Enhancing Biscuit Nutritional Value Through Apple and Sour Cherry Pomace Fortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Biscuit-Making Process
2.2. Determination of the Moisture Content and the Physical and Sensory Characteristics of Biscuits
2.3. Preparing the Necessary Extracts to Determine the Total Content of Sugars, Polyphenols, Flavonoids, Anthocyanins, and Anti-Radical Activity
2.4. Determination of Total Polyphenol Content
2.5. Determination of Total Flavonoid Content
2.6. Determination of Total Anthocyanin Content
2.7. Determination of Total Sugar Content
2.8. Determination of Antiradical Activity
2.9. FTIR Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Apple and Sour Cherry Pomace
3.2. Characterisation of Biscuits Fortified with Pomace
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassani, L.; Gomez-Zavaglia, A. Sustainable food systems in fruits and vegetables food supply chains. Front. Nutr. 2022, 9, 829061. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Despeisse, M.; Ball, P.D.; Evans, S. Strategies and ecosystem view for industrial sustainability. In Re-Engineering Manufacturing for Sustainability; Nee, A., Song, B., Ong, S.K., Eds.; Springer: Singapore, 2013. [Google Scholar] [CrossRef]
- Esturo, A.; Lizundia, E.; Sáez de Cámara, E. Fruit juice industry’s transition towards sustainability from the viewpoint of the producers. Sustainability 2023, 15, 3066. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Castangia, I.; Aroffu, M.; Fulgheri, F.; Abi Rached, R.; Corrias, F.; Sarais, G.; Bacchetta, G.; Argiolas, F.; Pinna, M.B.; Murru, M.; et al. From Field to Waste Valorization: A preliminary study exploring the impact of the wine supply chain on the phenolic profile of three sardinian pomace extracts. Foods 2024, 13, 1414. [Google Scholar] [CrossRef]
- Cifuentes-Faura, J. European Union policies and their role in combating climate change over the years. Air Qual. Atmos. Health 2022, 15, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.P.; Modak, D.; Sarkar, S.; Roy, S.K.; Sah, S.P.; Ghatani, K.; Bhattacharjee, S. Fruit Waste: A current perspective for the sustainable production of pharmacological, nutraceutical, and bioactive resources. Front. Microbiol. 2023, 14, 1260071. [Google Scholar] [CrossRef]
