Comprehensive Profiling of Coconut Oil Varieties: Fatty Acids Composition, Oxidative Stability, Bioactive Properties, and Sensory Attributes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Determination of Oil Quality
2.3.1. Acid Value Determination
2.3.2. Peroxide Value Determination
2.3.3. Oxidative Stability
2.4. Antioxidant Activity—DPPH Radical Scavenging Activity
2.5. Determination of Fatty Acid Methyl Esters (FAME) Content
2.6. Antimicrobial Activity Determination
2.7. Sensory Characteristic Determination
2.8. Statistical Analysis
3. Results
3.1. Oxidative Stability of the Oil Sample
3.2. DPPH Radical Scavenging Activity
3.3. Antimicrobial Activity
3.4. Fatty Acid Methyl Ester (FAME) Profile of Oil Samples
3.5. Sensory Analysis of Coconut Samples
3.6. Variable Interrelationships
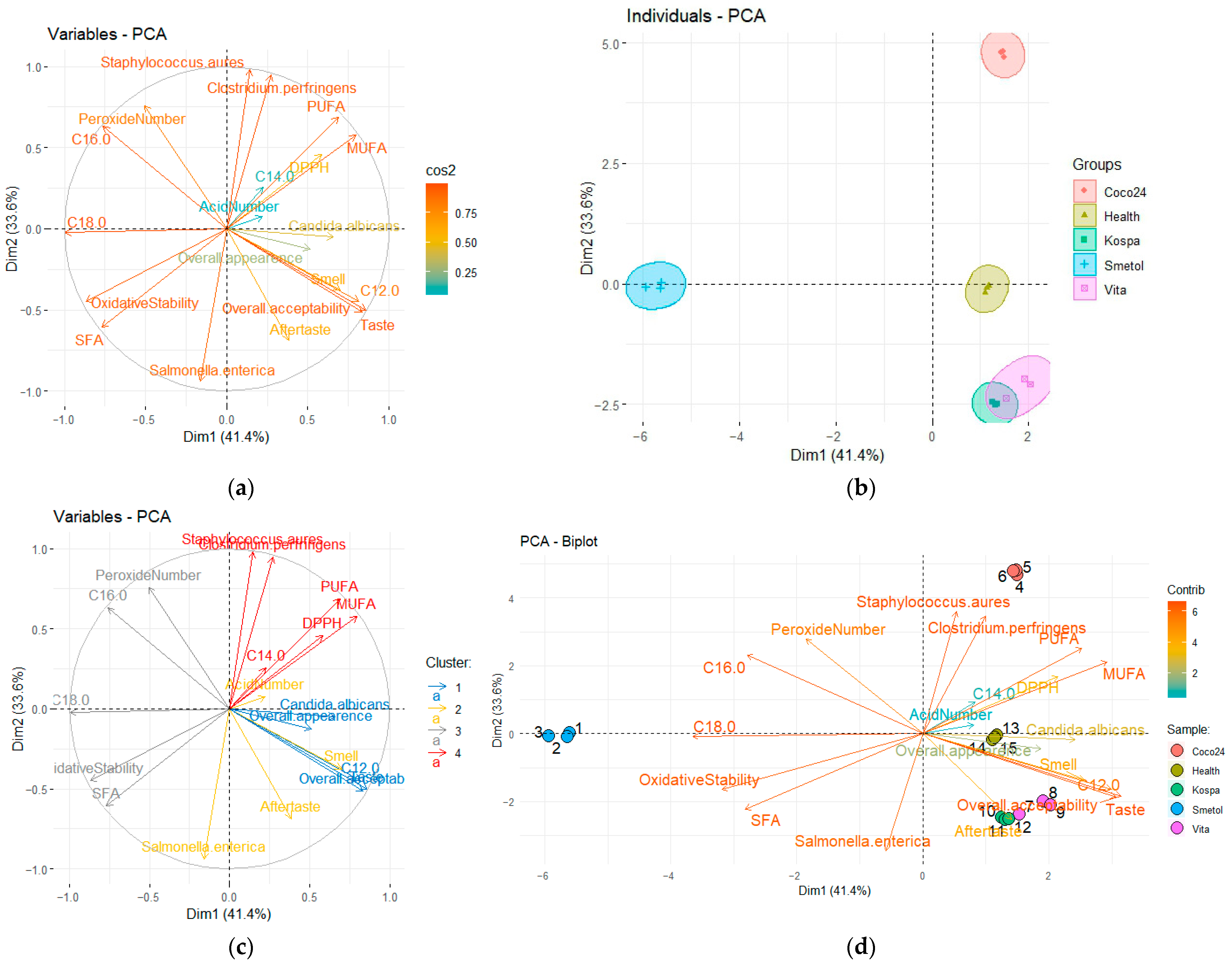
3.7. Principal Component Analysis (PCA)
4. Discussion
4.1. Oxidative Stability of the Oil Samples
4.2. DPPH Radical Scavenging Activity
4.3. Antimicrobial Activity
4.4. Fatty Acid Methyl Ester (FAME) Profile of Oil Samples
4.5. Sensory Characteristic of Coconut Samples
4.6. Variable Interrelationships
4.7. Principal Component Analysis (PCA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Marshall, S.; Abbott, K.; Cassettari, T.; Duve, E.; Fayet-Moore, F. How Should We Judge Edible Oils and Fats? An Umbrella Review of the Health Effects of Nutrient and Bioactive Components Found in Edible Oils and Fats. Crit. Rev. Food Sci. Nutr. 2022, 62, 5167–5182. [Google Scholar] [CrossRef]
- Boateng, L.; Ansong, R.; Owusu, W.; Steiner-Asiedu, M. Coconut Oil and Palm Oil’s Role in Nutrition, Health and National Development: A Review. Ghana Med. J. 2016, 50, 189–196. [Google Scholar] [CrossRef]
