The Dual Role of Metformin: Repurposing an Antidiabetic Drug for Cancer Therapy
Abstract
1. Introduction
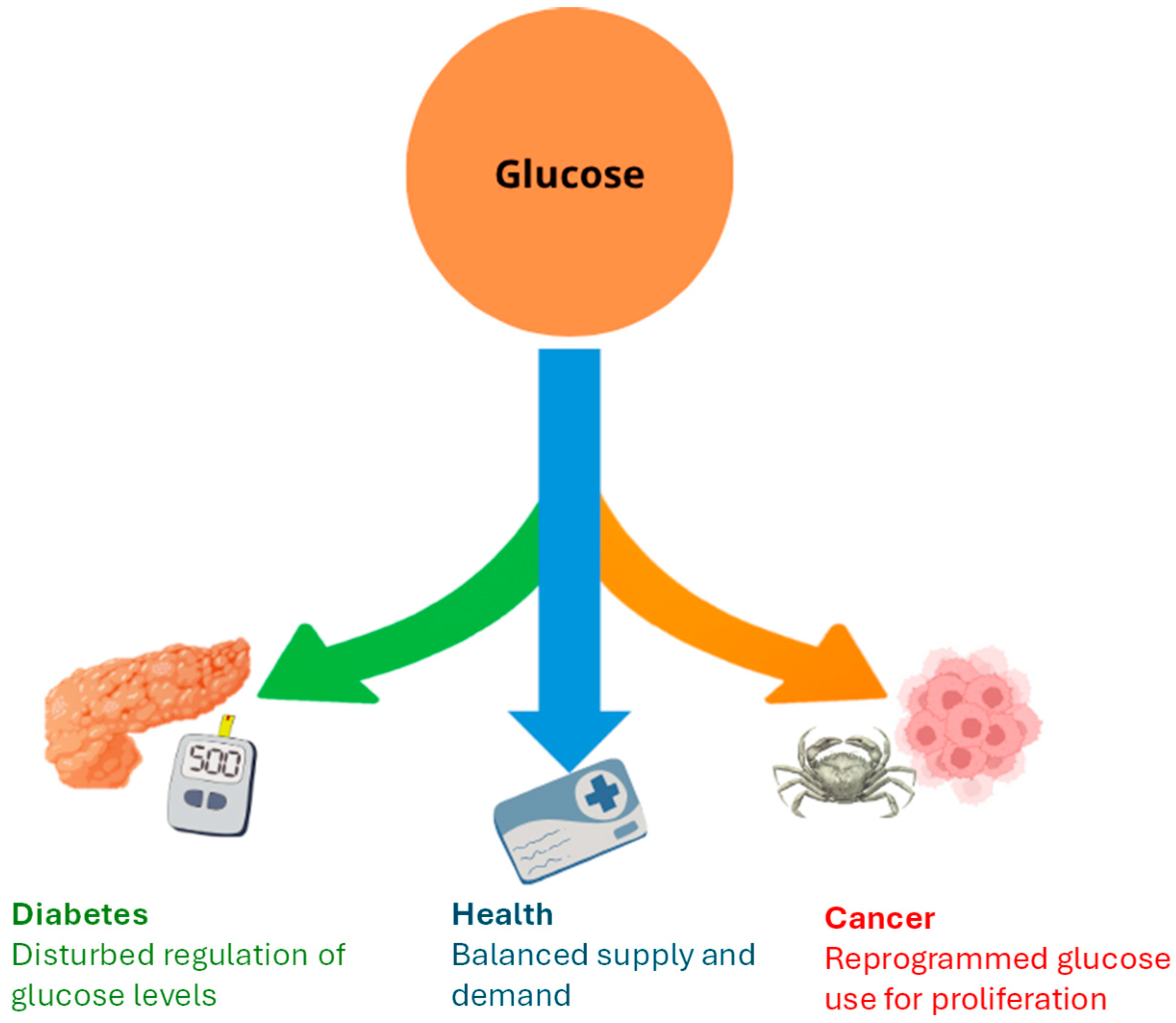
2. Disturbance of Glucose Homeostasis in Diabetes and Cancer
2.1. Diabetes
2.1.1. Insulin Signaling Pathway, PI3K/Akt Signaling, and Glucose Metabolism
2.1.2. Molecular Mechanisms of T2D Pathogenesis
2.1.3. T2D Treatment
2.2. Cancer
3. Metformin Targets and Mechanism of Action
3.1. Metformin Targets and Mechanism of Action as an Antidiabetic Drug
3.1.1. Metformin and Gut Microbiome
3.1.2. Metformin and Obesity
3.2. Metformin Targets and Mechanism of Action as an Anticancer Drug
3.2.1. Metformin-Induced Alterations in ROS Metabolism in Cancer Cells
3.2.2. The Role of PI3K/AKT/mTOR Pathway in Mediating the Anticancer Effects of Metformin
3.2.3. Metformin-Based Strategies to Enhance Antitumor Therapy
3.2.4. Metformin Encapsulation and Nanoparticle Delivery
3.2.5. Metformin in Combination Therapies
4. Clinical Trials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (U.S.). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998; Volume 97, pp. 20–21+24–25+27–37. [Google Scholar]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Cramer, M.J.; de Borst, G.J.; Visseren, F.L.J.; Westerink, J.; The SMART Study Group; van Petersen, R.; Dinther, B.G.F.; Algra, A.; van der Graaf, Y.; et al. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 220. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; De Curtis, A.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. A score of low-grade inflammation and risk of mortality: Prospective findings from the Moli-sani study. Haematologica 2016, 101, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, P.; Gong, J.; Liu, F.; Qiao, Y.; Si, C.; Wang, X.; Zhou, H.; Song, F. Association between the Finnish Diabetes Risk Score and cancer in middle-aged and older adults: Involvement of inflammation. Metabolism 2023, 144, 155586. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Calle, E.E.; Teras, L.R.; Petrelli, J.; Thun, M.J. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am. J. Epidemiol. 2004, 159, 1160–1167. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Becker, S.; Kaaks, R. Diabetes mellitus type 2—An independent risk factor for cancer? Exp. Clin. Endocrinol. Diabetes 2010, 118, 4–8. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, Y.-J.; Xue, C.-D.; Li, S.; Gao, Z.-N.; Qin, K.-R. Effects of T2DM on cancer progression: Pivotal precipitating factors and underlying mechanisms. Front. Endocrinol. 2024, 15, 1396022. [Google Scholar] [CrossRef]
- Ahmadabad, H.A.; Panah, S.M.; Ghasemnejad-Berenji, H.; Ghojavand, S.; Ghasemnejad-Berenji, M.; Khezri, M.R. Metformin and the PI3K/AKT signaling pathway: Implications for cancer, cardiovascular, and central nervous system diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 1035–1055. [Google Scholar] [CrossRef]
- Sarfstein, R.; Friedman, Y.; Attias-Geva, Z.; Fishman, A.; Bruchim, I.; Werner, H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PLoS ONE 2013, 8, e61537. [Google Scholar] [CrossRef] [PubMed]
- ScienceDirect. Phenformin—Medicine & Dentistry Topic Page. 2024. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/phenformin (accessed on 3 June 2025).
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef]
- Kumagai, A.K. Glucose transport in brain and retina: Implications in the management and complications of diabetes. Diabetes Metab. Res. Rev. 1999, 15, 261–273. [Google Scholar] [CrossRef]
- Ross, B.D.; Espinal, J.; Silva, P. Glucose metabolism in renal tubular function. Kidney Int. 1986, 29, 54–67. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, R.; van Solinge, W.W. The energy-less red blood cell is lost: Erythrocyte enzyme abnormalities of glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- Thau, L.; Sharma, S.G.J. Physiology, Cortisol. In StatPearls [Internet]; StatPearls, Ed.; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gerich, J.E. Glucose Counterregulation and Its Impact on Diabetes Mellitus. Diabetes 1988, 37, 1608–1617. [Google Scholar] [CrossRef]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef]
- Cameron, M.E.; Yakovenko, A.; Trevino, J.G. Glucose and Lactate Transport in Pancreatic Cancer: Glycolytic Metabolism Revisited. J. Oncol. 2018, 2018, 6214838. [Google Scholar] [CrossRef]
- Reckzeh, E.S.; Waldmann, H. Small-Molecule Inhibition of Glucose Transporters GLUT-1-4. Chembiochem 2020, 21, 45–52. [Google Scholar] [CrossRef]
- Holman, G.D. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflugers Arch. 2020, 472, 1155–1175. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Uddin, M.J.; Pang, M.-G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J. The Impact of Westernization on the Insulin/IGF-I Signaling Pathway and the Metabolic Syndrome: It Is Time for Change. Int. J. Mol. Sci. 2023, 24, 4551. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.C.; Dao, X.D.; Nguyen, D.V.; Luu, D.H.; Bui, T.M.H.; Le, T.H.; Nguyen, H.T.; Le, T.N.; Hosaka, T.; Nguyen, T.T.T. Insulin signaling and its application. Front. Endocrinol. 2023, 14, 1226655. [Google Scholar] [CrossRef]
- Perz, M.; Torlinska, T. Insulin receptor--structural and functional characteristics. Med. Sci. Monit. 2001, 7, 169–177. [Google Scholar]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- Youngren, J.F. Regulation of insulin receptor function. Cell Mol. Life Sci. 2007, 64, 873–891. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Lee, J.; Pilch, P.F. The insulin receptor: Structure, function, and signaling. Am. J. Physiol. 1994, 266, C319–C334. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Bai, X.C. The Activation Mechanism of the Insulin Receptor: A Structural Perspective. Annu. Rev. Biochem. 2023, 92, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Maji, S.; Sanghera, N.; Gopalasingam, P.; Gorbunov, E.; Tarasov, S.; Epstein, O.; Klein-Seetharaman, J. Structure and dynamics of the insulin receptor: Implications for receptor activation and drug discovery. Drug Discov. Today 2017, 22, 1092–1102. [Google Scholar] [CrossRef]
- Posner, B.I. Insulin Signalling: The Inside Story. Can. J. Diabetes 2017, 41, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Birnbaum, M.J. A role for the serine/threonine kinase, Akt, in insulin-stimulated glucose uptake. Biochem. Soc. Trans. 1997, 25, 981–988. [Google Scholar] [CrossRef]
- Holman, G.D.; Kasuga, M. From receptor to transporter: Insulin signalling to glucose transport. Diabetologia 1997, 40, 991–1003. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Taha, C.; Klip, A. The insulin signaling pathway. J. Membr. Biol. 1999, 169, 1–12. [Google Scholar] [CrossRef]
- Yudhani, R.D.; Sari, Y.; Nugrahaningsih, D.A.A.; Sholikhah, E.N.; Rochmanti, M.; Purba, A.K.R.; Khotimah, H.; Nugrahenny, D.; Mustofa, M. In Vitro Insulin Resistance Model: A Recent Update. J. Obes. 2023, 2023, 1964732. [Google Scholar] [CrossRef]
- Calejman, C.M.; Doxsey, W.; Fazakerley, D.; Guertin, D. Integrating adipocyte insulin signaling and metabolism in the multi-omics era. Trends Biochem. Sci. 2022, 47, 531–546. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Levi, R.; Fleischmann, B.K.; Gallo, M.P.; Malan, D. Insulin-Activated Signaling Pathway and GLUT4 Membrane Translocation in hiPSC-Derived Cardiomyocytes. Int. J. Mol. Sci. 2024, 25, 8197. [Google Scholar] [CrossRef]
- Kido, Y.; Nakae, J.; Accili, D. Clinical review 125: The insulin receptor and its cellular targets. J. Clin. Endocrinol. Metab. 2001, 86, 972–979. [Google Scholar] [PubMed]
- Gray, C.W.; Coster, A.C.F. From insulin to Akt: Time delays and dominant processes. J. Theor. Biol. 2020, 507, 110454. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Ko, H.J.; Barrett, T.; Kim, J.K.; Davis, R.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008, 322, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pathophysiology of Physical Inactivity-Dependent Insulin Resistance: A Theoretical Mechanistic Review Emphasizing Clinical Evidence. J. Diabetes Res. 2021, 2021, 7796727. [Google Scholar] [CrossRef]
