Abstract
A growing scientific interest in bioactive compounds from sea cucumbers is contributing to a broader recognition even in regions where their consumption is not common. This study evaluated the biological potential of a Holothuria forskali extract obtained through different extraction methods, including water extraction, ethanol–water extraction, and enzymatic hydrolysis. The hydrolysate (H), rich in low-molecular-weight peptides, yielded the highest antioxidant (30.6 ± 0.6 mg VitC Eq/g sample for ABTS and 10.7 ± 0.1 mg GAEs/g sample for Folin-reactive substances) and ACE-inhibitory (82.6%) activities. Based on these results, the hydrolysate was selected for encapsulation in two nanostructured delivery systems for comparative purposes: chitosan nanoparticles (NPs) and rapeseed lecithin liposomes (LPs). Both nanostructures were characterized in terms of size, ζ-potential, and polydispersity and subjected to simulated in vitro gastrointestinal digestion (GIDv) to assess their stability and mucoadhesive properties. After digestion, antioxidant activity increased in both systems, particularly in liposomes. Although encapsulation initially reduced ACE-inhibitory activity, gastrointestinal digestion restored or enhanced it, especially in liposomal formulations (≈37% inhibition). The mucoadhesive potential of the nanostructures after DGIv, focusing on their interactions with mucin, was assessed. Liposomal digests significantly increased viscosity in the presence of mucin, while chitosan nanoparticles decreased it, suggesting the formation of soluble complexes with reduced hydrodynamic volume. Electrostatic and hydrogen bonding interactions between chitosan and mucin were particularly evident in the NPH formulation. The rheological synergism parameter (Δη) revealed more negative values for NPs and NPHs, indicating stronger mucoadhesive interactions compared to controls and suggesting their suitability for mucosal delivery. These findings support the use of H. forskali hydrolysates as a source of functional bioactive compounds and highlight the potential of chitosan-based nanocarriers for enhancing their stability, bioaccessibility, and mucoadhesive properties in functional food or nutraceutical applications.
1. Introduction
The world’s population reached 8 billion people recently (November 2022) and is estimated to increase by 2 billion over the next 30 years [1]. In this context, feeding the population will be a major challenge, and the 2030 Agenda for Sustainable Development adopted by the UN in 2015 should be considered an opportunity for countries and their societies to embark on a new path toward improving the lives of many. In this regard, marine invertebrates represent a vast source of food and potential resources, as they are present in large numbers in the world’s oceans and seas [2,3,4]. Some of these species are exploited through aquaculture, while many others are obtained as by-catch and/or discarded, resulting in a significant environmental and economic impact. In some cases, the same species may be considered highly valuable or underutilized, depending on geographical areas and consumption habits. One such example is the sea cucumber: although Asian countries represent the predominant consumer market, in other cultures, it remains a species of limited interest and consumption. Nevertheless, sea cucumbers have recently attracted increasing attention due to their potential as a source of bioactive compounds.
Sea cucumber has a nutritional profile of interest, as it includes proteins (mostly collagen); lipids—including essential nutrients such as omega-3 and omega-6 fatty acids; vitamins A, B1 (thiamine), B2 (riboflavin), and B3 (niacin); and key minerals, particularly magnesium, zinc, calcium, and iron. Sea cucumber has long been recognized in Eastern cultures as a tonic and traditional remedy, valued for its effectiveness in treating conditions such as rheumatism, asthma, hypertension, impotence, constipation, cuts and burns, and others. Several studies show that it possesses unique biological and pharmacological activities, such as antitumor, anticoagulant, antihypertensive, anti-inflammatory, antimicrobial, and antioxidant effects, attributed to bioactive chemical compounds. These include triterpene glycosides (saponins), sterols (both glycosidic and sulfated forms), sulfated polysaccharides, chondroitin sulfates, glycosaminoglycans (GAGs), phenols, cerebrosides, lectins, neuropeptides, glycoproteins, glycosphingolipids, and essential fatty acids isolated from several holothurian species [2,3]. The body wall of the sea cucumber is the primary edible portion and contains the majority of the bioactive components [4,5].
Chitosan, obtained through the deacetylation of chitin—a major component of crustacean exoskeletons—is a molecule of significant interest due to its compatibility and multiple functional properties [6], including encapsulation potential in the form of chitosan nanoparticles, which act as emulsion stabilizers by forming an interfacial complex with adsorbed surface-active agents [7] and can serve as carriers of bioactive compounds, even masking undesirable flavor, in functional foods [8].
Liposomes are colloidal spherical vesicles formed by a lipid bilayer of phospholipids encompassing an aqueous core, which gives them an amphipathic character and the ability to incorporate bioactive compounds of different composition and nature with functional properties. Encapsulation in liposomes prevents degradation of the active compound through interactions with other compounds in the food matrix, protects it during processing, and influences its release. Their composition, rich in unsaturated fatty acids, may confer liposomes the status of a healthy ingredient [9]. Lecithin, rich in polyunsaturated fatty acids, including phosphatidylcholine, is commonly used to obtain liposomes, mainly from soybean due to its high availability; however, other sources of phospholipids have been explored for liposome production, such as rapeseed lecithin, thus avoiding problems of potential allergy issues [10]. Rapeseed lecithin has antioxidant properties due to its content of tocopherols, sterols, and carotenoids [11], which is advantageous in preventing oxidation problems caused by the high unsaturation of plant phospholipids—an advantage that can be enhanced by encapsulating antioxidant compounds rich in polyphenols such as those found in chia seeds [10].
Given the above, the use of an underutilized and/or discarded species such as the sea cucumber, together with the design of a system to protect its bioactive compounds, can contribute to obtaining a functional ingredient with potential dietary benefits and promote the sustainable use of marine resources. In relation to nanostructures and sea cucumbers, there are studies in which the encapsulating material is obtained from the holothurians themselves and/or they serve as the source of the bioactive compounds to be encapsulated. Accordingly, liposomes have been obtained from phospholipids of several holothurian species (Holothuria poli, H. tubulosa, H. arguinensis, and H. sanctori) encapsulating their own protein hydrolysates [12], as well as liposomes prepared from sea cucumber saponin [13] and liposomes composed of phospholipids and cerebrosides isolated from Cucumaria frondosa [14]. Nanoparticles made from chitosan extracted from sea cucumber Holothuria scabra [15], as well as fucoidan nanoparticles and liposomes from Holothuroidea [16], have also been reported. Other materials have also been used in combination with sea cucumber-derived compounds for nanostructures: for instance, egg lecithin nanoliposomes stabilized by sea cucumber-derived saponins [17]; egg yolk lecithin nanoliposomes in which cholesterol is partially replaced with sulfated sterols extracted from sea cucumber (mainly sulfated 24-methylene cholesterol and cholesterol sulfate) and in which fucoxanthin [18] and astaxanthin [19] are also encapsulated; and liposomes (composed of phosphatidylcholine, lipids, and cholesterol) loaded with triterpene saponin from the sea cucumber Cercodemas anceps Selenka [20]. In addition, extracts of Holothuria parva (in buffer phosphate) encapsulated in silver nanoparticles have been shown to inhibit α-amylase activity [21]. However, none of the aforementioned studies examine two encapsulation systems simultaneously or compare which method is most suitable for maintaining the properties of sea cucumber-derived bioactive compounds from before and after digestion, which constitutes a novel and original aspect of this study.
The aim of this work was to obtain a sea cucumber extract with biological properties using different extractions methods (water, ethanol–water, and enzymatic hydrolysis). Since the hydrolysate exhibited the highest antioxidant and antihypertensive activity, it was selected for encapsulation in two different systems for comparative purposes, chitosan nanoparticles and rapeseed lecithin liposomes, in order to protect the bioactive compounds and improve their stability. The effect of simulated in vitro gastrointestinal digestion of the lyophilized nanosystems on the particle characteristics, biological activity, and interaction with porcine mucin was also investigated.
2. Materials and Methods
2.1. Reagents
Chitosan was purchased from Guinama (Valencia, Spain), rapeseed lecithin was from Bungema (Rotterdam, The Netherlands), 2′2-azinobis-3-ethylbenzothiazol-6-sulfonic acid reagent (ABTS) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), and 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) was from Fluka (Thermo Fisher Scientific Inc. Waltham, MA, USA). All other reagents used for the different techniques were laboratory-grade.
2.2. Raw Material
Sea cucumbers (Holothuria forskali) from the Atlantic coast of A Coruña were supplied by the company Porto-Muiños (Cerceda, La Coruña, Spain), frozen and transported to ICTAN-CSIC in Madrid and kept at −80 °C until use. At the time of collection, holothurians were found in two distinct stages: naturally eviscerated or whole. The cucumbers were then thawed, gutted, washed, and refrozen as a preliminary step for freeze-drying. The resulting dried product was chopped and minced in an Osterizer mincer (cycle blend 10, Oster, Newell Brands, Atlanta, GA, USA) until a fine granulate was obtained.
Proximate Analysis
Proximate analyses of sea cucumber were conducted following the procedures established by the Association of Official Analytical Chemists (A.O.A.C., 2002) for moisture (method 950.46), ash (method 920.153), and total protein content (method 928.08). Crude fat was determined according to the method described by Bligh and Dyer [22], while carbohydrate content was calculated by difference. All analyses were performed in triplicate using three independent biological replicates.
2.3. Obtainment and Characterization of Extracts
2.3.1. Preparation of Extracts
Three different types of extracts were prepared, as described below (nomenclature of the extracts is detailed in Table 1).

Table 1.
Nomenclature of the extracts from Holothuria forskali.
Aqueous Extraction
Aqueous extraction (cold and hot) was performed according to Mamelona et al. [23] with slight modifications. For aqueous cold extraction, 2.5 g of freeze-dried sea cucumber was mixed with 100 mL of deionized water (1:40 w/v). The mixture was shaken in a magnetic stirrer (1.500 rpm) for 24 h at 4 °C. It was then centrifuged (Sorvall Lynx 6000, Thermo Fisher Scientific, Waltham, MA, USA, 10,000 g, 15 min, 4 °C) and filtered with filter paper. The resulting filtrate was frozen (24 h, −80 °C) and subsequently freeze-dried. For aqueous extraction with heat, the same proportions were kept: 2.5 g of freeze-dried sea cucumber and 100 mL of deionized water (1:40 w/v) were added. Subsequently, the mixture was homogenized in an ULTRA-TURRAX® dispersion device (Werke GmbH & Co. KG, Staufen, Germany, 1 min at 5000 rpm) and heated in a thermostatized stirring bath for 80 min at 98 °C. Then, the sample was centrifuged (10,000× g, 15 min, 4 °C) and filtered with filter paper, frozen (24 h, −80 °C), and freeze-dried. For both cold and hot extraction, other ratios (1:10, 1:20, 1:30 w/v) were previously tested but rejected because the resulting mixtures were not suitable (too thick). The extracts obtained in cold (WC) or hot (WT) water were reconstituted in water (WC/W or WT/W) or in ethanol–water (WC/E or WT/E), from sea cucumber naturally eviscerated (/E, without viscera) or without evisceration (/SE, with viscera).
Ethanol–Water Extraction
For this extract, 2.5 g of freeze-dried sea cucumber was weighed, to which 100 mL of a 70:30 (v/v) ethanol–water mixture was added. The mixture was placed in a thermostatically controlled bath with constant stirring at 40 °C for 4 h and subsequently centrifuged (10,000× g, 15 min, 4 °C), filtered through filter paper, and rotavapored in a Speed Vacuum (Savant SPD131DDA, Thermoscientific, Germany) in order to remove the organic solvent. The samples were frozen (24 h/−80 °C) and freeze-dried. The extracts obtained (E) were reconstituted in water (/W) or in ethanol–water (/E), from sea cucumber naturally eviscerated (/E, without viscera) or without evisceration (/SE, with viscera).
Preparation of Hydrolysates
Hydrolysates were obtained using the enzyme Alcalase 2.4 L (Protease from Bacillus licheniformis 2.59 AU/g, Sigma–Aldrich (Merck KGaA, Darmstadt, Germany) with an enzyme/substrate ratio of 1:20 (w:w). To this end, 2.5 g of freeze-dried sea cucumber was weighed, and 100 mL of deionized water was added. The mixture was kept under constant stirring during the whole process, and the temperature was adjusted to 50 °C and the pH to 8 (optimum conditions for the enzyme Alcalase). During the whole hydrolysis process (2 h), the conditions were kept constant to facilitate enzyme activity. To maintain the pH at 8, 1 M or 0.1 M NaOH was added. Finally, the enzyme was inactivated in a thermostatized bath at 90 °C/10 min. The samples were then centrifuged (10,000× g, 15 min, 4 °C), filtered with filter paper, frozen (24 h, −80 °C), and freeze-dried. The hydrolysates (H) were reconstituted in water (/W) or in ethanol–water (/E), from sea cucumber naturally eviscerated (/E, without viscera) or without evisceration (/SE, with viscera).
The hydrolysate obtained from sea cucumber eviscerated and reconstituted in water was further characterized in terms of the amino acid composition [24] and molecular weight distribution [25]. To determine the amino acid composition, the hydrolysate was dissolved in distilled water (5 mg/mL), and 20 μL of the sample was dried and hydrolyzed in vacuum-sealed glass tubes at 110 °C for 24 h using constant-boiling 6 N HCl containing 0.1% phenol. Norleucine (Sigma-Aldrich, St. Louis, MO, USA) was used as the internal standard. After hydrolysis, the samples were vacuum-dried again, reconstituted in application buffer, and injected into a Biochrom 20 amino acid analyzer (Pharmacia, Barcelona, Spain). A standard amino acid mixture was obtained from Sigma-Aldrich (St. Louis, MO, USA). For molecular weight (MW) distribution analysis, size-exclusion high-performance liquid chromatography (Shimadzu, SPE-MA10AVP, Kyoto, Japan) was performed according to Alemán et al. [25], using a Superdex 30 Increase 3.2/300 column (GE Healthcare Bio-Sciences, Barcelona, Spain). A 10 μL aliquot of the hydrolysate was loaded onto the column and eluted at a flow rate of 0.075 mL/min with phosphate-buffered saline (pH 7.4) as the mobile phase. MW standards included glycine (75 Da), hippuryl-L-histidyl-L-leucine (429 Da), vitamin B12 (1340 Da), aprotinin (6512 Da), and bovine serum albumin (67,000 Da). Optical density was monitored at 214 nm.
