Focused Ultrasound (FUS) and Pediatric Brain Tumors: Current Status and Future Directions
Abstract
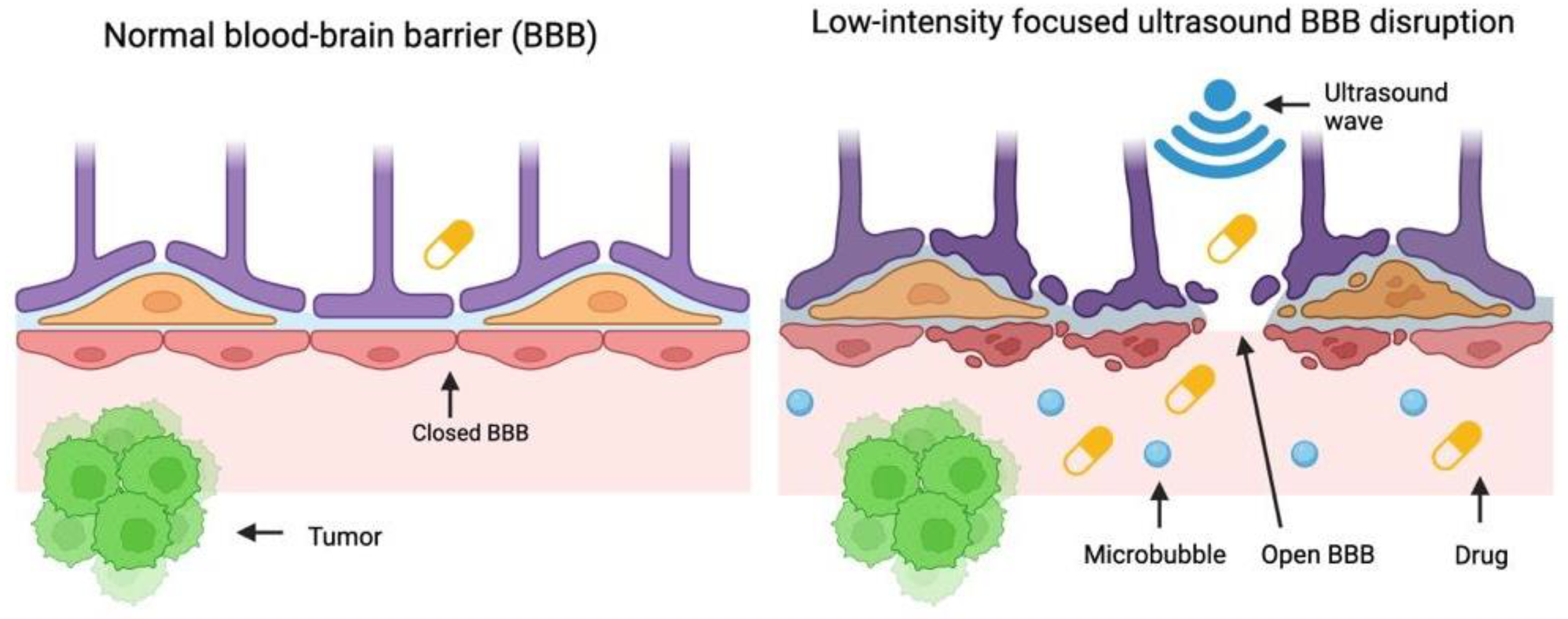
1. Introduction
2. Methodology
3. Results
4. Preclinical Studies Evaluating LIFU and DMG
4.1. Chemotherapy
Doxorubicin
4.2. Temozolomide (TMZ)
4.3. Epirubicin
4.4. Olaparib
4.5. Dordaviprone
4.6. Radiation Therapy
4.7. Immunotherapy
Monoclonal Anti-CD47 Antibody
4.8. Cetuximab
4.9. Immune Checkpoint Inhibitors
4.10. Biological Modifiers
Gambogic Acid
4.11. Commentary
5. Clinical Trials Involving Focused Ultrasound for Pediatric DMG
6. Future Research
7. Conclusions
Funding
Conflicts of Interest
References
- Chesney, K.M.; Keating, G.F.; Patel, N.; Kilburn, L.; Fonseca, A.; Wu, C.C.; Nazarian, J.; Packer, R.J.; Donoho, D.A.; Oluigbo, C.; et al. The role of focused ultrasound for pediatric brain tumors: Current insights and future implications on treatment strategies. Childs Nerv. Syst. 2024, 40, 2333–2344. [Google Scholar] [CrossRef]
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2022, 24 (Suppl. 3), iii1–iii38. [Google Scholar] [CrossRef]
- Parekh, K.; LeBlang, S.; Nazarian, J.; Mueller, S.; Zacharoulis, S.; Hynynen, K.; Powlovich, L. Past, present and future of Focused Ultrasound as an adjunct or complement to DIPG/DMG therapy: A consensus of the 2021 FUSF DIPG meeting. Neoplasia 2023, 37, 100876. [Google Scholar] [CrossRef]
- Janwadkar, R.; Leblang, S.; Ghanouni, P.; Brenner, J.; Ragheb, J.; Hennekens, C.H.; Kim, A.; Sharma, K. Focused Ultrasound for Pediatric Diseases. Pediatrics 2022, 149, e2021052714. [Google Scholar] [CrossRef]
- Hersh, A.M.; Jillala, R.; Kramer, P.; Keating, R.F.; Syed, H.R.; Oluigbo, C.O.; Groves, M.L.; Manbachi, A.; Theodore, N.; Woodworth, G.F.; et al. Focused ultrasound in pediatric neurosurgery: A scoping review of opportunities and challenges. Childs Nerv. Syst. 2025, 41, 210. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Piron, J.; Novell, A.; Bouakaz, A. Doxorubicin delivery into tumor cells with ultrasound and microbubbles. Mol. Pharm. 2011, 8, 799–806. [Google Scholar] [CrossRef]
- Sewing, A.C.; Lagerweij, T.; Van Vuurden, D.; Meel, M.; Veringa, S.; Carcaboso, A.; Gaillard, P.; Vandertop, W.; Wesseling, P.; Noske, D.; et al. Preclinical evaluation of convection-enhanced delivery of liposomal doxorubicin to treat pediatric diffuse intrinsic pontine glioma and thalamic high-grade glioma. J. Neurosurg. Pediatr. 