Featured Application
The potential application of intentionally exposing xenogeneic bone may benefit immediate implant placement in molar areas in specific conditions when a flapless approach with an intact buccal wall is granted. Outcomes after implant placement include maintenance of bone stability (both vertically and horizontally), at least 2 mm of keratinized mucosa around implants, and uneventful healing processes.
Abstract
This study evaluated the clinical and tomographic outcomes of socket healing. Immediate implants were placed in the molar area, and the gap was filled with either deproteinized bovine bone mineral (B) or collagen matrix (BM), n = 14/group. Scores of epithelization healing, immunoassay for VEGF, IL-1β, and FGF from wound exudate, keratinized mucosa variation (ΔKM), and bone levels were evaluated. The B group had slower tissue maturation than BM (p < 0.05), but gingival epithelialization was similar (p > 0.05). At the restorative phase, the B group exhibited greater ΔKM at prosthesis installation—1 to 2 months of postoperative (increase of 0.29 mm) compared to the BM group (reduction of −1.5 mm) (p < 0.05). Inflammatory tissue responses as well as vertical and horizontal bone remodeling were similar (p > 0.05). Crestal bone remodeling was limited to less than 0.8 mm for both groups. Taken together, the B and BM groups behaved similarly and promoted stable conditions for biomaterial incorporation in the socket healing after immediate implant placement in molar areas.
1. Introduction
Immediate implant placement fails to mitigate post-extraction bone resorption [1]; however, it remains a valuable technique for reducing the number of surgical interventions [2]. Alveolar ridge preservation techniques employing space-filling biomaterials have demonstrated a significant capacity to restrict bone remodeling following extraction and to preserve the alveolar ridge [3,4,5,6,7]. The deproteinized bovine bone mineral (DBBM) acts as a scaffold for new bone formation [8], exhibiting the ability to enhance soft tissue volume and preserve the alveolar volume when compared to natural healing [9].
A meta-analysis demonstrated that filling the gap resulted in a 54% decrease in horizontal buccal bone loss and significantly reduced soft tissue displacement [10]. Socket grafting with biomaterial prevents bone resorption with a mean difference of 1.99 mm in horizontal loss, 1.72 mm in vertical mid-buccal, and 1.16 mm in vertical mid-lingual dimensions, compared to tooth extraction alone [7]. Furthermore, alveolar ridge bone resorption in the posterior region diverges from those in the esthetic zone due to anatomical distinctions. Dimensional changes following tooth extraction in the posterior region of humans, when no preservation technique was applied, demonstrated a reduction in bone width of up to 50% during a one-year follow-up, with two-thirds of the total changes occurring within the first three months [11]. Thicker buccal bone and the larger gap fill in the posterior sockets lead to less pronounced ridge alterations [12].
Insufficient keratinized mucosa (KM) is associated with a higher susceptibility to increase the symptoms of inflammation, such as swelling, erythema, and bleeding on probing [13,14]. Implant sites with less than 2 mm of keratinized mucosa indicate a higher predisposition for the development of peri-implantitis, as patients demonstrated significantly greater marginal bone reduction, increased brushing sensitivity, and heightened plaque accumulation over a 4-year follow-up period [14].
Various surgical techniques and materials are employed to enhance soft tissues around dental implants to seal the socket opening, including autogenous tissue such as free gingival graft or sub-epithelial connective tissue graft, barrier membrane, or collagen matrices [2,7,15]. However, significant limitations persist in these techniques, including patient morbidity, limited donor tissue availability, infection risk, and the high biomaterial costs [2,15,16]. Exposing xenogeneic bone to the oral cavity during immediate implant placement offers a more simplified clinical protocol. However, the scientific literature on this specific technique remains limited [16,17], warranting further investigation.
Based on the previous study [16], related to the technique of xenogeneic bone left exposed, the implant gap is covered by a tissue resembling KM during the healing process, and there were no postoperative complications like contamination and rejection or biomaterial loss. Hence, the objective of this study was to investigate immediate dental implant placement in molars sites and to compare the use of collagen matrix and/or xenogeneic bone exposed to the oral environment. The null hypothesis was raised that both treatments are comparable, and there is no significant difference between the amount of KM, dimensional tissue changes, and quality of the healing process.