- Acosta-Martinez, M.; Cabail, M.Z. The PI3K/Akt Pathway in Meta-Inflammation. Int. J. Mol. Sci. 2022, 23, 15330. [Google Scholar] [CrossRef]
- Sanches, J.M.; Na Zhao, L.; Salehi, A.; Wollheim, C.B.; Kaldis, P. Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk. FEBS J. 2023, 290, 620–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-Z.; Li, Y.-R.; Zhang, D.; Yuan, J.-H.; Zhang, C.-S.; Liu, Y.; Song, L.-M.; Lin, Q.; Li, M.-W.; Dong, J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 1399. [Google Scholar] [CrossRef]
- Bassin, S.R.; Srinath, R. The Impact of Physical Activity in Patients With Type 2 Diabetes. Am. J. Lifestyle Med. 2025, 19, 147–161. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.B.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Hamdy, O.; Goodyear, L.J.; Horton, E.S. Diet and exercise in type 2 diabetes mellitus. Endocrinol. Metab. Clin. N. Am. 2001, 30, 883–907. [Google Scholar] [CrossRef]
- García-Molina, L.; Lewis-Mikhael, A.-M.; Riquelme-Gallego, B.; Cano-Ibáñez, N.; Oliveras-López, M.-J.; Bueno-Cavanillas, A. Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: A systematic review and meta-analysis. Eur. J. Nutr. 2020, 59, 1313–1328. [Google Scholar] [CrossRef]
- Campbell, D.J.T.; Campbell, D.B.; Ogundeji, Y.; Au, F.; Beall, R.; Ronksley, P.E.; Quinn, A.E.; Manns, B.J.; Hemmelgarn, B.R.; Tonelli, M.; et al. First-line pharmacotherapy for incident type 2 diabetes: Prescription patterns, adherence and associated costs. Diabet. Med. 2021, 38, e14622. [Google Scholar] [CrossRef]
- Ashcroft, F.M. Mechanisms of the glycaemic effects of sulfonylureas. Horm. Metab. Res. 1996, 28, 456–463. [Google Scholar] [CrossRef]
- Sibony, R.W.; Segev, O.; Dor, S.; Raz, I. Drug Therapies for Diabetes. Int. J. Mol. Sci. 2023, 24, 17147. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Á.D.; Sánchez-Aragó, M.; Giner-Sánchez, D.; Sánchez-Cenizo, L.; Willers, I.; Cuezva, J.M. Glucose avidity of carcinomas. Cancer Lett. 2009, 276, 125–135. [Google Scholar] [CrossRef]
- Zaal, E.A.; Berkers, C.R. The Influence of Metabolism on Drug Response in Cancer. Front. Oncol. 2018, 8, 500. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, A.; Zhou, X.; Wang, F. Suppression of pyruvate dehydrogenase kinase-2 re-sensitizes paclitaxel-resistant human lung cancer cells to paclitaxel. Oncotarget 2017, 8, 52642–52650. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, K.; Graulus, G.-J.; Mesotten, L.; Thomeer, M.; Derveaux, E.; Noben, J.-P.; Guedens, W.; Adriaensens, P. The Metabolic Landscape of Lung Cancer: New Insights in a Disturbed Glucose Metabolism. Front. Oncol. 2019, 9, 1215. [Google Scholar] [CrossRef]
- Kawatani, M.; Osada, H. Small-molecule inhibitors of glucose transporters. Vitam. Horm. 2025, 128, 213–242. [Google Scholar]
- Zhou, Z.; Li, Y.; Chen, S.; Xie, Z.; Du, Y.; Liu, Y.; Shi, Y.; Lin, X.; Zeng, X.; Zhao, H.; et al. GLUT1 promotes cell proliferation via binds and stabilizes phosphorylated EGFR in lung adenocarcinoma. Cell Commun. Signal 2024, 22, 303. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Q.; Fan, Y.; Fang, P.; Zhou, H.; Huang, J. OBHS Drives Abnormal Glycometabolis Reprogramming via GLUT1 in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7136. [Google Scholar] [CrossRef] [PubMed]
- Meziou, S.; Goulet, C.R.; Hovington, H.; Lefebvre, V.; Lavallée, E.; Bergeron, M.; Brisson, H.; Champagne, A.; Neveu, B.; Lacombe, D.; et al. GLUT1 expression in high-risk prostate cancer: Correlation with (18)F-FDG-PET/CT and clinical outcome. Prostate Cancer Prostatic Dis. 2020, 23, 441–448. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; He, J.; Li, W.; Zhang, A.; Li, N.; Zhou, G.; Sun, B. Glucose transporter 3 (GLUT3) promotes lactylation modifications by regulating lactate dehydrogenase A (LDHA) in gastric cancer. Cancer Cell Int. 2023, 23, 303. [Google Scholar] [CrossRef]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Panpan, S.I.; Wei, G.E.; Kaiming, W.U.; Zhang, R. O-GlcNAcylation of hexokinase 2 modulates mitochondrial dynamics and enhances the progression of lung cancer. Mol. Cell Biochem. 2025, 480, 2633–2643. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X. Glycolysis regulator PFKP induces human melanoma cell proliferation and tumor growth. Clin. Transl. Oncol. 2023, 25, 2183–2191. [Google Scholar] [CrossRef]
- Zheng, S.; Liu, Q.; Liu, T.; Lu, X. Posttranslational modification of pyruvate kinase type M2 (PKM2): Novel regulation of its biological roles to be further discovered. J. Physiol. Biochem. 2021, 77, 355–363. [Google Scholar] [CrossRef]
- Shu, Y.; Yue, J.; Li, Y.; Yin, Y.; Wang, J.; Li, T.; He, X.; Liang, S.; Zhang, G.; Liu, Z.; et al. Development of human lactate dehydrogenase a inhibitors: High-throughput screening, molecular dynamics simulation and enzyme activity assay. J. Comput. Aided Mol. Des. 2024, 38, 28. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of cancer stem cell metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Paredes, F.; Williams, H.C.; San Martin, A. Metabolic adaptation in hypoxia and cancer. Cancer Lett. 2021, 502, 133–142. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Li, J.; Eu, J.Q.; Kong, L.R.; Wang, L.; Lim, Y.C.; Goh, B.C.; Wong, A.L.A. Targeting Metabolism in Cancer Cells and the Tumour Microenvironment for Cancer Therapy. Molecules 2020, 25, 4831. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer. 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
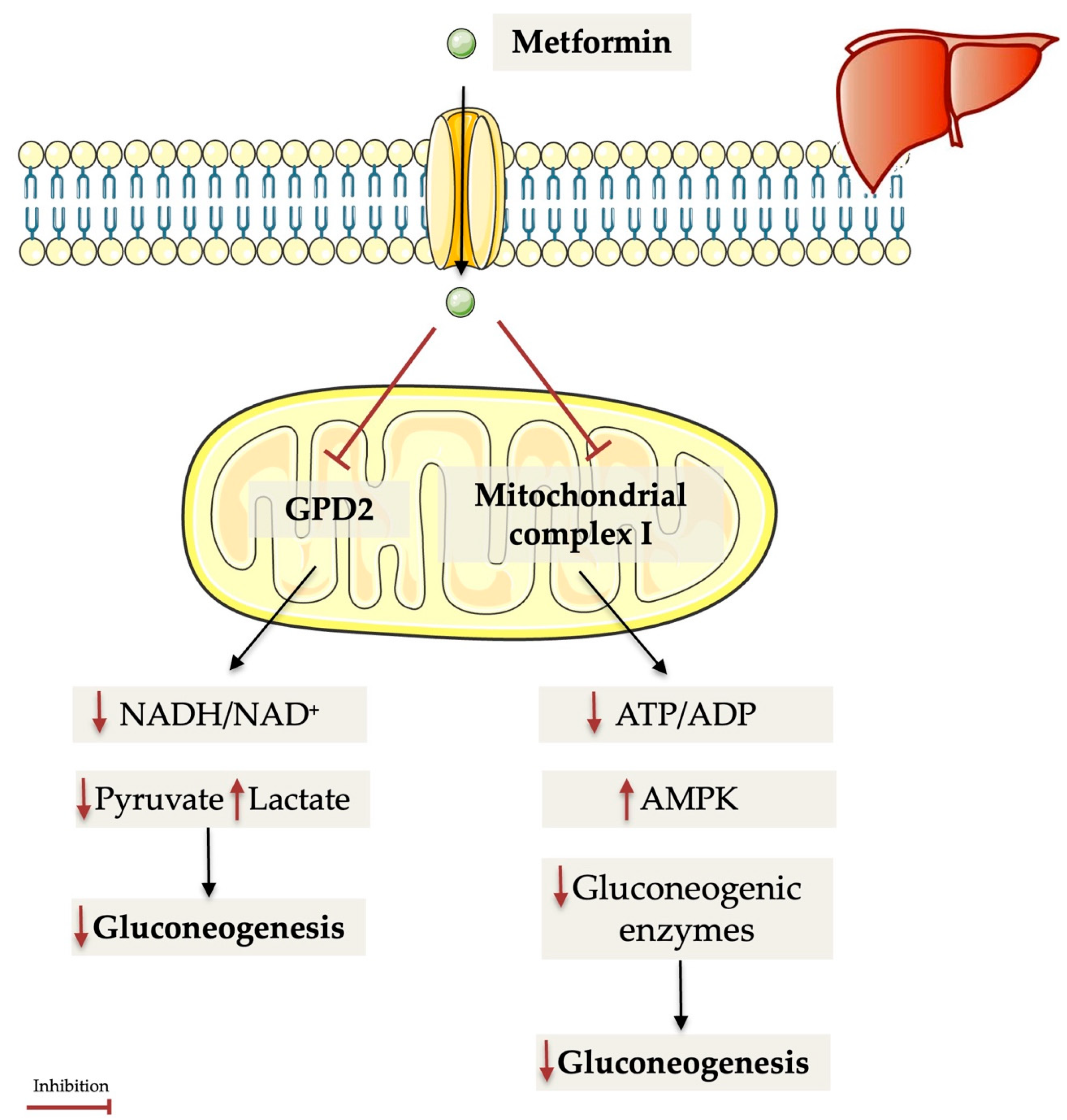
- Madiraju, A.K.; Qiu, Y.; Perry, R.J.; Rahimi, Y.; Zhang, X.-M.; Zhang, D.; Camporez, J.-P.G.; Cline, G.W.; Butrico, G.M.; Kemp, B.E.; et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat. Med. 2018, 24, 1384–1394. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Zhou, G.; Mayers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Metformin: Activation of 5’ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front. Genet. 2022, 13, 1022739. [Google Scholar] [CrossRef]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Induri, S.N.R.; Kansara, P.; Thomas, S.C.; Xu, F.; Saxena, D.; Li, X. The Gut Microbiome, Metformin, and Aging. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 85–108. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Maniar, K.; Moideen, A.; Bhattacharyya, R.; Banerjee, D. Metformin exerts anti-obesity effect via gut microbiome modulation in prediabetics: A hypothesis. Med. Hypotheses 2017, 104, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.N.; Yang, A.; Chu, N.; Chow, E. Current type 2 diabetes guidelines: Individualized treatment and how to make the most of metformin. Diabetes Obes. Metab. 2024, 26 (Suppl. S3), 55–74. [Google Scholar] [CrossRef]
- Whang, A.; Nagpal, R.; Yadav, H. Bi-directional drug-microbiome interactions of anti-diabetics. eBioMedicine 2019, 39, 591–602. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: New insights into old diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Rosell-Díaz, M.; Petit-Gay, A.; Molas-Prat, C.; Gallardo-Nuell, L.; Ramió-Torrentà, L.; Garre-Olmo, J.; Pérez-Brocal, V.; Moya, A.; Jové, M.; Pamplona, R.; et al. Metformin-induced changes in the gut microbiome and plasma metabolome are associated with cognition in men. Metabolism 2024, 157, 155941. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Molloy, M.P. Metformin, Microbiome and Protection Against Colorectal Cancer. Dig. Dis. Sci. 2021, 66, 1409–1414. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller, E.R., 3rd; Appel, L.J.; Yeh, H.-C. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, N. Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 5003–5014. [Google Scholar] [CrossRef]
