Injectable Hydrogel Systems for Targeted Drug Delivery: From Site-Specific Application to Design Strategy
Abstract
1. Introduction
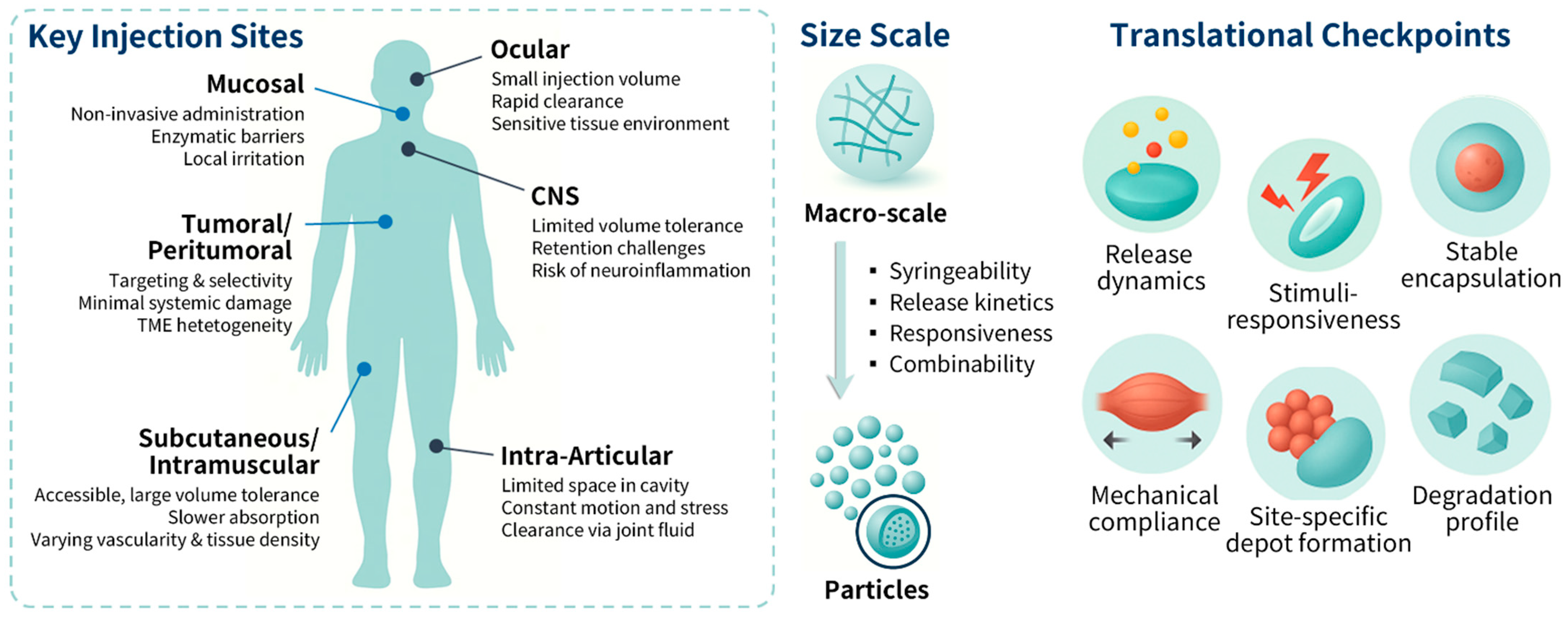
2. Hydrogels for Site-Specific Injectable Drug Delivery
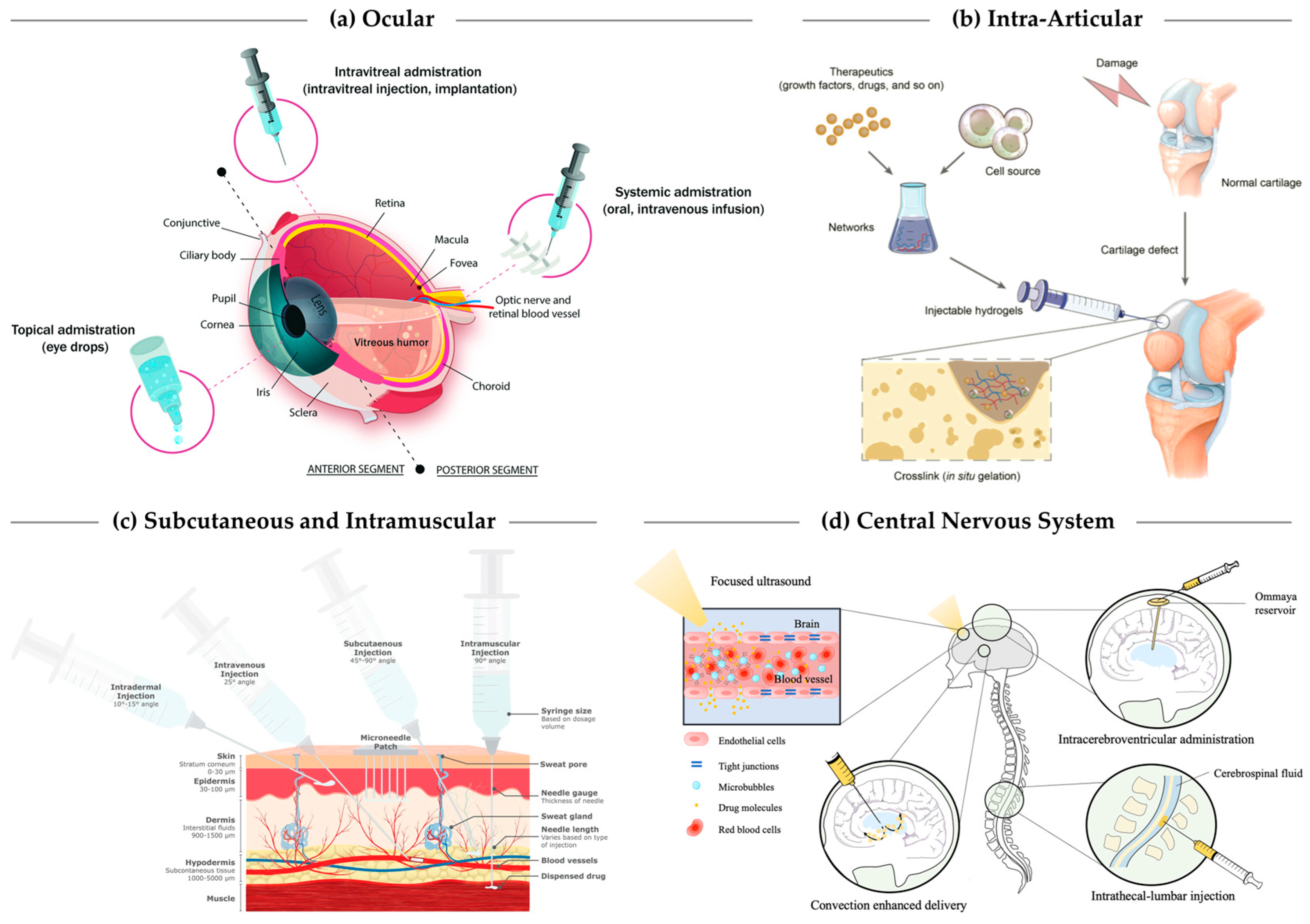
2.1. Ocular Delivery
2.2. Intra-Articular Injection
2.3. Subcutaneous, Dermal, and Intramuscular Depots
2.4. Tumoral and Peritumoral Injection
2.5. Central Nervous System Delivery
2.6. Mucosal Delivery
3. Design Principles Governing Hydrogel-Based DDS Formulations for Injectable Applications
3.1. Fabrication Techniques for Nanogels and Microgels
| Size Category/Forms | Typical Size Range | Fabrication Approaches | Drug Delivery Advantages | Injectability Constraints | Challenges/Limitations | Examples Formulated Agent | Refs. |
|---|---|---|---|---|---|---|---|
| Nanogels | 50–200 nm | Inverse microemulsion, nanoprecipitation, self-assembly, ionic gelation | Systemic or targeted delivery, intracellular uptake | Compatible with fine needles, require stable dispersion | Rapid clearance if <20 nm, surfactant/solvent residue, narrow crosslinking window | Small-molecule drugs (Doxorubicin, Ozoile) Nucleic acids (DNA, antisense oligonucleotides) | [118,119,120,121] |
| Small microgels | sub-µm to 5 µm | Microfluidics, batch emulsification, ionic gelation | Mucosal/ocular/topical retention, deformable and stimuli-responsive | Fine needle (27–30 G) compatible, shear-induced deformation during injection | Polydispersity, batch variability, formulation sensitivity | Proteins (FITC–BSA) Stem cells (mESCs) | [122,123,124,125] |
| Medium microgels | 5–50 µm | Microfluidics, W/O emulsification, electrospraying, jetting, micro-molding | Balanced retention–injectability, tunable depot formation | Small gauge needles, risk of clogging, moderate injection force | Shear/enzymatic degradation, moderate throughput | Small-molecule drugs (Ozoile) Metallic ions (Fe2+/Fe3+) | [120,126,127,128] |
| Large microgels | 50–200 µm | Jetting, extrusion dripping, top-down fragmentation, micro-molding | Robust depot formation, intra-articular and dermal administrations | Requires larger needles, limited dispersion, higher injection force | Broad size distribution, diffusion-limited release, mechanical heterogeneity | Stem cells, Nucleic acid (DNA, oligonucleotides) Small-molecule drugs (Doxorubicin) | [124,129,130,131,132] |
| Hybrid/modular composites | Multi-scale | Nanogel–polymer hybrid, microgel–nanoparticle conjugates, ionic coupling, etc. | Multi-drug treatment, modular and stimuli-responsive architectures | Matrix-dependent rheology, multi-phase stability | Complex multi-step synthesis, long-term storage stability | Small-molecule drugs (Amoxicillin, Ketoprofen) Proteins (FITC–BSA) Cells (MSCs, HepG2, HUVEC) | [123,131,133,134] |
3.1.1. Emulsion-Based Polymerization
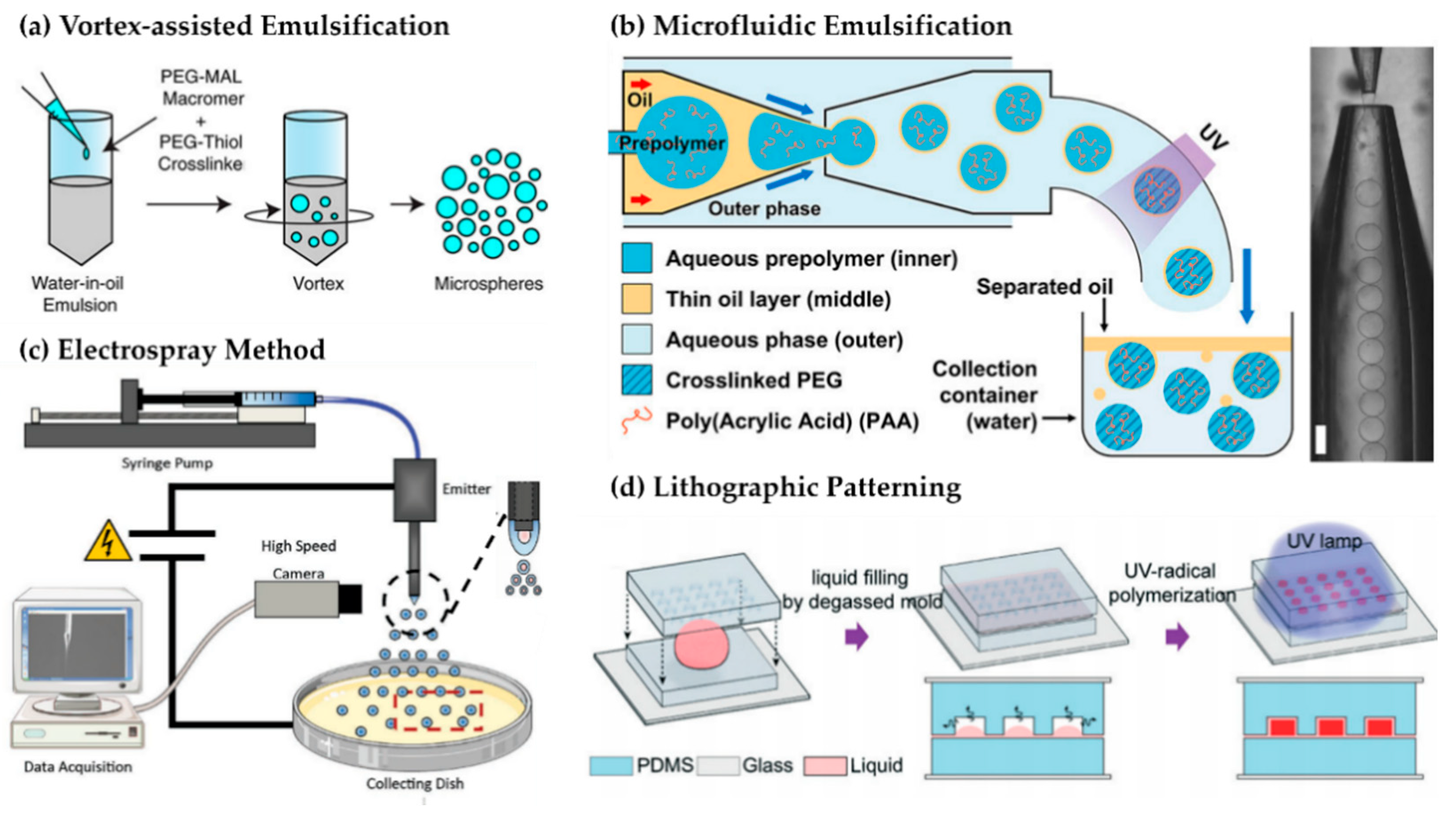
3.1.2. Microfluidic Droplet Generation
3.1.3. Electrospraying and Jetting
3.1.4. Photolithography and Micro-Molding
3.1.5. Top–Down Strategies
3.1.6. Physical Ionic Crosslinking (Ionotropic Crosslinking)
3.1.7. Macro-Scale Hydrogel Beads
3.2. Stimuli-Responsive Material Behavior
3.2.1. pH-Responsiveness

3.2.2. Enzyme-Responsiveness
3.2.3. Thermo-Responsiveness
3.2.4. Redox-Responsiveness
3.2.5. Light-Responsiveness
3.2.6. Multi-Responsive and Hybrid Strategies
3.3. Strategies for Drug Loading and Release Modulation
3.3.1. Drug−Particle Interactions and Loading Strategies
3.3.2. Release Kinetics and Stimulus-Modulated Delivery
4. Advanced Formulation Strategies for Programmable and Multi-Agent Microgel Delivery
4.1. Drug Cargos
4.1.1. Protein/Peptides
4.1.2. Small Molecules
| Category | Subtype | Unique Challenges | Integration with Hydrogel Systems | Representative Applications | Refs. |
|---|---|---|---|---|---|
| Drug Cargo | Proteins/Peptides | Aggregation, proteolysis, loss of bioactivity | Affinity binding; pH-/enzyme-responsive gels; composite depots | Insulin, cytokines, monoclonal antibodies | [267,269] |
| Small Molecules | Rapid clearance, burst release, poor solubility | Entrapment in hydrophobic domains; host–guest complexes | DOX, NSAIDs, antibiotics | [12,23,273] | |
| Nucleic Acids (DNA, siRNA, mRNA) | Nuclease degradation, poor uptake, immune activation | Cationic gels, LNP–hydrogel hybrids, DNA hydrogels | siRNA, mRNA vaccines, plasmid DNA | [7,16,37,274] | |
| Stem Cells | Low viability, shear stress, poor engraftment | ECM-mimetic hydrogels; peptide-functionalized microgels | Cartilage, spinal cord, myocardial regeneration | [275,276,277] | |
| DDS | Liposomes | Low viability, shear stress, poor engraftment | Entrapment in hydrogels; mucoadhesive or injectable composites | Ocular, dermal, and cancer therapies | [276,278] |
| Lipid Nanoparticles (LNPs) | Liver accumulation, transient expression, immune activation | Embedding LNPs in gels for local release and spatial targeting | mRNA cancer vaccines, regenerative medicine | [16,37] | |
| Polymeric Nanoparticles/Micelles | Rapid clearance, non-specific biodistribution | Physical embedding, hybrid gels for staged release | Anticancer agents, antibiotics | [44,270] |
4.1.3. Stem Cells
4.1.4. Nucleic Acids
4.2. Stimuli-Responsive and Controlled Release Systems
4.2.1. Single-Trigger Responsive Systems
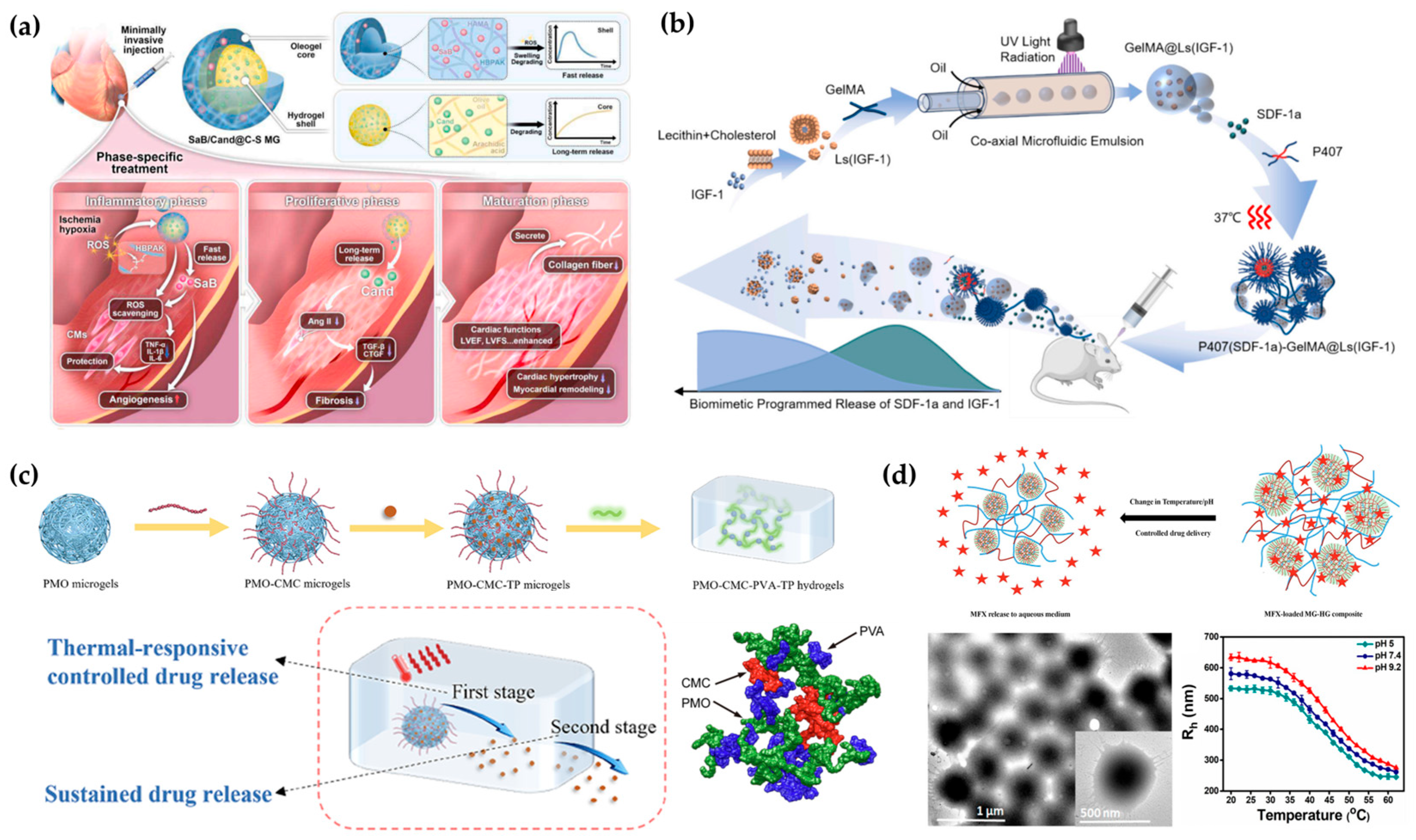
4.2.2. Multi-Stimuli Responsive Systems
4.2.3. Spatially Compartmentalized Systems
4.2.4. Translational Pathways from Bulk to Modular Hydrogels
4.3. Multi-Drug Delivery and Combination Therapy
5. Translational Outlook for Hydrogel Drug Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Yun, S.; Kim, H.Y.; Ko, S.; Islam, M.; Nam, K.W. Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration. Gels 2025, 11, 179. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 27–36. [Google Scholar] [CrossRef]
- Smeets, N.M.B.; Hoare, T. Designing Responsive Microgels for Drug Delivery Applications. J. Polym. Sci. A Polym. Chem. 2013, 51, 3027–3043. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The Development of Microgels/Nanogels for Drug Delivery Applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Y.; Zhang, Z.; Huang, H.; Xu, Y.; Shen, F.; Wang, L.; Sun, L. Injectable DNA Hydrogel-Based Local Drug Delivery and Immunotherapy. Gels 2022, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yung, B.C.; Qian, Z.; Chen, X. Improving Long-Term Subcutaneous Drug Delivery by Regulating Material-Bioenvironment Interaction. Adv. Drug Deliv. Rev. 2018, 127, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, D.; Maitland, D.; Hoare, T. Injectable Microgel-Hydrogel Composites for Prolonged Small-Molecule Drug Delivery. Biomacromolecules 2011, 12, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Paresishvili, T.; Kakabadze, Z. Challenges and Opportunities Associated with Drug Delivery for the Treatment of Solid Tumors. Oncol. Rev. 2023, 17, 10577. [Google Scholar] [CrossRef]
- Lee, H.; Noh, H. Advancements in Nanogels for Enhanced Ocular Drug Delivery: Cutting-Edge Strategies to Overcome Eye Barriers. Gels 2023, 9, 718. [Google Scholar] [CrossRef]
- Kidane, A.; Bhatt, P.P. Recent Advances in Small Molecule Drug Delivery. Curr. Opin. Chem. Biol. 2005, 9, 347–351. [Google Scholar] [CrossRef]
- Brako, F.; Boateng, J. Transmucosal Drug Delivery: Prospects, Challenges, Advances, and Future Directions. Expert Opin. Drug Deliv. 2025, 22, 525–553. [Google Scholar] [CrossRef]
- Gandhat, S.; Ugale, S.; Kumavat, S.; Gadge, K.; Dandawate, N.; Bhor, V. Microneedles in Transdermal Drug Delivery: A Comprehensive Review. J. Drug Deliv. Ther. 2025, 15, 201–209. [Google Scholar] [CrossRef]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Koh, H.Y.; Han, J.Y.; Seo, S. Synthesis of Hydrogel-Based Microgels and Nanogels Toward Therapeutic and Biomedical Applications. Appl. Sci. 2025, 15, 1368. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Coulter, S.M.; Pentlavalli, S.; An, Y.; Vora, L.K.; Cross, E.R.; Moore, J.V.; Sun, H.; Schweins, R.; McCarthy, H.O.; Laverty, G. In Situ Forming, Enzyme-Responsive Peptoid-Peptide Hydrogels: An Advanced Long-Acting Injectable Drug Delivery System. J. Am. Chem. Soc. 2024, 146, 21401–21416. [Google Scholar] [CrossRef]