2017, 19, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Filieri, S.; Miciaccia, M.; Armenise, D.; Baldelli, O.M.; Liturri, A.; Ferorelli, S.; Sardanelli, A.M.; Perrone, M.G.; Scilimati, A. Can Focused Ultrasound Overcome the Failure of Chemotherapy in Treating Pediatric Diffuse Intrinsic Pontine Glioma Due to a Blood-Brain Barrier Obstacle? Pharmaceuticals 2025, 18, 525. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Guo, Y.; Velalopoulou, A.; Leisen, J.; Motamarry, A.; Ramajayam, K.; Aryal, M.; Haemmerich, D.; Arvanitis, C.D. Closed-loop trans-skull ultrasound hyperthermia leads to improved drug delivery from thermosensitive drugs and promotes changes in vascular transport dynamics in brain tumors. Theranostics 2021, 11, 7276–7293. [Google Scholar] [CrossRef]
- Choi, H.J.; Han, M.; Jung, B.; Huh, H.; Lee, E.H.; Choi, J.R.; Park, J. Evaluation of blood-tumor barrier permeability and doxorubicin delivery in rat brain tumor models using additional focused ultrasound stimulation. Sci. Rep. 2025, 15, 6592. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound. Int. J. Nanomed. 2021, 16, 185–199. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, M.; Li, W.; Chen, Y.; Xu, Y.; Sun, L.; Liu, D.; Chen, S.; Gu, Y.; Ma, Y.; et al. Delivery of DNA octahedra enhanced by focused ultrasound with microbubbles for glioma therapy. J. Control. Release 2022, 350, 158–174. [Google Scholar] [CrossRef]
- Hart, E.t.; Bianco, J.; Bruin, M.A.C.; Derieppe, M.; Besse, H.C.; Berkhout, K.; Chin Joe Kie, L.A.; Su, Y.; Hoving, E.W.; Huitema, A.D.R.; et al. Radiosensitisation by olaparib through focused ultrasound delivery in a diffuse midline glioma model. J. Control. Release 2023, 357, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Woldegerima, A.; Wei, H.J.; Zhang, C.; Yadavillli, S.; Packer, R.; Wu, C.C.; Nazarian, J. DIPG-73. Focused Ultrasound for Treatment of Children Diagnosed with Diffuse Midline Gliomas. Neuro-Oncology 2024, 26 (Suppl. 4). [Google Scholar] [CrossRef]
- Chen, K.T.; Huang, C.Y.; Pai, P.C.; Yang, W.C.; Tseng, C.K.; Tsai, H.C.; Li, J.C.; Chuang, C.C.; Hsu, P.W.; Lee, C.C.; et al. Focused ultrasound combined with radiotherapy for malignant brain tumor: A preclinical and clinical study. J. Neuro-Oncol. 2023, 165, 535–545. [Google Scholar] [CrossRef]
- Fletcher, S.P.; Chisholm, A.; Lavelle, M.; Guthier, R.; Zhang, Y.; Power, C.; Berbeco, R.; McDannold, N. A study combining microbubble-mediated focused ultrasound and radiation therapy in the healthy rat brain and a F98 glioma model. Sci. Rep. 2024, 14, 4831. [Google Scholar] [CrossRef]