- Wallace, T.C. Health Effects of Coconut Oil—A Narrative Review of Current Evidence. J. Am. Coll. Nutr. 2019, 38, 97–107. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical Properties, Antioxidant Capacities, and Metal Contents of Virgin Coconut Oil Produced by Wet and Dry Processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Villarino, B.J.; Dy, L.M.; Lizada, M.C.C. Descriptive Sensory Evaluation of Virgin Coconut Oil and Refined, Bleached and Deodorized Coconut Oil. LWT-Food Sci. Technol. 2007, 40, 193–199. [Google Scholar] [CrossRef]
- Kappally, S.; Shirwaikar, A.; Shirwaikar, A. Coconut Oil—A Review of Potential Applications. Hygeia J. Drugs Med. 2015, 7, 34–41. [Google Scholar]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In Health Promotion and Disease Prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A Review on the Influence of Nutraceuticals and Functional Foods on Health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Seneviratne, K.N.; Sudarshana Dissanayake, D.M. Variation of Phenolic Content in Coconut Oil Extracted by Two Conventional Methods. Int. J. Food Sci. Technol. 2008, 43, 597–602. [Google Scholar] [CrossRef]
- Dayrit, F.M.; Nguyen, Q. Improving the Value of the Coconut with Biotechnology. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 29–50. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS: Champaign, IL, USA, 2004. [Google Scholar]
- AOCS. Official Method Cd 8-53. Peroxide Value. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; Firestone, D., Ed.; AOCS: Champaign, IL, USA, 2005. [Google Scholar]
- ISO 6886; International Standard ISO Animal and Vegetable Fats and Tocotrienol Contents by High-iTeh Standard Preview iTeh Standard Preview 1997. ISO: Geneva, Switzerland, 1997.
