Innovative Production of Bioactive White Clover Sprouts Under Microgravity: Towards Functional Foods Supporting Prostate Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sprouting Conditions
2.2. Extracts Preparation
2.3. Cell Cultures
2.4. Proliferation Assay
2.5. PSA and 5-α-Reductase Determination
2.6. Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results
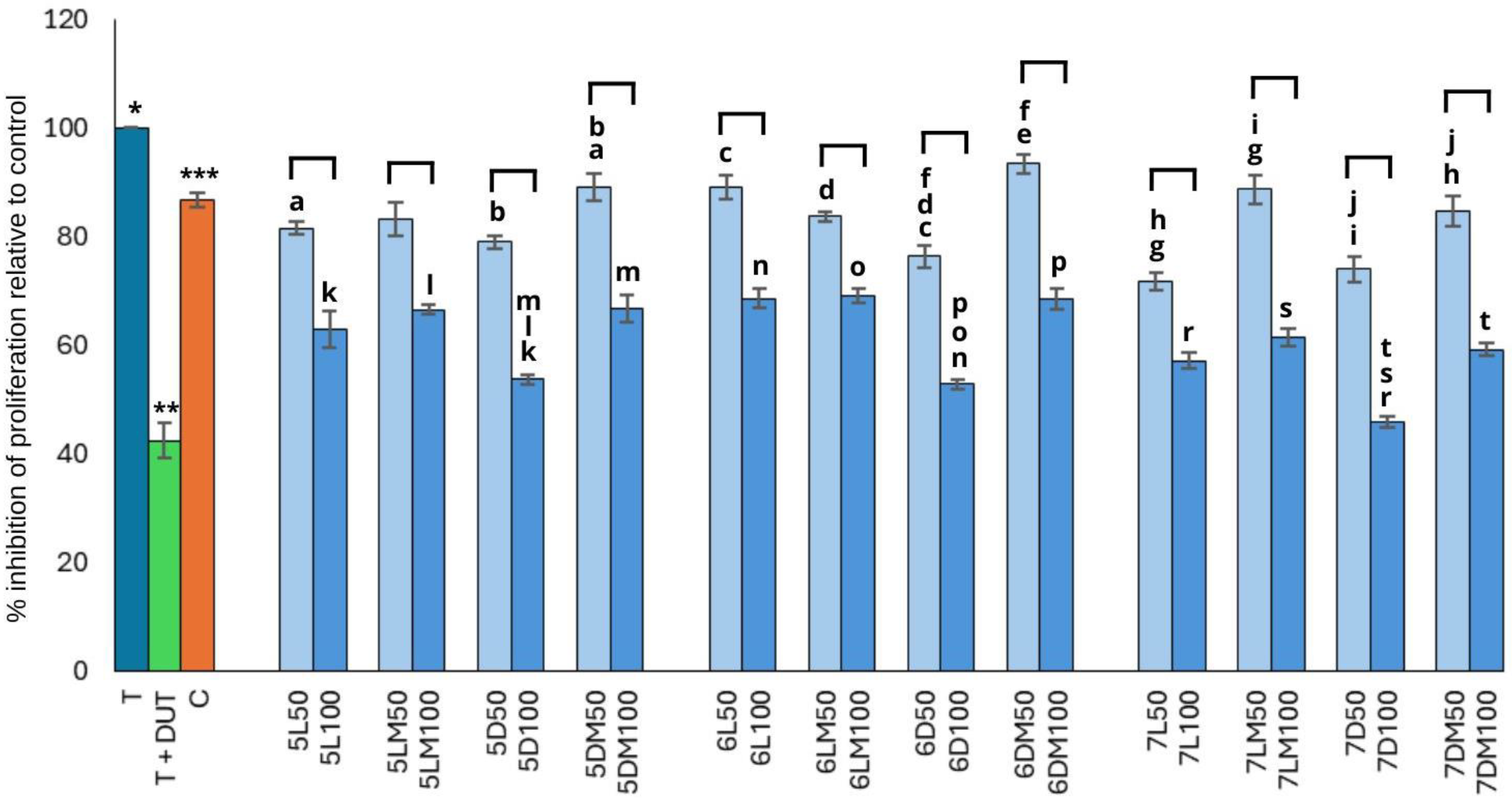
3.1. Anti-Proliferative Activity
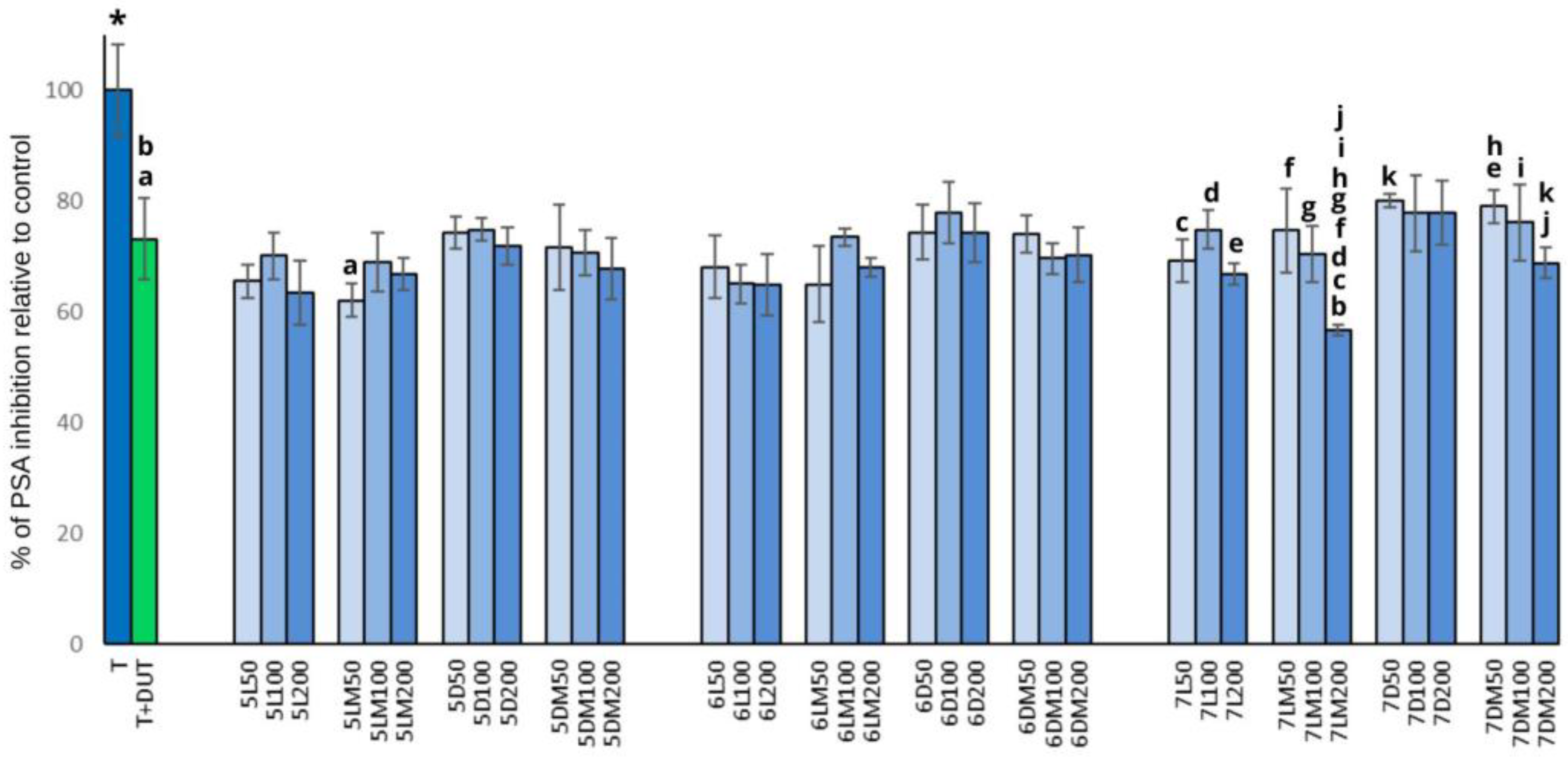
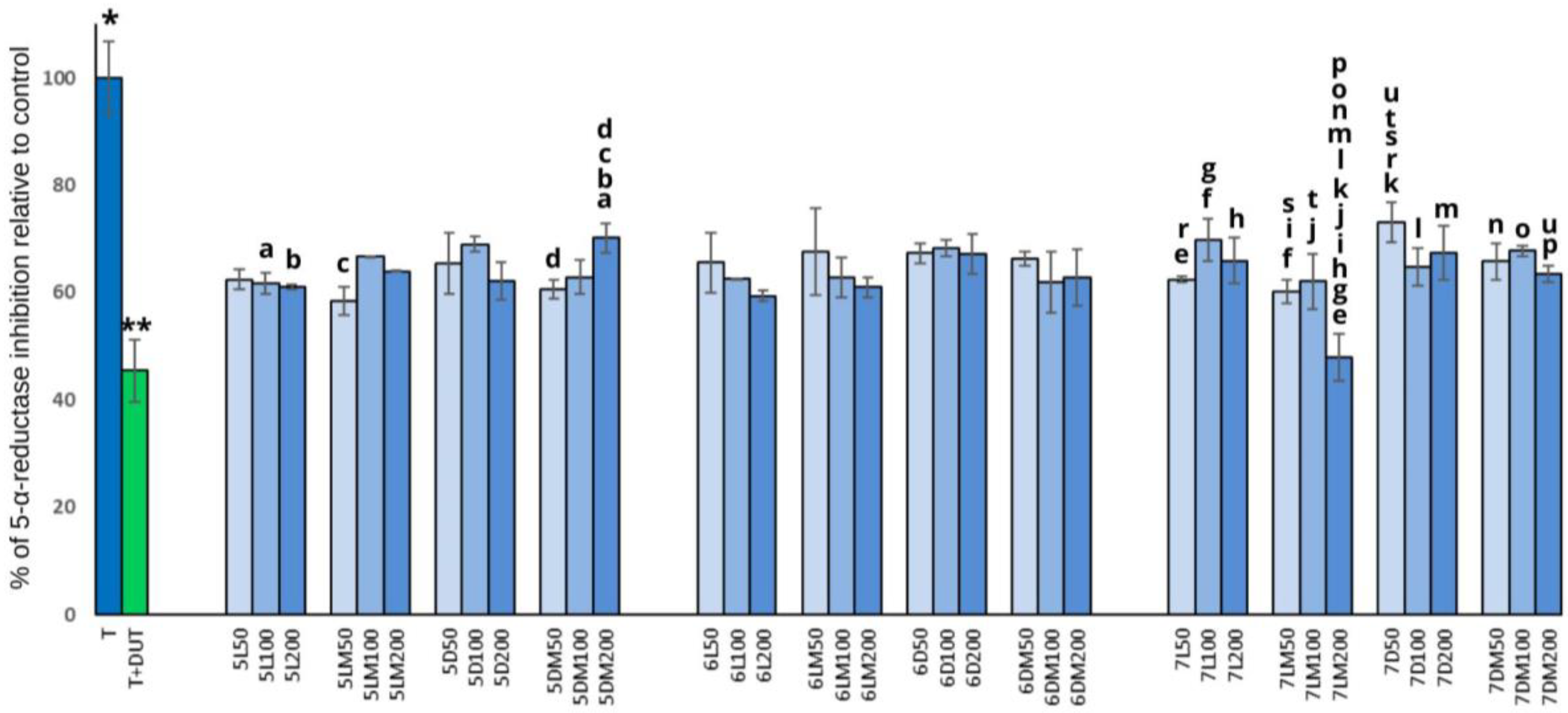
3.2. PSA Release and 5-α-Reductase Activity
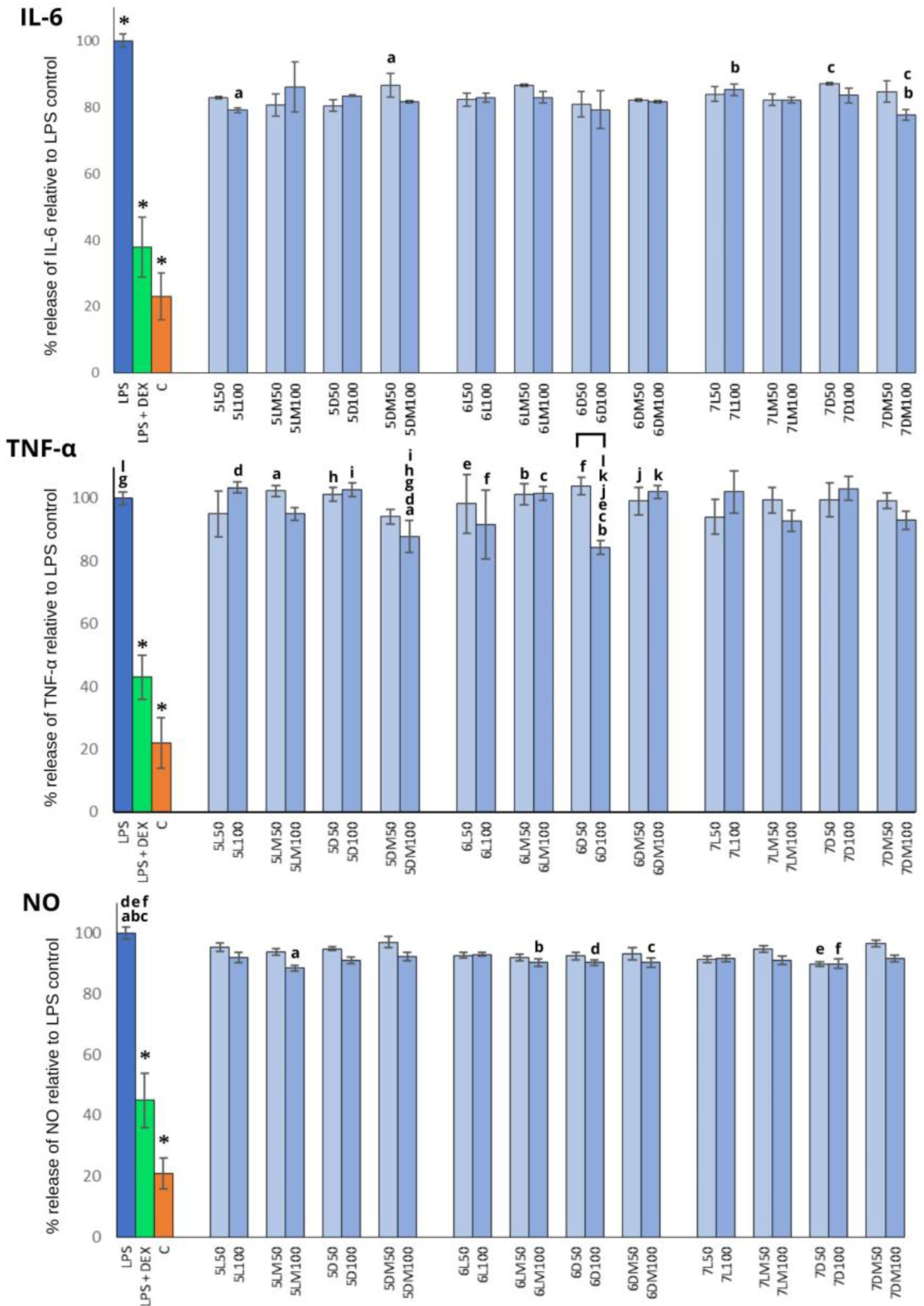
3.3. Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L | Standard light conditions |
| D | Darkness |
| LM | Standard light and microgravity |
| DM | Darkness and microgravity |
| T | Testosterone |
| C | Control conditions |
| DUT | Dutasteride |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin 6 |
| NO | Nitric oxide |
| LPS | Lipopolysaccharide |
| PSA | Prostate-specific antigen |
| BPH | Benign prostatic hyperplasia |
| DHT | Dihydrotestosterone |
| DEX | Dexamethasone |
References
- Galanty, A.; Prochownik, E.; Grudzińska, M.; Paśko, P. Chickpea Sprouts as a Potential Dietary Support in Different Prostate Disorders—A Preliminary In Vitro Study. Molecules 2024, 29, 1044. [Google Scholar] [CrossRef] [PubMed]