- Tonelli, F.; Evans, S.; Taticchi, P. Industrial sustainability: Challenges, perspectives, actions. Int. J. Bus. Innov. Res. 2013, 7, 143–163. [Google Scholar] [CrossRef]
- Idowu, A.T.; Igiehon, O.O.; Adekoya, A.E.; Idowu, S. Dates palm fruits: A review of their nutritional components, bioactivities and functional food applications. AIMS Agric. Food 2020, 5, 734–755. [Google Scholar] [CrossRef]
- Bernstein, M.; Muniz, N. Position of the Academy of Nutrition and Dietetics: Food and nutrition for older adults: Promoting health and wellness. J. Acad. Nutr. Diet. 2012, 112, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of agri-food by-products in the food industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Samynathan, R.; Ramasamy, P.; Kumar, M.S.; Thiruvengadam, M.; Khayrullin, M.; Shariati, M.A.; Nile, A.S.; Nile, S.H. Unveiling novel applications of fruit pomace for sustainable production of value-added products and health benefits: A review. Food Biosci. 2024, 61, 104533. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maciel, G.M.; Lima, N.P.; Lima, N.F.; Ribeiro, I.S.; Pinheiro, D.F.; Haminiuk, C.W.I. Valorization of bioactive compounds from juice industry waste: Applications, challenges, and future prospects. Trends Food Sci. Technol. 2024, 152, 104693. [Google Scholar] [CrossRef]
- Argun, M.E.; Ateş, H.; Argun, M.Ş.; Cakmakcı, Ö. Management of sour cherry processing industry waste water by super critical fluid method: Sequential recovery and treatment. Process Saf. Environ. Protect. 2025, 199, 107318. [Google Scholar] [CrossRef]
- Vidović, S.; Horecki, A.T.; Vladić, J.; Šumić, Z.; Gavarić, A.; Vakula, A. Apple. In Valorization of Fruit Processing by-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–42. [Google Scholar] [CrossRef]