2. Materials and Methods
2.1. Study Design and Patients
The present case–control study was recorded at clinicaltrials.gov with ID number NCT06191510. Every participant enrolled in the study provided written informed consent approved by the School of Dentistry of Ribeirao Preto Research Ethics Committee (protocol 21305419.1.0000.5419). The study followed the Helsinki Declaration principles and STROBE guidelines for manuscript preparation.
Patients were enrolled in private practice from 2019 to 2022 by an experienced oral surgeon (J.G.F.) (Table 1). The primary criteria for inclusion were molar extraction and an indication of immediate implant placement in a fresh socket with a preserved buccal socket wall. Further inclusion criteria were healthy adults (≥18 years old) demonstrating satisfactory plaque control and presence of interradicular septum, with a minimum of 5 mm intraosseous preparation for primary stability. Individuals were excluded if they smoked over 10 cigarettes daily, had uncontrolled systemic diseases or unmanaged metabolic disorders, had a history of chemotherapy or radiation treatment in the oral and maxillofacial region, were pregnant, had taken antibiotics or corticosteroids in the past 3 months, or were on medications that alter bone metabolism, including bisphosphonates. The estimation of the sample size was derived from the literature [18] involving post-extraction sockets after 4 months, using mean and standard deviation values for keratinized tissue. A total of 28 samples (14 per group) was calculated using GPower 3.1, with a significance level of 5%, 80% power, and a mean difference of 1.0 mm between groups. The control and test groups were randomly allocated using the randomization program (randomizer.org). All clinical procedures were performed by the same operator, and another independent blinded operator conducted the analyses (V.F.C.). The primary outcome of the study was the assessment of keratinized mucosa width, while the secondary outcomes included linear measurements of hard and soft tissue, performed on tomographic images in both horizontal and vertical directions.

Table 1.
Patient and implant characteristics.
2.2. Surgical Procedures
The surgical procedure and postoperative management were performed as previously described [16]. All surgeries followed uniform surgical procedures, consisting of careful minimally traumatic tooth extraction techniques, site preparation, implant placement using the flapless approach, and bone grafting of the gap. The implants (Tissue Level SP, SLActive®, TiZr, Straumann®, Basel, Switzerland) were placed with all the smooth surface junction in a subcrestal bone level, with at least 35 Ncm of insertion torque. The healing cap was placed to support the healing process around the implants, and DBBM particles (Geistlich Bio-Oss 0.25–1 mm, 0.25 g; Geistlich Pharma AG, Wolhusen, Switzerland) were firmly packed into the socket/implant gap up to the level of the marginal soft tissue. On the control sites, an additional step was performed with (BM) a collagen matrix placed (Geistlich Mucograft®) and adapted over the socket entrance, on top of the DBBM and healing cap, and sutured to the marginal gingiva with interrupted sutures (5-0 black polyamide, Ethicon/Johnson & Johnson, São José dos Campos/SP, Brazil). On the test sites, (B) the Bio-Oss graft was intentionally exposed to the oral cavity and anchored by a 5-0 polyamide cross-mattress suture after clinical visualization of a stable clot.
2.3. Keratinized Mucosa Measurement
The width of KM was evaluated by the same operator before extraction, after 1–2 months post-implant placement, and after the gingival emergence contour of a dental prosthesis was established. A period of 2 weeks was awaited after the installation of the implant-supported prosthesis to allow for gingival conditioning, after which the measurement of keratinized gingiva was performed. A periodontal probe was used on the buccal side to determine the distance between the gingival margin and the mucogingival junction by gently rolling the tissue coronally, allowing for precise identification of the mucogingival boundary [19]. If the measurement between the mucogingival junction and the gingival margin was positioned between the markings of the periodontal probe, it was considered as representing half a millimeter. This approach was adopted to enhance the precision of the measurement, accounting for the position relative to the probe’s standardized increments.