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Azevedo, E.P.P.; Alves, E.M.S.; Souza, J.R.B.; Araújo, K.S.; Khan, S.S.; Mendonça, C.E.A.; Maciel, M.I.S. Fatty Acid in Raw and Heated Coconut Oil in Eleven Coconut Oil Food Preparations Analysed by Gas Chromatography. Int. J. Gastron. Food Sci. 2021, 24, 100329. [Google Scholar] [CrossRef]
- Donaldson, J.R.; Warner, S.L.; Cates, R.G.; Young, D.G. Assessment of Antimicrobial Activity of Fourteen Essential Oils When Using Dilution and Diffusion Methods. Pharm. Biol. 2005, 8, 687–695. [Google Scholar] [CrossRef]
- Moigradean, D.; Poiana, M.-A.; Gogoasa, I. Quality Characteristics and Oxidative Stability of Coconut Oil during Storage. J. Agroaliment. Process. Technol. 2012, 18, 272–276. [Google Scholar]
- Gunstone, F.D. Reaction of Oxygen and Unsaturated Fatty Acids. J. Am. Oil Chem. Soc. 1984, 61, 441–447. [Google Scholar] [CrossRef]
- Ivanišová, E.; Juricová, V.; Árvay, J.; Kačániová, M.; Čech, M.; Kobus, Z.; Krzywicka, M.; Cichocki, W.; Kozłowicz, K. Physicochemical, Antioxidant, Antimicrobial, and Sensory Characteristics of Selected Kinds of Edible Oils. Appl. Sci. 2024, 14, 3630. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative Stability of Flaxseed Oil: Effect of Hydrophilic, Hydrophobic and Intermediate Polarity Antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Wathsala, S.C.; Perumpuli, P.; Abeysuriya, A. Physicochemical, Nutritional, Functional, and Sensory Properties of Coconut Oil Extracted by Different Methods. Ceylon J. Sci. 2025, 54, 845–853. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, X.; Liu, W.; Wang, T.; Zhang, T.; Wei, C. Prolonging the Oxidative Stability of Walnut Oil by Endogenous Antioxidants: Phytosterol Compounding for Improved Antioxidant Capacity. J. Food Compos. Anal. 2025, 137, 106931. [Google Scholar] [CrossRef]
- Osei, E.D.; Pokuah, A.A.; Atinpoore, R.A.; Faisal, E.S.; Amotoe-Bondzie, A.; Yussif, A.-M.; Akabanda, F.; Amagloh, F.K. Characterization of Soy Curd Residue and Full-Fat Soy Flour as Protein-Based Food Ingredients. Potravin. Slovak J. Food Sci. 2024, 18, 36–49. [Google Scholar] [CrossRef]
- Alimentarius, C. Codex Standard for Named Vegetable Oils. Codex Stan. 1999, 210, 1–13. [Google Scholar]
- O’brien, R.D. Fats and Oils: Formulating and Processing for Applications; CRC Press: Boca Raton, FL, USA, 2008; ISBN 1420061674. [Google Scholar]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; de Alencar, S.M.; Canniatti-Brazaca, S.G.; de Souza Vieira, T.M.F.; Shahidi, F. Chemical Changes and Oxidative Stability of Peanuts as Affected by the Dry-Blanching. J. Am. Oil Chem. Soc. 2016, 93, 1101–1109. [Google Scholar] [CrossRef]
- Negi, A.; Nimbkar, S.; Thirukumaran, R.; Moses, J.A.; Sinija, V.R. Impact of Thermal and Nonthermal Process Intensification Techniques on Yield and Quality of Virgin Coconut Oil. Food Chem. 2024, 434, 137415. [Google Scholar] [CrossRef] [PubMed]
- Anjali, N.V.P.; Algama, C.H.; Seneviratne, K.P.; Seneviratne, C.A.; Jayathilaka, N.; Seneviratne, K.N. A Comparison of Wet-extracted Coconut Oils Prepared under Hot and Cold Conditions. J. Am. Oil Chem. Soc. 2023, 100, 459–468. [Google Scholar] [CrossRef]
