Chronic Kidney Disease and Oral Health: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
- Diagnosis of CKD (stage G1–G5) under conservative therapy;
- Age ≥ 18 years;
- Both sexes;
- Acceptance and signature of informed consent.
- Cancer in the active phase;
- Positivity for human immunodeficiency virus (HIV), hepatitis B surface antigen (HBsAg), or hepatitis C virus (HCV);
- Inflammatory or infectious diseases in the acute phase;
- Refusal to provide informed consent.
2.1. Clinical–Instrumental Evaluation
2.2. Dental Evaluation
2.3. Statistical Analysis
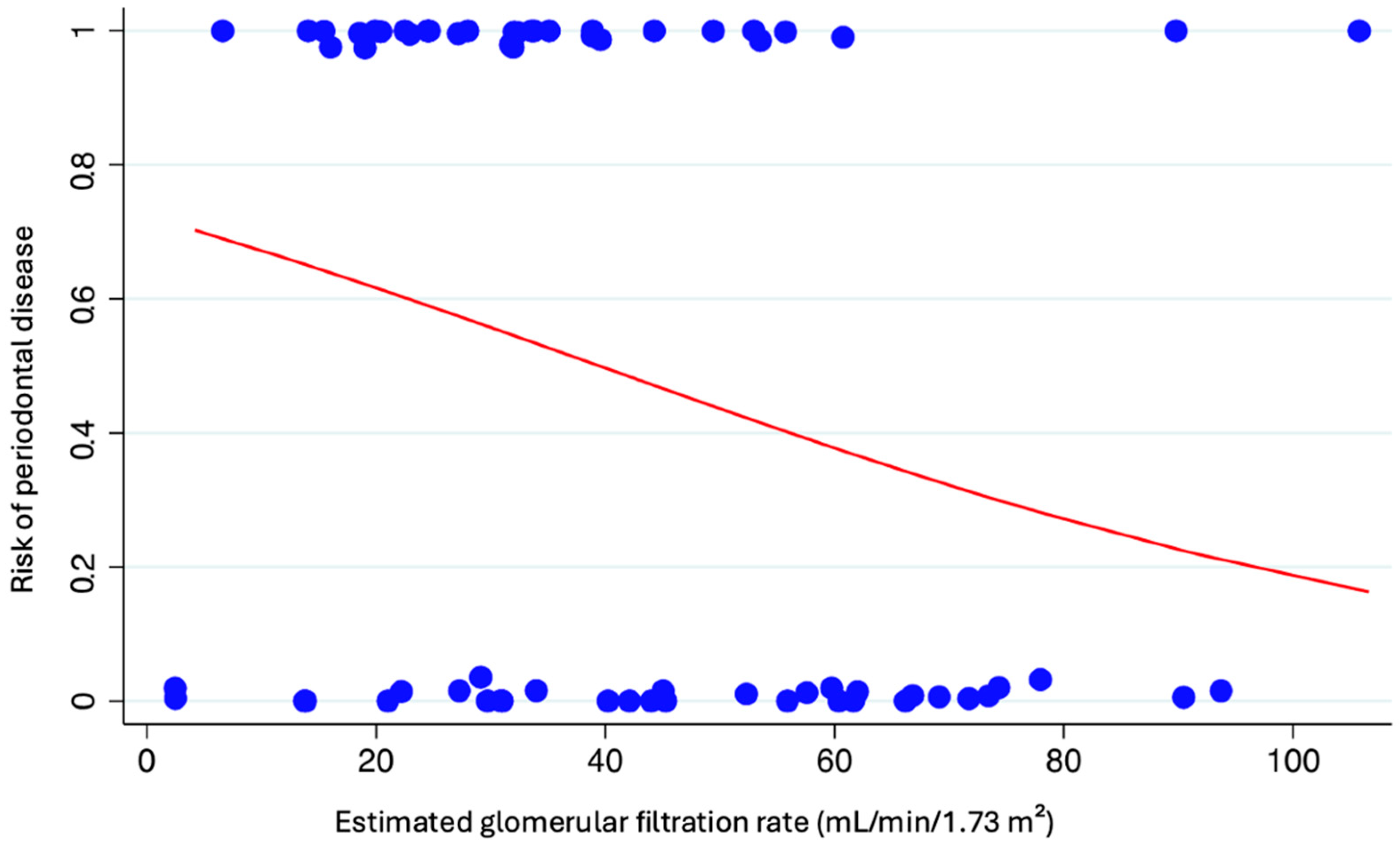
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Wilson Jones, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Ramadori, L.; Celotto, R.; Mitterhofer, A.P.; Di Daniele, N. Nutritional Approaches for the Management of Metabolic Acidosis in Chronic Kidney Disease. Nutrients 2021, 13, 2534. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Lamb, E.J.; Levey, A.S.; Stevens, P.E. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: Evolution not revolution. Clin. Chem. 2013, 59, 462–465. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Eriksen, B.O.; Ingebretsen, O.C. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006, 69, 375–382. [Google Scholar] [CrossRef]
- Evans, M.; Fryzek, J.P.; Elinder, C.G.; Cohen, S.S.; McLaughlin, J.K.; Nyren, O.; Fored, C.M. The natural history of chronic renal failure: Results from an unselected, population-based, inception cohort in Sweden. Am. J. Kidney Dis. 2005, 46, 863–870. [Google Scholar] [CrossRef]
- Grams, M.E.; Chow, E.K.; Segev, D.L.; Coresh, J. Lifetime incidence of CKD stages 3–5 in the United States. Am. J. Kidney Dis. 2013, 62, 245–252. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Canale, M.P.; Noce, A.; Di Lauro, M.; Marrone, G.; Cantelmo, M.; Cardillo, C.; Federici, M.; Di Daniele, N.; Tesauro, M. Gut Dysbiosis and Western Diet in the Pathogenesis of Essential Arterial Hypertension: A Narrative Review. Nutrients 2021, 13, 1162. