Sodium-Reduced Canned Dog Pâtés Enriched with Collagen Hydrolysate and Salicornia perennans: A Sustainable Strategy to Enhance Technological Quality and Oxidative Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation and Experimental Design
2.2. Raw Materials and Processing
2.3. Physicochemical Analyses
2.4. Texture Profile Analysis
2.5. Amino Acid Analysis
2.6. SDS–PAGE Analysis
2.7. Fatty Acid Analysis
2.8. Lipid and Protein Oxidation
2.9. Color and Light Stability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Texture Profile
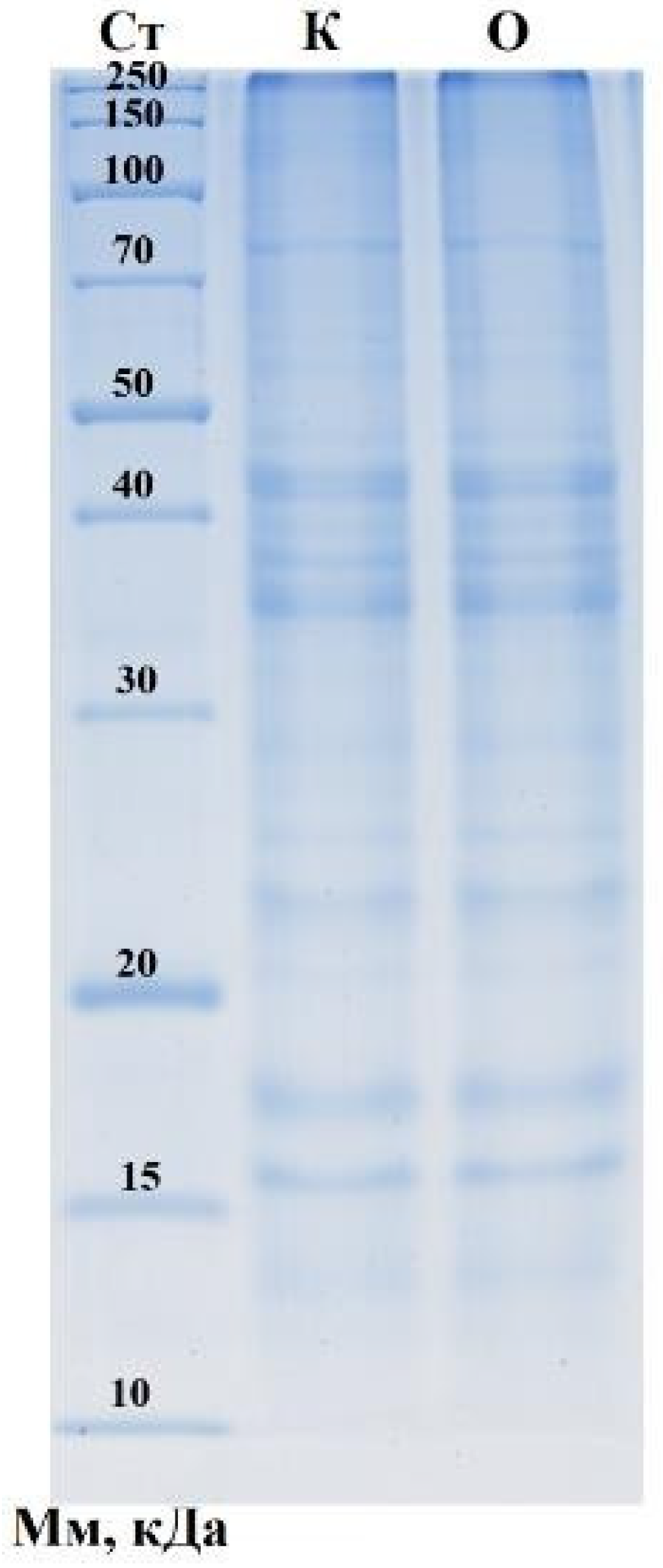
3.3. Amino-Acid Profile and SDS–PAGE
3.4. Fatty-Acid Profile
3.5. Dynamics of Lipid and Protein Oxidation During Storage
3.6. Thiobarbituric Acid Reactive Substances (TBARSs)
3.7. Dynamics of Lipid Oxidation Based on Acid Value
3.8. Color Characteristics and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazimierska, K.; Biel, W.; Witkowicz, R.; Kochel-Karakulska, J. The Nutritional Value Adequacy and Microbiological Quality of Canned Foods for Puppies and Adult Dogs. Appl. Sci. 2024, 14, 760. [Google Scholar] [CrossRef]
- Precedence Research. Wet Pet Food Market Size to Surpass USD 41.45 Billion by 2034. Precedence Research. Available online: https://www.precedenceresearch.com/wet-pet-food-market?utm_source=chatgpt.com (accessed on 5 August 2025).