- Kraciński, P.; Stolarczyk, P.; Czerwińska, W.; Nosecka, B. Fruit consumption habits and apple preferences of university students in Poland. Foods 2025, 14, 2073. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable use of apple pomace (AP) in different industrial sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, M.; Ottens, M. A structured approach to recover valuable compounds from agri-food side streams. Food Bioprocess Technol. 2021, 14, 1387–1406. [Google Scholar] [CrossRef]
- Kauser, S.; Murtaza, M.A.; Hussain, A.; Imran, M.; Kabir, K.; Najam, A.; An, Q.U.; Akram, S.; Fatima, H.; Batool, S.A.; et al. Apple pomace, a bioresource of functional and nutritional components with potential of utilization in different food formulations: A review. Food Chem. Adv. 2024, 4, 100598. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit seeds as sources of bioactive compounds: Sustainable production of high value-added ingredients from by-products within circular economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A. Possible uses of fruit pomaces in food technology as a fortifying additive—A review. Zesz. Probl. Postęp. Nauk. Rol. 2020, 600, 43–54. [Google Scholar] [CrossRef]
- Friant, M.C.; Vermeulen, W.J.V.; Salomone, R. Analysing European Union circular economy policies: Words versus actions. Sustain. Prod. Consum. 2021, 27, 337–353. [Google Scholar] [CrossRef]
- Kassim, F.O.; Thomas, C.L.P.; Afolabi, O.O.D. Integrated conversion technologies for sustainable agri-food waste valorization: A critical review. Biomass Bioenergy 2022, 156, 106314. [Google Scholar] [CrossRef]
- Ghadam, M.S.; Asl, M.R.S.; Sharifi, A.; Nia, A.P.; Armin, M. Effect of apple pomace powder addition on the physicochemical properties of oily cakes and ranking samples by Delphi Fuzzy Approach. J. Food Qual. 2023, 2023, 8111233. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of vegetable and fruit by-products as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef]
- Filipović, V.; Lončar, B.; Filipović, J.; Nićetin, M.; Knežević, V.; Šeregelj, V.; Košutić, M.; Bodroža Solarov, M. Addition of Combinedly Dehydrated Peach to the Cookies—Technological Quality Testing and Optimization. Foods 2022, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Mandache, M.; Topală, C.M.; Vijan, L.E.; Cosmulescu, S. The characterization of peach pomace and the influence of its incorporation on the chemical composition of biscuits. Appl. Sci. 2025, 15, 6983. [Google Scholar] [CrossRef]