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, S.; Noce, A.; Di Renzo, L.; Cianci, R.; Naticchia, A.; Giarrizzo, G.F.; Giordano, F.; Tozzo, C.; Splendiani, G.; De Lorenzo, A. Is rasburicase an effective alternative to allopurinol for management of hyperuricemia in renal failure patients? A double blind-randomized study. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 179–184. [Google Scholar] [PubMed]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubina, P.; Lahera, V.; Luno, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Santaella, N.G.; Maciel, A.P.; Simpione, G.; Santos, P.S. Halitosis, reduced salivary flow and the quality of life in pre-kidney transplantation patients. J. Clin. Exp. Dent. 2020, 12, e1045–e1049. [Google Scholar] [CrossRef]
- Adamowicz, K.; Lima Ribeiro, A.S.; Golda, A.; Wadowska, M.; Potempa, J.; Schmaderer, C.; Anders, H.J.; Koziel, J.; Lech, M. Bidirectional Interaction Between Chronic Kidney Disease and Porphyromonas gingivalis Infection Drives Inflammation and Immune Dysfunction. J. Immunol. Res. 2025, 2025, 8355738. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, E.; Davidovits, M.; Eidelman, E.; Schwarz, Z.; Bimstein, E. Pathophysiology, therapy, and oral implications of renal failure in children and adolescents: An update. Pediatr. Dent. 2005, 27, 98–106. [Google Scholar]
- Nikiforuk, G.; Fraser, D. The etiology of enamel hypoplasia: A unifying concept. J. Pediatr. 1981, 98, 888–893. [Google Scholar] [CrossRef]
- Tonnesen, H.H.; de Vries, H.; Karlsen, J.; Beijersbergen van Henegouwen, G. Studies on curcumin and curcuminoids. IX: Investigation of the photobiological activity of curcumin using bacterial indicator systems. J. Pharm. Sci. 1987, 76, 371–373. [Google Scholar] [CrossRef]
- Costacurta, M.; Di Lauro, M.; Cornali, K.; Docimo, R.; Noce, A. Developmental Defects of Enamel and Dental Caries in Pediatric Patients with Chronic Kidney Disease–Mineral Bone Disorders. Appl. Sci. 2025, 15, 1164. [Google Scholar] [CrossRef]
