Fruits of Polish Medicinal Plants as Potential Sources of Natural Antioxidants: Ellagic Acid and Quercetin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. Sample Preparation
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. UHPLC-ESI-MS/MS Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
| Fruit Matrix | Analyte | Content [μg/g] | Country of Origin | Source |
|---|---|---|---|---|
| Barberry | EA | n.d. | Poland | [29] |
| Q | 21.0–22.0 μg/g | China | [40] | |
| Blackthorn | EA | 94 μg/g | Romania | [41] |
| Q | 53 μg/g | Romania | [41] | |
| 207.0 μg/g fw | Sweden | [42] | ||
| Chokeberry | EA | n.d. | Bulgaria | [43] |
| 15.7 μg/g fw | Canada | [44] | ||
| 4.0 ± 0.1 μg/g | Croatia | [45] | ||
| n.d. | Croatia | [46] | ||
| 129 μg/g dw * | Romania | [47] | ||
| n.d. | Spain | [48] | ||
| Q | 148 μg/g dw | Bulgaria | [43] | |
| 2.1 μg/g fw | Canada | [44] | ||
| 92.2 μg/g * | Croatia | [45] | ||
| 437.8 μg/g fw | Croatia | [46] | ||
| 17.4 μg/g fw | Croatia | [49] | ||
| 89.0 μg/g * | Finland | [50] | ||
| 348.0 μg/g fw | Finland | [42] | ||
| 7 μg/g dw * | Romania | [47] | ||
| Elderberry | EA | 6.0 μg/g | Argentina | [51] |
| n.d. | Canada | [44] | ||
| n.d. | Croatia | [46] | ||
| n.d. | Germany | [48] | ||
| Q | 14.0 | Argentina | [51] | |
| n.d.—256.0 μg/g fw * | Canada | [6] | ||
| 3.3 μg/g fw | Canada | [44] | ||
| 144.0 μg/g | Croatia | [45] | ||
| 138.0 μg/g fw | Croatia | [46] | ||
| 5.5 μg/g * | Romania | [52] | ||
| 28.9–45.0 μg/g fw * | Slovenia | [53] | ||
| 331.0 μg/g fw | Sweden | [42] | ||
| Hawthorn | EA | 169 μg/g dw | Bulgaria | [43] |
| 303 μg/g | Romania | [41] | ||
| Q | n.d. | Bulgaria | [43] | |
| 0.015 μg/g | Romania | [41] | ||
| n.d.—0.1 μg/g dw | Spain | [28] | ||
| Lingonberry | EA | 13.6 μg/g fw | Canada | [[44] |
| Q | 2.3 μg/g fw | Canada | [44] | |
| 74.0–146.0 μg/g * | Finland | [50] | ||
| 131.0 μg/g fw | Finland | [42] | ||
| 2.0–14.1 μg/g * | USA | [54] | ||
| Rowanberry | EA | n.d. | Bulgaria | [43] |
| Q | n.d. | Bulgaria | [43] | |
| 12.0 μg/g dw | Estonia | [55] | ||
| 63.0 μg/g * | Finland | [50] | ||
| 510.0 μg/g dw | Poland | [56] | ||
| Sea-buckthorn | EA | n.d. | Canada | [57] |
| <LOD | Finland | [39] | ||
| 0.4–12.1 μg/g fw | Slovakia | [58] | ||
| Q | 67.0–175.0 μg/g fw * | Canada | [57] | |
| 62.0 μg/g * | Finland | [50] | ||
| 172.0 μg/g fw | Finland | [42] | ||
| 106.4–122.6 μg/g | Romania | [59] | ||
| n.d. | Slovakia | [58] |
| Sample | TPC | TFC | Country of Origin | Sources |
|---|---|---|---|---|
| barberry | 100.86 ± 1.97 mg GAE/g dw | 8.31 ± 0.51 mg QE/g dw | Italy/ Romania | [64] |
| barberry | 60.32 ± 0.21 mg GAE/g dw | 38.97 ± 1.60 mg CE/g dw | Iran | [65] |
| barberry | 184.10 ± 5.30 mg GAE/g dw | - | Iran | [66] |
| barberry | 100 mg GAE/g | - | Iran | [67] |
| barberry | 3.73–9.40 mg GAE/mL | 2.22–7.67 mg QE/mL | Iran | [68] |
| barberry | 10.24 ± 0.15 mg GAE/g fw | 0.86 ± 0.02 mg RE/g fw | Poland | [29] |
| barberry | 7.89 mg GAE/g fw | - | Türkiye | [69] |
| blackthorn | 14.02 mg GAE/g dw | 0.79 mg RE/g dw 0.45 mg QE/g dw | Bosnia and Herzegovina | [70] |
| [70] | ||||
| blackthorn | 23.19 ± 2.52 mg GAE/g dw | 2.96 ± 0.22 mg QE/g dw | Croatia | [71] |
| blackthorn, wild | 40.27 mg GAE/g fw | - | Poland | [62] |
| blackthorn | 83.40 mg GAE/g dw | 8.68 mg CE/g dw | Portugal | [7] |
| blackthorn | 1.51 ± 0.19 mg GAE/g dw | 3.29 ± 0.08 mg RE/g dw | Serbia | [60] |
| blackthorn | 327.02 ± 4.66 mg GAE/g dw | 127.16 ± 0.82 mg RE/g dw | Spain | [72] |
| chokeberry | 62.748 mg GAE/g dw | - | Bulgaria | [43] |
| chokeberry, wild | 9.09–10.39 mg GAE/g fw | - | Croatia | [49] |
| chokeberry, harvested | 8.56–12.06 mg GAE/g fw | - | Croatia | [49] |
| chokeberry, dried | 24.66 mg GAE/g dw | 13.94 mg GAE /g dw | Croatia | [73] |
| chokeberry, harvested | 7.78–12.85 mg GAE/g fw | - | Czechia | [74] |
| chokeberry | 10.49 mg GAE/g dw | - | Finland | [75] |
| chokeberry, dried | 19.54 mg GAE/g dw | 8.67 mg GAE/g dw | Germany | [73] |
| chokeberry, commercial | 11.39 ± 0.17 mg GAE/g dw | 7.75 ± 0.40 mg CE/g dw | Poland | [65] |