- KBV Research. Global Wet Pet Food Market Size, Share & Industry Trends Analysis Report by Pet (Dog, and Cat), by Source (Animal-Based, Plant-Derivatives, and Synthetic), by Distribution Channel, by Regional Outlook and Forecast, 2023–2030 [Report ID KBV-18441]. Available online: https://www.kbvresearch.com/wet-pet-food-market/?utm_source=chatgpt.com (accessed on 15 October 2023).
- Aldrich, G.; Koppel, K. Pet Food Palatability Evaluation: A Review of Standard Assay Techniques and Interpretation of Results with a Primary Focus on Limitations. Animals 2015, 5, 43–55. [Google Scholar] [CrossRef]
- Vasconcellos, R.S.; Henríquez, L.B.F.; Lourenço, P.d.S. Spray-Dried Animal Plasma as a Multifaceted Ingredient in Pet Food. Animals 2023, 13, 1773. [Google Scholar] [CrossRef]
- Kazimierska, K.; Biel, W. Comparative Analysis of Spray-Dried Porcine Plasma and Hydrolyzed Porcine Protein as Animal-Blood-Derived Protein Ingredients for Pet Nutrition. Molecules 2023, 28, 7917. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.-C.; Cámara, C.; Morera, S.; Saborido, N.; Polo, J. Technological Benefits Associated with the Use of Spray-Dried Animal Plasma in Fish-Based Chunks for Canned Pet Food. Animals 2023, 13, 3460. [Google Scholar] [CrossRef] [PubMed]
- Kazimierska, K.; Biel, W.; Iwański, R. Palatability Testing of Spray-Dried Animal Plasma-Infused Dog Foods and Treats. Appl. Sci. 2024, 14, 7671. [Google Scholar] [CrossRef]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.; Guo, C.; Ji, R. Proteomic Identification and Characterization of Collagen from Bactrian Camel (Camelus bactrianus) Hoof. Foods 2023, 12, 3303. [Google Scholar] [CrossRef]
- Banasaz, S.; Ferraro, V. Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review. Polymers 2024, 16, 1999. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation). Off. J. Eur. Union 2009, L 300, 1–33. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32009R1069 (accessed on 10 August 2025).
- European Commission. Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. Off. J. Eur. Union 2011, L 54, 1–254. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011R0142 (accessed on 10 August 2025).