2.4. Clinical Assessment of Epithelialization
Epithelialization score refers to the healing area covered by epithelium, graded from 1 to 5 [18]. Clinical assessments were performed by photographic documentation at 2 days, 1 week, and 1 month postoperatively. At each follow-up visit, a 0.05% methylene blue dye solution was applied to the surgical site to assess surface epithelialization [20]. The areas of exposed connective tissue were stained with the blue dye representing incomplete epithelium coverage of the healing site.
2.5. Immunoassay
Samples of wound exudate fluid from each patient post-implant installation were obtained using a sterile swab, with gentle rolling motions from one side to the other after light rinsing with sterile saline. The samples were collected at 2 days, 1 week, and 1 month postoperatively, placed in a 2 mL microtube, and frozen at −80 °C until protein extraction processing. The molecule content of each sample was measured by multiplex assay according to the manufacturer’s instructions (HCYTOMAG-60K-03, Merck Millipore, Burlington, MA, USA). The analyzed molecules were VEGF, IL-1β, and FGF-2, and their concentrations were quantified by MILLIPLEX Analyst 5.1 software (Merck Millipore, Burlington, MA, USA).
2.6. Cone Beam Computed Tomography Measurements
A cone beam computed tomography (CBCT) scan was performed using buccal retractors to isolate the soft tissue contours, with a field of view (FOV) of 7 × 15 cm, a 0.3 mm voxel size, and a resolution of 0.5 mm (scan time: 20 s, 14 bits grayscale) using an i-CAT Classic (Imaging Sciences International, Inc., Hatfield, PA, USA). The CBCTs were performed before tooth extraction, after the implant surgery (T1) and 4 months (T2) post-implant placement. To align the original DICOM data from the T1 and T2 CBCTs scans, a superimposition was performed following the methodology previously described in the literature [21], by the OnDemand3D software (Version 1.0.9.3223, CyberMed Inc., Seoul, Republic of Korea) and transformed into the (.gipl) format using the ITK-SNAP software (version 3.6.0, www.itksnap.org, accessed on 30 September 2020) to enable proper superimposition in the Slicer software (version 5.0.2, www.slicer.org, 30 September 2020). The linear measurements were made using the same reference points, in which the vertical and horizontal measurements followed the parallelism of a perpendicular line drawn on the implant. A horizontal reference line (RL) was determined at the implant platform with the smooth/rough surface junction [3]. A single-blinded and calibrated examiner performed all the measurements (Figure 1). Horizontal width hard tissue alteration changes between the T1 and T2 of the lingual/palatal and buccal bone wall (HBT) at five levels were measured: the RL and at 0 mm, −1 mm, and −2 mm above the RL; and 2 mm below the RL. The buccal and lingual bone level changes were determined by variation on vertical bone height between baseline (T1) and 4 months (T2) after implant placement (ΔVBH T1-T2). The buccal vertical soft tissue thickness was measured prior to tooth extraction using a CBCT scan. One of the major advantages of using a 3D Slicer is the ability to achieve transparency between T1 and T2 slices, allowing the same reference marking, traced only once, to be reproduced consistently across both analysis time points.
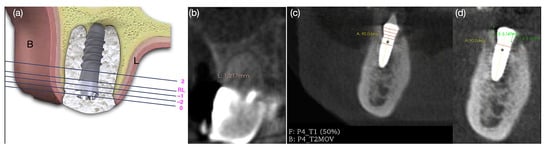
Figure 1.
(a) Illustrative diagram of horizontal bone thickness measurements from the reference line (RL); (b) vertical soft tissue measurement; (c) horizontal reference lines and superimposition of the DICOM (Digital Imaging and Communications in Medicine) of the two CBCT scans on T1 and T2; (d) vertical reference lines at the lingual and buccal bone wall.
2.7. Statistical Analysis
The sample size was based on a previous study [18] of at least 10 patients per group to identify a notable variance in the ridge width on the CBCT scans. Data were analyzed by parametric statistical tests for keratinized mucosa measurement and CBCT measurements. Multiple comparisons were made using a paired t-test. Categorical variables were summarized using descriptive statistics, including the interquartile range (IQR), standard deviation (SD), and mean. The easyanova package in the R environment was used for analysis of variance. The non-parametric tests were conducted using the rstatix package, and a Shapiro–Wilk normality test was performed using the Mann–Whitney U test using the differences between the groups applied for epithelialization and immunoassay. All tests employed significance at the 0.05 level.
