Study on the Adsorption Characteristics of Microbial-Reed Fiber and Its MICP Solidified Saline Soil Test
Abstract
1. Introduction
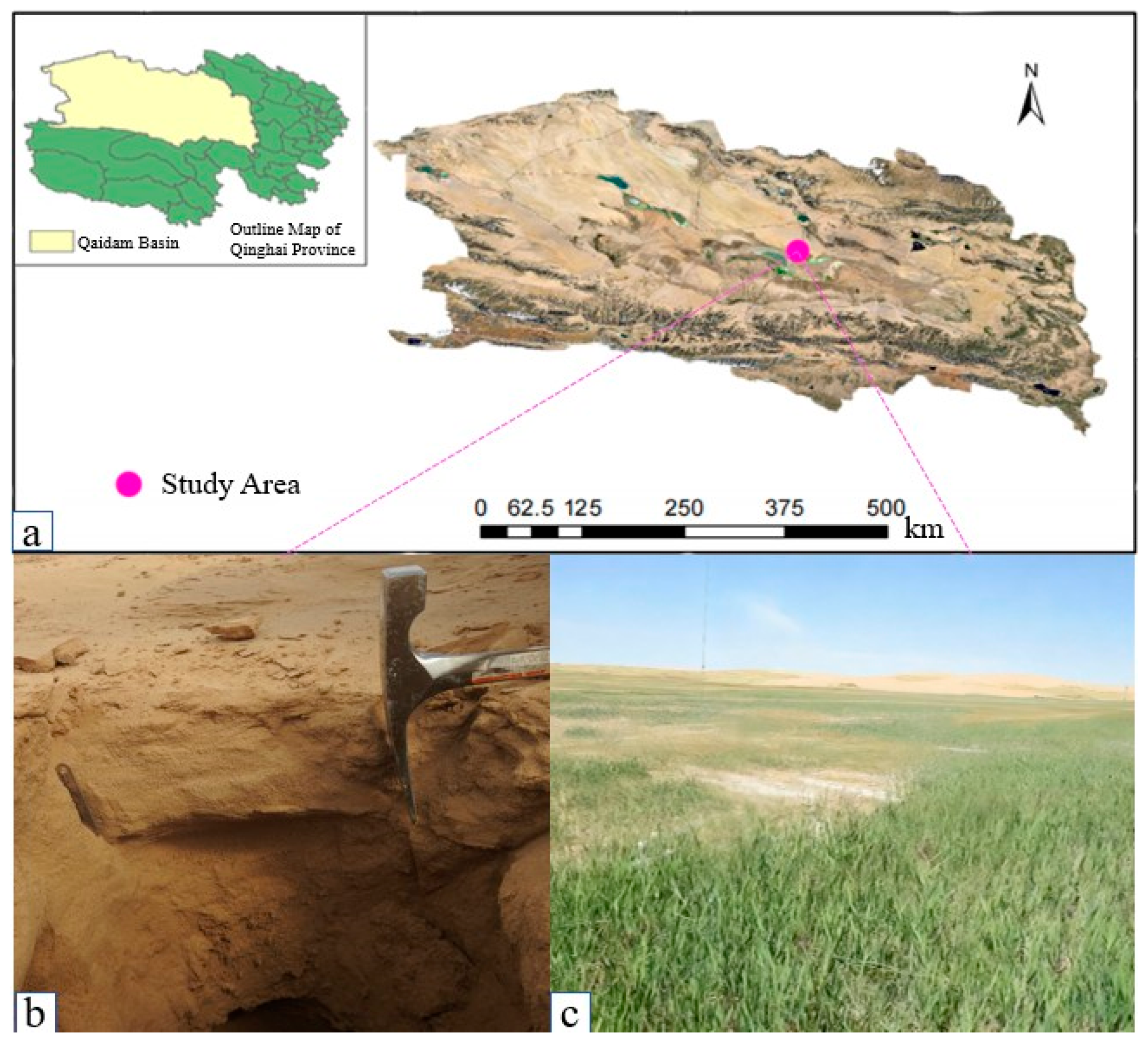
2. Overview of the Research Area
3. Experimental Materials and Methods
3.1. Experimental Materials
3.1.1. Basic Physical and Chemical Properties of Saline Soil
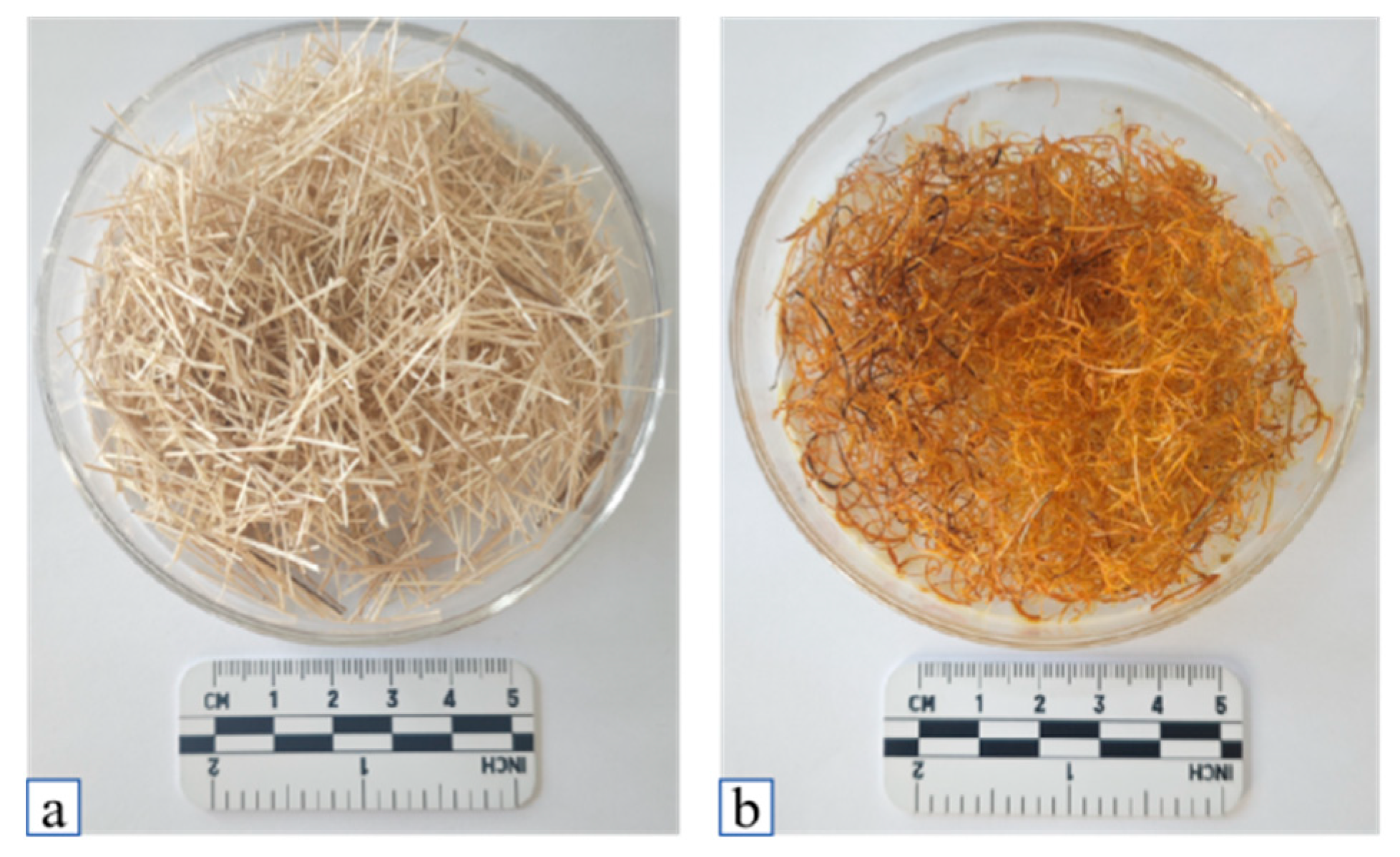
3.1.2. Reed Fiber Modification Process
3.1.3. Test Microorganisms
3.2. Experimental Design
- Fiber length (A): 10, 15, 20, 25, and 30 mm;
- Fiber content (B): 0.1%, 0.2%, 0.3%, 0.4%, and 0.5%, and 0.6% (mass ratio);
- Initial dry density (C): 1.405, 1.457, and 1.510 g/cm3;
- Binder solution concentration (D): 1.0, 2.0, and 3.0 mol/L.