- Lv, C.; Wang, Q.; Li, Z.; Jiang, X.; Liu, T.; Xu, X.; Feng, X. An Injectable Thermos-Sensitive Hydrogel for Sustained Release of α-Mangostin Promotes MRSA-Infected Wound Healing in Mice. Macromol. Res. 2025. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Z.; Deng, M.; Chen, L.; He, C.; Zhuang, X.; Chen, X. Thermo- and PH-Responsive Microgels for Controlled Release of Insulin. Polym. Int. 2012, 61, 1151–1157. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xue, W.; Wang, H.; Qiu, X.; Liu, Z. Thermo-Sensitive Hydrogel PLGA-PEG-PLGA as a Vaccine Delivery System for Intramuscular Immunization. J. Biomater. Appl. 2017, 31, 923–932. [Google Scholar] [CrossRef]
- Singh, N.; Aery, S.; Juneja, S.; Kumari, L.; Lone, M.S.; Dar, A.A.; Pawar, S.V.; Mehta, S.K.; Dan, A. Chitosan Hydrogels with Embedded Thermo- and PH-Responsive Microgels as a Potential Carrier for Controlled Release of Drugs. ACS Appl. Bio Mater. 2022, 5, 3487–3499. [Google Scholar] [CrossRef]
- Okuno, Y.; Iwasaki, Y. Microgel-Based Smart Materials: How Do You Design a Microgel? Langmuir 2025, 41, 7946–7964. [Google Scholar] [CrossRef] [PubMed]
- Alzanbaki, H.; Moretti, M.; Hauser, C.A.E. Engineered Microgels—Their Manufacturing and Biomedical Applications. Micromachines 2021, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.M.; Lu, Y.; Winnik, M.A. PEGMA-Based Microgels: A Thermoresponsive Support for Enzyme Reactions. Macromolecules 2016, 49, 8711–8721. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S.; Sharma, P.; Kumar, B.; Kumar, A. Single-, Dual-, and Multi-Stimuli-Responsive Nanogels for Biomedical Applications. Gels 2024, 10, 61. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable Hydrogels for Sustained Release of Therapeutic Agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Al Fatease, A.; Abdelkader, H. Recent Advances in Long-Acting Drug Delivery and Formulations. Pharmaceutics 2023, 15, 2519. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef]
- Marchenko, I.V.; Trushina, D.B. Local Drug Delivery in Bladder Cancer: Advances of Nano/Micro/Macro-Scale Drug Delivery Systems. Pharmaceutics 2023, 15, 2724. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for on-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yang, M.; Seo, J.H.; Jeong, D.I.; Hwang, C.; Kim, H.J.; Lee, J.; Lee, K.; Park, J.; Cho, H.J. Serially PH-Modulated Hydrogels Based on Boronate Ester and Polydopamine Linkages for Local Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 2189–2203. [Google Scholar] [CrossRef]
- Ahiabu, A.; Serpe, M.J. Rapidly Responding pH-and Temperature-Responsive Poly (N-Isopropylacrylamide)-Based Microgels and Assemblies. ACS Omega 2017, 2, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Marquez, M.; Cai, T.; Rosario, R.; Hu, Z.; Gust, D.; Hayes, M.; Vail, S.A.; Park, C. Do Photo-, Thermally, and PH-Responsive Microgels. Langmuir 2007, 23, 224–229. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic Synthesis of Lipid-Based Nanoparticles for Drug Delivery: Recent Advances and Opportunities. Lab Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Z.-C.; Li, S.; Ma, J.; Wei, C.; Yu, D.; Stelzel, J.L.; Ni, B.Y.X.; Miao, Y.; Van Batavia, K.; et al. An MRNA Lipid Nanoparticle-Incorporated Nanofiber-Hydrogel Composite for Cancer Immunotherapy. Nat. Commun. 2025, 16, 5707. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Fung, M.P.; Chen, Y.Q.; Chiu, Y.C. Development of Mucoadhesive Methacrylic Anhydride-Modified Hydroxypropyl Methylcellulose Hydrogels for Topical Ocular Drug Delivery. J. Drug Deliv. Sci. Technol. 2024, 93, 105450. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.Y.; Hong, H.K.; Son, J.Y.; Kim, B.; Chung, J.Y.; Woo, S.J.; Park, K.D. Intravitreal Long-Term Sustained Ranibizumab Delivery Using Injectable Microgel-Embedded Hydrogel. Asian J. Pharm. Sci. 2024, 19, 100947. [Google Scholar] [CrossRef]
- Anumolu, S.N.S.; DeSantis, A.S.; Menjoge, A.R.; Hahn, R.A.; Beloni, J.A.; Gordon, M.K.; Sinko, P.J. Doxycycline Loaded Poly(Ethylene Glycol) Hydrogels for Healing Vesicant-Induced Ocular Wounds. Biomaterials 2010, 31, 964–974. [Google Scholar] [CrossRef]
- Xi, L.; Wang, T.; Zhao, F.; Zheng, Q.; Li, X.; Luo, J.; Liu, J.; Quan, D.; Ge, J. Evaluation of an Injectable Thermosensitive Hydrogel as Drug Delivery Implant for Ocular Glaucoma Surgery. PLoS ONE 2014, 9, e100632. [Google Scholar] [CrossRef]
- Annala, A.; Ilochonwu, B.C.; Wilbie, D.; Sadeghi, A.; Hennink, W.E.; Vermonden, T. Self-Healing Thermosensitive Hydrogel for Sustained Release of Dexamethasone for Ocular Therapy. ACS Polym. Au 2023, 3, 118–131. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, Y.; Tao, Y.; Lin, W.; Wang, P. Intra-Articular Drug Delivery for Osteoarthritis Treatment. Pharmaceutics 2021, 13, 2166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, J.; Deng, G.; Zhong, G.; Zhao, J.; Lan, Q.; Meng, J.; Yu, Y.; Chen, F. Microgel Encapsulated Nanoparticles for Intra-Articular Disulfiram Delivery to Treat Osteoarthritis. Mol. Pharm. 2024, 21, 87–101. [Google Scholar] [CrossRef]
- Mancipe Castro, L.M.; Sequeira, A.; García, A.J.; Guldberg, R.E. Articular Cartilage and Synoviocyte-Binding Poly(Ethylene Glycol) Nanocomposite Microgels as Intra-Articular Drug Delivery Vehicles for the Treatment of Osteoarthritis. ACS Biomater. Sci. Eng. 2020, 6, 5084–5095. [Google Scholar] [CrossRef]
- Said, M.; Tavakoli, C.; Dumot, C.; Toupet, K.; Olivier, C.; Gilles, A.; Maumus, M.; Dong, Y.C.; Collomb, N.; Auxenfans, C.; et al. A Self-Healing Radiopaque Hyaluronic Acid Hydrogel as a New Injectable Biomaterial for Precision Medicine in Osteoarthritis. Theranostics 2025, 15, 4054–4073. [Google Scholar] [CrossRef] [PubMed]
- Vinikoor, T.; Dzidotor, G.K.; Le, T.T.; Liu, Y.; Kan, H.M.; Barui, S.; Chorsi, M.T.; Curry, E.J.; Reinhardt, E.; Wang, H.; et al. Injectable and Biodegradable Piezoelectric Hydrogel for Osteoarthritis Treatment. Nat. Commun. 2023, 14, 6257. [Google Scholar] [CrossRef] [PubMed]
- Dubbelboer, I.R.; Sjögren, E. Overview of Authorized Drug Products for Subcutaneous Administration: Pharmaceutical, Therapeutic, and Physicochemical Properties. Eur. J. Pharm. Sci. 2022, 173, 106181. [Google Scholar] [CrossRef]
- Yadav, J.; Uddin, S.; Civati, F.; Ma, W.; Liebminger, A.; Teschner, W.; André, G.; Trout, B.L.; Braatz, R.D.; Myerson, A.S. Developing Ultra-High Concentration Formulations of Human Immune Globulins for Subcutaneous Injectables. J. Pharm. Sci. 2025, 114, 1605–1614. [Google Scholar] [CrossRef]
- Kang, N.W.; Yoon, S.Y.; Kim, S.; Yu, N.Y.; Park, J.H.; Lee, J.Y.; Cho, H.J.; Kim, D.D. Subcutaneously Injectable Hyaluronic Acid Hydrogel for Sustained Release of Donepezil with Reduced Initial Burst Release: Effect of Hybridization of Microstructured Lipid Carriers and Albumin. Pharmaceutics 2021, 13, 864. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Cui, J.; Zhu, Y.; Sun, M.; Hamley, I.W.; Xiao, C.; Chen, L. An Injectable Antibacterial Hydrogel with Bacterial-Targeting Properties for Subcutaneous Suppuration Treatment. Chem. Eng. J. 2024, 488, 151137. [Google Scholar] [CrossRef]
- Lo, Y.W.; Sheu, M.T.; Chiang, W.H.; Chiu, Y.L.; Tu, C.M.; Wang, W.Y.; Wu, M.H.; Wang, Y.C.; Lu, M.; Ho, H.O. In Situ Chemically Crosslinked Injectable Hydrogels for the Subcutaneous Delivery of Trastuzumab to Treat Breast Cancer. Acta Biomater. 2019, 86, 280–290. [Google Scholar] [CrossRef] [PubMed]
- McCartan, A.; Mackay, J.; Curran, D.; Mrsny, R.J. Modelling Intramuscular Drug Fate in Vitro with Tissue-Relevant Biomimetic Hydrogels. Int. J. Pharm. X 2022, 4, 100125. [Google Scholar] [CrossRef]
- Wei, J.; Xue, W.; Yu, X.; Qiu, X.; Liu, Z. PH Sensitive Phosphorylated Chitosan Hydrogel as Vaccine Delivery System for Intramuscular Immunization. J. Biomater. Appl. 2017, 31, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Kim, J.S.; Lee, H.C.; Cheon, S.; Woo, M.R.; Woo, S.; Ku, S.K.; Yoo, H.H.; Kim, J.O.; Jin, S.G.; et al. Injectable Dual Thermoreversible Hydrogel for Sustained Intramuscular Drug Delivery. J. Control. Release 2024, 374, 590–605. [Google Scholar] [CrossRef]
- Soltani, M.; Chen, P. Effect of Tumor Shape and Size on Drug Delivery to Solid Tumors. J. Biol. Eng. 2012, 6, 4. [Google Scholar] [CrossRef]
- Chen, X.; Qian, H.; Qiao, H.; Dong, B.; Chen, E.; Huang, D.; Wang, T.; Chen, W. Tumor-Adhesive and PH-Degradable Microgels by Microfluidics and Photo-Cross-Linking for Efficient Antiangiogenesis and Enhanced Cancer Chemotherapy. Biomacromolecules 2020, 21, 1285–1294. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.Q.; Xu, W.; Zhang, J.Y.; Liu, R.; Huang, Y.C.; Xiao, C.; Du, J.Z. An Injectable Nanocomposite Hydrogel Improves Tumor Penetration and Cancer Treatment Efficacy. Acta Biomater. 2022, 147, 235–244. [Google Scholar] [CrossRef]