3. Results
3.1. Results: Characteristics of the Study
This study involved 26 patients and a total of 28 implants, whose allocation to the two registration groups (B and BM; n = 14) was randomized. Both groups exhibited similarities in terms of age, gender, implant diameter, position, length, and the need for immediate implant placement in the molar area (Table 1).
3.2. Keratinized Mucosa Measurement
The descriptive analysis of KM width is presented in Table 2 and Figure 2. When subdividing the data into maxilla and mandible, the only time point that exhibited statistical difference was in the interval between 1 and 2 months PO to the baseline (p = 0.03). In this variation, the mean KM width in the maxilla (2.71 ± 0.24 mm) was significantly higher than in the mandible (1.94 ± 0.24 mm), and in the maxilla, BM (3.42 ± 0.36 mm) was more representative than B (2.00 ± 0.31 mm). In BM, the maxilla exhibited a larger width of keratinized gingiva (3.41 ± 0.35 mm) than the mandible (1.88 ± 0.31 mm).

Table 2.
The width of keratinized mucosa in mm (Mean ± SD); n = 28.
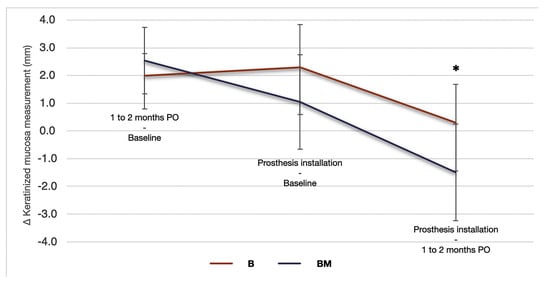
Figure 2.
Changes in the width of keratinized mucosa expressed in mm (n = 28). ∆ represents the net change in keratinized mucosa at different evaluation times: preoperative (baseline), after 1–2 months post-implant placement, and after the emergence contour of a provisional prosthesis. * indicate p < 0.05.
3.3. Clinical Assessment of Epithelialization
Wound healing proceeded typically without any complications, without any indications of infection or clinical manifestations observed in any of the patients (Figure 3). The median and interquartile range for clinical measurements, graded as 1 to 5, are shown in Table 3. In 30 days, the healing was not complete in both groups, with a small band of connective tissue dyed with methylene blue.
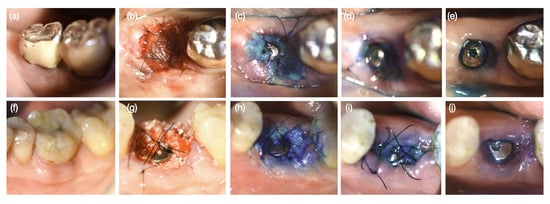
Figure 3.
Wound healing phases in sequence: (a,f) before tooth extraction, (b,g) immediately after implant placement, (c,h) 2 days postoperatively (PO), (d,i) 7 days PO, and (e,j) 30 days PO. a-e represents the Bio-Oss + Mucograft treatment group (BM), while (f–j) represents the Bio-Oss treatment group (B).

Table 3.
The width epithelialization degree (Median (IQR)); n = 28 (B = 14; BM = 14).
3.4. Immunoassay
The growth factors VEGF and FGF-2 remained constant during the healing process, with no statistical difference observed between time points and treatment types. However, IL-β decreased throughout the healing process, indicating a reduction in the inflammatory process, with B and BM showing similar trends (Figure 4).
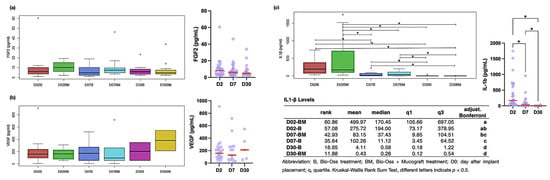
Figure 4.