- Ali, V.; Mandal, J.; Vyas, D. Insights into light-driven dynamics of phytochemicals in sprouts and microgreens. Plant Growth Regul. 2024, 105, 129–152. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Hassan, A.H.A.; Al Jaouni, S.K.; Alkhalifah, D.H.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H.; Khamis, G. Influence of elevated CO2 on nutritive value and health-promoting prospective of three genotypes of Alfalfa sprouts (Medicago sativa). Food Chem. 2021, 340, 128147. [Google Scholar] [CrossRef]
- Sathasivam, M.; Hosamani, R.K.; Swamy, B.; Kumaran, G.S. Plant responses to real and simulated microgravity. Life Sci. Space Res. 2021, 28, 74–86. [Google Scholar] [CrossRef]
- Paradiso, R.; Ceriello, A.; Pannico, A.; Sorrentino, S.; Palladino, M.; Giordano, M.; Fortezza, R.; De Pascale, S. Design of a Module for Cultivation of Tuberous Plants in Microgravity: The ESA Project “Precursor of Food Production Unit” (PFPU). Front. Plant Sci. 2020, 11, 417. [Google Scholar] [CrossRef]
- Nakajima, S.; Ogawa, Y.; Suzuki, T.; Kondo, N. Enhanced Antioxidant Activity in Mung Bean Seedlings Grown under Slow Clinorotation. Microgravity Sci. Technol. 2019, 31, 395–401. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Kamisaka, S.; Hoson, T. Changes in Levels of Cell Wall Constituents in Wheat Seedlings Grown under Continuous Hypergravity Conditions. Adv. Space Res. 2005, 36, 1292–1297. [Google Scholar] [CrossRef]
- Grudzińska, M.; Galanty, A.; Prochownik, E.; Kołodziejczyk, A.; Paśko, P. Can Simulated Microgravity and Darkness Conditions Influence the Phytochemical Content and Bioactivity of the Sprouts?—A Preliminary Study on Selected Fabaceae Species. Plants 2024, 13, 1515. [Google Scholar] [CrossRef]