- Sabino, I.J.; Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Injectable in Situ Forming Hydrogels Incorporating Dual-Nanoparticles for Chemo-Photothermal Therapy of Breast Cancer Cells. Int. J. Pharm. 2021, 600, 120510. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Qin, X.; Zhang, M.; Du, Q.; Luan, Y. An Injectable Hydrogel Reshaping Adenosinergic Axis for Cancer Therapy. Adv. Funct. Mater. 2022, 32, 2200801. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, L.; Zhu, Z.; Lu, H.; Shi, H.; Zhong, Q.; Oliveira, J.M.; Reis, R.L.; Gao, C.; Mao, Z. Conotoxin Loaded Dextran Microgel Particles Alleviate Effects of Spinal Cord Injury by Inhibiting Neuronal Excitotoxicity. Appl. Mater. Today 2021, 23, 101064. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Tang, X.; Meng, T.; Khutsishvili, D.; Xu, B.; Ma, S. CNS Organoid Surpasses Cell-Laden Microgel Assembly to Promote Spinal Cord Injury Repair. Research 2022, 2022, 9832128. [Google Scholar] [CrossRef]
- Kung, Y.; Chen, K.Y.; Liao, W.H.; Hsu, Y.H.; Wu, C.H.; Hsiao, M.Y.; Huang, A.P.H.; Chen, W.S. Facilitating Drug Delivery in the Central Nervous System by Opening the Blood-Cerebrospinal Fluid Barrier with a Single Low Energy Shockwave Pulse. Fluids Barriers CNS 2022, 19, 3. [Google Scholar] [CrossRef]
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Tsintou, M.; Dalamagkas, K.; Seifalian, A. Injectable Hydrogel versus Plastically Compressed Collagen Scaffold for Central Nervous System Applications. Int. J. Biomater. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, T.; Yang, S.; Doski, S.; Duncan, G.A. Hydrogels for Mucosal Drug Delivery. ACS Appl. Bio Mater. 2023, 6, 1684–1700. [Google Scholar] [CrossRef]
- Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Appl. Sci. 2019, 9, 1638. [Google Scholar] [CrossRef]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Cassano, R.; Di Gioia, M.L.; Trombino, S. Gel-Based Materials for Ophthalmic Drug Delivery. Gels 2021, 7, 130. [Google Scholar] [CrossRef]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal Hydrogels for Sustained Release of Therapeutic Proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Sunstrom, N.; Sunstrum, F.N. Wearable Devices for Subcutaneous Delivery of Large-Volume Biologics: Design, Use, and Regulatory Perspective. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable Thermoresponsive Hydrogels as Drug Delivery System for the Treatment of Central Nervous System Disorders: A Review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef]
- Wang, K.; Han, Z. Injectable Hydrogels for Ophthalmic Applications. J. Control. Release 2017, 268, 212–224. [Google Scholar] [CrossRef]
- Assiri, A.A.; Glover, K.; Mishra, D.; Waite, D.; Vora, L.K.; Thakur, R.R.S. Block Copolymer Micelles as Ocular Drug Delivery Systems. Drug Discov. Today 2024, 29, 104098. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Jain, S.; Mathur, R.; Mishra, P.; Mishra, A.K.; Velpandian, T. Sustained Ocular Drug Delivery from a Temperature and PH Triggered Novel in Situ Gel System. Drug Deliv. 2007, 14, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Bin Choy, Y.; Park, J.H.; Prausnitz, M.R. Mucoadhesive Microparticles Engineered for Ophthalmic Drug Delivery. J. Phys. Chem. Solids 2008, 69, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Fitaihi, R.; Abukhamees, S.; Orlu, M.; Craig, D.Q.M. Transscleral Delivery of Dexamethasone-Loaded Microparticles Using a Dissolving Microneedle Array. Pharmaceutics 2023, 15, 1622. [Google Scholar] [CrossRef]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic Nanoemulsions in Ophthalmic Drug Administration: Concept in Formulations and Characterization Techniques for Ocular Drug Delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, F.; Sheng, S.; Wei, Y.; Chen, X.; Su, J. Intra-Articular Nanodrug Delivery Strategies for Treating Osteoarthritis. Drug Discov. Today 2023, 28, 103482. [Google Scholar] [CrossRef]
- Kalairaj, M.S.; Pradhan, R.; Saleem, W.; Smith, M.M.; Gaharwar, A.K. Intra-Articular Injectable Biomaterials for Cartilage Repair and Regeneration. Adv. Healthc. Mater. 2024, 13, e2303794. [Google Scholar] [CrossRef]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-Articular Administration of Chitosan Thermosensitive in Situ Hydrogels Combined with Diclofenac Sodium-Loaded Alginate Microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained Low-Dose Dexamethasone Delivery via a PLGA Microsphere-Embedded Agarose Implant for Enhanced Osteochondral Repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef]
- Fang, W.; Yang, F.; Li, W.; Hu, Q.; Chen, W.; Yang, M.; Chen, J.; Qiu, L. Dexamethasone Microspheres and Celecoxib Microcrystals Loaded into Injectable Gels for Enhanced Knee Osteoarthritis Therapy. Int. J. Pharm. 2022, 622, 121802. [Google Scholar] [CrossRef]
- Shah, J.C.; Hong, J. Model for Long Acting Injectables (Depot Formulation) Based on Pharmacokinetics and Physical Chemical Properties. AAPS J. 2022, 24, 44. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Purslow, P.P. The Structure and Functional Significance of Variations in the Connective Tissue within Muscle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 947–966. [Google Scholar] [PubMed]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular Matrix: An Important Regulator of Cell Functions and Skeletal Muscle Development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G. Subcutaneous Administration of Therapeutic Monoclonal Antibody Drug Products Using a Syringe in Blinded Clinical Trials: Advances and Key Aspects Related to Blinding/Matching/Masking Strategies for Placebo Formulation. Mol. Pharm. 2025, 22, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Kittel, Y.; Kuehne, A.J.C.; De Laporte, L. Translating Therapeutic Microgels into Clinical Applications. Adv. Healthc. Mater. 2022, 11, e2101989. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Colaco, V.; Bandi, S.P.; Dhas, N.; Janardhanam, L.S.L.; Singh, S.; Vora, L.K. Stimuli-Responsive Self-Healing Ionic Gels: A Promising Approach for Dermal and Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2025, 11, 1338–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-Based in Situ Hydrogels for Controlled Delivery of Hydrophilic Macromolecules after Intramuscular Injection in Rats. Drug Deliv. 2015, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, J.; Jiang, L.; Luo, J.; Jing, L.; Li, X.; Jin, Y.; Dai, Z. Nanohybrid Liposomal Cerasomes with Good Physiological Stability and Rapid Temperature Responsiveness for High Intensity Focused Ultrasound Triggered Local Chemotherapy of Cancer. ACS Nano 2015, 9, 1280–1293. [Google Scholar] [CrossRef]
- She, W.; Li, N.; Luo, K.; Guo, C.; Wang, G.; Geng, Y.; Gu, Z. Dendronized Heparin-Doxorubicin Conjugate Based Nanoparticle as PH-Responsive Drug Delivery System for Cancer Therapy. Biomaterials 2013, 34, 2252–2264. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, Z.; Jamal, S.; Detamore, M.S.; Berkland, C. Hybrid Hydroxyapatite Nanoparticle Colloidal Gels Are Injectable Fillers for Bone Tissue Engineering. Tissue Eng. Part A 2013, 19, 2586–2593. [Google Scholar] [CrossRef]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Improvement of Survival in C6 Rat Glioma Model by a Sustained Drug Release from Localized PLGA Microspheres in a Thermoreversible Hydrogel. Int. J. Pharm. 2012, 427, 299–304. [Google Scholar] [CrossRef]
- Davis, A.J.; Tannock, I.F.; Tannock, I. Repopulation and Chemotherapy Review Repopulation of Tumour Cells between Cycles of Chemotherapy: A Neglected Factor. Lancet Oncol. 2000, 1, 86–93. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor Microenvironment Abnormalities: Causes, Consequences, and Strategies to Normalize. J. Cell Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Yang, G.; He, Y.; Li, X.; Du, T.; Xu, L.; Zhou, S. Uniformly Sized Hollow Microspheres Loaded with Polydopamine Nanoparticles and Doxorubicin for Local Chemo-Photothermal Combination Therapy. Chem. Eng. J. 2020, 379, 122317. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Ran, M.; Mustafa, R.A.; Luo, H.; Wang, J.; Smått, J.H.; Rosenholm, J.M.; Cui, W.; Lu, Y.; et al. Peritumoral Microgel Reservoir for Long-Term Light-Controlled Triple-Synergistic Treatment of Osteosarcoma with Single Ultra-Low Dose. Small 2021, 17, e2100479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Yan, M.; Feng, L.; Dong, S.; Hao, J. Drug Implants of Hydrogels via Collective Behavior of Microgel Colloids for On-Demand Cancer Therapy. ACS Appl. Bio Mater. 2019, 2, 1531–1541. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, J.; Xu, M.F.; Chen, J.; Gao, X.; Zhao, L.; Ding, F.; Wu, C.Z. One-Pot Preparation of PH- and Redox-Responsive Polymeric Microgel as an Efficient Carrier for Improved Breast Cancer Therapy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133320. [Google Scholar] [CrossRef]