| chokeberry | 78.49 mg GAE/g dw | - | Poland | [76] |
| chokeberry | 27.99 mg GAE/g dw | 5.23 mg CE/g dw | Romania | [47] |
| chokeberry, fresh | 52.22 mg GAE/g dw | 23.46 mg CE/g dw | Serbia | [63] |
| chokeberry, dried | 11.69–19.19 mg GAE/g dw | 8.00–10.37 mg CE/g dw | Serbia | [63] |
| chokeberry, wild | 25.56 mg GAE/g fw | - | United States | [61] |
| elderberry | 73.40 mg GAE/g dw | 8.60 mg QE/g dw | Iran | [77] |
| elderberry | 72.00–158.60 mg GAE/g of extract | 19.60–45.60 QE mg/g of extract | Iran | [78] |
| elderberry, wild | 5.36 mg GAE/g fw | - | Poland | [62] |
| elderberry | 89.74 ± 0.37 mg GAE/g dw of extract | - | Türkiye | [79] |
| hawthorn | 40.258 mg GAE/g dw | - | Bulgaria | [43] |
| hawthorn | 52.62–61.91 mg GAE/g dw | 44.25–55.96 mgCE/g dw | China | [80] |
| hawthorn | 12.26–12.82 mg GAE/g dw | n.d. mg RE/g dw | France | [81] |
| hawthorn | 23.37 ± 1.18 mg GAE/g dw | 2.58 ± 0.24 mg QE/g dw | Italy/ Romania | [64] |
| hawthorn | 132.14–204.29 mg GAE/g | - | Spain | [28] |
| lingonberry | 6.52 mg GAE/g fw | - | Canada | [61] |
| lingonberry | 6.21 mg GAE/g dw | - | Finland | [75] |
| lingonberry | 7.17 mg GAE/g fw 13.30–13.50 mg GAE/g dw | - | Finland Romania | [82] [83] |
| lingonberry | - | |||
| rowanberry | 21.482 mg GAE/g dw | - | Bulgaria | [43] |
| rowanberry, wild | 4.27 ± 0.59 mg GAE/g fw | 3.11 ± 0.27 mg RE/g fw | Czechia | [84] |
| rowanberry | 4.56 mg GAE/g dw | - | Finland | [75] |
| rowanberry | 5.50–10.14 mg GAE/g fw | - | Finland | [82] |
| rowanberry | 26.8 mg GAE/g dw | - | Poland | [56] |
| rowanberry, wild | 2.27 mg GAE/g fw | - | Poland | [62] |
| rowanberry, wild | 20.00 mg GAE/g dw | 3.50 mg RE/g dw | Serbia | [85] |
| sea-buckthorn, commercial | 5.14 ± 0.21 mg GAE/g dw | 2.70 ± 0.11 mg CE/g dw | Belarus | [65] |
| sea-buckthorn, harvested | 12.51–14.42 mg CE/g dw | - | Belarus | [86] |
| sea-buckthorn, wild | 32.20–33.51 mg GAE/g dw | 3.52–8.96 mg RE/g dw | China | [87] |
| sea-buckthorn, harvested | 11.80–15.95 mg GAE/g dw | 1.81–2.86 mg RE/g dw | China | [87] |
| sea-buckthorn, commercial | 8.48 ± 0.22 mg GAE/g dw | 1.81 ± 0.03 mg RE/g dw | China | [87] |
| sea-buckthorn, harvested | 8.62–14.17 mg GAE/g fw | 4.18–7.97 mg RE/g fw | Czechia | [88] |
| sea-buckthorn | 2.05–2.45 mg GAE/g dw | - | Finland | [75] |
| sea-buckthorn | 1.86–3.81 mg GAE/g dw | - | Hungary | [89] |
| sea-buckthorn | 8.82 ± 0.93 mg CE/g dw | - | Poland | [86] |
| sea-buckthorn | 140.14 mg GAE/g dw | 5.04 mg CE/g dw | Romania | [90] |
| sea-buckthorn, harvested | 10.12–18.66 mg GAE/g fw | 6.57–9.01 QE/g fw | Romania | [59] |
| sea-buckthorn | 0.70–3.62 mg GAE/g fw | 0.55–4.11 mg RE/g fw | Slovakia | [58] |
| sea-buckthorn, harvested | 21.31–55.38 mg GAE/g dw | - | Türkiye | [91] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, J.L.; Muñoz-Acevedo, A.; Rai, M. Ethnobotany: Application of Medicinal Plants, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2019; ISBN 978-0-429-45313-7. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Fatehi-Hassanabad, Z.; Jafarzadeh, M.; Tarhini, A.; Fatehi, M. The Antihypertensive and Vasodilator Effects of Aqueous Extract from Berberis vulgaris Fruit on Hypertensive Rats. Phytother. Res. 2005, 19, 222–225. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia Plants: A Review of Traditional Use, Biological Activities, and Perspectives for Modern Medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.; Applequist, W.L.; Finley, J.; Lister, P.; Townesmith, A.K.; Walker, K.M.; Brown, P.N. Variation of Select Flavonols and Chlorogenic Acid Content of Elderberry Collected throughout the Eastern United States. J. Food Compos. Anal. 2016, 47, 52–59. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-Tree, Blackthorn and Rose Fruits: Detailed Characterisation in Nutrients and Phytochemicals with Antioxidant Properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- EU CosIng—The Official Cosmetics Ingredient Database in EU Official Web Site. Available online: https://www.chemsafetypro.com/Topics/Cosmetics/EU_CosIng_Cosmetic_Ingredient_database.html (accessed on 1 April 2025).
