Quality Evaluation of Ostrich Semi-Fine Sausages with Reduced Sodium Nitrite Levels in the Context of Regulatory Changes
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Analysis
2.2.1. Production Efficiency Assessment
2.2.2. Basic Chemical Composition
2.2.3. pH Measurement
2.2.4. Color Parameters Measurement
2.2.5. Instrumental Texture Analysis
2.2.6. Determination of Residual Nitrite Levels
2.2.7. Volatile Compound Profile
2.2.8. Semi-Consumer Evaluation
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
3.1.1. Production Efficiency, Basic Composition and pH
3.1.2. Color Parameters
3.1.3. Texture Parameters
3.1.4. Residual Nitrite Level
3.2. Volatile Compound Profile
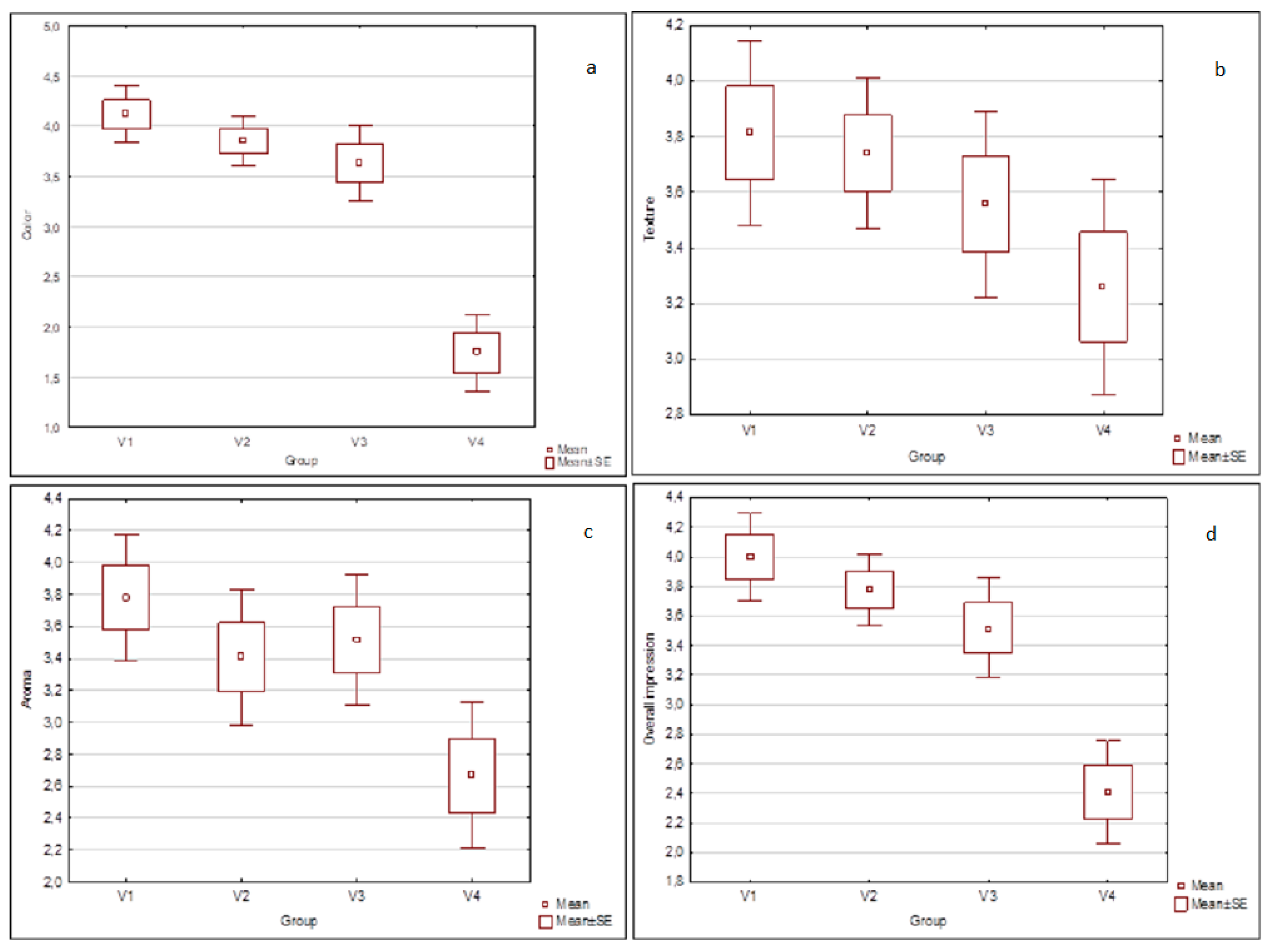
3.3. Semi-Consumer Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teixeira, A.; Rodrigues, S. Consumer perceptions towards healthier meat products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar] [CrossRef]
- Poławska, E.; Horbańczuk, J.O.; Pierzchała, M.; Strzałkowska, N.; Jóźwik, A.; Wójcik, A.; Pomianowski, J.; Gutkowska, K.; Wierzbicka, A.; Hoffman, L.C. Effect of dietary linseed and rapeseed supplementation on fatty acid profiles in the ostrich. Part 1. Muscles. Anim. Sci. Pap. Rep. 2013, 31, 239–248. [Google Scholar]
- Poławska, E.; Zdanowska-Sąsiadek, Ż.; Horbańczuk, J.; Pomianowski, J.F.; Jóźwik, A.; Tolik, D.; Raes, K.; De Smet, S. Effect of dietary organic and inorganic selenium supplementation on chemical, mineral and fatty acid composition of ostrich meat. CYTA-J. Food 2016, 14, 84–87. [Google Scholar] [CrossRef]
- Poławska, E.; Lisiak, D.; Jóźwik, A.; Pierzchała, M.; Strzałkowska, N.; Pomianowski, J.; Wojcik, A. The effect of the diet supplementation with linseed and rapeseed on the physico-chemical and sensory characteristics of ostrich meat. Anim. Sci. Pap. Rep. 2012, 30, 65–72. [Google Scholar]
- Hoffman, L.C. Value adding and processing of ratite meat: A review. Aust. J. Exp. Agric. 2008, 48, 1270–1275. [Google Scholar] [CrossRef]
- Manap, K.; Serikkyzy, M. Production of ostrich meat pâtés: Design of a food safety management system. Food Sci. Technol. Int. 2023, 29, 847–856. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Porębska, I.; Świder, O.; Sokołowska, B.; Szczepańska-Stolarczyk, J.; Lendzion, K.; Marszałek, K. The Impact of Plant Additives on the Quality and Safety of Ostrich Meat Sausages. Molecules 2024, 29, 3171. [Google Scholar] [CrossRef]
