Featured Application
The findings of this study provide a sustainable and cost-effective agricultural strategy for improving crop resilience in water-scarce regions. By using compost derived from olive pomace—an abundant agricultural waste product—farmers can enhance the soil’s water retention and nutrient content, thereby significantly improving barley’s drought tolerance. This application promotes progress towards a circular economy by valorizing agricultural by-products and offers a viable solution for maintaining crop productivity and food security in the face of climate change and increasing water scarcity.
Abstract
Drought stress, a major abiotic factor, significantly threatens global agricultural productivity and food security. This study evaluated the potential of olive pomace compost to alleviate water stress in barley (Hordeum vulgare L.). A pot experiment compared well-watered (80% FC) and drought-stressed plants (40% FC), with compost applied at recommended (40 tons/ha) and double doses (80 tons/ha). Water stress reduced growth (fresh (−28.6%) and dry biomass, (−49.9%) shoot length (−20.45%)), photosynthetic pigments (chlorophyll a (−16.9%), chlorophyll b, (−52.16%) and carotenoids (−24.67%)), and water content, while impairing water relations, as shown by lower relative water content and higher water saturation deficit and consumption. Drought-stressed plants also exhibited elevated oxidative stress, indicated by increased malondialdehyde levels (+68.42%), and a modulated antioxidant defense system, with higher DPPH inhibition (+12.30%), with total phenolic content increasing by 220.70% and FRAP and ORAC values increasing by 55.18% and 37.44%, respectively. The application of olive pomace compost effectively mitigated these adverse effects, resulting in improved growth (ranging from 30% to 66%), pigment content (especially with double dose), and water relations; a lowering of oxidative stress (−37.5%); and moderation of the antioxidant response, indicating a reduced overall stress burden. The study findings show that olive pomace compost provides a sustainable and cost-effective strategy for improving crop resilience in water-scarce regions. By using this abundant agricultural waste, farmers can enhance soil health and maintain food security in the face of climate change.
1. Introduction
Barley (Hordeum vulgare L.), an annual plant belonging to the grass family (Poaceae), stands as one of the earliest domesticated crops, representing an ancient agricultural cornerstone [1]. Globally, barley ranks as the fourth most important cereal crop in terms of both production area and yield [2]. Its cultivation spans a broad range of geographical and climatic zones, from subarctic latitudes like Norway to subtropical fringes near the Sahara, and from sea level to high altitudes [3]. This distribution reflects its remarkable adaptability [4]. The majority of the global harvest, around 75%, is utilized for animal feed, while the remaining 25% is for human consumption, either as food grain or, significantly, for malting processes used in beverages and food industries [5].
Barley is also a critical food source in regions with marginal agricultural conditions. Despite its inherent resilience, however, its production faces significant environmental constraints, primarily drought stress, i.e., a shortage of available water that causes detrimental morphological, biochemical, physiological, and molecular changes in plants [6], severely limiting growth and productivity. This issue is expected to worsen as global climate change is expected to increase the frequency of droughts.
Drought negatively impacts barley through various mechanisms: it restricts root growth, reducing water and nutrient uptake [7]; decreases tiller number, plant height, and grains per ear, lowering thousand-kernel weight and overall yield [8]; causes visible symptoms like wilting, yellowing, and leaf senescence [9]; triggers stomatal closure; and limits CO2 uptake and photosynthesis [10]. It also alters grain composition, often increasing protein concentration while reducing starch content and affecting kernel size, which can negatively impact quality for uses such as malting [11,12].
Improving yield stability, especially in drought-prone and semi-arid regions, remains a goal for farmers. Applying compost can provide a sustainable method to bolster plant tolerance to water stress through soil improvement [13]. The high organic matter content in compost significantly increases the soil water-holding capacity, allowing the soil to absorb more moisture and gradually release it to plants [14]. Among the various organic wastes available for composting, olive pomace presents a valuable resource due to its rich organic composition, which makes it particularly effective in improving soil structure and water retention. Additionally, compost also improves soil structure by binding particles, which enhances water infiltration, minimizes runoff, and creates vital pore spaces, thus preventing compaction [15]. This improved soil environment supports deeper, more extensive root systems, allowing plants to access water across a larger soil volume [16]. Furthermore, the slow release of nutrients from compost promotes plant health and drought resilience [17].
The use of biofertilizers to improve crop physiological parameters is a well-established practice in modern agriculture. For instance, studies have shown that marine macroalgae such as Enteromorpha intestinalis and Corallina elongata can enhance the growth and chlorophyll content of Zea mays L., highlighting the potential of organic amendments to act as a nutrient source and growth promoter under abiotic stress [18].
This study analyzes the impact of drought stress on barley cultivation and the capacity of olive pomace compost to enhance the tolerance of water-stressed barley plants. Morpho-physiological and biochemical parameters, including fresh biomass, dry biomass, shoot length, pigment content, water level status, malondialdehyde (MDA) content, and antioxidant activity, such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), total phenolic content (TPC), ferric reducing antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC), were adopted as measures of barley’s response to experimental factors. The application of compost is hypothesized to enhance the drought tolerance of barley plants. This study aimed to demonstrate this by a favorable modulation of key morpho-physiological and biochemical parameters under water deficit conditions. Specifically, it was anticipated that compost-treated plants would show a significant increase in fresh biomass, dry biomass, shoot length, and pigment content, alongside maintenance of higher water levels. This enhanced tolerance was thought to be biochemically reflected by a reduction in malondialdehyde (MDA) and an increase in antioxidant activity.
2. Materials and Methods
2.1. Growth Conditions and Treatments
A pot experiment was conducted in a non-climate-controlled greenhouse at the Institute of Agriculture and Tourism, Poreč, Croatia (45°13′22.1″ N, 13°36′13.1″ E), from March to May 2025. The experimental design was a completely randomized design with six treatments and four biological replicates per treatment, for a total of 24 experimental units. Each unit consisted of a plastic pot (20 cm depth, 15 cm diameter) containing 2 kg of sandy loam soil.