- Parsegian, K.; Randall, D.; Curtis, M.; Ioannidou, E. Association between periodontitis and chronic kidney disease. Periodontol. 2000 2022, 89, 114–124. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kozak, M.; Pawlik, A. The Role of the Oral Microbiome in the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 5231. [Google Scholar] [CrossRef]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Fisher, M.A.; Taylor, G.W.; Shelton, B.J.; Jamerson, K.A.; Rahman, M.; Ojo, A.O.; Sehgal, A.R. Periodontal disease and other nontraditional risk factors for CKD. Am. J. Kidney Dis. 2008, 51, 45–52. [Google Scholar] [CrossRef]
- Chen, P.; Lin, X.; Zhang, C.; Xie, Y.; Guo, Z.; Ren, F. Fusobacterium nucleatum-infected periodontitis promotes renal interstitial fibrosis in rats through the TGF-beta/SMAD2/3 and beta-catenin signaling pathways. Gene 2024, 927, 148729. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.L.; Liu, X.Y.; Meng, X.; Zhao, R.Q.; Ou, L.L.; Li, B.Z.; Xing, T. Periodontitis Exacerbates and Promotes the Progression of Chronic Kidney Disease Through Oral Flora, Cytokines, and Oxidative Stress. Front. Microbiol. 2021, 12, 656372. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Chu, H.; Xia, W.; Zou, X.; Wang, D.; Rong, M. Periodontitis regulates renal impairment in obese mice via TGF-beta/Smad pathway. Am. J. Transl. Res. 2021, 13, 12523–12535. [Google Scholar] [PubMed]
- American Academy of Periodontology. Comprehensive periodontal therapy: A statement by the American Academy of Periodontology. J. Periodontol. 2011, 82, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Akar, H.; Akar, G.C.; Carrero, J.J.; Stenvinkel, P.; Lindholm, B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 218–226. [Google Scholar] [CrossRef]
- Silva, D.F.; Oliveira, I.C.R.; Medeiros, S.A.; Baeder, F.M.; Albuquerque, A.C.L.; Lima, E.O. Oral health challenges in patients with chronic kidney disease: A comprehensive clinical assessment. Saudi Dent. J. 2024, 36, 364–367. [Google Scholar] [CrossRef]