- Chuppava, B.; Siebert, D.-C.; Visscher, C.; Kamphues, J.; Abd El-Wahab, A. Impact of Animal By-Products on Diet Digestibility and Fecal Quality in Beagle Dogs. Life 2023, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, P.; Bauer, S.; Bauer, F.; Dicakova, Z. Contents of Polyamines and Biogenic Amines in Canned Pet (Dogs and Cats) Food on the Austrian Market. Foods 2021, 10, 2365. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, W.; Łukasiewicz-Mierzejewska, M.; Damaziak, K.; Bień, D. Biogenic Amines in Poultry Meat and Poultry Products: Formation, Appearance, and Methods of Reduction. Animals 2022, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.F.D.; Monteiro, C.F.C.; Bortolo, M.; Marx, F.R.; Model, J.F.A.; Vinagre, A.S.; Trevizan, L. Effects of Diets Based on Hydrolyzed Chicken Liver and Different Protein Concentrations on the Formation and Deamination of Biogenic Amines and Total Antioxidant Capacity of Dogs. Animals 2023, 13, 2578. [Google Scholar] [CrossRef]
- Wei, L.; Ren, Y.; Huang, L.; Ye, X.; Li, H.; Li, J.; Cao, J.; Liu, X. Quality, Thermo-Rheology, and Microstructure Characteristics of Cubic Fat Substituted Pork Patties with Composite Emulsion Gel Composed of Konjac Glucomannan and Soy Protein Isolate. Gels 2024, 10, 111. [Google Scholar] [CrossRef]
- Benjakul, S.; Chantakun, K.; Karnjanapratum, S. Impact of retort process on characteristics and bioactivities of herbal soup based on hydrolyzed collagen from seabass skin. J. Food Sci. Technol. 2018, 55, 3779–3791. [Google Scholar] [CrossRef]
- Syukri, D.; Rini Fiana, R.M.; Jonrinaldi Lita, R.P.; Aisyah, Q.; Sukma, H.H.; Azzahra, Y.; Tarumiyo, A.A.; Dermawan, D. Trends and Innovations in Sterilization of Ready-to-Eat Food Products: Challenges and Future Directions. OnLine J. Biol. Sci. 2025, 25, 373–382. [Google Scholar] [CrossRef]
- Pascall, M.A.; DeAngelo, K.; Richards, J.; Arensberg, M.B. Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability. Foods 2022, 11, 3043. [Google Scholar] [CrossRef]
- Fardella, M.; Ramírez, C.; Caballero, E.; Sánchez, E.; Pinto, M.; Núñez, H.; Valencia, P.; Almonacid, S.; Simpson, R. Variable Retort Temperature Profiles (VRTPs) and Retortable Pouches as Tools to Minimize Furan Formation in Thermally Processed Food. Foods 2021, 10, 2205. [Google Scholar] [CrossRef]
- D’Almeida, A.P.; de Albuquerque, T.L. Innovations in Food Packaging: From Bio-Based Materials to Smart Packaging Systems. Processes 2024, 12, 2085. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; Heikkilä, T.; Pernu, N.; Kovanen, S.; Hielm-Björkman, A.; Kivistö, R. Raw Meat-Based Diets in Dogs and Cats. Vet. Sci. 2017, 4, 33. [Google Scholar] [CrossRef]
- Klinmalai, P.; Kamonpatana, P.; Sodsai, J.; Promhuad, K.; Srisa, A.; Laorenza, Y.; Kovitvadhi, A.; Areerat, S.; Seubsai, A.; Harnkarnsujarit, N. Modern Palatant Strategies in Dry and Wet Pet Food: Formulation Technologies, Patent Innovations, and Market Evolution. Foods 2025, 14, 2824. [Google Scholar] [CrossRef]
- Koppel, K. Sensory analysis of pet foods. J. Sci. Food Agric. 2014, 94, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Stefani, V.; Lucke, A.; Zebeli, Q. Buffering Capacity of Various Commercial and Homemade Foods in the Context of Gastric Canine Digestion. Animals 2023, 13, 3662. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Lee, Y. Instrumental Analysis or Human Evaluation to Measure the Appearance, Smell, Flavor, and Physical Properties of Food. Foods 2023, 12, 3453. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020.