The soil’s physical and chemical properties were analyzed prior to the experiment. Its texture was determined to be sandy loam (54.21% sand, 34.60% silt, 11.19% clay), with a field capacity (FC) of 42% as determined by the gravimetric method. The soil’s chemical properties included a pH of 6.93, 2.58% organic matter, 23.87 mg of available P2O5 per 100 g of soil, 0.201% total N, and 25.5 mg of available K2O per 100 g of soil.
The compost used had an electrical conductivity (EC) of 4.67 mS/cm. However, this high EC was effectively managed as the compost was thoroughly mixed with a much larger volume of soil (1 part compost to 10 parts soil), diluting its effect and preventing any osmotic stress to the plants.
The greenhouse conditions were dependent on the prevailing climatic conditions. These were continuously monitored using a data logger (HOBO M×2301, Onset, Bourne, MA, USA). The mean daytime temperature was 30.30 ± 4.64 °C with 22.54 ± 4.98% relative humidity, while the mean nighttime temperature was 8.1 ± 1.6 °C with 85 ± 8.6% relative humidity. The photosynthetically active radiation (PAR) ranged from 1.2 ± 0.21 to 1243.7 ± 66.78 µmol m−2 s−1, consistent with a natural photoperiod, no supplemental lighting was used.
In this study, 15 seeds of barley (Hordeum vulgare L.) (var. Barun) were sown in each pot and irrigated to field capacity to ensure uniform germination. Subsequently, thinning was conducted after full emergence, leaving five healthy seedlings per pot. The pots were subjected to two water regimes: well-watered maintained at 80% field capacity, and drought-stressed, maintained at 40% field capacity throughout the growing season. The well-watered and water-stress pots received water every two days to reach the target moisture level. The well-watered and water-stressed pots were maintained at their target moisture levels of 80% and 40% of field capacity (FC), respectively. To maintain these levels, all pots were weighed daily using a digital balance, and deionized water was added to restore them to their predetermined target weight. To prevent water loss and nutrient runoff, pots were placed on individual trays. This approach ensured precise and consistent control over the soil moisture content throughout the experiment. Within each water regime, two concentrations of olive pomace compost were applied: a standard dose of 10 g/kg soil (compost) and a 2-fold higher dose (compost × 2), in addition to a no-compost (control) (Table 1). This resulted in six distinct treatments. The standard dose was based on an organic matter application rate of 40 tons per hectare [19] (10 g/kg soil). For the composting process, a 1-ton pilot-scale bioreactor was used to co-compost olive pomace with barley straw. The barley straw, added at a specific C:N ratio of 50 served as a bulking agent to improve porosity and air circulation. The composting pile was monitored daily for temperature and moisture content and turned weekly to ensure optimal conditions. The thermophilic phase, characterized by temperatures reaching 53.2–55.6 °C, was sustained for 15–20 days, which is crucial for pathogen inactivation. After six months of careful management, the process concluded with a maturation phase, resulting in a high-quality and stable compost that met the standards for agricultural use. The final analysis showed the following characteristics: Ash: 14.5%; Organic Matter: 85.4%; Electrical Conductivity (EC): 4.67 mS/cm; pH: 6.93; Total Carbon (C): 35.3%; Total Nitrogen (N): 2.11%; C/N Ratio: 17; Total Phenols: 3.42 mg gallic acid equivalents (GAE)/g DW; and Oil Content: 0.05%.

Table 1.
Experimental treatments and their respective components.
2.2. Plant Sampling, Measurements, and Analyses
After two months, the experiment concluded, and the plants were harvested. The harvested plants were divided into two groups: the first group was used to determine biomass, including fresh weight, dry weight, and plant length measurements. The second group was used for the analysis of other physiological and biochemical parameters, as described below.
2.2.1. Growth Parameter Determination
Biomass was determined by measuring fresh weight and dry weight. Fresh weight was measured immediately after harvest using a laboratory balance. For dry weight, samples were placed in a forced-air oven at 80 °C and dried to a constant weight, which was then measured using the same balance. Plant length was also measured.
2.2.2. Pigment Content
Fresh leaf matter (100 mg) was used to assess the content of photosynthetic pigments, including chlorophyll a, chlorophyll b, total chlorophyll and carotenoids following Bouhadi et al. [20]. Briefly, 0.1 g of fresh leaf was ground using 1.5 mL of 80% acetone. After centrifugation at 16,000× g for 10 min, the absorbance was measured at 470, 645, and 662 nm using a Tecan Infinite 200 Pro M Nano+ spectrophotometer (Männedorf, Switzerland).
2.2.3. Water Level Status
To assess the leaf water status of barley plants, including relative water content (RWC), water saturation deficit (WSD), and water uptake capacity (WUC), a standardized procedure was followed using fully expanded young leaves. These were harvested, and their fresh weight (FW) was immediately recorded. Subsequently, these leaves were hydrated in darkness at room temperature for 24 h using distilled water. Following this saturation, turgid weight (TW) was determined after carefully removing surface moisture. The dry weight (DW) was then obtained after oven-drying the leaves at 72 °C for 48 h. The fresh weight (FW), turgid weight (TW), and dry weight (DW) were used to calculate RWC, WSD, and WUC as follows:
2.2.4. Malondialdehyde (MDA) Content
The malondialdehyde (MDA) content was quantified using the protocol adapted from [21]. In brief, 30 mg of dried plant material was extracted in 1.5 mL of 0.1% trichloroacetic acid (TCA). The homogenate was then centrifuged (16,000× g for 10 min) to remove insoluble material. A 400 µL portion of the resulting supernatant was reacted with a 0.5% thiobarbituric acid (TBA) solution by heating at 95 °C, promoting the formation of a colored MDA-TBA adduct. Following cooling, the mixture was centrifuged again at 16,000× g for 10 min. The absorbance of the supernatant was measured at 532 nm and 600 nm using a Tecan Infinite 200 Pro, and MDA concentration was calculated and expressed as nanomoles per gram of dry weight using its extinction coefficient of 155 mM−1 cm−1.