- McDonough, C.M.; Alviola, J.N.; Waniska, R.D. Preservatives: Extending shelf life and shelf stability. In Tortillas; AACC International Press: St. Paul, MN, USA, 2015; pp. 195–200. [Google Scholar]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.V.; Olika Keyata, E. Potentials of natural preservatives to enhance food safety and shelf life: A review. Sci. World J. 2022, 2022, 9901018. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Sodium nitrite in processed meat and poultry meats: A review of curing and examining the risk/benefit of its use. Am. Meat Sci. Assoc. White Pap. Ser. 2011, 3, 1–14. [Google Scholar]
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chem. 2018, 240, 430–436. [Google Scholar] [CrossRef]
- Majou, D.; Christieans, S. Mechanisms of the bactericidal effects of nitrate and nitrite in cured meats. Meat Sci. 2018, 145, 273–284. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological hazards associated with salt and nitrite reduction in cured meat products: Control strategies based on antimicrobial effect of natural ingredients and protective microbiota. Curr. Opin. Food Sci. 2021, 38, 32–39. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Granby, K. Occurrence of volatile and non-volatile N-nitrosamines in processed meat products and the role of heat treatment. Food Control 2015, 48, 163–169. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zeng, X.; Sun, Z.; Wu, A.; He, J.; Dang, Y.; Pan, D. Production of a safe cured meat with low residual nitrite using nitrite substitutes. Meat Sci. 2020, 162, 108027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and nitrate in meat processing: Functions and alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Green alternatives to nitrates and nitrites in meat-based products–a review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2133–2148. [Google Scholar] [CrossRef]
- IARC Working Group. Monographs on the Evaluation of Carcinogenic Risks to Humans; The Lancet Oncology: Lyon, France, 2018. [Google Scholar]
- Crowe, W.; Elliott, C.T.; Green, B.D.; Kuhnle, G.G. Dietary inclusion of nitrite-containing frankfurter exacerbates colorectal cancer pathology and alters metabolism in APCmin mice. NPJ Sci. Food 2022, 6, 35. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/2108 of 6 October 2023 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council and the Annex to Commission Regulation (EU) No 231/2012 as Regards Food Additives Nitrites (E 249-250) and Nitrates (E 251-252). Available online: https://eur-lex.europa.eu/eli/reg/2023/2108/oj/eng (accessed on 15 October 2025).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/4792 (accessed on 12 November 2025).