- Zhai, M.; Wu, P.; Liao, Y.; Wu, L.; Zhao, Y. Polymer Microspheres and Their Application in Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 6556. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, T.L.; Zhang, H.J.; Gao, J.; Yang, P.F. A Promising Application of Injectable Hydrogels in Nerve Repair and Regeneration for Ischemic Stroke. Int. J. Nanomed. 2024, 19, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.K.; Kim, H.W. Clinical and Experimental Advances in Regeneration of Spinal Cord Injury. J. Tissue Eng. 2010, 1, 650857. [Google Scholar] [CrossRef]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef]
- Niu, G.; Jin, Z.; Zhang, C.; He, D.; Gao, X.; Zou, C.; Zhang, W.; Ding, J.; Das, B.C.; Severinov, K.; et al. An Effective Vaginal Gel to Deliver CRISPR/Cas9 System Encapsulated in Poly (β-Amino Ester) Nanoparticles for Vaginal Gene Therapy. EBioMedicine 2020, 58, 102897. [Google Scholar] [CrossRef]
- Permana, A.D.; Asri, R.M.; Amir, M.N.; Himawan, A.; Arjuna, A.; Juniarti, N.; Utami, R.N.; Mardikasari, S.A. Development of Thermoresponsive Hydrogels with Mucoadhesion Properties Loaded with Metronidazole Gel-Flakes for Improved Bacterial Vaginosis Treatment. Pharmaceutics 2023, 15, 1529. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Momoh, M.A.; Nnamani, P.O.; Umeyor, C.E.; Uronnachi, E.M.; Dias, M.L.; Ibezim, E.C.; Attama, A.A. Dual-Responsive Micellar Microgels Matrixed with Surface-Engineered Lipids: A New Approach for Controlled Vaginal Drug Delivery. J. Pharm. Innov. 2022, 17, 821–839. [Google Scholar] [CrossRef]
- Gosecka, M.; Gosecki, M. Antimicrobial Polymer-Based Hydrogels for the Intravaginal Therapies—Engineering Considerations. Pharmaceutics 2021, 13, 1393. [Google Scholar] [CrossRef]
- Feng, Q.; Li, D.; Li, Q.; Cao, X.; Dong, H. Microgel Assembly: Fabrication, Characteristics and Application in Tissue Engineering and Regenerative Medicine. Bioact. Mater. 2022, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.K.; Chien, Y.W. Hydrogel-Based Lontotherapeutic Delivery Devices for Transdermal Delivery of peptide/protein Drugs. Pharm. Res. 1993, 10, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.; Randhawa, G.; Kim, D.; Simpson, M.; Hoare, T. Microgels and Nanogels for the Delivery of Poorly Water-Soluble Drugs. Mol. Pharm. 2022, 19, 1704–1721. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication Methods of Biopolymeric Microgels and Microgel-Based Hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- McAllister, K.; Sazani, P.; Adam, M.; Cho, M.J.; Rubinstein, M.; Samulski, R.J.; DeSimone, J.M. Polymeric Nanogels Produced via Inverse Microemulsion Polymerization as Potential Gene and Antisense Delivery Agents. J. Am. Chem. Soc. 2002, 124, 15198–15207. [Google Scholar] [CrossRef]
- Sartipzadeh, O.; Naghib, S.M.; Haghiralsadat, F.; Shokati, F.; Rahmanian, M. Microfluidic-Assisted Synthesis and Modeling of Stimuli-Responsive Monodispersed Chitosan Microgels for Drug Delivery Applications. Sci. Rep. 2022, 12, 8382. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Russo, T.; Toto, E.; Santonicola, M.G. Fabrication of Alginate/Ozoile Gel Microspheres by Electrospray Process. Gels 2024, 10, 52. [Google Scholar] [CrossRef]
- Ahmed, H.; Stokke, B.T. Fabrication of Monodisperse Alginate Microgel Beads by Microfluidic Picoinjection: A Chelate Free Approach. Lab Chip 2021, 21, 2232–2243. [Google Scholar] [CrossRef]
- Chen, M.; Bolognesi, G.; Vladisavljević, G.T. Crosslinking Strategies for the Microfluidic Production of Microgels. Molecules 2021, 26, 3752. [Google Scholar] [CrossRef]
- Kim, H.U.; Lim, Y.J.; Lee, H.J.; Lee, N.J.; Bong, K.W. Degassed Micromolding Lithography for Rapid Fabrication of Anisotropic Hydrogel Microparticles with High-Resolution and High Uniformity. Lab Chip 2020, 20, 74–83. [Google Scholar] [CrossRef]
- Panda, P.; Ali, S.; Lo, E.; Chung, B.G.; Hatton, T.A.; Khademhosseini, A.; Doyle, P.S. Stop-Flow Lithography to Generate Cell-Laden Microgel Particles. Lab Chip 2008, 8, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.J.M.; Linkhorst, J.; Göttlich, T.; Savinsky, J.; Krüger, A.J.D.; De Laporte, L.; Wessling, M. Soft Temperature-Responsive Microgels of Complex Shape in Stop-Flow Lithography. Lab Chip 2020, 20, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Asadikorayem, M.; Weber, P.; Surman, F.; Puiggalí-Jou, A.; Zenobi-Wong, M. Foreign Body Immune Response to Zwitterionic and Hyaluronic Acid Granular Hydrogels Made with Mechanical Fragmentation. Adv. Healthc. Mater. 2025, 14, e2402890. [Google Scholar] [CrossRef] [PubMed]
- Hei, X.; Li, S.; Liu, Z.; Wu, C.; Ma, X.; Jiao, B.; Hu, H.; Zhu, J.; Adhikari, B.; Wang, Q.; et al. Characteristics of Pickering Emulsions Stabilized by Microgel Particles of Five Different Plant Proteins and Their Application. Food Chem. 2024, 449, 139187. [Google Scholar] [CrossRef]
- Duffy, C.; O’Sullivan, M.; Jacquier, J.C. Preparation of Novel Chitosan Iron Microgel Beads for Fortification Applications. Food Hydrocoll. 2018, 84, 608–615. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Liu, H.; Su, W.; Chen, W.; Qin, J. One-Step Generation of Core–Shell Gelatin Methacrylate (GelMA) Microgels Using a Droplet Microfluidic System. Adv. Mater. Technol. 2019, 4, 1800632. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, J.; Li, T.; Duan, R.; Xia, F.; Willner, I. Two-Photon Lithographic Patterning of DNA-Coated Single-Microparticle Surfaces. Nano Lett. 2019, 19, 618–625. [Google Scholar] [CrossRef]
- Naqvi, S.M.; Vedicherla, S.; Gansau, J.; McIntyre, T.; Doherty, M.; Buckley, C.T. Living Cell Factories—Electrosprayed Microcapsules and Microcarriers for Minimally Invasive Delivery. Adv. Mater. 2016, 28, 5662–5671. [Google Scholar] [CrossRef]
- Wang, H.; Deng, H.; Gao, M.; Zhang, W. Self-Assembled Nanogels Based on Ionic Gelation of Natural Polysaccharides for Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 703559. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Alginate-Based Emulsion Micro-Gel Particles Produced by an External/Internal O/W/O Emulsion-Gelation Method: Formation, Suspension Rheology, Digestion, and Application to Gel-in-Gel Beads. Food Hydrocoll. 2021, 120, 106926. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Jadach, B.; Wojtyłko, M.; Tatarek, A.; Białek, A.; Krysztofiak, J.; Gackowski, M.; Otto, F.; Osmałek, T. Ionotropic Gelation and Chemical Crosslinking as Methods for Fabrication of Modified-Release Gellan Gum-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 108. [Google Scholar] [CrossRef]
- Butté, A.; Storti, G.; Morbidelli, M. Microgel Formation in Emulsion Polymerization. Macromol. Theory Simul. 2007, 16, 441–457. [Google Scholar] [CrossRef]
- Widener, A.E.; Duraivel, S.; Angelini, T.E.; Phelps, E.A. Injectable Microporous Annealed Particle Hydrogel Based on Guest–Host-Interlinked Polyethylene Glycol Maleimide Microgels. Adv. Nanobiomed Res. 2022, 2, 2200030. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.; Sarkar, A. Design of Novel Emulsion Microgel Particles of Tuneable Size. Food Hydrocoll. 2017, 71, 47–59. [Google Scholar] [CrossRef]
- Torres, O.; Andablo-Reyes, E.; Murray, B.S.; Sarkar, A. Emulsion Microgel Particles as High-Performance Bio-Lubricants. ACS Appl. Mater. Interfaces 2018, 10, 26893–26905. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.; Murray, B.; Sarkar, A. Emulsion Microgel Particles: Novel Encapsulation Strategy for Lipophilic Molecules. Trends Food Sci. Technol. 2016, 55, 98–108. [Google Scholar] [CrossRef]
- Choi, Y.; Park, S.R.; Lee, S.J.; Choi, C.H. Microfluidic Production of Polyacrylic Acid Functionalized PEG Microgels for Efficient Biomolecular Conjugation. Front. Sens. 2022, 3, 1016791. [Google Scholar] [CrossRef]
- Torres, O.; Tena, N.M.; Murray, B.; Sarkar, A. Novel Starch Based Emulsion Gels and Emulsion Microgel Particles: Design, Structure and Rheology. Carbohydr. Polym. 2017, 178, 86–94. [Google Scholar] [CrossRef]
- Ngai, T.; Auweter, H.; Behrens, S.H. Environmental Responsiveness of Microgel Particles and Particle-Stabilized Emulsions. Macromolecules 2006, 39, 8171–8177. [Google Scholar] [CrossRef]
