Chemical and Biochemical Properties of Verjuice Obtained from Vitis labrusca Grapes by Using Different Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.1.1. Grapes
2.1.2. Chemicals
2.2. Extraction Procedures
2.2.1. Pressing Extraction (PE)
2.2.2. Steam Extraction (SE)
2.2.3. Centrifuge Juicer Extraction (CJE)
2.3. Yield
2.4. Oenological Parameters
2.5. Total Polyphenolics, Antioxidant and Anti-Hypertensive Activities
2.6. Determination of Volatile Organic Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yield
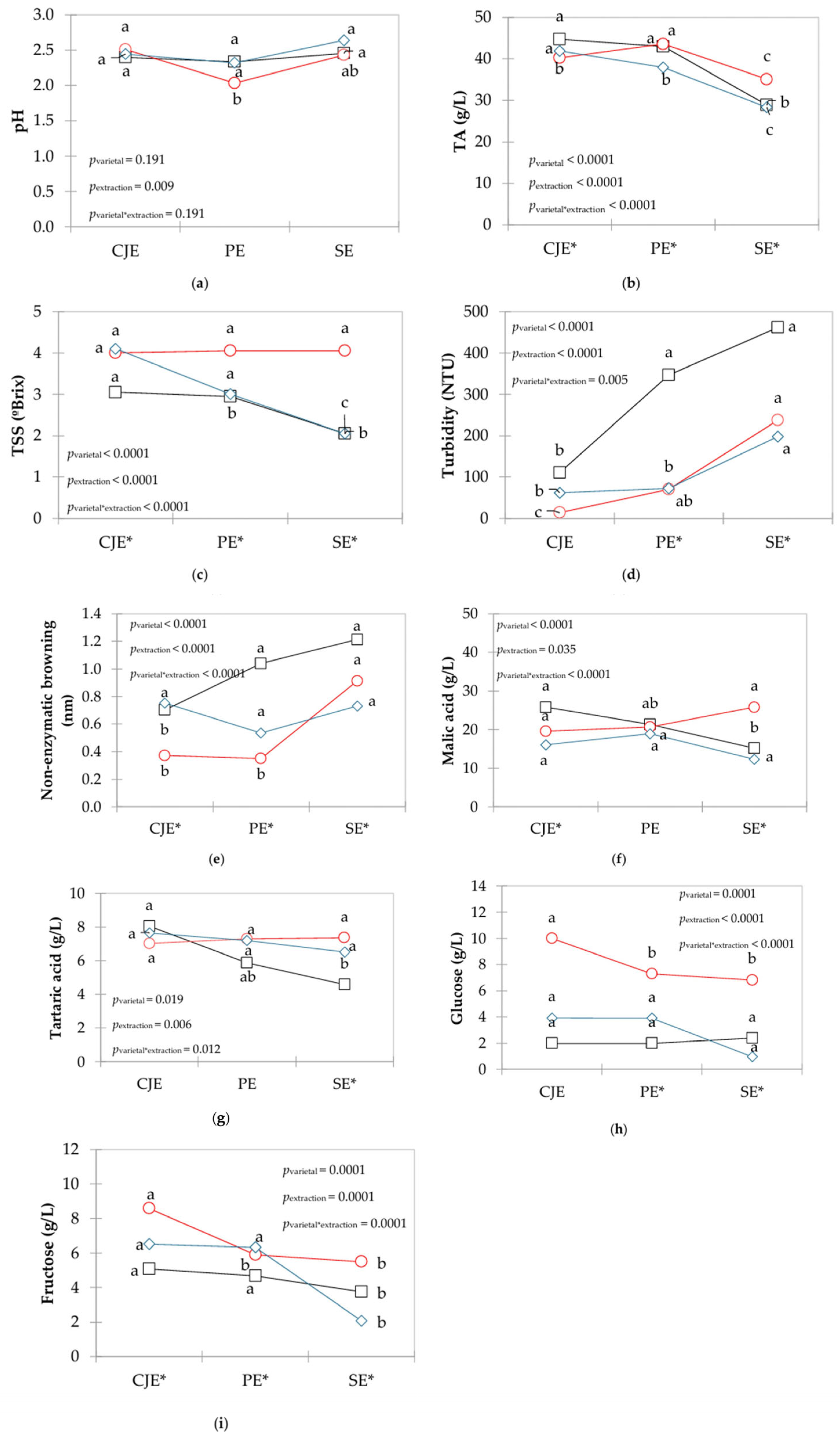
3.2. Oenological Parameters
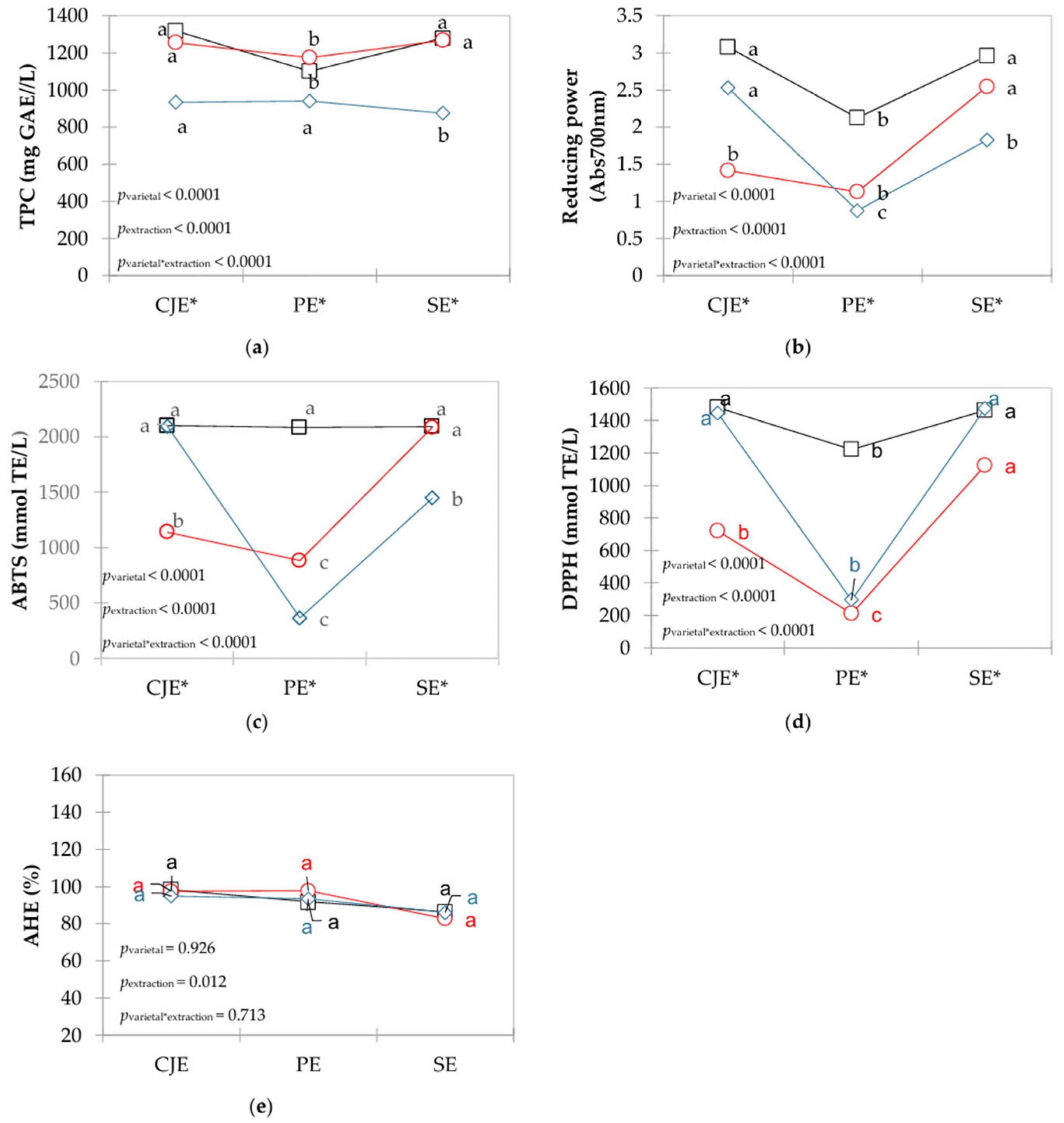
3.3. Biological Properties
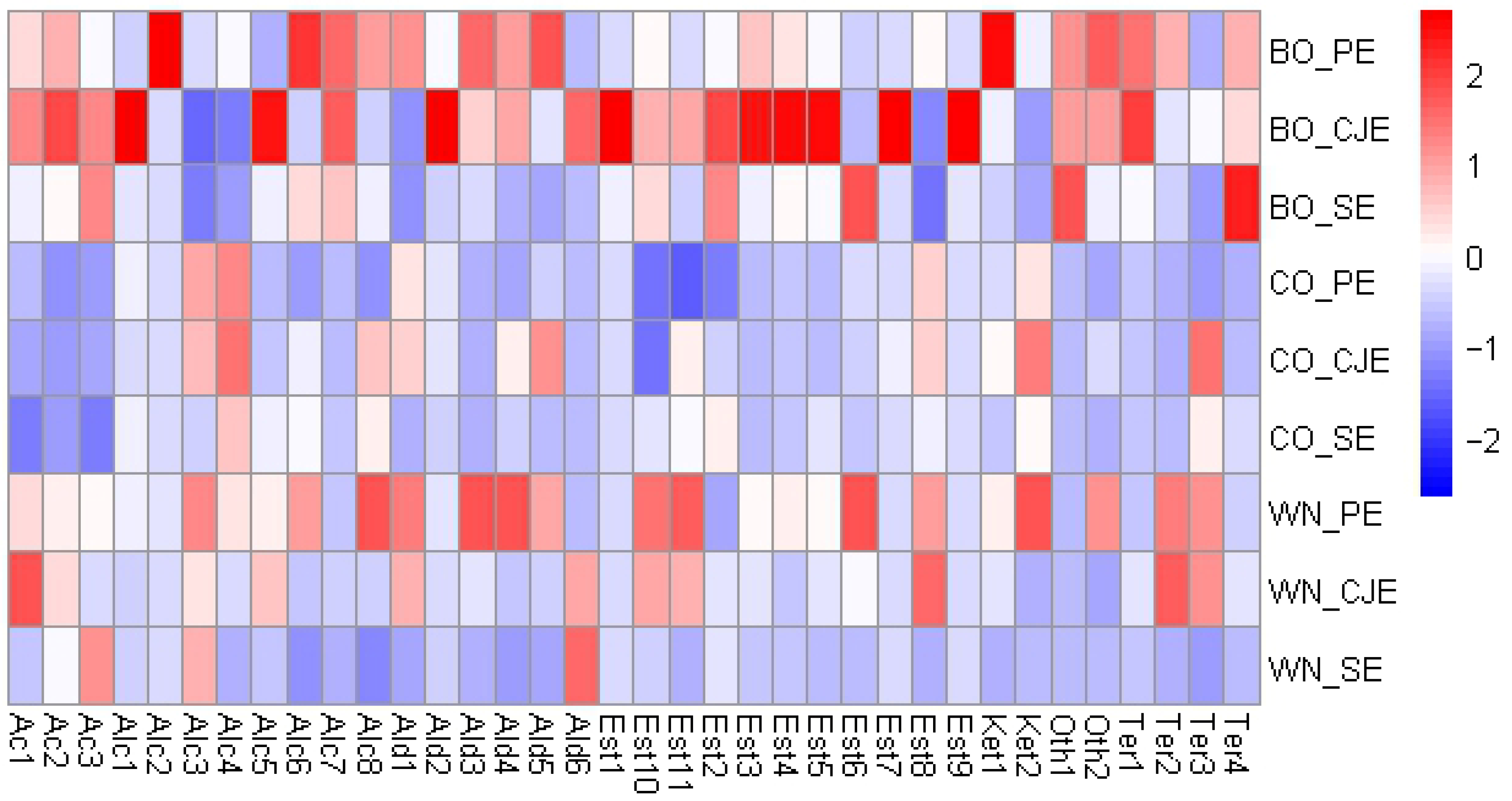
3.4. VOC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | Concord grape varietal |
| BO | Bordô grape varietal |
| WN | White Niágara grape varietal |
| PE | Pressing extraction |
| CJE | Centrifuge juicer extraction |
| SE | Steam extraction |
| TPC | Total phenolic compounds |
| ABTS | Antioxidant activity measured by the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) method |
| DPPH | Antioxidant activity measured by 2,2-difenil-1-picrilhidrazil method |