- Ferreira, I.; Leite, A.; Vasconcelos, L.; Rodrigues, S.; Mateo, J.; Munekata, P.E.S.; Teixeira, A. Sodium Reduction in Traditional Dry-Cured Pork Belly Using Glasswort Powder (Salicornia herbacea) as a Partial NaCl Replacer. Foods 2022, 11, 3816. [Google Scholar] [CrossRef]
- Šopík, T.; Lazárková, Z.; Salek, R.N.; Talár, J.; Purevdorj, K.; Buňková, L.; Foltin, P.; Jančová, P.; Novotný, M.; Gál, R.; et al. Changes in the Quality Attributes of Selected Long-Life Food at Four Different Temperatures over Prolonged Storage. Foods 2022, 11, 2004. [Google Scholar] [CrossRef]
- Šiška, L.; Gál, R.; Štefunko, F.; Polášek, Z.; Lazárková, Z.; Pětová, M.; Trvdoň, Z.; Salek, R.N. Quality Evaluation of Chicken Liver Pâté Affected by Algal Hydrocolloids Addition: A Textural and Rheological Approach. Animals 2024, 14, 2715. [Google Scholar] [CrossRef]
- Nuñez, S.M.; Cárdenas, C.; Valencia, P.; Pinto, M.; Silva, J.; Pino-Cortés, E.; Almonacid, S. Effect of Adding Bovine Skin Gelatin Hydrolysates on Antioxidant Properties, Texture, and Color in Chicken Meat Processing. Foods 2023, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kong, L.; Yu, P.; Wen, R.; Yu, X.; Xu, X.; Peng, X. Whey Protein Hydrolysates Improved the Oxidative Stability and Water-Holding Capacity of Pork Patties by Reducing Protein Aggregation during Repeated Freeze–Thaw Cycles. Foods 2022, 11, 2133. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, M.J.; Alfaia, C.M.; Rodrigues, S.S.; Teixeira, A. Strategies to Reduce Salt Content: PDO and PGI Meat Products Case. Foods 2024, 13, 2681. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Zaky, A.A.; Lorenzo, J.M.; Camiña, M.; Franco, D. A review on bioactive peptides derived from meat and by-products: Extraction methods, biological activities, applications and limitations. Meat Sci. 2023, 204, 109278. [Google Scholar] [CrossRef]
- Xing, L.; Liu, R.; Cao, S.; Zhang, W.; Guanghong, Z. Meat protein based bioactive peptides and their potential functional activity: A review. Int. J. Food Sci. Technol. 2019, 54, 1956–1966. [Google Scholar] [CrossRef]
- Al Hajj, W.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. Hydrolyzed collagen: Exploring its applications in the food and beverage industries and assessing its impact on human health—A comprehensive review. Heliyon 2024, 10, e36433. [Google Scholar] [CrossRef]
- Chuang, L.; Jiyong, S.; Chenguang, Z.; Xiaowei, H.; Xiaodong, Z.; Zhikun, Y.; Zhihua, L.; Xuetao, H.; Yanxiao, L.; Jianbo, X.; et al. Effects of sodium chloride substitutes on physicochemical properties of salted beef. Food Chem. X 2023, 20, 100885. [Google Scholar] [CrossRef]
- Zou, T. Salt Reduction in Processed Meats: Current Advances and Future Directions. Agric. Sci. Food Process. 2024, 1, 4–20. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, T.; Zhang, Y.; Jiang, L.; Sui, X. Potential of hydrolyzed wheat protein in soy-based meat analogues: Rheological, textural and functional properties. Food Chem. X 2023, 20, 100921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.; Park, J.Y.; Park, I.-H.; Kang, K.S.; Shin, M.-S. The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-Glucoside on Obesity. Processes 2023, 11, 977. [Google Scholar] [CrossRef]