- Tazhibi, M.; McQuillan, N.; Wei, H.J.; Gallitto, M.; Bendau, E.; Webster Carrion, A.; Berg, X.; Kokossis, D.; Zhang, X.; Zhang, Z.; et al. Focused ultrasound-mediated blood-brain barrier opening is safe and feasible with moderately hypofractionated radiotherapy for brainstem diffuse midline glioma. J. Transl. Med. 2024, 22, 320. [Google Scholar] [CrossRef] [PubMed]
- Gallitto, M.; Zhang, X.; De Los Santos, G.; Wei, H.J.; Fernández, E.C.; Duan, S.; Sedor, G.; Yoh, N.; Kokossis, D.; Angel, J.C.; et al. Targeted delivery of napabucasin with radiotherapy improves outcomes in diffuse midline glioma. Neuro-Oncology 2025, 27, 795–810. [Google Scholar] [CrossRef]
- Sheybani, N.D.; Breza, V.R.; Paul, S.; McCauley, K.S.; Berr, S.S.; Miller, G.W.; Neumann, K.D.; Price, R.J. ImmunoPET-informed sequence for focused ultrasound-targeted mCD47 blockade controls glioma. J. Control. Release 2021, 331, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Porret, E.; Kereselidze, D.; Dauba, A.; Schweitzer-Chaput, A.; Jegot, B.; Selingue, E.; Tournier, N.; Larrat, B.; Novell, A.; Truillet, C. Refining the delivery and therapeutic efficacy of cetuximab using focused ultrasound in a mouse model of glioblastoma: An (89)Zr-cetuximab immunoPET study. Eur. J. Pharm. Biopharm. 2023, 182, 141–151. [Google Scholar] [CrossRef]
- Lee, H.; Guo, Y.; Ross, J.L.; Schoen, S., Jr.; Degertekin, F.L.; Arvanitis, C. Spatially targeted brain cancer immunotherapy with closed-loop controlled focused ultrasound and immune checkpoint blockade. Sci. Adv. 2022, 8, eadd2288. [Google Scholar] [CrossRef]
- Dong, L.; Li, N.; Wei, X.; Wang, Y.; Chang, L.; Wu, H.; Song, L.; Guo, K.; Chang, Y.; Yin, Y.; et al. A Gambogic Acid-Loaded Delivery System Mediated by Ultrasound-Targeted Microbubble Destruction: A Promising Therapy Method for Malignant Cerebral Glioma. Int. J. Nanomed. 2022, 17, 2001–2017. [Google Scholar] [CrossRef]
- Bae, S.; Liu, K.; Pouliopoulos, A.N.; Ji, R.; Jiménez-Gambín, S.; Yousefian, O.; Kline-Schoder, A.R.; Batts, A.J.; Tsitsos, F.N.; Kokossis, D.; et al. Transcranial blood-brain barrier opening in Alzheimer’s disease patients using a portable focused ultrasound system with real-time 2-D cavitation mapping. Theranostics 2024, 14, 4519–4535. [Google Scholar] [CrossRef]
- Columbia University. Non-Invasive Focused Ultrasound (FUS) with Oral Panobinostat in Children with Progressive Diffuse Midline Glioma (DMG). Available online: https://clinicaltrials.gov/study/NCT04804709?cond=%22Diffuse%20Intrinsic%20Pontine%20Glioma%22&intr=%22Panobinostat%22&viewType=Table&rank=5 (accessed on 13 May 2025).