- Tabesh, A.; Sadat Abtahi, M.; Narimany, R.; Sadat Abtahi, M. Oral health-related quality of life in chronic kidney disease patients. Dent. Res. J. 2022, 19, 73. [Google Scholar] [CrossRef]
- Basilicata, M.; Pieri, M.; Marrone, G.; Nicolai, E.; Di Lauro, M.; Paolino, V.; Tomassetti, F.; Vivarini, I.; Bollero, P.; Bernardini, S.; et al. Saliva as Biomarker for Oral and Chronic Degenerative Non-Communicable Diseases. Metabolites 2023, 13, 889. [Google Scholar] [CrossRef]
- Taccone-Gallucci, M.; Noce, A.; Bertucci, P.; Fabbri, C.; Manca-di-Villahermosa, S.; Della-Rovere, F.R.; De Francesco, M.; Lonzi, M.; Federici, G.; Scaccia, F.; et al. Chronic treatment with statins increases the availability of selenium in the antioxidant defence systems of hemodialysis patients. J. Trace Elem. Med. Biol. 2010, 24, 27–30. [Google Scholar] [CrossRef]
- Basha, M.M.; Al-Kadasi, B.A.; Al-Hajri, M.; Al-Sharani, H.M.; Elayah, S.A. Exploring the correlation between periodontal disease and serum biomarkers in haemodialysis patients. BMC Oral. Health 2024, 24, 1066. [Google Scholar] [CrossRef]
- Dembowska, E.; Jaron, A.; Raslawska-Socha, J.; Gabrysz-Trybek, E.; Bladowska, J.; Gacek, S.; Trybek, G. The Evaluation of the Periodontal Status of Hemodialysis Patients with End-Stage Renal Disease. J. Clin. Med. 2022, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shu, C.J.; Wang, C.J.; Chen, K. Meta-analysis of the association between chronic periodontitis and chronic kidney disease. World J. Clin. Cases 2024, 12, 5094–5107. [Google Scholar] [CrossRef]
- Belluz, M.; Longhi, E. Periodontal Disease. In Managing Psychosexual Consequences in Chronic Diseases; Springer International Publishing: Cham, Switzerland, 2023; pp. 329–336. [Google Scholar]
- Ouanounou, A. Xerostomia in the Geriatric Patient: Causes, Oral Manifestations, and Treatment. Compend. Contin. Educ. Dent. 2016, 37, 306–311. [Google Scholar] [PubMed]
