Sensor-Based Analysis of Upper Limb Motor Coordination After Stroke: Insights from EMG, ROM, and Motion Data During the Wolf Motor Function Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
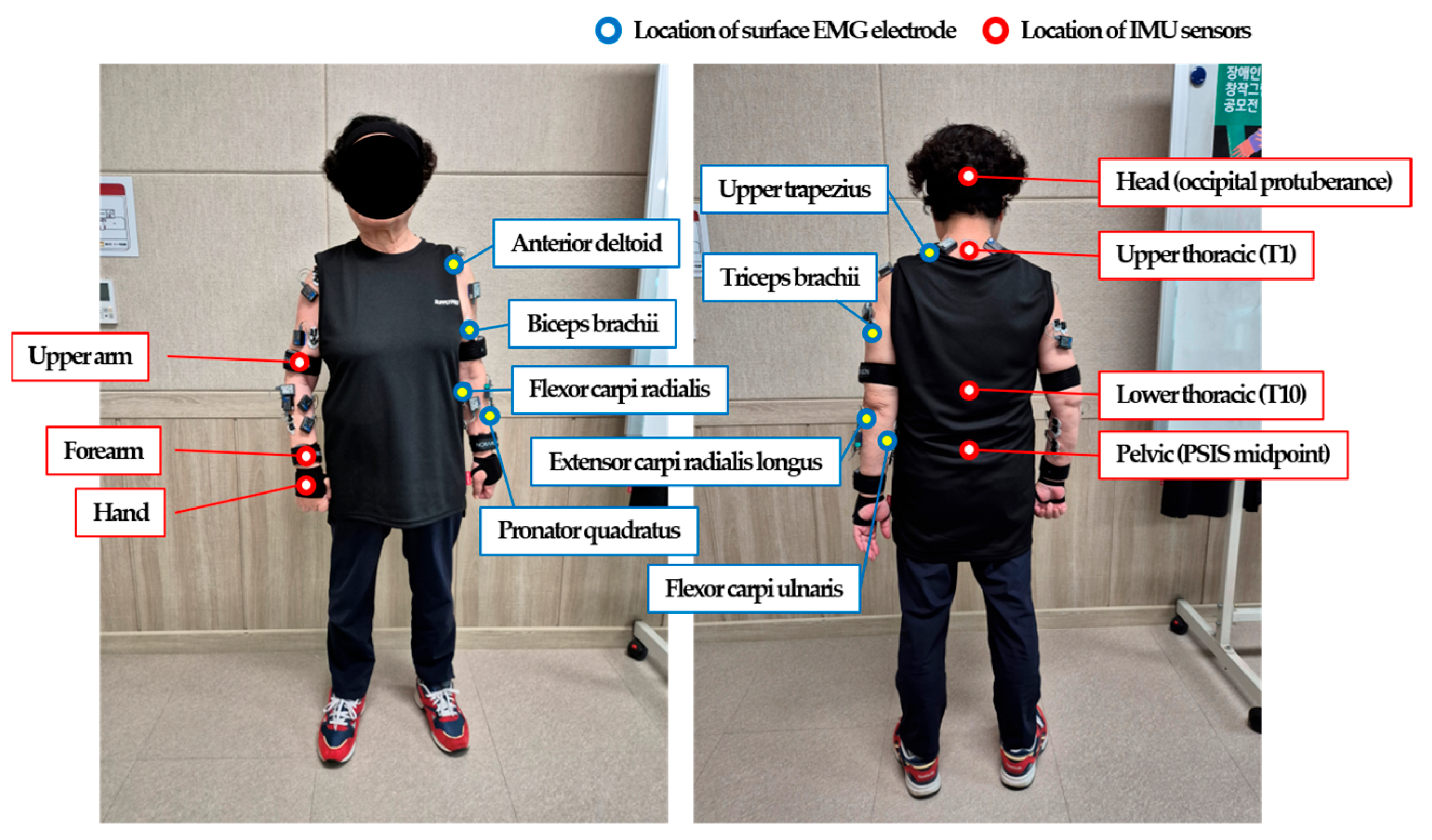
2.2. Experimental Procedure
2.3. Data Analysis
3. Results
3.1. Muscle Activation
3.2. Joint Mobility
3.3. Movement Amplitude
3.4. Correlation Analysis
3.4.1. Correlations Among EMG Variables
3.4.2. Correlations Among ROM Variables
3.4.3. Correlations Among RMS Variables
3.4.4. Correlations Among EMG, ROM, and RMS Variables
4. Discussion
5. Conclusions
- Practical implications: This sensor-based, domain-specific approach quantifies both absolute impairments (e.g., 20–30% lower EMG activity, 15–25° ROM reduction) and inter-modality associations. As a result, it enables precise detection of deficits that conventional WMFT scoring may overlook. It further supports individualized rehabilitation strategies that address proximal compensation and distal coordination together.
