Impact of Purification Methods on the Antioxidant Properties of Tannin-Rich Extracts Obtained from Berry Fruit By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pomace Production
2.3. Extraction of Crude Extracts
2.4. Extract Purification
2.4.1. Purification Using Amberlite XAD 1600N Adsorbent Resin
2.4.2. Purification via Size-Exclusion Chromatography
2.5. Polyphenol Determination
2.5.1. Ellagitannin Quantification
2.5.2. Determination of Flavanols
2.5.3. Determination of Anthocyanins
2.6. Total Polyphenol Content (TPC)
2.7. Determination of Antioxidant Capacity
2.7.1. Ferric Reducing Antioxidant Power (FRAP)
2.7.2. DPPH Radical Scavenging Activity
2.8. Statistical Analysis
3. Results and Discussion
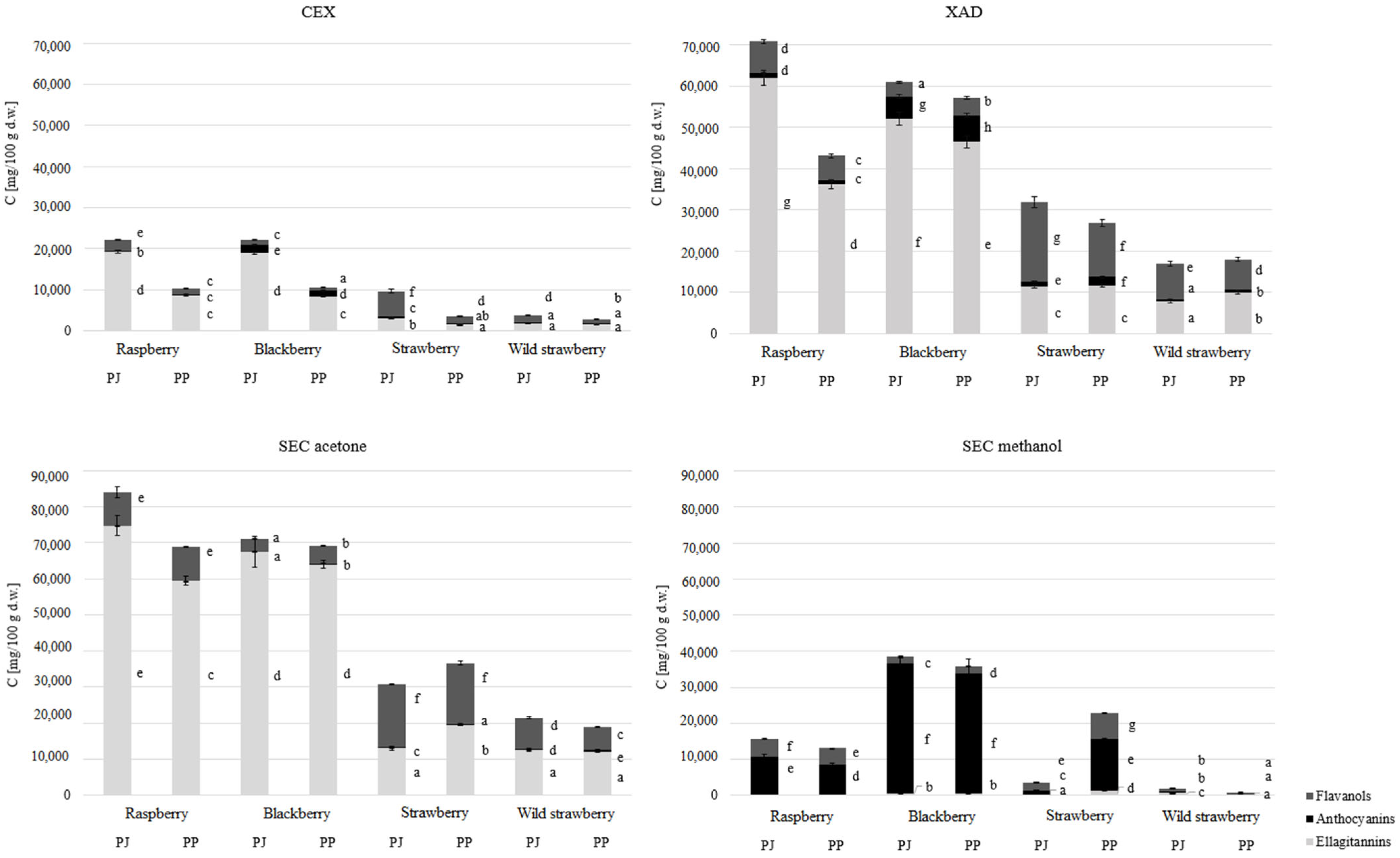
3.1. Extract Characterization
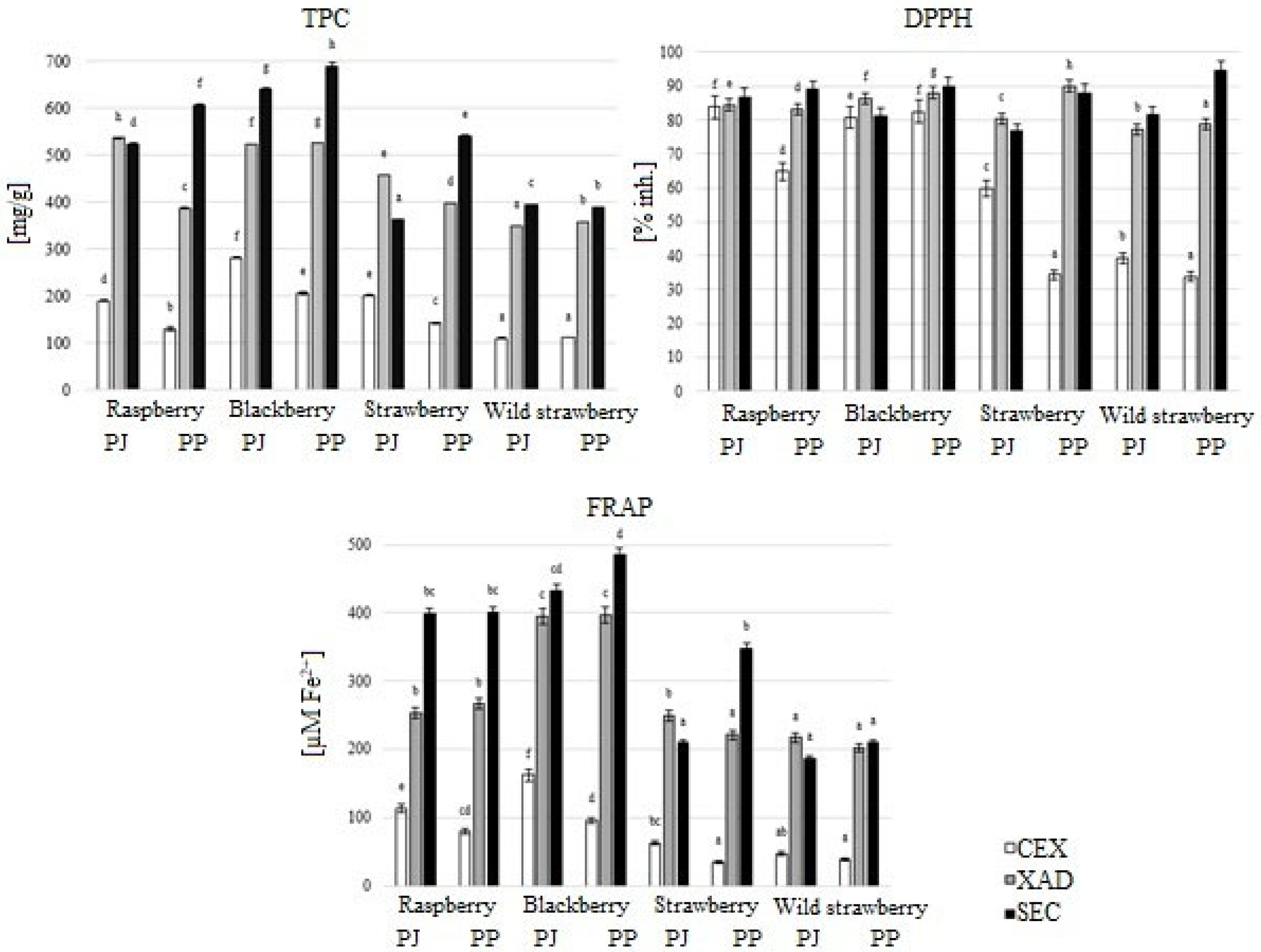
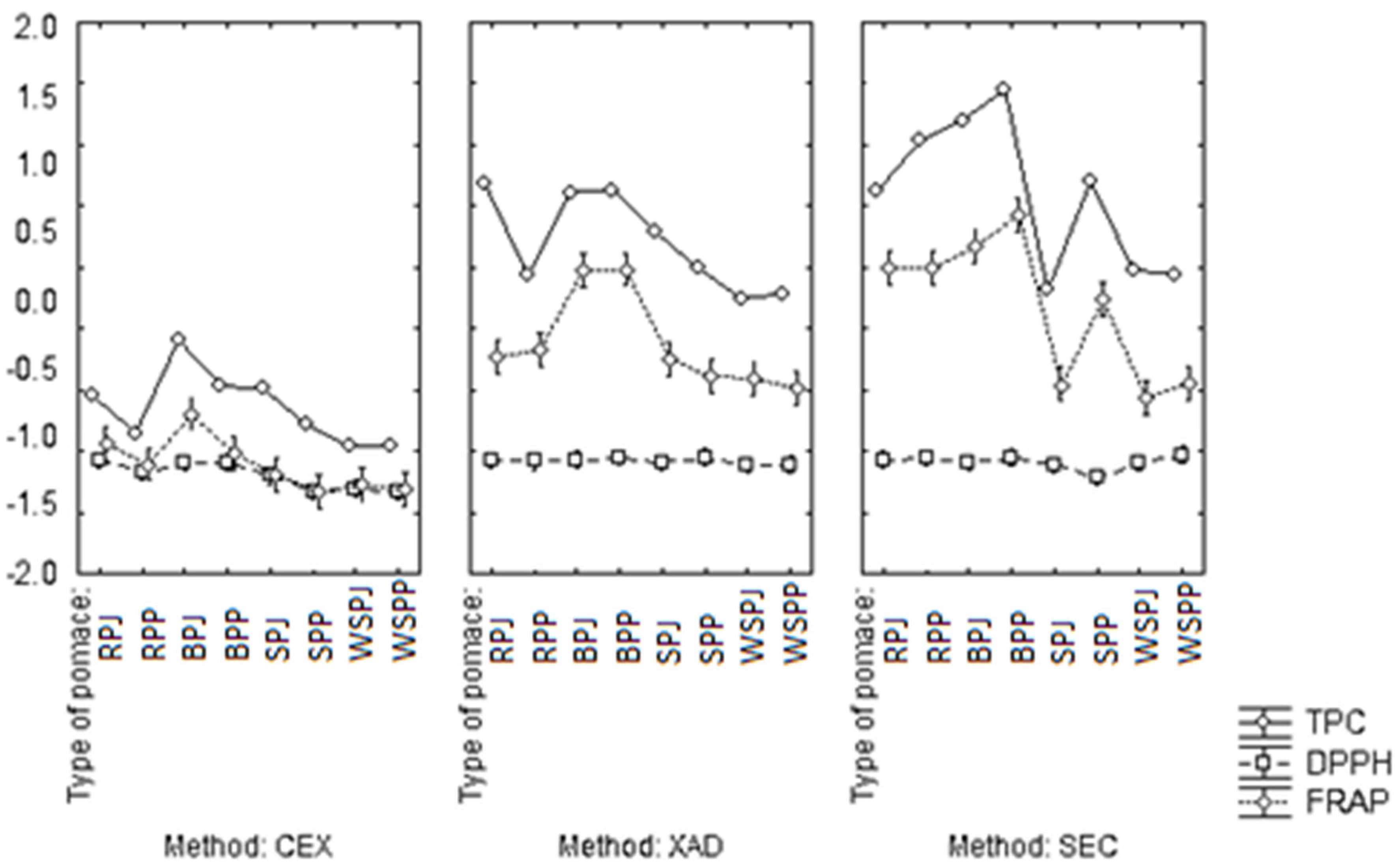
3.2. Antioxidant Properties of Extracts with Different Degrees of Purification
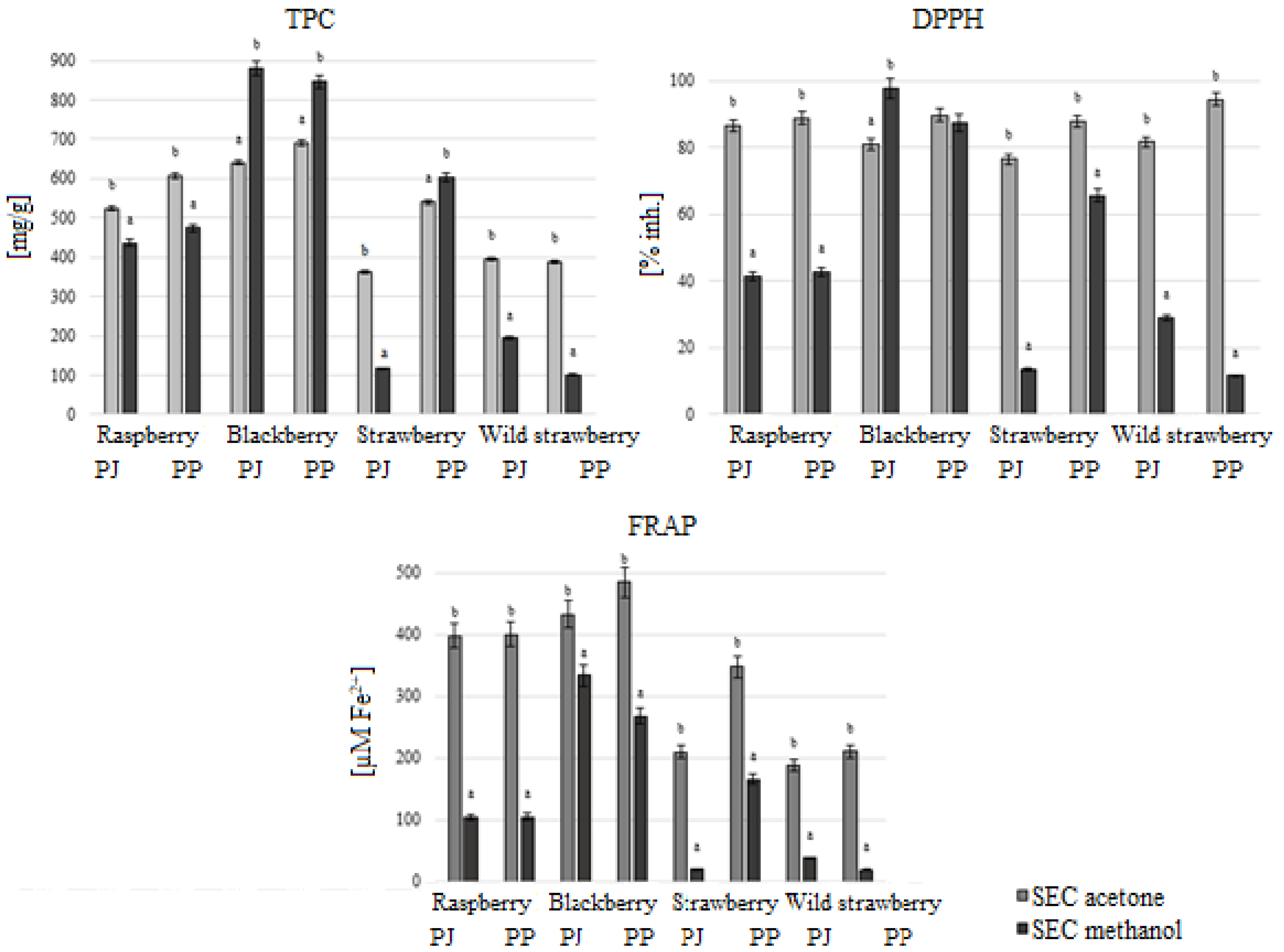
3.3. Antioxidant Activity of Acetone and Methanol Extracts After Purification by Size-Exclusion Chromatography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Standard | Calibration Curve | R2 | Standard Error |
|---|---|---|---|