- Columbia University. FUS Etoposide for DMG. Available online: https://www.clinicaltrials.gov/study/NCT05762419 (accessed on 13 May 2025).
- InSightec. Blood Brain Barrier (BBB) Disruption Using Exablate Focused Ultrasound with Doxorubicin for Treatment of Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG). Available online: https://clinicaltrials.gov/study/NCT05630209 (accessed on 13 May 2025).
- Englander, Z.K.; Wei, H.J.; Pouliopoulos, A.N.; Bendau, E.; Upadhyayula, P.; Jan, C.I.; Spinazzi, E.F.; Yoh, N.; Tazhibi, M.; McQuillan, N.M.; et al. Focused ultrasound mediated blood-brain barrier opening is safe and feasible in a murine pontine glioma model. Sci. Rep. 2021, 11, 6521. [Google Scholar] [CrossRef] [PubMed]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Focused Ultrasound Foundation. Focused Ultrasound for Diffuse Intrinsic Pontine Glioma (DIPG) [White Paper]. Available online: https://www.fusfoundation.org/content/images/pdf/Focused_Ultrasound_Foundation_DIPG_Workshop_White_Paper_2021.pdf (accessed on 13 May 2025).
- Weisbrod, L.J.; Thiraviyam, A.; Vengoji, R.; Shonka, N.; Jain, M.; Ho, W.; Batra, S.K.; Salehi, A. Diffuse intrinsic pontine glioma (DIPG): A review of current and emerging treatment strategies. Cancer Lett. 2024, 590, 216876. [Google Scholar] [CrossRef]
- Syed, H.R.; Kilburn, L.; Fonseca, A.; Nazarian, J.; Oluigbo, C.; Myseros, J.S.; Packer, R.J.; Keating, R.F. First-in-human sonodynamic therapy with ALA for pediatric diffuse intrinsic pontine glioma: A phase 1/2 study using low-intensity focused ultrasound: Technical communication. J. Neuro-Oncol. 2023, 162, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Balasubramanyian, V.; de Groot, J.F.; Majd, N.K. Preclinical Models of Low-Grade Gliomas. Cancers 2023, 15, 596. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e525. [Google Scholar] [CrossRef]
- Roberts, J.W.; Powlovich, L.; Sheybani, N.; LeBlang, S. Focused ultrasound for the treatment of glioblastoma. J. Neuro-Oncol. 2022, 157, 237–247. [Google Scholar] [CrossRef]
- Chen, K.T.; Chai, W.Y.; Lin, Y.J.; Lin, C.J.; Chen, P.Y.; Tsai, H.C.; Huang, C.Y.; Kuo, J.S.; Liu, H.L.; Wei, K.C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef]
- Sarica, C.; Nankoo, J.F.; Fomenko, A.; Grippe, T.C.; Yamamoto, K.; Samuel, N.; Milano, V.; Vetkas, A.; Darmani, G.; Cizmeci, M.N.; et al. Human Studies of Transcranial Ultrasound neuromodulation: A systematic review of effectiveness and safety. Brain Stimul. 2022, 15, 737–746. [Google Scholar] [CrossRef]
- Fry, W.J.; Barnard, J.W.; Fry, E.J.; Krumins, R.F.; Brennan, J.F. Ultrasonic lesions in the mammalian central nervous system. Science 1955, 122, 517–518. [Google Scholar] [CrossRef]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology: Advances in Diagnosis and Treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; Niazi, T.N. Clinical Trials of Focused Ultrasound for Brain Tumors. Cancers 2025, 17, 513. [Google Scholar] [CrossRef] [PubMed]
- Young, C.C.; Narsinh, K.H.; Chen, S.R.; Ansari, S.A.; Hetts, S.W.; Lang, F.F.; Wintermark, M.; Kan, P.T. State of Practice: A Report from the Inaugural SNIS Neurointerventional Oncology Summit. Am. J. Neuroradiol. 2025, ajnr.A8902. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, L.; Patel, T.; Miclea, M.; Schwark, K.; Ajaero, D.; Momen, F.; Clausen, M.; Adam, T.; Aittaleb, R.; Wadden, J.; et al. Updates in Diagnostic Techniques and Experimental Therapies for Diffuse Intrinsic Pontine Glioma. Cancers 2025, 17, 931. [Google Scholar] [CrossRef]