- Scientific implications: Integrating EMG, ROM, and RMS measures offers a robust, multidimensional framework for quantifying motor coordination after stroke. This approach also reveals mechanisms underlying compensatory strategies. Together, these insights advance understandings of motor control deficits and guide the development of evidence-based rehabilitation protocols.
- Societal implications: This approach allows earlier detection and more precise targeting of persistent impairments, even long after stroke onset. These benefits may improve long-term functional independence. They may also help reduce disability and ease the societal and economic burden of chronic stroke.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langhorne, F.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Roby-Brami, A.; Jarrasse, N.; Parry, R. Impairment and compensation in dexterous upper-limb function after stroke. From the direct consequences of pyramidal tract lesions to behavioral involvement of both upper-limbs in daily activities. Front. Hum. Neurosci. 2021, 15, 662006. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, G.; Zhang, X.; Huang, S.; Zheng, H.; Ma, K.; Xie, L. Detecting compensatory movements of stroke survivors using pressure distribution data and machine learning algorithms. J. Neuroeng. Rehabil. 2019, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Martino Cinnera, A.; Picerno, P.; Bisirri, A.; Koch, G.; Morone, G.; Vannozzi, G. Upper limb assessment with inertial measurement units according to the international classification of functioning in stroke: A systematic review and correlation meta-analysis. Top. Stroke Rehabil. 2024, 31, 66–85. [Google Scholar] [CrossRef]
- Repnik, E.; Puh, U.; Goljar, N.; Munih, M.; Mihelj, M. Using inertial measurement units and electromyography to quantify movement during action research arm test execution. Sensors 2018, 18, 2767. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; MacDonald, J.R.; Reisman, D.S.; Boyd, L.; Kimberley, T.J.; Schindler-Ivens, S.M.; Hornby, T.G.; Ross, S.A.; Scheets, P.L. Observation of amounts of movement practice provided during stroke rehabilitation. Arch. Phys. Med. Rehabil. 2009, 90, 1692–1698. [Google Scholar] [CrossRef]
- Schwarz, A.; Kanzler, C.M.; Lambercy, O.; Luft, A.R.; Veerbeek, J.M. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke 2019, 50, 718–727. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, A.E.P.; Durairaj, P.; Tan, K.K.; Verawaty, V. Development of a compensation-aware virtual rehabilitation system for upper extremity rehabilitation in community-dwelling older adults with stroke. J. Neuroeng. Rehabil. 2023, 20, 56. [Google Scholar] [CrossRef]
- Villepinte, C.; Verma, A.; Dimeglio, C.; De Boissezon, X.; Gasq, D. Responsiveness of kinematic and clinical measures of upper-limb motor function after stroke: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2021, 64, 101366. [Google Scholar] [CrossRef]
- Konieczny, M.; Pakosz, P.; Domaszewski, P.; Błaszczyszyn, M.; Kawala-Sterniuk, A. Analysis of upper limbs target-reaching movement and muscle co-activation in patients with first time stroke for rehabilitation progress monitoring. Appl. Sci. 2022, 12, 1551. [Google Scholar] [CrossRef]
- Bhagubai, M.M.; Wolterink, G.; Schwarz, A.; Held, J.P.; Van Beijnu, B.J.F.; Veltink, P.H. Quantifying pathological synergies in the upper extremity of stroke subjects with the use of inertial measurement units: A pilot study. IEEE J. Transl. Eng. Health Med. 2020, 9, 2100211. [Google Scholar] [PubMed]
- Wang, X.; Zhang, J.; Xie, S.Q.; Shi, C.; Li, J.; Zhang, Z.Q. Quantitative upper limb impairment assessment for stroke rehabilitation: A review. IEEE Sens. J. 2024, 24, 7432–7447. [Google Scholar] [CrossRef]
- Sheng, B.; Chen, X.; Cheng, J.; Zhang, Y.; Tao, J.; Duan, C. A novel scoring approach for the Wolf Motor Function Test in stroke survivors using motion-sensing technology and machine learning: A preliminary study. Comput. Methods Programs Biomed. 2024, 243, 107887. [Google Scholar]