- Xie, X.; Li, W.; Xiong, Z.; Xu, J.; Liao, T.; Sun, L.; Xu, H.; Zhang, M.; Zhou, J.; Xiong, W.; et al. Metformin reprograms tryptophan metabolism via gut microbiome-derived bile acid metabolites to ameliorate depression-Like behaviors in mice. Brain Behav. Immun. 2025, 123, 442–455. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Saigal, A.; Szamosi, J.C.; Hammill, J.A.; Bezverbnaya, K.; Wang, D.; Gautam, J.; Tsakiridis, E.E.; Di Pastena, F.; McNicol, J.; et al. Metformin-induced reductions in tumor growth involves modulation of the gut microbiome. Mol. Metab. 2022, 61, 101498. [Google Scholar] [CrossRef]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef]
- Lee, A.; Morley, J.E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes. Res. 1998, 6, 47–53. [Google Scholar] [CrossRef]
- Haber, R.; Zarzour, F.; Ghezzawi, M.; Saadeh, N.; Bacha, D.S.; Al Jebbawi, L.; Chakhtoura, M.; Mantzoros, C.S. The impact of metformin on weight and metabolic parameters in patients with obesity: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2024, 26, 1850–1867. [Google Scholar] [CrossRef]
- Pu, R.; Shi, D.; Gan, T.; Ren, X.; Ba, Y.; Huo, Y.; Bai, Y.; Zheng, T.; Cheng, N. Effects of metformin in obesity treatment in different populations: A meta-analysis. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820926000. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.L.; Guppy, M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004, 313, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Silva, P.M.A.; Sarmento, B.; Queirós, O. Targeting Glucose Metabolism in Cancer Cells as an Approach to Overcoming Drug Resistance. Pharmaceutics 2023, 15, 2610. [Google Scholar] [CrossRef]
- Tavares-Valente, D.; Granja, S.; Baltazar, F.; Queirós, O. Bioenergetic modulators hamper cancer cell viability and enhance response to chemotherapy. J. Cell Mol. Med. 2018, 22, 3782–3794. [Google Scholar] [CrossRef]
- Yendapally, R.; Sikazwe, D.; Kim, S.S.; Ramsinghani, S.; Fraser-Spears, R.; Witte, A.P.; La-Viola, B. A review of phenformin, metformin, and imeglimin. Drug Dev. Res. 2020, 81, 390–401. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Andrzejewski, S.; Siegel, P.M.; St-Pierre, J. Metabolic Profiles Associated with Metformin Efficacy in Cancer. Front. Endocrinol. 2018, 9, 372. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.; Ren, M.; Chen, Y.; Zhang, J.; Li, J.; Gao, F.; Bao, Y.; Huang, Y.; Yang, X.; et al. Metformin Induces Apoptosis and Ferroptosis of Ovarian Cancer Cells Under Energy Stress Conditions. Cells 2025, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Sikka, A.; Kaur, M.; Agarwal, C.; Deep, G.; Agarwal, R. Metformin suppresses growth of human head and neck squamous cell carcinoma via global inhibition of protein translation. Cell Cycle 2012, 11, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yeerna, H.; Goto, Y.; Ando, T.; Wu, V.H.; Zhang, X.; Wang, Z.; Amornphimoltham, P.; Murphy, A.N.; Tamayo, P.; et al. Metformin Inhibits Progression of Head and Neck Squamous Cell Carcinoma by Acting Directly on Carcinoma-Initiating Cells. Cancer Res. 2019, 79, 4360–4370. [Google Scholar] [CrossRef] [PubMed]
- Shameem, M.; Bagherpoor, A.J.; Nakhi, A.; Dosa, P.; Georg, G.; Kassie, F. Mitochondria-targeted metformin (mitomet) inhibits lung cancer in cellular models and in mice by enhancing the generation of reactive oxygen species. Mol. Carcinog. 2023, 62, 1619–1629. [Google Scholar] [CrossRef]
- Teng, M.; Li, Z.; Gu, Y.; Fan, Y.; Wang, D.; Liu, M.; Li, Y.; Wei, G.; Huang, Y. Real-time monitoring of glucose metabolism and effects of metformin on HepG2 cells using (13)C in-cell NMR spectroscopy. Biochem. Biophys. Res. Commun. 2024, 694, 149383. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Jamieson, J.; Marroquin, L.; Nadanaciva, S.; Billis, P.A.; Will, Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol. Appl. Pharmacol. 2008, 233, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Kim, J.; Lazim, R.; Lee, J.Y.; Kim, J.Y.; Gosu, V.; Lee, Y.; Choi, S.; Kwon, H.J. The anticancer effect of metformin targets VDAC1 via ER-mitochondria interactions-mediated autophagy in HCC. Exp. Mol. Med. 2024, 56, 2714–2725. [Google Scholar] [CrossRef]
- Lee, J.O.; Kang, M.J.; Byun, W.S.; Kim, S.A.; Seo, I.H.; Han, J.A.; Moon, J.W.; Kim, J.H.; Kim, S.J.; Lee, E.J.; et al. Metformin overcomes resistance to cisplatin in triple-negative breast cancer (TNBC) cells by targeting RAD51. Breast Cancer Res. 2019, 21, 115. [Google Scholar] [CrossRef]
- Zhuang, Y.; Miskimins, W.K. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J. Mol. Signal 2008, 3, 18. [Google Scholar] [CrossRef]
- Alimova, I.N.; Liu, B.; Fan, Z.; Edgerton, S.M.; Dillon, T.; Lind, S.E.; Thor, A.D. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009, 8, 909–915. [Google Scholar] [CrossRef]
- Amaral, I.; Silva, C.; Correia-Branco, A.; Martel, F. Effect of metformin on estrogen and progesterone receptor-positive (MCF-7) and triple-negative (MDA-MB-231) breast cancer cells. Biomed. Pharmacother. 2018, 102, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, E.; Cioffi, M.; Sancho, P.; Sanchez-Ripoll, Y.; Trabulo, S.M.; Dorado, J.; Balic, A.; Hidalgo, M.; Heeschen, C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS ONE 2013, 8, e76518. [Google Scholar] [CrossRef]
- Karnevi, E.; Said, K.; Andersson, R.; Rosendahl, A.H. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer 2013, 13, 235. [Google Scholar] [CrossRef]
- Velez, J.; Pan, R.; Lee, J.T.; Enciso, L.; Suarez, M.; Duque, J.E.; Jaramillo, D.; Lopez, C.; Morales, L.; Bornmann, W.; et al. Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget 2016, 7, 51435–51449. [Google Scholar] [CrossRef]
- Scotland, S.; Saland, E.; Skuli, N.; de Toni, F.; Boutzen, H.; Micklow, E.; Sénégas, I.; Peyraud, R.; Peyriga, L.; Théodoro, F.; et al. Mitochondrial energetic and AKT status mediate metabolic effects and apoptosis of metformin in human leukemic cells. Leukemia 2013, 27, 2129–2138. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Laurent, K.; Giuliano, S.; Larbret, F.; Ponzio, G.; Gounon, P.; Le Marchand-Brustel, Y.; Giorgetti-Peraldi, S.; Cormont, M.; Bertolotto, C.; et al. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010, 70, 2465–2475. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- El-Mir, M.-Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- Warkad, M.S.; Kim, C.-H.; Kang, B.-G.; Park, S.-H.; Jung, J.-S.; Feng, J.-H.; Inci, G.; Kim, S.-C.; Suh, H.-W.; Lim, S.S.; et al. Metformin-induced ROS upregulation as amplified by apigenin causes profound anticancer activity while sparing normal cells. Sci. Rep. 2021, 11, 14002. [Google Scholar] [CrossRef] [PubMed]
- Westhaus, A.; Blumrich, E.M.; Dringen, R. The Antidiabetic Drug Metformin Stimulates Glycolytic Lactate Production in Cultured Primary Rat Astrocytes. Neurochem. Res. 2017, 42, 294–305. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar] [CrossRef]
- Liu, S.; Washio, J.; Sato, S.; Abiko, Y.; Shinohara, Y.; Kobayashi, Y.; Otani, H.; Sasaki, S.; Wang, X.; Takahashi, N. Rewired Cellular Metabolic Profiles in Response to Metformin under Different Oxygen and Nutrient Conditions. Int. J. Mol. Sci. 2022, 23, 989. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Chandel, N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef]
- Drahota, Z.; Palenickova, E.; Endlicher, R.; Milerova, M.; Brejchova, J.; Vosahlikova, M.; Svoboda, P.; Kazdova, L.; Kalous, M.; Cervinkova, Z.; et al. Biguanides inhibit complex I, II and IV of rat liver mitochondria and modify their functional properties. Physiol. Res. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef]