- Topală, C.M.; Ducu, C. Spectroscopic study of sea buckthorn extracts. Curr. Trends Nat. Sci. 2014, 3, 48–53. [Google Scholar]
- Topală, C.M.; Rusea, I. Analysis of leaves using FTIR spectroscopy and principal component analysis. Discrimination of different plant samples. Curr. Trends Nat. Sci. 2018, 7, 286–291. [Google Scholar]
- Mandache, M.B.; Vijan, L.E.; Cosmulescu, S. The influence of extraction time on concentration of bioactive compounds in apple and sour cherry pomace: A need for optimization. AgroLife Sci. J. 2025, 14, 98–107. [Google Scholar]
- Topală, C.M.; Vîjan, L.E.; Giura, S.; Botu, M. Attenuated total reflection Fourier transform infrared (ATR-FTIR): A method for the biochemical study of walnut leaves. Curr. Trends Nat. Sci. 2020, 9, 266–272. [Google Scholar] [CrossRef]
- Stamin, F.D.; Vijan, L.E.; Topală, C.M.; Cosmulescu, S.N. The influence of genotype, environmental factors, and location on the nutraceutical profile of Rosa canina L. fruits. Agronomy 2024, 14, 2847. [Google Scholar] [CrossRef]
- Pancerz, M.; Ptaszek, A.; Sofińska, K.; Barbasz, J.; Szlachcic, P.; Kucharek, M.; Łukasiewicz, M. Colligative and hydrodynamic properties of aqueous solutions of pectin from cornelian cherry and commercial apple pectin. Food Hydrocoll. 2019, 89, 406–415. [Google Scholar] [CrossRef]
- Gomravi, Y.; Karimi, A.; Azimi, H. Adsorption of heavy metal ions via apple waste low-cost adsorbent: Characterization and performance. Korean J. Chem. Eng. 2021, 38, 1843–1858. [Google Scholar] [CrossRef]
- Athmaselvi, K.A.; Kumar, C.; Balasubramanian, M.; Roy, I. Thermal, structural, and physical properties of freeze dried tropical fruit powder. J. Food Process. 2014, 2014, 524705. [Google Scholar] [CrossRef]