- Baden, M.Y.; Liu, G.; Satija, A.; Li, Y.; Sun, Q.; Fung, T.T.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Changes in plant-based diet quality and total and cause-specific mortality. Circulation 2019, 140, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M.K.; Koraqi, H.; Ali, A.; Lima, C.M.G.; Alansari, W.S.; Eskandrani, A.A.; et al. Extraction of Bioactive Compounds from Different Vegetable Sprouts and Their Potential Role in the Formulation of Functional Foods against Various Disorders: A Literature-Based Review. Molecules 2022, 27, 7320. [Google Scholar] [CrossRef]
- Khoja, K.K.; Howes, M.R.; Hider, R.; Sharp, P.A.; Farrell, I.W.; Latunde-Dada, G.O. Cytotoxicity of Fenugreek Sprout and Seed Extracts and Their Bioactive Constituents on MCF-7 Breast Cancer Cells. Nutrients 2022, 14, 784. [Google Scholar] [CrossRef]
- Galanty, A.; Niepsuj, M.; Grudzińska, M.; Zagrodzki, P.; Podolak, I.; Paśko, P. In the Search for Novel, Isoflavone-Rich Functional Foods—Comparative Studies of Four Clover Species Sprouts and Their Chemopreventive Potential for Breast and Prostate Cancer. Pharmaceuticals 2022, 15, 806. [Google Scholar] [CrossRef]
- Grela, E.R.; Kiczorowska, B.; Samolińska, W.; Matras, J.; Kiczorowski, P.; Rybiński, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—Content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Grabowska, K.; Paśko, P.; Zagrodzki, P.; Podolak, I. Multidirectional Effects of Terpenoids from Sorbus intermedia (EHRH.) PERS Fruits in Cellular Model of Benign Prostate Hyperplasia. Pharmaceuticals 2023, 16, 965. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Zagrodzki, P.; Gdula-Argasińska, J.; Grabowska, K.; Koczurkiewicz-Adamczyk, P.; Wróbel-Biedrawa, D.; Podolak, I.; Pękala, E.; Paśko, P. A Comparative Survey of Anti-Melanoma and Anti-Inflammatory Potential of Usnic Acid Enantiomers—A Comprehensive In Vitro Approach. Pharmaceuticals 2021, 14, 945. [Google Scholar] [CrossRef]
- Nakayama, A.; Ide, H.; Lu, Y.; Takei, A.; Fukuda, K.; Osaka, A.; Arai, G.; Horie, S.; Okada, H.; Saito, K. Effects of Curcumin Combined with the 5-alpha Reductase Inhibitor Dutasteride on LNCaP Prostate Cancer Cells. In Vivo 2021, 35, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, D.K.; Pruthi, R.S. Prostate-specific antigen. Expert Opin. Pharmacother. 2000, 1, 1399–1411. [Google Scholar] [CrossRef]
- Tindall, D.J.; Rittmaster, R.S. The rationale for inhibiting 5α-reductase isoenzymes in the prevention and treatment of prostate cancer. J. Urol. 2008, 179, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (bph). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Oliveira, M.B.P.P. Evaluating the impact of sprouting conditions on the glucosinolate content of Brassica oleracea sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.M. Sprout Garden: Indoor Grower’s Guide to Gourmet Sprouts; Book Publishing Co.: Summertown, TN, USA, 1999. [Google Scholar]