3.2.1. Determination of Strain Enzyme Activity
3.2.2. Calculation of Microbial Strain Retention Rate
3.2.3. Microbial Adsorption Capacity Test for Calcium
3.2.4. Interface Performance Test
3.2.5. MICP Curing Test of Saline Soil Columns
3.2.6. Performance Test of Solidified Soil Mass
- (1)
- Unconfined Compressive Strength Test (UCS) and Unconsolidated Undrained Shear Strength Test (UU)
- (2)
- Quantification of Calcium Carbonate Content
4. Results and Analysis
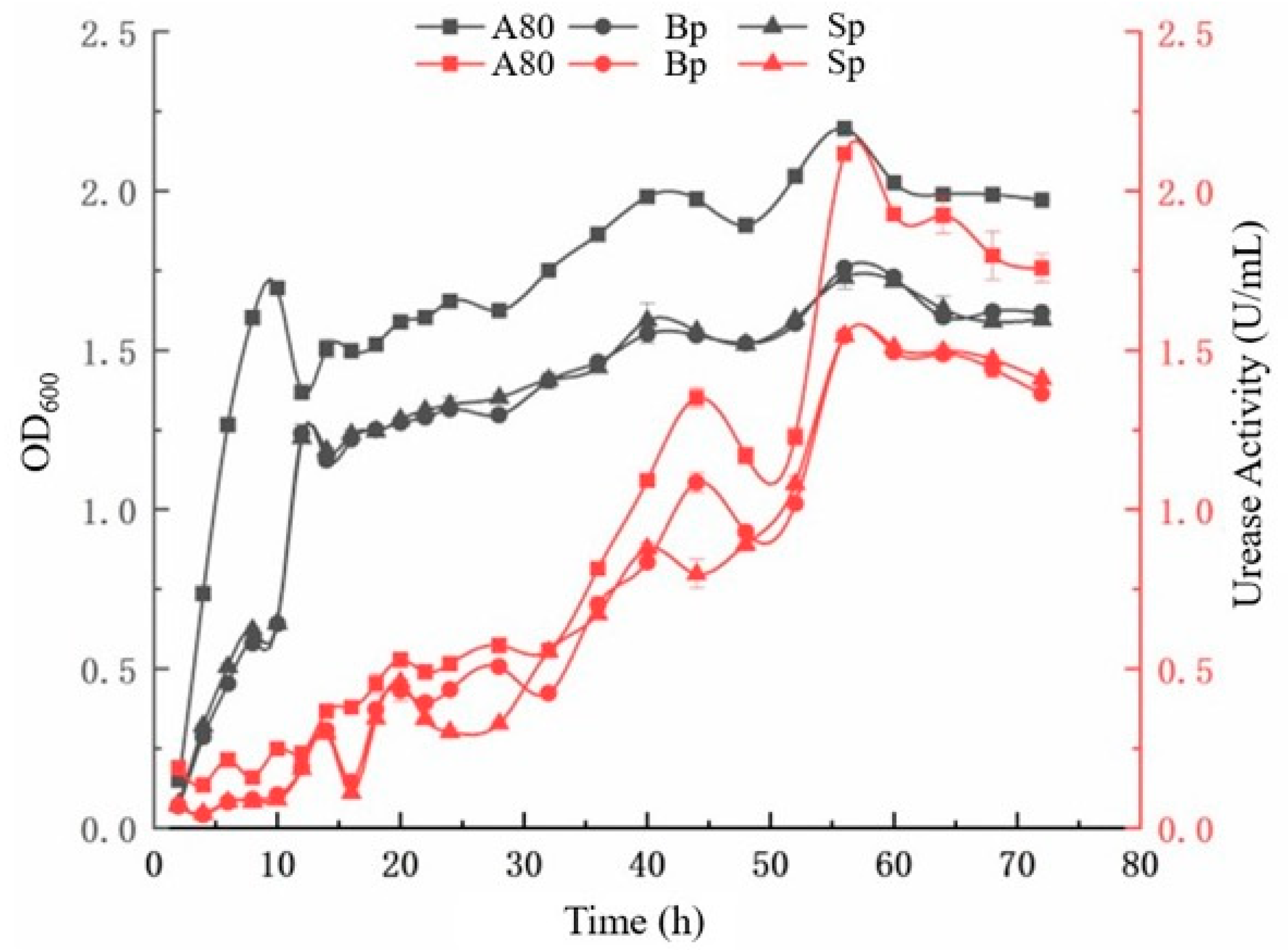
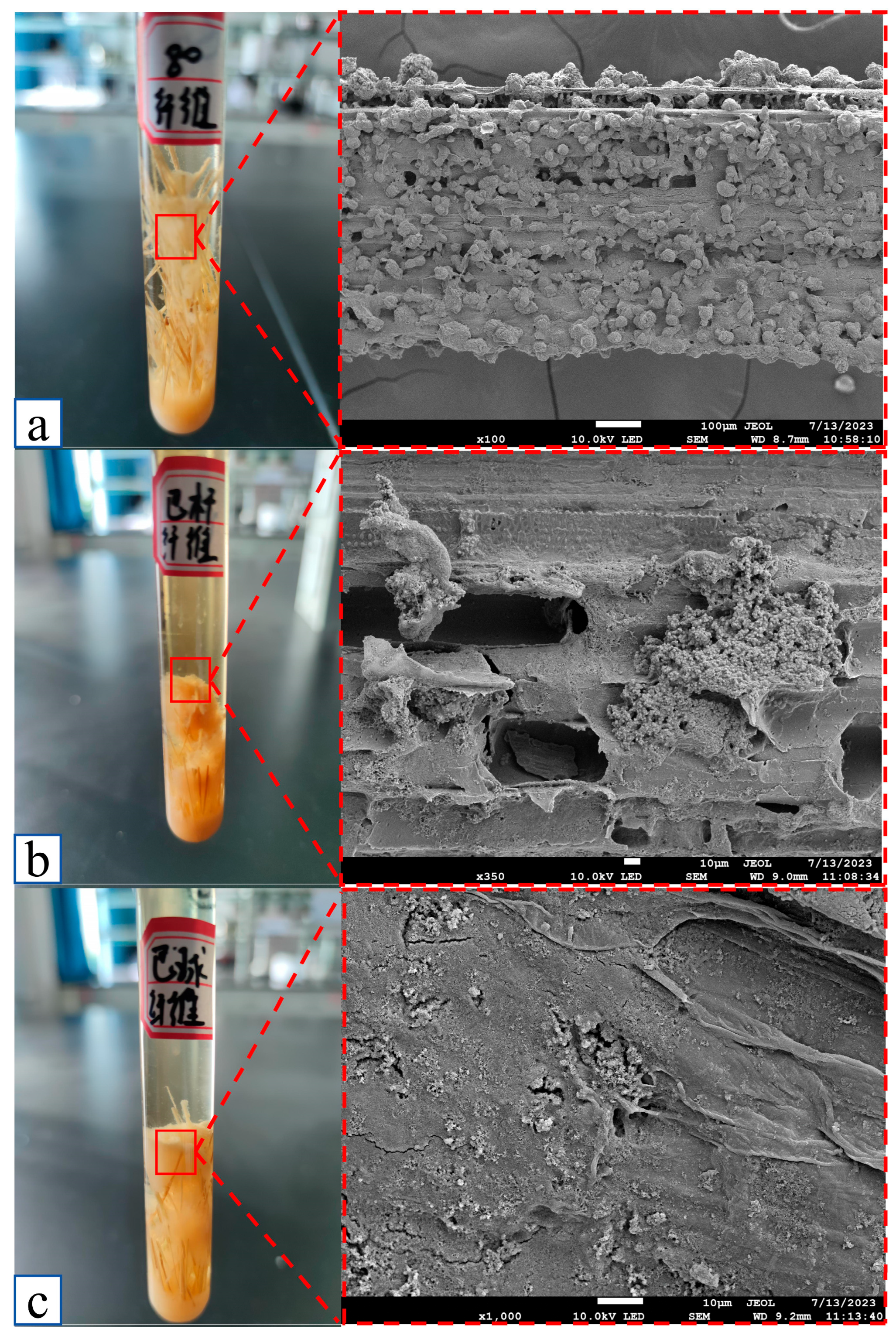
4.1. Growth Characteristics of A80 Strain in a Saline Environment
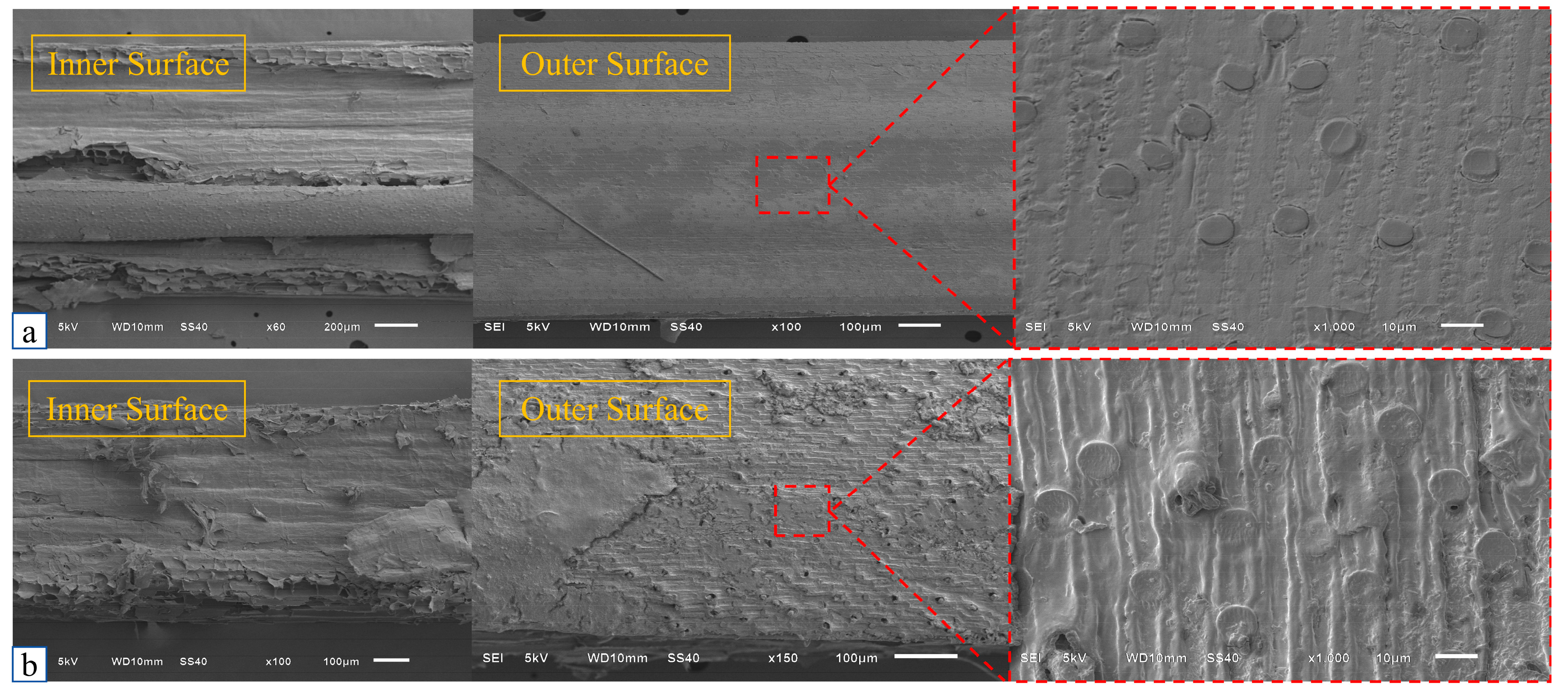
4.2. Effect of Fiber Surface Roughness on Calcium Carbonate Adhesion
4.2.1. Research and Analysis of Strain and Reed Fiber Adsorption
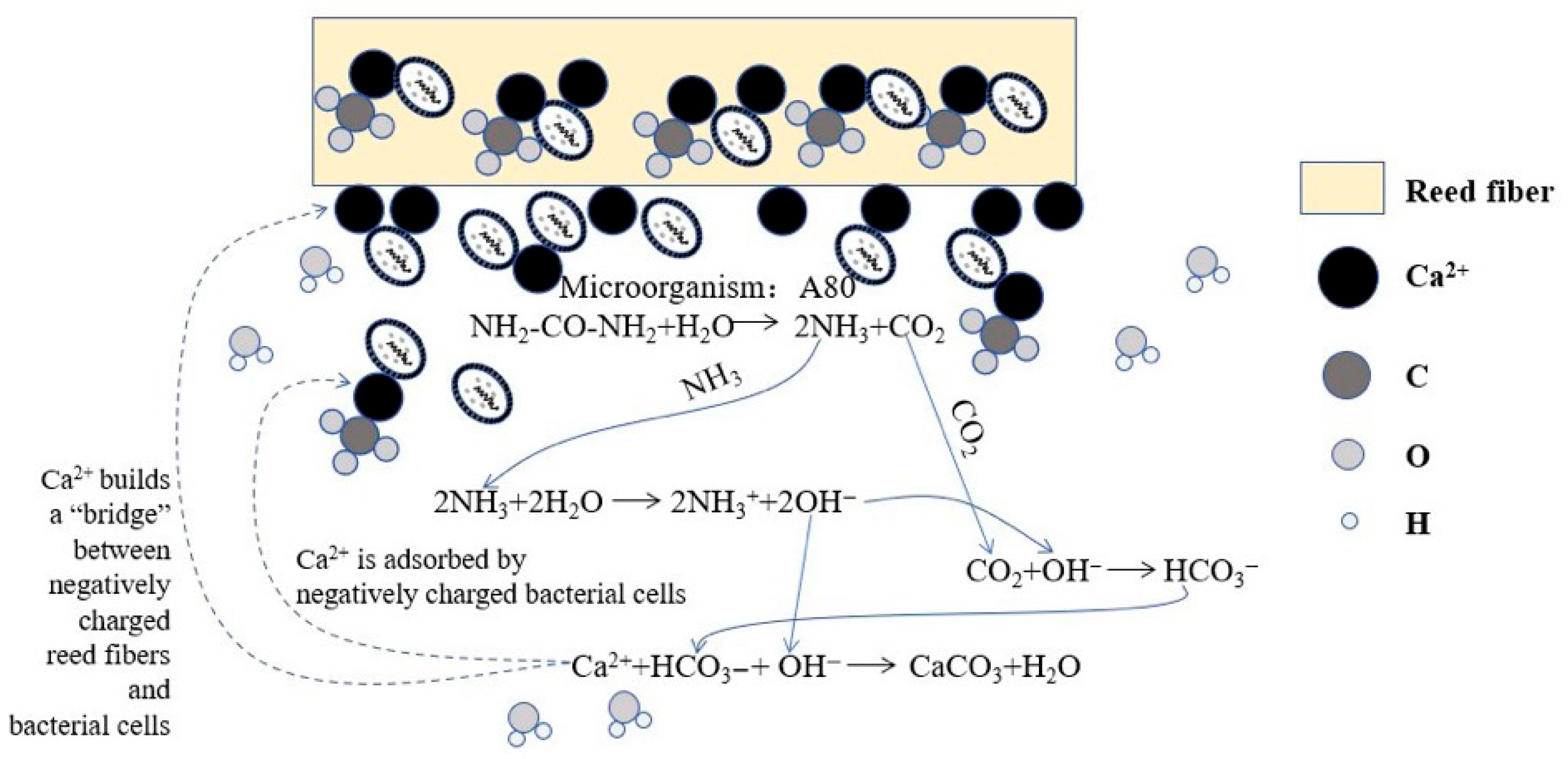
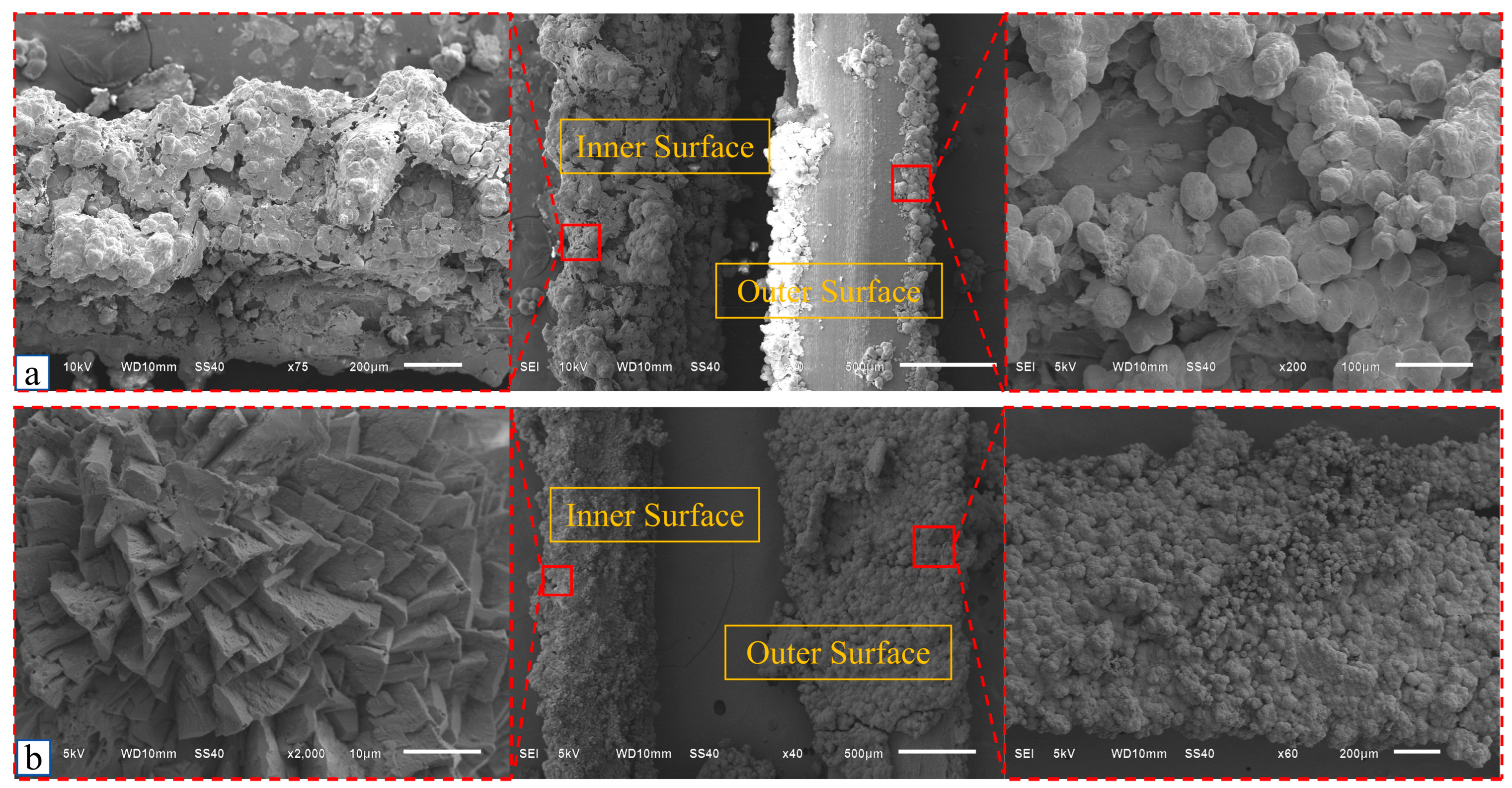
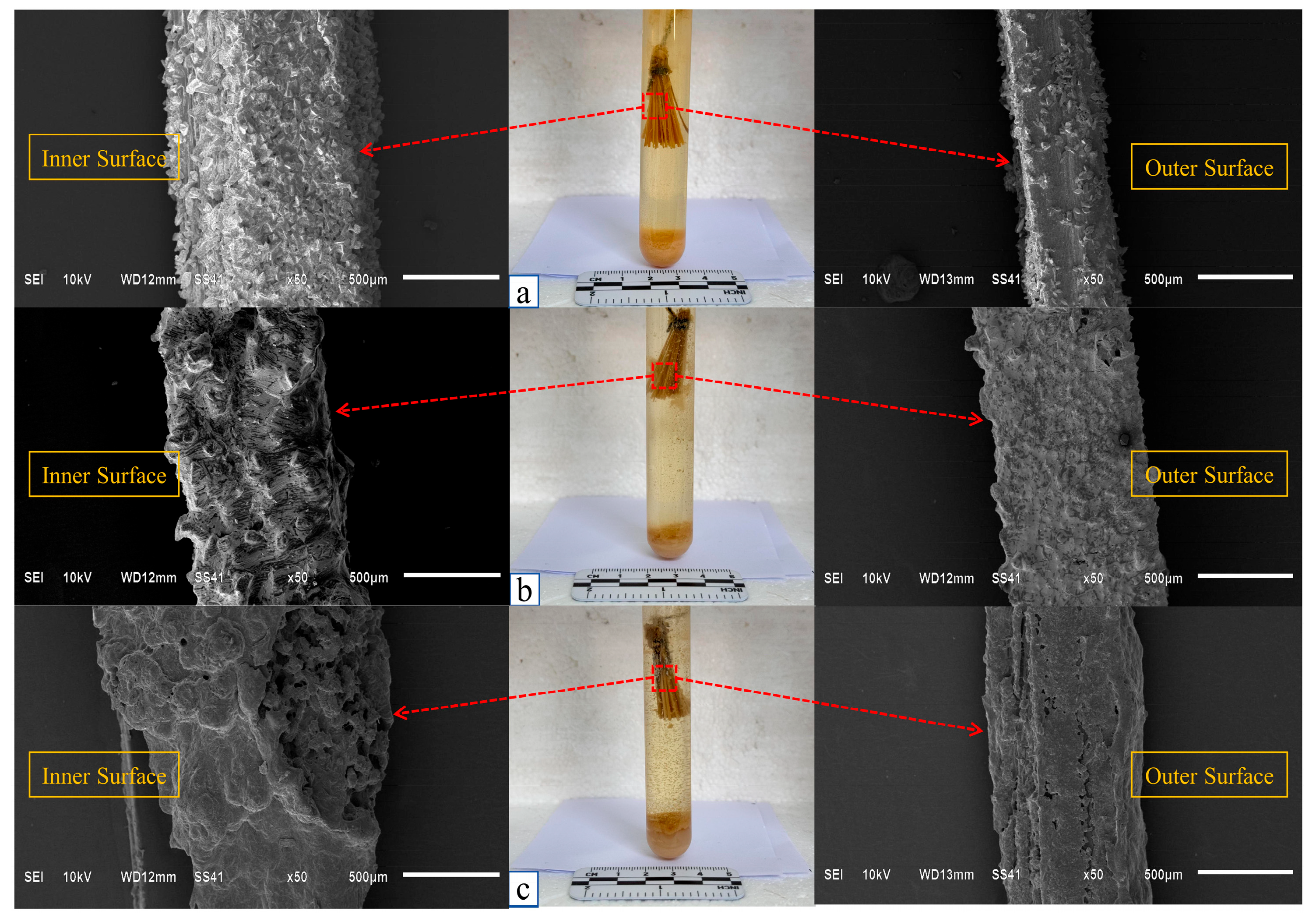
4.2.2. Microanalysis of Calcium Adsorption by Strains
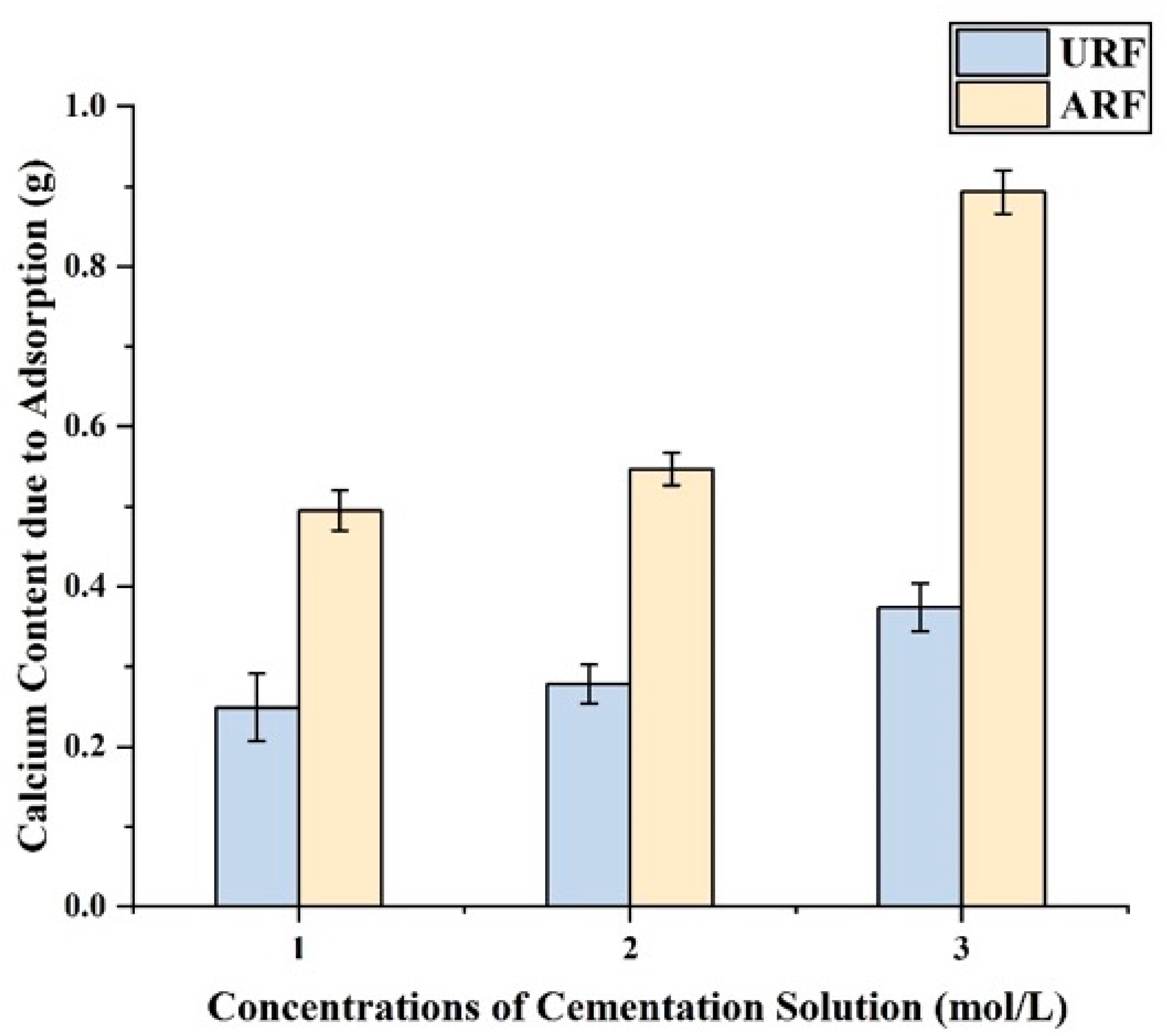
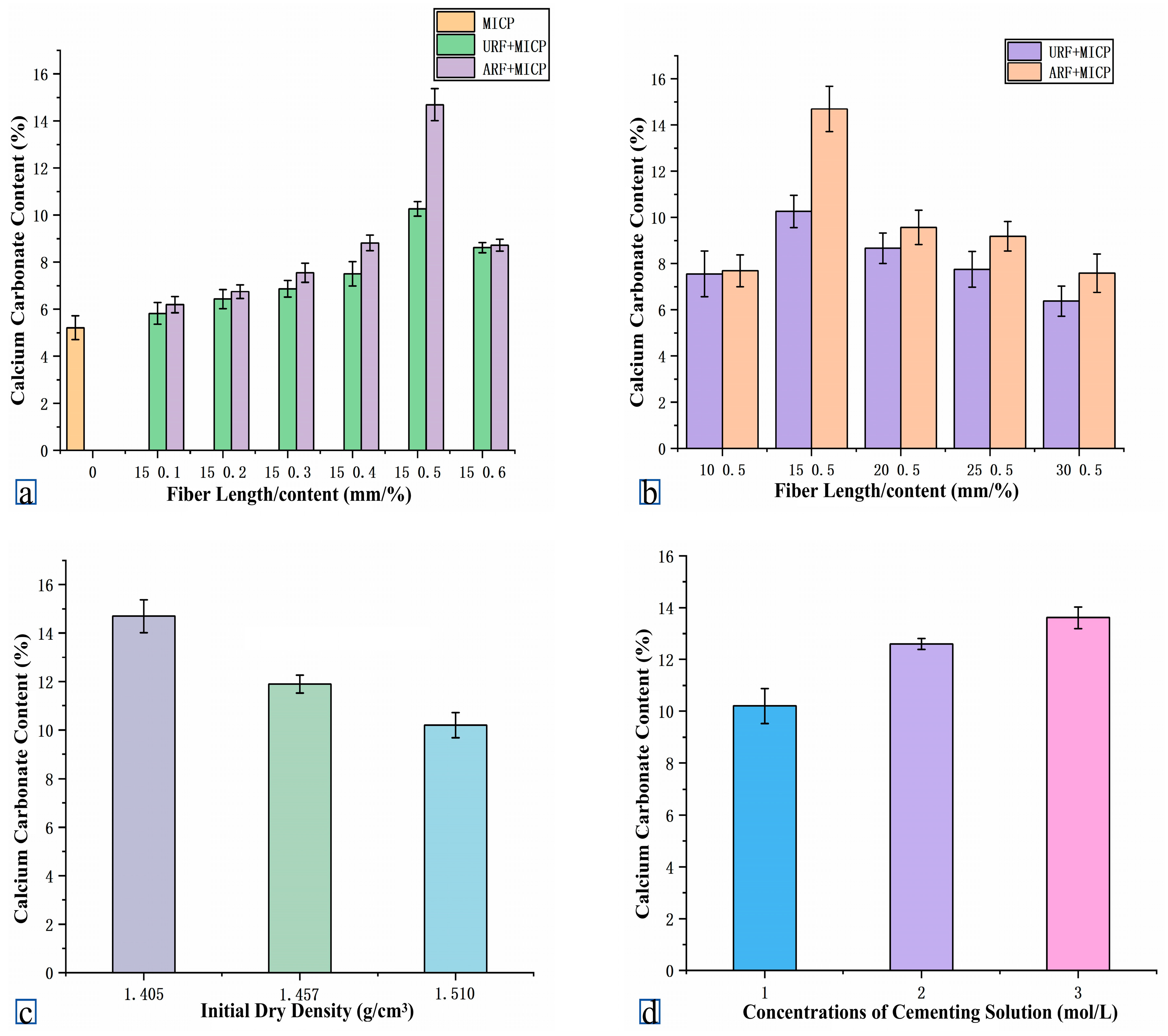
