C32), with IC50 values as low as 0.104 ± 0.015 µL/mL. In contrast, T. canadensis seed EO displayed minimal effects on cell viability, resembling the milder impact observed for A. concolor [9] and P. pungens [14]. Interestingly, IC50 values for Tsuga cone in both melanoma cells lines are significantly lower than in fibroblasts and amounted to 0.155 ± 0.022 μL/mL in A375 cells and 0.104 ± 0.015 μL/mL in C32 cells. When compared to standard chemotherapeutic agents, such as cisplatin and paclitaxel, this cytotoxic effect is significant. For instance, the Genomics of Drug Sensitivity in Cancer (GDSC) database reports that cisplatin exhibits IC50 values in various cancer cell lines ranging from 0.177 to 1.02 µM. Similarly, paclitaxel shows IC50 values between 0.00126 and 12.1 µM across different cell lines [25]. These comparisons suggest that the cytotoxic potency of T. canadensis cone EO is comparable to these established chemotherapeutic agents. In contrast, viability of fibroblasts treated with 0.2 μL/mL Tsuga cone decreased only by 24%. However, the viability of keratinocytes is reduced by Tsuga cone similarly to viability of melanoma cells, and its IC50 value was 0.134 ± 0.019 μL/mL. The inclusion of fibroblasts in the study allowed us to understand that OEs used as, for example, fragrance ingredients in cosmetics can be used without significant damage to healthy cells. The study showed that cone EO reduced fibroblast viability by only 24% at the highest tested concentration, indicating its relatively low toxicity to healthy cells compared to melanoma cells. These results reinforce the importance of cone EO, especially as a potential source of bioactive compounds or for possible applications as additional substances as anticancer drug development. The radar plot (Supplementary Figure S2) highlights the potent cytotoxicity of T. canadensis cone EO against melanoma cell lines (IC50: 0.104 ± 0.015 µL/mL for C32 cells) compared to milder effects observed in seed EO and related species.

Figure 2.
Cytotoxic effects of Tsuga canadensis seed and cone EOs on various cell lines. Viability of fibroblasts, keratinocytes, A375 melanoma cells, and C32 melanoma cells was assessed after treatment with different concentrations (0.012, 0.025, 0.05, 0.1, and 0.2 µL/mL) of Tsuga seed (white bars) and cone (gray bars) EOs. Cell viability is expressed as a percentage of the control. IC50 values for each cell line are provided in the table inset. Data are presented as mean ± SEM. Asterisks (*) indicate statistically significant differences (p < 0.05) compared to the control group.
3.4. Antimicrobial Activities of T. canadensis Seed and Cone EOs
EOs from both cones and seeds of the T. canadensis exhibited medium antimicrobial properties against Staphylococcus aureus (ATCC 29213) (Table 3). The minimum inhibitory concentration (MIC) for the cone EO was found to be 1.95 µL/mL, surprisingly almost as low as for biological active volatiles—thymol. While for the seed EO, it was slightly higher at 3.91 µL/mL. The minimum bactericidal concentration (MBC), which is the lowest concentration required to kill the bacteria, was 13.02 µL/mL for the cone EO and 31.25 µL/mL for the seed EO. The MBC/MIC ratios for both EOs were 7 and 8, respectively, indicating their bacteriostatic properties rather than bactericidal. In comparison with the control antibiotic, gentamycin, a known antibiotic, showed bactericidal properties with an MIC of 0.31 µg/mL, an MBC of 0.61 µg/mL, and an MBC/MIC ratio of 2. The antimicrobial properties of T. canadensis EOs against S. aureus align with findings from other coniferous species, such as A. concolor [9], P. pungens, and P. orientalis [14], which also demonstrated mild antimicrobial activity. In T. canadensis, cone EO exhibited higher antimicrobial potency than seed EO, with lower MIC (1.95 µL/mL vs. 3.91 µL/mL) and MBC (13.02 µL/mL vs. 31.25 µL/mL) values, a trend consistent with the stronger antimicrobial effects observed in cone EO of P. orientalis [14]. However, both seed and cone EOs of T. canadensis were less effective than the control antibiotic gentamycin.

Table 3.
Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), MBC/MIC ratio and effectiveness of the analyzed substances against Staphylococcus aureus ATCC 29213.