| VOC | Volatile organic compounds |
| GAE/L | Galic acid equivalent per liter |
| mmol TE/L | Mili mol Trolox equivalent per liter |
| TSS | Total soluble solids |
| TA | Titratable acidity |
References
- Guidoni, S.; Ferrandino, A.; Novello, V. Effects of seasonal and agronomical practices on skin anthocyanin profile of Nebbiolo grapes. Am. J. Enol. Vitic. 2008, 59, 22–29. [Google Scholar] [CrossRef]
- Öncül, N.; Karabiyikli, Ş. Factors affecting the quality attributes of unripe grape functional food products. J. Food Biochem. 2015, 39, 689–695. [Google Scholar] [CrossRef]
- Fia, G.; Bucalossi, G.; Proserpio, C.; Vincenzi, S. Unripe grapes: An overview of the composition, traditional and innovative applications, and extraction methods of a promising waste of viticulture. Aust. J. Grape Wine Res. 2022, 28, 8–26. [Google Scholar] [CrossRef]
- Simone, G.V.; Montevecchi, G.; Masino, F.; Matrella, V.; Imazio, S.A.; Antonelli, A.; Bignami, C. Ampelographic and chemical characterization of Reggio Emilia and Modena (northern Italy) grapes for two traditional seasonings: ‘saba’ and ‘agresto’. J. Sci. Food Agric. 