- Yao, J.; Wang, Z.; Wang, R.; Wang, Y.; Xu, J.; He, X. Anti-proliferative and anti-inflammatory prenylated isoflavones and coumaronochromones from the fruits of Ficus altissima. Bioorg. Chem. 2021, 113, 104996. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, Y.; Shen, L.; Li, D.; Xu, J.; He, X.; Wang, Y. Isoflavones isolated from the fruits of Ficus altissima and their anti-proliferative activities. Fitoterapia 2024, 175, 105966. [Google Scholar] [CrossRef]
- Yin, J.; Heo, J.H.; Hwang, Y.J.; Le, T.T.; Lee, M.W. Inhibitory Activities of Phenolic Compounds Isolated from Adina rubella Leaves Against 5α-Reductase Associated with Benign Prostatic Hypertrophy. Molecules 2016, 21, 887. [Google Scholar] [CrossRef]
- Engelhardt, P.F.; Riedl, C.R. Effects of One-Year Treatment with Isoflavone Extract from Red Clover on Prostate, Liver Function, Sexual Function, and Quality of Life in Men with Elevated PSA Levels and Negative Prostate Biopsy Findings. Urology 2008, 71, 185–190. [Google Scholar] [CrossRef]
- Evans, B.A.J.; Griffiths, K.; Morton, M.S. Inhibition of 5α-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar] [CrossRef]
- Bae, M.; Woo, M.; Kusuma, I.W.; Arung, E.T.; Yang, C.H.; Kim, Y. Inhibitory Effects of Isoflavonoids on Rat Prostate Testosterone 5α-Reductase. Innov. Acupunct. Med. 2012, 5, 319–322. [Google Scholar] [CrossRef]
- Ratha, P.; Neumann, T.; Schmidt, C.A.; Schneidewind, L. Can Isoflavones Influence Prostate Specific Antigen Serum Levels in Localized Prostate Cancer? A Systematic Review. Nutr. Cancer 2020, 73, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; McCaskill-Stevens, W.; Lampe, J.W. Addressing the soy and breast cancer relationship: Review, commentary, and workshop proceedings. J. Natl. Cancer Inst. 2006, 98, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Peternac, D.; Klima, I.; Cecchini, M.G.; Schwaninger, R.; Studer, U.E.; Thalmann, G.N. Agents used for chemoprevention of prostate cancer may influence PSA secretion independently of cell growth in the LNCaP model of human prostate cancer progression. Prostate 2008, 68, 1307–1318. [Google Scholar] [CrossRef]
- Sivonová, M.K.; Kaplán, P.; Tatarková, Z.; Lichardusová, L.; Dušenka, R.; Jureceková, J. Androgen receptor and soy isoflavones in prostate cancer. Mol. Clin. Oncol. 2019, 10, 191–204. [Google Scholar] [PubMed]
- Al-Shami, A.S.; Essawy, A.E.; Elkader, H.A.E.A. Molecular mechanisms underlying the potential neuroprotective effects of Trifolium pratense and its phytoestrogen-isoflavones in neurodegenerative disorders. Phytother. Res. 2023, 37, 2693–2737. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rasul, A.; Batool, R.; Sarfraz, I.; Hussain, G.; Tahir, M.M.; Qin, T.; Selamoglu, Z.; Ali, M.; Li, J.; et al. Potential Anticancer Properties and Mechanisms of Action of Formononetin. BioMed Res. Int. 2019, 2019, 5854315. [Google Scholar] [CrossRef]
- Lee, S.A.; Park, B.R.; Moon, S.M.; Han, S.H.; Kim, C.S. Anti-inflammatory potential of Trifolium pratense L. leaf extract in LPS-stimulated RAW264.7 cells and in a rat model of carrageenan-induced inflammation. Arch. Physiol. Biochem. 2018, 126, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, P.; Wang, Y.; Yang, C.; Wu, X.; Wu, C.; Luo, L.; Wang, Q.; Niu, C.; Yao, J. Structural characterization and anti-inflammatory activity evaluation of chemical constituents in the extract of Trifolium repens L. J. Food Biochem. 