| Lambertianic C | Y = 21.659x − 21,768 | 0.9999 | 7.2806 |
| Sanguiin H-6 | Y = 26.265x − 3.287 | 0.9999 | 5.2067 |
| Agrimoniin | Y = 21.910x + 13.179 | 0.9990 | 6.8407 |
| Ellagic acid | Y = 101.77x − 135.79 | 0.9994 | 84.9484 |
Appendix B
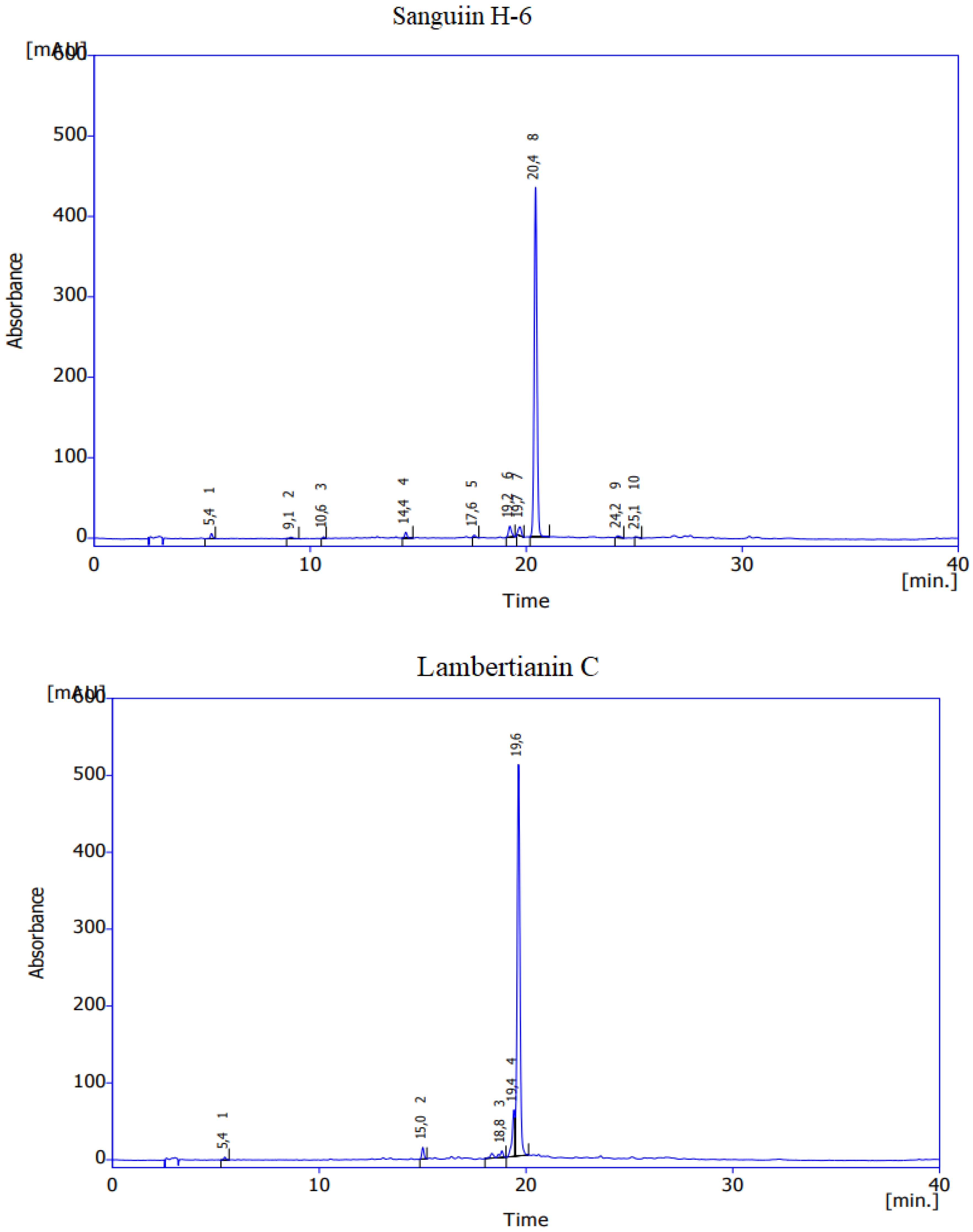
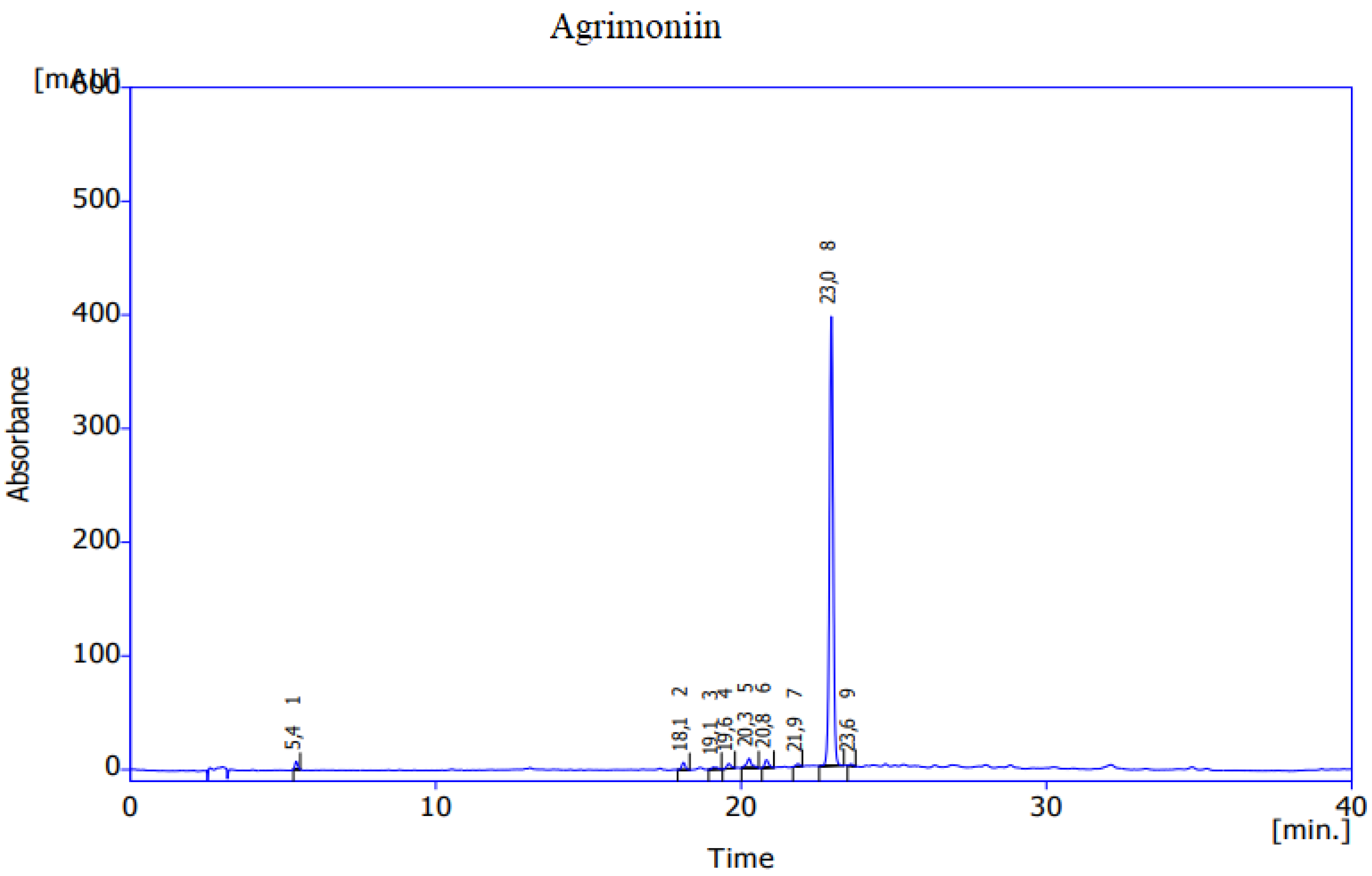
References
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowski, J.; Stochmal, A. Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxidative Med. Cell. Longev. 2016, 2016, 4692486. [Google Scholar] [CrossRef]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.M.; Morris, C. Association of strawberries and anthocyanidin intake with Alzheimer’s Dementia Risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.; Yeşiloğlu, Y.; Bayrak, Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef]
- Selva, M.; Carole, T.; John, S.; Xingqian, Y.; Xue, S.J. Ellagic acid in strawberry (Fragaria spp.): Biological, technological, stability, and human health aspects. Food Qual. Saf. 2017, 1, 227–252. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Petkovsek-Mikulic, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Gündoğdu, M.; Kan, T.; Canan, I. Bioactive and antioxidant characteristics of blackberry cultivars from East Anatolia. Turk. J. Agric. For. 2016, 40, 344–351. [Google Scholar] [CrossRef]