- Singh, M.L.; Papas, A. Oral implications of polypharmacy in the elderly. Dent. Clin. N. Am. 2014, 58, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Fabrini, R.; Dessi, M.; Bocedi, A.; Santini, S.; Rovella, V.; Pastore, A.; Tesauro, M.; Bernardini, S.; Di Daniele, N.; et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014, 51, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018, 87, 697–724. [Google Scholar] [CrossRef] [PubMed]
| Alterations Related to CKD | Oral Injuries | Reference |
|---|---|---|
| The urea and other uremic toxin accumulation in the saliva. | Xerostomia and uremic halitosis. Indoxyl sulfate enhances the survival and responses of macrophages and lymphocytes, inducing alveolar bone loss. | [16,17] |
| The normochromic and normocytic anemia. | Paleness of the gingival mucosa. | [18] |
| Calcium–phosphorus metabolism impairments. | Dental hard tissue anomalies (including hypoplasia of the dental enamel and increased susceptibility to tooth decay) or oral bone tissue alteration (including demineralization of the alveolar bone, fracture of the jaw, abnormal bone healing after an extraction, and tooth mobility due to a bone substance loss). | [19] |
| Uremic gastritis. | Gastroesophageal reflux, gastrointestinal lesions, and Helicobacter pylori infection that contribute to the onset of oral lesions and dental caries. | [20,21] |
| Chronic low-grade inflammation and oxidative stress. | High risk to develop periodontal disease. | [22] |
| CKD STAGE | tot. (n) | % | Gender (Male/Female) | Mean Age (Years) * |
|---|---|---|---|---|
| G1-2 | 14 | 18.7 | (10/4) | 54.14 ± 11.51 |
| G3a | 10 | 13.3 | (6/4) | 70.50 ± 8.54 |
| G3b | 23 | 30.7 | (11/12) | 72.04 ± 11.72 |
| G4 | 19 | 25.3 | (7/12) | 72.84 ± 11.11 |
| G5 | 9 | 12.0 | (6/3) | 57.00 ± 19.78 |
| Parameter | Stage G1–2 | Stage G3a | Stage G3b | Stage G4 | Stage G5 |
|---|---|---|---|---|---|