- Semenza, G.L. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today 2007, 12, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Saladini, S.; Aventaggiato, M.; Barreca, F.; Morgante, E.; Sansone, L.; Russo, M.A.; Tafani, M. Metformin Impairs Glutamine Metabolism and Autophagy in Tumour Cells. Cells 2019, 8, 49. [Google Scholar] [CrossRef]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Yumashev, A.; Hjazi, A.; Toama, M.A.; AbdRabou, M.A.; Gehlot, A.; Alwaily, E.R.; Shirsalimi, N.; Yadav, P.K.; et al. Effects of metformin on cancers in experimental and clinical studies: Focusing on autophagy and AMPK/mTOR signaling pathways. Cell Biochem. Funct. 2024, 42, e4071. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2011, 122, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxidative Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Okon, I.S.; Zou, M.-H. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol. Res. 2015, 100, 170–174. [Google Scholar] [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Zielonka, J.; Kalyanaraman, B.; Hartley, R.C.; Murphy, M.P.; Avadhani, N.G. Mitochondria-targeted paraquat and metformin mediate ROS production to induce multiple pathways of retrograde signaling: A dose-dependent phenomenon. Redox Biol. 2020, 36, 101606. [Google Scholar] [CrossRef]
- Mu, W.; Jiang, Y.; Liang, G.; Feng, Y.; Qu, F. Metformin: A Promising Antidiabetic Medication for Cancer Treatment. Curr. Drug Targets 2023, 24, 41–54. [Google Scholar] [CrossRef]
- Jara, J.A.; López-Muñoz, R. Metformin and cancer: Between the bioenergetic disturbances and the antifolate activity. Pharmacol. Res. 2015, 101, 102–108. [Google Scholar] [CrossRef]
- Gou, S.; Qiu, L.; Yang, Q.; Li, P.; Zhou, X.; Sun, Y.; Zhou, X.; Zhao, W.; Zhai, W.; Li, G.; et al. Metformin leads to accumulation of reactive oxygen species by inhibiting the NFE2L1 expression in human hepatocellular carcinoma cells. Toxicol. Appl. Pharmacol. 2021, 420, 115523. [Google Scholar] [CrossRef]
- Cheng, G.; Lanza-Jacoby, S. Metformin decreases growth of pancreatic cancer cells by decreasing reactive oxygen species: Role of NOX4. Biochem. Biophys. Res. Commun. 2015, 465, 41–46. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, A.K.; Zaman, A.; Das, P.K. Metformin-Loaded Hyaluronic Acid-Derived Carbon Dots for Targeted Therapy against Hepatocellular Carcinoma by Glutamine Metabolic Reprogramming. Mol. Pharm. 2023, 20, 6391–6406. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Morales, N.; Rovira-Llopis, S.; Bañuls, C.; Lopez-Domenech, S.; Escribano-Lopez, I.; Veses, S.; Jover, A.; Rocha, M.; Hernandez-Mijares, A.; Victor, V.M. Does Metformin Protect Diabetic Patients from Oxidative Stress and Leukocyte-Endothelium Interactions? Antioxid. Redox Signal 2017, 27, 1439–1445. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Kang, L.; Li, J.; Yang, L.; Zhang, J.; Liu, J.; Zhu, M.; Zhang, Q.; Shen, Y.; et al. Metformin attenuates myocardial ischemia-reperfusion injury via up-regulation of antioxidant enzymes. PLoS ONE 2017, 12, e0182777. [Google Scholar] [CrossRef]
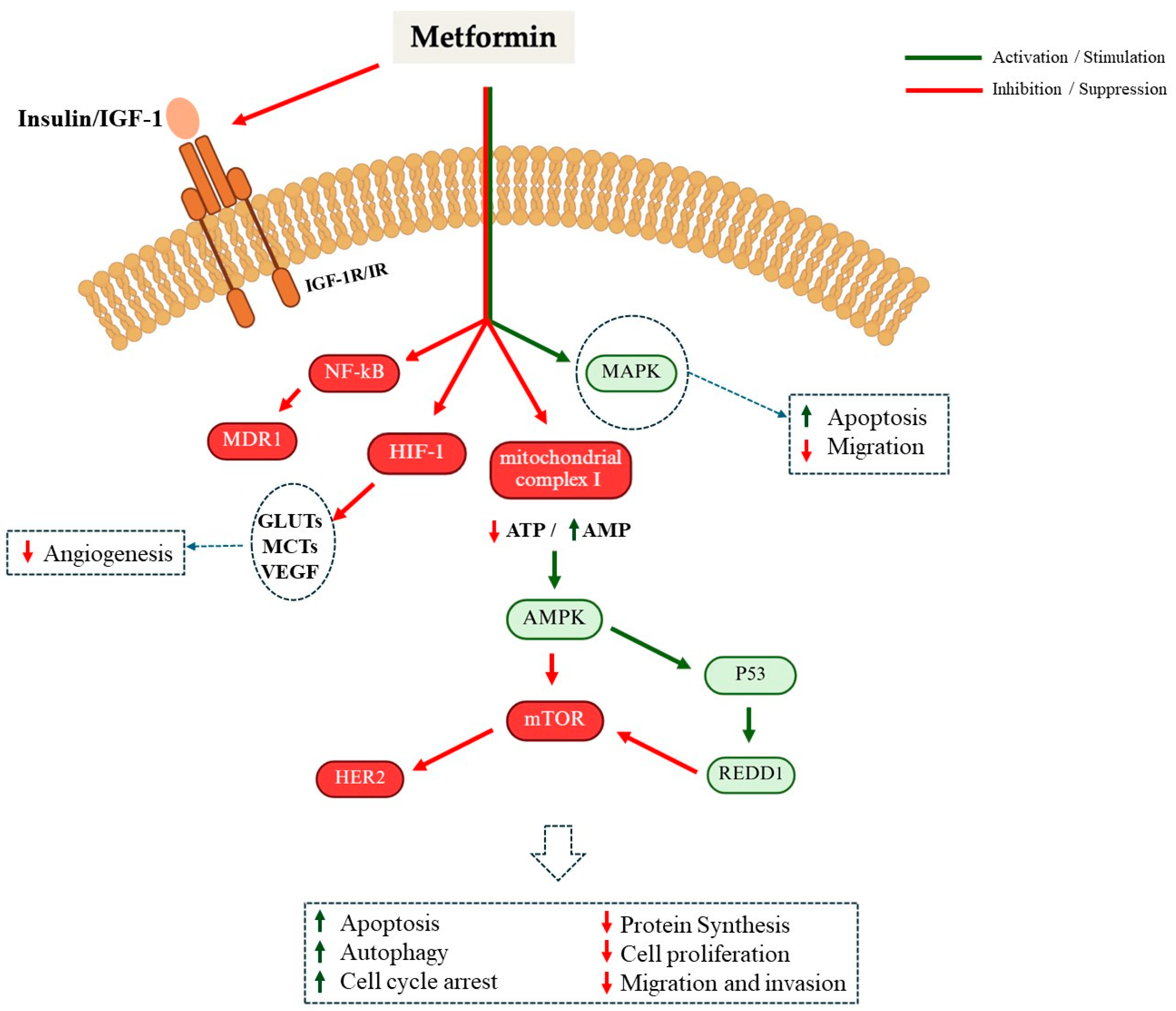
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Bhagwat, I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Ikhlas, S.; Ahmad, M. Metformin: Insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 2017, 185, 53–62. [Google Scholar] [CrossRef]
- Palma, F.R.; Ratti, B.A.; Paviani, V.; Coelho, D.R.; Miguel, R.; Danes, J.M.; Zaichik, S.V.; de Abreu, A.L.; Silva, S.O.; Chen, Y.; et al. AMPK-deficiency forces metformin-challenged cancer cells to switch from carbohydrate metabolism to ketogenesis to support energy metabolism. Oncogene 2021, 40, 5455–5467. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Griss, T.; Vincent, E.E.; Egnatchik, R.; Chen, J.; Ma, E.H.; Faubert, B.; Viollet, B.; DeBerardinis, R.J.; Jones, R.G. Metformin Antagonizes Cancer Cell Proliferation by Suppressing Mitochondrial-Dependent Biosynthesis. PLoS Biol. 2015, 13, e1002309. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- Hardie, D.G. Molecular Pathways: Is AMPK a Friend or a Foe in Cancer? Clin. Cancer Res. 2015, 21, 3836–3840. [Google Scholar] [CrossRef]
- Vara-Ciruelos, D.; Russell, F.M.; Hardie, D.G. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? Open Biol. 2019, 9, 190099. [Google Scholar] [CrossRef]
- Sadria, M.; Seo, D.; Layton, A.T. The mixed blessing of AMPK signaling in Cancer treatments. BMC Cancer 2022, 22, 105. [Google Scholar] [CrossRef]
- Ling, N.X.Y.; Kaczmarek, A.; Hoque, A.; Davie, E.; Ngoei, K.R.W.; Morrison, K.R.; Smiles, W.J.; Forte, G.M.; Wang, T.; Lie, S.; et al. mTORC1 directly inhibits AMPK to promote cell proliferation under nutrient stress. Nat. Metab. 2020, 2, 41–49. [Google Scholar] [CrossRef]
- Vara-Ciruelos, D.; Dandapani, M.; Hardie, D.G. AMP-Activated Protein Kinase: Friend or Foe in Cancer? Annu. Rev. Cancer Biol. 2020, 4, 1–16. [Google Scholar] [CrossRef]
- Cai, X.; Hu, X.; Tan, X.; Cheng, W.; Wang, Q.; Chen, X.; Guan, Y.; Chen, C.; Jing, X. Metformin Induced AMPK Activation, G0/G1 Phase Cell Cycle Arrest and the Inhibition of Growth of Esophageal Squamous Cell Carcinomas In Vitro and In Vivo. PLoS ONE 2015, 10, e0133349. [Google Scholar] [CrossRef]
- Keerthana, C.K.; Rayginia, T.P.; Shifana, S.C.; Anto, N.P.; Kalimuthu, K.; Isakov, N.; Anto, R.J. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1114582. [Google Scholar] [CrossRef]
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupuy, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. AMPK Is a Negative Regulator of the Warburg Effect and Suppresses Tumor Growth In Vivo. Cell Metab. 2013, 17, 113–124. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef]
- Chomanicova, N.; Gazova, A.; Adamickova, A.; Valaskova, S.; Kyselovic, J. The role of AMPK/mTOR signaling pathway in anticancer activity of metformin. Physiol. Res. 2021, 70, 501–508. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Role of mTOR Signaling as a Therapeutic Target in Cancer. Int. J. Mol. Sci. 2021, 22, 1743. [Google Scholar] [CrossRef]
- Daugan, M.; Wojcicki, A.D.; D’hAyer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef]
- Sathe, A.; Chalaud, G.; Oppolzer, I.; Wong, K.Y.; von Busch, M.; Schmid, S.C.; Tong, Z.; Retz, M.; Gschwend, J.E.; Schulz, W.A.; et al. Parallel PI3K, AKT and mTOR inhibition is required to control feedback loops that limit tumor therapy. PLoS ONE 2018, 13, e0190854. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Nguyen, H.G.; Wen, L.; Edlind, M.P.; Carroll, P.R.; Kim, W.; Ruggero, D. Cell type-specific abundance of 4EBP1 primes prostate cancer sensitivity or resistance to PI3K pathway inhibitors. Sci. Signal. 2015, 8, ra116. [Google Scholar] [CrossRef]
- Hervieu, A.; Kermorgant, S. The Role of PI3K in Met Driven Cancer: A Recap. Front. Mol. Biosci. 2018, 5, 86. [Google Scholar] [CrossRef]
- Ferreira, G.D.; Germeyer, A.; Machado, A.d.B.; Nascimento, T.L.D.; Strowitzki, T.; Brum, I.S.; Corleta, H.v.E.; Capp, E. Metformin modulates PI3K and GLUT4 expression and Akt/PKB phosphorylation in human endometrial stromal cells after stimulation with androgen and insulin. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 157–162. [Google Scholar] [CrossRef]
- Tzatsos, A.; Kandror, K.V. Nutrients Suppress Phosphatidylinositol 3-Kinase/Akt Signaling via Raptor-Dependent mTOR-Mediated Insulin Receptor Substrate 1 Phosphorylation. Mol. Cell. Biol. 2006, 26, 63–76. [Google Scholar] [CrossRef]
- Jang, S.-K.; Hong, S.-E.; Lee, D.-H.; Kim, J.-Y.; Hong, J.; Park, I.-C.; Jin, H.-O. Inhibition of AKT Enhances the Sensitivity of NSCLC Cells to Metformin. Anticancer. Res. 2021, 41, 3481–3487. [Google Scholar] [CrossRef]
- Papa, A.; Pandolfi, P.P. The PTEN–PI3K Axis in Cancer. Biomolecules 2019, 9, 153. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chan, D.K.; Haugrud, A.B.; Miskimins, W.K. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS ONE 2014, 9, e108444. [Google Scholar] [CrossRef]