- Rojas-Ocampo, E.; Torrejón-Valqui, L.; Muñóz-Astecker, L.D.; Medina-Mendoza, M.; Mori-Mestanza, D.; Castro-Alayo, E.M. Antioxidant capacity, total phenolic content and phenolic compounds of pulp and bagasse of four Peruvian berries. Heliyon 2021, 7, e07787. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Velázquez, O.A.; Montes-Ávila, J.; Milán-Carrillo, J.; Reyes-Moreno, C.; Mora-Rochin, S.; Cuevas-Rodríguez, E.-O. Characterization of tannins from two wild blackberries (Rubus spp.) by LC–ESI–MS/MS, NMR and antioxidant capacity. J. Food Meas. Charact. 2019, 13, 2265–2274. [Google Scholar] [CrossRef]
- Schulz, M.; Chim, J.F. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the Interaction of Tannins with Co-existing Substances. VI.: Effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Kahkonen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.-P. Biological activity of ellagitannins: Effects as antioxidants, pro-oxidants and metal chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Study on the active polyphenol constituents in differently colored Rubus Chingii Hu and the structure-activity relationship of the main ellagitannins and ellagic acid. LWT 2020, 121, 108967. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Sójka, M.; Macierzyński, J.; Zaweracz, W.; Buczek, M. Transfer and mass balance of ellagitannins, anthocyanins, flavan-3-ols, and flavonols during the processing of red raspberries (Rubus idaeus L.) to juice. J. Agric. Food Chem. 2016, 64, 5549–5563. [Google Scholar] [CrossRef]
- Milczarek, A.; Sójka, M.; Klewicki, R. Transfer of ellagitannins to unclarified juices and purees in the processing of selected fruits of the Rosaceae family. Food Chem. 2021, 344, 128684. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. For. 2016, 40, 855–867. [Google Scholar] [CrossRef]
- Yang, J.W.; Choi, I.S. Comparison of the phenolic composition and antioxidant activity of Korean black raspberry, Bokbunja, (Rubus coreanus Miquel) with those of six other berries. CyTA J. Food 2016, 15, 110–117. [Google Scholar]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Strawberry agro-industrial by-products as a source of bioactive compounds: Effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour. Bioprocess. 2021, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.-I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Klimczak, E.; Król, B. Oznaczanie zawartości różnych form kwasu elagowego w ubocznych produktach przerobu truskawek. Żywn. Nauka Technol. Jakość 2010, 4, 81–94. [Google Scholar]