2.3.2. Biological Properties
Antioxidant Activity
Antioxidant activity was measured by the ABTS radical method and reducing power (FRAP) [25]. The results are expressed as mg vitamin C Eq. per g of extract or L of liposomal/nanoparticle dispersion based on a standard curve for the ABTS method and mmol FeSO4·7H2O equivalents/g of sample based on a standard curve for FRAP.
Folin-reactive substances (FRSs) were quantified spectrophotometrically at 750 nm using the Folin–Ciocalteu reagent and gallic acid as the standard [26], employing a Shimadzu spectrophotometer (model UV-1601 Double Beam UV-Vis, Kyoto, Japan). The results are expressed as milligrams of gallic acid equivalents per gram of sample (mg GAEs/g). All determinations were performed in triplicate using three independent biological replicates.
ACE-Inhibitory Activity
Angiotensin-converting enzyme (ACE)-inhibitory activity was assessed using reversed-phase high-performance liquid chromatography (RP-HPLC), by monitoring the elution of hippuric acid (HA) and hippuryl-L-histidyl-L-leucine (HHL) at 228 nm, as described by Alemán et al. [25]. All determinations were performed in triplicate. The IC50 is defined as the concentration of hydrolysate (mg/mL) required to reduce the HA peak area by 50%, indicating 50% inhibition of ACE activity.
Antimicrobial Activity
The antimicrobial activity of the extracts was evaluated using the agar diffusion method [27]. Sterile filter paper discs (5 mm diameter, Whatman No. 1) were impregnated with 40 μL of each solution, adjusted to pH 5, and placed on the surface of agar plates previously inoculated with the selected microorganisms. Discs soaked in a 0.15 M lactic acid solution served as controls. The activity was tested against 26 microbial strains, selected either for their relevance to human health (e.g., probiotics and pathogens) or for their role in food spoilage. All strains were obtained from the CECT (Spanish Type Culture Collection). After incubation, the diameter of the inhibition zones—used as an indicator of antimicrobial activity—was measured using a Scan 1200 device (Interscience, Saint Nom la Bretèche, France). The results are expressed as mm of inhibition growth. Each determination was performed in triplicate.
2.4. Encapsulation in Nanostructures
2.4.1. Preparation of Chitosan Nanoparticles and Rapeseed Lecithin Liposomes
For nanoparticle synthesis [8], 1.8 g of commercial chitosan was dissolved in 600 mL of 1% acetic acid under stirring overnight with pH adjustment to 5. Separately, a solution of sodium tripolyphosphate (TPP) (0.24 g in 240 mL water) was prepared as a crosslinking agent. Then, using the Micro Tube Pump MP-3 (Rikakikai Co., Ltd., Tokyo, Japan), which works continuously, 80 mL of the TPP solution was passed dropwise through each 200 mL of chitosan solution, which remained under continuous agitation for 5 min at 750 rpm (MM30E, Ovan, Badalona, Spain). Subsequently, the mixture was subjected to ultrasound with a probe-tip sonicator (Q Sonica 700) for 4 min in 1 min cycles (the container was kept on ice to avoid temperature rise). Empty nanoparticles (NPs) were obtained. Nanoparticles with the H. forskali hydrolysate (NPHs) were made in the same way but adding 0.36 g of hydrolysate (final concentration 1.3 mg/mL) to the chitosan solution after pH adjustment, before adding the TTP.
To prepare the liposomes, 1.8 g of rapeseed lecithin was suspended in 10 mL of 10 mM phosphate buffer, pH 7, with gentle agitation. Subsequently, 38.5 mL of the phosphate buffer and 1.5 mL of glycerol were added with gentle stirring, and the mixture was kept in a bath at 80 °C for 2 h. The resulting dispersion was shaken well in a Vortex for 5 min and then subjected to ultrasound with a sonicator (Q Sonica 700), 5 cycles of 1 min with 1 min breaks at 90% amplitude, 700 W power, kept on ice. Empty liposomes (L) were obtained. Liposomes with the sea cucumber hydrolysate (LHs) were synthesized in the same way but adding 0.36 g of the hydrolysate (final concentration 7.2 mg/mL) before introducing the suspension to the bath at 80 °C for 2 h.
The nanoparticles and liposomes (empty and loaded with the hydrolysate) were frozen at −80 °C and then freeze-dried for stabilization and moisture content standardization.
2.4.2. Characterization of Nanostructures
Nanostructure characterization was performed using a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK) to measure the particle size (z-average), polydispersity index (PDI), and ζ-potential, as previously described [25]. The z-average and PDI of nanoparticle and liposomal dispersions were measured by dynamic light scattering (DLS), while the ζ-potential was determined by laser Doppler velocimetry. All measurements were taken 5 times at room temperature after dilution (1:100) in deionized water.
2.4.3. Encapsulation Efficiency
The encapsulation efficiency was evaluated for both nanostructures in their fresh state. The amount of entrapped hydrolysate was calculated as the difference between the total hydrolysate used in the preparation of nanoparticles and liposomes and the unencapsulated (free) hydrolysate, which was quantified using the BCA Protein Assay Kit (Thermo Scientific). Free hydrolysate was separated from the nanostructures using a 10 kDa membrane filter (Amicon® Ultra-15, Merck Millipore Ltd., Cork, Ireland) and centrifuged at 5000× g for 40 min at 4 °C (Multifuge 3 LR, Heraeus, Madrid, Spain). This procedure was also applied to unloaded nanoparticles and liposomes as blanks to confirm that no material passed through the filter. The entrapment efficiency (EE) of the hydrolysate in both nanoparticles and liposomes was calculated in triplicate according to Equation (1).
EE (%) = [(Total hydrolysate − Non-encapsulated hydrolysate)/Total hydrolysate] × 100
2.4.4. In Vitro Gastrointestinal Digestion
Simulated gastrointestinal digestion was performed with the freeze-dried nanoparticles and liposomes, as an estimation of bioaccessibility, based on the harmonized INFOGEST protocol [28]. Dried samples (1 g) were placed in 50 mL Falcon tubes, and solutions corresponding to the oral phase were first added: 3.5 mL of simulated oral fluid, 25 µL of 0.3 M CaCl2(H2O)2, 975 µL of H2O, and 0.5 mL of α-amylase solution (15,000 U/mL). These were mixed in a Roto-therm mini plus rotating incubator (Benchmark) for 2 min at 37 °C. After 2 min, the gastric phase solutions were added: 7.5 mL of simulated gastric fluid and 5 µL of 0.3 M CaCl2(H2O)2 were added, the pH was adjusted to 3 by adding 1 M HCl, and finally, 1.6 mL of pepsin solution (25,000 U/mL) was added. The pH was adjusted at each stage for the correct action of the enzymes. Subsequently, the tubes were returned to the Roto-therm mini plus (Benchmark) rotating incubator for 2 h at 37 °C. Then, the enzyme solution was added to the incubator. Thereafter, the intestinal phase solutions: 11 mL of simulated intestinal fluid, 2.5 mL of bile (160 mM), and 40 µL of 0.3 M CaCl2(H2O)2 were added, the pH was adjusted to 7 by adding 1 M NaOH, and then 5 mL of pancreatin solution (800 U/mL) was added. Simulated oral, gastric, and intestinal fluids were prepared as per the INFOGEST protocol [28]. The samples were then placed in the Roto-therm mini plus rotating incubator (Benchmark) for 2 h at 37 °C. Afterward, the digestion was terminated by inactivating the enzymes by heating for 5 min at 90 °C. Samples were centrifuged at 8000× g for 30 min using a Sorvall Combi Plus ultracentrifuge (Thermo Fisher Scientific, Waltham, MA, USA).
2.4.5. Mucin Interaction
The mucoadhesive properties were determined by assessing the interaction of mucin with the nanostructure dispersions in vitro. Steady-shear viscosity measurements were performed at 37 °C and a 100 s−1 shear rate in a TA Instruments Discovery HR 10 Hybrid rheometer (Waters TA Instruments, Milford, MA, USA), using a Peltier temperature control system and a cone–plate stainless-steel geometry (40 mm diameter, 2° angle, 53 µm gap). Porcine gastric mucin (Sigma, Darmstadt, Germany) was dispersed in distilled water at 2% (w/v) for 2 h at room temperature and subsequently mixed with the corresponding whole (non-centrifuged) nanoparticle and liposome digests at a ratio of 1:1 (v/v) in a Vortex. The mixtures were incubated in a waterbath at 37 °C for 30 min. For comparison, sample digests and mucin (2%) were diluted 1:1 with distilled water.
The rheological synergism parameter was calculated using apparent viscosity values (at 100 s−1) as follows:
where η(mixcienccture) is the apparent viscosity (mPa.s) of the digest–mucin mixture, η(mucin) is the apparent viscosity (mPa.s) of the mucin dispersion (at 1% w/v), and η (digest) is the apparent viscosity (mPa.s) of the digest (diluted 1:1 with water).
Δη = η(mixture) − (η(mucin) + η(digests))
Particle size and ζ-potential measurements of whole digests and digest–mucin mixtures were analyzed as described in the previous section.
2.5. Statistical Analyses
Statistical analyses were conducted using SPSS software (version 15.0; IBM SPSS Statistics, Chicago, IL, USA). A one-way analysis of variance (ANOVA) was applied, followed by Tukey’s post hoc test to assess differences between groups, with statistical significance set at p < 0.05.
3. Results and Discussion
3.1. Proximate Composition of Holothurians
The proximate composition of whole holothurians was 40.75% protein, 41.24% ash, 8.85% fat, and 9.16% carbohydrate (on a dry weight basis), with a moisture content of 89.4%. The natural spontaneous evisceration process observed in live holothurians resulted in an increase in protein (53.5%) and carbohydrate (12.7%) concentrations, while the ash and fat contents decreased (31.8% and 1.8%, respectively). The moisture content of eviscerated individuals was 88.7%. Evisceration refers to the ability of sea cucumbers to spontaneously expel their internal organs when stressed or threatened, and it negatively influences their value due to the significant energy expenditure it entails for the organism [29]. In the present study, the difference in the proximate composition between the two stages (naturally eviscerated or whole, upon capture) is mainly reflected in the fat content (8.85% vs. 1.84%), which could be related to the increased energy demand, and in protein (40.7 vs. 53.5%). Similarly, Zhong et al. [30] reported that fresh sea cucumbers with internal organs had higher moisture and fat contents but a significantly lower protein content than those with organs removed. Although the proximate composition of holothurians depends on the species [2], 60.96% protein, 0.79% fat, and 30.3% ash (dry weight) was found in cleaned Holothuria tubulosa specimens (in which the internal organs were manually removed) [31], reflecting a high percentage of protein and ash, consistent with the present findings.