2.2.5. Antioxidant Activity, Total Polyphenol Content, and Total Flavonoid Content
Following the lyophilization of barley leaves (Labogene Coolsafe 95-15 Pro, Allerød, Denmark), a co-extraction protocol was applied according to Major et al. [22]. Approximately 30 mg of the lyophilized leaves in 80% aqueous methanol was sonicated for 10 min, followed by centrifugation at 16,000 rpm for 10 min. The methanol extract was then used to quantify total phenols (TPC) and antioxidant activities (DPPH, FRAP, and ORAC).
The Folin–Ciocalteu method was used to quantify TPC. Briefly, 20 µL of methanolic extract was mixed with 140 µL of Folin–Ciocalteu reagent and 140 µL of 6% sodium carbonate, then incubated for 60 min at 25 °C. Absorbance was recorded at 750 nm using a Tecan Infinite 200 Pro. Results, based on a gallic acid calibration curve, are reported as mg gallic acid per gram dry weight.
Radical-scavenging activity was assessed using the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, following Brand-Williams et al. [23]. A 100 µL portion of methanolic extract was combined with 200 µL of 0.02 M DPPH solution and incubated for 30 min at 25 °C. Absorbance was measured at 517 nm, and DPPH radical scavenging capacity was determined from a Trolox calibration curve and expressed as µmol Trolox per gram of dry weight.
The FRAP assay was performed according to Benzie and Strain [24], with minor modifications. A 100 µL portion of methanolic extract was mixed with 200 µL of freshly prepared FRAP reagent and incubated for 10 min at 25 °C. Absorbance was measured at 593 nm, and antioxidant activity was expressed as µmol Trolox per gram of dry weight.
The ORAC assay was performed following Ou et al. [25]. Methanolic extract (37.5 µL) was added to 225 µL of 4 µM fluorescein solution and incubated for 30 min at 37 °C. Fresh AAPH (37.5 µL) was then added, and fluorescence was recorded for 120 min (excitation: 485 nm, emission: 528 nm) using a microplate reader Tecan Infinite 200 Pro. ORAC values were calculated against a Trolox standard curve and expressed as µmol Trolox equivalents (TE) per gram of dry weight.
2.3. Statistical Analysis
Statistical analysis of the data was performed using two-way Analysis of Variance (ANOVA) to evaluate the independent and interaction effects of the water regime and compost dose on the morpho-physiological and biochemical parameters of barley plants. The experiment was designed with four biological replicates for each treatment combination. Before analysis, the data were confirmed to meet the assumptions of normality and homogeneity of variance. When significant variations were detected (p < 0.05), Tukey’s post hoc test was applied to identify specific group differences. All statistical analyses were conducted using SPSS 25. All graphical representations were created using OriginPro 9.
3. Results
3.1. Growth Parameters and Pigment Content
Barley plants under water stress consistently showed a reduction in fresh biomass, dry biomass, and shoot length by 28.6%, 49.9%, and 20.45%, respectively, compared to well-watered plants (Figure 1). This finding highlights the significant negative impact of drought on barley growth, underscoring the necessity of effective mitigation strategies in arid regions.
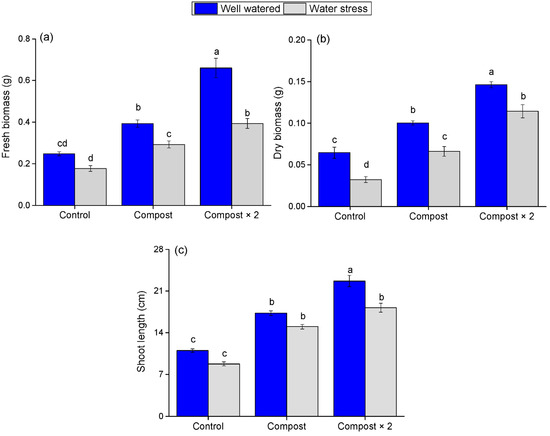
Figure 1.
Impact of water stress and compost application on barley growth parameters, (a) fresh biomass, (b) dry biomass, and (c) shoot length. Data are presented as mean ± standard error. Different letters (a, b, ...) indicate significant differences (p < 0.05) based on a post hoc analysis following a two-way ANOVA.
Furthermore, the application of olive pomace compost significantly mitigated these adverse effects, leading to a notable increase in all three parameters for both well-watered and water-stressed plants. Notably, while water stress negatively affected plants in all treatments, the fresh biomass of plants receiving the standard compost dose under drought conditions was comparable to that of unfertilized, well-watered plants. The two-way ANOVA revealed a significant main effect of compost (F = 82.631; p < 0.01) and water stress (F = 53.048; p < 0.01) for fresh biomass, but no significant interaction between the two factors (F = 9.226; p = 0.971, Table 2). This key finding highlights the compost’s profound ability to buffer against drought-induced growth losses. This improvement was particularly evident in the Compost × 2 treatment, where plants exhibited the highest fresh biomass, dry biomass, and shoot length. These results demonstrate that a higher concentration of olive pomace compost can provide superior benefits, likely by offering a more robust improvement in soil properties and nutrient availability.

Table 2.
Two-way ANOVA results for the effects of compost and water stress on barley morpho-physiological and biochemical parameters.
The data reveals that barley plants subjected to water stress show lower concentrations of pigments than well-watered plants (Figure 2, Controls), with significantly (F = 62.12, 19.696, and 34.403; p < 0.001, Table 2) lower levels of chlorophyll a (−16.9%), chlorophyll b (−52.16%) and carotenoids (−24.67%). This reduction in photosynthetic pigments is a classic physiological response to drought, as plants actively degrade chlorophyll to minimize oxidative damage and conserve water.