- Marquez-Lopez, A.; Ayala-Flores, F.; Macias-Pureco, S.; Chavez-Parga, M.D.; Valencia Flores, D.C.; Maya-Yescas, R.; Gonzalez-Hernandez, J.C. Extract of ellagitannins starting with strawberries (Fragaria sp.) and blackberries (Rubus sp.). Food Sci. Technol. 2020, 40, 430–439. [Google Scholar] [CrossRef]
- Setlhodi, R.; Mashile, B.; Izu, G.O.; Gbashi, S.; Mashele, S.S.; Bonnet, S.L.; Makhafola, T.J.; Chukwuma, C.I. Effect of solvent extraction on the antioxidant and phytochemical profiles of ellagitannins from “wonderful” pomegranate peel: An advanced chemometrics analysis. Eur. Food Res. Technol. 2023, 249, 1807–1820. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Fotschki, B.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Grzelak-Błaszczyk, K.; Markiewicz, L. Metabolism of strawberry mono- and dimeric ellagitannins in rats fed a diet containing fructo-oligosaccharides. Eur. J. Nutr. 2017, 56, 853–864. [Google Scholar] [CrossRef]
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Development of a fractionation method for the detection and identification of oak ellagitannins in red wines. Anal. Chim. Acta 2010, 660, 171–176. [Google Scholar] [CrossRef]