| Reference | Treatment | Dose | Animal model | Ultrasound Intensity or Frequency | BBB Permeability Measure | Results | Median Survival | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Kim et al. 2021 [10] | Doxorubicin | 8 mg/kg | GL261 glioma-bearing mice and F98 glioma-bearing rats | 1.7 MHz, 10 min at 41.5 °C | T2-weighted MR images | FUS-induced hyperthermia significantly enhanced the delivery of thermosensitive liposomal doxorubicin to tumore, improved vascular permeability, and prolonged survival of glioma-bearing rodents. | 16 days (vs. 9 days in the TSL-Dox alone) | Hemorrhage in the brain near the skull surface when hyperthermia was applied at 42.5 °C for 10 min in mice. Lower hyperthermia (41.5 °C) was safe in rodents, with no damage seen. |
| Choi et al., 2025 [11] | Doxorubicin | 5.67 mg/kg | Male Sprague Dawley rats implanted with intracranial 9L gliosarcoma tumors | 1 MHz, 120 s duration, acoustic pressure of 0.5, 1, 2, or 0.72 Mpa | T1-weighted or Dynamic contrast-enhanced MRI, Evans blue dye staining | FUS stimulation without microbubbles followed by BBB disruption with microbubbles significantly increased blood-tumor permeability and doxorubicin delivery compared to BBB disruption alone. Dynamic contrast-enhanced MRI showed a 2.65-fold increase in signal intensity and a 2.08-fold increase in permeability in tumor regions. | N/A | None detected |
| Yang et al. 2021 [12] | Temozolomide (TMZ) | 50 mg/kg daily for 5 days | Orthotopic glioblastoma-bearing nude mice implanted with human T98G glioblastoma cells | 1.84 W, 3–5 m duration | Evans blue dye staining | FUS improved accumulation of lipid-polymer hybrid nanoparticles (LPHNs) in tumor regions and enabled CRISPR/Cas9-mediated MGMT knockdown, restoring sensistivity to TMZ. This combination therapy inhibited tumor growth and significantly extended survival in glioma-bearing mice. | 43 days (vs. 22–30 days in other groups) | At higher FUS/MB doses: erythrocyte extravasation and hemorrhage during BBB opening. |
| Shen et al., 2022 [13] | Epirubicin | 2 mg/kg | Nude mice baring intracranial glioma xenografts | 0.971 MHz, 60 s duration, 0.52 MPa | In vivo fluorescence imaging using an IVIS imaging system | FUS treatment significantly increased epirubicin accumulation in glioma tissue (4.4-fold enhancement over controls). In vivo imaging and histology confirmed deep tumore penetration and prolonged survival (50% increase in median survival). | 36.5 days (vs. 25 days in other groups) | None detected |
| Hart et al., 2023 [14] | Olaparib | 10 or 100 mg/kg | Patient-derived xenograft (PDX) DMG mouse model. | 1 MHz, 1.6 Hz pulse repetition frequency, 400 kPa pressure, 120 s duration | Evans blue dye staining | Effects of PARP1 inhibition were evaluated in vitro using viability, clonogenic, and neurosphere assays. In vivo olaparib extravasation and pharmacokinetic profiling following FUS-BBBO was measured by LC-MS/MS. Survival benefit of FUS-BBBO combined with olaparib and RT was assessed. | N/A | None detected |