3.2. Biological Activities of Extracts
3.2.1. Antioxidant Activity
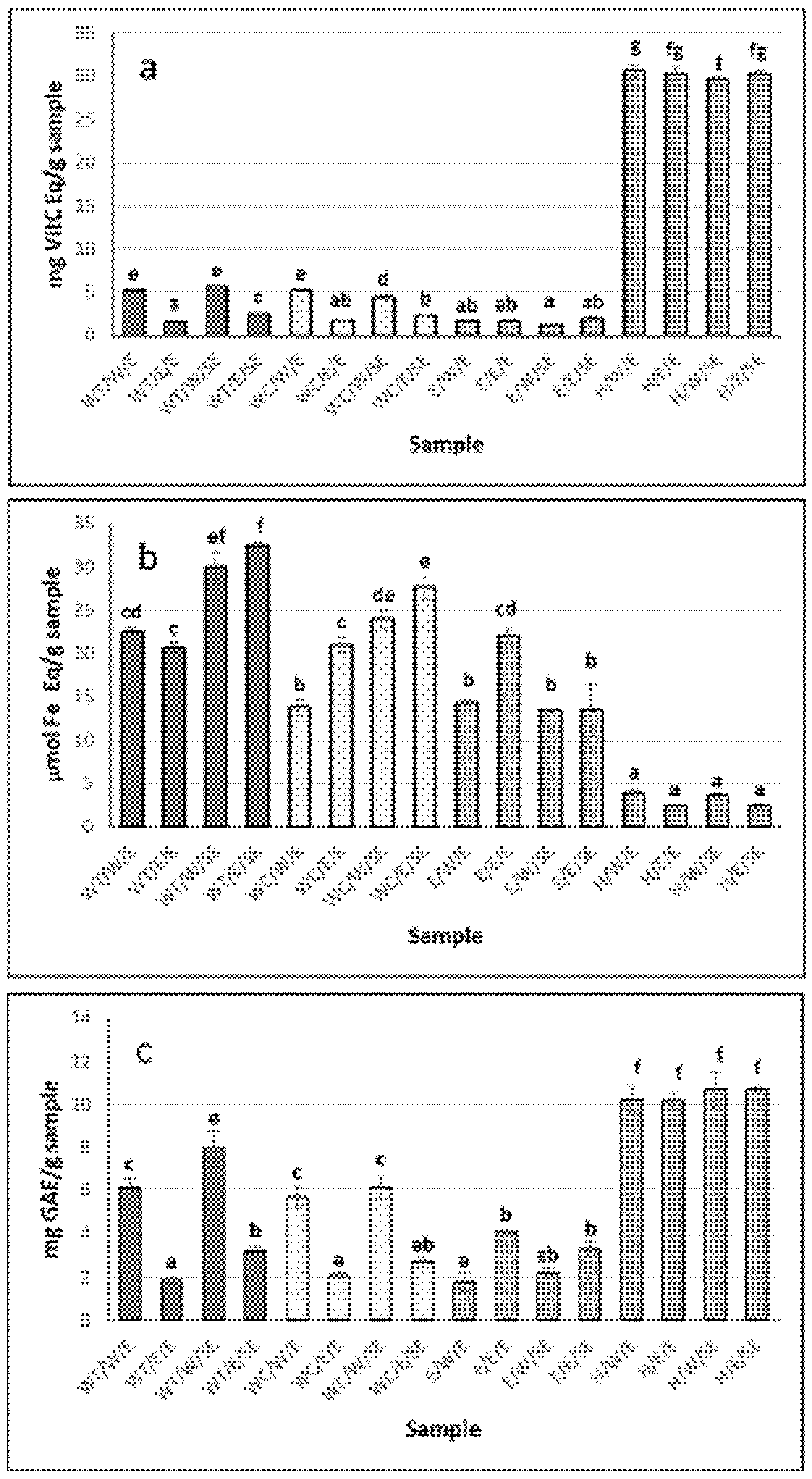
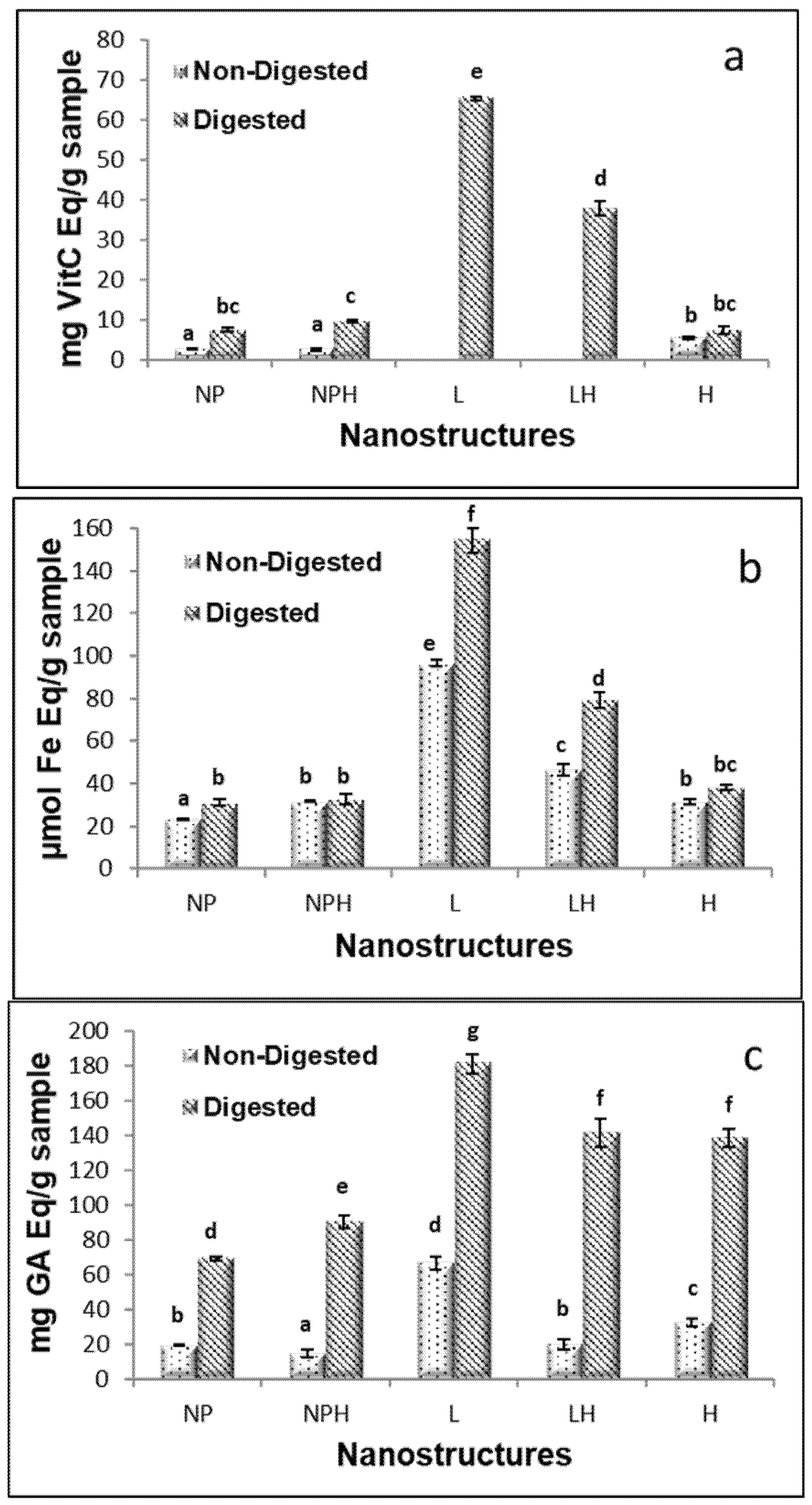
The antioxidant capacity of the extracts is shown in Figure 1. The extracts with the highest antioxidant capacity, as determined by the ABTS method (Figure 1a), were the enzymatic hydrolysates H/W and H/E (reconstituted in water and ethanol–water, respectively). However, most of the extracts exhibited antioxidant capacity by the FRAP method (Figure 1b); this activity varied depending on the state prior to collection of the cucumber (eviscerated or non-eviscerated) and the extraction method, with maximum values of 30 ± 2.8 and 32.5 ± 0.3 μmol Fe Eq/g for the aqueous extract from whole sea cucumber treated with heat (WT), reconstituted in both water and ethanol–water (WT/W/SE and WT/E/SE, respectively) (p ≤ 0.05). Lower values were found by Carletti et al. [32], with 7, 6.2, and 8.9 μmol Fe/g sample in aqueous extracts from Holothuria arguinensis, Holothuria mammata, and H. forskali, respectively. The same authors found that the antioxidant activity was reduced in ethanol–water extracts, reporting 2, 1.5, and 2.1 μmol Fe Eq/g sample, respectively, for the same species. A similar trend was observed in the present study, with maximum values of 22 ± 0.8 μmol Fe/g in ethanol–water extracts reconstituted in this same solvent. Moreover, the extraction temperature appeared to influence the antioxidant capacity measured by FRAP, with generally higher values observed for aqueous extraction conducted at 98 °C compared to room temperature, although the difference was not always statistically significant (Figure 1b). In methanol extracts, Nursid et al. [33] reported values ranging from 11 to 46.9 μmol Fe/g in three sea cucumber (Sticophus herrmanni, Pearsonothuria graeffei, and Holothuria edulis), highlighting the importance of species differences in determining the antioxidant activity. These authors also noted that the antioxidant activity of holothurias was relatively low compared to ascorbic acid due to the absence of -OH groups and aromatic rings, which play a key role in the antioxidant properties. In the present study, for the aqueous extracts, Holothuria forskali-SE extracts showed higher values than Holothuria forskali-E extracts, indicating that the state prior to collection also influenced the reducing capacity of the sample (p < 0.05).
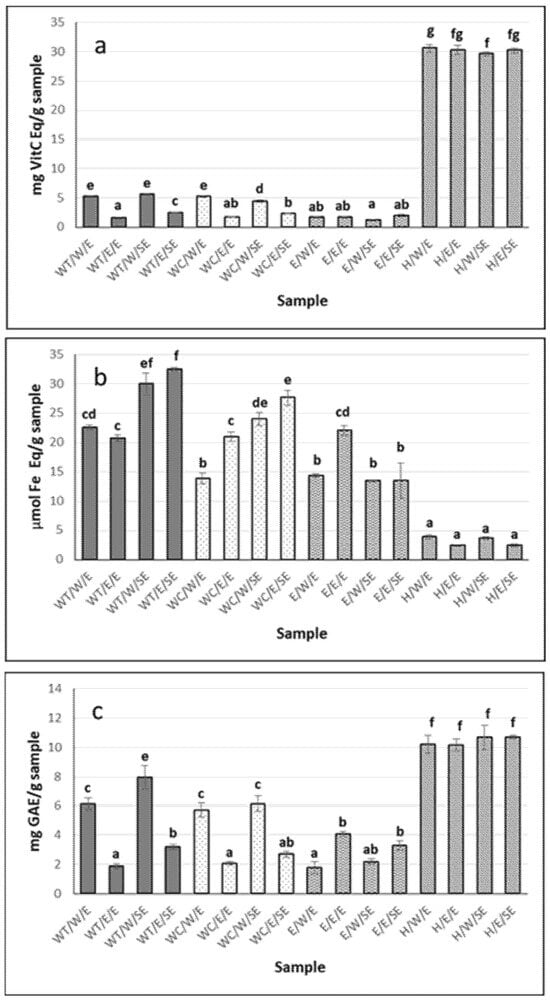
Figure 1.
Antioxidant activity of H. forskali extracts: (a) ABTS assay, (b) FRAP assay, and (c) Folin-reactive substances. Nomenclature of the extracts is detailed in Table 1. Different letters (a, b, c…) indicate significant differences (p < 0.05) among the different extracts.
Hydrolysates from both eviscerated and non-eviscerated sea cucumber, regardless of whether they were reconstituted in water or ethanol–water, showed the highest values for Folin-reactive substances, 10.7 ± 0.1 mg GAEs/g sample (Figure 1c). Similar values were reported in aqueous extracts of Holothuria leucospilota, Holothuria scabra, and Stichopus chloronotus [34] and slightly lower in Holothuria edulis and Stichopus horrens [35], once again highlighting interspecies differences among sea cucumbers. Moreover, extract activity has been shown to vary depending on the anatomical region of the holothurian [23]. In the present work, differences between extracts were mainly attributed to the type of solvent/processing used, rather than to whether or not the individual had previously expelled its viscera.
Although hydrolysates presented the most Folin-reactive substances, aqueous extracts reconstituted in water showed higher values than those reconstituted in ethanol–water (i.e., 6.15 ± 0.4 vs. 1.87 mg GAEs/g sample for WT/W/E and WT/E/E, respectively) as well as compared to ethanol–water extracts (p < 0.05) (Figure 1c). The Folin-Ciocalteu method is used to determine the total phenol content; however, it should be noted that the reagent is not specific to phenolic compounds, as other compounds such as proteins can also react with it [36]. Regarding aqueous extracts, heat may facilitate the release of phenolic compounds from non-phenolic structures, resulting in the formation of molecules that contribute to antioxidant activity [37]. The solvent used also influenced the activity of the extract. Extracts from various sea cucumber species obtained using organic solvents have shown lower levels of Folin-reactive substances compared to aqueous extracts [34,35], suggesting that most antioxidant compounds of sea cucumbers may be hydrophilic in nature. Similar results were reported in three holothurian species (H. arguinensis, H. forskali, and H. mammata) [32] and for Holothuria arguinensis, which exhibited 14.2 mg GAEs/100 g dry weight in aqueous extracts, while no FRSs were detected in the 80% ethanolic extract [38].
The antioxidant capacity measured by ABTS correlated with the Folin-reactive substances in H. forskali (Figure 1a,c). In this context, the total phenolic content (TPC), determined using the Folin–Ciocalteu method, ranged from 22.5 to 236.0 mg GAEs/100 g dry matter (dm), in Cucumaria frondosa, depending on the type of extract and anatomical area [23]. These authors found no correlation between the TPC and antioxidant capacity (measured by ORAC) but a significant correlation between flavonoids and ORAC, suggesting that this polyphenolic group may contribute to the redox reactions, along with others such as easily assimilated antioxidant phenols (e.g., anthocyanins, anthocyanidins, tannins, etc.), since flavonoids accounted for 20% of the total phenols. However, Zhong et al. [30] reported no correlation between the radical scavenging capacity and TPC, indicating that other components—such as vitamin E, carotenoids, and terpenoids—could contribute to the antioxidant activity of sea cucumber.
Overall extracts reconstituted in water exhibited higher activity by ABTS and Folin-reactive substances than their counterparts reconstituted in ethanol–water (p < 0.05), in contrast to what was observed by FRAP (Figure 1a–c).