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A Review of the Chemistry of the Genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Kasper-Pakosz, R.; Pietras, M.; Łuczaj, Ł. Wild and Native Plants and Mushrooms Sold in the Open-Air Markets of South-Eastern Poland. J. Ethnobiol. Ethnomed. 2016, 12, 45. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Köhler, P.; Pirożnikow, E.; Graniszewska, M.; Pieroni, A.; Gervasi, T. Wild Edible Plants of Belarus: From Rostafiński’s Questionnaire of 1883 to the Present. J. Ethnobiol. Ethnomed. 2013, 9, 21. [Google Scholar] [CrossRef]
- Łuczaj, Ł. Archival Data on Wild Food Plants Used in Poland in 1948. J. Ethnobiol. Ethnomed. 2008, 4, 4. [Google Scholar] [CrossRef]
- Kujawska, M.; Klepacki, P.; Łuczaj, Ł. Fischer’s Plants in Folk Beliefs and Customs: A Previously Unknown Contribution to the Ethnobotany of the Polish-Lithuanian-Belarusian Borderland. J. Ethnobiol. Ethnomed. 2017, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Bartoszek, M. The Study of Antioxidant Capacity of Varieties of Nalewka, a Traditional Polish Fruit Liqueur, Using EPR, NMR and UV–Vis Spectroscopy. J. Food Compos. Anal. 2015, 40, 114–119. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with Antiglycation Activity and Mechanisms of Action: A Review of Recent Findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Microbial Production of Ellagic Acid and Biodegradation of Ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Wu, J.-C.; Lai, C.-S.; Tsai, M.-L.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Chemopreventive Effect of Natural Dietary Compounds on Xenobiotic-Induced Toxicity. J. Food Drug Anal. 2017, 25, 176–186. [Google Scholar] [CrossRef]
- Afifi, N.A.; Ibrahim, M.A.; Galal, M.K. Hepatoprotective Influence of Quercetin and Ellagic Acid on Thioacetamide-Induced Hepatotoxicity in Rats. Can. J. Physiol. Pharmacol. 2018, 96, 624–629. [Google Scholar] [CrossRef]
- Chen, W.-J.; Tsai, J.-H.; Hsu, L.-S.; Lin, C.-L.; Hong, H.-M.; Pan, M.-H. Quercetin Blocks the Aggressive Phenotype of Triple Negative Breast Cancer by Inhibiting IGF1/IGF1R-Mediated EMT Program. J. Food Drug Anal. 2021, 29, 98–112. [Google Scholar] [CrossRef]
- Stavric, B. Quercetin in Our Diet: From Potent Mutagen to Probable Anticarcinogen. Clin. Biochem. 1994, 27, 245–248. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of Antioxidant Properties of Honey by a Combination of Spectrophotometric/Fluorimetric Assays and Chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of Drying on the Bioactive Compounds, Antioxidant, Antibacterial and Antityrosinase Activities of Pomegranate Peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef]
- Szmagara, A.; Krzyszczak, A.; Sadok, I.; Karczmarz, K.; Staniszewska, M.M.; Stefaniak, E.A. Determination of Ellagic Acid in Rose Matrix by Spectrofluorimetry. J. Food Compos. Anal. 2019, 78, 91–100. [Google Scholar] [CrossRef]
- Stüber, M.; Reemtsma, T. Evaluation of Three Calibration Methods to Compensate Matrix Effects in Environmental Analysis with LC-ESI-MS. Anal. Bioanal. Chem. 2004, 378, 910–916. [Google Scholar] [CrossRef]
- Özgen, M.; Saraçoğlu, O.; Geçer, E.N. Antioxidant Capacity and Chemical Properties of Selected Barberry (Berberis vulgaris L.) Fruits. Hortic. Environ. Biotechnol. 2012, 53, 447–451. [Google Scholar] [CrossRef]
- Abuashwashi, M.A.; Palomino, O.M.; Gómez-Serranillos, M.P. Geographic Origin Influences the Phenolic Composition and Antioxidant Potential of Wild Crataegus monogyna from Spain. Pharm. Biol. 2016, 54, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Pyrkosz-Biardzka, K.; Kucharska, A.; Sokół-Łętowska, A.; Strugała, P.; Gabrielska, J. A Comprehensive Study on Antioxidant Properties of Crude Extracts from Fruits of Berberis vulgaris L., Cornus Mas L. and Mahonia Aquifolium Nutt. Pol. J. Food Nutr. Sci. 2014, 64, 91–99. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Talcott, S.T.; Percival, S.S. Low Concentrations of Quercetin and Ellagic Acid Synergistically Influence Proliferation, Cytotoxicity and Apoptosis in MOLT-4 Human Leukemia Cells. J. Nutr. 2003, 133, 2669–2674. [Google Scholar] [CrossRef]