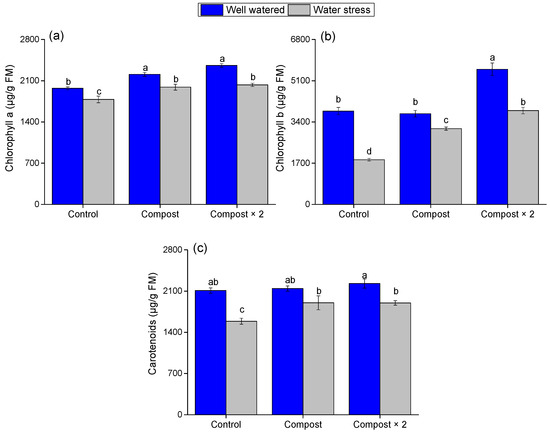
Figure 2.
Impact of water stress and compost application on (a) chlorophyll a, (b) chlorophyll b, and (c) carotenoid content in barley leaves. Data are presented as mean ± standard error. Different letters (a, b, ...) indicate significant differences (p < 0.05) based on a post hoc analysis following a two-way ANOVA.
Notably, under both conditions, barley plants grown in soil with added olive pomace compost have higher levels of chlorophyll a (F = 35.072, p < 0.001), chlorophyll b (F = 56.951, p < 0.001), and carotenoids than the control group. This finding suggests that the compost helped maintain the plant’s photosynthetic capacity. While the effect of compost on chlorophyll a was consistent across both water regimes (F = 1.769, p = 0.199), a significant interaction effect was found for chlorophyll b (F = 3.848, p = 0.041) and carotenoids (F = 0.478, p = 0.004). This effect was especially pronounced at double the recommended dose (Compost × 2), highlighting the dose-dependent benefits of the compost in enhancing the plant’s overall physiological health and drought resilience.
3.2. Water Status and MDA Content
Figure 3 shows how water stress significantly reduces relative water content (RWC) (Figure 3a) by 21.81% and increases water saturation deficit (WSD) (Figure 3b) in plants. The ANOVA test confirmed that the water stress factor had a highly significant effect on both RWC and WSD (F = 5.137, p = 0.043; F = 374.134, p < 0.0001, respectively). The application of compost, particularly at the double dose, effectively mitigated these effects, resulting in higher RWC and lower WSD under stress conditions. The significant interaction effect between compost and water stress for both RWC (F = 5.165, p = 0.024) and WSD (F = 108.056, p < 0.0001, Table 2) suggests that the compost improved the plant’s ability to maintain cellular hydration by enhancing the soil’s water-holding capacity, thereby enabling the plant to better regulate its water balance.
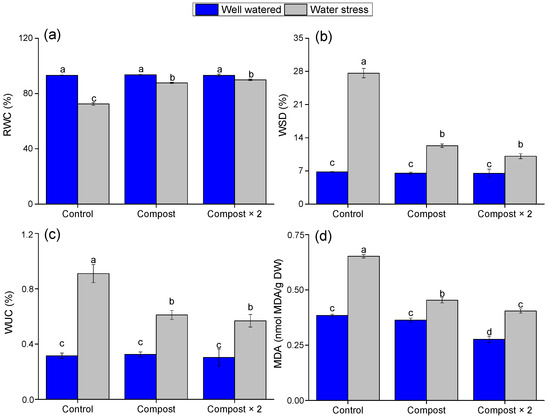
Figure 3.
Impact of water stress and compost application on (a–c) water status and (d) MDA content in barley plants. Data are presented as mean ± standard error. Different letters (a, b, ...) indicate significant differences (p < 0.05) based on a post hoc analysis following a two-way ANOVA.
Interestingly, while water-stressed control plants exhibited a high water uptake capacity (WUC) (Figure 3c), compost-treated plants showed lower WUC under stress conditions. This seemingly counterintuitive result is a key positive indicator. With a more consistent supply of moisture from the improved soil, compost-amended plants did not need to close their stomata as tightly to conserve water. The significant interaction between compost and water stress for WUC (F = 11.539, p < 0.001, Table 2) further confirms this, indicating a fundamental shift in how the plants manage water. Rather than struggling to cope with water deficit, the compost-treated plants were able to maintain stomatal opening to facilitate photosynthesis, leading to higher overall growth despite a lower WUC value.
Water stress also significantly increased malondialdehyde MDA content by 0.27 nmol/g d.w (Figure 3d); however, both compost treatments significantly (p < 0.001) reduced MDA levels by 0.20 nmol and 0.26 nmol/d.w under water stress, respectively, compared with the control group without compost. The significant main effect of compost (F = 153.316, p < 0.0001) and the significant interaction effect (F = 41.837, p < 0.0001, Table 2) indicate that compost application helped to alleviate oxidative damage in the plants, likely by providing nutrients that bolster their antioxidant defense systems.
3.3. Antioxidant Activities
The data show that water stress triggers a pronounced defensive response in barley (Figure 4). This stress led to a substantial increase in overall antioxidant capacity, with the water stress factor having a highly significant effect on DPPH, FRAP, ORAC, and TPC (p < 0.0001) values increasing by 12.30%, 55.18%, and 37.44%, respectively. This surge in antioxidant activity is a natural plant mechanism to combat the oxidative damage induced by drought. This increase was accompanied by a significant increase (p < 0.0001) in levels of protective phenolic compounds, as reflected by a 220.70% increase in TPC levels. Although compost-treated plants still enhanced their antioxidant defenses under water stress, the application of olive pomace compost effectively moderated this response, resulting in DPPH, TPC, FRAP, and ORAC levels lower than those of the stressed control group (Figure 4). The significant interaction effects for TPC, FRAP, and ORAC (p < 0.0001, p = 0.015, and p = 0.009, respectively; see Table 2) confirm that the compost’s mitigating effect on the antioxidant system is dependent on the water regime. This is a key finding, suggesting that the compost-treated plants were under less severe stress than the control group. Instead of needing to mount a robust, resource-intensive defense, they were better able to manage the stress from the outset, likely due to improved soil conditions and nutrient availability provided by the compost.