| Red blood cells (millions/μL) | 5.01 ± 0.46 | 4.77 ± 0.76 | 4.18 ± 0.64 | 3.97 ± 0.44 | 3.88 ± 0.97 |
| Hemoglobin (g/dL) | 14.99 ± 0.98 | 13.93 ± 1.95 | 12.38 ± 2.58 | 11.48 ± 1.39 | 11.32 ± 2.02 |
| White blood cells (thousands/μL) | 6.39 ± 1.98 | 6.26 ± 1.90 | 7.86 ± 5.70 | 7.45 ± 2.33 | 5.55 ± 1.00 |
| Platelets (thousands/μL) | 233.29 ± 96.18 | 248.00 ± 71.57 | 234.61 ± 69.31 | 228.84 ± 69.35 | 193.11 ± 43.37 |
| Creatinine (mg/dL) | 10.04 ± 0.20 | 1.19 ± 0.12 | 1.68 ± 0.30 | 2.19 ± 0.66 | 6.72 ± 4.15 |
| e-GFR (mL/min/1.73 m2) | 76.46 ± 13.82 | 54.89 ± 3.62 | 36.10 ± 5.40 | 23.54 ± 3.56 | 10.34 ± 4.40 |
| Azotemia (mg/dL) | 42.31 ± 9.65 | 47.00 ± 12.56 | 72.62 ± 23.67 | 90.52 ± 38.71 | 130.22 ± 41.20 |
| Glycaemia (mg/dL) | 91.79 ± 19.01 | 88.60 ± 12.25 | 104.62 ± 22.04 | 95.20 ± 24.41 | 98.38 ± 27.14 |
| Uric acid (mg/dL) | 6.07 ± 1.53 | 4.43 ± 1.11 | 5.28 ± 2.22 | 5.03 ± 1.91 | 6.38 ± 1.15 |
| Albumin (mg/dL) | 4.39 ± 0.42 | 4.53 ± 0.65 | 4.53 ± 0.56 | 4.20 ± 0.26 | 4.29 ± 0.49 |
| Sodium (mmol/L) | 140.69 ± 1.84 | 141.67 ± 2.06 | 140.57 ± 2.84 | 139.20 ± 4.95 | 141.22 ± 3.30 |
| Potassium (mmol/L) | 4.55 ± 0.42 | 4.64 ± 0.48 | 4.64 ± 0.52 | 4.69 ± 0.66 | 5.36 ± 0.78 |
| Calcium (mmol/L) | 9.54 ± 0.51 | 8.86 ± 2.05 | 9.47 ± 0.59 | 9.10 ± 1.20 | 8.89 ± 0.85 |
| Phosphorus (mmol/L) | 3.12 ± 0.40 | 3.24 ± 0.53 | 3.36 ± 0.84 | 3.66 ± 0.57 | 4.90 ± 1.27 |
| Total cholesterol (mg/dL) | 191.00 ± 41.78 | 186.50 ± 44.93 | 172.74 ± 51.03 | 167.00 ± 26.87 | 151.2 ± 37.35 |
| HDL-cholesterol (mg/dL) | 49.55 ± 14.31 | 50.87 ± 12.76 | 47.79 ± 15.22 | 54.30 ± 26.26 | 43.80 ± 7.79 |
| LDL-cholesterol (mg/dL) | 117.88 ± 38.54 | 109.00 ± 34.67 | 96.79 ± 37.87 | 93.10 ± 36.03 | 89.12 ± 29.35 |
| Triglycerides (mg/dL) | 112.92 ± 46.81 | 108.20 ± 40.41 | 135.06 ± 93.36 | 114.27 ± 46.22 | 122.4 ± 62.63 |
| Parathyroid hormone (pmol/L) | 89.75 ± 68.29 | 105.87 ± 33.25 | 86.57 ± 57.01 | 151.75 ± 89.99 | 604.27 ± 954.74 |
| Parameter | Stage G1–2 | Stage G3a | Stage G3b | Stage G4 | Stage G5 |
|---|---|---|---|---|---|