2013, 93, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Cheikh-Ali, H.; Hijazi, A.; Merah, O.; Al-Rekaby, A.E.A.N.; Awada, R. Phytochemical profile, antioxidant andantitumor activities of green grape juice. Processes 2020, 8, 507. [Google Scholar] [CrossRef]
- Mansour, B.; Shaheen, N.; Kmail, A.; Haggag, N.; Saad, S.; Sadiq, O.; Zaid, R.; Saad, B. Anti-Inflammatory and Anti-Adipogenesis Effects of Alchemilla vulgaris L., Salvia officinalis L., and Vitis vinifera L. in THP-1-Derived Macrophages and 3T3-L1 Cell Line. Immuno 2023, 3, 148–159. [Google Scholar] [CrossRef]
- Dupas De Matos, A.; Curioni, A.; Bakalisnki, A.T.; Marangon, M.; Pasini, G.; Vincenzzi, S. Chemical and sensory analysis of verjuice: An acidic food ingredient obtained from unripe grape berries. Innov. Food Sci. Emerg. Technol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- Coombe, B. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 100–110. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Reis, M.G.; Maggs, R.; Hort, J. Understanding consumer acceptability of verjuice, its potential applications and sensory and chemical drivers of liking. Food Res. Int. 2024, 188, 114480. [Google Scholar] [CrossRef]