- Gong, J.; Su, Y.; Lei, J.; Zhu, S.; He, Y.; Tan, C.P.; Liu, Y.; Xu, Y.J. Construction and Characterization of Pickering Emulsion Gels Stabilized by β-Glucans Microgel Particles. Food Hydrocoll. 2024, 151, 109778. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.S.; Sarkar, A. Overcoming in Vitro Gastric Destabilisation of Emulsion Droplets Using Emulsion Microgel Particles for Targeted Intestinal Release of Fatty Acids. Food Hydrocoll. 2019, 89, 523–533. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, Z.; Meng, Z. Study on Oil Body Emulsion Gels Stabilized by Composited Polysaccharides through Microgel Particles Compaction and Natural Gelation. Food Hydrocoll. 2023, 135, 108146. [Google Scholar] [CrossRef]
- Lu, B.; Tarn, M.D.; Pamme, N.; Georgiou, T.K. Microfluidically Fabricated PH-Responsive Anionic Amphiphilic Microgels for Drug Release. J. Mater. Chem. B 2016, 4, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Wang, Q.; Zhang, Z.; Wang, H.; Wang, S.; Chi, Z.; Shang, L.; Wang, W.; Shu, Y. Microfluidic Preparation of Gelatin Methacryloyl Microgels as Local Drug Delivery Vehicles for Hearing Loss Therapy. ACS Appl. Mater. Interfaces 2022, 14, 46212–46223. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Bianchi, J.R.; Carvalho, B.G.; Carvalho, H.F.; de la Torre, L.G. Microfluidic-Based Gelatin Methacrylate Microgel as a Scaffold to Create Reverse-Polarity HT29 Spheroids. Int. J. Biol. Macromol. 2025, 305, 140824. [Google Scholar] [CrossRef]
- Chung, C.H.Y.; Cui, B.; Song, R.; Liu, X.; Xu, X.; Yao, S. Scalable Production of Monodisperse Functional Microspheres by Multilayer Parallelization of High Aspect Ratio Microfluidic Channels. Micromachines 2019, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rohne, F.; Vasquez-Muñoz, D.; Jung, S.H.; Lomadze, N.; Pich, A.; Santer, S.; Bekir, M. Selective Segregation of Thermo-Responsive Microgels via Microfluidic Technology. Small Methods 2024, 8, e2400226. [Google Scholar] [CrossRef]
- Seyfoori, A.; Seyyed Ebrahimi, S.A.; Samandari, M.; Samiei, E.; Stefanek, E.; Garnis, C.; Akbari, M. Microfluidic-Assisted CTC Isolation and In Situ Monitoring Using Smart Magnetic Microgels. Small 2023, 19, 2205320. [Google Scholar] [CrossRef]
- Seiffert, S.; Weitz, D.A. Microfluidic Fabrication of Smart Microgels from Macromolecular Precursors. Polymer 2010, 51, 5883–5889. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Ng, K. Electrospray Alginate-Konjac Glucomannan Microgel as Efficient Colon-Targeted Delivery Vehicle for Quercetin. Food Biosci. 2023, 56, 103307. [Google Scholar] [CrossRef]
- He, J.; Wang, Z.; Wei, L.; Ye, Y.; Din, Z.U.; Zhou, J.; Cong, X.; Cheng, S.; Cai, J. Electrospray-Assisted Fabrication of Dextran–Whey Protein Isolation Microcapsules for the Encapsulation of Selenium-Enriched Peptide. Foods 2023, 12, 1008. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.F.; Koocheki, A.; Ghorani, B.; Mohebbi, M. Formation of Alginate/Alyssum Homolocarpum Seed Gum (AHSG) Microgels through Electrospraying Technique to Encapsulate and Release Curcumin. Food Hydrocoll. Health 2024, 5, 100177. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Liu, Y.; Shao, C.; Bian, F.; Zhao, Y. Biomimetic Enzyme Cascade Reaction System in Microfluidic Electrospray Microcapsules. Sci. Adv. 2018, 4, eaat2816. [Google Scholar] [CrossRef]
- Liu, S.; Loo, Y.T.; Zhang, Y.; Ng, K. Electrospray Alginate Microgels Co-Encapsulating Degraded Konjac Glucomannan and Quercetin Modulate Human Gut Microbiota in Vitro. Food Chem. 2024, 434, 137508. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S.T.; Keam, S.J. Triamcinolone Acetonide Extended-Release: A Review in Osteoarthritis Pain of the Knee. Drugs 2019, 79, 455–462. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, Z.C.; Ding, Q.; Choi, J.J.; Ahmad, Z.; Chang, M.W.; Li, J.S. Tri-Needle Coaxial Electrospray Engineering of Magnetic Polymer Yolk-Shell Particles Possessing Dual-Imaging Modality, Multiagent Compartments, and Trigger Release Potential. ACS Appl. Mater. Interfaces 2017, 9, 21485–21495. [Google Scholar] [CrossRef]
- Paulsen, K.S.; Di Carlo, D.; Chung, A.J. Optofluidic Fabrication for 3D-Shaped Particles. Nat. Commun. 2015, 6, 6976. [Google Scholar] [CrossRef]
- Martino, C.; Vigolo, D.; Solvas, X.C.I.; Stavrakis, S.; deMello, A.J. Real-Time PEGDA-Based Microgel Generation and Encapsulation in Microdroplets. Adv. Mater. Technol. 2016, 1, 1600028. [Google Scholar] [CrossRef]
- Habasaki, S.; Lee, W.C.; Yoshida, S.; Takeuchi, S. Vertical Flow Lithography for Fabrication of 3D Anisotropic Particles. Small 2015, 11, 6391–6396. [Google Scholar] [CrossRef]
- Chai, N.; Zhang, J.; Zhang, Q.; Du, H.; He, X.; Yang, J.; Zhou, X.; He, J.; He, C. Construction of 3D Printed Constructs Based on Microfluidic Microgel for Bone Regeneration. Compos. B Eng. 2021, 223, 109100. [Google Scholar] [CrossRef]
- Doan, D.; Kulikowski, J.; Gu, X.W. Diffusion of Anisotropic Colloidal Microparticles Fabricated Using Two-Photon Lithography. Part. Part. Syst. Charact. 2021, 38, 2100033. [Google Scholar] [CrossRef]
- Keutgen, A.; Klein, I.; Shi, F.; Kuehne, A.J.C. Mesoscopic Supramolecular Assembly of Stereolithographically Printed Microgels. Adv. Funct. Mater. 2024, 34, 2310835. [Google Scholar] [CrossRef]
- Chen, Y.S.; Ke, L.Y.; Wei, S.Y.; Poddar, M.S.; Liu, C.H. Optofluidic Thin-Film Lithography for Photocrosslinking Hydrogel-Based Microarchitectures and the Assembling of Modular Cell-Embedded Microarchitectures. Sens. Actuators B Chem. 2022, 352, 131048. [Google Scholar] [CrossRef]
- Stubley, S.J.; Cayre, O.J.; Murray, B.S.; Torres, I.C.; Farrés, I.F. Enzyme Cross-Linked Pectin Microgel Particles for Use in Foods. Food Hydrocoll. 2021, 121, 107045. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Zhang, H.; Liu, T.; Wu, G. Fabrication of Soy Protein Microgels via Two Top-down Methods and Characterization of the Foaming Behavior. Food Biosci. 2024, 59, 103950. [Google Scholar] [CrossRef]
- Ishii, T.; Matsumiya, K.; Aoshima, M.; Matsumura, Y. Microgelation Imparts Emulsifying Ability to Surface-Inactive Polysaccharides—Bottom-up vs Top-down Approaches. NPJ Sci. Food 2018, 2, 15. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, Q.; Zhang, H. Microgel Particles of Chlorella Pyrenoidosa Protein to Fabricate Camellia Oil-Based Oleogels by Pickering Emulsion-Templated Approach. ACS Food Sci. Technol. 2024, 4, 1501–1510. [Google Scholar] [CrossRef]
- Lefroy, K.S.; Murray, B.S.; Ries, M.E.; Curwen, T.D. A Natural, Cellulose-Based Microgel for Water-in-Oil Emulsions. Food Hydrocoll. 2021, 113, 106408. [Google Scholar] [CrossRef]
- Siegel, R.A.; Nuxoll, E.E.; Hillmyer, M.A.; Ziaie, B. Top-Down and Bottom-Up Fabrication Techniques for Hydrogel Based Sensing and Hormone Delivery Microdevices. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 232–235. [Google Scholar]
- Polley, N.; Werner, P.; Balderas-Valadez, R.F.; Pacholski, C. Bottom, Top, or in Between: Combining Plasmonic Nanohole Arrays and Hydrogel Microgels for Optical Fiber Sensor Applications. Adv. Mater. Interfaces 2022, 9, 2102312. [Google Scholar] [CrossRef]
- Sharratt, W.N.; Lopez, C.G.; Sarkis, M.; Tyagi, G.; O’connell, R.; Rogers, S.E.; Cabral, J.T. Ionotropic Gelation Fronts in Sodium Carboxymethyl Cellulose for Hydrogel Particle Formation. Gels 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Jakubowska, E.; Jadach, B.; Gadziński, P.; Osmałek, T. Natural Gums in Drug-Loaded Micro- and Nanogels. Pharmaceutics 2023, 15, 759. [Google Scholar] [CrossRef]
- Smeraldo, A.; Ponsiglione, A.M.; Netti, P.A.; Torino, E. Tuning of Hydrogel Architectures by Ionotropic Gelation in Microfluidics: Beyond Batch Processing to Multimodal Diagnostics. Biomedicines 2021, 9, 1551. [Google Scholar] [CrossRef]
- Shen, C.; Liu, F.; Wu, L.; Yu, C.; Yu, W. Dripping, Jetting and Regime Transition of Droplet Formation in a Buoyancy-Assisted Microfluidic Device. Micromachines 2020, 11, 962. [Google Scholar] [CrossRef]
- Marra, F.; De Vivo, A.; Sarghini, F. Virtualization of Fluid-Dynamics in Micro-Air Assisted Extruders for Food Microfluidic Based Encapsulation. J. Food Eng. 2017, 213, 89–98. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Goshima, T.; Kai, T.; Kobaru, S.; Ohzuno, Y.; Nii, S.; Kiyoyama, S.; Yoshida, M.; Takei, T. Preparation of Alginate Hydrogel Beads on a Superhydrophobic Surface with Calcium Salt Powder to Enhance the Mechanical Strength and Encapsulation Efficiency of Ingredients. Materials 2024, 17, 6027. [Google Scholar] [CrossRef]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef]