- Horbańczuk, O.K.; Wierzbicka, A. Technological and nutritional properties of ostrich, emu, and rhea meat quality. J. Vet. Res. 2016, 60, 279–286. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E.; Dekowska, A.; Sokołowska, B.; Połaska, M.; Lendzion, K. Evaluation of the Spoilage-Related Bacterial Profiles of Vacuum-Packaged Chilled Ostrich Meat by Next-Generation DNA Sequencing Approach. Processes 2021, 9, 803. [Google Scholar] [CrossRef]
- Asif, A.M.; Faiz-ul, H.; Ahmet, O.F.; Bahar, K.G.; Zarnain, M.; Ahmed, B.J.; Ali, F.S. Ostrich Meat: A Review on Nutritional Properties and Health Benefits. Food Sci. Anim. Resour. 2025, 45, 965–980. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Malak, N.M.L. The effect of different cooking methods on sensory attributes, physicochemical properties, and microbial safety of ostrich meat (Struthio camelus). J. Adv. Vet. Anim. Res. 2024, 11, 194–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcinkowska-Lesiak, M.; Zalewska, M.; Kazem, A.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Półtorak, A. Production of restructured beef jerky using blood plasma solutions activated by non-thermal atmospheric plasma. Anim. Sci. Pap. Rep. 2023, 41, 195–218. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Półtorak, A. Application of atmospheric pressure cold plasma activated plant protein preparations solutions as an alternative curing method for pork sausages. Meat Sci. 2022, 187, 1–9. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głabska, D.; Brodowska, M.; Sun, D.W.; Wierzbicka, A. Volatile compounds and fatty acids profile in Longissimus dorsi muscle from pigs fed with feed containing bioactive components. LWT—Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Guzek, D.; Sun, D.W.; Wierzbicka, A. Differentiation of chill-stored and frozen pork necks using electronic nose with ultra-fast gas chromatography. J. Food Process Eng. 2017, 40, e12540. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Mellett, F.D. Quality characteristics of low fat ostrich meat patties formulated with either pork lard or modified corn starch, soya isolate and water. Meat Sci. 2003, 65, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Sayas-Barberá, E.; Navarro, C.; Sendra, E.; Pérez-Alvarez, J.A. Physical, chemical, and sensory properties of Bologna sausage made with ostrich meat. J. Food Sci. 2003, 68, 1511–1515. [Google Scholar] [CrossRef]