- Kumar, P.S.; Keran, D.A.; Pushpavalli, S.; Shiva, K.N.; Uma, S. Effect of cellulose and gum derivatives on physicochemical, microstructural and prebiotic properties of foam-mat driedred banana powder. Int. J. Biol. Macromol. 2022, 218, 44–56. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Stamin, F.D.; Topală, C.M.; Mazilu, I.C.; Badea, G.I.; Vijan, L.E.; Cosmulescu, S. Optimization of phenolic compounds extraction from Crataegi fructus. Appl. Sci. 2025, 15, 9525. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, S.; Mehmood, A.; Bilal, M.; Patil, P.J.; Akram, K.; Farooq, U. Effect of apple pomace on nutrition, rheology of dough and cookies quality. J. Food Sci. Technol. 2020, 57, 3244–3251. [Google Scholar] [CrossRef]
- Naseem, Z.; Bhat, N.A.; Mir, S.A. Valorisation of apple pomace for the development of high-fibre and polyphenol-rich wheat flour cookies. Sci. Rep. 2024, 14, 25912. [Google Scholar] [CrossRef]
- Bustos, M.C.; Perez, G.T.; Leon, A.E. Structure and quality of pasta enriched with functional ingredients. RSC Adv. 2015, 5, 30780–30792. [Google Scholar] [CrossRef]
- De Toledo, N.M.V.; Nunes, L.P.; Da Silva, P.P.M.; Spoto, M.H.F.; Canniatti-Brazaca, S.G. Influence of pineapple, apple and melon by-products on cookies: Physicochemical and sensory aspects. Int. J. Food Sci. Technol. 2017, 52, 1185–1192. [Google Scholar] [CrossRef]
- Mamat, H.; Hardan, M.O.A.; Hill, S.E. Physicochemical properties of commercial semi-sweet biscuit. Food Chem. 2010, 121, 1029–1038. [Google Scholar] [CrossRef]
- Salehi, F.; Aghajanzadeh, S. Effect of dried fruits and vegetables powder on cakes quality: A review. Trends Food Sci. Tech. 2020, 95, 162–172. [Google Scholar] [CrossRef]
- Zarroug, Y.; Sriti, J.; Sfayhi, D.; Slimi, B.; Allouch, W.; Zayani, K.; Hammami, K.; Sowalhia, M.; Kharrat, M. Effect of addition of Tunisian Zizyphus lotus L. fruits on nutritional and sensory qualities of cookies. Ital. J. Food Sci. 2021, 33, 84–97. [Google Scholar] [CrossRef]
- Tyagi, P.; Chauhan, A.K.; Aparna. Optimization and characterization of functional cookies with addition of Tinospora cordifolia as a source of bioactive phenolic antioxidants. LWT—Food Sci. Technol. 2020, 130, 109639. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of apple pomace on quality characteristics of brown rice based cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef]