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and Functional Properties of Collagen Hydrolysates from Spanish Mackerel Skin as Influenced by Average Molecular Weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, S.Y.; Lee, D.Y.; Kim, J.H.; Yun, S.H.; Lee, J.; Mariano, E., Jr.; Moon, S.S.; Hur, S.J. Analytical Methods and Effects of Bioactive Peptides Derived from Animal Products: A Mini-Review. Food Sci. Anim. Resour. 2024, 44, 533–550. [Google Scholar] [CrossRef]
- Eneji, C.A. The effect of heat treatment on the chemical composition of canned meat. Glob. J. Pure Appl. Sci. 2001, 7, 49–56. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.A.; Kong, C.-S.; Um, Y.R.; Lim, S.-Y.; Yea, S.S.; Seo, Y. Evaluation of Salicornia herbacea as a Potential Antioxidant and Anti-Inflammatory Agent. J. Med. Food 2009, 12, 661–668. [Google Scholar] [CrossRef]
- Aprilia, G.H.S.; Kim, H.S. Development of strategies to manufacture low-salt meat products—A review. J. Anim. Sci. Technol. 2022, 64, 218–234. [Google Scholar] [CrossRef]
- Yu, C.; Hu, W.; Chen, L.; Ouyang, K.; Chen, H.; Lin, S.; Wang, W. Basic Amino Acids as Salt Substitutes in Low-Salt Gel-Based Meat Products: A Comprehensive Review of Mechanisms, Benefits, and Future Perspectives. Foods 2025, 14, 637. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Yasemi, M. Protein Hydrolysates from Fisheries Processing Byproducts: Production, Characteristics, Food Applications and Challenges. Foods 2023, 12, 4470. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Xing, L.; Li, G.; Toldrá, F.; Zhang, W. The physiological activity of bioactive peptides obtained from meat and meat by-products. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; pp. 147–185. [Google Scholar] [CrossRef]
- Fredsgaard, M.; Kaniki, S.E.K.; Antonopoulou, I.; Chaturvedi, T.; Thomsen, M.H. Phenolic Compounds in Salicornia spp. and Their Potential Therapeutic Effects on H1N1, HBV, HCV, and HIV: A Review. Molecules 2023, 28, 5312. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954. [Google Scholar] [CrossRef] [PubMed]
- González-Osuna, M.F.; Bernal-Mercado, A.T.; Wong-Corral, F.J.; Ezquerra-Brauer, J.M.; Soto-Valdez, H.; Castillo, A.; Rodríguez-Figueroa, J.C.; Del-Toro-Sánchez, C.L. Bioactive Peptides and Protein Hydrolysates Used in Meat and Meat Products’ Preservation—A Review. ACS Food Sci. Technol. 2024, 4, 1003–1016. [Google Scholar] [CrossRef]
- Dragoev, S.G. Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health. Foods 2024, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Atzori, G.; Garcia Caparros, P.; Castagna, A.; Custódio, L.; Lazazzara, V.; Madsen, C.K.; Menicucci, F.; Rodrigues, M.J.; Solymosi, K.; Acosta Motos, J.R. Salt-induced nutritional and metabolic shifts in halophytes: Implications for food security. J. Sci. Food Agric. 2025, 1, 1–20. [Google Scholar] [CrossRef]