4.2.3. Comparison of Calcium Adsorption Amount Between URF Group and ARF Group
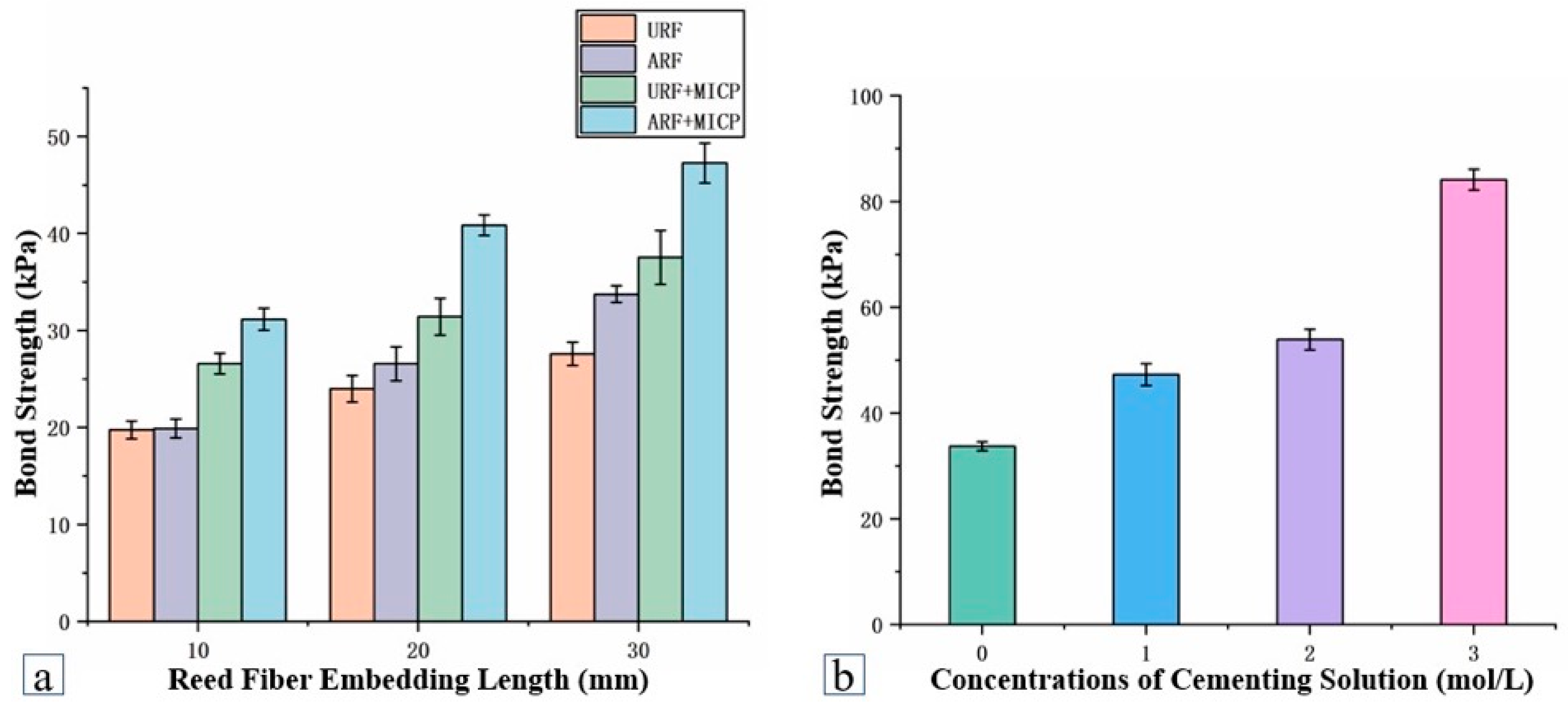
4.3. Reinforcement Mechanism of Fiber–MICP Interface Bond Strength
4.4. The Influence of Fiber Parameters on the Mechanical Properties of Cured Soil
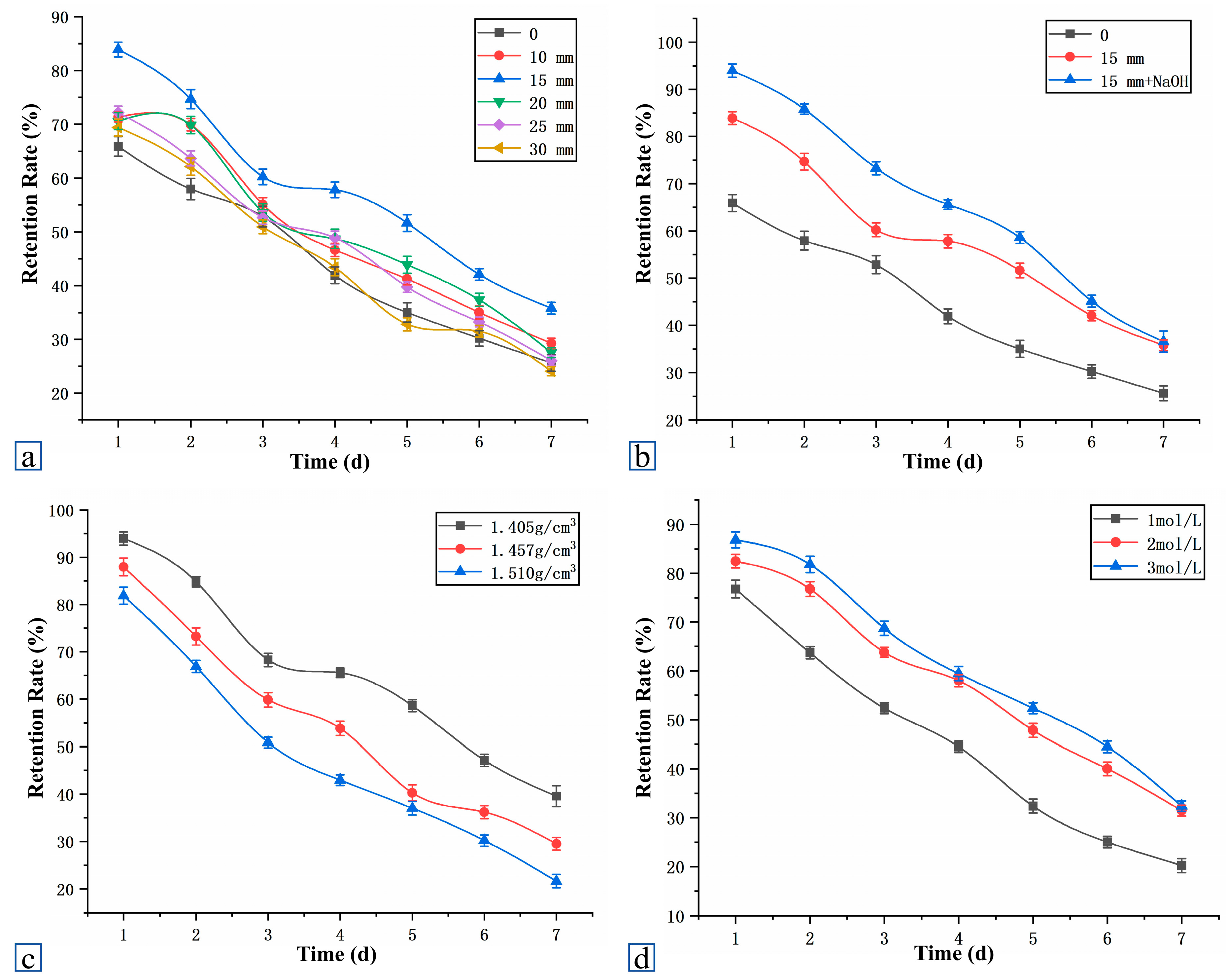
4.4.1. Analysis of Microbial Retention Rate
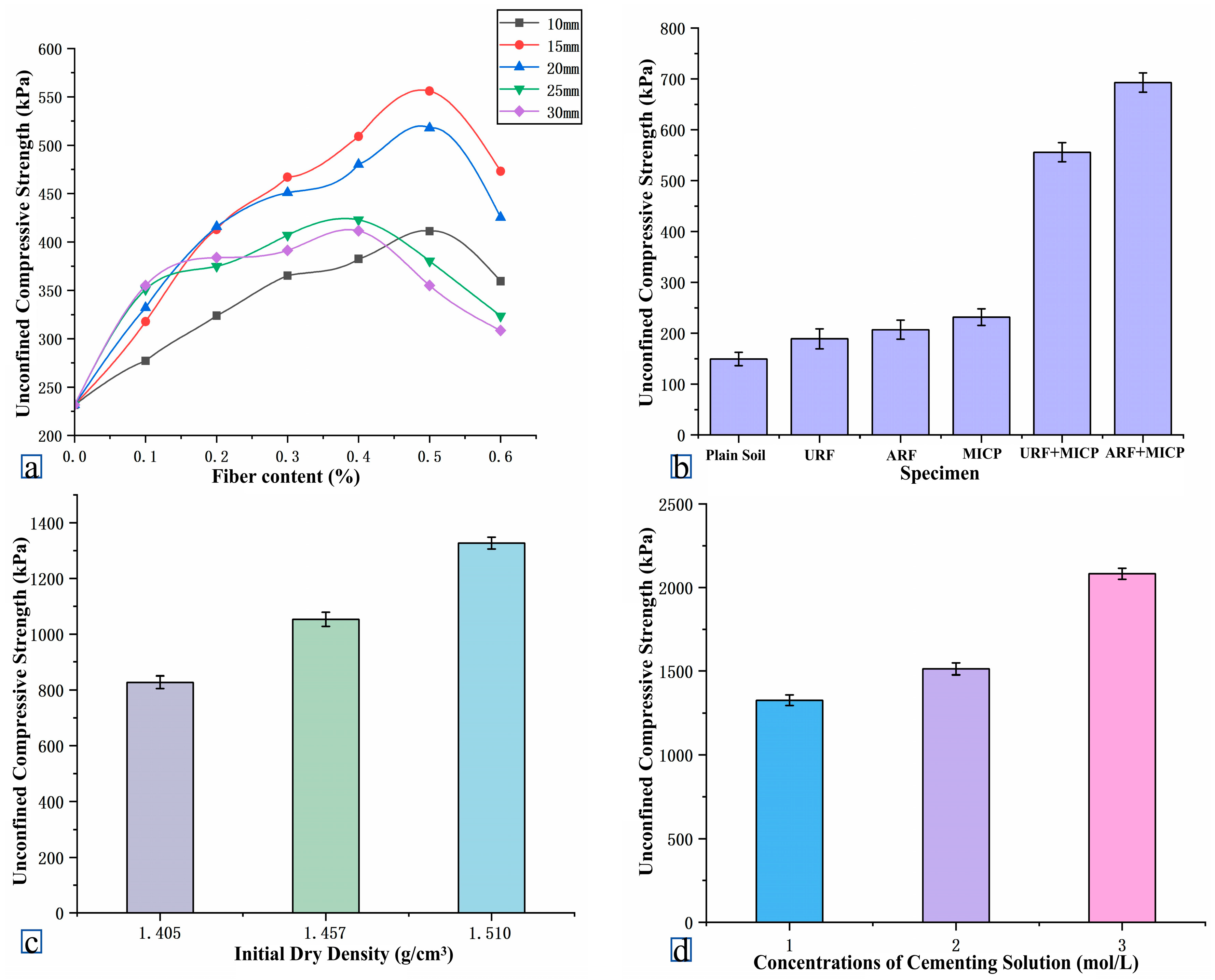
4.4.2. Threshold Effects of Fiber Length and Dosage
4.4.3. The Effect of Fiber Addition on Sample Strength
4.4.4. The Failure Pattern of Unconfined Compressive Strength
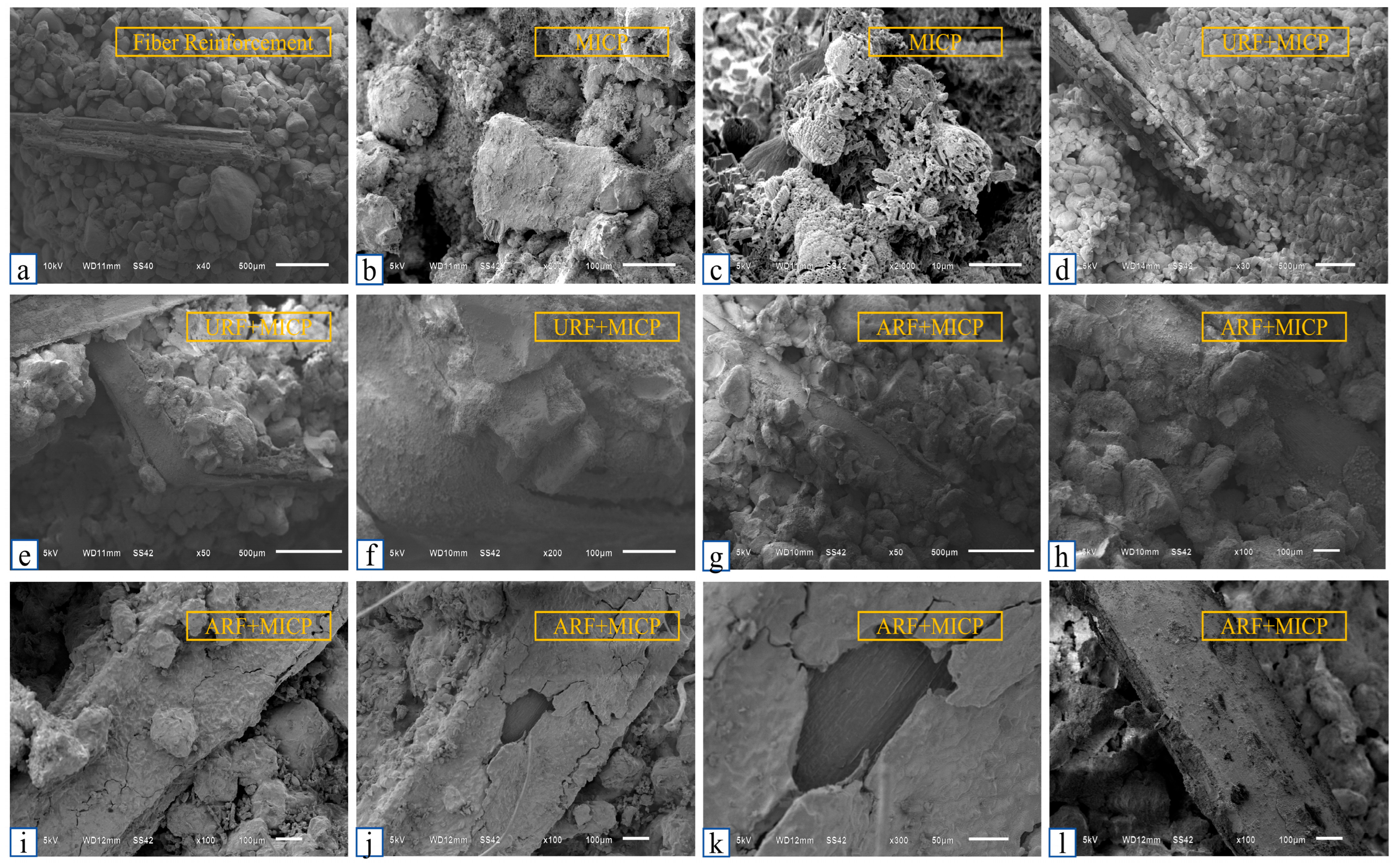
4.5. Fiber-MICP Reinforced Soil Microstructure Enhancement Mechanism
4.6. Comparative Analysis
5. Conclusions
- Improvement of interface performance: Alkaline modification significantly enhances the interfacial bonding performance between reed fibers and sand particles. The peak tensile strength of the ARF group reached 12.45 N, which was 26.7% higher than that of unmodified fibers (URF). SEM analysis showed that the surface roughness of the modified fibers (Ra = 1.23 μm) and the coverage of calcium carbonate (85.3%) were significantly improved, providing a good interface foundation for synergistic curing.