3.2.2. ACE-Inhibitory Capacity
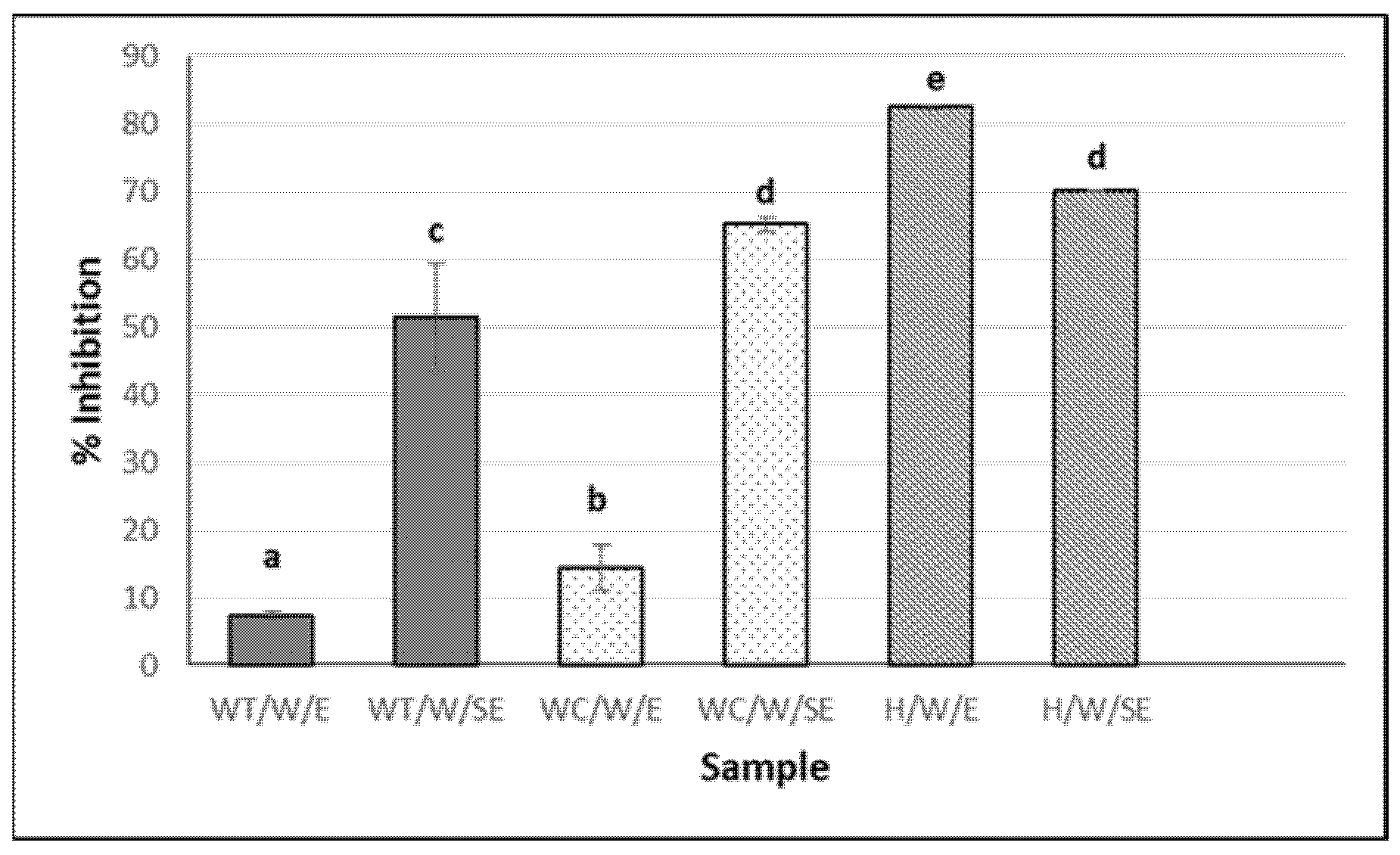
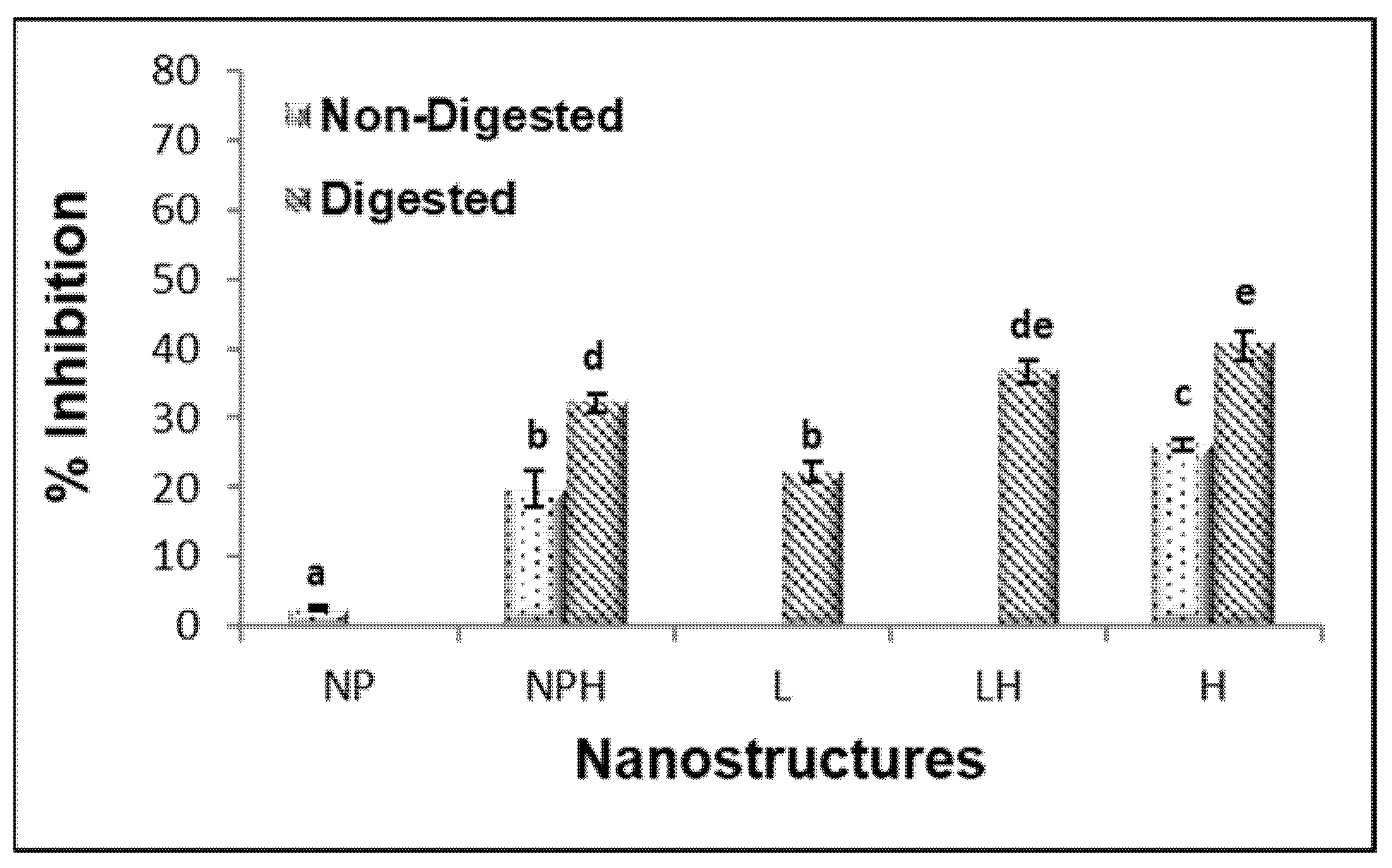
The highest percentage of ACE inhibition was observed in the hydrolysates, particularly in the eviscerated sea cucumber (82.6 ± 0.2%) (Figure 2). However, the activity of the aqueous extracts (with and without heat) was significantly higher in the non-eviscerated sea cucumber (e.g., 51.5 ± 7.9 vs. 7.4 ± 0.5% for WT/W/SE and WT/W/E, respectively) (p ≤ 0.05). Ethanolic extracts showed no activity. ACE-inhibitory activity has been reported in hydrolysates from Cucumaria frondosa tissues obtained using various food-grade enzymes, highlighting their potential value in functional foods and nutraceuticals, among other applications [39]. In the present study, the hydrolysates showed higher antioxidant activity by ABTS and stronger ACE-inhibitory capacity (Figure 1a and Figure 2). Ghanbari et al. [40] found a positive correlation between antioxidant and ACE-inhibitory activities, suggesting that peptides with similar structures may exhibit this dual functionality.
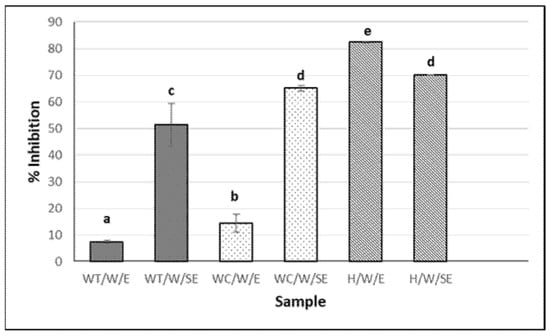
Figure 2.
ACE-inhibitory activity of H. forskali extracts (1 mg/mL). Nomenclature of the extracts is detailed in Table 1. Different letters (a, b, c…) indicate significant differences (p < 0.05) among the different extracts.
3.2.3. Antimicrobial Activity
In this study, neither the aqueous extracts nor the hydrolysates exhibited antimicrobial activity. The hydrophilic compounds extracted with water (e.g., sugars, certain proteins) may lack such activity, while hydrolysates may contain peptides or amino acids that do not necessarily exhibit antimicrobial properties. However, the ethanol–water extract (reconstituted in water) of eviscerated and non-eviscerated Holothuria forskali (E/W/E and E/W/SE, respectively) showed antimicrobial capacity (Table 2). Among the most sensitive microorganisms were B. coagulans (21.43 ± 4.29 and 23.03 ± 1.26 mm) and molds and yeasts such as C. lindemuthianum (21.96 ± 4.54 and 10.25 ± 1.62 mm) and D. hansenii (21.17 ± 1.63 and 20.33 ± 1.91 mm), for E/W/E and E/W/SE, respectively. In contrast, P. aeruginosa showed very limited inhibition, with halos of 7.8 mm. These extracts did not inhibit the growth of some microorganisms, such as V. parahaemolyticus or S. pyogenes. However, although complete inhibition was not observed, a decrease in microbial growth around the disc was noted in P. fluorescens (Table 2).

Table 2.
Antimicrobial activity (mm) against selected microorganisms.
Among the most important microorganisms in terms of food hygiene and safety are Salmonella spp., C. perfringes, B. cereus, S. aureus, L. monocytogenes, and E. coli. In the present study, some of these microorganisms were sensitive to H. forskali extracts. Additionally, Photobacterium phosphoreum exhibited inhibition zones of 11.93 mm and 9.9 mm for E/W/E and E/W/SE, respectively, and both extracts also inhibited the growth of Shewanella putrefaciens (Table 2). This is particularly relevant since S. putrefaciens and P. phosphoreum are known spoilage organisms in chilled fish products [27]. Nugroho et al. [41] evaluated 21 different sea cucumber species (extracted by maceration in methanol) against various microbial strains and observed inhibition halos against S. aureus (in 14 species) and B. subtilis (in 4 species), while E. coli showed no inhibition. However, inhibition of microorganisms such as E. coli, B. cereus, and P. aeruginosa was reported in an ethyl acetate extract of Holothuria forskali [42].
In the present study, most of the microorganisms tested were sensitive to ethanolic extracts of H. forskali, suggesting their potential as candidates for natural pharmaceutical supplements with antimicrobial activity. Moreover, few differences were observed between eviscerated and non-eviscerated sea cucumbers (Table 2). This is likely due to ethanol’s ability to extract a broader spectrum of secondary metabolites (e.g., phenolic compounds, alkaloids, terpenoids, etc.) [26] with known antimicrobial properties [43]. Additionally, ethanol may alter bacterial membrane permeability, enhancing the uptake of active compounds. In this context, Abraham et al. [43] investigated 90% ethanolic extracts from four sea cucumber species (Actinopyga echinites, A. miliaris, Holothuria atra, and H. scabra) and reported inhibition of A. hydrophila, P. aeruginosa, and S. aureus, as well as antifungal activity against Candida albicans, Aspergillus niger, and Tricophyton mentagrophytes. These authors noted that the inhibitory capacity of saponins was comparable to that of the unsaponifiable fraction of H. atra, suggesting the presence of antimicrobial steroidal sapogenins in this fraction and attributing part of the antimicrobial activity to these compounds.
Accordingly, a triterpene glycoside isolated from the methanolic extract of the sea cucumber Actinopyga lecanora has been shown to possess in vitro antifungal capacity against 20 strains, including species from the genera Aspergillus, Penicillum, and Candida [44]. While most sea cucumber extracts with antimicrobial activity are alcohol-based, the reaction products of 3-polyphenol oxidase purified from Apostichopus japonicus coelomocytes with L-DOPA/dopamine have also been reported to inhibit the growth of some Gram-positive and Gram-negative bacteria [45]. Furthermore, the antimicrobial activity of Actinopyga lecanora hydrolysates produced with bromelain and papain against E. coli, S. aureus, Pseudomonas sp., and P. aeruginosa has also been described [46]. In the present study, Alcalase hydrolysates exhibited no activity, suggesting that compounds obtained during ethanolic extraction—possibly of triterpenoid nature—were responsible for the antimicrobial effects observed (Table 2).
Since the hydrolysates exhibited the highest antioxidant activity (ABTS and Folin-reactive substances) and ACE-inhibitory activity, the hydrolysate reconstituted in water from eviscerated sea cucumber (H/W/E) was selected for further characterization. Additionally, this hydrolysate was encapsulated in two different nanosystems: chitosan nanoparticles and liposomes.
3.3. Characterization of Hydrolysate Reconstituted in Water from Eviscerated Sea Cucumber
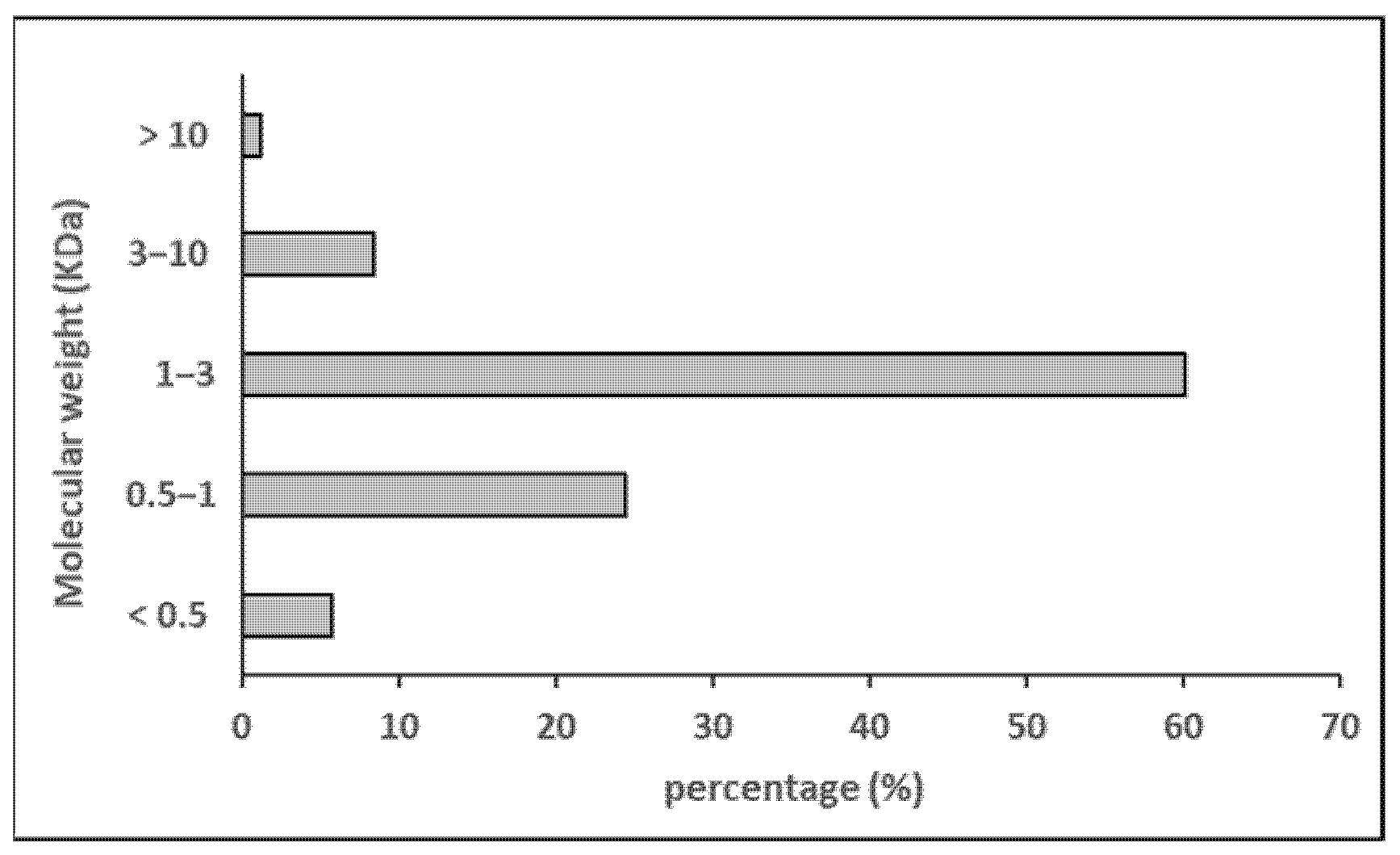
The molecular weight distribution and amino acid composition of the hydrolysate (H/W/E) are shown in Figure 3 and Table 3, respectively. The hydrolysate was primarily composed of peptides with a molecular weight below 3 kDa (≈90%), with a substantial proportion between 3 and 1 kDa (≈60%). The most abundant amino acid residues were glycine (19.4%), hydroxylysine (13.0%), threonine (8.2%), and alanine (8.2%). The total amino acid content (proline + hydroxyproline) was 8.2%, and hydrophobic residues accounted for 29% of the total amino acid profile. In most studies on sea cucumbers, glycine is reported as the major amino acid, followed by glutamic acid, proline, and alanine, whereas hydroxylysine is not usually measured [47].