- Silvestri, A.; Palumbo, F.; Rasi, I.; Posca, D.; Pavlidou, T.; Paoluzi, S.; Castagnoli, L.; Cesareni, G. Metformin Induces Apoptosis and Downregulates Pyruvate Kinase M2 in Breast Cancer Cells Only When Grown in Nutrient-Poor Conditions. PLoS ONE 2015, 10, e0136250. [Google Scholar] [CrossRef]
- Yeşildağ, A.; Kızıloğlu, H.T.; Dirican, E.; Erbaş, E.; Gelen, V.; Kara, A. Anticarcinogenic Effects of Gold Nanoparticles and Metformin Against MCF-7 and A549 Cells. Biol. Trace. Elem. Res. 2024, 202, 4494–4507. [Google Scholar] [CrossRef]
- De, A.; Wadhwani, A.; Sauraj; Roychowdhury, P.; Kang, J.H.; Ko, Y.T.; Kuppusamy, G. WZB117 Decorated Metformin-Carboxymethyl Chitosan Nanoparticles for Targeting Breast Cancer Metabolism. Polymers 2023, 15, 976. [Google Scholar] [CrossRef]
- Abdolahinia, E.D.; Nadri, S.; Rahbarghazi, R.; Barar, J.; Aghanejad, A.; Omidi, Y. Enhanced penetration and cytotoxicity of metformin and collagenase conjugated gold nanoparticles in breast cancer spheroids. Life Sci. 2019, 231, 116545. [Google Scholar] [CrossRef]
- Jafari-Gharabaghlou, D.; Dadashpour, M.; Khanghah, O.J.; Salmani-Javan, E.; Zarghami, N. Potentiation of Folate-Functionalized PLGA-PEG nanoparticles loaded with metformin for the treatment of breast Cancer: Possible clinical application. Mol. Biol. Rep. 2023, 50, 3023–3033. [Google Scholar] [CrossRef]
- Chen, C.; Yang, L.; Peng, Y.; Zhang, W.J.; Yang, X.X.; Zhou, W. Autophagic blockage by metformin-loaded PLGA nanoparticles causes cell cycle arrest of HepG2 cells. Nanomedicine 2024, 19, 43–58. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Rezaeiroshan, A.; Babaei, A.; Khanali, A.; Aghajanshakeri, S.; Farmoudeh, A.; Nokhodchi, A. Hyaluronic Acid-Coated Chitosan/Gelatin Nanoparticles as a New Strategy for Topical Delivery of Metformin in Melanoma. Biomed. Res. Int. 2023, 2023, 3304105. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Long, Y.; Peng, H. Targeted Delivery of Metformin Against Lung Cancer Cells Via Hyaluronan-Modified Mesoporous Silica Nanoparticles. Appl. Biochem. Biotechnol. 2023, 195, 4067–4083. [Google Scholar] [CrossRef]
- Snima, K.; Jayakumar, R.; Unnikrishnan, A.; Nair, S.V.; Lakshmanan, V.-K. O-carboxymethyl chitosan nanoparticles for metformin delivery to pancreatic cancer cells. Carbohydr. Polym. 2012, 89, 1003–1007. [Google Scholar] [CrossRef]
- Shiridokht, F.; Dadashi, H.; Vandghanooni, S.; Eskandani, M.; Farajollahi, A. Metformin-loaded chitosan nanoparticles augment silver nanoparticle-induced radiosensitization in breast cancer cells during radiation therapy. Colloids Surf. B Biointerfaces 2025, 245, 114220. [Google Scholar] [CrossRef]
- Sun, L.; Yao, H.-J.; Li, J.-C.; Zhao, B.-Q.; Wang, Y.-A.; Zhang, Y.-G. Activated Carbon nanoparticles Loaded with Metformin for Effective Against Hepatocellular Cancer Stem Cells. Int. J. Nanomed. 2023, 18, 2891–2910. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Abdelaziz, A.M.; Shaker, O.G.; Ayeldeen, G. Metformin-loaded lecithin nanoparticles induce colorectal cancer cytotoxicity via epigenetic modulation of noncoding RNAs. Mol. Biol. Rep. 2021, 48, 6805–6820. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Kotak, D.J.; Devarajan, P.V. Design and Characterization of Metformin-Loaded Solid Lipid Nanoparticles for Colon Cancer. AAPS PharmSciTech 2017, 18, 358–368. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Chen, Q.; You, Y.; Li&Ast, X.; Chen, T. Functionalized Selenium Nanoparticles Synergizes With Metformin to Treat Breast Cancer Cells Through Regulation of Selenoproteins. Front. Bioeng. Biotechnol. 2021, 9, 758482. [Google Scholar] [CrossRef]
- Parikh, A.B.; Kozuch, P.; Rohs, N.; Becker, D.J.; Levy, B.P. Metformin as a repurposed therapy in advanced non-small cell lung cancer (NSCLC): Results of a phase II trial. Investig. New Drugs 2017, 35, 813–819. [Google Scholar] [CrossRef]
- Fatehi-Agdam, M.; Vatankhah, M.A.; Panahizadeh, R.; Jeddi, F.; Najafzadeh, N. Efficacy of Metformin and Chemotherapeutic Agents on the Inhibition of Colony Formation and Shh/Gli1 Pathway: Metformin/Docetaxel Versus Metformin/5-Fluorouracil. Drug Res. 2021, 71, 17–25. [Google Scholar] [CrossRef]
- Mlicka, A.; Mlicki, P.; Niewiadomski, P.; Zielińska, W.; Hałas-Wiśniewska, M.; Izdebska, M. Synergistic effect of metformin and doxorubicin on the metastatic potential of T24 cells. Acta Histochem. 2023, 125, 151975. [Google Scholar] [CrossRef]
- Patel, P.J.; Shah, J.S. Metformin pretreatment potentiates the antiproliferative action of doxorubicin against breast cancer. Ann. Pharm. Fr. 2023, 81, 636–652. [Google Scholar] [CrossRef]
- Sahu, P.; Camarillo, I.G.; Sundararajan, R. Enhanced Antiproliferation Potency of Electrical Pulse-Mediated Metformin and Cisplatin Combination Therapy on MDA-MB-231 Cells. Appl. Biochem. Biotechnol. 2022, 194, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Shanchun, H.; You, P.; Sujuan, N.; Xuebing, Z.; Yijie, B.; Xiaohui, X.; Jianming, H.; La, N.; Zhehui, B.; Qi, L.; et al. Integrative analyses of biomarkers and pathways for metformin reversing cisplatin resistance in head and neck squamous cell carcinoma cells. Arch. Oral. Biol. 2023, 147, 105637. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, T.C.; Tamura, R.E.; Junqueira, M.d.S.; Mororó, J.d.S.; Bustos, S.O.; Natalino, R.J.M.; Russell, S.; Désaubry, L.; Strauss, B.E.; Chammas, R. Metformin-induced chemosensitization to cisplatin depends on P53 status and is inhibited by Jarid1b overexpression in non-small cell lung cancer cells. Aging 2021, 13, 21914–21940. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.P.; Jr, T.C.T.; Pavan, I.C.B.; Silva, F.R.; Granato, D.C.; Peruca, G.F.; Pauletti, B.A.; Domingues, R.R.; Bezerra, R.M.N.; De Moura, L.P.; et al. Metformin impairs cisplatin resistance effects in A549 lung cancer cells through mTOR signaling and other metabolic pathways. Int. J. Oncol. 2021, 58, 28. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.M.; Lee, Y.-H.; Jang, S.; Yi, H.-K. Synergistic anti-cancer effects of metformin and cisplatin on YD-9 oral squamous carcinoma cells via AMPK pathway. J. Appl. Oral. Sci. 2025, 33, e20240385. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, X.; Yang, D.; Wei, W.; Gan, J.; Xia, X.; Chen, Q.; Jiang, J.; Feng, X. Metformin overcomes chemoresistance by regulating stemness via KLF4 in oral squamous cell carcinoma. Oral Dis. 2025, 31, 468–481. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, Z.; Zhu, S.; Zeng, Y.; Lin, Q.; Luo, L.; Hong, X. Effects of different combined regimens of cisplatin, metformin, and quercetin on nasopharyngeal carcinoma cells and subcutaneous xenografts. Sci. Rep. 2021, 11, 1040. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Wu, T.; Zhang, L.; Kang, J.; Liao, J.; Jiang, D.; Hu, Z.; Han, Z.; Zhou, B. Metformin combined with cisplatin reduces anticancer activity via ATM/CHK2-dependent upregulation of Rad51 pathway in ovarian cancer. Neoplasia 2024, 57, 101037. [Google Scholar] [CrossRef]
- Jafarzadeh, E.; Montazeri, V.; Aliebrahimi, S.; Sezavar, A.H.; Ghahremani, M.H.; Ostad, S.N. Targeting Cancer Stem Cells and Hedgehog Pathway: Enhancing Cisplatin Efficacy in Ovarian Cancer with Metformin. J. Cell Mol. Med. 2025, 29, e70508. [Google Scholar] [CrossRef]
- Xiao, Y.-Y.; Xiao, J.-X.; Wang, X.-Y.; Wang, T.; Qu, X.-H.; Jiang, L.-P.; Tou, F.-F.; Chen, Z.-P.; Han, X.-J. Metformin-induced AMPK activation promotes cisplatin resistance through PINK1/Parkin dependent mitophagy in gastric cancer. Front. Oncol. 2022, 12, 956190. [Google Scholar] [CrossRef]
- Fang, C.-W.; Yang, J.-S.; Chiang, J.-H.; Shieh, P.-C.; Tsai, F.-J.; Tsai, C.-W.; Chang, W.-S. Metformin induces autophagy of cisplatin-resistant human gastric cancer cells in addition to apoptosis. Biomedicine 2023, 13, 14–23. [Google Scholar] [CrossRef]
- Duan, G.; Qi, M.; Xun, L.; An, Y.; Zuo, Z.; Luo, Y.; Song, Z. Metformin Enhances the Chemosensitivity of Gastric Cancer to Cisplatin by Downregulating Nrf2 Level. Anal. Cell Pathol. 2025, 2025, 5714423. [Google Scholar] [CrossRef] [PubMed]