- Casalini, T.; Perale, G. From Microscale to Macroscale: Nine Orders of Magnitude for a Comprehensive Modeling of Hydrogels for Controlled Drug Delivery. Gels 2019, 5, 28. [Google Scholar] [CrossRef]
- Ko, J. Multi-Functional Hydrogel Electrodes for Emerging Electronic and Robotic Applications. Korean J. Chem. Eng. 2023, 40, 3106–3129. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, H.J. Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology. Cells Tissues Organs 2025, 214, 128–147. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Teow, Y.H.; Takriff, M.S.; Ahmad, A.L.; Atieh, M.A. Recent Developments in Stimuli-Responsive Polymer for Emerging Applications: A Review. Results Eng. 2025, 25, 103900. [Google Scholar] [CrossRef]
- Kunene, S.C.; Lin, K.-S.; Weng, M.-T.; Carrera Espinoza, M.J.; Lin, Y.-S.; Wu, C.-M.; Tsai, W.-C. Dual Stimuli-Responsive Polymeric Microgels for Enhanced Doxorubicin Delivery to Hepatocellular Carcinoma. J. Drug Deliv. Sci. Technol. 2023, 87, 104776. [Google Scholar] [CrossRef]
- Morris, G.; Goodman, S.; Sorzabal Bellido, I.; Milanese, C.; Girella, A.; Pallavicini, P.; Taglietti, A.; Gaboardi, M.; Jäckel, F.; Diaz Fernandez, Y.A.; et al. Temperature and PH Stimuli-Responsive System Delivers Location-Specific Antimicrobial Activity with Natural Products. ACS Appl. Bio Mater. 2024, 7, 131–143. [Google Scholar] [CrossRef]
- Annegarn, M.; Dirksen, M.; Hellweg, T. Importance of Ph in Synthesis of Ph-Responsive Cationic Nano-and Microgels. Polymers 2021, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Dong, H.; Qiu, Y.; Chang, A.; Zhu, H. PH-Responsive Deacetylated Sphingan WL Gum-Based Microgels for the Oral Delivery of Ciprofloxacin Hydrochloride. ACS Omega 2024, 9, 46397–46407. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Suhail, M.; Li, X.R.; Liu, J.Y.; Hsieh, W.C.; Lin, Y.W.; Wu, P.C. Fabrication of Alginate Based Microgels for Drug-Sustained Release: In-Vitro and in-Vivo Evaluation. Int. J. Biol. Macromol. 2021, 192, 958–966. [Google Scholar] [CrossRef]
- Zengin, A.; Karakose, G.; Caykara, T. Poly(2-(Dimethylamino)Ethyl Methacrylate) Brushes Fabricated by Surface-Mediated RAFT Polymerization and Their Response to PH. Eur. Polym. J. 2013, 49, 3350–3358. [Google Scholar] [CrossRef]
- Amalvy, J.I.; Wanless, E.J.; Li, Y.; Michailidou, V.; Armes, S.P.; Duccini, Y. Synthesis and Characterization of Novel PH-Responsive Microgels Based on Tertiary Amine Methacrylates. Langmuir 2004, 20, 8992–8999. [Google Scholar] [CrossRef]
- Klinger, D.; Landfester, K. Stimuli-Responsive Microgels for the Loading and Release of Functional Compounds: Fundamental Concepts and Applications. Polymer 2012, 53, 5209–5231. [Google Scholar] [CrossRef]
- Rashid, Z.; Ranjha, N.M.; Rashid, F.; Raza, H. Pharmacokinetic Evaluation of Microgels for Targeted and Sustained Delivery of Acid Labile Active Pharmaceutical Agent in Animal Model. J. Drug Deliv. Sci. Technol. 2020, 57, 101770. [Google Scholar] [CrossRef]
- Ishak, W.H.W.; Jia, O.Y.; Ahmad, I. PH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers 2020, 12, 1932. [Google Scholar] [CrossRef]
- Chen, K.; Wang, J.; Cao, J.; Liu, F.; Fang, J.; Zheng, W.; Liu, S.; Zhao, Y.; Shuai, X.; Huang, J.; et al. Enzyme-Responsive Microgel with Controlled Drug Release, Lubrication and Adhesion Capability for Osteoarthritis Attenuation. Acta Biomater. 2024, 190, 191–204. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, E.J.; Lim, J.H.; Cho, H.K.; Hong, W.J.; Jeon, H.H.; Chung, B.G. Dual Stimuli-Triggered Nanogels in Response to Temperature and PH Changes for Controlled Drug Release. Nanoscale Res. Lett. 2019, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Schneider, S.; Plamper, F.; Pich, A. Responsive Supramolecular Microgels with Redox-Triggered Cleavable Crosslinks. Macromolecules 2020, 53, 1043–1053. [Google Scholar] [CrossRef]
- Hu, C.; Sun, Y.; van Wissen, G.; Peng, Y.; Pich, A. Visible Light-Responsive Microgels Modified with Donor-Acceptor Stenhouse Adducts. Chem. Mater. 2022, 34, 4774–4784. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-Responsive Polymer Hydrogels for Therapeutic Delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix Metalloproteinases as Novel Biomarkers and Potential Therapeutic Targets in Human Cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef]
- Schötz, S.; Griepe, A.K.; Goerisch, B.B.; Kortam, S.; Vainer, Y.S.; Dimde, M.; Koeppe, H.; Wedepohl, S.; Quaas, E.; Achazi, K.; et al. Esterase-Responsive Polyglycerol-Based Nanogels for Intracellular Drug Delivery in Rare Gastrointestinal Stromal Tumors. Pharmaceuticals 2023, 16, 1618. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, G.; Zhan, W.; Wang, J.; Wang, C.; Yue, Q.Y.; Huang, Z.; Wang, Y. Hyaluronidase-Responsive Hydrogel Loaded with Magnetic Nanoparticles Combined with External Magnetic Stimulation for Spinal Cord Injury Repair. Mater. Today Bio 2025, 30, 101378. [Google Scholar] [CrossRef]
- Brouns, J.E.P.; Dankers, P.Y.W. Introduction of Enzyme-Responsivity in Biomaterials to Achieve Dynamic Reciprocity in Cell-Material Interactions. Biomacromolecules 2021, 22, 4–23. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, J.; Tu, M.; Zeng, R.; Rong, J. Hyaluronan Microgel as a Potential Carrier for Protein Sustained Delivery by Tailoring the Crosslink Network. Mater. Sci. Eng. C 2014, 36, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, G.; Su, W.K.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-Tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef]
- Torres-Herrero, B.; Armenia, I.; Ortiz, C.; de la Fuente, J.M.; Betancor, L.; Grazú, V. Opportunities for Nanomaterials in Enzyme Therapy. J. Control. Release 2024, 372, 619–647. [Google Scholar] [CrossRef]
- Wei, Q.; Jiang, S.; Zhu, R.; Wang, X.; Wang, S.; Wang, Q. Injectable Peptide Hydrogel Enables Integrated Tandem Enzymes’ Superactivity for Cancer Therapy. iScience 2019, 14, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Tang, Y.; He, X.; Liu, K.; Qin, L.; Wang, X.; Wang, Q. Enzyme-Integrated Hydrogels for Advanced Biological Applications. Polym. Sci. Technol. 2025. [Google Scholar] [CrossRef]
- Kaniewska, K.; Mackiewicz, M.; Smutok, O.; Gonchar, M.; Katz, E.; Karbarz, M. Enzymatically Triggered Drug Release from Microgels Controlled by Glucose Concentration. ACS Biomater. Sci. Eng. 2024, 10, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Wang, B.; Kokufuta, E. Enzyme-Regulated Microgel Collapse for Controlled Membrane Permeability. Langmuir 2001, 17, 4704–4707. [Google Scholar] [CrossRef]
- Rosi, B.P.; Tavagnacco, L.; Comez, L.; Sassi, P.; Ricci, M.; Buratti, E.; Bertoldo, M.; Petrillo, C.; Zaccarelli, E.; Chiessi, E.; et al. Thermoresponsivity of Poly(N-Isopropylacrylamide) Microgels in Water-Trehalose Solution and Its Relation to Protein Behavior. J. Colloid. Interface Sci. 2021, 604, 705–718. [Google Scholar] [CrossRef]
- Sanzari, I.; Buratti, E.; Huang, R.; Tusan, C.G.; Dinelli, F.; Evans, N.D.; Prodromakis, T.; Bertoldo, M. Poly(N-Isopropylacrylamide) Based Thin Microgel Films for Use in Cell Culture Applications. Sci. Rep. 2020, 10, 6126. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Marcelo, G.; Areias, L.R.P.; Viciosa, M.T.; Martinho, J.M.G.; Farinha, J.P.S. PNIPAm-Based Microgels with a UCST Response. Polymer 2017, 116, 261–267. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive Nanocomposite Hydrogels Based on Gelatin/Poly (N–Isopropylacrylamide) (PNIPAM) for Controlled Drug Delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Shang, Y.; Yi, X.; Xiang, D.; Zhou, L. Nanoagent-Mediated Photothermal Therapy: From Delivery System Design to Synergistic Theranostic Applications. Int. J. Nanomed. 2025, 20, 6891–6927. [Google Scholar] [CrossRef]
- Welsch, N.; Wittemann, A.; Ballauff, M. Enhanced Activity of Enzymes Immobilized in Thermoresponsive Core-Shell Microgels. J. Phys. Chem. B 2009, 113, 16039–16045. [Google Scholar] [CrossRef]
- Fan, X.; Teng, C.P.; Yeo, J.C.C.; Li, Z.; Wang, T.; Chen, H.; Jiang, L.; Hou, X.; He, C.; Liu, J. Temperature and PH Responsive Light-Harvesting System Based on AIE-Active Microgel for Cell Imaging. Macromol. Rapid. Commun. 2021, 42, 2000716. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Tavano, L.; Muzzalupo, R. Thermo-Sensitive Vesicles in Controlled Drug Delivery for Chemotherapy. Pharmaceutics 2018, 10, 150. [Google Scholar] [CrossRef]