- Saunders, F.; Oommen, M.; Lapjitkusol, R.; Amankwatia, E.; Kaminski, L.; Swanson, A.; Mitchell, A.; Wallace, H.M. Potentiation of the Cyotoxic Effects of Natural Chemopreventative Agents, Ellagic Acid and Quercetin. Toxicology 2009, 262, 21. [Google Scholar] [CrossRef]
- Celikler Ozer, O.; Orhan, I.E.; Çalışkan, B.; Senol Deniz, F.S.; Gokbulut, A.; Gur Maz, T.; Aysal, A.; Emerce, E.; Shekfeh, S.; Kahraman, A.; et al. Exploration of Anti-Tyrosinase Effect of Geranium glaberrimum Boiss. & Heldr. with in Silico Approach and Survey of 21 Geranium Species. J. Herb. Med. 2021, 27, 100431. [Google Scholar] [CrossRef]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McClees, S.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)Phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S. How to Increase Dietary Polyphenol Bioavailability: Understanding the Roles of Modulating Factors, Lifestyle and Gut Microbiota. Food Humanit. 2025, 4, 100602. [Google Scholar] [CrossRef]
- Flores, G.; del Castillo, M.L.R. Variations in Ellagic Acid, Quercetin and Myricetin in Berry Cultivars after Preharvest Methyl Jasmonate Treatments. J. Food Compos. Anal. 2015, 39, 55–61. [Google Scholar] [CrossRef]
- Gbylik-Sikorska, M.; Gajda, A.; Burmańczuk, A.; Grabowski, T.; Posyniak, A. Development of a UHPLC-MS/MS Method for the Determination of Quercetin in Milk and Its Application to a Pharmacokinetic Study. J. Vet. Res. 2019, 63, 87–91. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Hu, X.; You, L.; Khan, R.A.A.; Yu, Y. Phenolic Contents, Organic Acids, and the Antioxidant and Bio Activity of Wild Medicinal Berberis Plants- as Sustainable Sources of Functional Food. Molecules 2022, 27, 2497. [Google Scholar] [CrossRef]
- Oancea, A.-G.; Saracila, M.; Vlaicu, P.A.; Varzaru, I.; Untea, A.E.; Dragomir, C. Assessment of the Antioxidant Potential of Blackthorns and Hawthorns: Comparative Analysis and Potential Use in Ruminants’ Nutrition. Separations 2024, 11, 275. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and Contents of Phenolic Compounds in Eighteen Scandinavian Berry Species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological Activities of Selected Polyphenol-Rich Fruits Related to Immunity and Gastrointestinal Health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Dubé, P.; Anhê, F.F.; Pilon, G.; Marette, A.; Lemire, M.; Harris, C.; Dewailly, E.; Desjardins, Y. Comprehensive Analysis of Phenolic Compounds and Abscisic Acid Profiles of Twelve Native Canadian Berries. J. Food Compos. Anal. 2015, 44, 214–224. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidovi, M. Flavonols, Phenolic Acids and Antioxidant Activity of Some Red Fruits. Dtsch. Lebensm.-Rundsch. 2007, 103, 369–378. [Google Scholar]
- Jakobek, L.; Seruga, M. Influence of Anthocyanins, Flavonols and Phenolic Acids on the Antiradical Activity of Berries and Small Fruits. Int. J. Food Prop. 2012, 15, 122–133. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Oancea, A.G.; Varzaru, I.; Vlaicu, P.A. Comparative Analysis of Black Chokeberry (Aronia melanocarpa L.) Fruit, Leaves, and Pomace for Their Phytochemical Composition, Antioxidant Potential, and Polyphenol Bioaccessibility. Foods 2024, 13, 1856. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A. Evaluation of Commercial Red Fruit Juice Concentrates as Ingredients for Antioxidant Functional Juices. Eur. Food Res. Technol. 2004, 219, 133–141. [Google Scholar] [CrossRef]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic Acids, Flavonols, Anthocyanins and Antiradical Activity of “Nero”, “Viking”, “Galicianka” and Wild Chokeberries. Sci. Hortic. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the Flavonols Quercetin, Myricetin, and Kaempferol in 25 Edible Berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Mattson, M.L.G.; Corfield, R.; Bajda, L.; Pérez, O.E.; Schebor, C.; Salvatori, D. Potential Bioactive Ingredient from Elderberry Fruit: Process Optimization for a Maximum Phenolic Recovery, Physicochemical Characterization, and Bioaccesibility. J. Berry Res. 2021, 11, 51–68. [Google Scholar] [CrossRef]