- Yakushiji, H.; Sakurai, N.; Morinaga, K. Changes in cell-wall polysaccharides from the mesocarp of grape berries during veraison. Physiol. Plantar 2001, 111, 188–195. [Google Scholar] [CrossRef]
- Seymor, G.B.; Taylor, J.E.; Tucker, G.A. Biochemistry of Fruit Ripening, 1st ed.; Chapman and Hall: New York, NY, USA, 1993; 454p. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Trattato di Enologia 1. In Microbiologia del Vino e Vinificazioni, 2nd ed.; Edagricole: Bologna, Italy, 2005; 563p. [Google Scholar]
- Souza, P.V.; Foschesato, M.L. Emprego da poda verde para a obtenção de duas safras por ciclo vegetativo em ‘Niagara Branca’. Bragantia 2007, 66, 611–617. [Google Scholar] [CrossRef]
- Lassen, R.S.; Sant’Anna, V.; Leães, F.L.; Lima Filho, T.; Dupas de Matos, A. Exploring the Use of Verjuice for Reduced Sodium Pickle Production: Determination of Hedonic and Rejection Thresholds. J. Sens. Stud. 2025, 40, e70030. [Google Scholar] [CrossRef]
- Grape Observatory. Observatório da Uva. Available online: https://www.embrapa.br/observatorio-da-uva (accessed on 7 June 2024).