2019, 43, e12981. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.Y.; Park, S.J. Effects of Trifolium repens and Trifolium pratense on the LPS-induced Inflammatory Responses in RAW 264.7 Macrophages. J. Agric. Life Environ. Sci. 2021, 33, 409–419. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markiewicz, M.; Galanty, A.; Prochownik, E.; Kołodziejczyk, A.; Paśko, P. Innovative Production of Bioactive White Clover Sprouts Under Microgravity: Towards Functional Foods Supporting Prostate Health. Appl. Sci. 2025, 15, 11668. https://doi.org/10.3390/app152111668
Markiewicz M, Galanty A, Prochownik E, Kołodziejczyk A, Paśko P. Innovative Production of Bioactive White Clover Sprouts Under Microgravity: Towards Functional Foods Supporting Prostate Health. Applied Sciences. 2025; 15(21):11668. https://doi.org/10.3390/app152111668
Chicago/Turabian StyleMarkiewicz, Marta, Agnieszka Galanty, Ewelina Prochownik, Agata Kołodziejczyk, and Paweł Paśko. 2025. "Innovative Production of Bioactive White Clover Sprouts Under Microgravity: Towards Functional Foods Supporting Prostate Health" Applied Sciences 15, no. 21: 11668. https://doi.org/10.3390/app152111668
APA StyleMarkiewicz, M., Galanty, A., Prochownik, E., Kołodziejczyk, A., & Paśko, P. (2025). Innovative Production of Bioactive White Clover Sprouts Under Microgravity: Towards Functional Foods Supporting Prostate Health. Applied Sciences, 15(21), 11668. https://doi.org/10.3390/app152111668