- Anton, A.M.; Pintea, A.M.; Rugin, D.O.; Scon, Z.M.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary Studies on the Chemical Characterization and Antioxidant Capacity of Polyphenols from Sambucus sp. Dig. J. Nanomater. Biostructures 2013, 8, 973–980. [Google Scholar]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Leiner, R.H.; Holloway, P.S.; Neal, D.B. Antioxidant Capacity and Quercetin Levels in Alaska Wild Berries. Int. J. Fruit Sci. 2006, 6, 83–91. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Rätsep, R.; Aluvee, A.; Kazernavičiūtė, R.; Bhat, R. Antioxidants Characterization of the Fruit, Juice, and Pomace of Sweet Rowanberry (Sorbus aucuparia L.) Cultivated in Estonia. Antioxidants 2021, 10, 1779. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Michel, P. Antioxidant Activity of Inflorescences, Leaves and Fruits of Three Sorbus Species in Relation to Their Polyphenolic Composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Kesari, V.; Watt, I.; Wishart, D.; Todd, J.F.; Schroeder, W.R.; Paliyath, G.; Krishna, P. Metabolite Profiling and Expression Analysis of Flavonoid, Vitamin C and Tocopherol Biosynthesis Genes in the Antioxidant-Rich Sea Buckthorn (Hippophae rhamnoides L.). Phytochemistry 2015, 118, 181–191. [Google Scholar] [CrossRef]
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of Phenolic Compounds and Vitamins C and E on Antioxidant Activity of Sea Buckthorn (Hippophaë rhamnoides L.) Berries and Leaves of Diverse Ripening Times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A.C.; Bunea, A.; Pripon Furtuna, F.R.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae rhamnoides L.) Varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef]
- Stanković, M.I.; Savić, V.L.; Živković, J.V.; Stanojević, L.P.; Tadić, V.M.; Arsić, I.A. Tyrosinase Inhibitory and Antioxidant Activity of Wild Prunus Spinosa L. Fruit Extracts as Natural Source of Bioactive Compounds. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 651–657. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Zalewska-Korona, M.; Kalbarczyk, J. Antioxidant Capacity, Ascorbic Acid and Phenolics Content in Wild Edible Fruits. J. Fruit Ornam. Plant Res. 2009, 17, 115–120. [Google Scholar]
- Petković, M.; Đurović, I.; Miletić, N.; Radovanović, J. Effect of Convective Drying Method of Chokeberry (Aronia melanocarpa L.) on Drying Kinetics, Bioactive Components and Sensory Characteristics of Bread with Chokeberry Powder. Period. Polytech. Chem. Eng. 2019, 63, 600–608. [Google Scholar] [CrossRef]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.R.; Tanase, C.; Dias, M.I.; Pereira, C.; et al. Development of an Optimized Drying Process for the Recovery of Bioactive Compounds from the Autumn Fruits of Berberis Vulgaris L. and Crataegus monogyna Jacq. Antioxidants 2021, 10, 1579. [Google Scholar] [CrossRef]
- Rybicka, I.; Kiewlicz, J.; Kowalczewski, P.Ł.; Gliszczyńska-Świgło, A. Selected Dried Fruits as a Source of Nutrients. Eur. Food Res. Technol. 2021, 247, 2409–2419. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The Antioxidant and Chemical Properties of Berberis Vulgaris and Its Cytotoxic Effect on Human Breast Carcinoma Cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Motalleb, G.; Hanachi, P.; Kua, S.H.; Fauziah, O.; Asmah, R. Evaluation of Phenolic Content and Total Antioxidant Activity in Berberis vulgaris Fruit Extract. J. Biol. Sci. 2005, 5, 648–653. [Google Scholar] [CrossRef]