- Aytac, E. Comparison of Extraction Methods of Virgin Coconut Oil: Cold Press, Soxhlet and Supercritical Fluid Extraction. Sep. Sci. Technol. 2022, 57, 426–432. [Google Scholar] [CrossRef]
- Guo, X.; Huang, H.; Jiang, X.; Chang, C.; Gao, P.; Zhong, W.; Hu, C.; He, D.; Yin, J. Comparative Analysis of Cold-Pressed and Hot-Pressed Coconut Oil Extraction: Implications for Quality and Antioxidant Capacity. LWT 2025, 221, 117614. [Google Scholar] [CrossRef]
- Gilbraith, W.E.; Carter, J.C.; Adams, K.L.; Booksh, K.S.; Ottaway, J.M. Improving Prediction of Peroxide Value of Edible Oils Using Regularized Regression Models. Molecules 2021, 26, 7281. [Google Scholar] [CrossRef]
- Dabbour, I.R.; Al-Ismail, K.M.; Takruri, H.R.; Azzeh, F.S. Chemical Characteristics and Antioxidant Content Properties of Cold Pressed Seed Oil of Wild Milk Thistle Plant Grown in Jordan. Pak. J. Nutr. 2014, 13, 67. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Shehzad, Q.; Yu, L.; Tian, A.; Wang, S.; Ma, L.; Zheng, L.; Xu, L. Comparative Study on Quality Characteristics of Bischofia polycarpa Seed Oil by Different Solvents: Lipid Composition, Phytochemicals, and Antioxidant Activity. Food Chem. X 2023, 17, 100588. [Google Scholar] [CrossRef] [PubMed]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Samir, Z.T.; Jassim, M.A.; Saeed, S.K. Effect of Different Extraction Methods on Physicochemical Properties, Antioxidant Activity, of Virgin Coconut Oil. Mater. Today Proc. 2021, 42, 2000–2005. [Google Scholar] [CrossRef]
- Medeiros de Azevedo, W.; Ferreira Ribeiro de Oliveira, L.; Alves Alcântara, M.; Tribuzy de Magalhães Cordeiro, A.M.; Florentino da Silva Chaves Damasceno, K.S.; Kelly de Araújo, N.; Fernandes de Assis, C.; Sousa Junior, F.C.D. Physicochemical Characterization, Fatty Acid Profile, Antioxidant Activity and Antibacterial Potential of Cacay Oil, Coconut Oil and Cacay Butter. PLoS ONE 2020, 15, e0232224. [Google Scholar] [CrossRef]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. In Protein and Sugar Export and Assembly in Gram-Positive Bacteria; Bagnoli, F., Rappuoli, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–44. ISBN 978-3-319-56014-4. [Google Scholar]
- Grenda, T.; Jarosz, A.; Sapała, M.; Grenda, A.; Patyra, E.; Kwiatek, K. Clostridium Perfringens—Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance. Pathogens 2023, 12, 768. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nitti, F.; Jumina, J.; Detha, A.I.R. Antimicrobial Properties of Lauric Acid and Monolaurin in Virgin Coconut Oil: A Review. ChemBioEng Rev. 2022, 9, 442–461. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Selvarajan, V.S.; Selvarajan, R.; Pandiyan, J.; Abia, A.L.K. Unveiling the Potency and Harnessing the Antibacterial Activities of Plant Oils against Foodborne Pathogens. Microbiol. Res. 2023, 14, 1291–1300. [Google Scholar] [CrossRef]