- MAPA. SIVIBE. Available online: https://mapa-indicadores.agricultura.gov.br/publico/extensions/SIVIBE/SIVIBE.html (accessed on 1 May 2023).
- Villa, F.; Potrich, C.; Dall’oglio, P.A. Sensory profile and physical-chemical analysis of integral grape juice prepared through steam extraction process. Sci. Agrar. Parana. 2018, 17, 300–304. Available online: https://e-revista.unioeste.br/index.php/scientiaagraria/article/view/19663 (accessed on 15 May 2025).
- Mota, R.V.; Glória, M.B.A.; de Souza, B.S.; Peregrino, I.; Pimentel, R.M.A.; Dias, F.A.N.; de Souza, L.C.; de Souza, A.L.; Regina, M.A. Bioactive compounds and juice quality from selected grape cultivars. Bragantia 2018, 77, 62–73. [Google Scholar] [CrossRef]
- Bender, A.; Souza, A.L.K.; Caliari, V.; Malgarin, M.B.; Andrade, S.B. Perfil físico-químico e sensorial de sucos de uva brancos produzidos por extração a quente. Rev. Eletr. Cient. UERGS 2018, 4, 743–751. [Google Scholar] [CrossRef]
- Lopes, M.L.M.; Miguel, M.A.L.; Fialho, E.; Valente-Mesquita, V.L. Grape juice obtained using steam extraction and other small-scale extraction methods: Phenolic content, antioxidant capacity and stability during storage. Int. J. Food Sci. Technol. 2016, 51, 1696–1702. [Google Scholar] [CrossRef]
- Silva, G.G.; Dutra, M.C.P.; de Oliveira, J.B.; Rybka, A.C.P.; Pereira, G.E.; dos Santos Lima, M. Processing methods with heat increases bioactive phenolic compounds and antioxidant activity in grape juices. J. Food Biochem. 2018, 43, e12732. [Google Scholar] [CrossRef]
- Ide, W.; Sabando, C.; Castaño, J.; Pettinelli, N.; Bustos, R.; Linares, A.; Mora, L.; Müller, N.; Pascual, G.; Rodríguez-Llamazares, S. Grape (Vitis vinifera L. cv. País) Juices Obtained by Steam Extraction. Processes 2021, 9, 1670. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Brandelli, A.; Lopes, C.G.H.L. Polyphenoloxidase activity, browning potential and phenolic content of peaches during postharvest ripening. J. Food Biochem. 2005, 29, 624–637. [Google Scholar] [CrossRef]
- Dartora, B.; Sant’Anna, V.; Hickert, L.R.; Fabricio, M.F.; Ayub, M.A.Z.; Flôres, S.H.; Perez, K.J. Factors influencing kombucha production: Effects of tea composition, sugar and SCOBY. Food Sci. Technol. 2023, 43, e8123. [Google Scholar] [CrossRef]
- Burdzaki, L.N.; Wagner, R.; Furlan, J.M.; Müller, G.; Hickert, L.R.; Sant’Anna, V. Production of low-alcoholic and low-gluten beer: Physicochemical properties and volatile compounds. Braz. J. Food Technol. 2024, 27, e20244038. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult 1965, 20, 144–158. Available online: https://www.ajevonline.org/content/16/3/144 (accessed on 10 April 2025). [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Panala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wu, K.-C.; Chiang, S.-H. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007, 100, 1537–1543. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Pherobase The Pherobase: Database of Pheromones and Semiochemicals|The World Largest Database of Behavioural Modifying Chemicals. Available online: https://pherobase.com/ (accessed on 10 June 2024).