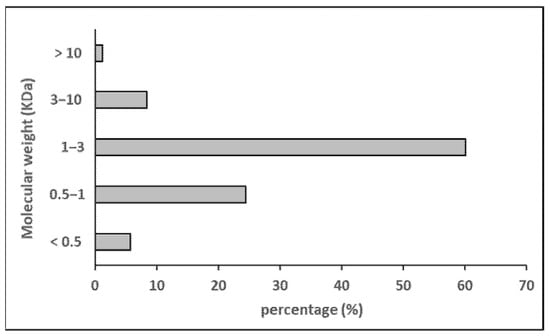
Figure 3.
Molecular weight distribution of sea cucumber hydrolysate.

Table 3.
Amino acid composition of sea cucumber hydrolysate.
It has been reported that marine invertebrates such as sea cucumbers can absorb phenolic compounds from algae and/or microalgae (possibly from phytoplankton), including flavonoids, anthocyanins, anthocyanidins, and tannins [30]. For instance, Esmat et al. [48] identified phenolic components in organic solvents extracts of H. atra, which exhibited high nitric oxide (NO-)∙ scavenging activity (≈93%) but low DPPH radical scavenging activity (≈17%) and moderate for iron chelating activity (≈57%), when compared to commonly used standards. Similarly, Mamelona et al. [23] found that organic extracts from the digestive tract of C. frondosa showed the highest antioxidant activity compared to aqueous and hydroalcoholic water/organic extracts. In the present study, the highest antioxidant activity (ABTS and FRSs) was observed in the hydrolysates (Figure 1a,c); this enhanced antioxidant and ACE-inhibitory activity appears to be mainly attributed to the release of peptides generated during hydrolysis, rather than the presence of other compounds.
In gelatin hydrolysates from the holothurian Paracaudina chinens var. obtained using bromelain, Zeng et al. [49] observed that the <5 kDa fraction was the most active, with scavenging rates of 75.41% for hydroxyl radicals (•OH) and 29.02% for superoxide anions (O2−). When compared with other marine species, tuna gelatin hydrolysate exhibited an antioxidant capacity of 15 mg VitC Eq/g and squid gelatin hydrolysate of 30 mg VitC Eq/g [24], values similar to those obtained for sea cucumber hydrolysates in the present study (Figure 1a). Moreover, no statistically significant differences (p < 0.05) were found between the two stages of H. forskali examined (E—eviscerated; SE—uneviscerated).
However, the hydrolysates showed lower activity in the FRAP assay (<4 µmol Fe Eq/g sample), likely because the peptides responsible for antioxidant activity act through different mechanisms. In gelatin from various marine vertebrate species, Alemán et al. [24] observed that hydrolysis with Alcalase resulted in a greater increase in radical scavenging capacity (ABTS) than in reducing power (FRAP). Although both assays are based on electron transfer mechanisms, the ABTS method primarily assesses the ability of compounds to neutralize hydrophilic radicals by donating electrons or hydrogen atoms, thereby stabilizing reactive species. In contrast, the FRAP assay measures the capacity of antioxidants to reduce ferric (Fe3+) to ferrous (Fe2+) ions via electron donation, reflecting their potential to terminate free radical chain reactions through redox activity [50]. In this regard, the antioxidant activity of aqueous extracts from H. arguinensis, H. mammata, and H. forskali, as measured by ABTS, was higher than the values obtained with FRAP [32].
Regarding ACE-inhibitory activity, as expected, the highest activity in hydrolysates (e.g., 85.6 ± 0.2% for H/W/E) was due to the formation of specific antihypertensive peptides released by hydrolysis [51]. Alcalase hydrolysates from Holothuria atra, Holothuria leucospilota, and Bohadschia marmorata showed ACE-inhibitory activity; H. atra was the most active ACE inhibitor compared with hydrolysates produced using bromelain and/or the sequential or combined use of bromelain and Alcalase [52]. These authors found ACE inhibition ranging from 21 to 63%, with an IC50 value of 0.32 mg/mL. The most active fraction for ACE inhibition had a molecular weight below 3 KDa, indicating that the low-molecular-weight peptides were more active than the high-molecular-weight peptides. The molecular weight of the peptides and the peptide sequence of the hydrolysates are determining factors in their ability to inhibit ACE, although comparison with results in the literature is sometimes not easy due to variations among sea cucumber species and hydrolysis conditions (e.g., enzyme, time, temperature, etc.). Zhang et al. [53] demonstrated that peptides derived from Apostichopus japonicus through trypsin hydrolysis—such as Gly-Lys and Ala-Pro-Arg—can interact with the active site of ACE, showing strong binding affinity within its pocket region. Peptides with potent ACE-inhibitory activity often feature branched aliphatic residues at the N-terminus and/or aromatic, positively charged or proline residues at the C-terminus [54]. ACE-inhibitory activity is usually favored in hydrolysates prepared with Alcalase because it is an enzyme with a certain preference for aromatic groups [55], and the existence of aromatic amino acids at the C-terminal end of the peptides facilitates the binding of these with ACE, causing the inhibition of its activity [56].
3.4. Encapsulation of Hydrolysate in Two Nanosystems: Chitosan Nanoparticles and Liposomes
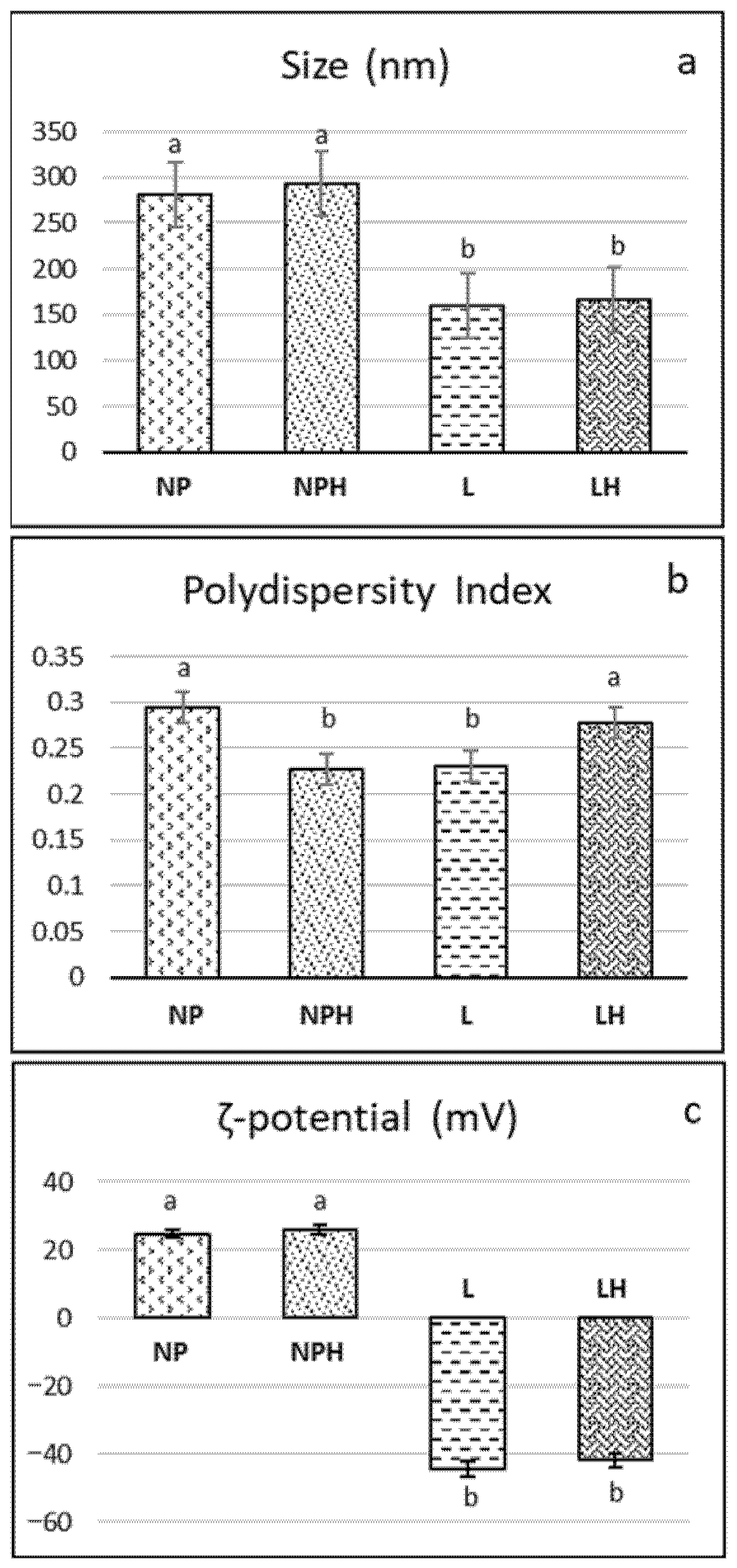
After characterization, the H/W/E hydrolysate was encapsulated in two nanostructures. Following preparation, the size of the liposomes was smaller (160–167 nm) than that of the chitosan nanoparticles, both with and without hydrolysates (280–293 nm) (Figure 4a). Similar sizes (150–220 nm) were obtained in liposomes from rapeseed lecithin with and without encapsulated tilapia viscera protein hydrolysate [57] and liposomes from partially purified phospholipids from rapeseed lecithin (200–220 nm), where size was found to be related to the pH of the dispersion [10]. Since liposomes of an appropriate size can be obtained without purifying the phospholipids and removing components such as sterols (stigmasterol, β-sitosterol), tocopherols (γ, δ, and α), and amino acids [10], in the present study, liposomes were prepared from whole rapeseed lecithin. When compared to soybean liposomes (a widely studied raw material in liposome production), Sepúlveda et al. [58] reported that soybean lecithin liposomes were smaller (150 nm), while those from mixtures of soybean and rapeseed lecithin had an intermediate size (190 nm). The same authors observed that the size of the liposomes decreased when tilapia protein hydrolysates were encapsulated, reaching values between 147 and 177 nm, which would indicate that the materials were compacted due to interaction by electrical charges. Accordingly, a reduction in the size of soy lecithin liposomes after casein encapsulation was reported by Panja et al. [59]. Conversely, a higher concentration of peptides—i.e., a larger number of entrapped peptides—resulted in a larger mean particle diameter of liposomes [60]. In the present study, no change in size was observed due to the presence of the hydrolysate in liposomes and nanoparticles (Figure 4a). Chitosan NPs are formed by intermolecular crosslinking and electrostatic interaction between the phosphoric groups of TPP and the amino (−NH3+) groups of chitosan [61]. The size of the chitosan nanoparticles obtained in the present work is similar to that of those reported by Lima et al. [8] in chitosan nanoparticles encapsulating a hydrolysate of stripped weakfish (Cynoscion guatucupa) by-products using Protamex (~265 nm).
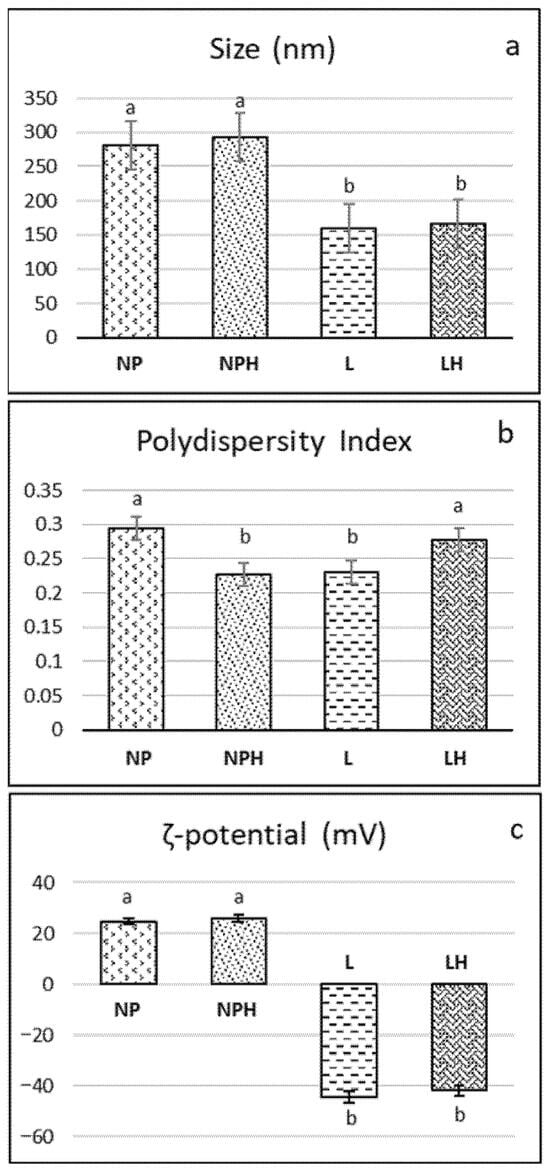
Figure 4.