- Laowansiri, M.; Suwanchote, S.; Wannigama, D.L.; Badavath, V.N.; Hongsing, P.; Edwards, S.W.; Suratannon, N.; Chatchatee, P.; Lertpichitkul, P.; Rerknimitr, P. Monolaurin Inhibits Antibiotic-Resistant Staphylococcus aureus in Patients with Atopic Dermatitis. Sci. Rep. 2025, 15, 23180. [Google Scholar] [CrossRef]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Siswanta, D.; Sholikhah, E.N.; Fitriastuti, D. Synthesis and Antibacterial Activity 1-Monolaurin. Orient. J. Chem. 2018, 34, 863. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Peterson, M.L. Glycerol Monolaurate Antibacterial Activity in Broth and Biofilm Cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrımsson, O.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial Effects of Virgin Coconut Oil and Its Medium-Chain Fatty Acids on Clostridium Difficile. J. Med. Food 2013, 16, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable Concentrated Emulsions of the 1-Monoglyceride of Capric Acid (Monocaprin) with Microbicidal Activities against the Food-Borne Bacteria Campylobacter Jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 522–526. [Google Scholar] [CrossRef]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Ogbolu, D.O.; Oni, A.A.; Daini, O.A.; Oloko, A.P. In Vitro Antimicrobial Properties of Coconut Oil on Candida Species in Ibadan, Nigeria. J. Med. Food 2007, 10, 384–387. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, W.; Johnson, E.T.; Xie, Y.; Zhao, R. Antifungal Effects of Three Natural Branched Medium-Chain Fatty Acids and Their Potential as Fumigants against Aspergillus flavus in Stored Peanut Seeds. Food Control 2025, 168, 110950. [Google Scholar] [CrossRef]
- Gutierrez-Perez, C.; Cramer, R.A. Targeting Fungal Lipid Synthesis for Antifungal Drug Development and Potentiation of Contemporary Antifungals. npj Antimicrob. Resist. 2025, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Tjin, L.D.; Setiawan, A.S.; Rachmawati, E. Exposure Time of Virgin Coconut Oil against Oral Candida albicans. Padjadjaran J. Dent. 2016, 89–94. [Google Scholar] [CrossRef]
- Perna, M.; Hewlings, S. Saturated Fatty Acid Chain Length and Risk of Cardiovascular Disease: A Systematic Review. Nutrients 2022, 15, 30. [Google Scholar] [CrossRef]
- Shameena Beegum, P.P.; Manikantan, M.R.; Sharma, M.; Pandiselvam, R.; Gupta, R.K.; Hebbar, K.B. Optimization of Processing Variables for the Development of Virgin Coconut Oil Cake Based Extruded Snacks. J. Food Process Eng. 2019, 42, e13048. [Google Scholar] [CrossRef]
- Ben Hammouda, I.; Triki, M.; Matthäus, B.; Bouaziz, M. A Comparative Study on Formation of Polar Components, Fatty Acids and Sterols during Frying of Refined Olive Pomace Oil Pure and Its Blend Coconut Oil. J. Agric. Food Chem. 2018, 66, 3514–3523. [Google Scholar] [CrossRef]
- Van Nguyen, L.; Shahidi, F. Fatty Acid Distribution and Oxidative Stability of DHA/EPA-Enriched Structured Lipids From Virgin Coconut Oil. J. Am. Oil Chem. Soc. 2025, 102, 1439–1452. [Google Scholar] [CrossRef]
- Chatterjee, P.; Fernando, M.; Fernando, B.; Dias, C.B.; Shah, T.; Silva, R.; Williams, S.; Pedrini, S.; Hillebrandt, H.; Goozee, K. Potential of Coconut Oil and Medium Chain Triglycerides in the Prevention and Treatment of Alzheimer’s Disease. Mech. Ageing Dev. 2020, 186, 111209. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.S.; El-Sayed, E.M. Beneficial Effects of Coconut Oil in Treatment of Parkinson’s Disease. Neurophysiology 2020, 52, 169–175. [Google Scholar] [CrossRef]
- Ervin, R.B. Dietary Intake of Fats and Fatty Acids for the United States Population: 1999–2000; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; CDC: Atlanta, Georgia, 2004.