- Andrejko, D.; Blicharz-Kania, A.; Krajewska, M.; Sagan, A.; Pastusiak, M.; Ociesa, M. The influence of the use of carrot and apple pomace on changes in the physical characteristics and nutritional quality of oat cookies. Processes 2024, 12, 2063. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Kohajdová, Z.; Karovičová, J.; Magala, M.; Kuchtová, V. Effect of apple pomace powder addition on farinographic properties of wheat dough and biscuits quality. Chem. Pap. 2014, 68, 1059–1065. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Uchoa, A.M.A.; Correia da Costa, J.M.; Maia, G.A.; Meira, T.R.; Sousa, P.H.M.; Montenegro Brasil, I. Formulation and physicochemical and sensorial evaluation of biscuit-type cookies supplemented with fruit powders. Plant Foods Hum. Nutr. 2009, 64, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Das Chagas, E.G.L.; Vanin, F.M.; Dos Santos Garcia, V.A.; Yoshida, C.M.P.; De Carvalho, R.A. Enrichment of antioxidants compounds in cookies produced with camu-camu (Myrciaria dubia) coproducts powders. LWT 2021, 137, 110472. [Google Scholar] [CrossRef]
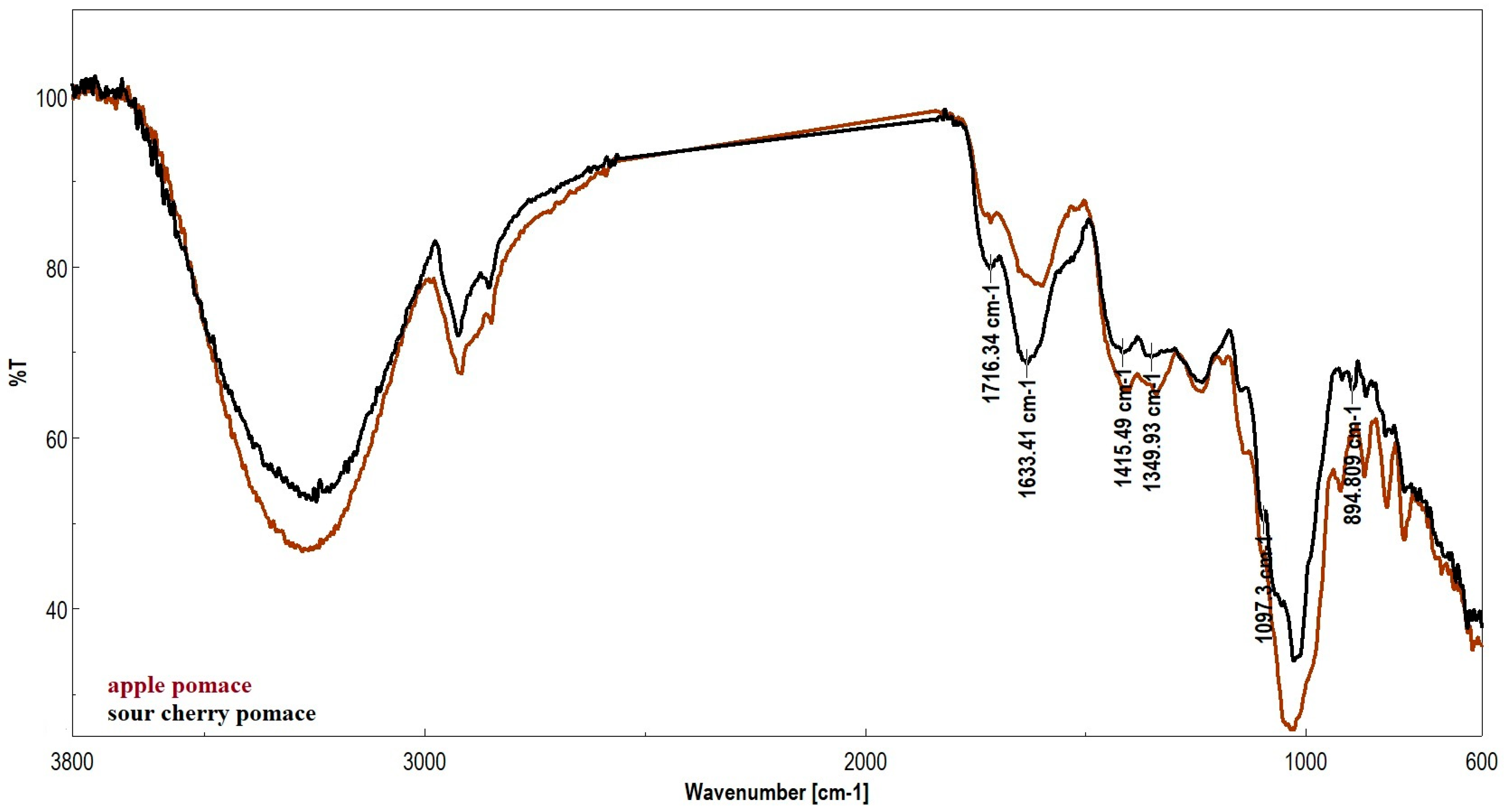
| Samples Wavenumber (cm−1) | Attributions | |||
|---|---|---|---|---|
| Apple | Sour Cherry | Functional Grouping | Vibration Type | Corresponding Compounds |
| 3278 | 3256 | –OH (Hydroxyl) | Stretching | Water, alcohols, polyphenols, cellulose, pectins |
| 2916 2848 | 2924 2855 | C–H (aliphatic) | Stretching (symmetrical/asymmetrical) | Lignin waxes, lipids, aliphatic chains |
| 1715 | 1716 1633 | C=O (carbonyl) | Stretching | Carboxylic acids, esters (pectin), aldehydes |
| 1599 | 1554 | C=C (aromatic) | Stretching | Lignin, polyphenols |
| 1411 1399 | 1415 1349 | C–H and C=C (aromatic) | Deformation and stretching | Lignin, phenolic compounds |
| 1236 1105 1026 | 1235 1097 1026 | C–O, C–O–C, C-O-C glycosidic bond | Stretching | Cellulose, hemicellulose, pectins, sugars |
| 921 885 815 777 | 916 894 862 777 | C–H (aromatic out-of-plane) | Deformation from the plane | Lignin, phenolic structures |
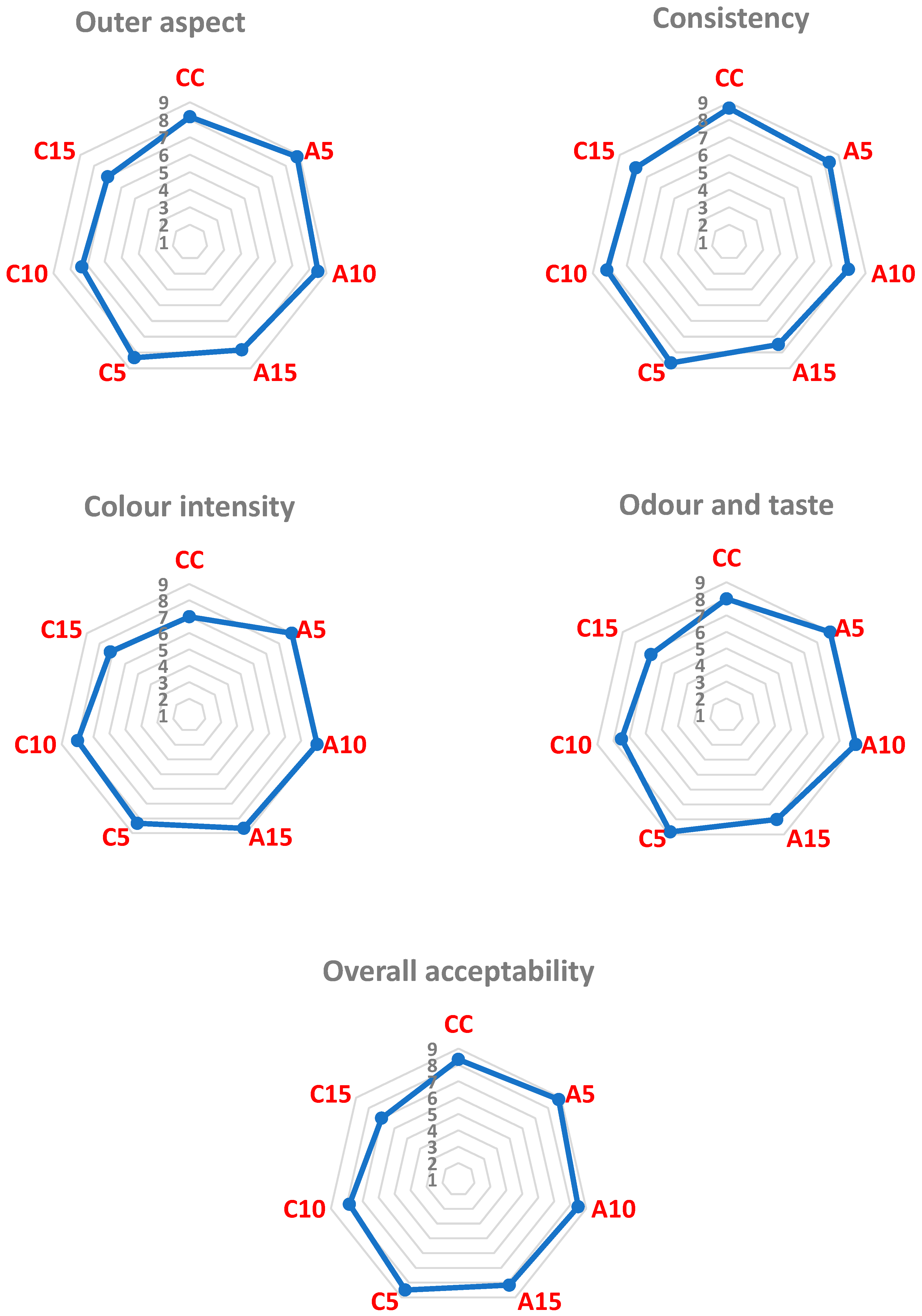
| Sample | Pomace Weight | Moisture Content (%) | Height (mm) | Diameter (mm) | Diameter/Height Ratio |