- Gholizadeh-Moghadam, N.; Hosseini, B.; Alirezalu, A. Classification of Barberry Genotypes by Multivariate Analysis of Biochemical Constituents and HPLC Profiles. Phytochem. Anal. 2019, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Çalişir, S.; Marakoğlu, T.; Çoklar, H. Some Physicomechanical and Nutritional Properties of Barberry (Berberis vulgaris L.) Fruits. J. Food Process Eng. 2009, 32, 497–511. [Google Scholar] [CrossRef]
- Tahirović, A.; Bašić, N.; Čopra-Janićijević, A. Effect of Solvents on Phenolic Compounds Extraction and Antioxidant Activity of Prunus spinosa L. Fruits. Bull. Chem. Technol. Bosnia Herzeg. 2018, 50, 19–24. [Google Scholar]
- Velickovic, I.; Zizak, Z.; Rajcevic, N.; Ivanov, M.; Sokovic, M.; Marin, P.; Grujic, S. Examination of the Polyphenol Content and Bioactivities of Prunus Spinosa L. Fruit Extracts. Arch. Biol. Sci. 2020, 72, 105–115. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic Compounds of Blackthorn (Prunus spinosa L.) and Influence of in Vitro Digestion on Their Antioxidant Capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Tolić, M.-T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Valsikova, M.; Sochor, J.; Reznicek, V.; Kramarova, D. Phenolic Content, Antioxidant Capacity, Radical Oxygen Species Scavenging and Lipid Peroxidation Inhibiting Activities of Extracts of Five Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Cultivars. J. Med. Plants Res. 2010, 4, 2431–2437. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.-L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and Antibacterial Activities of Aqueous Ethanol Extracts of Berries, Leaves, and Branches of Berry Plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa Phenolics and Their Antioxidant Activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Mahboubi, A. Total Phenolic Content, Antioxidant and Antimicrobial Activity of Sambucus nigra L. J. Biol. Act. Prod. Nat. 2012, 2, 275–283. [Google Scholar] [CrossRef]
- Azari, B.; Siami, A.; Ebrahimzadeh, M.A.; Khan, B.A. Antioxidants Activity of Extracts from Sambucus Nigra, Efficiency of Different Extraction Methods. Lat. Am. Appl. Res.-Int. J. 2015, 45, 139–144. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In Vitro Antioxidant Properties and Anthocyanin Compositions of Elderberry Extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Wen, L.; You, L. Bioactive Compounds of Different Hawthorn Cultivars and Their Antioxidant Activities. Mod. Food Sci. Technol. 2017, 33, 91–95. [Google Scholar] [CrossRef]
- Froehlicher, T.; Hennebelle, T.; Martin-Nizard, F.; Cleenewerck, P.; Hilbert, J.-L.; Trotin, F.; Grec, S. Phenolic Profiles and Antioxidative Effects of Hawthorn Cell Suspensions, Fresh Fruits, and Medicinal Dried Parts. Food Chem. 2009, 115, 897–903. [Google Scholar] [CrossRef]
- Hukkanen, A.T.; Pölönen, S.S.; Kärenlampi, S.O.; Kokko, H.I. Antioxidant Capacity and Phenolic Content of Sweet Rowanberries. J. Agric. Food Chem. 2006, 54, 112–119. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic Compounds and Antioxidant Activity of Lingonberry (Vaccinium vitis-Idaea L.) Leaf, Stem and Fruit at Different Harvest Periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O.; Jurikova, T.; Sochor, J.; Fisera, M.; Balla, S.; Baron, M.; Hrabe, J. Bioactive Compounds in Sweet Rowanberry Fruits of Interspecific Rowan Crosses. Open Life Sci. 2014, 9, 1078–1086. [Google Scholar] [CrossRef]