- Grabowski, T.; Kijowski, J. Mięso i Przetwory Drobiowe (pl); WNT: Łódź, Poland, 2004. [Google Scholar]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of Sodium Nitrite Reduction on Lipid Oxidation and Antioxidant Properties of Cooked Meat Products. Antioxidants 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Półtorak, A. Plasma-activated milk powder as a sodium nitrite alternative in pork sausages. Meat Sci. 2022, 192, 1–10. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Piovesan, N.; Terra, L.D.M.; Lovato, M.; Terra, N.N.; Fries, L.L.M. Colorimetric and sensory characteristics of fermented cured sausage with Brazilian ostrich meat addition. Food Sci. Technol. 2013, 33, 660–665. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Stasiak, D.M.; Kęska, P. The Influence of Different Levels of Sodium Nitrite on the Safety, Oxidative Stability, and Color of Minced Roasted Beef. Sustainability 2019, 11, 3795. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. The influence of nitrite and nitrate on microbial, chemical and sensory parameters of slow dry fermented sausage. Meat Sci. 2006, 73, 660–673. [Google Scholar] [CrossRef]
- Deniz, E.E.; Serdaroğlu, M. Effects of nitrite levels, endpoint temperature and storage on pink color development in turkey rolls. Eur. Food Res. Technol. 2003, 217, 471–474. [Google Scholar] [CrossRef]
- Al Marazzeq, K.; Haddadin, M.; Al Abdullah, B.; Angor, M. Effect of Nitrite Substitution with Olive Leaves Extract on Color and Sensory Properties of Beef Mortadella. J. Agric. Sci. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- King, A.M.; Glass, K.A.; Milkowski, A.L.; Seman, D.L.; Sindelar, J.J. Modeling the Impact of Ingoing Sodium Nitrite, Sodium Ascorbate, and Residual Nitrite Concentrations on Growth Parameters of Listeria monocytogenes in Cooked, Cured Pork Sausage. J. Food Prot. 2016, 79, 184–193. [Google Scholar] [CrossRef]
- Dong, Q.L.; Tu, K.; Guo, L.Y.; Yang, J.L.; Wang, H.; Chen, Y.Y. The effect of sodium nitrite on the textural properties of cooked sausage during cold storage. J. Texture Stud. 2007, 38, 537–554. [Google Scholar] [CrossRef]
- Dissanayake, K.; Rifky, M.; Zokirov, K.; Jesfar, M.; Farmonov, J.; Ermat, S.; Makhmayorov, J.; Samadiy, M. Impact of curing salt (nitrites) on the processed meat products and its alternatives: A review. New Mater. Compd. Appl. 2024, 8, 254–264. [Google Scholar] [CrossRef]
- Domaradzki, P.; Florek, M. Mięso i przetwory mięsne. In Towaroznawstwo Surowców i Produktów Zwierzęcych z Elementami Przetwórstwa (pl); Litwińczuk, Z., Ed.; PWRiL, State Agricultural and Forestry Publishing House: Warsaw, Poland, 2012; pp. 287–391. [Google Scholar]
- Deng, S.; Bai, X.; Li, Y.; Wang, B.; Kong, B.; Liu, Q.; Xia, X. Changes in moisture, colour, residual nitrites and N-nitrosamine accumulation of bacon induced by nitrite levels and dry-frying temperatures. Meat Sci. 2021, 181, 108604. [Google Scholar] [CrossRef] [PubMed]