|---|---|---|---|---|---|
| Control sample | 0% | 6.83 ± 0.75 a | 10.65 ± 0.27 a | 55.77 ± 0.45 a | 5.24 |
| Biscuits with added apple pomace | 5% | 8.07 ± 0.89 a | 10.07 ± 0.21 ab | 54.50 ± 0.60 b | 5.41 |
| 10% | 8.33 ± 0.72 a | 9.33 ± 0.42 bc | 52.67 ± 0.38 cd | 5.65 | |
| 15% | 8.65 ± 0.95 a | 8.73 ± 0.21 c | 52.03 ± 0.47 de | 5.96 | |
| Biscuits with added sour cherry pomace | 5% | 7.12 ± 0.78 a | 9.90 ± 0.44 ab | 53.33 ± 0.75 bc | 5.39 |
| 10% | 7.20 ± 0.79 a | 8.43 ± 0.55 cd | 51.97 ± 0.21 de | 6.16 | |
| 15% | 7.27 ± 0.80 a | 7.57 ± 0.31 d | 50.80 ± 0.36 f | 6.71 | |
| F-value | 1.11 | 12.85 | 17.83 |
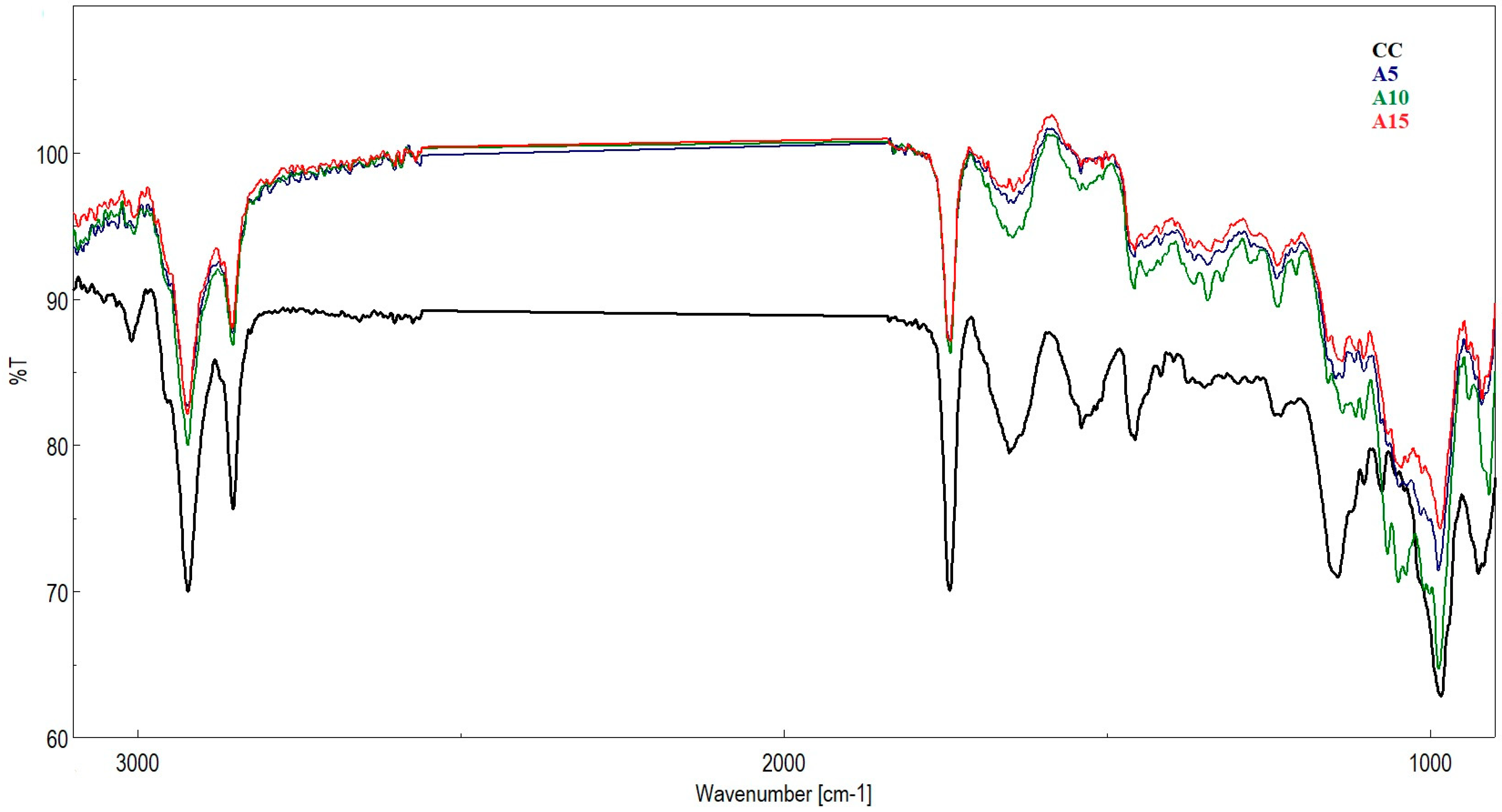
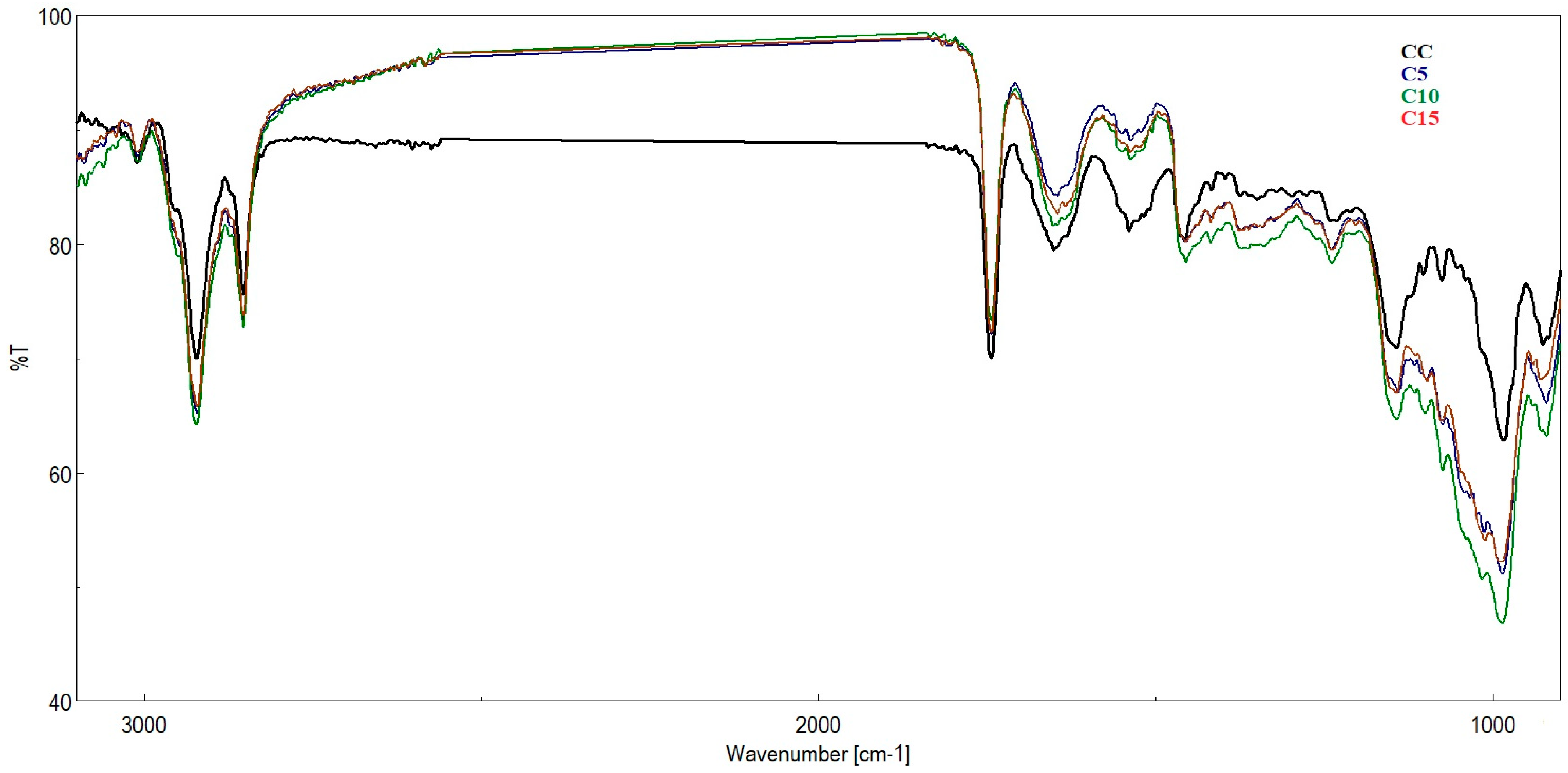
| Samples Wavenumber (cm−1) | Attribution | ||||||
|---|---|---|---|---|---|---|---|
| Control Sample [30] | Biscuits with Added Apple Pomace | Biscuits with Added Sour Cherry Pomace | |||||
| 5% | 10% | 15% | 5% | 10% | 15% | ||
| 3009 | 3018 | 3005 | 3006 | 3009 | 3008 | 3010 | N–H stretching and the =C–H groups are associated with bands characteristic of olefins or unsaturated fatty acids |
| 2922 | 2922 | 2922 | 2922 | 2921 | 2922 | 2920 | Asymmetric stretching vibration of CH2 L |
| 2853 | 2853 | 2853 | 2854 | 2853 | 2853 | 2853 | Symmetric stretching vibration of CH2 L |
| 1743 | 1743 | 1742 | 1743 | 1743 | 1743 | 1743 | C=O stretching (lipids) L |
| 1651 | 1644 | 1645 | 1644 | 1645 | 1652 | 1645 | Amide I (C = O stretching) P |
| 1539 | 1540 | 1541 | 1507 | 1537 | 1538 | 1538 | Amide II (N-H bending, C-N stretching) P |
| 1456 | 1458 1418 | 1458 1439 | 1457 1415 | 1455 1415 | 1455 1418 | 1454 1418 | CH3 bending vibration (lipids and proteins) L, P Stretching C-N, deformation N-H, deformation C-H |
| 1345 | 1345 | 1340 | 1374 | 1362 | 1376 | Stretching C-O, in-plane C-O stretching vibration combined with the ring stretch of phenyl C | |
| 1231 | 1238 | 1236 | 1237 | 1239 | 1239 | 1239 | Amide III (C-N stretching, N-H bending) P |
| 1143 | 1147 | 1208 | 1136 | 1140 | 1142 | 1143 | C-O stretching vibration Oligosaccharide C-O bond C |