| BMI (kg/m2) | 26.88 ± 5.39 | 27.28 ± 4.98 | 28.89 ± 6.18 | 27.42 ± 6.31 | 25.46 ± 3.54 |
| Resistance (Ω) | 498.86 ± 69.84 | 500.10 ± 74.43 | 485.77 ± 100.24 | 509.24 ± 95.73 | 487.50 ± 75.95 |
| Reactance (Ω) | 52.14 ± 16.24 | 40.20 ± 11.19 | 43.94 ± 11.10 | 42.49 ± 13.29 | 39.19 ± 12.33 |
| Phase Angle (°) | 5.98 ± 1.85 | 4.69 ± 1.35 | 5.13 ± 1.41 | 4.73 ± 1.03 | 4.53 ± 1.09 |
| TBW % | 54.28 ± 7.83 | 54.12 ± 6.22 | 53.17 ± 6.26 | 52.81 ± 6.43 | 55.84 ± 9.33 |
| ICW % | 52.59 ± 7.74 | 46.07 ± 9.39 | 49.08 ± 7.24 | 48.32 ± 4.76 | 44.95 ± 8.07 |
| ECW % | 47.00 ± 7.61 | 51.91 ± 12.04 | 51.09 ± 7.32 | 53.14 ± 6.53 | 51.24 ± 14.26 |
| FM % | 26.22 ± 9.85 | 29.02 ± 9.48 | 28.21 ± 7.54 | 30.51 ± 9.04 | 24.23 ± 10.42 |
| FFM % | 73.78 ± 9.85 | 70.98 ± 9.48 | 71.79 ± 7.54 | 69.49 ± 9.04 | 66.48 ± 23.61 |
| BCM % | 52.54 ± 8.44 | 44.97 ± 10.05 | 48.06 ± 7.88 | 45.86 ± 6.92 | 44.53 ± 7.32 |
| BMR (Kcal/day) | 1620.56 ± 258.0 | 1426.30 ± 214.58 | 1506.41 ± 254.03 | 1391.48 ± 151.93 | 1445.18 ± 138.48 |
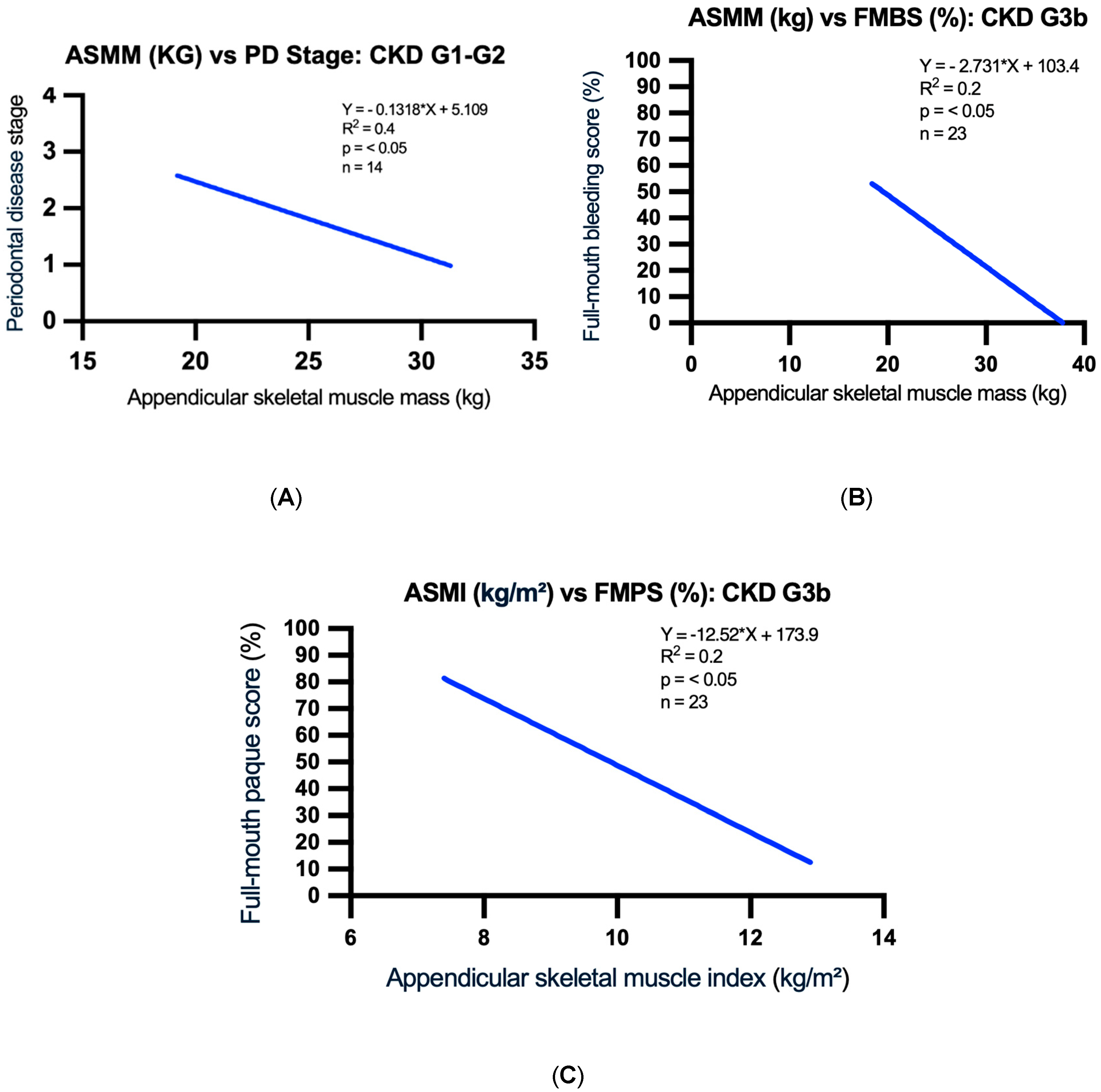
| ASMM (kg) | 26.3 ± 3.9 | 25.7 ± 3.3 | 26.3 ± 5.2 | 24.7 ± 3.6 | 26.5 ± 4.1 |
| ASMI (kg/m2) | 9.1 ± 0.9 | 9.8 ± 1.0 | 10.2 ± 1.2 | 10.0 ± 1.5 | 9.3 ± 1.6 |
| CKD Stage | Total Number of Patients | Number of Edentulous Patients | % of Sites with Periodontal Pocket Depth > 3 mm | % of Sites With Gingival Recessions > 1 mm |
|---|---|---|---|---|
| G1–2 | 14 | 1 | 10.86 | 19.53 |
| G3a | 10 | 1 | 31.88 | 32.44 |
| G3b | 23 | 3 | 19.75 | 32.20 |
| G4 | 19 | 4 | 23.81 | 38.37 |
| G5 | 9 | 1 | 19.63 | 45.88 |
| CKD Stage | % Localized Gingivitis (10% < FMBS < 30%) | % Generalized Gingivitis (FMBS > 30%) | % Plaque Index |
|---|---|---|---|
| G1–2 | 14 | 64 | 45 |
| G3a | 50 | 40 | 86 |
| G3b | 20 | 67 | 75 |
| G4 | 26 | 52 | 85 |
| G5 | 20 | 80 | 77 |
| PD Classification | Percentage (%) | |
|---|---|---|
| PD Stage | Stage I | 31.3 |
| Stage II | 19.4 | |
| Stage III | 19.4 | |
| Stage IV | 28.3 | |
| No PD | 1.6 | |