| Woldegerima et al., 2024 [15] | Dordaviprone (ONC201) | N/A | Syngeneic diffuse midline glioma mouse | N/A | Gadolinium-enhanced MRI | FUS enhanced ONC201 delivery, leading to greater biomarker response, increased ROS, and reduced tumor burden compared to ONC201 alone. | N/A | None detected |
| Chen et al., 2023 [16] | Radiotherapy (RT) | Whole brain, 2 and 5 Gy at a rate of 3.3 Gy/min | Mouse glioblastoma (GBM) model | 500 kHz, 0.4–0.56 mechanical index (MI), duty cycle of 1%, burst period of 1 s, 120 s duration | MRI | Mice recieving FUS prior to RT (2 Gy) exhibited significantly longer survival compared to those recieving RT (2 Gy) alone or no treatment, though survival was comparable to the RT (5 Gy) group. | N/A | None detected across 24 RT-FUS sessions; one grade-3 radiation necrosis attributed to re-irradiation (RT) |
| Fletcher et al., 2024 [17] | Radiotherapy (RT) | 4, 8, 15 Gy | Healthy rats and rats bearing F98 glioma tumors | 220 kHz, 102–444 kPa | Contrast enhanced T1-weighted MRI | In healthy rats, the combination of FUS and RT at 8 and 15 Gy induced ablative lesions detectable by MRI within 72 h, persisting up to 21 days. In the F98 glioma model, FUS combined with 4 Gy RT reduced tumore volumes by 45–57% compared to controls. However, survival benefits were minimal. Histological analysis showed significant increases in apoptosis and vessel-associated ceramin in the FUS-RT group compared to FUS or RT alone. | 28 days (vs. 27 days in control and RT only groups) | Transient edema on MRI; rare minor T2 change (1/13 rats); no motor/neurologic deficits. FUS+RT at high dose: MRI-visible lesions and histologic scarring; authors caution on MB dose/pressure escalation. |
| Tazhibi et al., 2024 [18] | Radiotherapy (RT) | 39 Gy in 13 fractions | Non–tumor-bearing mice and syngeneic DMG murine model | 0.5 MHz, peak-negative pressure 0.3 Mpa, 5 Hz repetition time, 120 s duration | MRI | Demonstrated that repeated brainstem FUS during RT is safe, feasible, and well-tolerated; progression still occurred post-RT | 54 days (vs. 28 days in control) | None detected |
| Gallitto et al., 2025 [19] | Napabucasin + Radiotherapy (RT) | 2 Gy in 5 fractions; Napabucasin 80 μM | Patient-derived DMG cultures; orthotopic DMG mouse | N/A | Gadolinium contrast enhancement on MRI | Napabucasin acted as a potent radiosensitizer; CED delivery improved survival in vivo. | 46 days (vs. 33 days RT alone and 26 days napabucasin alone) | None detected |
| Sheybani et al. 2021 [20] | Monoclonal anti-CD47 antibody (mcD47) | 8 or 32 mg/kg every 3 days for 3 doses | Orthotopic murine glioma model using GL261 cells implanted in C57BL/6 mice | 1.1 MHz, 0.5% duty cycle, 0.4 MPa, 2 m duration | Contrast-enhanced MR imaging | Post-FUS delivery of Zirconium-89 labeled mCD47 led to significantly enhanced antibody accumulation in gliomas, compared to pre-FUS administration. This treatment sequence suppressed tumor growth and prolonged survival using less antibody than prior methods. | Increased 14 day survival by 40% | None detected |
| Porret et al., 2023 [21] | Cetuximab | N/A | Orthotopic U251 glioblastoma xenografts in nude mice | 1 MHz, 0.5% duty cycle, 60 s duration | Evans blue dye staining | FUS significantly increased early deliver and homogenization of cetuximab in the brain, including tumor regions. However, it did not enhance long-term accumulation or retention of the antibody in tumors. There was no significant difference in survival between FUS-treated and control groups. | N/A | None detected |