Furthermore, we assessed the antimicrobial activity of these EOs against Escherichia coli (ATCC 8739) (Table 4). The MIC for the cone EO was found to be 7.81 µL/mL, while for the seed EO, it was slightly higher at 13.02 µL/mL. The MBC was 62.50 µL/mL for both the cone and seed EOs. The results exhibited that the cone EO had greater antibacterial activity against E. coli than that isolated from seeds. The MBC/MIC ratios for both EOs were 8 and 5, respectively, again confirming their bacteriostatic nature rather than bactericidal properties. What is more, in comparison to the thymol and gentamicin sulfate (positive controls), the seed EO exhibited higher antibacterial activities than cone EO, but both EOs significantly lower than reference substances.

Table 4.
Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), MBC/MIC ratio, and the effectiveness of the analyzed substances against Escherichia coli ATCC 8739.
4. Conclusions
The essential oils of Tsuga canadensis seeds and cones differ significantly in composition, especially in the proportions of the main components. Notably, the seed EO is primarily composed of oxygenated monoterpenes, with bornyl acetate being the most prevalent, while the essential oil from the cones shows cases a richer profile of monoterpene hydrocarbons, evident in compounds such as α- and β-pinene and myrcene.
The yield of oils from both organs of the Canadian hemlock is low, taking into account our previous studies, where the yield of oil from fir seeds reaches even 18%.
Additionally, the seed lipophilic extract isolated with 35% yield, is characterized by its abundance of beneficial mono- and polyunsaturated fatty acids and antioxidants like β-tocopherol, which can bolster the assertion of Tsuga canadensis’s considerable potential value. β-Tocopherol, a form of vitamin E, accounts for 0.029 mg per 1 g of seed extract. In terms of phytosterols, β-sitosterol was the most prevalent, making up 1.53 mg per 1 g of seed extract, followed by campesterol. This reveals a rich composition of fatty acids, tocopherols, and phytosterols. The presence of these compounds is significant as they are known to exhibit antioxidant properties, especially vitamin E, which protects cell membranes from oxidative damage by neutralizing harmful free radicals.
Analysis of the effect of EOs on cancer cells (melanoma lines A375 and C32) allows for determining their ability to selectively inhibit their growth. This study showed that cone EO is characterized by a lower IC50 for melanoma cells than for fibroblasts, which suggests its possible potential, as supporting substances use in anticancer therapy; however, cone OE cytotoxicity testing should be extended.
Furthermore, the antimicrobial properties of the EOs revealed that the cone EO exhibited greater antibacterial activity against tested bacterial strains than the seed essential oil. However, both EOs are bacteriostatic rather than bactericidal.
This is the first study on the chemical compositions and biological properties of essential oils from Canadian hemlock seeds and cones; however, the biological activity, including their antioxidant properties measure with methods based on the ability to neutralize free radicals (DPPH, ABTS, ORAC) as well antimicrobial properties using other bacterial and yeast-like fungi strains, should be further investigated. Previous studies of seed extracts were based only on the identification of fatty acids. In this work, the seed studies were extended to the identification of forms of vitamin E and phytosterols.
Supplementary Materials
The following supporting information can be downloaded at www.mdpi.com/10.3390/app15041713/s1: Figure S1: Comparison of major chemical compounds in essential oils (EOs) from T. canadensis (seed and cone), Abies alba, and Picea orientalis. Bars represent the relative composition (%) of key components (bornyl acetate, α-pinene, limonene, myrcene, and β-pinene) with standard deviation (SD) values shown as error bars. The hatching and grayscale patterns differentiate the EOs from various sources. This visualization highlights the distinct dominance of bornyl acetate in T. canadensis seed EO and the varying profiles of other conifer species. Figure S2: Comparison of major chemical compounds in essential oils (EOs) from T. canadensis (seed and cone), Abies alba, and Picea orientalis. Bars represent the relative composition (%) of key components (bornyl acetate, α-pinene, limonene, myrcene, and β-pinene) with standard deviation (SD) values shown as error bars. The hatching and grayscale patterns differentiate the EOs from various sources. This visualization highlights the distinct dominance of bornyl acetate in T. canadensis seed EO and the varying profiles of other conifer species.