- van Rooijen, M.A.; Mensink, R.P. Palmitic Acid versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients 2020, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.J.; Tham, P.E.; Khoo, K.S.; Cheng, C.K.; Chew, K.W.; Show, P.L. A Comprehensive Review on the Techniques for Coconut Oil Extraction and Its Application. Bioprocess Biosyst. Eng. 2021, 44, 1807–1818. [Google Scholar] [CrossRef]
- Petersen, K.S.; Maki, K.C.; Calder, P.C.; Belury, M.A.; Messina, M.; Kirkpatrick, C.F.; Harris, W.S. Perspective on the Health Effects of Unsaturated Fatty Acids and Commonly Consumed Plant Oils High in Unsaturated Fat. Br. J. Nutr. 2024, 132, 1039–1050. [Google Scholar] [CrossRef]
- Rozani, M.A.S.; Hamid, H.A.A.; Hadzir, N.M.; Latif, M.A.M.; Som, A.M. Virgin Coconut Oil-Based Emulsion and Its Benefits: A Review. Malays. J. Anal. Sci. 2024, 28, 1087–1101. [Google Scholar]


| Sample | Acid Number (mg KOH/g) | Peroxide Number (mmol O2/kg) | Oxidative Stability (h) |
|---|---|---|---|
| Coco24 | 0.340 ± 0.017 b | 1.20 ± 0.010 a | 25.8 ± 0.22 d |
| Health | 0.087 ± 0.006 d | 0.51 ± 0.006 d | 54.6 ± 1.28 c |
| Kospa | 0.418 ± 0.006 a | 0.57 ± 0.015 d | 59.1 ± 0.90 c |
| Smetol | 0.203 ± 0.013 c | 1.11 ± 0.015 b | 124.5 ± 0.98 a |
| Vita | 0.213 ± 0.006 c | 0.64 ± 0.046 c | 72.2 ± 0.57 b |
| Shapiro–Wilk | 0.0866 | 0.1679 | 0.3017 |
| Bartlett Test | 0.4452 | 0.0921 | 0.3775 |
| Durbin-Watson | 0.3663 | 0.3164 | 0.1038 |
| Box–Cox (λ) | – | – | – |
| Tukey′s HSD test | Yes | Yes | Yes |
| Kruskal–Wallis test | No | No | No |
| Sample | Bacteria | |||||
|---|---|---|---|---|---|---|
| Bacillus subtilis CCM 2010 | Staphylococcus aureus CCM 2461 | Clostridium perfringens CCM 4991 | Salmonella enterica CCM 3807 | Haemophilus influenzae CCM 4454 | Yersinia enterocolitica CCM 5671 | |
| Coco24 | 1.29 ± 0.055 a | 2.01 ± 0.010 a | 1.01 ± 0.006 d | 0.08 ± 0.006 e | 1.02 ± 0.006 b | 1.03 ± 0.015 c |
| Health | 1.35 ± 0.006 a | 1.37 ± 0.021 b | 0.24 ± 0.012 a | 0.67 ± 0.021 d | 1.10 ± 0.021 a | 1.08 ± 0.030 c |
| Kospa | 0.31 ± 0.020 b | 1.15 ± 0.010 d | 0.08 ± 0.006 e | 1.51 ± 0.012 a | 0.57 ± 0.010 d | 1.90 ± 0.017 a |
| Smetol | 0.08 ± 0.006 c | 1.28 ± 0.061 c | 0.14 ± 0.006 c | 1.05 ± 0.055 c | 0.42 ± 0.012 e | 0.81 ± 0.027 d |
| Vita | 0.32 ± 0.010 b | 1.09 ± 0.015 d | 0.16 ± 0.006 b | 1.13 ± 0.010 b | 0.66 ± 0.010 c | 1.16 ± 0.017 b |
| Shapiro–Wilk | <0.0001 | 0.0997 | 0.0232 | 0.0828 | 0.7535 | 0.5678 |
| Bartlett Test | <0.0001 | 0.0718 | 0.8077 | 0.0409 | 0.5876 | 0.8769 |
| Durbin-Watson | 0.9914 | 0.0276 | 0.9615 | 0.3101 | 0.2444 | 0.7296 |
| Box–Cox (λ) | −0.2626 | −2.00 | 0.5454 | 0.6666 | -- | -- |
| Tukey’s HSD test | No | Yes | No | Yes | Yes | Yes |
| Kruskal–Wallis test | Yes | No | Yes | No | No | No |
| Sample | Fungi | ||
|---|---|---|---|
| Candida albicans CCM 8186 | Candida glabrata CCM 8185 | Candida tropicalis CCM 8184 | |
| Coco24 | 0.56 ± 0.035 c | 1.17 ± 0.015 b | 2.13 ± 0.017 c |
| Health | 1.02 ± 0.006 a | 1.53 ± 0.046 a | 3.67 ± 0.012 a |
| Kospa | 0.52 ± 0.010 c | 0.45 ± 0.025 d | 1.52 ± 0.006 d |
| Smetol | 0.27 ± 0.067 d | 0.67 ± 0.027 c | 0.87 ± 0.059 e |
| Vita | 0.66 ± 0.015 b | 0.64 ± 0.032 c | 2.81 ± 0.127 b |
| Shapiro–Wilk | 0.1434 | 0.9158 | 0.0330 |