- Winning, D.; Wychowaniec, J.K.; Wu, B.; Heise, A.; Rodriguez, B.J.; Brougham, D.F. Thermoresponsiveness Across the Physiologically Accessible Range: Effect of Surfactant, Cross-Linker, and Initiator Content on Size, Structure, and Transition Temperature of Poly(N-Isopropylmethacrylamide) Microgels. ACS Omega 2024, 9, 36185–36197. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in Redox-Responsive Drug Delivery Systems of Tumor Microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Dagdelen, S.; Marcisz, K.; Waleka-Bargiel, E.; Stojek, Z.; Karbarz, M. Redox-Degradable Microgel Based on Poly(Acrylic Acid) as Drug-Carrier with Very High Drug-Loading Capacity and Decreased Toxicity against Healthy Cells. Polym. Degrad. Stab. 2021, 190, 109652. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P. Synthesis Strategies for Disulfide Bond-Containing Polymer-Based Drug Delivery System for Reduction-Responsive Controlled Release. Front. Mater. Sci. 2015, 9, 211–226. [Google Scholar] [CrossRef]
- Shee, M.; Lal Banerjee, S.; Dey, A.; Das Jana, I.; Basak, P.; Mandal, M.; Mondal, A.; Kumar Das, A.; Das, N.C. PH-Induced Fluorescent Active Sodium Alginate-Based Ionically Conjugated and REDOX Responsive Multi-Functional Microgels for the Anticancer Drug Delivery. Int. J. Pharm. 2024, 662, 124490. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ling, L.; Ismail, M.; He, W.; Xia, Q.; Zhou, W.; Yao, C.; Li, X. Redox Sensitive Lipid-Camptothecin Conjugate Encapsulated Solid Lipid Nanoparticles for Oral Delivery. Int. J. Pharm. 2018, 549, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Radecka, I.; Trzebicka, B.; Jiao, C.; Obst, F.; Geisler, M.; Che, Y.; Richter, A.; Appelhans, D.; Gaitzsch, J.; Voit, B. Reversible Protein Capture and Release by Redox-Responsive Hydrogel in Microfluidics. Polymers 2022, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Dagdelen, S.; Mackiewicz, M.; Osial, M.; Waleka-Bargiel, E.; Romanski, J.; Krysinski, P.; Karbarz, M. Redox-Responsive Degradable Microgel Modified with Superparamagnetic Nanoparticles Exhibiting Controlled, Hyperthermia-Enhanced Drug Release. J. Mater. Sci. 2023, 58, 4094–4114. [Google Scholar] [CrossRef]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-Sensitive Polymers and Bioconjugates for Biomedical Applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Wang, W.; Su, Y.Q.; Hensen, E.J.M.; Serpe, M.J. Biological Imaging and Sensing with Multiresponsive Microgels. Chem. Mater. 2016, 28, 259–265. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, J.; Liu, X.; Cao, P.; Song, P.; Wang, R.; Xiong, Y. Poly(Ionic Liquid)-Based Nanogels and Their Reversible Photo-Mediated Association and Dissociation. Polym. Chem. 2017, 8, 1146–1154. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR Light-Responsive Nanocarriers for Controlled Release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Schimka, S.; Lomadze, N.; Rabe, M.; Kopyshev, A.; Lehmann, M.; Von Klitzing, R.; Rumyantsev, A.M.; Kramarenko, E.Y.; Santer, S. Photosensitive Microgels Containing Azobenzene Surfactants of Different Charges. Phys. Chem. Chem. Phys. 2017, 19, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Colaco, R.; Appiah, C.; Staubitz, A. Controlling the LCST-Phase Transition in Azobenzene-Functionalized Poly (N-Isopropylacrlyamide) Hydrogels by Light. Gels 2023, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Schimka, S.; Gordievskaya, Y.D.; Lomadze, N.; Lehmann, M.; Von Klitzing, R.; Rumyantsev, A.M.; Kramarenko, E.Y.; Santer, S. Communication: Light Driven Remote Control of Microgels’ Size in the Presence of Photosensitive Surfactant: Complete Phase Diagram. J. Chem. Phys. 2017, 147, 031101. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Bradley, M.; Vincent, B. Photo-Responsive Properties of Poly(NIPAM-Co-AAc) Microgel Particles with Absorbed, Hydrophobically Modified Organic Salts. J. Colloid Interface Sci. 2012, 368, 287–291. [Google Scholar] [CrossRef]
- Dolgopolov, A.V.; Grafskaia, K.N.; Bovsunovskaya, P.V.; Melnikova, E.R.; Ivanov, D.A.; Pich, A.; Zhu, X.; Möller, M. Aqueous Microgels Modified with Photosensitive Wedge-Shaped Amphiphilic Molecules: Synthesis, Structure and Photochemical Behaviour. Photochem. Photobiol. Sci. 2019, 18, 1709–1715. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, M.; Wu, S.; Wang, W.; Lian, Q.; Saunders, B.R. Triply Responsive Coumarin-Based Microgels with Remarkably Large Photo-Switchable Swelling. Polym. Chem. 2019, 10, 2516–2526. [Google Scholar] [CrossRef]
- Jangizehi, A.; Eckhardt, J.; Borkowska, K.I.; Dallos, Z.; Bauer, M.; Salehi, H.; Razavi, R.; Shakeri, A.; Hashemifard, S.A.; Seiffert, S. Thermo- and Light Responsive Microgels for Efficient Brackish and Seawater Forward Osmosis Desalination. Desalination 2025, 595, 118314. [Google Scholar] [CrossRef]
- Klinger, D.; Landfester, K. Photo-Sensitive PMMA Microgels: Light-Triggered Swelling and Degradation. Soft Matter 2011, 7, 1426–1440. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Jia, N.; Sotto, A.; Zhao, Y.; Shen, J.; Gao, C.; Van Der Bruggen, B. Engineering of Thermo-/PH-Responsive Membranes with Enhanced Gating Coefficients, Reversible Behaviors and Self-Cleaning Performance through Acetic Acid Boosted Microgel Assembly. J. Mater. Chem. A Mater. 2018, 6, 11874–11883. [Google Scholar] [CrossRef]
- Ruiz, A.L.; Ramirez, A.; McEnnis, K. Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery. Pharmaceutics 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, Q.; Wu, M.; Wang, W.; Gao, Y.; Liu, X.; Sun, Y.; Yang, D.; Peng, Q.; Wang, T.; et al. A PH and Temperature Dual-Responsive Microgel-Embedded, Adhesive, and Tough Hydrogel for Drug Delivery and Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 19560–19573. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Gordon, M.R.; Ventura, J.; Li, L.; Thayumanavan, S. Multi-Stimuli Responsive Macromolecules and Their Assemblies. Chem. Soc. Rev. 2013, 42, 7421–7435. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Kim, D.K.; Kalva, N.; Eom, K.H.; Kim, J.H.; Kim, I. Multi-Stimuli-Responsive Nanomicelles Fabricated Using Synthetic Polymer Polylysine Conjugates for Tumor Microenvironment Dependent Drug Delivery. J. Mater. Chem. B 2020, 8, 5745–5755. [Google Scholar] [CrossRef]
- Alkanawati, M.S.; Machtakova, M.; Landfester, K.; Thérien-Aubin, H. Bio-Orthogonal Nanogels for Multiresponsive Release. Biomacromolecules 2021, 22, 2976–2984. [Google Scholar] [CrossRef]
- Xiang, D.; Wu, X.; Cao, W.; Xue, B.; Qin, M.; Cao, Y.; Wang, W. Hydrogels with Tunable Mechanical Properties Based on Photocleavable Proteins. Front. Chem. 2020, 8, 7. [Google Scholar] [CrossRef]
- Huynh, C.T.; Nguyen, M.K.; Tonga, G.Y.; Longé, L.; Rotello, V.M.; Alsberg, E. Photocleavable Hydrogels for Light-Triggered SiRNA Release. Adv. Healthc. Mater. 2016, 5, 305–310. [Google Scholar] [CrossRef]
- Han, I.K.; Chung, T.; Han, J.; Kim, Y.S. Nanocomposite Hydrogel Actuators Hybridized with Various Dimensional Nanomaterials for Stimuli Responsiveness Enhancement. Nano Converg. 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Kim, J.W.; Fernández-Nieves, A.; Weitz, D.A. Highly Responsive Hydrogel Scaffolds Formed by Three-Dimensional Organization of Microgel Nanoparticles. Nano Lett. 2008, 8, 168–172. [Google Scholar] [CrossRef]
- Xiao, X.; Ji, J.; Wang, H.; Nangia, S.; Wang, H.; Libera, M. Self-Defensive Antimicrobial Surfaces Using Polymyxin-Loaded Poly(Styrene Sulfonate) Microgels. ACS Biomater. Sci. Eng. 2022, 8, 4827–4837. [Google Scholar] [CrossRef]
- Spencer, D.S.; Shodeinde, A.B.; Beckman, D.W.; Luu, B.C.; Hodges, H.R.; Peppas, N.A. Cytocompatibility, Membrane Disruption, and SiRNA Delivery Using Environmentally Responsive Cationic Nanogels. J. Control. Release 2021, 332, 608–619. [Google Scholar] [CrossRef]
- Ma, J.; He, X.; Wang, L.; Pang, J. Magnetic Fucoidan Aerogel Microspheres: Cationic Drug Loading Kinetics and Electrostatic-Magnetic Synergy. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137843. [Google Scholar] [CrossRef]
- Hriberšek, P.; Kogej, K. Effect of Multivalent Cations on Intermolecular Association of Isotactic and Atactic Poly(Methacrylic Acid) Chains in Aqueous Solutions. Polymers 2019, 11, 605. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Advances in the Development of Cyclodextrin-Based Nanogels/Microgels for Biomedical Applications: Drug Delivery and Beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef]
- Pillarisetti, S.; Vijayan, V.; Rangasamy, J.; Bardhan, R.; Uthaman, S.; Park, I.K. A Multi-Stimuli Responsive Alginate Nanogel for Anticancer Chemo-Photodynamic Therapy. J. Ind. Eng. Chem. 2023, 123, 361–370. [Google Scholar] [CrossRef]