| 1075 | 1103 1048 | 1104 1066 1050 | 1103 1046 | 1098 1010 | 1100 1015 | 1097 | Carbohydrates ν(CO), ν(CC), ring (polysaccharides, pectin) C |
| 983 | 987 | 987 | 985 | 987 | 986 | 989 | Stretching OCH3 |
| Sample | Pomace Weight | Polyphenols (mg GAE/100 g) | Flavonoids (mg CE/100 g) | Anthocyanins (mg C3GE/100 g) | Sugars (g GluE/100 g) | RSA (%) |
|---|---|---|---|---|---|---|
| Control sample | 0% | 390.21 ± 0.39 e | 120.13 ± 0.46 g | 18.78 ± 0.30 f | 39.16 ± 0.36 e | 18.77 ± 0.63 f |
| Biscuits with added apple pomace | 5% | 439.52 ± 0.73 d | 164.17 ± 0.30 f | 24.84 ± 0.25 e | 44.50 ± 0.66 c | 20.20 ± 0.74 e. |
| 10% | 441.19 ± 0.39 d | 170.33 ± 0.42 e | 25.45 ± 0.10 d | 45.37 ± 0.02 b | 25.60 ± 0.28 c | |
| 15% | 453.25 ± 2.17 b | 180.23 ± 0.66 c | 26.58 ± 0.25 c | 46.31 ± 0.91 a | 30.80 ± 0.53 a | |
| Biscuits with added sour cherry pomace | 5% | 445.34 ± 1.46 c | 178.80 ± 0.46 d | 26.72 ± 0.28 c | 43.57 ± 0.17 d | 22.79 ± 0.50 d |
| 10% | 450.69 ± 2.79 b | 187.09 ± 0.42 b | 27.88 ± 0.23 b | 43.99 ± 0.33 cd | 23.33 ± 0.78 d | |
| 15% | 475.16 ± 1.11 a | 204.10 ± 0.66 a | 28.58 ± 0.34 a | 44.63 ± 0.11 c | 26.57 ± 0.64 b | |
| F-value | 1619.92 | 8935.36 | 470.13 | 75.60 | 143.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandache, M.B.; Topală, C.M.; Vijan, L.E.; Cosmulescu, S. Enhancing Biscuit Nutritional Value Through Apple and Sour Cherry Pomace Fortification. Appl. Sci. 2025, 15, 11823. https://doi.org/10.3390/app152111823
Mandache MB, Topală CM, Vijan LE, Cosmulescu S. Enhancing Biscuit Nutritional Value Through Apple and Sour Cherry Pomace Fortification. Applied Sciences. 2025; 15(21):11823. https://doi.org/10.3390/app152111823
Chicago/Turabian StyleMandache, Maria Bianca, Carmen Mihaela Topală, Loredana Elena Vijan, and Sina Cosmulescu. 2025. "Enhancing Biscuit Nutritional Value Through Apple and Sour Cherry Pomace Fortification" Applied Sciences 15, no. 21: 11823. https://doi.org/10.3390/app152111823
APA StyleMandache, M. B., Topală, C. M., Vijan, L. E., & Cosmulescu, S. (2025). Enhancing Biscuit Nutritional Value Through Apple and Sour Cherry Pomace Fortification. Applied Sciences, 15(21), 11823. https://doi.org/10.3390/app152111823