| Bartlett Test | 0.0306 | 0.7405 | 0.0031 |
| Durbin-Watson | 0.1753 | 0.2341 | 0.3186 |
| Box–Cox (λ) | 0.5454 | -- | 0.5454 |
| Tukey’s HSD test | No | Yes | No |
| Kruskal–Wallis test | Yes | No | Yes |
| Sample | C6:0 | C8:0 | C10:0 | C12:0 | C14:0 | C16:0 | C18:0 | C18:1trans n-9 | C18:1cis n-9 | C18:2cis n-6 | C20:0 | PUFA | MUFA | SFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coco24 | 0.533 ± 0.015 d | 7.12 ± 0.015 d | 5.72 ± 0.015 d | 46.50 ± 0.25 b | 19.40 ± 0.20 a | 9.57 ± 0.07 b | 2.76 ± 0.17 b | 0 ± 0 | 6.45 ± 0.116 a | 1.61 ± 0.021 a | 0.030 ± 0.010 c | 1.63 ± 0.006 a | 6.46 ± 0.098 a | 91.70 ± 0.159 c |
| Health | 0.640 ± 0.01 c | 8.15 ± 0.07 c | 6.38 ± 0.006 a | 50.21 ± 0.02 a | 18.54 ± 0.02 a | 7.86 ± 0.01155 c | 2.99 ± 0.02 b | 0 ± 0 | 4.46 ± 0.021 b | 0.73 ± 0.015 b | 0.073 ± 0.006 b | 0.73 ± 0.012 c | 4.35 ± 0.095 b | 94.94 ± 0.155 b |
| Kospa | 0.677 ± 0.0058 a | 9.00 ± 0.10 a | 6.30 ± 0.011 b | 49.99 ± 0.16 a | 19.22 ± 0.27 a | 7.09 ± 0.0693 d | 2.85 ± 0.04 b | 0 ± 0 | 4.37 ± 0.017 b | 0.78 ± 0.006 b | 0.063 ± 0.006 b | 0.77 ± 0.015 b | 4.34 ± 0.046 b | 94.8 ± 0.015 b |
| Smetol | 0.470 ± 0.01 e | 6.53 ± 0.01 e | 5.38 ± 0.0153 e | 43.75 ± 1.44 c | 18.59 ± 0.06 a | 10.53 ± 0.29 a | 10.70 ± 0.17 a | 0.837 ± 0.015 a | 1.24 ± 0.130 c | 0.13 ± 0.020 c | 0.137 ± 0.006 a | 0.15 ± 0.006 d | 2.19 ± 0.067 c | 97.6 ± 0.067 a |
| Vita | 0.687 ± 0.0058 a | 8.66 ± 0.03 b | 6.03 ± 0.015 c | 49.23 ± 0.267 a | 18.66 ± 1.71 a | 7.45 ± 0.04 d | 2.96 ± 0.02 b | 0 ± 0 | 4.37 ± 0.021 b | 0.75 ± 0.030 b | 0.063 ± 0.012 b | 0.72 ± 0.006 c | 4.37 ± 0.021 b | 94.64 ± 0.202 b |
| Shapiro–Wilk | 0.7238 | 0.3915 | 0.1779 | 0.0055 | 0.0008 | 0.0073 | 0.0084 | <0.0001 | 0.0740 | 0.8988 | 0.3199 | 0.2297 | 0.3861 | 0.8996 |
| Bartlett Test | 0.6899 | 0.0492 | 0.7909 | <0.0001 | <0.0001 | 0.0058 | 0.0072 | <0.0001 | 0.0280 | 0.4696 | 0.8025 | 0.5534 | 0.4264 | 0.1074 |
| Durbin-Watson | 0.7798 | 0.3607 | 0.6279 | 0.1614 | 0.4143 | 0.0113 | 0.5564 | 0.0186 | 0.4677 | 0.8078 | 0.9741 | 0.8516 | 0.8051 | 0.6005 |
| Box–Cox (λ) | – | 0.6667 | – | 2.00 | 2.00 | −2.00 | −2.00 | 1.3535 | 1.3535 | – | – | – | – | – |
| Tukey’s HSD test | Yes | Yes | Yes | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Kruskal–Wallis test | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Correlations | DPPH | Peroxide Number | Oxidative Stability | S. aureus | C. perfringens | S. enterica | C. albicans | C12:0 | C16:0 | C18:0 | PUFA | MUFA | Smell | Taste | Aftertaste |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidative Stability | −0.707 | 0.122 | |||||||||||||
| Staphylococcus aureus | 0.505 | 0.674 | −0.587 | ||||||||||||
| C. perfringens | 0.496 | 0.647 | −0.658 | 0.972 | |||||||||||
| S. enterica | −0.697 | −0.563 | 0.53 | −0.924 | −0.906 | ||||||||||
| C. albicans | 0.859 | −0.648 | −0.541 | 0.021 | 0.022 | −0.251 | |||||||||
| C12:0 | 0.436 | −0.875 | −0.535 | −0.302 | −0.25 | 0.247 | 0.766 | ||||||||
| C16:0 | −0.194 | 0.902 | 0.403 | 0.503 | 0.403 | −0.477 | −0.566 | −0.92 | |||||||
| C18:0 | −0.609 | 0.502 | 0.893 | −0.177 | −0.29 | 0.177 | −0.669 | −0.817 | 0.753 | ||||||
| PUFA | 0.577 | 0.248 | −0.924 | 0.791 | 0.872 | −0.695 | 0.261 | 0.207 | −0.087 | −0.705 | |||||