- Simunović, S.; Đorđević, V.Ž.; Simunović, S.; Franco, D.; Stajić, S.; Tomašević, I. Sensory Quality, Oxidative Stability, Textural and Fatty Acid Profile of Nitrite-Reduced Kulen Fermented Sausage During Ripening. Meat Technol. 2023, 64, 212–217. [Google Scholar] [CrossRef]
- Pavanello, A.C.L.; Catarino, R.P.F.; Portela, C.d.S.; da Silva, J.B.M.D.; Mascareli, V.A.B.; da Costa, V.L.L.; Vicenzi, B.G.; Mendonça, F.J.; Prudencio, S.H.; Pimentel, T.C.; et al. Hydrolyzed collagen as a partial fat substitute in chicken burgers produced with white striping meat. Poult. Sci. 2025, 104, 104622. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of Sodium Nitrite Reduction on Lipid Oxidation and Antioxidant Properties of Cooked Meat Products. Antioxidants 2019, 9, 9. [Google Scholar] [CrossRef]
- Ren, G.; He, Y.; Liu, L.; Wu, Y.; Jiao, Q.; Liu, J.; Cai, X.; Zhu, Y.; Huang, Y.; Huang, M.; et al. Effects of collagen hydrolysate on the stability of anthocyanins: Degradation kinetics, conformational change and interactional characteristics. Food Chem. 2025, 464, 141513. [Google Scholar] [CrossRef]
- Jo, H.-G.; Chilakala, R.; Kim, M.-J.; Sin, Y.-S.; Lee, K.-S.; Cheong, S.-H. Assessment of the Effects of Salt and Salicornia herbacea L. on Physiochemical, Nutritional, and Quality Parameters for Extending the Shelf-Life of Semi-Dried Mullets (Chelon haematocheilus). Foods 2022, 11, 597. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, R.; Biswas, S.; Maheswarappa, N.B.; Govindaiah, P.M.; Thegalapalle, M. Bioactive nanoliposome-encapsulated chicken collagen hydrolysate integrated into gelatin-alginate films for antioxidant and antimicrobial meat packaging. Int. J. Biol. Macromol. 2025, 330, 147994. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Stasiak, D.M.; Kęska, P. The Influence of Different Levels of Sodium Nitrite on the Safety, Oxidative Stability, and Color of Minced Roasted Beef. Sustainability 2019, 11, 3795. [Google Scholar] [CrossRef]
| Ingredient | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) | Control (No Hydrolysate, 2% NaCl) |
|---|---|---|
| Turkey filet | 29.0 | 29.0 |
| Beef liver | 9.0 | 9.0 |
| Beef lung | 9.0 | 9.0 |
| Carrot | 7.0 | 7.0 |
| Buckwheat groats (pre-gelatinized) | 8.0 | 8.0 |
| Flaxseed oil | 3.7 | 3.7 |
| Protein hydrolysate (sheep/camel/bovine) | 3.0 | — |
| NaCl | 1.0 | 2.0 |
| Salicornia extract | 1.0 | — |
| Water (q.s. to 100%) | 29.3 | 32.3 |
| Total | 100.0 | 100.0 |
| Metric | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|
| Moisture (%) | 76.4 ± 0.3 a | 76.1 ± 0.4 a |
| Crude protein (%) | 8.8 ± 0.2 a | 9.2 ± 0.2 a |
| Crude fat (%) | 5.2 ± 0.2 a | 5.0 ± 0.2 a |
| Ash (%) | 2.10 ± 0.07 a | 2.14 ± 0.06 a |
| Carbohydrate (%) | 7.5 ± 0.4 a | 7.6 ± 0.4 a |
| pH | 6.31 ± 0.03 a | 6.34 ± 0.03 a |
| a_w | 0.983 ± 0.001 a | 0.975 ± 0.001 a |
| Buffering capacity (mmol H+·kg−1·pH−1) | 790 ± 30 a | 510 ± 25 b |
| ME (metabolizable energy, kcal·100 g−1; Atwater mod.) | 101 ± 3 a | 102 ± 3 a |
| Centrifugal syneresis (% w/w) * | 3.1 ± 0.4 a | 1.8 ± 0.3 b |
| Parameter | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|