- Stanković, M.I.; Savić, V.L.; Živković, J.V.; Tadić, V.M.; Arsić, I.A. Tyrosinase Inhibitory and Antioxidant Activity of Wild Rosa Canina L. and Sorbus Aucuparia L. Fruit Extracts. Acta Pol. Pharm.-Drug Res. 2019, 76, 523–533. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szałkiewicz, M. Composition of Phenolic Acids in Sea Buckthorn (Hippophae rhamnoides L.) Berries. J. Am. Oil Chem. Soc. 2005, 82, 175–179. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Yang, K.; He, Q.; Wang, Y.; Sun, Y.; He, C.; Xiao, P. Impact of Drying Methods on Phenolic Components and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Berries from Different Varieties in China. Molecules 2021, 26, 7189. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Ercisli, S.; Mlcek, J.; Jurikova, T.; Hoza, I. Antioxidant and Radical Scavenging Activities in Fruits of 6 Sea Buckthorn (Hippophae rhamnoides L.) Cultivars. Turk. J. Agric. For. 2014, 38, 224–232. [Google Scholar] [CrossRef]
- Ficzek, G.; Mátravölgyi, G.; Furulyás, D.; Rentsendavaa, C.; Jócsák, I.; Papp, D.; Simon, G.; Végvári, G.; Stéger-Máté, M. Analysis of Bioactive Compounds of Three Sea Buckthorn Cultivars (Hippophaë rhamnoides L. ‘Askola’, ‘Leikora’, and ‘Orangeveja’) with HPLC and Spectrophotometric Methods. Eur. J. Hortic. Sci. 2019, 84, 31–38. [Google Scholar] [CrossRef]
- Ursache, F.-M.; Ghinea, I.O.; Turturică, M.; Aprodu, I.; Râpeanu, G.; Stănciuc, N. Phytochemicals Content and Antioxidant Properties of Sea Buckthorn (Hippophae rhamnoides L.) as Affected by Heat Treatment—Quantitative Spectroscopic and Kinetic Approaches. Food Chem. 2017, 233, 442–449. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E.; Ozdemir, O.; Sengul, M. The Genotypic Effects on the Chemical Composition and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Berries Grown in Turkey. Sci. Hortic. 2007, 115, 27–33. [Google Scholar] [CrossRef]

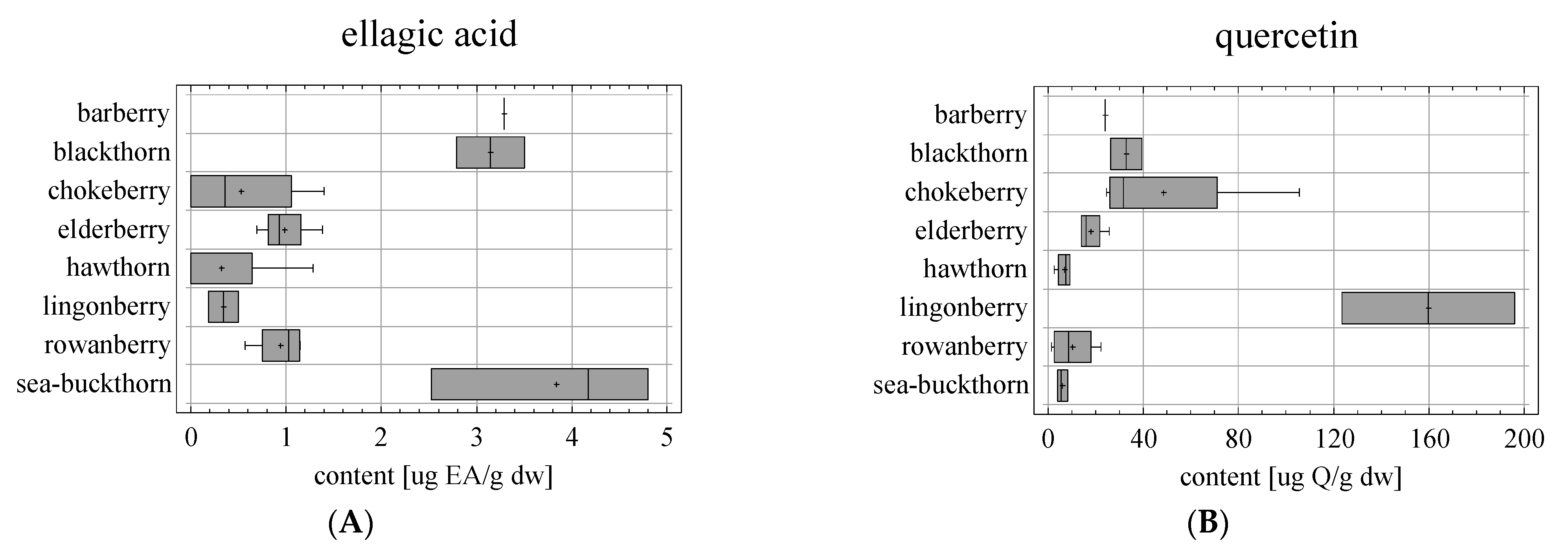
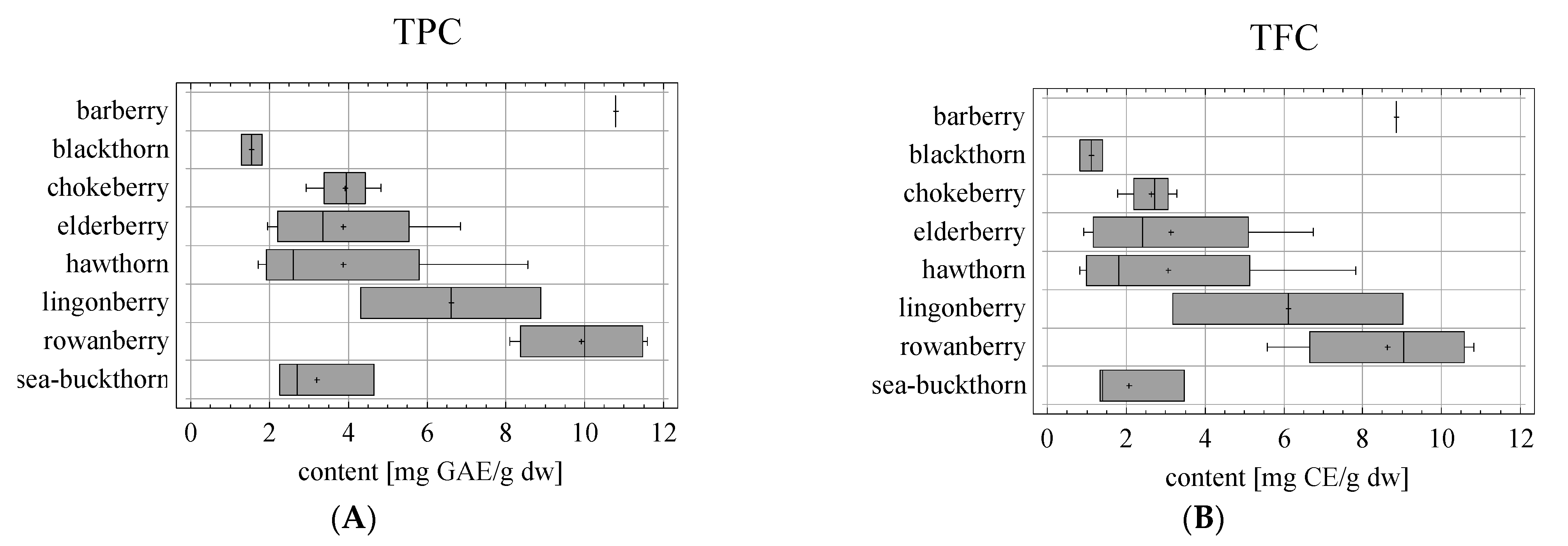
| Sample | Fruit | Latin Name | Type of Drying | Fineness | Producer |
|---|---|---|---|---|---|
| S1 | barberry | Berberis vulgaris | dried | whole fruit | EkoHerba |
| S2 | blackthorn | Prunus spinosa | dried | whole fruit | Rafex |
| S3 | blackthorn | Prunus spinosa | dried | whole fruit | EkoHerba |
| S4 | chokeberry | Aronia melanocarpa | lyophilized | powder | Premium Rosa |
| S5 | chokeberry | Aronia melanocarpa | dried | powder | Rafex |
| S6 | chokeberry | Aronia melanocarpa | lyophilized | whole fruit | Rafex |
| S7 | chokeberry | Aronia melanocarpa | dried | whole fruit | Kawon |
| S8 | elderberry | Sambucus nigra | lyophilized | powder | Premium Rosa |
| S9 | elderberry | Sambucus nigra | lyophilized | whole fruit | Rafex |
| S10 | elderberry | Sambucus nigra | dried | whole fruit | Rafex |
| S11 | elderberry | Sambucus nigra | dried | whole fruit | Kawon |
| S12 | hawthorn | Crataegus monogyna | dried | whole fruit | Rafex |
| S13 | hawthorn | Crataegus monogyna | dried | whole fruit | Kawon |
| S14 | hawthorn | Crataegus monogyna | dried | whole fruit | Dary Natury |
| S15 | hawthorn | Crataegus monogyna | dried | whole fruit | EkoHerba |
| S16 | lingonberry | Vaccinium vitis-idaea | lyophilized | powder | Premium Rosa |
| S17 | lingonberry | Vaccinium vitis-idaea | dried | whole fruit | Rafex |
| S18 | rowanberry | Sorbus aucuparia | dried | whole fruit | Rafex |
| S19 | rowanberry | Sorbus aucuparia | dried | whole fruit | Dary Natury |
| S20 | rowanberry | Sorbus aucuparia | dried | whole fruit | Flos |
| S21 | rowanberry | Sorbus aucuparia | dried | whole fruit | EkoHerba |
| S22 | sea-buckthorn | Hippophae rhamnoides | lyophilized | powder | Premium Rosa |
| S23 | sea-buckthorn | Hippophae rhamnoides | dried | whole fruit | Rafex |
| S24 | sea-buckthorn | Hippophae rhamnoides | dried | whole fruit | Farmvit |
| Type of Fruit Matrix | Analyte | LR [μg/g] | EC | R2 | LOD [μg/g] | LOQ [μg/g] |
|---|---|---|---|---|---|---|
| Barberry | EA Q | 1.51–151.10 1.51–151.10 | y = 24.9x − 57.3 y = 579.9x − 1274.3 | 0.997 0.992 | 0.45 0.45 | 1.36 1.51 |
| Blackthorn | EA Q | 1.51–151.10 7.55–151.10 | y = 35.4x − 83.6 y = 880.6x − 8440.7 | 0.989 0.986 | 0.45 2.42 | 1.51 7.10 |
| Chokeberry | EA Q | 1.51–151.10 1.51–151.10 | y = 46.7x + 42.2 y = 690.5x − 892.9 | 0.997 0.992 | 0.45 0.45 | 1.51 1.36 |