- Ferri, V.C.; Sainz, R.L.; Bandeira, P.S. Aceitação de blends de uvas ‘Bordô’ e ‘Isabel’ em sucos. Braz. J. Food Res. 2017, 8, 88–101. [Google Scholar] [CrossRef]
- Bresolin, B.; Gularte, M.A.; Manfroi, V. Exogenous water in grape juice obtained through the steam extraction method. Rev. Bras. Tecnol. Agroind. 2013, 7, 922–933. [Google Scholar] [CrossRef]
- Charnock, H.M.; Pickering, G.J.; Kemp, B.S. The Maillard reaction in traditional method sparkling wine. Front. Microbiol. 2022, 13, 979866. [Google Scholar] [CrossRef]
- Göğüş, F.; Bozkurt, H.; Eren, S. Kinetics of Maillard Reactions Between the Major Sugars and Amino Acids of Boiled Grape Juice. LWT 1998, 31, 196–200. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Brandelli, A.; Marczak, L.D.F.; Tessaro, I.C. Kinetic modeling of total polyphenol extraction from grape marc and characterization of the extracts. Separ Purif. Technol. 2012, 100, 82–87. [Google Scholar] [CrossRef]
- Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Natural Angiotensin Converting Enzyme (ACE) Inhibitors with Antihypertensive Properties. Natural Products Targeting Clinically Relevant Enzymes. In Natural Products Targeting Clinically Relevant Enzymes, 1st ed.; Wiley: London, UK, 2017; pp. 45–67. [Google Scholar] [CrossRef]
- Fleck, N.; de Oliveira, W.C.; Padilha, R.L.; Brandelli, A.; Sant’Anna, V. Antimicrobial effect of phenolic-rich jaboticaba peel aqueous extract on Staphylococcus aureus and Escherichia coli. Braz. J. Food Technol. 2023, 26, e2022087. [Google Scholar] [CrossRef]
- Martins, C.C.; Kahmann, A.; Anzanello, M.J.; Rodrigues, R.; Rodrigues, E.; Mercali, G.D. Acid hydrolysis conditions do affect the non-extractable phenolic compounds composition from grape peel and seed. Food Res. Int. 2023, 174, 113636. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Shadnoush, M.; Khorshidian, N.; Mortazavian, A.M. Insights to potential antihypertensive activity of berry fruits. Phytother. Res. 2020, 35, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Shu, G.; Yuan, J.; Zhang, J.; Qin, S.; Li, J. Enhanced antihypertensive potential of fermented pomegranate juice: The contribution of phenolic compounds biotransformation and the resultant angiotensin-I-converting enzyme inhibition mechanism. Food Chem. 2023, 404, 134745. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Mohammadi, E.; Babaie Beigi, M.A.; Mirzamohammadi, F.; Salehi, O. The effects of unripe grape juice on lipid profile improvement. Cholesterol 2012, 2012, 42–45. [Google Scholar] [CrossRef]
- Hickert, L.R.; Cattani, A.; Manfroi, L.; Wagner, R.; Furlan, J.M.; Sant’Anna, V. Strategies on aroma formation in Chardonnay sparkling base wine: Different Saccharomyces cerevisiae strains, co-inoculation with Torulaspora delbrueckii and utilization of bentonite. Biotechnol. Appl. Biochem. 2024, 71, 96–109. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Maggs, R.; Hort, J. Exploring consumer and producer views of verjuice: A grape-based product made from viticultural waste. Aust. J. Grape Wine Res. 2023, 2023, 5548698. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Analysis of volatile compounds from Iberian hams: A review. Grasas Y Aceites 2012, 63, 432–452. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Garching (University of Munich) Publisher: Lichtenbergstraße, Germany, 1998; 68p. [Google Scholar]