Characterization of nanostructures: (a) size, (b) polydispersity, and (c) ζ-potential. NPs: chitosan nanoparticles (empty); NPHs: chitosan nanoparticles loaded with H. forskali hydrolysate; L: liposomes (empty); LHs: liposomes loaded with H. forskali hydrolysate. Different letters (a, b, c…) indicate significant differences (p < 0.05) among the different nanostructures.
Both liposomes and nanoparticles exhibited optimal polydispersity values (<0.3) (Figure 4b), within the range reported for soy phosphatidylcholine liposomes, regardless of whether they encapsulated a collagen hydrolysate or not [9], and for chitosan nanoparticles [8,62]. Moreover, in the latter, polydispersity was found to decrease with increasing TPP concentration.
Regarding the stability of the colloidal system as measured by the ζ-potential (Figure 4c), the liposomes (pH 7) were notably electronegative, with values of −44.5 ± 1.22 mV and −41.86 ± 0.66 mV for LPs and LPHs, respectively. Empty liposomes formulated with soy lecithin, rapeseed lecithin, and a blend of both exhibited similar ζ-potential values, close to +46 mV. [58]. Liposomes composed of soy lecithin and cholesterol (4:1) with a ζ-potential close to or greater than ±30 mV were reported to be stable in suspension in terms of both size and charge, as the surface charge prevents particle aggregation [63]. In this regard, the negative ζ-potential in liposomes increased with a rising peptide concentration, suggesting that the net surface charge is strongly influenced by the electrical properties and hydrophobicity of the encapsulated amino acid residues. Higher ζ-potential values promote increased electrostatic repulsion between liposomal carriers in aqueous media, helping to prevent particle aggregation [60]. In contrast, the nanoparticles (pH 5) exhibited an electropositive ζ-potential below 30 mV (~25–26 mV; Figure 4c) due to the cationic nature of chitosan under acidic conditions. Particles with a ζ-potential value of between 20 and 25 mV can be considered relatively stable [61]. These authors reported values ranging from 14 to 33 mV in chitosan nanoparticles loaded with various concentrations of peptides from common kilka (Clupeonella cultriventris caspia). In this sense, Lima et al. [62] observed that increasing TPP concentrations led to greater crosslinking, neutralizing the protonated groups of chitosan upon interaction with TPP and thereby decreasing the ζ-potential. A reduction in ζ-potential was also observed in peptide-loaded nanoparticles as the concentration of fish hydrolysate increased from 2 mg/mL to 5 mg/mL, attributed to interactions between NH3+ groups and the increasing peptide content, which modulates the surface charge characteristics of the nanoparticles [61].
The encapsulation efficiency (EE) of the hydrolysate was 26.6 ± 1.35% and 50.45 ± 0.67% in chitosan nanoparticles and liposomes, respectively. The EE was low for both types of nanostructures, especially for NPHs, which could be attributed to the high concentration of hydrolysate added (20%), making encapsulation more difficult. In liposomes made from a rapeseed and soybean lecithin mixture, the EE decreased with an increasing concentration of protein hydrolysate, reaching values of 90, 60, and 50% when 5%, 10%, and 20% of hydrolysate were encapsulated, respectively [58], in agreement with the results obtained in the present work. A higher EE (61.32%) was achieved when ethanol–water extracts of chia seed were encapsulated in rapeseed lecithin liposomes [10]. However, Pavlovic et al. [60] reported that the EE of soy protein concentrate hydrolysates encapsulated in liposomes (prepared with a mixture of phosphatidylcholine, lysophosphatidylcholine, and tocopherol) increased with a higher peptide concentration (in the range of 50–200 mg peptide/g phospholipids). Regarding the EE in chitosan nanoparticles loaded with protein compounds, increases from 38.7 to 72.5% were reported when the concentration of bovine serum albumin encapsulated was raised from 0.25 to 1.50 mg/mL [64]. EE values of around 56–59% were obtained by Lima et al. [8] in chitosan nanoparticles encapsulating stripped weakfish protein hydrolysate at concentrations of 0.57 mg/mL. These values were higher than those found in the present work, with chitosan nanoparticles encapsulating holothurian hydrolysate at a concentration of 0.43 mg/mL. However, an EE of around 22%, similar to this research work, was reported for chitosan nanoparticles encapsulating the peptide Phe-Pro-Leu [65]. Several factors influence the encapsulation efficiency, in addition to the amount of bioactive compounds to be encapsulated, such as the molecular weight and composition of the peptides in the hydrolysate, the pH of the solution, the concentration of chitosan, and the concentration of the crosslinking agent during nanoparticle formation [62], as well as the purity and type of phospholipid material and the encapsulation method and conditions employed [60].
3.5. Antioxidant and ACE-Inhibitory Activities of Nanostructures Before and After Simulated Digestion
Antioxidant and ACE-inhibitory activities were determined before and after in vitro gastrointestinal digestion (GIDv) to assess whether the activity remained after the simulated process. Prior to digestion, nanoparticles and liposomes were freeze-dried to stabilize the nanostructures [66,67]; for example, liposomes can be unstable due to oxidation and hydrolysis of lipids, rupture and fusion of colloidal particles, and leakage of their hydrophilic cores [60]. In addition, freeze-drying is a suitable method to avoid excessive dilution in the samples when incorporating digestive fluids and to facilitate comparison between the two nanosystems. Therefore, these liposomes and nanoparticles may be more appropriate for use as food ingredients in their lyophilized form rather than in their fresh form.
The freeze-dried NPs showed almost no antioxidant activity, as determined by ABTS, irrespective of the presence of the hydrolysate. In freeze-dried liposomes, no activity was observed (Figure 5a). After in vitro GID, the activity increased in both nanostructures, especially in liposomes (L) (65.5 ± 0.5 mg VitC Eq/g sample) and to a lesser extent in liposomes with the hydrolysate (LHs) (37.8 ± 1.78 g VitC Eq/g sample). This effect was attributed to the breakdown or dissolution of structures and the release of antioxidant compounds, such as the breaking of bonds between phenols and proteins, allowing reactive functional groups to act. In this regard, the activity of Holothuria leucospilota polysaccharide hydrolysates has been shown to improve after gastric and intestinal digestion [68]. Xu et al. [69] reported that after digestion, the ABTS of encapsulated oyster protein hydrolysates was higher than that of the free ones because the small peptides (90.46% had a molecular weight <1 kDa) can be degraded during digestion into compounds with a lower antioxidant activity or even destroyed. This finding suggests that encapsulation may protect peptides from gastrointestinal digestion. In the present work, GIDv did not modify the ABTS radical scavenging capacity of the hydrolysate.
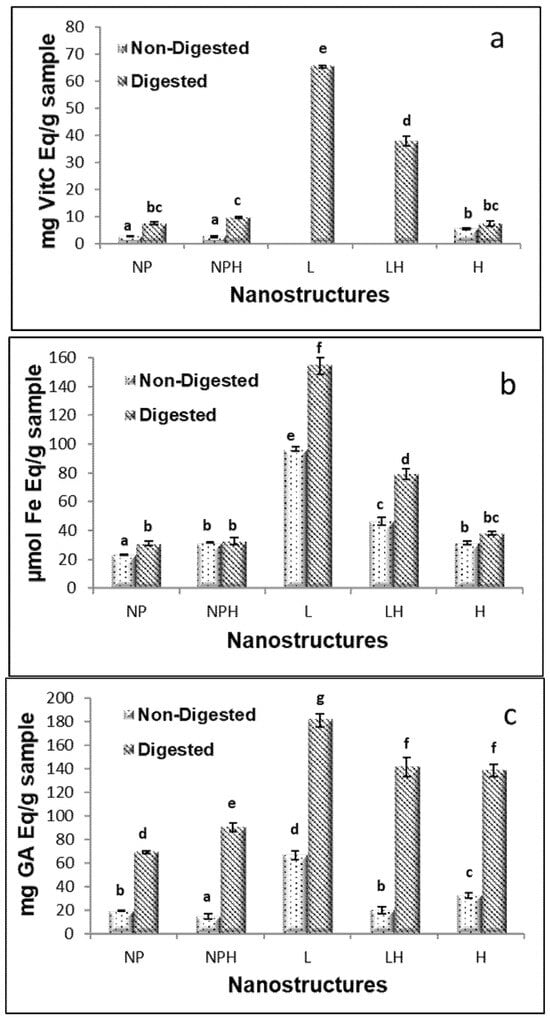
Figure 5.
Antioxidant activity of freeze-dried hydrolysates from H. forskali before and after in vitro gastrointestinal digestion (GIDv): (a) ABTS assay, (b) FRAP assay, and (c) Folin-reactive substances. NPs: chitosan nanoparticles (empty); NPHs: chitosan nanoparticles loaded with hydrolysate; L: liposomes (empty); LHs: liposomes loaded with hydrolysate. Different letters (a, b, c…) indicate significant differences (p < 0.05) among the different samples.
Freeze-dried NPs, both empty and hydrolysate-loaded, showed very low values for antioxidant activity measured by FRAP (Figure 5b). Similarly, freeze-dried liposomes exhibited low activity. After GIDv, the activity was maintained in the NPs with (31.5 ± 0.3 vs. 32.6 ± 2.4 µmol Fe Eq/g sample, before and after DGIv, respectively) or without hydrolysate (23.2 ± 0.19 vs. 30.9 ± 1.7 µmol Fe Eq/g sample, before and after DGIv, respectively), as well as in the free hydrolysate (31.14 ± 1.3 vs. 38.3 ± 1.2 µmol Fe Eq/g sample, before and after DGIv, respectively). However, the antioxidant activity increased markedly in liposomes (approximately 60% in empty and 70% in hydrolysate-loaded systems). This fact could be attributed to a release of lecithin-derived compounds due to liposome rupture and to some other antioxidant compounds which were not part of the membrane. After GIDv, liposomes do not always break but sometimes remain intact, and the contents inside them can be released [70].
Regarding Folin–Ciocalteu-reactive substances, after in vitro GID, the activity was significantly improved in all cases, including the free hydrolysate (H), showing the higher availability of the active compounds after digestion (Figure 5c).
The ACE-inhibitory activity of the freeze-dried hydrolysate was 26% (0.43 mg/mL). Encapsulation of the hydrolysate in nanoparticles reduced this activity to 19.7%, whereas no inhibition was detected in liposomes regardless of the presence of hydrolysate (Figure 6), possibly due to the interaction of some peptides with the components of the nanostructures. After digestion, the ACE-inhibitory activity increased for all hydrolysate samples (H, NPHs, and LHs). The low-molecular-weight peptides released during gastrointestinal digestion were likely responsible for the increase in ACE-inhibitory activity observed for the hydrolysate (both free and encapsulated). These results are consistent with those reported by Pérez-Vega et al. [71], who observed an increase in ACE-inhibitory activity in boiled sea cucumber hydrolysate after treatment with gastrointestinal enzymes. Rapeseed liposomes (L) without hydrolysate also exhibited ACE-inhibitory activity after digestion, possibly due to the release of certain peptides or other bioactive compounds present in the unpurified rapeseed lecithin upon liposome disruption by bile salts or enzymatic action. Similarly, Marín-Peñalver et al. [9] reported that ACE-inhibitory activity increased more markedly after digestion of liposomes encapsulating squid gelatin hydrolysate compared to digestion of the non-encapsulated hydrolysate.
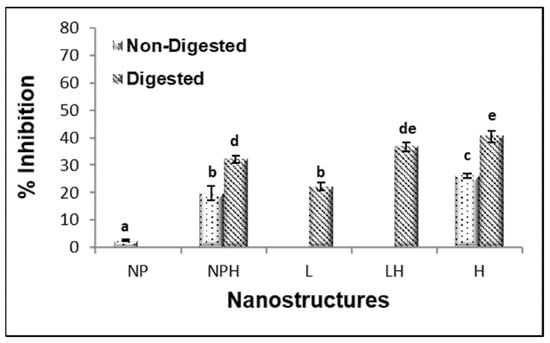
Figure 6.
ACE-inhibitory activity of freeze-dried hydrolysates from H. forskali before and after in vitro gastrointestinal digestion (GIDv). NPs: chitosan nanoparticles (empty); NPHs: chitosan nanoparticles loaded with hydrolysate; L: liposomes (empty); LHs: liposomes loaded with hydrolysate. Different letters (a, b, c…) indicate significant differences (p < 0.05) among the different samples.