Levels of cytokines and growth factors. Molecules were measured by a Luminex assay in patients after implant placement. (a) FGF-2; (b) VEGF; (c) IL1-β. Abbreviation: B, Bio-Oss treatment; BM, Bio-Oss + Mucograft treatment; D: day after implant placement. * indicate p < 0.05.
3.5. Cone Beam Computed Tomography Measurements
The total number of evaluated tomographic analyses was 64 DICOM files. An overall count of 22 vertical soft tissue thickness measurements (mm) was analyzed prior to tooth extraction, with 11 corresponding to each group. Due to technical issues with T1 and T2 alignment, one case was discarded. Thus, in the analyses requiring DICOM overlap, a total of 10 image pairs were obtained for the B group and 11 for the BM group. The outcomes of the CBCT assessment are shown in Table 4 and Table 5, which delineate the changes in horizontal bone thickness and height from baseline to 4 months after implant placement.

Table 4.
Variations in ridge width from baseline to 4 months, as determined by CBCT measurements and expressed in mm.

Table 5.
Statistical analysis of bone levels (mm), between the variation on T1 and T2 derived from CBCT.
The initial horizontal bone thickness, regardless of the bone level (RL, 0, −1, −2 mm, RL and 2), was statistically similar between the two groups (p > 0.05). Both Groups B and BM showed similar behavior, with no statistically significant difference observed in horizontal bone thickness and vertical bone height. The 0 level experienced the greatest buccal bone loss, with a reduction of 0.74 mm in Group B and 1.11 mm in Group BM, though no statistical difference was found (p > 0.05). The linear correlation between initial vertical soft tissue measurement and buccal vertical bone height (ΔT1-T2) was 0.2818 (p = 0.27).
4. Discussion
This case–control study experiment showed no statistically significant differences among the DBBM intentionally uncovered in the oral cavity or sealed by the collagen matrix in the change in alveolar bone dimensions, including width and height, from baseline to 4 months post implant placement. Therefore, the null hypothesis of no difference between healing groups was accepted, which lends positive support to the utilization of the DBBM exposed to the oral environment in clinical practices under specific circumstances as outlined in this study. Immediate implant insertion to fill the gap with grafts resulted in 0.59 mm less horizontal bone loss [10]. The buccal bone wall thickness (>1 mm) [7,12] and the larger horizontal gap significantly influenced a reduction in hard tissue alteration [12,17]. In the present study, given that it involves a molar area, the patient profiles have these two characteristics, in which the mean initial thickness on the buccal side, at reference 0, was 2.44 ± 0.51 mm for B and 2.74 ± 0.51 mm for BM, all exhibiting a thick phenotype, which may contribute to HBT stability. It is important to highlight that in this study, an implant was used to replace the third molar in a single patient. However, this was a specific case, despite being an unusual procedure, where the patient had the presence of the antagonist tooth and expressed a desire for such a treatment.
In the present investigation, horizontal buccal bone changes above the RL, in mm, from baseline to 4 months postoperatively in B were 0.74, 0.44, and 0.20; and in BM were 1.11, 0.92, and 0.70; for line 0, −1, and −2, respectively. The findings of this study are consistent with the existing literature, and also in molar area and the same implant type in a follow-up from 1 to 5 years, which showed changes above the RL that were 0.84, 0.55, and 0.15 mm [3]. Furthermore, according to another study [22], a notable horizontal bone alteration (0.88 ± 0.25; p < 0.05) was observed at the most cervical level. These disparities could be impacted by the positioning of the reference line and the large implant and cap, reducing the gap and providing little space for the DBBM and socket overfilling.
The vertical dimension changes from the most coronal measurement at the buccal side were 0.74 ± 0.32 mm [22] and 0.18 ± 1.82 mm [3]. In a meta-analysis, buccal bone height changed ranging from 0.1 to 1.3 mm after immediate implant placement with socket grafting [10]. Our results are consistent, with vertical bone levels less than 1.00 mm, with no difference in the treatment group, specific to bone remodeling [23], as no bone loss occurred below the RL. The initial gingival tissue thickness (more than 2 mm) on placed implants is important to maintain crestal bone level [23,24,25]. Our results did not show a linear correlation between vertical soft tissue measurement and vertical bone level, possibly due to the average soft tissue height among patients being consistently close (2.25 ± 0.48 mm), with a tendency toward a thick phenotype; additionally, the small sample size for this analysis may have influenced the outcome.