- Optimization of mechanical properties: Orthogonal experimental results showed that when the fiber length as 15 mm, the dosage was 0.5%, the dry density was 1.55 g/cm3, and the concentration of the binder was 3.0 mol/L, the unconfined compressive strength (UCS) of the solidified soil reached 2082.85 kPa, which was 2.99 times higher than that of the traditional MICP group. The stress–strain curve showed that the peak strain (ε = 4.12%) of the ARF-MICP group significantly increased, and the failure mode changed from brittle fracture to ductile failure.
- Based on the experimental results, a collaborative reinforcement model of “fiber bridging calcium carbonate filling” was proposed, revealing the bidirectional reinforcement mechanism of fiber constrained calcium carbonate distribution and calcium carbonate-reinforced fiber anchoring, providing a theoretical basis for the ecological restoration of plateau saline soil. Recommended process parameters include a fiber length of 15 mm, dosage of 0.5%, bonding solution concentration of 3.0 mol/L, and dry density of 1.55 g/cm3. Construction process: layered compaction → bacterial solution injection → bonding and solidification → curing for 7 days. Scope of application: suitable for high-altitude saline soil areas with salt content ≤ 5% and freeze–thaw cycles ≤ 10 times/year.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, L. Deserts and sandlands in China (Part I). Encycl. Knowl. 2021, 17, 40–41. [Google Scholar]
- Ma, M.; Li, X.T.; Tang, C.P. Current situation, causes, and prevention strategies of desertification. Mod. Hortic. 2014, 8, 154–155. [Google Scholar] [CrossRef]
- Luo, Y.D. Discussion on characteristics of saline soil and its foundation treatment methods. Miner. Explor. 2019, 10, 1214–1218. [Google Scholar] [CrossRef]
- Li, P.F.; Yang, Y.L.; Lan, T.; Guo, S.W.; Zhang, K.; Han, J.W.; Zhang, Q. Soil physicochemical properties and water retention characteristics of improved coastal saline soil in Tianjin. Trans. Chin. Soc. Agric. Eng. 