- Hassani, E.; Mozzendizaji, S.; Shafiei-Irannejad, V.; Mohammadzadeh, A. Metformin boosts doxorubicin efficacy and increases CD8+ T cell frequency in mouse breast cancer. Clin. Transl. Oncol. 2025, 27, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ibarra, K.C.; López, J.Y.S.; Razo, T.D.P.; Lozano, J.R.C.; Ortiz-Tamayo, B.G.; Palafox-Mariscal, L.A.; Arreola, R.M.G.; González-García, J.R.; Ortiz-Lazareno, P.C. Metformin in combination with chemotherapy increases apoptosis in gastric cancer cells and counteracts senescence induced by chemotherapy. Oncol. Lett. 2024, 28, 457. [Google Scholar] [CrossRef] [PubMed]
- Asghariazar, V.; Ojarood, M.V.; Vatankhah, M.A.; Panahizadeh, R.; Haris, H.M.; Najafzadeh, N.; Khakbaz, P.; Soozangar, N.; Jeddi, F. Metformin reverses 5-FU chemoresistance by downregulating DKK1, WNT5A, and ABCB1 expressions in gastric cancer: An experimental and bioinformatic study. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 10155–10167. [Google Scholar] [CrossRef]
- Sang, J.; Tang, R.; Yang, M.; Sun, Q. Metformin Inhibited Proliferation and Metastasis of Colorectal Cancer and presented a Synergistic Effect on 5-FU. Biomed. Res. Int. 2020, 2020, 9312149. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Zhao, Q.; Chen, H.; Du, S.; Zeng, Z. Metformin reverses 5-FU resistance induced by radiotherapy through mediating folate metabolism in colorectal cancer. Mol. Med. 2025, 31, 199. [Google Scholar] [CrossRef]
- Ling, S.; Tian, Y.; Zhang, H.; Jia, K.; Feng, T.; Sun, D.; Gao, Z.; Xu, F.; Hou, Z.; Li, Y.; et al. [Corrigendum] Metformin reverses multidrug resistance in human hepatocellular carcinoma Bel-7402/5-fluorouracil cells. Mol. Med. Rep. 2022, 25, 175. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Mehrabian, A.; Mirzavi, F.; Rezayat, S.M.; Mashreghi, M.; Farhoudi, L.; Kharrazi, S.; Sadri, K.; Jaafari, M.R. Docetaxel in combination with metformin enhances antitumour efficacy in metastatic breast carcinoma models: A promising cancer targeting based on PEGylated liposomes. J. Pharm. Pharmacol. 2022, 74, 1307–1319. [Google Scholar] [CrossRef]
- Catapano, J.; Luty, M.; Wróbel, T.; Pudełek, M.; Piwowarczyk, K.; Kędracka-Krok, S.; Siedlar, M.; Madeja, Z.; Czyż, J. Acquired drug resistance interferes with the susceptibility of prostate cancer cells to metabolic stress. Cell Mol. Biol. Lett. 2022, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Wang, B.; Zhai, J. Metformin Affects Paclitaxel Sensitivity of Ovarian Cancer Cells Through Autophagy Mediated by Long Noncoding RNASNHG7/miR-3127–5p Axis. Cancer Biother. Radiopharm. 2022, 37, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, Y.; Yu, Z.; Luo, Z. Metformin Suppresses Stemness of Non-Small-Cell Lung Cancer Induced by Paclitaxel through FOXO3a. Int. J. Mol. Sci. 2023, 24, 16611. [Google Scholar] [CrossRef] [PubMed]
- Al-Kofahi, T.; Altrad, B.; Amawi, H.; A Aljabali, A.; Abul-Haija, Y.M.; A Obeid, M. Paclitaxel-loaded niosomes in combination with metformin: Development, characterization and anticancer potentials. Ther. Deliv. 2024, 15, 109–118. [Google Scholar] [CrossRef]
- Feng, S.-W.; Chang, P.-C.; Chen, H.-Y.; Hueng, D.-Y.; Li, Y.-F.; Huang, S.-M. Exploring the Mechanism of Adjuvant Treatment of Glioblastoma Using Temozolomide and Metformin. Int. J. Mol. Sci. 2022, 23, 8171. [Google Scholar] [CrossRef]
- Ohno, M.; Kitanaka, C.; Miyakita, Y.; Tanaka, S.; Sonoda, Y.; Mishima, K.; Ishikawa, E.; Takahashi, M.; Yanagisawa, S.; Ohashi, K.; et al. Metformin with Temozolomide for Newly Diagnosed Glioblastoma: Results of Phase I Study and a Brief Review of Relevant Studies. Cancers 2022, 14, 4222. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.L. Drug repurposing for cancer therapy. Signal Transduct. Target Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef]
- Bakry, H.M.; Mansour, N.O.; ElKhodary, T.R.; Soliman, M.M. Efficacy of metformin in prevention of paclitaxel-induced peripheral neuropathy in breast cancer patients: A randomized controlled trial. Front. Pharmacol. 2023, 14, 1181312. [Google Scholar] [CrossRef]
- Martin-Castillo, B.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; Amillano, K.; Domínguez, S.; et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: The METTEN study. Oncotarget 2018, 9, 35687–35704. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, C.; Wang, Z.; Zhang, J.; Wang, L.; Zhang, S.; Cao, J.; Tao, Z.; Li, T.; Wang, B.; et al. A randomized phase II study of aromatase inhibitors plus metformin in pre-treated postmenopausal patients with hormone receptor positive metastatic breast cancer. Oncotarget 2017, 8, 84224–84236. [Google Scholar] [CrossRef]
- Fenn, K.; Maurer, M.; Lee, S.M.; Crew, K.D.; Trivedi, M.S.; Accordino, M.K.; Hershman, D.L.; Kalinsky, K. Phase 1 Study of Erlotinib and Metformin in Metastatic Triple-Negative Breast Cancer. Clin. Breast Cancer 2020, 20, 80–86. [Google Scholar] [CrossRef]
- Rocca, A.; Cortesi, P.; Cortesi, L.; Gianni, L.; Matteucci, F.; Fantini, L.; Maestri, A.; Giunchi, D.C.; Cavanna, L.; Ciani, R.; et al. Phase II study of liposomal doxorubicin, docetaxel and trastuzumab in combination with metformin as neoadjuvant therapy for HER2-positive breast cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835920985632. [Google Scholar] [CrossRef]
- Gennari, A.; Foca, F.; Zamarchi, R.; Rocca, A.; Amadori, D.; De Censi, A.; Bologna, A.; Cavanna, L.; Gianni, L.; Scaltriti, L.; et al. Insulin-like growth factor-1 receptor (IGF-1R) expression on circulating tumor cells (CTCs) and metastatic breast cancer outcome: Results from the TransMYME trial. Breast Cancer Res. Treat. 2020, 181, 61–68. [Google Scholar] [CrossRef]
- Akce, M.; Farran, B.; Switchenko, J.M.; Rupji, M.; Kang, S.; Khalil, L.; Ruggieri-Joyce, A.; Olson, B.; Shaib, W.L.; Wu, C.; et al. Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer. J. Immunother. Cancer 2023, 11, e007235. [Google Scholar] [CrossRef]
- Ahmed, J.; Stephen, B.; Khawaja, M.R.; Yang, Y.; Salih, I.; Barrientos-Toro, E.; Raso, M.G.; Karp, D.D.; Piha-Paul, S.A.; Sood, A.K.; et al. A phase I study of temsirolimus in combination with metformin in patients with advanced or recurrent endometrial cancer. Gynecol. Oncol. 2025, 193, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Desai, J.; Palackdharry, S.M.; Morris, J.C.; Zhu, Z.; Jandarov, R.; Riaz, M.K.; Takiar, V.; Mierzwa, M.; Gutkind, J.S.; et al. Phase 1 dose-finding study of metformin in combination with concurrent cisplatin and radiotherapy in patients with locally advanced head and neck squamous cell cancer. Cancer 2020, 126, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Barrón, F.; Padilla, M.Á.S.; Avilés-Salas, A.; Ramírez-Tirado, L.A.; Jiménez, M.J.A.; Vergara, E.; Zatarain-Barrón, Z.L.; Hernández-Pedro, N.; Cardona, A.F.; et al. Effect of Metformin Plus Tyrosine Kinase Inhibitors Compared With Tyrosine Kinase Inhibitors Alone in Patients with Epidermal Growth Factor Receptor-Mutated Lung Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, e192553. [Google Scholar] [CrossRef] [PubMed]
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naive Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865. [Google Scholar] [CrossRef]
- Skinner, H.; Hu, C.; Tsakiridis, T.; Santana-Davila, R.; Lu, B.; Erasmus, J.J.; Doemer, A.J.; Videtic, G.M.; Coster, J.; Yang, A.X.; et al. Addition of Metformin to Concurrent Chemoradiation in Patients with Locally Advanced Non-Small Cell Lung Cancer: The NRG-LU001 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- IRCCS San Raffaele. Combination Chemotherapy with or Without Metformin Hydrochloride in Treating Patients with Metastatic Pancreatic Cancer, NCT Number NCT01167738. 2015. Available online: https://clinicaltrials.gov/study/NCT01167738 (accessed on 26 October 2025).