One of the major concerns regarding leaving the DBBM exposed is the biomaterial becoming contaminated, leading to surgical procedure failure. However, the literature reveals successful cases [8,9,16], suggesting it is a safe technique with over 6 years of follow-up [16]. Furthermore, the results show no difference in the pro-inflammatory cytokine between the types of treatment in immunoassay. The increase in IL-1β is indicative of delayed healing [26], although this cytokine decreased during the healing periods. Besides the uneventful healing with no infection, maintaining the biomaterial in the grafted site is critical. A diligent follow-up with rigorous postoperative instruction is required, and it was reinforced at the recall visits during sample collections.
Another concern about exposing the DBBM to the oral environment is maintaining the material in position during the healing phases. Based on the follow-up tomography performed at 4 months postoperatively, where the presence of xenogeneic bone particles was clearly visible, it is possible to verify that the biomaterial was kept in place. Additionally, this finding is supported by the results of the horizontal and vertical linear bone measurements taken at 4 months, which were similar in both the exposed group and the group that used collagen matrix to seal the wound, suggesting that the bone was well maintained, comparable to the control group.
The grafted sites with the DBBM and membrane exhibited a superior band of KM compared to non-grafted sites [19]. A collagen matrix can speed up the initial phases of soft tissue healing and potentially result in increased soft tissue thickness [2]. Recovery was faster in the BM group, with almost complete healing achieved in 7 days, whereas in the B group, this outcome was seen after 7 days. Considering the healing time, it is possible to justify why in the maxilla, the width of keratinized gingiva was notably higher in the BM group (3.42 ± 0.36 mm) compared to the B group (2.00 ± 0.31 mm) during the baseline to 1–2 months post-implant placement variation period (p < 0.05). In the earlier assessment, the B group was still in a slower maturation phase, resulting in this difference, whereas in a longer evaluation period, from baseline variation due to gingival conditioning, the B group already has a greater width of keratinized gingiva.
A KM width measuring under 2 mm was significantly associated with discomfort during brushing, a predisposition for the development of peri-implantitis, and a reduction in vestibular depth [13,14]. It is important to emphasize the significance of the extension of this band and to highlight that in both study groups, there was an increase in KM compared to baseline. The DBBM could potentially enhance the soft tissue appearance over time, potentially attributed to the integration of particles into the soft tissue, mitigating bone loss and consequently leading to enhanced long-term esthetics [27]. Upon exposure of the DBBM to the oral cavity, the mucosal tissue that was formed is very similar to KM, suggesting a potential increase in this type of mucosa [16]. This may potentially justify the significant difference in a greater width of KM in the ∆ 1 to 2 months postoperative provisional contour in B when compared to BM (p = 0.006).
One of the limitations of this study was the potential influence of metal artifacts from the crown and the implant in the CBCT images could impact the results [28], potentially leading to inaccuracies in the measurements. Therefore, we chose not to perform soft tissue measurements due to the radiopacity of the implant and biomaterial in the gap. Future studies should focus on soft tissue measurements and further investigation into the histological characteristics of the tissue to deepen the understanding of these outcomes. Another limitation we must emphasize is the reliability of the assessment being constrained by the fact that the measurements were conducted by a single researcher. Furthermore, the results should be interpreted with caution, as despite the sample size calculations, the number of patients included is small and may not be sufficient to draw robust conclusions. We suggest future studies with a larger sample size and a split-mouth design to enhance internal validity and strengthen the reliability of the findings.
The strength of this study lies in its comprehensive evaluation of clinical and tomographic outcomes in socket healing after immediate molar implant placement. A key differentiator and the novelty of the study is the intentional exposure of xenogeneic bone, which was specifically assessed. Additionally, no implant loss was observed with this technique, further supporting the stability and effectiveness of the approach. Also, it is important to mention that deliberate exposure of the biomaterial is not a clinical recommendation. In this case, the importance of clot stabilization by bone wall support, sutures, and adequate use of restorative appliances and their components was addressed to secure favorable clinical outcomes.