| Hardness (N) | 32.1 ± 1.8 a | 29.4 ± 1.5 b |
| Cohesiveness (–) | 0.41 ± 0.02 a | 0.46 ± 0.02 b |
| Springiness (mm) | 4.9 ± 0.2 a | 5.0 ± 0.2 a |
| Gumminess (N) | 13.2 ± 0.9 a | 13.6 ± 0.8 a |
| Chewiness (N·mm) | 64.7 ± 4.1 a | 68.0 ± 3.9 a |
| Adhesiveness (mJ) | 0.82 ± 0.06 a | 0.68 ± 0.05 b |
| Amino Acid | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|
| Aspartic acid | 2.23 ± 0.32 b | 2.97 ± 0.43 a |
| Glutamic acid | 3.86 ± 0.59 a | 3.42 ± 0.51 b |
| Serine | 0.57 ± 0.09 a | 0.53 ± 0.09 a |
| Threonine | 0.71 ± 0.11 a | 0.51 ± 0.08 b |
| Glycine | 2.39 ± 0.35 a | 2.34 ± 0.35 a |
| Arginine | 2.45 ± 0.37 a | 2.25 ± 0.34 a |
| Alanine | 2.51 ± 0.38 a | 2.47 ± 0.37 a |
| Tyrosine | 0.47 ± 0.07 b | 0.55 ± 0.08 a |
| Cystine | 0.23 ± 0.03 a | 0.22 ± 0.03 a |
| Valine | 0.76 ± 0.12 a | 0.83 ± 0.13 a |
| Methionine | 0.19 ± 0.03 b | 0.29 ± 0.04 a |
| Isoleucine | 0.56 ± 0.09 a | 0.54 ± 0.08 a |
| Phenylalanine | 0.56 ± 0.08 b | 0.67 ± 0.09 a |
| Leucine | 1.45 ± 0.22 a | 1.34 ± 0.21 a |
| Proline | 0.44 ± 0.07 a | 0.40 ± 0.06 a |
| Lysine | 1.39 ± 0.21 a | 1.22 ± 0.18 a |
| Histidine | 0.42 ± 0.06 a | 0.33 ± 0.05 b |
| Tryptophan | 0.18 ± 0.04 b | 0.25 ± 0.05 a |
| Hydroxyproline | 0.14 ± 0.02 a | 0.10 ± 0.01 b |
| Group/FA | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|
| SFA (Σ) | 56.4 ± 3.1 a | 52.8 ± 2.9 b |
| C14:0 Myristic | 4.5 ± 0.4 a | 4.1 ± 0.3 b |
| C16:0 Palmitic | 27.1 ± 2.1 a | 25.2 ± 1.9 b |
| C18:0 Stearic | 21.2 ± 2.1 a | 19.0 ± 1.7 b |
| (minor SFA C15:0, C17:0, C20:0–C23:0) | 3.6 ± 0.9 a | 4.5 ± 1.0 a |
| MUFA (Σ) | 38.1 ± 2.3 a | 39.5 ± 2.4 a |
| C18:1 cis (oleic) | 30.0 ± 2.1 a | 31.2 ± 2.2 a |
| C18:1 trans (elaidic) | 3.8 ± 0.4 a | 3.5 ± 0.3 b |
| (other MUFA C15:1, C16:1, C17:1, C20:1, C22:1, C24:1) | 4.3 ± 1.0 a | 4.8 ± 1.1 a |
| PUFA n-3 (Σ) | 0.8 ± 0.4 b | 1.5 ± 0.5 a |
| C18:3 n-3 (ALA) | 0.8 ± 0.4 b | 1.5 ± 0.5 a |
| EPA + DHA | <0.1 | <0.1 |
| PUFA n-6 (Σ) | 4.5 ± 0.6 a | 4.2 ± 0.5 a |
| C18:2 n-6 (LA) | 4.0 ± 0.4 a | 3.3 ± 0.3 b |
| (others: C18:2 trans, C20:2, C20:4) | 0.5 ± 0.4 b | 0.9 ± 0.5 a |
| PUFA total (n-3 + n-6) | 5.3 ± 0.8 b | 5.7 ± 0.9 a |
| n-6:n-3 ratio | 5.6 ± 0.3 a | 3.8 ± 0.2 b |
| Parameter | Day | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|---|
| Peroxide value, meq/kg | 0 | 3.8 ± 0.4 a | 3.5 ± 0.4 a |
| 2 | 4.1 ± 0.4 a | 4.3 ± 0.4 a | |
| 4 | 5.9 ± 0.3 a | 5.5 ± 0.3 b | |
| 6 | 7.1 ± 0.4 a | 7.0 ± 0.4 a | |
| 8 | 10.1 ± 0.5 a | 9.7 ± 0.5 b | |
| 10 | 14.0 ± 0.9 a | 13.0 ± 0.9 b | |
| Carbonyl compounds, nmol/mg protein | 0 | 99.19 ± 4.1 a | 95.73 ± 3.9 a |
| 2 | 112.5 ± 5.0 a | 105.2 ± 4.7 b | |
| 4 | 130.6 ± 5.8 a | 119.0 ± 5.2 b | |
| 6 | 151.2 ± 6.7 a | 135.4 ± 5.9 b | |
| 8 | 180.5 ± 8.1 a | 158.3 ± 6.8 b | |
| 10 | 215.3 ± 9.6 a | 185.9 ± 8.1 b |
| Parameter | Day | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|---|
| Thiobarbituric acid value, mg MDA/kg | 0 | <0.039 a | <0.039 a |
| 2 | <0.039 a | <0.039 a | |
| 4 | 0.117 ± 0.012 a | 0.140 ± 0.014 a | |
| 6 | 1.719 ± 0.120 a | 0.766 ± 0.054 b | |
| 8 | 3.289 ± 0.230 a | 1.910 ± 0.134 b | |