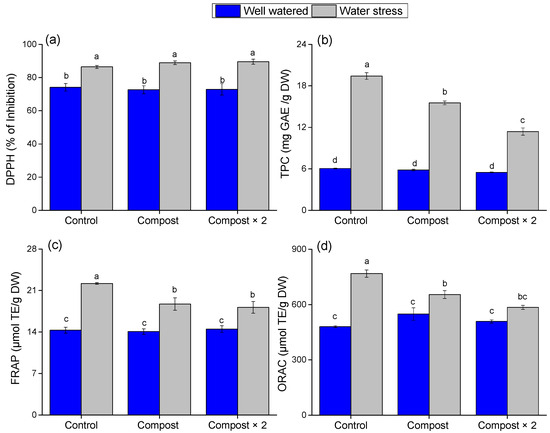
Figure 4.
Effect of water stress and compost application on (a) DPPH, (b) TPC, (c) FRAP, and (d) ORAC activity in barley plants. Data are presented as mean ± standard error. Different letters (a, b, ...) indicate significant differences (p < 0.05) based on a post hoc analysis following a two-way ANOVA.
4. Discussion
The present study investigated the physiological, biochemical, and growth responses of barley to water stress and the application of compost derived from olive pomace. The data (Figure 1) show how water stress leads to a significant reduction in key growth parameters, including fresh biomass, dry biomass, and shoot length. This response is well documented in cereal crops, as water scarcity limits cell expansion, turgor pressure, and overall metabolic activity [26]. The mitigating effect of adding olive pomace compost was evident for all these parameters, with a clear dose-dependent trend. Plants grown with the double dose of compost exhibited the greatest biomass and shoot length under both well-watered and water-stressed conditions with more than 50% compared to control plants. This improvement can be attributed to the compost’s ability to improve soil structure, increase nutrient availability, and enhance the soil’s water-holding capacity [27], creating a more favorable environment for root growth and nutrient uptake [28]. Compost application leads to outcomes consistent with its direct effects on soil properties, which in turn support more resilient plant growth. Beyond its physical effects, compost acts as a vital vector for delivering essential nutrients—the fundamental building blocks for plant biomass. It provides a slow-release source of key macronutrients such as nitrogen (N), phosphorus (P), and potassium (K), all of which are critical for robust plant growth [29]. For instance, nitrogen is a core constituent of chlorophyll and amino acids, which are the structural components of proteins essential for cell division and overall physiological development [30]. The observed increase in photosynthetic pigment content in compost-amended plants can be directly attributed to the availability of other essential micronutrients, including magnesium (Mg) and iron (Fe) [31]. These elements function as critical cofactors in chlorophyll synthesis and are integral to the electron transport chain, a key process in photosynthesis. Consequently, the enhanced biomass and shoot length observed in compost-treated plants are a direct result of this improved nutrient availability, in addition to better water relations.
The beneficial effects of compost were also evident in the plants’ photosynthetic machinery (Figure 2). Water stress led to a marked decline in the concentrations of chlorophyll a, chlorophyll b, and carotenoids. This reduction in photosynthetic pigments is a classic stress symptom, as plants reduce their photosynthetic surface area and efficiency to conserve water [32,33,34]. For example, Wasaya et al. [35] show that drought significantly reduces net photosynthesis, stomatal conductance, relative water content, 100-grain weight, and grain yield in bread wheat (Triticum aestivum L.). However, compost application helped preserve these pigments, with treated plants maintaining significantly higher chlorophyll and carotenoid levels than the stressed control group. This finding suggests that the compost improved the soil’s moisture and nutrient availability, enabling plants to sustain a more robust photosynthetic apparatus, which is critical for energy production and growth even under adverse conditions [36]. In addition, nutrients supplied by compost, such as nitrogen (N), are crucial for synthesizing chlorophyll and enzymes within the chloroplasts, the sites of photosynthesis [37]. A stable supply of water, facilitated by compost’s ability to improve soil water retention, is equally vital. Adequate hydration maintains the turgor pressure in plant cells, keeping leaves fully exposed to sunlight and allowing stomata to remain open for the continuous uptake of CO2 needed for the Calvin cycle [38,39,40]. Similarly, El Amerany et al. [41] show that the application compost on tomato plant (Solanum lycopersicum) improve chlorophyll fluorescence (Fv/Fm) and leaf area by 17% and 393%, respectively, by comparison with control plants.
The internal physiological data provide a deeper understanding of the mechanisms behind the observed growth patterns (Figure 3). Water stress severely compromised the plant’s water status, resulting in a decrease in the RWC and a corresponding increase in WSD. The elevated WUC further indicates that the stressed plants were struggling to maintain an efficient water balance. In addition, the most critical finding was the significant increase in MDA levels under water stress, which serves as a key biomarker for oxidative stress and cellular membrane damage [42].
The compost application effectively countered these adverse effects. Plants treated with compost demonstrated better water relations, with higher RWC and lower WSD and WUC, indicating that the compost improved the soil’s capacity to store water and make it available to the plants [43]. Furthermore, the compost significantly reduced the levels of MDA, both in well-watered and water-stressed conditions (<0.50 nmol MDA/g DW), which suggests that the compost not only improved water availability but also provided protective compounds or nutrients that enhanced the plant’s ability to manage stress and protect its cellular integrity [44]. The presence of phenolic compounds and other antioxidants in the compost itself may have played an additional, direct role in reducing oxidative stress and lipid peroxidation, a key point that distinguishes this study from those using standard composts [44]. Additionally, the findings agree with those of Filippou et al. [45] and Wang et al. [46], who observed that water stress increased lipid peroxidation in Medicago truncatula and Medicago sativa roots. On the other hand, the results of Abdou et al. [47] demonstrate that applying compost improves the content of osmoprotectants in Nigella sativa plants under moderate drought stress (I50), including total free amino acids (135.5 mg·g−1), total soluble sugars (377.66 mg·g−1), carbohydrates (2.76%), and proline (0.73 µmol·g−1). The accumulation of osmoprotectants helps the plants to cope with harmful environmental conditions like drought stress by maintaining the cell’s osmotic balance and protecting cellular structures like membranes and proteins from damage. Which allows metabolic processes to continue under stressful conditions.