5. Conclusions
This study investigated the outcome of a non-recommended clinical situation where xenogeneic bone is kept exposed to the oral environment after immediate implant placement. Although this is not its conventional usage, this approach may lead to bone stability and wider keratinized mucosa in certain cases of the molar area. However, it is important to note that this approach should only be considered when diligent clinical oversight is provided. Furthermore, these results should be interpreted with caution, as additional clinical studies with longer follow-up periods are necessary, along with complementary animal studies to provide histological insights.
Author Contributions
The authors confirm their contribution to the paper as follows: study conception and design: J.G.-F., V.F.C. and M.T.J.; experiments and data collection: J.G.-F. and V.F.C.; analysis and interpretation of results: V.F.C., R.O., P.B.F. and P.L.S.; draft manuscript preparation: V.F.C., A.B.N.J., M.R.M. and M.T.J.; funding acquisition: V.F.C. and M.T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation—FAPESP, Research Project, grant number 2020/12740-2.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the School of Dentistry of Ribeirao Preto Research Ethics Committee (protocol 21305419.1.0000.5419; 10 December 2019). The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Carral, C.; Argibay, O.; Linares, A. Implant placement in fresh extraction sockets. Periodontology 2000 2019, 79, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, M.; Lambert, F.; Knafo, B.; Popelut, A.; Vandenberghe, B.; Finelle, B. Immediate implant in the posterior region combined with alveolar ridge preservation and sealing socket abutment: A retrospective 3D radiographic analysis. Clin. Implant Dent. Relat. Res. 2020, 23, 61–72. [Google Scholar] [CrossRef]
- Araujo, M.G.; Linder, E.; Lindhe, J. Bio-Oss collagen in the buccal gap at immediate implants: A 6-month study in the dog. Clin. Oral Implant. Res. 2011, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sanchez, I.; Sanz-Martin, I.; Ortiz-Vigon, A.; Molina, A.; Sanz, M. Complications in bone-grafting procedures: Classification and management. Periodontology 2000 2022, 88, 86–102. [Google Scholar] [CrossRef]
- Yuenyongorarn, P.; Kan, J.Y.K.; Rungcharassaeng, K.; Matsuda, H.; Roe, P.; Lozada, J.L.; Caruso, J. Facial Gingival Changes With and Without Socket Gap Grafting Following Single Maxillary Anterior Immediate Tooth Replacement: One-Year Results. J. Oral Implantol. 2020, 46, 496–505. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 195–223. [Google Scholar] [CrossRef]
- Thalmair, T.; Fickl, S.; Schneider, D.; Hinze, M.; Wachtel, H. Dimensional alterations of extraction sites after different alveolar ridge preservation techniques—A volumetric study. J. Clin. Periodontol. 2013, 40, 721–727. [Google Scholar] [CrossRef]
- Thoma, D.S.; Bienz, S.P.; Lim, H.C.; Lee, W.Z.; Hammerle, C.H.F.; Jung, R.E. Explorative randomized controlled study comparing soft tissue thickness, contour changes, and soft tissue handling of two ridge preservation techniques and spontaneous healing two months after tooth extraction. Clin. Oral Implant. Res. 2020, 31, 565–574. [Google Scholar] [CrossRef]
- Seyssens, L.; Eeckhout, C.; Cosyn, J. Immediate implant placement with or without socket grafting: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 339–351. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Ferrus, J.; Cecchinato, D.; Pjetursson, E.B.; Lang, N.P.; Sanz, M.; Lindhe, J. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin. Oral Implant. Res. 2010, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Blasi, G. Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J. Periodontol. 2019, 90, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Perussolo, J.; Souza, A.B.; Matarazzo, F.; Oliveira, R.P.; Araujo, M.G. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: A 4-year follow-up study. Clin. Oral Implant. Res. 2018, 29, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pacheco, A.; Soto-Penaloza, D.; Gomez, M.; Penarrocha-Oltra, D.; Alarcon, M.A. Socket seal surgery techniques in the esthetic zone: A systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. Int. J. Implant Dent. 2021, 7, 13. [Google Scholar] [CrossRef]