| Elderberry | EA Q | 0.76–151.10 1.51–151.10 | y = 34.3x − 16.4 y = 655.3x + 6699.2 | 0.999 0.998 | 0.30 0.45 | 0.76 1.36 |
| Hawthorn | EA Q | 0.76–151.10 0.76–151.10 | y = 59.5x + 25.0 y = 723.2x − 701.9 | 0.998 0.994 | 0.24 0.21 | 0.76 0.66 |
| Lingonberry | EA Q | 1.51–151.10 1.51–151.10 | y = 39.1x + 29.5 y = 398.0x + 768.7 | 0.998 0.996 | 0.45 0.45 | 1.36 1.51 |
| Rowanberry | EA Q | 1.51–151.10 1.51–151.10 | y = 32.3x − 0.5 y = 451.1x + 9411.4 | 0.990 0.993 | 0.45 0.45 | 1.36 1.21 |
| Sea-buckthorn | EA Q | 0.76–151.10 0.76–151.10 | y = 52.6x + 104.1 y = 520.3x + 3036.8 | 0.998 0.994 | 0.15 0.30 | 0.60 0.76 |
| Sample | Fruit | TPC ± SD [mg GAE/g dw] n = 6 | TFC ± SD [mg CE/g dw] n = 6 | EA ± SD [μg/gdw] n = 3 | Q ± SD [μg/gdw] n = 3 |
|---|---|---|---|---|---|
| S1 | barberry (D) | 17.78 ± 0.13 s | 8.85 ± 0.22 m | 3.29 ± 0.24 j | 24.01 ± 0.25 i |
| S2 | blackthorn (D) | 1.28 ± 0.04 a | 0.81 ± 0.01 a | 2.79 ± 0.10 i | 26.29 ± 0.68 j |
| S3 | blackthorn (D) | 1.81 ± 0.05 b | 1.40 ± 0.01 c | 3.50 ± 0.16 j | 39.36 ± 0.45 l |
| S4 | chokeberry (L) | 8.56 ± 0.03 q | 7.82 ± 0.10 l | 1.40 ± 0.38 g | 27.19 ± 1.18 j |
| S5 | chokeberry (D) | 3.06 ± 0.06 i | 2.46 ± 0.05 e | 0.71 ± 0.07 bcd | 105.69 ± 2.45 m |
| S6 | chokeberry (L) | 8.89 ± 0.21 r | 9.03 ± 0.15 n | nd a | 24.51 ± 1.41 i |
| S7 | chokeberry (D) | 4.31 ± 0.18 l | 2.94 ± 0.00 h | nd a | 36.20 ± 0.20 k |
| S8 | elderberry (L) | 11.36 ± 0.15 t | 10.34 ± 0.03 o | 1.38 ± 0.26 g | 13.84 ± 0.16 f |
| S9 | elderberry (L) | 11.59 ± 0.20 u | 10.83 ± 0.25 p | 0.92 ± 0.02 de | 17.81 ± 0.17 g |
| S10 | elderberry (D) | 8.64 ± 0.03 q | 7.75 ± 0.18 l | 0.92 ± 0.09 de | 25.65 ± 0.17 ij |
| S11 | elderberry (D) | 8.10 ± 0.16 p | 5.58 ± 0.16 j | 0.69 ± 0.04 bc | 13.88 ± 0.30 f |
| S12 | hawthorn (D) | 4.82 ± 0.09 n | 3.29 ± 0.13 h | 1.28 ± 0.05 fg | 9.17 ± 0.11 e |
| S13 | hawthorn (D) | 3.86 ± 0.02 j | 1.78 ± 0.10 d | nd a | 6.09 ± 0.07 d |
| S14 | hawthorn (D) | 2.93 ± 0.09 h | 2.83 ± 0.12 g | nd a | 8.90 ± 0.02 e |
| S15 | hawthorn (D) | 4.05 ± 0.04 k | 2.61 ± 0.05 f | nd a | 2.63 ± 0.04 ab |
| S16 | lingonberry (L) | 6.85 ± 0.03 o | 6.75 ± 0.08 k | 0.18 ± 0.05 a | 123.42 ± 1.70 n |
| S17 | lingonberry (D) | 4.23 ± 0.08 l | 3.44 ± 0.01 i | 0.50 ± 0.05 b | 196.20 ± 3.10 o |
| S18 | rowanberry (D) | 1.95 ± 0.02 c | 0.92 ± 0.02 a | 0.92 ± 0.06 cde | 3.71 ± 0.51 bc |
| S19 | rowanberry (D) | 2.47 ± 0.02 f | 1.39 ± 0.01 c | 1.15 ± 0.26 ef | 22.246 ± 1.06 h |
| S20 | rowanberry (D) | 2.13 ± 0.02 d | 1.18 ± 0.01 b | 0.57 ± 0.05 b | 13.52 ± 0.64 f |
| S21 | rowanberry (D) | 1.71 ± 0.02 b | 0.81 ± 0.02 a | 1.13 ± 0.11 ef | 1.44 ± 0.43 a |
| S22 | sea-buckthorn (L) | 4.66 ± 0.15 m | 3.48 ± 0.02 i | 4.80 ± 0.18 l | 4.06 ± 0.30 bc |
| S23 | sea-buckthorn (D) | 2.71 ± 0.03 g | 1.40 ± 0.03 c | 2.53 ± 0.11 h | 8.32 ± 0.38 e |
| S24 | sea-buckthorn (D) | 2.25 ± 0.07 e | 1.33 ± 0.04 c | 4.18 ± 0.15 k | 5.36 ± 0.21 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmagara, A.; Krzyszczak-Turczyn, A.; Sadok, I. Fruits of Polish Medicinal Plants as Potential Sources of Natural Antioxidants: Ellagic Acid and Quercetin. Appl. Sci. 2025, 15, 6094. https://doi.org/10.3390/app15116094
Szmagara A, Krzyszczak-Turczyn A, Sadok I. Fruits of Polish Medicinal Plants as Potential Sources of Natural Antioxidants: Ellagic Acid and Quercetin. Applied Sciences. 2025; 15(11):6094. https://doi.org/10.3390/app15116094
Chicago/Turabian StyleSzmagara, Agnieszka, Agnieszka Krzyszczak-Turczyn, and Ilona Sadok. 2025. "Fruits of Polish Medicinal Plants as Potential Sources of Natural Antioxidants: Ellagic Acid and Quercetin" Applied Sciences 15, no. 11: 6094. https://doi.org/10.3390/app15116094
APA StyleSzmagara, A., Krzyszczak-Turczyn, A., & Sadok, I. (2025). Fruits of Polish Medicinal Plants as Potential Sources of Natural Antioxidants: Ellagic Acid and Quercetin. Applied Sciences, 15(11), 6094. https://doi.org/10.3390/app15116094