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathôt, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef]
- Martin, M.P.; Borchiellini, D.; Thamphya, B.; Guillot, A.; Paoli, J.B.; Besson, D.; Hilgers, W.; Priou, F.; El Kouri, C.; Hoch, B.; et al. TAXOMET: A French Prospective Multicentric Randomized Phase II Study of Docetaxel Plus Metformin Versus Docetaxel Plus Placebo in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, 501–509. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients with Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- De, A.; Kuppusamy, G. Metformin in breast cancer: Preclinical and clinical evidence. Curr. Probl. Cancer. 2020, 44, 100488. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.-C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; Baglioni, M.; Bondarenko, M.; Laluce, N.C.; Rozados, V.; André, N.; Carré, M.; Scharovsky, O.G.; Márquez, M.M. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget 2017, 8, 2874–2889. [Google Scholar] [CrossRef] [PubMed]
- Falah, R.R.; Talib, W.H.; Shbailat, S.J. Combination of metformin and curcumin targets breast cancer in mice by angiogenesis inhibition, immune system modulation and induction of p53 independent apoptosis. Ther. Adv. Med. Oncol. 2017, 9, 235–252. [Google Scholar] [CrossRef]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 2015, 6, 25677–25695. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Amirabadizadeh, A.; Aramjoo, H.; Llorens, S.; Roshanravan, B.; Saeedi, F.; Talebi, M.; Shakibaei, M.; Samarghandian, S. Impact of Metformin on Cancer Biomarkers in Non-Diabetic Cancer Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Curr. Oncol. 2021, 28, 1412–1423. [Google Scholar] [CrossRef]
- Laskov, I.; Drudi, L.; Beauchamp, M.-C.; Yasmeen, A.; Ferenczy, A.; Pollak, M.; Gotlieb, W.H. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecol. Oncol. 2014, 134, 607–614. [Google Scholar] [CrossRef]
- Wen, K.-C.; Sung, P.-L.; Wu, A.T.H.; Chou, P.-C.; Lin, J.-H.; Huang, C.-Y.F.; Yeung, S.-C.J.; Lee, M.-H. Neoadjuvant metformin added to conventional chemotherapy synergizes anti-proliferative effects in ovarian cancer. J. Ovarian Res. 2020, 13, 95. [Google Scholar] [CrossRef]
- Bragagnoli, A.C.; Araujo, R.L.C.; Ferraz, M.W.; dos Santos, L.V.; Abdalla, K.C.; Comar, F.; Santos, F.A.; Oliveira, M.A.; Carvalheira, J.B.C.; Cárcano, F.M.; et al. Metformin plus lrinotecan in patients with refractory colorectal cancer: A phase 2 clinical trial. Br. J. Cancer 2021, 124, 1072–1078. [Google Scholar] [CrossRef]
- Gutkind, J.S.; Molinolo, A.A.; Wu, X.; Wang, Z.; Nachmanson, D.; Harismendy, O.; Alexandrov, L.B.; Wuertz, B.R.; Ondrey, F.G.; Laronde, D.; et al. Inhibition of mTOR signaling and clinical activity of metformin in oral premalignant lesions. JCI Insight 2021, 6, e147096. [Google Scholar] [CrossRef] [PubMed]
- van Eijck, C.W.F.; Vadgama, D.; van Eijck, C.H.J.; Wilmink, J.W.; for The Dutch Pancreatic Cancer Group (DPCG). Metformin boosts antitumor immunity and improves prognosis in upfront resected pancreatic cancer: An observational study. J. Natl. Cancer Inst. 2024, 116, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorg. Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Usha, T.; Middha, S.K.; Kukanur, A.A.; Shravani, R.V.; Anupama, M.N.; Harshitha, N.; Rahamath, A.; Kulkarni, S.S.; Goyal, A.K. Drug Repurposing Approaches: Existing Leads for Novel Threats and Drug Targets. Curr. Protein Pept. Sci. 2021, 22, 251–271. [Google Scholar] [CrossRef]
- Al Khzem, A.H.; Gomaa, M.S.; Alturki, M.S.; Tawfeeq, N.; Sarafroz, M.; Alonaizi, S.M.; Al Faran, A.; Alrumaihi, L.A.; Alansari, F.A.; Alghamdi, A.A. Drug Repurposing for Cancer Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 12441. [Google Scholar] [CrossRef]
- Lord, S.R.; Harris, A.L. Is it still worth pursuing the repurposing of metformin as a cancer therapeutic? Br. J. Cancer 2023, 128, 958–966. [Google Scholar] [CrossRef]

| Cancer Type | In Vitro Cell Lines/In Vivo Model and Dose Range | Effects on Cancer Characteristics | Mechanism | Metabolic Effects | References |
|---|---|---|---|---|---|
| Ovarian | In vitro studies with SKOV3 and A2780 cell lines | Reduces cell viability and proliferation; induces apoptosis and ferroptosis | Inhibition of mitochondrial complex I; energy depletion; ferroptosis under stress | Mitochondrial dysfunction and decreased ATP production; altered glucose metabolism | [130] |
| Head and Neck Squamous Cell Carcinoma (HNSCC) | In vitro studies with FaDU, Detroit 562 and HNSCC cell lines | Reduces growth and colony formation; cell cycle arrest | Complex I inhibition; loss of NAD+/NADH homeostasis | Reduced ATP levels; increased metabolic stress markers (increased AMP/ATP ratio) | [131,132] |
| Lung | In vitro studies with BEAS-2B, 1170 and NSCLC H522, H2030, H1299, H2009, H838, A549 and H1975 cell lines In vivo studies with A/J mice (200 mg metformin /kg by intraperitoneal injection) | Decreases proliferation and colony formation; induces apoptosis through oxidative stress | Complex I inhibition causing energy depletion; increased mitochondrial ROS | Lower ATP production; altered lactate levels | [133] |
| Liver | In vitro studies with LO2, HepG2, Huh-7, LX-2 cell lines and with primary human hepatocytes | Reduces proliferation; induces autophagy and mitochondrial dysfunction; inhibition of cell cycle progression | Mitochondrial dysfunction; inhibition of mitochondrial complex I; disruption of mitochondrial voltage-dependent anion channel 1 (VDAC1) | Decreased ATP production; increased lactate production; influence on metabolite levels of lactate, alanine, glycerol-3-phosphate, glycerol, glycine, and glutamate, derived from glucose | [134,135,136] |
| Breast | In vitro studies with MCF-7, BT-474, SKBR-3 and TNBC Hs 578T and MDA-MB-231 cell lines | Suppresses proliferation; activates apoptosis; reduces cell growth and colony formation; potentiates the effect of conventional anticancer drugs; cell cycle arrest | Direct inhibition of complex I leading to metabolic stress; decreased mitochondrial respiration and increased aerobic glycolysis | Increased glucose consumption and lactate production; short-term exposure to metformin reduces cellular glucose uptake, but long-term exposure leads to the opposite | [137,138,139,140] |
| Pancreatic | In vitro studies with AsPC-1, BxPC-3, PANC-1 and MIAPaCa-2 cell lines and with primary pancreatic CSC In vivo studies with mice xenografted with pancreatic carcinomas (150 mg metformin/kg by intraperitoneal injection) | Limits tumor progression and proliferation; specifically eliminates pancreatic cancer stem cells | Complex I inhibition, reducing oxidative phosphorylation | Decreased ATP production; impaired mitochondrial metabolism; hyperglycemia protects against the metformin-induced growth inhibition | [141,142] |
| Leukemia | In vitro studies with REL NALM-6, HL60, MOLM14 and U937 cell lines | Induces apoptosis and energy stress; induces superoxide generation and oxidative stress; blockage of cell cycle and inhibition of cell proliferation and colony formation of leukemic cells | Inhibition of mitochondrial electron transport | Reduced oxygen consumption and mitochondrial ATP synthesis; increased glycolysis and lactate production, pentose phosphate pathway and fatty acid metabolism | [143,144] |
| Prostate | In vitro studies with LNCaP, P69, PC-3 and DU145 cell lines | Decreases proliferation and migration; induces energetic crisis; synergic effect with 2DG | Complex I inhibition induces energetic crisis | Decreased ATP levels; metabolic crisis indicators | [145] |
| Colorectal | In vitro studies with HCT116 p53−/− cell line In vivo studies with mice xenografted with this cell line (drinking water containing 1–5 mg/mL of metformin) | Sensitizes cells to chemotherapy; reduces proliferation, migration, and invasion | Complex I inhibition causes energy depletion and enhances chemosensitivity | Reduced ATP; altered glucose metabolism | [146] |
| Nanoparticle Type | Composition/Carrier | Target/Indication | Purpose | Mechanism/Outcome | References |
|---|---|---|---|---|---|
| Functionalized polymeric NPs | Folate-PLGA-PEG | Breast cancer (MDA-MB-231) | Targeted delivery, enhanced apoptosis and gene modulation | The effect of metformin was enhanced through increased cellular internalization, activation of the AMPK pathway, and inhibition of the mTOR pathway, promoting apoptosis and cell cycle arrest | [211] |
| Functionalized chitosan NPs | WZB117-OCMC | Breast cancer | Target GLUT1-overexpressing breast cancer cells and enhance metabolic disruption via dual delivery | Dual inhibition of GLUT1 and mTOR pathways enhances metformin uptake, reduces glycolysis, and promotes apoptosis and cell cycle arrest | [209] |
| O-CMC (O-carboxymethyl chitosan) | Pancreatic cancer | Improve intracellular retention and controlled release of metformin | NPs enhance metformin’s cellular uptake and retention, enabling sustained drug release and increased apoptosis in pancreatic cancer cells | [215] | |
| Polymeric NPs | PLGA | Liver cancer (HepG2 cells) | Deliver metformin efficiently to hepatocellular carcinoma cells and enhance radiosensitization | NPs enhance metformin’s antitumor effect by blocking autophagy, inhibiting mTOR/p53/HIF1A signaling, and inducing cell cycle arrest in HepG2 cells | [212] |
| Dual gold NP system | MET-GNPs + COL-GNPs | Breast cancer spheroids | Facilitate deep tumor penetration and selective delivery of metformin in dense tumor ECM | NPs enhance tumor penetration by degrading ECM and improve metformin delivery, leading to increased apoptosis in breast cancer spheroids | [210] |
| Hybrid NPs | Chitosan + Silver NPs | Breast cancer (radiation therapy) | Radiosensitization enhancement during radiotherapy | NPs enhance radiosensitization by increasing oxidative stress, impairing DNA repair, and promoting apoptosis in breast cancer cells | [216] |
| Carbon-based NPs | Activated Carbon NPs | Hepatocellular CSCs | Target hepatocellular CSCs with sustained metformin delivery and selective cytotoxicity | Activated carbon nanoparticles improve metformin delivery to hepatocellular CSCs, enhancing apoptosis and suppressing proliferation via AMPK activation and mTOR inhibition | [217] |