| PD Grade | Grade I | 53.7 |
| Grade II | 35.8 | |
| Grade III | 8.9 | |
| No PD | 1.6 | |
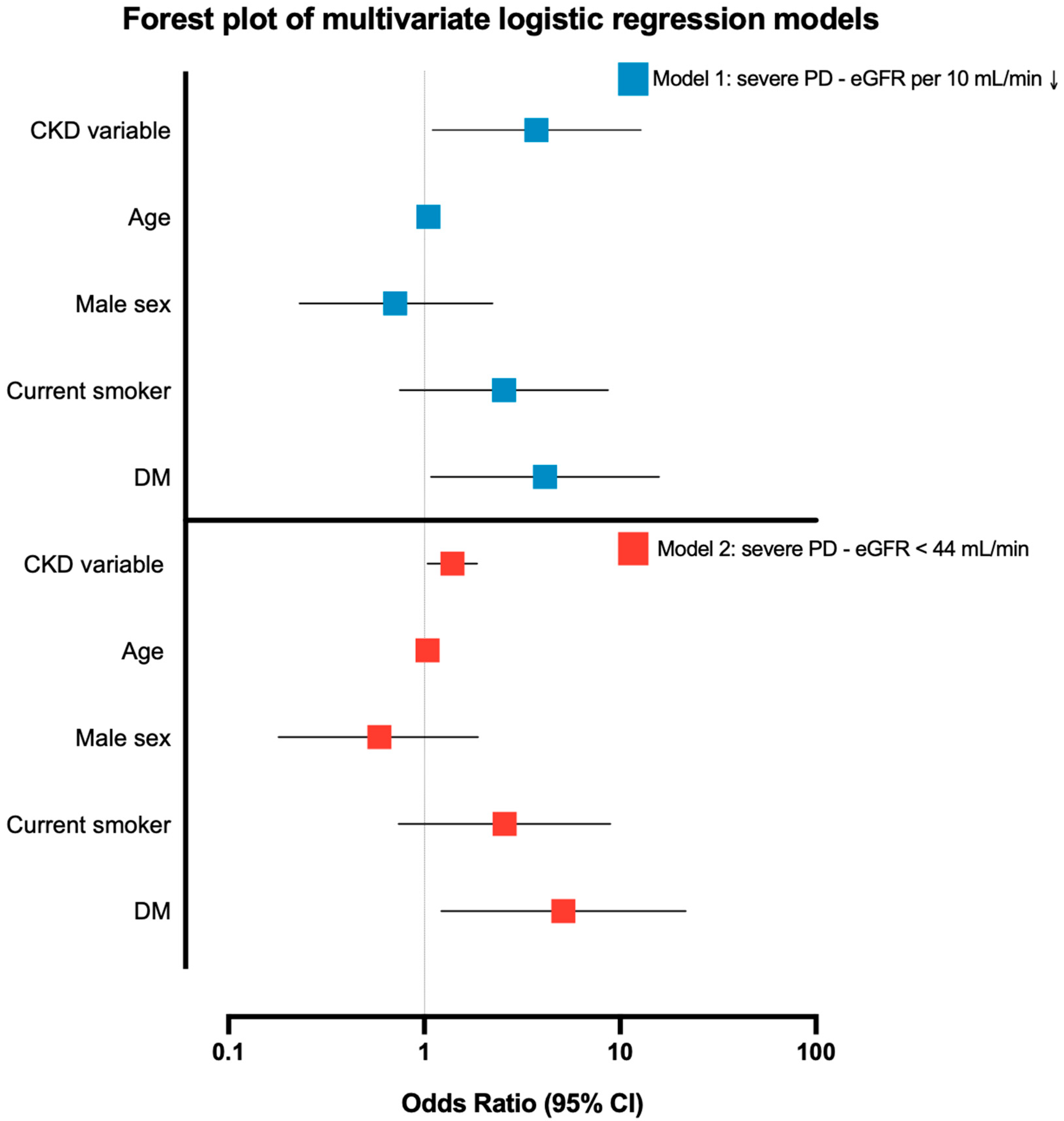
| Predictor | Model 1: Severe PD ~ eGFR per 10↓ (N = 67) | Model 2: Severe PD ~ eGFR < 44 (N = 67) |
|---|---|---|
| CKD variable | OR 1.39 (95% CI 1.04–1.86), p = 0.027 * | OR 3.74 (95% CI 1.10–12.77), p = 0.035 * |
| Age (per year) | OR 1.04 (0.998–1.09), p = 0.060 | OR 1.05 (1.001–1.095), p = 0.045 * |
| Male sex | OR 0.59 (0.18–1.88), p = 0.370 | OR 0.71 (0.23–2.23), p = 0.556 |
| Current smoker | OR 2.57 (0.74–8.90), p = 0.136 | OR 2.55 (0.75–8.69), p = 0.134 |
| Diabetes | OR 5.13 (1.22–21.6), p = 0.026 * | OR 4.13 (1.08–15.8), p = 0.038 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basilicata, M.; Di Lauro, M.; Bruno, G.; Cornali, K.; Marrone, G.; Masci, C.; Troiano, G.; Manca di Villahermosa, S.; Mitterhofer, A.P.; Bollero, P.; et al. Chronic Kidney Disease and Oral Health: A Cross-Sectional Study. Appl. Sci. 2025, 15, 10804. https://doi.org/10.3390/app151910804
Basilicata M, Di Lauro M, Bruno G, Cornali K, Marrone G, Masci C, Troiano G, Manca di Villahermosa S, Mitterhofer AP, Bollero P, et al. Chronic Kidney Disease and Oral Health: A Cross-Sectional Study. Applied Sciences. 2025; 15(19):10804. https://doi.org/10.3390/app151910804
Chicago/Turabian StyleBasilicata, Michele, Manuela Di Lauro, Giovanni Bruno, Kevin Cornali, Giulia Marrone, Claudia Masci, Giuseppe Troiano, Simone Manca di Villahermosa, Anna Paola Mitterhofer, Patrizio Bollero, and et al. 2025. "Chronic Kidney Disease and Oral Health: A Cross-Sectional Study" Applied Sciences 15, no. 19: 10804. https://doi.org/10.3390/app151910804
APA StyleBasilicata, M., Di Lauro, M., Bruno, G., Cornali, K., Marrone, G., Masci, C., Troiano, G., Manca di Villahermosa, S., Mitterhofer, A. P., Bollero, P., & Noce, A. (2025). Chronic Kidney Disease and Oral Health: A Cross-Sectional Study. Applied Sciences, 15(19), 10804. https://doi.org/10.3390/app151910804