Under water stress, the plants’ antioxidant capacity was significantly upregulated, a key defense mechanism to counteract the damaging effects of ROS. Many studies report that abiotic stress, such as salinity and drought, increases ROS (reactive oxygen species) production [48,49]. This causes damage to cell structures, DNA, nucleic acids, and proteins, and leads to the peroxidation of membrane lipids and ultimately, cellular death [50]. Consequently, this results in a decline in plant growth and development [51].
This enhanced antioxidant capacity was evidenced by a marked increase in DPPH, FRAP, and ORAC values. Moreover, a significant accumulation of protective compounds was observed, with total phenolic content (TPC) showing a substantial increase. The coordinated activation of both enzymatic and non-enzymatic antioxidant systems is a well-documented plant strategy to maintain cellular homeostasis and mitigate oxidative damage caused by stress [52]. For instance, reduced RWC and increased WSD and WUC compromise plant water status, prompting stomatal closure that limits CO2 uptake and photosynthesis. Excess light energy absorbed under these conditions results in the overproduction of ROS, which can severely damage cellular components [53]. The resulting oxidative stress causes cellular damage, a condition indicated by a significant increase in MDA levels (Figure 3). In a defensive response, the plant activates its antioxidant system, increasing the production of protective compounds (high TPC) and boosting its overall antioxidant capacity (increased DPPH, FRAP, and ORAC). This energy-demanding response diverts resources away from growth and photosynthesis, ultimately resulting in the stunted growth and reduced pigment concentrations observed in the water-stressed plants (Figure 1 and Figure 2).
The application of olive pomace compost effectively breaks this negative cycle. By enhancing the soil’s water-holding capacity, the compost improves the plant’s water status, allowing it to maintain better stomatal regulation and continue efficient photosynthesis, reducing the overproduction of ROS, leading to less oxidative stress and significantly lower MDA levels.
With the initial stress mitigated, the plant does not need to mount such an extreme antioxidant defense, which is reflected in the moderated levels of TPC, DPPH, FRAP, and ORAC in the compost-treated, stressed plants. This interpretation is quantitatively supported by the inverse relationship between the moderated antioxidant levels and the reduced MDA content, providing strong evidence of a lower oxidative load rather than an inadequate defense. Furthermore, our soil analysis data (pH 6.93, 2.58% organic matter, and sufficient P, N, and K) rules out a nutritional deficiency as a cause for the lower antioxidant activity. By lessening the stress burden, the compost allows the plant to reallocate energy and resources back to growth and other vital processes, resulting in the improved biomass and shoot length observed.
This study provides compelling evidence that olive pomace compost improves the growth and drought resilience of barley plants. However, it is important to acknowledge certain limitations, as the use of a greenhouse did not ensure fully controlled conditions. The observed results could have been influenced by natural fluctuations in key variables like solar radiation, temperature, and humidity, which were not kept constant. Therefore, while the results are promising, future research should aim to validate these findings under strictly controlled laboratory conditions to isolate the precise effects of the compost. Additionally, multi-season field trials are recommended to assess the long-term impacts on crop productivity and soil health under real-world agricultural conditions.
Additionally, while our study elucidated the physiological and biochemical mechanisms of the compost’s effects, the underlying molecular mechanisms remain to be fully explored. We observed significant changes in antioxidant and phenolic compounds content, but the specific genes and proteins that regulate these responses are not yet identified. Future research should utilize advanced molecular techniques, such as transcriptome and proteome analysis, to investigate gene expression and protein-level changes in response to compost application under stress. This would provide a more holistic understanding of how olive pomace compost enhances plant resilience at the genetic and cellular levels. Furthermore, studies on the composition of the compost itself could better isolate which components are most beneficial and how they interact with the plant and soil.
The findings of this study on the efficacy of olive pomace compost for enhancing barley drought tolerance hold significant practical implications. Scalability is highly feasible, as olive pomace is an abundant and often underutilized byproduct of the olive oil industry. Repurposing this agricultural waste into a valuable soil amendment presents an economically viable solution for both waste management and crop production, particularly in arid and semi-arid regions where these industries often overlap.
5. Conclusions
The findings from this study confirm that olive pomace compost serves as a highly effective soil amendment for enhancing drought tolerance in barley. Its addition to the soil significantly enhanced the plant’s physical growth and photosynthetic efficiency. At the physiological level, it improved the plant’s water relations and mitigated cellular damage from oxidative stress. Importantly, the dose-dependent nature of these benefits was a key finding; a double dose of compost provided a superior, more robust effect on all measured parameters. Furthermore, the compost regulated the plant’s antioxidant defense system, allowing it to manage stress more efficiently. These comprehensive effects suggest that olive pomace compost provides a dual benefit—it not only improves soil conditions but also directly enhances the plant’s intrinsic resilience, positioning it as a superior bio-stimulant for stress-prone environments. These effects underscore the potential of repurposing olive pomace waste as a valuable agricultural input to enhance crop resilience in arid and semi-arid regions.