| Topical polymeric NPs | HA-coated chitosan/gelatin | Melanoma (topical) | Enable topical delivery of metformin to melanoma cells with enhanced skin penetration and retention | HA-coated chitosan/gelatin NPs enhance topical metformin delivery by improving skin penetration, cellular uptake, and cytotoxicity against melanoma cells | [213] |
| Functionalized mesoporous silica NPs | HA-coated MSNs | Lung cancer (A549) | Target CD44-positive lung cancer cells via HA-modified silica NPs for selective metformin delivery | HA-modified mesoporous silica NPs enhance metformin delivery to A549 cells via CD44 targeting, promoting AMPK activation, mTOR inhibition, and apoptosis | [214] |
| Lipid-based NPs | Lecithin | Colorectal cancer | Improve metformin bioavailability and enable epigenetic modulation | Lecithin NPs enhance metformin’s cytotoxicity in colorectal cancer cells via epigenetic modulation of noncoding RNAs, leading to apoptosis and reduced cell viability | [218] |
| Colon cancer | Enhance metformin stability and sustained release for colorectal cancer therapy | NPs improve metformin’s stability and sustained release, enhancing cellular uptake and cytotoxicity against colorectal cancer cells. | [219] | ||
| Functionalized selenium NPs | TW80-SeNPs + Metformin | Breast cancer (MCF-7) | Combine selenium NPs with metformin to synergistically target breast cancer cells | NPs synergize with metformin by modulating selenoproteins, increasing oxidative stress, and promoting apoptosis in breast cancer cell | [220] |
| Gold NPs | AuNPs + Metformin | Breast and lung cancer (MCF-7, A549) | Evaluate synergistic antitumor effects of metformin and gold NPs | Nps synergize with metformin to enhance apoptosis, modulate BAX/BCL2 expression, and PI3K/Akt/mTOR pathway inhibition, reducing viability in breast and lung cancer cells | [208] |
| Chemotherapeutic Agent | Cancer Type | Observed Effect | Mechanism of Synergy | References |
|---|---|---|---|---|
| Carboplatin + Pemetrexed | Advanced NSCLC, non-squamous | ORR of 23%, PFS of 3.9 months, OS of 11.7 months. No significant improvement over historical controls | Potential metabolic modulation via AMPK activation and mTOR inhibition. No LKB1/STK11 mutations identified in patients | [221] |
| Cisplatin | NSCLC | Metformin sensitized p53 wild-type NSCLC cells (A549, HCC827) to cisplatin; no effect in p53-null cells (H1299, H358) | Chemosensitization is p53-dependent; inhibited by Jarid1b overexpression. Metformin alters p53 localization to mitochondria and reverses cisplatin-induced resistance | [227] |
| Metformin restored cisplatin sensitivity in resistant cells (A549); reduced cell viability and colony formation; enhanced apoptosis | Inhibition of mTOR signaling; modulation of apoptosis, oxidative stress, and G2/M transition; regulation of transcription and RNA processing pathways | [228] | ||
| Triple-negative breast cancer (MDA-MB-231 cells) | Cell viability reduced to ~25.9% with 30 µM cisplatin + 5 mM metformin after 24 h; enhanced antiproliferative effect compared to drugs alone | Electrical pulses enhanced drug uptake; metformin suppressed glucose metabolism and increased ROS, promoting apoptosis | [225] | |
| HNSCC | Metformin reversed cisplatin resistance; enhanced apoptosis and reduced proliferation in resistant cells | Transcriptomic analysis revealed modulation of base excision repair pathway and other DNA damage response genes; reduced self-repair capacity after chemotherapy | [226] | |
| Oral squamous cell carcinoma | Synergistic reduction in cell viability and proliferation; increased apoptosis and ROS; inhibition of EMT and migration | Activation of AMPK pathway; inhibition of EMT; confirmed by reversal with AMPK inhibitor (Compound C) | [229] | |
| Synergistic inhibition of cell proliferation and increased apoptosis in chemoresistant cells | Metformin downregulates cancer stemness via suppression of KLF4 expression, enhancing cisplatin sensitivity | [230] | ||
| Nasopharyngeal carcinoma (Sune-1 cells and xenografts) | The combinations significantly inhibited cell proliferation and tumor growth | Synergistic inhibition via distinct anticancer mechanisms; metformin contributed to metabolic stress and enhanced cisplatin efficacy | [231] | |
| Ovarian cancer | Combination reduced apoptosis and DNA damage; induced resistance to cisplatin | Metformin activated the ATM/CHK2 pathway, leading to upregulation of Rad51, which enhanced DNA repair and reduced cisplatin efficacy | [232] | |
| Synergistic inhibition of cell viability, proliferation, and colony formation; enhanced apoptosis and S-phase arrest | Downregulation of pluripotency factors (Oct-4, Sox2, Nanog); inhibition of Hedgehog signaling; suppression of MDR1 and ERCC1; modulation of autophagy and DNA damage response | [233] | ||
| Gastric cancer | Metformin reduced cisplatin cytotoxicity; increased cell survival and resistance | AMPK activation induced PINK1/Parkin-dependent mitophagy, promoting mitochondrial quality control and reducing apoptosis | [234] | |
| Metformin reduced viability and confluence of resistant cells; induced both autophagy and apoptosis | Induction of autophagy markers (Atg5, Atg12, LC3-II); increased caspase-3 and -7 activity; effect reversed by 3-MA, confirming autophagy involvement | [235] | ||
| Increased chemosensitivity to cisplatin; reduced cell viability and metabolic activity; enhanced apoptosis and oxidative stress | Downregulation of Nrf2; activation of p53 and AMPK pathways; reversal of effects by Nrf2 overexpression or AMPK/p53 inhibition | [236] | ||
| Doxorubicin | Bladder cancer (T24 cells) | Synergistic inhibition of metastatic potential; reduced migration and invasion | Likely via modulation of EMT and metabolic stress pathways | [223] |
| Breast cancer (4T1 mouse model) | Enhanced antitumor efficacy; increased CD8+ T cell frequency in tumor microenvironment; reduced toxicity | Immunomodulation via increased CD8+ T cell infiltration; modulation of HIF-1α and STAT3 expression; reduced doxorubicin toxicity | [237] | |
| Breast cancer (DMBA-induced in rats) | Reduced tumor incidence and volume; improved survival; reduced organ toxicity | Enhanced antioxidant and anti-inflammatory activity; reduced Ki67 expression; decreased IL-6, IL-1β, and NF-κB levels | [224] | |
| 5-Fluorouracil | Gastric cancer | Inhibition of tumor colony formation; reduced expression of Gli1 and TWIST1 | Suppression of the Shh/Gli1 signaling pathway; EMT modulation | [222] |
| Increased apoptosis; reduced chemotherapy-induced senescence | Loss of mitochondrial membrane potential; activation of caspase-dependent apoptosis pathways | [238] | ||
| Reversal of 5-FU resistance; increased apoptosis and cytotoxicity | Downregulation of DKK1, WNT5A, ABCB1 (MDR1), P-gp, and CD44; inhibition of drug resistance pathways | [239] | ||
| Colorectal cancer | Inhibition of proliferation and metastasis; enhanced anti-tumor effect in vivo | Synergistic suppression of tumor growth and invasion; possibly via modulation of cell cycle and apoptosis pathways | [240] | |
| Reversal of 5-FU resistance induced by radiotherapy; enhanced chemosensitivity | Restoration of folate metabolism via HM13-GGH-5,10-CH2-THF axis; modulation of gene expression linked to drug resistance | [241] | ||
| Hepatocellular carcinoma | Reversal of multidrug resistance; enhanced anti-proliferative and pro-apoptotic effects | Activation of AMPK/mTOR pathway; suppression of HIF-1α; downregulation of P-gp and MRP1 | [242] | |
| Docetaxel | Gastric cancer | Inhibition of tumor colony formation; reduced expression of Gli1 and TWIST1 | Suppression of the Shh/Gli1 signaling pathway; EMT modulation | [222] |
| Breast cancer (metastatic) | Enhanced antitumor efficacy; prolonged survival in vivo | Dual targeting of tumor bulk and cancer stem-like cells; improved delivery via PEGylated liposomes | [243] | |
| Prostate cancer | Reduced proliferation, motility, and viability in wild-type cells; partial reversal of resistance | Inactivation of ABC drug transporters; metabolic imbalance; Warburg effect induction; lineage-specific EMT responses | [244] | |
| Paclitaxel | Ovarian cancer | Reversal of paclitaxel resistance; reduced cell viability, migration, and invasion | Inhibition of SNHG7/miR-3127-5p axis; suppression of autophagy; promotion of apoptosis | [245] |
| NSCLC | Suppression of cancer stemness; reduced self-renewal and tumorigenicity in resistant cells | Activation of AMPK; upregulation of FOXO3a; inhibition of Akt and MEK pathways; downregulation of stemness markers (c-MYC, Oct4, Nanog, Notch) | [246] | |
| Breast cancer (T47D cells) | Significant reduction in IC50 of paclitaxel when combined with metformin; enhanced cytotoxicity | Enhanced cellular uptake via niosomes; metformin-induced sensitization; potential reduction in required paclitaxel dose | [247] | |
| Temozolomide | Glioblastoma | Enhanced inhibition of proliferation and migration; increased apoptosis | Suppression of mitochondrial biogenesis, EMT, and MGMT expression; increased ROS production and mitochondrial dysfunction | [248] |
| Well-tolerated combination; promising progression-free survival and overall survival | Activation of AMPK-FOXO3a pathway; differentiation of glioma stem-like cells; suppression of tumor formation | [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, F.; Cunha, A.; Barbosa, J.; Faria, J.; Queirós, O. The Dual Role of Metformin: Repurposing an Antidiabetic Drug for Cancer Therapy. Appl. Sci. 2025, 15, 11576. https://doi.org/10.3390/app152111576
Barbosa F, Cunha A, Barbosa J, Faria J, Queirós O. The Dual Role of Metformin: Repurposing an Antidiabetic Drug for Cancer Therapy. Applied Sciences. 2025; 15(21):11576. https://doi.org/10.3390/app152111576
Chicago/Turabian StyleBarbosa, Flávia, Andrea Cunha, Joana Barbosa, Juliana Faria, and Odília Queirós. 2025. "The Dual Role of Metformin: Repurposing an Antidiabetic Drug for Cancer Therapy" Applied Sciences 15, no. 21: 11576. https://doi.org/10.3390/app152111576
APA StyleBarbosa, F., Cunha, A., Barbosa, J., Faria, J., & Queirós, O. (2025). The Dual Role of Metformin: Repurposing an Antidiabetic Drug for Cancer Therapy. Applied Sciences, 15(21), 11576. https://doi.org/10.3390/app152111576