3.6. Physical Properties of In Vitro GID Digests
The viscosity of whole digests with empty (NPs) and hydrolysate-loaded (NPHs) chitosan nanoparticles (1.4 and 1.6 mPa.s, respectively) was noticeably higher compared to the control digest sample (C) (0.6 mPa.s), especially the latter (p ≤ 0.05) (Table 4). The presence of empty liposomes (L) also led to a significant increase (p ≤ 0.05) in viscosity, although to a much lesser extent than NPs (p ≤ 0.05). In contrast, the liposomes loaded with the hydrolysate (LHs), as well as the free hydrolysate (H), exhibited values similar to C (≈0.7 mPa.s). Loading with the hydrolysate, thus, resulted in an opposite effect in nanoparticles compared to liposomes, which could be partly explained by differences in their surface charges, even after digestion (Table 4). The elevated viscosity observed for chitosan nanoparticles and empty liposomes may enhance the residence time of bioactive compounds in the gastrointestinal tract, promoting prolonged interaction with the mucosal surface and potentially improving absorption. In contrast, liposomes loaded with hydrolysates did not significantly alter the viscosity, suggesting a different interaction mechanism with the digestive environment. In the case of nanoparticles, the relatively high amount of non-entrapped hydrolysate, which originally had an electronegative ζ-potential (−40.63 ± 3.33 mV), may further interact with positively charged chitosan side groups through electrostatic attractions upon freeze-drying, favoring the formation of chitosan–protein complexes, which can be further stabilized by hydrophobic interactions [72]. The formation of such complexes, which remained in a soluble colloidal form after GIDv, would be the main factor responsible for the increase in viscosity in the NPH digest, in agreement with Ye and Chen [73]. In the case of liposomes, which originally showed an electronegative ζ-potential, the non-encapsulated fraction of the hydrolysate would favor electrostatic repulsions, increasing the fluidity of the system. In this sense, the digested hydrolysate (H) (−19.4 ± 2.4 mV) led to a significant increase (p ≤ 0.05) in the electronegative ζ-potential compared to the C digest (−15.6 ± 0.5 mV), and to a greater extent in LHs (−22.7 ± 2.5 mV) compared to L (−16.5 ± 0.8 mV) (p ≤ 0.05), confirming the greater electrostatic repulsions induced by the hydrolysate (Table 4). The negative zeta potential of C was largely due to the presence of anionic bile salts in the simulated intestinal fluid [74]. The electronegative zeta potentials, as in liposomal digests, could prevent aggregation and facilitate dispersion, enhancing the systemic bioavailability of bioactive compounds [74]. Unlike liposomes, the ζ-potential of NP and NPH digests showed less electronegative values as compared to the control, in line with the original positive surface charge of chitosan nanoparticles. In addition to the presence of bile salts, this effect was favored by the deprotonation of chitosan at the elevated pH of the intestinal fluid. As a consequence, chitosan nanoparticles may undergo partial dissociation, promoting the formation of aggregates by their interaction with the anionic bile salts [75], and could affect colloidal stability and bioavailability of encapsulated compounds. In this case, the presence of the hydrolysate in NPHs did not change the zeta potential compared to NPs (2.4 ± 0.2 and 2.6 ± 0.1 mV, respectively), suggesting its involvement in the aggregates formed. The results of particle size determination (ζ-average) showed a sharp increase in both NP and NPH digests as compared to the rest of samples, which, in fact, did not show any significant difference (p > 0.05) from each other, including the control of digestion (Table 4). Although these results are not conclusive, since they exceeded the detection limit of the equipment (≈5 µm), they provided a clear sign of the great aggregation of both NP and NPH digests, also being compatible with the presence of a certain amount of dissolved chitosan, which could also contribute to increasing the viscosity. The hypothesized co-existence of chitosan in the polymeric form could be supported by the high value of the PDI in NP and NPH digests (0.63 and 0.67, respectively), higher than in C (0.58), where no particles were present. It should be noted that both NP and liposome colloidal suspensions were entirely lyophilized prior to in vitro GID, which means that no previous separation from bulk suspension by centrifugation was carried out. In the case of liposomes, crude phospholipids were not expected to remain in the suspension due to their water-insoluble nature. However, a certain amount of dissolved non-crosslinked chitosan chains was more likely to be present in the acid suspension. In addition, the acid environment during the exposure to the simulated gastric fluid could have also led to some particle disintegration by increased chitosan solubility and possible leakage of the loaded hydrolysate [76]. In contrast, the particle size (492 and 554 nm) and PDI (0.37 and 0.38) of liposomal digests (L and LHs, respectively) were compatible with the persistence of nanovesicles, which after GI digestion increased their mean size. In this connection, an increase in particle size and slight variations in lamellarity with the concomitant emergence of multivesicular structures was previously observed in liposomes embedded in a carboxymethylcellulose film subjected to simulated GIDv [9].

Table 4.
Physical properties of sample digests and their interaction with mucin.
3.7. Study of Interaction Between Mucin and Digests
In order to evaluate the mucoadhesive potential of the nanostructures, the interaction of the nanostructures after DGIv with mucin in solution was determined by different methodologies. The study of steady-shear measurement of viscosity (η) was used to evaluate the interaction between the liposomal and nanoparticle digests with mucin. Both liposome (L and LH) digests were the only samples that significantly increased the viscosity in the presence of mucin as compared to the control digest, reaching 1.07 and 1.04 mPa·s for L and LHs, respectively (p ≤ 0.05) (Table 4). The relatively high viscosity in both liposomal–mucin mixtures diverged from the markedly lower values in the corresponding single digests, while the opposite effect occurred with both NPs and NPHs, with values of 0.94 and 0.89 mPa·s, respectively. The decrease in viscosity in the nanoparticle–mucin–digest mixtures could be an indicator of associative interactions of chitosan with mucin by forming soluble complexes which lowered the hydrodynamic volume of the mixture [77]. A decrease in specific viscosity has previously been reported as an indicator of interaction product formation [78]. In contrast, an excess of repulsive interactions causing phase segregation between negatively surface charged liposomes, mucin, and anionic bile salts would explain the increase in viscosity in the mucin–liposomal mixtures [77]. The cationic nature of chitosan and chitosan nanoparticles has been widely reported to be a key feature for interacting electrostatically with the negatively charged sialic acid groups of mucin, especially at the acid pH where amino groups are mostly protonated [79]. The ζ-potential of both digested NPs and NPHs became more electronegative in the presence of mucin (−10.1 ± 1.6 and −12.7 ± 1.4 mV, respectively) (Table 4), which could be the result of chitosan–mucin interactions, likely being favored by partial deprotonation at the neutral pH at the end of the simulated intestinal digestion. This notable variation was not observed in the case of the corresponding liposomal–mucin mixtures, whose ζ-potential values remained more electronegative compared to both nanoparticle digests (−18.7 ± 1.2 and 17.9 ± 0.6 mV, for L and LHs, respectively) (p ≤ 0.05). The particle size measurement, again, showed opposite behavior between nanoparticles and liposomes with respect to their interaction with mucin (Table 4). In the case of NPs (6951 ± 712 vs. 2135 ± 121 mm) and NPHs (6687 ± 534 vs. 1529 ± 121 mm), for whole digest and mucin–digest, respectively, the incubation with mucin led to a noticeable decrease in average particle size, which would agree with the chitosan–mucin interaction causing the observed decline in viscosity, more pronouncedly in the NPH sample. Furthermore, at the neutral pH in the simulated intestinal fluid, the chitosan deprotonated amino groups would greatly participate in hydrogen bonding with mucin hydroxyl groups [80]. In contrast, an increase in the mean particle size in mucin mixtures, as compared to their corresponding mucin-free samples, was recorded equally for L (1397 ± 114 vs. 492.3 ± 42.9), C (1275 ± 66 vs. 458.7 ± 25.6), and H digests (1313 ± 202 vs. 369 ± 15.9) (p < 0.05), strongly suggesting that it was due mainly to mucin aggregation, and no evident interactions between empty liposomes and mucin took place (Table 4). An increasing trend in particle size was also observed for the LH digest–mucin mixture, although rendering smaller-sized aggregates, likely due to possible interference of the non-encapsulated fraction of the hydrolysate.
A rheological synergism parameter (∆η) was calculated based on differences in viscosity between the sample digest–mucin mixture ηmix and the theoretical additive effect of the mucin blank solution (ηmuc) and digest sample (ηdigest) separately, all of them incubated in the same conditions and at the right comparable concentrations [81]. This rheological method evaluates the potential mucoadhesive effect based on the premise that interactions between nanostructures and mucin lead to deviations from the expected additive viscosity behavior of mucin and nanostructure-containing dispersions [82]. As shown in Table 4, a negative ∆η value was found with all sample digests, including the control C and the plain hydrolysate H, resulting from the lower viscosity values registered in the mucin–sample digest component compared to the sum of individual viscosities of the mucin solution and sample digests. Typically, positive ∆η values have been reported for mucoadhesive biopolymers by increasing polymer entanglements with mucin, causing an increase in the viscosity of the mixture; however, negative values can be also expected working with dilute nanoparticle suspensions [82], or depending on a number of factors, such as the polymer/mucin ratio, salt concentration, or shear rate [77]. The highest negative ∆η values, i.e., those that deviated largely from ∆η = 0 (no interaction), were obtained with NP and NPH digests, especially the latter. Negative ∆η values resulting from the decreased viscosity in the chitosan nanoparticle–mucin mixtures were previously reported, most likely due to increased electrostatic interactions and hydrogen bonding with mucin molecules, causing a reduction in the overall hydrodynamic volume [83]. The higher negative values with chitosan nanoparticles than with liposomes could be also attributed to aggregation phenomena due to the greater adsorption of mucin onto the nanoparticles’ surface [82]. By comparing the rheological synergism with that registered by the control of digestion C, in which denatured enzymes and bile salts might play a role in their interaction with mucin chains, NPs and NPHs increased the mucoadhesive component by 28% and 42%, respectively. In contrast, the liposomal digests decreased the relative synergism well below the control value, suggesting the formation of a segregated phase in the mixture, which would act to the detriment of a proper mucoadhesive effect. The digested hydrolysate (H) not only did not contribute to an increase in mucoadhesion with respect to C but reduced it by approximately 5%. Therefore, the increase in mucoadhesion in NPHs with respect to NPs was more likely due to the chitosan–hydrolysate interaction effect than to the non-encapsulated hydrolysate fraction.
4. Conclusions
The enzymatic hydrolysate of Holothuria forskali showed the highest antioxidant (ABTS and FRSs) and ACE-inhibitory activities, making it a promising candidate for functional food applications. Its encapsulation in rapeseed lecithin liposomes and chitosan nanoparticles demonstrated advantages: liposomes enhanced antioxidant activity after digestion, while chitosan nanoparticles exhibited strong mucoadhesive properties, suggesting their potential for targeted mucosal delivery. Although encapsulation initially reduced ACE-inhibitory activity, digestion restored or even enhanced it, particularly in liposomal systems. These findings highlight the value of underutilized marine resources and the effectiveness of nanostructured carriers in improving the stability, bioaccessibility, and functionality of bioactive compounds.
Author Contributions
Conceptualization, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M.; methodology, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M.; validation, formal analysis, and investigation, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M.; resources, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M.; writing—original draft preparation, A.A., M.E.L.-C. and M.d.C.G.-G.; writing—review and editing, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M.; project administration, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M.; funding acquisition, A.A., M.E.L.-C., M.d.C.G.-G. and M.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
Grant PID2020-116142RB-100 funded by MCIN/AEI/10.13039/501100011033; Grant PID2023-148723OB-100 funded by MCIN/AEI/10.13039/501100011033 and “ERDF, A way to make Europe”; Grant PID2023-146278OB-100 funded by MCIN/AEI/10.13039/501100011033 and “ERDF, A way to make Europe”; Grant 202570E183 funded by CSIC.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available on request from the authors.
Acknowledgments
Authors thank the company Porto-Muiños, S.A., for supplying the sea cucumber.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- UN. 2023. Available online: http://www.un.org>global-issues>ageing (accessed on 4 February 2025).