- Lei, B.; Chen, M.; Wang, Y.; Zhang, J.; Xu, S.; Liu, H. Double Security Drug Delivery System DDS Constructed by Multi-Responsive (PH/Redox/US) Microgel. Colloids Surf. B Biointerfaces 2020, 193, 111022. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody–Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, Q.; Ren, P.; Liu, Q.; Zhang, N.; Ji, Y.; Liu, J. Zinc Ions Coordinated Carboxymethyl Chitosan-Hyaluronic Acid Microgel for Pulmonary Drug Delivery. Int. J. Biol. Macromol. 2021, 193, 1043–1049. [Google Scholar] [CrossRef]
- Al-Tikriti, Y.; Hansson, P. Drug-Eluting Polyacrylate Microgels: Loading and Release of Amitriptyline. J. Phys. Chem. B 2020, 124, 2289–2304. [Google Scholar] [CrossRef]
- Gao, Y.; Wong, K.Y.; Ahiabu, A.; Serpe, M.J. Sequential and Controlled Release of Small Molecules from Poly(N -Isopropylacrylamide) Microgel-Based Reservoir Devices. J. Mater. Chem. B 2016, 4, 5144–5150. [Google Scholar] [CrossRef] [PubMed]
- Birlik Demirel, G.; Bayrak, Ş. Ultrasound/Redox/PH-Responsive Hybrid Nanoparticles for Triple-Triggered Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 71, 103267. [Google Scholar] [CrossRef]
- Lin, X.; Deng, S.; Fu, T.; Lei, Y.; Wang, Y.; Yao, J.; Lu, Y.; Huang, Y.; Shang, J.; Chen, J.; et al. Hyaluronic Acid-Based Hydrogel Microspheres with Multi-Responsive Properties for Antibacterial Therapy and Bone Regeneration in Staphylococcus Aureus-Infected Skull Defects. Mater. Today Bio 2025, 32, 101676. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and Recent Advances in Peptide and Protein Drug Delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Abune, L.; Wang, Y. Affinity Hydrogels for Protein Delivery. Trends Pharmacol. Sci. 2021, 42, 300–312. [Google Scholar] [CrossRef]
- Chen, L.; Khan, S.A. Programmable Protein Delivery from Microgel/Hydrogel Composites (MHCs) via Discrete Combinations of Multi-State Protein-Loaded Unit Ingredients. RSC Pharm. 2024, 1, 689–704. [Google Scholar] [CrossRef]
- Li, J.; Weber, E.; Guth-Gundel, S.; Schuleit, M.; Kuttler, A.; Halleux, C.; Accart, N.; Doelemeyer, A.; Basler, A.; Tigani, B.; et al. Tough Composite Hydrogels with High Loading and Local Release of Biological Drugs. Adv. Healthc. Mater. 2018, 7, e1701393. [Google Scholar] [CrossRef]
- Song, L.; Zheng, W.; Wang, S.; Zhai, Z.; Li, S.; Ding, J.; Shen, L.; Zhang, J.; Zhu, Y.; Gao, C. ROS-Responsive Core–Shell Microgels for Phase-Specific Treatment of Myocardial Infarction via Programmed Drug Delivery. Chem. Eng. J. 2025, 507, 160295. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Dagdelen, S.; Waleka-Bargiel, E.; Karbarz, M. A Polyampholyte Core-Shell Microgel as an Environmentally Sensitive Drug Carrier. Arab. J. Chem. 2024, 17, 105464. [Google Scholar] [CrossRef]
- Koscielniak, P.; Sawicka, M.; Sterin, I.; Marcisz, K.; Kaniewska, K.; Karbarz, M.; Katz, E.; Smutok, O. Cystamine-Crosslinked and Nanozyme Decorated Polyacrylic Acid-Based Composite Microgel of Dual Functionality: Delivery and Controlled Doxorubicin Release. Appl. Surf. Sci. Adv. 2025, 27, 100773. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.X.; Zhong, H.; Li, X.R.; Jun, Y.L.; Wang, Q.L.; Ding, L.S.; Cheng, Z.P.; Qian, H.Y. Folic Acid Conjugated Palygorskite/Au Hybrid Microgels: Temperature, PH and Light Triple-Responsive and Its Application in Drug Delivery. Colloids Surf. B Biointerfaces 2023, 229, 113432. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the Delivery of RNA Therapeutics: From Concept to Clinical Reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a Bioactive Material to Regulate Stem Cell Fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Han, Y.; Li, J.; Huang, Y.; Yu, D.; Liu, Y.; Wang, Y.; Fan, C.; Huang, Y.; Guan, W.; et al. Topical Delivery of Cultivated Limbal Stem Cells via Porous Photocurable Hydrogel: A Noninvasive, Convenient, and Efficient Stem Cells Delivery Strategy for Limbal Stem Cell Deficiency. J. Control. Release 2025, 384, 113900. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chan, A.; Morad, L.; Kornblum, H.I.; Fan, G.; Carmichael, S.T. Hydrogel Matrix to Support Stem Cell Survival after Brain Transplantation in Stroke. Neurorehabil. Neural Repair 2010, 24, 636–644. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, D.; Mai, H.; Chen, R.; Xu, Y.; Lei, M.; Xie, J.; Tang, Z.; Fu, J.; Chen, Y.; et al. Injectable Thermo-Responsive Poloxamer Hydrogel/Methacrylate Gelatin Microgels Stimulates Bone Regeneration through Biomimetic Programmed Release of SDF-1a and IGF-1. Int. J. Biol. Macromol. 2024, 271, 132742. [Google Scholar] [CrossRef]
- Sponchioni, M.; O’Brien, C.T.; Borchers, C.; Wang, E.; Rivolta, M.N.; Penfold, N.J.W.; Canton, I.; Armes, S.P. Probing the Mechanism for Hydrogel-Based Stasis Induction in Human Pluripotent Stem Cells: Is the Chemical Functionality of the Hydrogel Important? Chem. Sci. 2020, 11, 232–240. [Google Scholar] [CrossRef]
- Steinle, H.; Ionescu, T.M.; Schenk, S.; Golombek, S.; Kunnakattu, S.J.; Özbek, M.T.; Schlensak, C.; Wende, H.P.; Avci-Adali, M. Incorporation of Synthetic MRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-Based Biohybrid Injectable Hydrogel for Cardiac Tissue Engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Huang, Y.; Li, J.; Dong, R.; Yun, X.; Ren, Y.; Liu, X.; Hui, H.; Wu, L.; et al. Thermal-Responsive Microgels Incorporated PVA Composite Hydrogels: Integration of Two-Stage Drug Release and Enhanced Self-Healing Ability for Chronic Wound Treatment. Chem. Eng. J. 2025, 506, 159813. [Google Scholar] [CrossRef]
| Target Site/Route | Clinical/Application Context | Injection Volume/Depot Limits | Formulation Considerations | Refs. |
|---|---|---|---|---|
| Ocular (topical, subconjunctival, intravitreal) | Dry eye, conjunctivitis, uveitis, age-related macular degeneration, diabetic retinopathy | Limited ocular retention; intravitreal ≤ 50 µL | Mucoadhesion for surface retention; vitreous depot stability; optical clarity | [38,39,40,41,42] |
| Intra-articular | Osteoarthritis, rheumatoid arthritis, inflammatory joint diseases | 1–2 mL typical; high shear; rapid synovial turnover | Shear-compliant, bioadhesive; tunable degradation; cartilage penetration | [43,44,45,46,47] |
| Subcutaneous (SC) | Monoclonal antibodies, insulin analogs, long-acting biologics | ≤1.5 mL; enzymatic degradation; lymphatic clearance | In situ gelling depots; resistance to lymphatic clearance; soft mechanics | [8,48,49,50,51,52] |
| Intramuscular (IM) | Hormone analogs, antipsychotics, long-acting injectables, vaccines | ≤5 mL tolerated; dense ECM; anisotropic spread | ECM-mimetic stiffness; reproducible distribution; stability under contractile stress | [22,53,54,55] |
| Tumoral/Peritumoral injection | Intratumoral ablation; localized immunotherapy; stromal targeting | Tumor-size dependent; irregular ECM; proximity to vasculature | Bio-responsive hydrogels; sustained retention; stromal-matched mechanics; prevent burst release | [56,57,58,59,60,61] |
| Central Nervous System | Spinal cord injury, glioma, neuropathic pain, neuroinflammation | ≤100 µL; reflux risk; rapid CSF turnover | Soft, conformal hydrogels; resist CSF clearance; controlled degradation | [62,63,64,65,66] |
| Mucosal (nasal, vaginal, rectal) | Anti-infective, anti-inflammatory, vaccine, supportive therapies | ≤1 mL; clearance by mucus, cilia, or peristalsis | Mucoadhesion; pH/enzymatic responsiveness; depot formation in confined space | [13,67,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Kim, M.; Kim, N.; Byun, S.; Seo, S.; Han, J.Y. Injectable Hydrogel Systems for Targeted Drug Delivery: From Site-Specific Application to Design Strategy. Appl. Sci. 2025, 15, 11599. https://doi.org/10.3390/app152111599
Lee Y, Kim M, Kim N, Byun S, Seo S, Han JY. Injectable Hydrogel Systems for Targeted Drug Delivery: From Site-Specific Application to Design Strategy. Applied Sciences. 2025; 15(21):11599. https://doi.org/10.3390/app152111599
Chicago/Turabian StyleLee, Yeji, Minji Kim, Nurihan Kim, Seonyeong Byun, Soonmin Seo, and Jung Y. Han. 2025. "Injectable Hydrogel Systems for Targeted Drug Delivery: From Site-Specific Application to Design Strategy" Applied Sciences 15, no. 21: 11599. https://doi.org/10.3390/app152111599
APA StyleLee, Y., Kim, M., Kim, N., Byun, S., Seo, S., & Han, J. Y. (2025). Injectable Hydrogel Systems for Targeted Drug Delivery: From Site-Specific Application to Design Strategy. Applied Sciences, 15(21), 11599. https://doi.org/10.3390/app152111599