- Xue, H.; Shen, L.; Wang, X.; Liu, C.; Liu, C.; Liu, H.; Zheng, X. Isolation and purification of anthocyanin from blueberry using macroporous resin combined Sephadex LH-20 techniques. Food Sci. Technol. Res. 2019, 25, 29–38. [Google Scholar] [CrossRef]
- Cuevas-Rodríguez, E.O.; Dia, V.P.; Yousef, G.G.; Garcia Saucedo, P.A.; López-Medina, J.; Paredes-López, O.; Gonzalez de Mejia, E.; Lila, M.A. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J. Agric. Food Chem. 2010, 58, 9542–9548. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from raspberry (Rubus idaeus L.) fruit as natural inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef]
- Srivastava, A.; Greenspan, P.; Hartle, D.K.; Hargrove, J.L.; Amarowicz, R.; Pegg, R.B. Antioxidant and anti-inflammatory activities of polyphenolics from Southeastern U.S. Range Blackberry Cultivars. J. Agric. Food Chem. 2010, 58, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Kołodziejczyk, K.; Milala, J.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Composition and properties of the polyphenolic extracts obtained from industrial plum pomaces. J. Funct. Foods 2015, 12, 168–178. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Sachett, A.; Gallas-Lopes, M.; Conterato, G.M.M.; Herrmann, A.P.; Piato, A. Antioxidant Activity by FRAP Assay: In Vitro Protocol. 2021. Available online: https://www.protocols.io/view/antioxidant-activity-by-frap-assay-in-vitro-protoc-j8nlk4n35g5r/v1 (accessed on 5 December 2024).
- Sachett, A.; Gallas-Lopes, M.; Conterato, G.M.M.; Herrmann, A.P.; Piato, A. Antioxidant Activity by DPPH Assay: In Vitro Protocol. 2021. Available online: https://www.protocols.io/view/antioxidant-activity-by-dpph-assay-in-vitro-protoc-q26g783n9lwz/v1 (accessed on 5 December 2024).
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. Comparison of the total phenolic content, total anthocyanin content and antioxidant activity of polyphenol-rich fruits grown in Chile. Cienc. Investig. Agrar. 2014, 41, 49–60. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
| Purification Method | Pomace Type | Ellagitannins | Anthocyanins | Flavanols | |
|---|---|---|---|---|---|
| TPC | 0.8087 * | −0.3033 * | 0.8363 * | −0.0329 | 0.5386 * |
| DPPH | 0.5103 * | −0.3355 * | 0.6609 * | 0.2206 | 0.2665 |
| FRAP | 0.7625 * | −0.3997 * | 0.8734 * | −0.0231 | 0.4653 * |
| Type of Extract | Ellagitannins | Anthocyanins | Flavanols | |