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Ismail, S.-E.; Hussein, N.A.; Rashad, M.M.; El-Sikaily, A.M.; Hassanin, A.E.-L.A.; Fakharany, E.M.E. Sea cucumber sulphated polysaccharides extract potentiates the anticancer effect of 5- fluorouracil on hepatocellular carcinoma cells. Sci. Rep. 2025, 15, 20255. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Resoles, J.J.A.; Yu, E.T. The neuropeptidomes of the sea cucumbers Stichopus cf. horrens and Holothuria scabra. Sci. Rep. 2025, 15, 7032. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Colloid Interface Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- Lima, K.O.; da Rocha, M.; Alemán, A.; López-Caballero, M.E.; Tovar, C.A.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Yogurt fortification by the addition of microencapsulated strippedweakfish (Cynoscion guatucupa) protein hydrolysate. Antioxidants 2021, 10, 1567. [Google Scholar] [CrossRef]
- Marín-Peñalver, D.; Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Carboxymethyl cellulose films containing nanoliposomes loaded with an angiotensin-converting enzyme inhibitory collagen hydrolysate. Food Hydrocoll. 2019, 94, 553–560. [Google Scholar] [CrossRef]
- Alemán, A.; Pérez-García, S.; Fernández de Palencia, P.; Montero, M.P.; Gómez-Guillén, M.D.C. Physicochemical, antioxidant, and anti-inflammatory properties of rapeseed lecithin liposomes loading a chia (Salvia hispanica L.) seed extract. Antioxidants 2021, 10, 693. [Google Scholar] [CrossRef]
- Butina, E.A.; Gerasimenko, E.O.; Bugaets, I.A.; Kopteva, A.A. Comparative assessment of plant lecithins as the technologically functional components for creating encapsulated biologically active substances. Int. J. Pharm. Res. 2018, 10, 297–304. [Google Scholar]
- Mecheta, A.; Hanachi, A.; Jeandel, C.; Arab-Tehrany, E.; Bianchi, A.; Velot, E.; Mezali, K.; Linder, M. Physicochemical Properties and Liposomal Formulations of Hydrolysate Fractions of Four Sea Cucumbers (Holothuroidea: Echinodermata) from the Northwestern Algerian Coast. Molecules 2020, 25, 2972. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Dong, P.; Li, Z.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea cucumber saponin liposomes ameliorate obesity-induced inflammation and insulin resistance in high-fat-diet-fed mice. Food Funct. 2018, 9, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.-H.; Xu, J.; Wang, Y.-M.; Xue, C.-H.; Kurihara, H.; Takahashi, K. Transport and uptake effects of marine complex lipid liposomes in small intestinal epithelial cell models. Food Funct. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Sumarto Hasan b Karnila, R.; Sukmiwati, M. Characteristics of chitosan nanoparticles extracted from sea cucumber (Holothuria scabra) as source materials for glucosamine. Pertanika J. Sci. Technol. 2019, 27, 2409–2425. [Google Scholar]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.Y.; Li, Z.J.; Xue, C.-H.; Dong, P.; Huang, Q.R.; Wang, Y.-M.; Zhang, T.T. Characterization and Absorption Kinetics of a Novel Multifunctional Nanoliposome Stabilized by Sea Cucumber Saponins Instead of Cholesterol. J. Agric. Food Chem. 2020, 68, 642–651. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, Y.; Chang, Y.; Xue, C.; Zhang, T. Preparation and properties of fucoxanthin-loaded liposomes stabilized by sea cucumber derived cholesterol sulfate instead of cholesterol. J. Biosci. Bioeng. 2022, 135, 160–166. [Google Scholar] [CrossRef]
- Srihera, N.; Li, Y.; Zhang, T.-T.; Wang, Y.M.; Yanagita, T.; Waiprib, Y.; Xue, C.-H. Preparation and characterization of astaxanthin-loaded liposomes stabilized by sea cucumber sulfated sterols instead of cholesterol. J. Oleo Sci. 2022, 71, 401–410. [Google Scholar] [CrossRef]
- Nga, N.T.; Phuong, D.T.; Cuc, N.T.; Phuong, T.H.; Huong, P.T.M.; Cuong, N.X.; Tai, B.H.T.; Kiem, P.V.; Thao, D.T. Nanoliposomal Cercodemasoide A and Its Improved Activities Against NTERA-2 Cancer Stem Cells. Nat. Prod. Commun. 2020, 15, 1934578X20982108. [Google Scholar] [CrossRef]
- Edrispour, Z.; Homaei, A. Exploring in vitro effect of silver nanoparticles and Holothuria parva extracts on kinetics and stability of α-amylase. Biotechnol. Appl. Biochem. 2022, 70, 885–894. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Antioxidant activity of several marine skin gelatins. LWT Food Sci. Technol. 2011, 44, 407–413. [Google Scholar] [CrossRef]
- Alemán, A.; Mastrogiacomo, I.; López-Caballero, M.E.; Ferrari, B.; Montero, P.; Gómez-Guillén, M.C. A Novel Functional Wrapping Design by Complexation of ε-Polylysine with Liposomes Entrapping Bioactive Peptides. Food Bioprocess Technol. 2016, 9, 1113–1124. [Google Scholar] [CrossRef]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, P.; Gómez-Guillén, M.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef] [PubMed]
- Alemán, A.; González, F.; Arancibia, M.Y.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Comparative study between film and coating packaging based on shrimp concentrate obtained from marine industrial waste for fish sausage preservation. Food Control 2016, 70, 325–332. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct 2014, 5, 113–124. [Google Scholar] [CrossRef]
- Jenzri, M.; Gharred, C.; Bouraoui, Z.; Guerbej, H.; Jebali, J.; Gharred, T. Evisceration of Holothuria poli by mechanical, chemical and hypoxia stress methods and its bioremediation potentials for the pisciculture wastewater. Aquac. Res. 2022, 53, 3309–3317. [Google Scholar] [CrossRef]
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional characteristics and antioxidant properties of fresh and processed sea cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 55, 1188–1192. [Google Scholar] [CrossRef]
- Bilgin, S.; Tanrikulu, H.Ö. The changes in chemical composition of Holothuria tubulosa (Gmelin, 1788) with ambient-drying and oven-drying methods. Food Sci. Nutr. 2018, 6, 1456–1461. [Google Scholar] [CrossRef]
- Carletti, A.; Cardoso, C.; Lobo-Arteaga, J.; Sales, S.; Juliao, D.; Ferreira, I.; Chainho, P.; Dionísio, M.A.; Gaudêncio, M.J.; Afonso, C.; et al. Antioxidant and Anti-inflammatory Extracts From Sea Cucumbers and Tunicates Induce a Pro-osteogenic Effect in Zebrafish Larvae. Front. Nutr. 2022, 9, 888360. [Google Scholar] [CrossRef]
- Nursid, M.; Hadiati, D.A.; Winanto, T. Antioxidant capacity of dry sea cucumber Holothuria edulis, Pearsonothuria graeffei, and Stichopus herrmanni from Boalemo waters, Gorontalo, Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 967, pp. 12–27. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Hashim, R.B.; Taher, M.; Daud, J.M.; Ikeda, M.-A. In Vitro Antioxidant and Antiproliferative Activities of Three Malaysian Sea Cucumber Species. Eur. J. Sci. Res. 2009, 37, 376–387. [Google Scholar]
- Althunibat, O.; Ridzwan, B.; Taher, M.; Daud, J.; Jauhari Arief Ichwan, S.; Qaralleh, H. Antioxidant and cytotoxic properties of two sea cucumbers, Holothuria edulis Lesson and Stichopus horrens Selenka. Acta Biol. Hung. 2013, 64, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Giménez, B.; Moreno, S.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Antioxidant properties of green tea extract incorporated to fish gelatin films after simulated gastrointestinal enzymatic digestion. LWT Food Sci. Technol. 2013, 53, 445–451. [Google Scholar] [CrossRef]
- Jallali, I.; Megdiche, W.; M’Hamdi, B.; Oueslati, S.; Smaoui, A.; Abdelly, C.; Ksouri, R. Changes in phenolic composition and antioxidant activities of the edible halophyte Crithmum maritimum L. with physiological stage and extraction method. Acta Physiol. Plant 2012, 34, 1451–1459. [Google Scholar] [CrossRef]
- Roggatz, C.C.; González-Wangüemert, M.; Pereira, H.; João Rodrigues, M.; Moreira da Silva, M.; Barreira, L.; Varela, J.; Custódio, L. First report of the nutritional profile and antioxidant potential of Holothuria arguinensis, a new resource for aquaculture in Europe. Nat. Prod. Res. 2016, 30, 2034–2040. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Antioxidant potential and physicochemical properties of protein hydrolysates from body parts of North Atlantic sea cucumber (Cucumaria frondosa). Food Prod. Process. Nutr. 2021, 3, 3. [Google Scholar] [CrossRef]
- Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870–28885. [Google Scholar] [CrossRef]
- Nugroho, A.; Harahap, I.A.; Ardiansyah, A.; Bayu, A.; Rasyid, A.; Murniasih, T.; Setyastuti, A.; Putra, M.Y. Antioxidant and antibacterial activities in 21 species of Indonesian sea cucumbers. J. Food Sci. Technol. 2021, 59, 239–248. [Google Scholar] [CrossRef]
- Telahigue, K.; Ghali, R.; Nouiri, E.; Labidi, A.; Hajji, T. Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J. Mar. Biol. Assoc. UK 2020, 100, 229–237. [Google Scholar] [CrossRef]
- Abraham, T.J.; Nagarajan, J.; Shanmugam, S.A. Antimicrobial substances of potential biomedical importance from holothurian species. Indian J. Mar. Sci. 2002, 31, 161–164. [Google Scholar]
- Kumar, R.; Chaturvedi, A.K.; Shuklab, P.K.; Lakshmia, V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorganic Med. Chem. Lett. 2007, 17, 4387–4391. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, Z.; Dong, Y.; Guan, X.; Wang, B.; Jiang, B.; Yang, A.; Chen, Z.; Gao, S.; Sun, H. Characterization of phenoloxidase from the sea cucumber Apostichopus japonicas. Immunobiology 2014, 219, 450–456. [Google Scholar] [CrossRef]
- Ghanbari, R.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Actinopyga lecanora Hydrolysates as Natural Antibacterial Agents. Int. J. Mol. Sci. 2012, 13, 16796–16811. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of bioactive peptides from sea cucumber and its potential health benefits: A comprehensive review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Esmat, A.Y.; Said, M.M.; Soliman, A.A.; El-Masry, K.S.H.; Badiea, E.A. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition 2013, 29, 258–267. [Google Scholar] [CrossRef]
- Zeng, M.; Xiao, F.; Zhao, Y.; Liu, Z.; Li, B.; Dong, S. Study on the free radical scavenging activity of sea cucumber (Paracaudina chinens var.) gelatin hydrolysate. J. Ocean Univ. China 2007, 6, 255–258. [Google Scholar] [CrossRef]
- Sun, T.; Tang, J.; Powers, J.R.; Chen, F. Antioxidant activity and sensory quality of asparagus affected by microwave-circulated water combination and conventional cooking methods. Food Chem. 2011, 128, 1087–1093. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Liu, Z.; Dong, S.; Zhao, X.; Zeng, M. Antihypertensive effect and purification of an ACE inhibitory peptide from sea cucumber gelatin hydrolysate. Process Biochem. 2007, 42, 1586–1591. [Google Scholar] [CrossRef]
- Dewi, A.S.; Patantis, G.; Fawzya, Y.N.; Irianto, H.E.; Sa’diah, S. Angiotensin-converting enzyme (ace) inhibitory activities of protein hydrolysates from Indonesian sea cucumbers. Int. J. Pept. Res. Ther. 2020, 26, 485–2493. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Wang, L.; Zhang, S.; Wang, F.; Lin, H.; Gao, S.; Li, X.; Liu, K. Anti-inflammatory peptides and metabolomics-driven biomarkers discovery from sea cucumber protein hydrolysates. J. Food Sci. 2021, 86, 3540–3549. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine Macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Doucet, D.; Otter, D.E.; Gauthier, S.F.; Foegeding, E.A. Enzyme-induced gelation of extensively hydrolyzed whey proteins by alcalase: Peptide identification and determination of Enzyme Specificity. J. Agric. Food Chem. 2003, 51, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.A.; FitzGerald, R.J. Angiotensin Converting Enzyme Inhibitory Peptides Derived from Food Proteins: Biochemistry, Bioactivity and Production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Sepúlveda, C.T.; Alemán, A.; Zapata, J.E.; Montero, P.; Gómez-Guillén, M.C. Characterization and storage stability of spray dried soy-rapeseed lecithin/trehalose liposomes loaded with a tilapia viscera hydrolysate. Innov. Food Sci. Emerg. Technol. 2021, 139, 102708. [Google Scholar] [CrossRef]
- Sepúlveda, C.T.; Zapata, J.E.; Martínez-Álvarez, O.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. The preferential use of a soy-rapeseed lecithin blend for the liposomal encapsulation of a tilapia viscera hydrolysate. Innov. Food Sci. Emerg. Technol. 2021, 71, 110530. [Google Scholar] [CrossRef]
- Panja, S.; Khatua, D.K.; Halder, M. Effect of casein on pure lecithin liposome: Mixed biomacromolecular system for providing superior stabilization to hydrophobic molecules. Colloids Surf. B Biointerfaces 2019, 180, 298–305. [Google Scholar] [CrossRef]
- Pavlović, N.; Mijalković, J.; Đorđević, V.; Pecarski, D.; Bugarski, B.; Knezević-Jugović, Z. Ultrasonication for production of nanoliposomes with encapsulated soy protein concentrate hydrolysate: Process optimization, vesicle characteristics and in vitro digestion. Food Chem. X 2022, 15, 100370. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Soleimani, M.R.; Nikkhah, M. Chitosan/sodium tripolyphosphate nanoparticles as efficient vehicles for antioxidant peptidic fraction from common kilka. Int. J. Biol. Macromol. 2018, 111, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.; Mauricio, C.; Alemán, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, M.P.; Prentice, C. Characterization, bioactivity and application of chitosan-based nanoparticles in a food emulsion model. Polymers 2021, 13, 3331. [Google Scholar] [CrossRef] [PubMed]
- Batalla Mayoral, J.; Cuadros Moreno, A.; San Martín-Martínez, E. Potencial zeta en la determinación de carga superficial de liposomas. Lat. Am. J. Phys. Educ. 2014, 8, 4319-1–4319-6. Available online: http://www.lajpe.org/dec14/4319_San_Martin.pdf (accessed on 5 June 2023).
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Xiong, Y.-M.; Feng, Y.-X.; Chang, M.; Wang, Q.; Yin, S.-N.; Jian, L.-Y.; Ren, D.-F. Formulated chitosan-sodium tripolyphosphate nanoparticles for co-encapsulation of ellagic acid and anti-inflammatory peptide: Characterization, stability and anti-inflammatory activity. J. Sci. Food Agric. 2023, 103, 3447–3456. [Google Scholar] [CrossRef]
- Umerska, A.; Paluch, K.J.; Santos-Martínez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Liofilización de nanopartículas complejas de polielectrolitos: Efecto de la composición de nanopartículas y selección de crioprotectores. Int. J. Pharm. 2018, 552, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, D.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Protein aggregation, water binding and thermal gelation of salt-ground hake muscle in the presence of wet and dried soy phosphatidylcholine liposomes. Food Hydrocoll. 2018, 82, 466–477. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, C.; Zheng, Q.; Wu, J.; Zhu, K.; Shen, X.; Cao, J. Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity, molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota). Food Hydrocoll. 2019, 89, 735–741. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, S.; Liu, L.; Zhao, Y.; Zeng, M. Encapsulation of oyster protein hydrolysates in nanoliposomes: Vesicle characteristics, storage stability, in vitro release, and gastrointestinal digestion. J. Food Sci. 2023, 86, 960–968. [Google Scholar] [CrossRef]
- Alemán, A.; Marín-Peñalver, D.; Fernández de Palencia, P.; Gómez-Guillén, M.C.; Montero, M.P. Anti-Inflammatory properties, bioaccessibility and intestinal absorption of sea fennel (Crithmum maritimum) extract encapsulated in soy phosphatidylcholine liposomes. Nutriens 2022, 14, 210. [Google Scholar] [CrossRef]
- Pérez-Vega, J.A.; Olivera-Castillo, L.; Gómez-Ruiz, J.A.; Hernández-Ledesma, B. Release of multifunctiona l peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J. Funct. Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- Bekale, L.; Agudelo, D.; Tajmir-Riahi, H.A. Effect of polymer molecular weight on chitosan-protein interaction. Colloids Surf. B Biointerfaces 2015, 125, 309–317. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H. Characterization of the interactions between chitosan/whey protein at different conditions. Food Sci. Technol. 2019, 39, 163–169. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Perez-Ciordia, S.; Marin-Arroyo, M.R.; McClements, D.J. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: Impact of protein-carbohydrate Maillard conjugation. J. Agric. Food Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef]
- Niaz, T.; Imran, M.; Mackie, A. Improving carvacrol bioaccessibility using core-shell carrier-systems under simulated gastrointestinal digestion. Food Chem. 2021, 353, 129505. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Mackie, A.R.; Goycoolea, F.M.; Menchicchi, B.; Caramella, C.M.; Saporito, F.; Lee, S.; Stephansen, K.; Chronakis, I.S.; Hiorth, M.; Adamczak, M.; et al. Innovative methods and applications in mucoadhesion research. Macromol. Biosci. 2017, 17, 1600534. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Characterization of chitosan hydrochloride–mucin interaction by means of viscosimetric and turbidimetric measurements. Eur. J. Pharm. Sci. 2000, 10, 251–257. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.E.; Gallo, J.M. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Ferrari, F.; Rossi, S.; Larghi, V.; Zambito, Y.; Caramella, C. Comparison of different in vitro and ex vivo methods to evaluate mucoadhesion of glycol-palmitoyl chitosan micelles. J. Drug Deliv. Sci. Technol. 2010, 20, 419–424. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).