- Garcez-Filho, J.; Carvalho, V.F.; Taba, M., Jr. Usage of xenogeneic bone intentionally left exposed to the oral environment after immediate implant placement: A case report with six-year follow-up. J. Int. Acad. Periodontol. 2023, 25, 120–129. [Google Scholar] [CrossRef]
- Levine, R.A.; Dias, D.R.; Wang, P.; Araujo, M.G. Effect of the buccal gap width following immediate implant placement on the buccal bone wall: A retrospective cone-beam computed tomography analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 403–413. [Google Scholar] [CrossRef]
- Jung, G.-U.; Jeon, T.-H.; Kang, M.-H.; Um, I.-W.; Song, I.-S.; Ryu, J.-J.; Jun, S.-H. Volumetric, Radiographic, and Histologic Analyses of Demineralized Dentin Matrix Combined with Recombinant Human Bone Morphogenetic Protein-2 for Ridge Preservation: A Prospective Randomized Controlled Trial in Comparison with Xenograft. Appl. Sci. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Barone, A.; Ricci, M.; Tonelli, P.; Santini, S.; Covani, U. Tissue changes of extraction sockets in humans: A comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin. Oral Implant. Res. 2013, 24, 1231–1237. [Google Scholar] [CrossRef]
- Kohale, B.R.; Agrawal, A.A.; Raut, C.P. Effect of low-level laser therapy on wound healing and patients’ response after scalpel gingivectomy: A randomized clinical split-mouth study. J. Indian Soc. Periodontol. 2018, 22, 419–426. [Google Scholar] [CrossRef]
- de Souza, M.M.; Martinez, C.J.H.; Carvalho, V.F.; Furlaneto, F.A.C.; Palioto, D.B.; Messora, M.R.; Scombatti de Souza, S.L.; Novaes, A.B., Jr.; Taba, M.J. Alveolar ridge and keratinized gingiva preservation using collagen matrix and inorganic bone substitute in flapless extractions: A case series of exposed biomaterials. J. Int. Acad. Periodontol. 2022, 24, 10. [Google Scholar]
- Chen, Z.; Li, J.; Wang, H.L.; Yu, H. Initial Bone Volume Changes After Immediate Implant Placement Associated with Filling the Gap Using Bovine Bone in Molar Sites. Int. J. Oral Maxillofac. Implant. 2019, 34, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Puisys, A.; Linkevicius, R.; Alkimavicius, J.; Gineviciute, E.; Linkeviciene, L. The influence of submerged healing abutment or subcrestal implant placement on soft tissue thickness and crestal bone stability. A 2-year randomized clinical trial. Clin. Implant Dent. Relat. Res. 2020, 22, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Puisys, A.; Steigmann, M.; Vindasiute, E.; Linkeviciene, L. Influence of Vertical Soft Tissue Thickness on Crestal Bone Changes Around Implants with Platform Switching: A Comparative Clinical Study. Clin. Implant Dent. Relat. Res. 2015, 17, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Zukauskas, S.; Puisys, A.; Andrijauskas, P.; Zaleckas, L.; Vindasiute-Narbute, E.; Linkevicius, T. Influence of Implant Placement Depth and Soft Tissue Thickness on Crestal Bone Stability Around Implants With and Without Platform Switching: A Comparative Clinical Trial. Int. J. Periodontics Restor. Dent. 2021, 41, 347–355. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S6–S22. [Google Scholar] [CrossRef]
- Zaki, J.; Yusuf, N.; El-Khadem, A.; Scholten, R.; Jenniskens, K. Efficacy of bone-substitute materials use in immediate dental implant placement: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2021, 23, 506–519. [Google Scholar] [CrossRef]
- Razavi, T.; Palmer, R.M.; Davies, J.; Wilson, R.; Palmer, P.J. Accuracy of measuring the cortical bone thickness adjacent to dental implants using cone beam computed tomography. Clin. Oral Implant. Res. 2010, 21, 718–725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).