2017, 33, 149–156. [Google Scholar] [CrossRef]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities, and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Montoya, B.M.; Dejong, J.T. Stress-strain behavior of sands cemented by microbially induced calcite precipitation. J. Geotech. Geoenvironmental Eng. 2015, 141, 04015019. [Google Scholar] [CrossRef]
- Liu, L.; Shen, Y.; Liu, H.L.; Chu, J. Application research of microbial cementation in prevention of dam failure. Rock Soil Mech. 2016, 37, 3410–3416. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Zheng, J.J.; Song, Y.; Lai, H.J.; Cui, M.J.; Wu, C.C. Experimental study on shear strength of microbially induced calcite precipitation (MICP)-treated fiber-reinforced sand. J. Civ. Environ. Eng. 2019, 41, 15–21. [Google Scholar]
- Yin, L.Y.; Tang, C.S.; Zhang, L. Mechanical properties of calcareous sand modified by MICP combined with fiber reinforcement. Geol. J. China Univ. 2021, 27, 679–686. [Google Scholar]
- Jiang, Z.; Peng, J.; Xu, P.X.; Wei, R.J.; Li, L.L. Experimental study on high strength of calcareous sand reinforced by microbes combined with carbon fiber. J. Civ. Environ. Eng. 2024, 46, 64–73. [Google Scholar]
- Li, L.; Zhao, Q.; Zhang, H.; Amini, F.; Li, C. A full contact flexible mold for preparing samples based on microbial-induced calcite precipitation technology. Geotech. Test. J. 2014, 37, 917–921. [Google Scholar] [CrossRef]
- Chen, H.J.; Peng, C.F.; Tang, C.W.; Chen, Y.T. Self-healing concrete by biological substrate. Materials 2019, 12, 4099. [Google Scholar] [CrossRef]
- Choi, S.-G.; Wang, K.; Chu, J. Properties of biocemented, fiber reinforced sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar] [CrossRef]
- Li, W.C.; Jiang, Y.M.; Qi, Y.X. Research overview of reed fiber and its reinforced composites. Knitt. Ind. 2022, 11, 73–76. [Google Scholar]
- Lu, J.; Liu, Z.H.; Si, Y.B.; Xu, J.J.; Du, Y.F.; Dong, L. Characteristics, development and utilization, and control methods of reed. Weed Sci. 2007, 3, 7–8,24. [Google Scholar]
- GB/T 50123-2019; Standard for Soil Test Methods. China Standards Press: Beijing, China, 2019.