Author Contributions
Conceptualization, M.B.; methodology, T.K.K. and M.B.; software, Q.J.; validation, N.M., D.B. and D.H.; formal analysis, N.M. and D.B.; writing—original draft preparation, M.B.; writing—review and editing, S.G.B., D.H. and M.Č.; visualization, Q.J.; supervision, D.B. and M.Č.; project administration, M.Č.; funding acquisition, M.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation under the project numbers HRZZ-IPS-2022-02-2099 and HRZZ-MOBDOL-2023-08-5800.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors would like to express their sincere gratitude to Igor Pasković and the technicians at the Institute for Agriculture and Tourism, Poreč, for their invaluable assistance and patience. Their support was crucial during the preparation of the olive pomace compost and the transportation of the experimental pots to the greenhouse.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| TPC | Total Phenolic Content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| ORAC | Oxygen Radical Absorbance Capacity |
| MDA | Malondialdehyde |
| RWC | Relative Water Content |
| WSD | Water Saturation Deficit |
| WUC | Water Uptake Capacity |
| ROS | Reactive Oxygen Species |
| FC | Field Capacity |
References
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Gous, P.W.; Warren, F.; Mo, O.W.; Gilbert, R.G.; Fox, G.P. The effects of variable nitrogen application on barley starch structure under drought stress. J. Inst. Brew. 2015, 121, 502–509. [Google Scholar] [CrossRef]
- Högy, P.; Poll, C.; Marhan, S.; Kandeler, E.; Fangmeier, A. Impacts of temperature increase and change in precipitation pattern on crop yield and yield quality of barley. Food Chem. 2013, 136, 1470–1477. [Google Scholar] [CrossRef]
- Tricase, C.; Amicarelli, V.; Lamonaca, E.; Rana, R.L. Economic analysis of the barley market and related uses. Grasses Food Feed 2018, 10, 25–46. [Google Scholar]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Yu, X.; Chen, M.; Lu, W.; Wu, Y.; Xiong, F. Novel insights into the effect of drought stress on the development of root and caryopsis in barley. PeerJ 2020, 8, e8469. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajlouni, Z.I.; Al-Abdallat, A.M.; Al-Ghzawi, A.L.A.; Ayad, J.Y.; Abu Elenein, J.M.; Al-Quraan, N.A.; Baenziger, P.S. Impact of pre-anthesis water deficit on yield and yield components in barley (Hordeum vulgare L.) plants grown under controlled conditions. Agronomy 2016, 6, 33. [Google Scholar] [CrossRef]
- Grašič, M.; Dobravc, M.; Golob, A.; Vogel-Mikuš, K.; Gaberščik, A. Water shortage reduces silicon uptake in barley leaves. Agric. Water Manag. 2019, 217, 47–56. [Google Scholar] [CrossRef]
- Robertson, B.C.; He, T.; Li, C. The Genetic Control of Stomatal Development in Barley: New Solutions for Enhanced Water-Use Efficiency in Drought-Prone Environments. Agronomy 2021, 11, 1670. [Google Scholar] [CrossRef]
- Gous, P.W.; Gilbert, R.G.; Fox, G.P. Drought-proofing barley (Hordeum vulgare) and its impact on grain quality: A review. J. Inst. Brew. 2015, 121, 19–27. [Google Scholar] [CrossRef]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite Profiling of Barley Grains Subjected to Water Stress: To Explain the Genotypic Difference in Drought-Induced Impacts on Malting Quality. Front. Plant Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 162024. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K. Enhancing Soil Physical Quality with Compost Amendments: Effects of Particle Size and Additives. Agronomy 2025, 15, 458. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Mosnegutu, E.; Tomozei, C.; Chitimus, D.; Irimia, O. Assessment of Manure Compost Used as Soil Amendment—A Review. Processes 2023, 11, 1167. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Qi, Y.; Shahid, M.; Hussain, S.; Masood, H.A.; Xu, L.; Ali, H.M.; Negm, S.F.A.; Yao, Y.; et al. Fertilization of Microbial Composts: A Technology for Improving Stress Resilience in Plants. Plants 2023, 12, 3550. [Google Scholar] [CrossRef] [PubMed]
- Bouhadi, M.; Cherifi, O.; Bahammou, N.; Cherifi, K.; Talbi, M.; Elkouali, M.H.; Fougrach, H. The effect of Enteromorpha intestinalis and Corallina elongata on physiological parameters of Zea mays L. Arab Gulf J. Sci. Res. 2021, 39, 303–313. [Google Scholar] [CrossRef]
- Sirousmehr, A.; Arbabi, J.; Asgharipour, M.R. Effect of drought stress levels and organic manures on yield, essential oil content and some morphological characteristics of sweet basil (Ocimum basilicum). Adv. Environ. Biol. 2014, 8, 880–885. [Google Scholar]
- Bouhadi, M.; El Kouali, M.H.; Samir, K.; Elbouhmadi, K.; Talbi, M.; Fougrach, H. Exogenous Application of Thiamine and Nicotinic Acid Improves Tolerance and Morpho-physiological Parameters of Lens culinaris Under Lead (Pb) Exposure. J. Plant Growth Regul. 2024, 43, 4185–4198. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Major, N.; Perković, J.; Palčić, I.; Bažon, I.; Horvat, I.; Ban, D.; Goreta Ban, S. The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent. Antioxidants 2022, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Noor, R.S.; Hussain, F.; Abbas, I.; Umair, M.; Sun, Y. Effect of compost and chemical fertilizer application on soil physical properties and productivity of sesame (Sesamum Indicum L.). Biomass Conv. Bioref. 2023, 13, 905–915. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. Control. Release Fertil. Sustain. Agric. 2020, 13, 231–245. [Google Scholar] [CrossRef]
- Sandhu, N.; Sethi, M.; Kumar, A.; Dang, D.; Singh, J.; Chhuneja, P. Biochemical and Genetic Approaches Improving Nitrogen Use Efficiency in Cereal Crops: A Review. Front. Plant Sci. 2021, 12, 657629. [Google Scholar] [CrossRef]