- Duda, Z. Wybrane zagadnienia stosowania azotynu w przetwórstwie mięsa. Żywność Technol. Jakość 1998, 3, 5–42. [Google Scholar]
- Bak, K.H.; Bauer, S.; Eisenreich, C.; Paulsen, P. Residual Nitrite, Nitrate, and Volatile N-Nitrosamines in Organic and Conventional Ham and Salami Products. Foods 2025, 14, 112. [Google Scholar] [CrossRef]
- Sheng, S.; Silva, E.M.; Tarté, R.; Claus, J.R. Residual nitrite and nitrate in processed meats and meat analogues in the United States. Sci. Rep. 2025, 15, 3269. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Han, W.; Zhang, J.; Hou, J. Analysis of the monitoring status of residual nitrite in meat products in China from 2000 to 2011. Meat Sci. 2018, 136, 30–34. [Google Scholar] [CrossRef]
- Houra, R.; Khadijeh, A.; Zahra, P.; Hosseini, H.; Abdorreza, M. Volatile N-nitrosamine, residual nitrite, and ascorbic acid levels in sausages during storage. Foods Raw Mater. 2020, 8, 107–114. [Google Scholar] [CrossRef]
- Ledezma-Zamora, K.; Sánchez-Gutiérrez, R.; Ramírez-Leiva, A.; Mena-Rivera, L. Residual nitrite in processed meat products in Costa Rica: Method validation, long-term survey and intake estimations. Food Chem. 2021, 361, 130082. [Google Scholar] [CrossRef]
- Oral, Z.F.Y.; Sallan, S. Evaluation of Quality Characteristics of Commercial Fermented Sausages (Sucuk and Heat-Treated Sucuk). Turk. J. Agric. Food Sci. Technol. 2023, 11, 1855–1861. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Rocha, Y.J.P.; de Noronha, R.L.F.; Trindade, M.A. Understanding the consumer’s perception of traditional frankfurters and frankfurters with healthy attributes through sorting task and hard laddering techniques. Meat Sci. 2019, 149, 70–78. [Google Scholar] [CrossRef] [PubMed]

| Ingredients (%) | Variant 1 | Variant 2 | Variant 3 | Variant 4 |
|---|---|---|---|---|
| Ostrich meat | 70 | 70 | 70 | 70 |
| Pork yowl | 30 | 30 | 30 | 30 |
| Salt | 2.5 | 2.5 | 2.5 | 2.5 |
| NaNO2 | 0.015 | 0.012 | 0.006 | - |
| Sodium polyphosphate | 0.25 | 0.25 | 0.25 | 0.25 |
| Fresh garlic | 0.15 | 0.15 | 0.15 | 0.15 |
| Ground white pepper | 0.07 | 0.07 | 0.07 | 0.07 |
| Nutmeg | 0.03 | 0.03 | 0.03 | 0.03 |
| Ice water | 10 | 10 | 10 | 10 |
| Item | Group | Influence | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time of Storage, Day | V1 1 | V2 | V3 | V4 | Time of Storage (TS) | Group (G) | TS × G | ||
| pH | 1 | Mean | 6.30 aA 2 | 6.30 aBA | 6.32 aBA | 6.32 aA | ** 3 | ** | *** |
| ±SD | 0.01 | 0.01 | 0.01 | 0.01 | |||||
| 7 | Mean | 6.30 aA | 6.31 aA | 6.32 aA | 6.34 bB | ||||