|---|---|---|---|---|
| TPC | −0.1422 | 0.3592 * | 0.1774 | 0.0585 |
| DPPH | −0.5821 * | 0.5595 * | −0.0591 | 0.3493 * |
| FRAP | −0.5734 * | 0.7595 * | −0.2622 | 0.4043 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejduk, A.; Sójka, M.; Klewicki, R. Impact of Purification Methods on the Antioxidant Properties of Tannin-Rich Extracts Obtained from Berry Fruit By-Products. Appl. Sci. 2025, 15, 11701. https://doi.org/10.3390/app152111701
Hejduk A, Sójka M, Klewicki R. Impact of Purification Methods on the Antioxidant Properties of Tannin-Rich Extracts Obtained from Berry Fruit By-Products. Applied Sciences. 2025; 15(21):11701. https://doi.org/10.3390/app152111701
Chicago/Turabian StyleHejduk, Agnieszka, Michał Sójka, and Robert Klewicki. 2025. "Impact of Purification Methods on the Antioxidant Properties of Tannin-Rich Extracts Obtained from Berry Fruit By-Products" Applied Sciences 15, no. 21: 11701. https://doi.org/10.3390/app152111701
APA StyleHejduk, A., Sójka, M., & Klewicki, R. (2025). Impact of Purification Methods on the Antioxidant Properties of Tannin-Rich Extracts Obtained from Berry Fruit By-Products. Applied Sciences, 15(21), 11701. https://doi.org/10.3390/app152111701