| Lee et al., 2022 [22] | Immune checkpoint inhibitors (anti-PD-1 and anti-CTLA-4 antibodies | 100 mg/kg | Orthotopic GL261 glioma bearing C57BL/6 mice | 1.64 MHz, 10-ms pulse length, 1-Hz pulse repetition frequency, 120 s duration, 70-kPa peak negative pressure | Dynamic contrast-enhanced MRI | Closed-loop controlled FUS precisely opened the BBB, enhancing delivery of immune checkpoint inhibitors to brain tumores. This combination therapy increased infiltration of cyctotoxic T cells, reduced tumor growth, and improved survival compared to immune checkpoint blockade alone. | N/A | Minimal inflammatory effects outside tumor; one animal death due to anesthesia (not FUS-related) |
| Dong et al. 2022 [23] | Gambogic Acid | 50 μL (1.5 μmol/L) | U87 and U251 glioma-bearing mice | 900 W, 0.6% duty cycle, 2 m duration, 0.63 Mpa | Fluorescence signal was detected with a live animal imaging system (IVIS) | FUS enhanced delivery of Gamogic acid-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles to glioma cells, increasing drug uptake and tumor inhibition. | 59 days (vs. 38-56 days in other groups) | Higher intensities (0.76–0.88 MPa) caused erythrocyte extravasation and apoptosis in adjacent tissue |
| Title | NCT | Status | Location | Size | Device | Drug |
|---|---|---|---|---|---|---|
| Non-Invasive Focused Ultrasound (FUS) With Oral Panobinostat in Children With Progressive Diffuse Midline Glioma (DMG) | NCT04804709 | Active, not recruiting | New York, NY | 3 | Focused Ultrasound with neuro-navigator-controlled sonication | Panobinostat |
| A Feasibility Study Examining the Use of Non-Invasive Focused Ultrasound (FUS) With Oral Etoposide Administration in Children With Progressive Diffuse Midline Glioma (DMG) | NCT05762419 | Recruiting | New York, NY | 10 | Focused ultrasound with neuro-navigator-controlled sonication | Etoposide |
| Blood Brain Barrier (BBB) Disruption Using Exablate Focused Ultrasound With Doxorubicin for Treatment of Pediatric Diffuse Intrinsic Pontine Gliomas (DIPG) | NCT05630209 | Recruiting | Washington, DC and Miami, FL | 10 | Exablate | Doxorubicin |
| Blood Brain Barrier (BBB) Disruption Using Exablate Focused Ultrasound With Doxorubicin for Treatment of Pediatric DIPG | NCT05615623 | Recruiting | Toronto, ON | 10 | Exablate | Doxorubicin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinknecht, S.; Fox, K.; Tsitsos, F.; Zacharoulis, S. Focused Ultrasound (FUS) and Pediatric Brain Tumors: Current Status and Future Directions. Appl. Sci. 2025, 15, 11322. https://doi.org/10.3390/app152111322
Kleinknecht S, Fox K, Tsitsos F, Zacharoulis S. Focused Ultrasound (FUS) and Pediatric Brain Tumors: Current Status and Future Directions. Applied Sciences. 2025; 15(21):11322. https://doi.org/10.3390/app152111322
Chicago/Turabian StyleKleinknecht, Sarah, Kristen Fox, Fotios Tsitsos, and Stergios Zacharoulis. 2025. "Focused Ultrasound (FUS) and Pediatric Brain Tumors: Current Status and Future Directions" Applied Sciences 15, no. 21: 11322. https://doi.org/10.3390/app152111322
APA StyleKleinknecht, S., Fox, K., Tsitsos, F., & Zacharoulis, S. (2025). Focused Ultrasound (FUS) and Pediatric Brain Tumors: Current Status and Future Directions. Applied Sciences, 15(21), 11322. https://doi.org/10.3390/app152111322