| ±SD | 0.01 | 0.01 | 0.01 | 0.01 | |||||
| 14 | Mean | 6.29 aA | 6.34 bB | 6.42 bD | 6.37 cC | ||||
| ±SD | 0.01 | 0.01 | 0.01 | 0.01 | |||||
| Item | Time of Storage, Day | Group | Influence | Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| V1 1 | V2 | V3 | V4 | Time of Storage (TS) | Group (G) | TS × G | |||
| L* | 1 | Mean | 52.72 | 53.76 | 55.86 | 56.25 | NS 3 | NS | NS |
| ±SD | 1.86 | 2.47 | 2.38 | 1.95 | |||||
| 7 | Mean | 52.42 | 54.63 | 53.92 | 54.88 | ||||
| ±SD | 0.72 | 2.11 | 1.72 | 0.86 | |||||
| 14 | Mean | 54.71 | 54.38 | 54.47 | 55.06 | ||||
| ±SD | 1.27 | 2.03 | 3.66 | 1.72 | |||||
| a* | 1 | Mean | 21.21 2aB | 19.89 aB | 20.66 bB | 12.56 aA | * | *** | *** |
| ±SD | 0.30 | 1.66 | 0.88 | 0.63 | |||||
| 7 | Mean | 18.59 aB | 16.89 aB | 14.07 aB | 8.50 aA | ||||
| ±SD | 2.68 | 1.85 | 1.48 | 4.46 | |||||
| 14 | Mean | 7.46 aB | 6.73 aB | 6.05 aB | 7.09 aB | ||||
| ±SD | 0.18 | 0.90 | 1.10 | 0.54 | |||||
| b* | 1 | Mean | 7.46 b | 6.73 a | 6.05 a | 7.09 a | * | NS | NS |
| ±SD | 0.18 | 0.90 | 1.10 | 0.54 | |||||
| 7 | Mean | 7.04 a | 7.29 ab | 7.24 ab | 7.59 a | ||||
| ±SD | 0.41 | 0.82 | 0.20 | 0.23 | |||||
| 14 | Mean | 7.23 ab | 8.02 b | 8.38 b | 9.35 b | ||||
| ±SD | 0.68 | 1.0 | 1.71 | 2.56 | |||||
| C* | 1 | Mean | 23.30 aB | 22.50 aB | 21.12 bB | 13.72 aA | NS | *** | *** |
| ±SD | 0.31 | 1.2 | 1.67 | 0.57 | |||||
| 7 | Mean | 22.35 aB | 21.19 aB | 21.90 bB | 14.68 aA | ||||
| ±SD | 0.39 | 1.84 | 0.85 | 0.66 | |||||
| 14 | Mean | 19.9 aB | 18.74 aB | 16.47 aAB | 13.25 aA | ||||
| ±SD | 2.33 | 1.29 | 0.79 | 1.70 | |||||
| H° | 1 | Mean | 0.33 aA | 0.30 aA | 0.29 aA | 0.54 aA | * | *** | *** |
| ±SD | 0.01 | 0.03 | 0.04 | 0.02 | |||||
| 7 | Mean | 0.32 aA | 0.35 aA | 0.34 aA | 0.54 aA | ||||
| ±SD | 0.02 | 0.01 | 0.02 | 0.01 | |||||
| 14 | Mean | 0.38 aA | 0.45 aA | 0.54 aAB | 0.86 aB | ||||
| ±SD | 0.08 | 0.09 | 0.13 | 0.36 | |||||
| Item | Group | Influence | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time of Storage, Day | V1 1 | V2 | V3 | V4 | Time of Storage (TS) | Group (G) | TS × G | ||
| Hardness (N) | 1 | Mean | 17.39 2aA | 22.44 aA | 19.92 aA | 19.50 aA | NS 3 | *** | *** |
| ±SD | 1.50 | 1.67 | 2.67 | 5.05 | |||||
| 7 | Mean | 13.947 aA | 28.59 aB | 25.6 aB | 34.25 abC | ||||
| ±SD | 1.37 | 2.74 | 4.92 | 7.56 | |||||
| 14 | Mean | 16.96 aA | 31.95 aB | 34.55 aB | 41.57 bB | ||||
| ±SD | 0.64 | 9.50 | 12.46 | 5.46 | |||||
| Adhesiveness (J/cm2) | 1 | Mean | −0.04 bA | −0.05 aA | −0.04 aA | −0.03 aA | NS | *** | *** |
| ±SD | 0.00 | 0.01 | 0.00 | 0.01 | |||||
| 7 | Mean | −0.06 abA | −0.06 aA | −0.05 aA | −0.02 aB | ||||