- Ikan, C.; Soussani, F.-E.; Ouhaddou, R.; Ech-Chatir, L.; Errouh, F.; Boutasknit, A.; Assouguem, A.; Ali, E.A.; Ullah, R.; Ait Barka, E.; et al. Use of Biofertilizers as an Effective Management Strategy to Improve the Photosynthetic Apparatus, Yield, and Tolerance to Drought Stress of Drip-Irrigated Wheat in Semi-Arid Environments. Agronomy 2024, 14, 1316. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Khanna, K.; Bhardwaj, R. Scrutinizing the impact of water deficit in plants: Transcriptional regulation, signaling, photosynthetic efficacy, and management. Physiol. Plant. 2021, 172, 935–962. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Lamsaadi, N.; Bouhadi, M.; Abchir, O.; Chtita, S.; Samir, K.; El Rasafi, T.; Ghoulam, C.; Farissi, M. Uptake and competition between cadmium nanoparticles and essential nutrients (Fe, Mg and Mn) in Phaseolus vulgaris L. using a molecular docking approach. Euro-Mediterr. J. Environ. Integr. 2025, 1–11. [Google Scholar] [CrossRef]
- Falcioni, R.; de Oliveira, C.A.; Vedana, N.G.; Mendonça, W.A.; Gonçalves, J.V.F.; da Silva Haubert, D.d.F.; de Matos, D.H.S.; Reis, A.S.; Antunes, W.C.; Crusiol, L.G.T.; et al. Progressive Water Deficit Impairs Soybean Growth, Alters Metabolic Profiles, and Decreases Photosynthetic Efficiency. Plants 2025, 14, 2615. [Google Scholar] [CrossRef]
- Wasaya, A.; Manzoor, S.; Yasir, T.A.; Sarwar, N.; Mubeen, K.; Ismail, I.A.; Raza, A.; Rehman, A.; Hossain, A.; EL Sabagh, A. Evaluation of Fourteen Bread Wheat (Triticum aestivum L.) Genotypes by Observing Gas Exchange Parameters, Relative Water and Chlorophyll Content, and Yield Attributes under Drought Stress. Sustainability 2021, 13, 4799. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Zhou, Q.; Wang, X.; Song, S.; Dong, S. Physiological Response of Soybean Plants to Water Deficit. Front. Plant Sci. 2022, 12, 809692. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, M.; Cheng, Z.; Yang, L. Effects of Nitrogen Deficiency on the Photosynthesis, Chlorophyll a Fluorescence, Antioxidant System, and Sulfur Compounds in Oryza sativa. Int. J. Mol. Sci. 2024, 25, 10409. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Cheddadi, I.; Landrein, B.; Long, Y. Revisiting the relationship between turgor pressure and plant cell growth. New Phytol. 2023, 238, 62–69. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2023, 13, 1808. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Padilla, Y.G.; Álvarez, S.; Calatayud, Á.; Colmenero-Flores, J.M.; Gómez-Bellot, M.J.; Hernández, J.A.; Martínez-Alcalá, I.; Penella, C.; Pérez-Pérez, J.G.; et al. Advancements in Water-Saving Strategies and Crop Adaptation to Drought: A Comprehensive Review. Physiol. Plant. 2025, 177, e70332. [Google Scholar] [CrossRef]
- El Amerany, F.; Rhazi, M.; Wahbi, S.; Taourirte, M.; Meddich, A. The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci. Hortic. 2019, 261, 109015. [Google Scholar] [CrossRef]
- Mohideen, K.; Sudhakar, U.; Balakrishnan, T.; Almasri, M.A.; Al-Ahmari, M.M.; Al Dira, H.S.; Suhluli, M.; Dubey, A.; Mujoo, S.; Khurshid, Z.; et al. Malondialdehyde, an Oxidative Stress Marker in Oral Squamous Cell Carcinoma—A Systematic Review and Meta-Analysis. Curr. Issues Mol. Biol. 2021, 43, 1019–1035. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.Y.; Sayre, J.M.; Schmidt, R.; Fonte, S.J.; Rodrigues, J.L.; Scow, K.M. Compost amendment maintains soil structure and carbon storage by increasing available carbon and microbial biomass in agricultural soil—A six-year field study. Geoderma 2022, 427, 116117. [Google Scholar] [CrossRef]
- Lerma-Moliz, R.; López-González, J.; Suárez-Estrella, F.; Martínez-Gallardo, M.; Jurado, M.; Estrella-González, M.; Toribio, A.; Jiménez, R.; López, M. Antioxidant and biofertilizing effect of compost extracts on horticultural crops to minimize the use of agrochemicals. Environ. Technol. Innov. 2024, 36, 103776. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal. Behav. 2011, 6, 270–277. [Google Scholar] [CrossRef]
- Wang, Y.; Long, S.; Zhang, J.; Wang, P.; Zhao, L. Evaluation of Growth, Physiological, and Biochemical Responses of Different Medicago sativa L. Varieties Under Drought Stress. Plants 2024, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Abdou, N.M.; Roby, M.H.; Abdulkreem, A.; Elkelish, A.; Sayed, A.A.; Alharbi, B.M.; Mahdy, H.A.; Badawy, A.I. Compost Improving Morphophysiological and Biochemical Traits, Seed Yield, and Oil Quality of Nigella sativa under Drought Stress. Agronomy 2023, 13, 1147. [Google Scholar] [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Han, C.; Wang, S.; Bai, Y.; Song, P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Bouhadi, M.; El Abbassi, A.; El Hajjouji, H.; El Kouali, M.H.; Talbi, M.; Fougrach, H. The effect of chromium (VI) on stress related genes expression in the roots of Vicia faba L. Biologia 2025, 80, 1967–1976. [Google Scholar] [CrossRef]
- Bouhadi, M.; Abchir, O.; Yamari, I.; El Hamsas El Youbi, A.; Azgaoui, A.; Chtita, S.; El Hajjouji, H.; El Kouali, M.; Talbi, M.; Fougrach, H. Genotoxic effects and mitosis aberrations of chromium (VI) on root cells of Vicia faba and its molecular docking analysis. Plant Physiol. Biochem. 2024, 207, 108361. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Zeng, Y.; Yang, D.; Zhou, Z.; Zheng, Z.; Xiao, P.; Ding, X.; Li, Q.; Chen, J.; et al. Nanotherapies Based on ROS Regulation in Oral Diseases. Adv. Sci. 2025, 12, 2409087. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).