Author Contributions
Conceptualization, A.W.-B.; methodology, experiments, and analysis, A.W.-B., Ł.S., P.K. and E.M.; writing—original draft, A.W.-B., S.N.M. and Ł.S.; writing—review and editing, A.W.-B., S.N.M., Ł.S. and P.B.; visualization, A.W.-B., S.N.M. and Ł.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article and supplementary material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin, K.L.; Goebel, P.C. The foundation species influence of Eastern hemlock (Tsuga canadensis) on biodiversity and ecosystem function on the Unglaciated Allegheny Plateau. For. Ecol. Manag. 2013, 289, 143–152. [Google Scholar] [CrossRef]
- Garr, H.D.; Éwe, G.E. Hemlock bark (Tsuga canadensis) for pharmaceutical purposes. J. Am. Pharm. Assoc. 1920, 9.6, 567–573. [Google Scholar]
- Ömer, K.; Kocak, A. Volatile constituents of Juniperus communis L., Taxus canadensis Marshall. and Tsuga canadensis (L.) Carr. from Canada. J. Agric. Sci. Technol. B 2014, 4, 135–140. [Google Scholar]
- Craft, J.D.; Setzer, W.N. Leaf essential oil composition of Tsuga canadensis growing wild in North Alabama and Northwest Georgia. Am. J. Essent. Oils Nat. Prod. 2017, 5, 26–29. [Google Scholar]
- Hofmann, T.; Albert, L.; Németh, L.; VrSanská, M.; Schlosserová, N.; Voběrková, S.; Visi-Rajczi, E. Antioxidant and Antibacterial Properties of Norway Spruce (Picea abies H. Karst.) and Eastern Hemlock (Tsuga canadensis (L.) Carriè re) Cone Extracts. Forests 2021, 12, 1189. [Google Scholar] [CrossRef]
- Royer, M.; Houde, R.; Viano, Y.; Stevanovic, T. Non-wood forest products based on extractives—A new opportunity for the Canadian forest industry part 1: Hardwood forest species. J. Food Res. 2012, 1, 8. [Google Scholar] [CrossRef]
- Hofmann, T.; Visi-Rajczi, E.; Albert, L. Antioxidant properties assessment of the cones of conifers through the combined evaluation of multiple antioxidant assays. Ind. Crop. Prod. 2020, 145, 111935. [Google Scholar] [CrossRef]
- Thanh, T.T.; Vergnes, M.; Kaloustian, J.; El-Moselhy, T.F.; Amiot-Carlin, M.; Portugal, H. Effect of storage and heating on phytosterol concentrations in vegetable oils determined by GC/MS. J. Sci. Food Agric. 2006, 86, 220–225. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Szoka, Ł.; Karna, E.; Wiktorowska-Owczarek, A.; Sienkiewicz, M. Abies concolor seeds and cones as new source of essential composition and biological activity. Molecules 2017, 22, 1880. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Smeds, A.; Willfor, S. Chemical Composition and Content of Lipophilic Seed Extractives of Some Abies and Picea Species by Chem. Biodiversity 2016, 13, 1194–1201. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciąg, A.; Szoka, Ł.; Karna, E. Chemical composition and biological activity of Abies alba and A. koreana seed and cone essential oils and characterization of their seed hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wajs-Bonikowska, A.; Szoka, Ł.; Karna, E.; Wiktorowska-Owczarek, A.; Sienkiewicz, M. Composition and biological activity of Picea pungens and Picea orientalis seed and cone essential oils. Chem. Biodivers. 2017, 14, e1600264. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Németh-Zámboriné, E. Natural variability of essential oil components. In Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 87–125. [Google Scholar]
- Bohm, B.A.; Bruce, A.B. The Geography of Phytochemical Races; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef]
- Wolff, R.L.; Lavialle, O.; Frédérique, P.; Aitzetmüller, K. Abietoid Seed Fatty Acid Compositions—A Review of the Genera Abies, Cedrus, Hesperopeuce, Keteleeria, Pseudolarix, and Tsuga and Preliminary Inferences on the Taxonomy of Pinaceae. Lipids 2002, 36, 1–8. [Google Scholar] [CrossRef]
- Wolff, R.L.; Destaillats, F.; Angers, P. α-Linolenic acid and its Δ5-desaturation product, coniferonic acid, in the seed lipids of Tsuga and Hesperopeuce as a taxonomic means to differentiate the two genera. Lipids 2001, 36, 211–213. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Ambrozova GPekarova, M.; Lojek, A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw macrophages. Eur. J. Nutr. 2010, 49, 133–139. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Genomics of Drug Sensitivity in Cancer On-Line Database, Sanger Institute and Massachusetts General Hospital Cancer Centre. Available online: https://www.cancerrxgene.org/ (accessed on 25 January 2025).
- Syazwani Ridzuan, A.; Mohd Amin, I.; Goot Heah, K.; Zulkapli, R. Vitamin E isomers and cancer research: A review. Asia Pac. J. Mol. Biol. Biotechnol. 2022, 30, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).