| ±SD | 0.01 | 0.00 | 0.01 | 0.00 | |||||
| 14 | Mean | −0.07 aA | −0.07 aA | −0.06 aA | −0.02 aB | ||||
| ±SD | 0.01 | 0.02 | 0.01 | 0.00 | |||||
| Springiness (-) | 1 | Mean | 0.54 aB | 0.47 aB | 0.49 aB | 0.22 aA | NS | *** | *** |
| ±SD | 0.05 | 0.07 | 0.03 | 0.00 | |||||
| 7 | Mean | 0.48 aB | 0.51 aB | 0.51 aB | 0.27 abA | ||||
| ±SD | 0.04 | 0.02 | 0.05 | 0.04 | |||||
| 14 | Mean | 0.47 aAB | 0.50 aB | 0.49 aAB | 0.38 bA | ||||
| ±SD | 0.04 | 0.00 | 0.03 | 0.04 | |||||
| Cohesiveness (-) | 1 | Mean | 0.60 aB | 0.62 aB | 0.58 aB | 0.45 aA | NS | *** | *** |
| ±SD | 0.04 | 0.08 | 0.04 | 0.03 | |||||
| 7 | Mean | 0.59 aB | 0.60 aB | 0.59 aB | 0.41 aA | ||||
| ±SD | 0.08 | 0.02 | 0.01 | 0.02 | |||||
| 14 | Mean | 0.55 aB | 0.57 aB | 0.56 aB | 0.36 aA | ||||
| ±SD | 0.02 | 0.01 | 0.02 | 0.03 | |||||
| Item | Group | Influence | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time of Storage, Day | V1 1 | V2 | V3 | V4 | Time of Storage (TS) | Group (G) | TS × G | ||
| Nitrate ions (mg/kg) | 1 | Mean | 41.17 cD2 | 36.29 cC | 16.90 cB | 0.22 aA | ** 3 | ** | *** |
| ±SD | 0.88 | 0.59 | 0.30 | 0.03 | |||||
| 7 | Mean | 39.80 bD | 35.11 bC | 15.19 bB | 0.13 aA | ||||
| ±SD | 0.17 | 0.30 | 0.09 | 0.04 | |||||
| 14 | Mean | 35.28 aD | 31.84 aC | 13.13 aB | 0.15 aA | ||||
| ±SD | 0.23 | 0.37 | 0.28 | 0.01 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowska-Lesiak, M.; Wrzosek, A.; Wojtasik-Kalinowska, I.; Półtorak, A.; Pierzchała, M.; Poławska, E. Quality Evaluation of Ostrich Semi-Fine Sausages with Reduced Sodium Nitrite Levels in the Context of Regulatory Changes. Appl. Sci. 2025, 15, 12504. https://doi.org/10.3390/app152312504
Marcinkowska-Lesiak M, Wrzosek A, Wojtasik-Kalinowska I, Półtorak A, Pierzchała M, Poławska E. Quality Evaluation of Ostrich Semi-Fine Sausages with Reduced Sodium Nitrite Levels in the Context of Regulatory Changes. Applied Sciences. 2025; 15(23):12504. https://doi.org/10.3390/app152312504
Chicago/Turabian StyleMarcinkowska-Lesiak, Monika, Andrzej Wrzosek, Iwona Wojtasik-Kalinowska, Andrzej Półtorak, Mariusz Pierzchała, and Ewa Poławska. 2025. "Quality Evaluation of Ostrich Semi-Fine Sausages with Reduced Sodium Nitrite Levels in the Context of Regulatory Changes" Applied Sciences 15, no. 23: 12504. https://doi.org/10.3390/app152312504
APA StyleMarcinkowska-Lesiak, M., Wrzosek, A., Wojtasik-Kalinowska, I., Półtorak, A., Pierzchała, M., & Poławska, E. (2025). Quality Evaluation of Ostrich Semi-Fine Sausages with Reduced Sodium Nitrite Levels in the Context of Regulatory Changes. Applied Sciences, 15(23), 12504. https://doi.org/10.3390/app152312504