- Schieberle, P.; Grosch, W. Evaluation of the flavour of wheat and rye bread crusts by aroma extract dilution analysis. Z. Lebensm. Unters. Forsch. 1987, 185, 111–113. [Google Scholar] [CrossRef]
- Burdok, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, NW, USA, 2009; 2160p. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part1. Chemical components and vinicultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Frölech, D.B.; de Assis, A.M.; Oliveira, B.A.; Nadal, M.C.; de Mello, L.L.; Lessa, F.d.O.; Schuch, M.W. Physical-chemical and sensory characterization of two important grape cultivars in Brazil. Braz. J. Dev. 2020, 6, 39958–39970. [Google Scholar] [CrossRef]
- Adedeji, J.; Hartman, T.G.; Rosen, R.T.; Ho, C.T. Free and glycosidically bound aroma compounds in hog plum (Spondias mombins L.). J. Agric. Food Chem. 1991, 39, 1494–1497. [Google Scholar] [CrossRef]
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma Compound Analysis of Eruca sativa (Brassicaceae) SPME Headspace Leaf Samples Using GC, GC−MS, and Olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef]
- Marin, A.B.; Acree, T.E.; Barnard, J. Variation in odor detection thresholds determined by charm analysis. Chem. Sens. 1998, 13, 435–444. [Google Scholar] [CrossRef]
| Grape Varietal | Yield * (%) | ||
|---|---|---|---|
| PE | CJE | SE | |
| Concord (CO) | 41.18 | 47.34 | 57.38 |
| Bordô (BO) | 32.40 | 23.12 | 31.80 |
| White Niagara (WN) | 33.23 | 30.13 | 42.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, L.d.S.; Sant’Anna, V.; Leães, F.L.; Weber, F.H.; Furlan, J.M.; Clerici, N.J.; Brandelli, A.; Crepalde, L.T.; Dupas de Matos, A. Chemical and Biochemical Properties of Verjuice Obtained from Vitis labrusca Grapes by Using Different Extraction Methods. Appl. Sci. 2025, 15, 11531. https://doi.org/10.3390/app152111531
Soares LdS, Sant’Anna V, Leães FL, Weber FH, Furlan JM, Clerici NJ, Brandelli A, Crepalde LT, Dupas de Matos A. Chemical and Biochemical Properties of Verjuice Obtained from Vitis labrusca Grapes by Using Different Extraction Methods. Applied Sciences. 2025; 15(21):11531. https://doi.org/10.3390/app152111531
Chicago/Turabian StyleSoares, Letícia da Silva, Voltaire Sant’Anna, Fernanda Leal Leães, Fernanda Hart Weber, Júnior Mendes Furlan, Naiara Jacinta Clerici, Adriano Brandelli, Ludmylla Tamara Crepalde, and Amanda Dupas de Matos. 2025. "Chemical and Biochemical Properties of Verjuice Obtained from Vitis labrusca Grapes by Using Different Extraction Methods" Applied Sciences 15, no. 21: 11531. https://doi.org/10.3390/app152111531
APA StyleSoares, L. d. S., Sant’Anna, V., Leães, F. L., Weber, F. H., Furlan, J. M., Clerici, N. J., Brandelli, A., Crepalde, L. T., & Dupas de Matos, A. (2025). Chemical and Biochemical Properties of Verjuice Obtained from Vitis labrusca Grapes by Using Different Extraction Methods. Applied Sciences, 15(21), 11531. https://doi.org/10.3390/app152111531