- Wang, W.; Sun, C.; Cui, J.D. Adsorption properties of composite modified reed fiber for cationic dyes. Wool Text. J. 2022, 50, 55–61. [Google Scholar]
- Fang, X.; Yang, Y.; Chen, Z.; Liu, H.; Xiao, Y.; Shen, C. Influence of fiber content and length on engineering properties of MICP-treated coral sand. Geomicrobiol. J. 2020, 37, 582–594. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, X.; Wang, J.; Wang, Y.; Feng, D.; Zhu, C. Influence of fiber type and length on mechanical properties of MICP-treated sand. Materials 2022, 15, 4017. [Google Scholar] [CrossRef]
- Zhang, D.; Shahin, M.A.; Yang, Y.; Liu, H.L.; Cheng, L. Effect of microbially induced calcite precipitation treatment on the bonding properties of steel fiber in ultra-high performance concrete. J. Build. Eng. 2022, 50, 10413. [Google Scholar] [CrossRef]
- Hou, F.X.; Zhao, Y.; Hu, P.S.; Li, B.; Zhang, W. Screening and identification of new salt-tolerant urease microorganisms in cold and arid regions. J. Arid. Land Resour. Environ. 2021, 35, 178–183. [Google Scholar]
- Feng, N.; Guo, X.; Liang, S. Enhanced Cu(II) adsorption by orange peel modified with sodium hydroxide. Trans. Nonferrous Met. Soc. China 2010, 20, s146–s152. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.P.; Xu, Y.W. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef]
- Gupta, M.K.; Srivastava, R.K.; Bisaria, H. Potential of jute fibre reinforced polymer composites: A review. Int. J. Fiber Text. Res. 2015, 5, 30–38. [Google Scholar]
- Imran, M.A.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. The influence of the addition of plant-based natural fibers (jute) on biocemented sand using MICP method. Materials 2020, 13, 4198. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Patel, R.; Patel, S. Influence of Shear Strength Parameters on Loose Fine Sand Treated with Coir Fiber and Microbially Induced Calcite Precipitation. Springer Proc. Mater. 2022, 2, 68. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, H. Experimental Investigation on Mechanical Behavior of Sands Treated by Enzyme-Induced Calcium Carbonate Precipitation with Assistance of Sisal-Fiber Nucleation. Front. Earth Sci. 2022, 10, 992474. [Google Scholar] [CrossRef]
- Li, Q.F.; Xing, Z.G.; Dang, B.; Peng, E.X.; Hu, X.Y. Study on the Mechanical Properties of Lignin Fiber-MICP Solidified Silt under Freezing and Thawing Cycles. J. Glaciol. Geocryol. 2024, 46, 1828–1838. [Google Scholar]
- Chen, H.; Li, X.; Zhang, Q. Performance of MICP-Treated Soil against Environmental Deterioration. In GeoHunan International Conference; ASCE: Reston, VA, USA, 2023; pp. 16–23. [Google Scholar] [CrossRef]






| Sampling Depth (m) | pH | Types of Easily Soluble Salts (g/kg) | Total Salt Content (g/kg) | Types of Saline Soil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO32− | HCO3− | Cl− | SO42− | K+ | Na+ | Ca2+ | Mg2+ | ||||
| 0.0 | 8.15 | 0 | 0.46 | 12.43 | 1.80 | 1.92 | 0.81 | 1.73 | 2.46 | 39.60 | Chloride Saline Soil |
| Name | Color | Length (mm) | Thickness (mm) | Tensile Strength (MPa) | Moisture Content (%) | Cross-Sectional Shape |
|---|---|---|---|---|---|---|
| Reed Fiber | Light Yellow | 60 | 0.19~0.21 | 610.13 | 3.6% | Rectangular |
| TSA Medium Components | Dosage/(g·L−1) |
|---|---|
| Tryptone | 17 g |
| Soy peptone | 3 g |
| NaCl | 5 g |
| K2PHO4 | 2.5 g |
| Glucose | 2.5 g |
| Source of Study | Soil Type Improved | Fiber Type and Modification Method | Key Parameters (Fiber/Curing System) | Core Mechanical Property Indicators | Interface and Microscopic Characteristics | Failure Mode |
|---|---|---|---|---|---|---|
| Present Study | Plateau saline soil | Alkali-modified reed fiber (ARF) | Length: 15 mm, content: 0.5%; cementing solution: 3.0 mol/L, dry density: 1.55 g/cm3 | Unconfined compressive strength (UCS) = 2082.85 kPa, 2.99 times higher than conventional MICP group; peak strain = 4.12% | Fiber tensile strength = 12.45 N (26.7% higher than unmodified reed fiber (URF)); surface roughness Ra = 1.23 μm; CaCO3 coverage rate = 85.3% | Ductile failure |
| Imran, A.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. (2020) [26] | Quartz sand | Unmodified jute fiber | Length: 15 mm, content: 3.0%; cementing solution concentration not specified | Optimal UCS ≈ 610 kPa, approximately 2.0 times higher than pure MICP group; peak strain not reported | Fibers provide attachment sites for CaCO3 precipitation, reducing specimen brittleness | Quasi-ductile failure |
| Patel, C.; Patel, R.; Patel, S. (2022) [27] | Fine sand | Unmodified coir fiber | Aspect ratio: 182, content: 0.3%; cementing solution: 3.0 mol/L | Cohesion increased by 6 times compared with pure MICP group; internal friction angle = 42° (1.4 times higher than pure MICP group); UCS not measured | CaCO3 fills pores, and fibers enhance inter-particle constraint | Brittleness reduction |
| Patel, C.; Patel, R.; Patel, S. (2022) [28] | Quartz sand | Unmodified sisal fiber | Length: 10 mm, content: 0.2%; enzyme-induced calcium carbonate precipitation (EICP) curing system | Optimal UCS ≈ 450 kPa, 4.0 times higher than pure EICP group; peak strain data fluctuated and was not quantified | Fibers provide nucleation sites on their surface, forming a “bridging network”; CaCO3 crystals are interwoven | Enhanced ductile characteristics |
| Li, Q.F.; Xing, Z.G.; Dang, B.; (2024) [29] | Silt in seasonal frozen regions | Unmodified lignin fiber | Content: 1.5%; cementing solution concentration not specified, dry density not specified | UCS = 420 kPa (after 10 freeze–thaw cycles), 17.5% higher retention rate than pure MICP group; peak strain = 1.8% | CaCO3 content was 426.6% higher than pure MICP group; a dense cemented structure formed among fibers, CaCO3, and soil particles | Semi-brittle failure |
| Chen, H.; Li, X.; Zhang, Q. (2023) [30] | Sand | Unmodified natural fiber | Content not specified; cementing solution concentration not specified | Peak strain = 1.6% after 5 wet–dry cycles (300% higher than non-fiber group); UCS retention rate = 65% | Fibers inhibited the spalling of CaCO3 crystals and reduced the increase in porosity | Ductile failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Bai, Z.; Wang, X.; Wang, R.; Zhang, W. Study on the Adsorption Characteristics of Microbial-Reed Fiber and Its MICP Solidified Saline Soil Test. Appl. Sci. 2025, 15, 11198. https://doi.org/10.3390/app152011198
Du Y, Bai Z, Wang X, Wang R, Zhang W. Study on the Adsorption Characteristics of Microbial-Reed Fiber and Its MICP Solidified Saline Soil Test. Applied Sciences. 2025; 15(20):11198. https://doi.org/10.3390/app152011198
Chicago/Turabian StyleDu, Yimo, Zhenyu Bai, Xiaoli Wang, Ruze Wang, and Wen Zhang. 2025. "Study on the Adsorption Characteristics of Microbial-Reed Fiber and Its MICP Solidified Saline Soil Test" Applied Sciences 15, no. 20: 11198. https://doi.org/10.3390/app152011198
APA StyleDu, Y., Bai, Z., Wang, X., Wang, R., & Zhang, W. (2025). Study on the Adsorption Characteristics of Microbial-Reed Fiber and Its MICP Solidified Saline Soil Test. Applied Sciences, 15(20), 11198. https://doi.org/10.3390/app152011198