| 10 | 4.865 ± 0.315 a | 2.673 ± 0.189 b |
| Parameter | Day | Control (No Hydrolysate, 2% NaCl) | Treatment (+3% Hydrolysate, 1% Salicornia, 1% NaCl) |
|---|---|---|---|
| Acid value, mg KOH/g | 0 | 2.4 ± 0.2 a | 2.0 ± 0.2 a |
| 2 | 3.0 ± 0.2 a | 2.6 ± 0.2 b | |
| 4 | 3.6 ± 0.3 a | 3.1 ± 0.2 b | |
| 6 | 4.2 ± 0.3 a | 3.9 ± 0.3 b | |
| 8 | 6.5 ± 0.5 a | 5.2 ± 0.3 b | |
| 10 | 8.5 ± 0.6 a | 7.0 ± 0.5 b |
| Day | Sample | L* (Lightness) | a* (Redness) | b* (Yellowness) | Color Stability, % |
|---|---|---|---|---|---|
| 0 | Control (no hydrolysate, 2% NaCl) | 58.37 ± 0.44 a | 7.38 ± 0.13 a | 25.31 ± 0.35 a | 100.0 |
| Treatment (+3% hydrolysate, 1% Salicornia, 1% NaCl) | 51.20 ± 0.51 b | 7.48 ± 0.58 a | 24.35 ± 0.83 a | 100.0 | |
| 2 | Control (no hydrolysate, 2% NaCl) | 58.10 ± 0.43 a | 7.25 ± 0.18 a | 25.42 ± 0.37 a | 99.3 |
| Treatment (+3% hydrolysate, 1% Salicornia, 1% NaCl) | 51.15 ± 0.50 b | 7.40 ± 0.54 a | 24.45 ± 0.81 a | 99.8 | |
| 4 | Control (no hydrolysate, 2% NaCl) | 57.82 ± 0.46 a | 7.11 ± 0.15 b | 25.62 ± 0.36 a | 97.2 |
| Treatment (+3% hydrolysate, 1% Salicornia, 1% NaCl) | 51.05 ± 0.49 b | 7.32 ± 0.51 a | 24.61 ± 0.79 a | 98.6 | |
| 6 | Control (no hydrolysate, 2% NaCl) | 57.40 ± 0.47 a | 6.97 ± 0.20 b | 25.81 ± 0.39 a | 96.3 |
| Treatment (+3% hydrolysate, 1% Salicornia, 1% NaCl) | 51.00 ± 0.50 b | 7.25 ± 0.50 a | 24.78 ± 0.77 a | 97.8 | |
| 8 | Control (no hydrolysate, 2% NaCl) | 57.12 ± 0.48 a | 6.85 ± 0.22 b | 25.98 ± 0.40 a | 95.4 |
| Treatment (+3% hydrolysate, 1% Salicornia, 1% NaCl) | 50.98 ± 0.52 b | 7.18 ± 0.49 a | 24.90 ± 0.75 a | 97.2 | |
| 10 | Control (no hydrolysate, 2% NaCl) | 56.80 ± 0.50 a | 6.73 ± 0.25 b | 26.10 ± 0.42 a | 94.8 |
| Treatment (+3% hydrolysate, 1% Salicornia, 1% NaCl) | 50.95 ± 0.53 b | 7.10 ± 0.49 a | 24.95 ± 0.76 a | 96.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoman, A.; Tokysheva, G.; Makangali, K. Sodium-Reduced Canned Dog Pâtés Enriched with Collagen Hydrolysate and Salicornia perennans: A Sustainable Strategy to Enhance Technological Quality and Oxidative Stability. Appl. Sci. 2025, 15, 11575. https://doi.org/10.3390/app152111575
Shoman A, Tokysheva G, Makangali K. Sodium-Reduced Canned Dog Pâtés Enriched with Collagen Hydrolysate and Salicornia perennans: A Sustainable Strategy to Enhance Technological Quality and Oxidative Stability. Applied Sciences. 2025; 15(21):11575. https://doi.org/10.3390/app152111575
Chicago/Turabian StyleShoman, Aruzhan, Gulzhan Tokysheva, and Kadyrzhan Makangali. 2025. "Sodium-Reduced Canned Dog Pâtés Enriched with Collagen Hydrolysate and Salicornia perennans: A Sustainable Strategy to Enhance Technological Quality and Oxidative Stability" Applied Sciences 15, no. 21: 11575. https://doi.org/10.3390/app152111575
APA StyleShoman, A., Tokysheva, G., & Makangali, K. (2025). Sodium-Reduced Canned Dog Pâtés Enriched with Collagen Hydrolysate and Salicornia perennans: A Sustainable Strategy to Enhance Technological Quality and Oxidative Stability. Applied Sciences, 15(21), 11575. https://doi.org/10.3390/app152111575