| MUFA | 0.627 | 0.094 | −0.963 | 0.694 | 0.79 | −0.627 | 0.379 | 0.353 | −0.238 | −0.808 | 0.986 | ||||
| SFA | −0.594 | −0.147 | 0.942 | −0.712 | −0.814 | 0.64 | −0.324 | −0.299 | 0.195 | 0.777 | −0.99 | −0.994 | |||
| Smell | −0.132 | −0.392 | −0.4 | −0.265 | −0.069 | 0.349 | 0.064 | 0.555 | −0.711 | −0.659 | 0.333 | 0.424 | |||
| Taste | 0.277 | −0.799 | −0.502 | −0.377 | −0.236 | 0.319 | 0.592 | 0.905 | −0.958 | −0.836 | 0.242 | 0.393 | 0.813 | ||
| Aftertaste | −0.459 | −0.556 | −0.087 | −0.567 | −0.478 | 0.767 | −0.12 | 0.533 | −0.727 | −0.375 | −0.075 | 0.005 | 0.767 | 0.645 | |
| Overall acceptability | 0.201 | −0.751 | −0.461 | −0.395 | −0.236 | 0.344 | 0.513 | 0.855 | −0.933 | −0.804 | 0.232 | 0.378 | 0.867 | 0.993 | 0.668 |
| Features | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| DPPH | 0.5850 | 0.4580 | −0.6472 |
| Acid number | 0.2205 | 0.0732 | 0.9460 |
| Peroxide number | −0.5050 | 0.7597 | 0.3132 |
| Oxidative stability | −0.8689 | −0.4500 | −0.0550 |
| Staphylococcus aureus | 0.1456 | 0.9804 | 0.0564 |
| Clostridium perfringens | 0.2729 | 0.9482 | 0.1265 |
| Salmonella enterica | −0.1596 | −0.9410 | 0.2858 |
| Candida albicans | 0.6592 | −0.0499 | −0.7183 |
| C12.0 | 0.8163 | −0.4480 | −0.2062 |
| C14.0 | 0.2254 | 0.2546 | 0.4765 |
| C16.0 | −0.7599 | 0.6299 | 0.0216 |
| C18.0 | −0.9955 | −0.0250 | 0.0062 |
| PUFA | 0.6896 | 0.6881 | 0.2135 |
| MUFA | 0.7971 | 0.5783 | 0.1540 |
| SFA | −0.7688 | −0.6049 | −0.1918 |
| Overall appearance | 0.5124 | −0.1234 | −0.2847 |
| Smell | 0.7011 | −0.3747 | 0.4776 |
| Taste | 0.8590 | −0.5011 | −0.0286 |
| Aftertaste | 0.3849 | −0.6866 | 0.5965 |
| Overall acceptability | 0.8354 | −0.5124 | 0.0347 |
| Eigen values | 8.28 | 6.72 | 3.07 |
| Variance, % | 41.41 | 33.60 | 15.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanišová, E.; Osei, E.D.; Amotoe-Bondzie, A.; Encina-Zelada, C.R.; Šípkovský, A.; Kačániová, M.; Gálik, B.; Afoakwah, N.A. Comprehensive Profiling of Coconut Oil Varieties: Fatty Acids Composition, Oxidative Stability, Bioactive Properties, and Sensory Attributes. Appl. Sci. 2025, 15, 11070. https://doi.org/10.3390/app152011070
Ivanišová E, Osei ED, Amotoe-Bondzie A, Encina-Zelada CR, Šípkovský A, Kačániová M, Gálik B, Afoakwah NA. Comprehensive Profiling of Coconut Oil Varieties: Fatty Acids Composition, Oxidative Stability, Bioactive Properties, and Sensory Attributes. Applied Sciences. 2025; 15(20):11070. https://doi.org/10.3390/app152011070
Chicago/Turabian StyleIvanišová, Eva, Emmanuel Duah Osei, Anthony Amotoe-Bondzie, Christian R. Encina-Zelada, Adam Šípkovský, Miroslava Kačániová, Branislav Gálik, and Newlove Akowuah Afoakwah. 2025. "Comprehensive Profiling of Coconut Oil Varieties: Fatty Acids Composition, Oxidative Stability, Bioactive Properties, and Sensory Attributes" Applied Sciences 15, no. 20: 11070. https://doi.org/10.3390/app152011070
APA StyleIvanišová, E., Osei, E. D., Amotoe-Bondzie, A., Encina-Zelada, C. R., Šípkovský, A., Kačániová, M., Gálik, B., & Afoakwah, N. A. (2025). Comprehensive Profiling of Coconut Oil Varieties: Fatty Acids Composition, Oxidative Stability, Bioactive Properties, and Sensory Attributes. Applied Sciences, 15(20), 11070. https://doi.org/10.3390/app152011070