- Schwarz, A.; Bhagubai, M.M.; Nies, S.H.; Held, J.P.; Vltink, P.H.; Buurke, J.H.; Luft, A.R. Characterization of stroke-related upper limb motor impairments across various upper limb activities by use of kinematic core set measures. J. Neuroeng. Rehabil. 2022, 19, 2. [Google Scholar]
- Parnandi, A.; Uddin, J.; Nilsen, D.M.; Schambra, H. Pragmatic classification of movement primitives for stroke rehabilitation. arXiv 2019, arXiv:1902.08697. [Google Scholar] [CrossRef]
- Zaidi, K.F.; Harris-Love, M. A novel Procrustes analysis method to quantify multi-joint coordination of the upper extremity after stroke. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023. [Google Scholar]
- Nordin, N.; Xie, S.Q.; Wünsche, B. Assessment of movement quality in robot-assisted upper limb rehabilitation after stroke: A review. J. Neuroeng. Rehabil. 2014, 11, 137. [Google Scholar] [CrossRef]
- Alt Murphy, M.; Häger, C.K. Kinematic analysis of the upper extremity after stroke—How far have we reached and what have we grasped? Phys. Ther. Rev. 2015, 20, 137–155. [Google Scholar] [CrossRef]
- Zaidi, K.F.; Harris-Love, M. Upper extremity kinematics: Development of a quantitative measure of impairment severity and dissimilarity after stroke. PeerJ 2023, 11, e16374. [Google Scholar] [CrossRef]
- Goffredo, M.; Proietti, S.; Pournajaf, S.; Galafate, D.; Cioeta, M.; Le Pera, D.; Posteraro, F.; Franceschini, M. Baseline robot-measured kinematic metrics predict discharge rehabilitation outcomes in individuals with subacute stroke. Front. Bioeng. Biotechnol. 2022, 10, 1012544. [Google Scholar] [CrossRef]
- Hwang, D.; Shin, J.H.; Kwon, S. Kinematic assessment to measure change in impairment during active and active-assisted type of robotic rhhabilitation for patients with stroke. Sensors 2021, 21, 7055. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Catlin, P.A.; Ellis, M.; Archer, A.L.; Morgan, B.; Piacentino, A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001, 32, 1635–1639. [Google Scholar] [CrossRef]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook, E.W., III; Taub, E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef]
- Arya, K.N.; Pandian, S.; Verman, R.; Garg, R.K. Movement therapy induced neural reorganization and motor recovery in stroke: A review. J. Bodyw. Mov. Ther. 2011, 15, 528–537. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of robot-assisted therapy on upper limb recovery after stroke: A systematic review. Neurorehabilit. Neural Repair 2008, 22, 111–121. [Google Scholar] [CrossRef]
- Bogard, K.; Wolf, S.; Zhang, Q.; Thompson, P.; Morris, D.; Nichols-Larsen, D. Can the wolf motor function test be streamlined? Neurorehabilit. Neural Repair 2009, 23, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Han, S.; Leigh, J.H.; Bang, M.S. Smartwatch-based functional assessment for upper extremity impairment after musculoskeletal injuries: A pilot study. Hong Kong J. Occup. Ther. 2024, 37, 31–41. [Google Scholar] [CrossRef]
- Woodbury, M.; Velozo, C.A.; Thompson, P.A.; Light, K.; Uswatte, G.; Taub, E.; Winstein, C.J.; Morris, D.; Blanton, S.; Nichols-Larsen, D.S.; et al. Measurement structure of the Wolf Motor Function Test: Implications for motor control theory. Neurorehabilit. Neural Repair 2020, 24, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Feldner, H.A.; Papazian, C.; Peters, K.M.; Creutzfeldt, C.J.; Steele, K.M. Clinical use of surface electromyography to track acute upper extremity muscle recovery after stroke: A descriptive case study of a single patient. Appl. Syst. Innov. 2021, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, S.H.; Xu, D.; Ren, Y.; Lee, S.J.; Zhang, L.Q. EMG-based continuous and simultaneous estimation of arm kinematics in able-bodied individuals and stroke survivors. Front. Neurosci. 2017, 11, 480. [Google Scholar] [CrossRef]
- Lv, W.; Liu, K.; Zhou, P.; Huang, F.; Lu, Z. Surface EMG analysis of weakness distribution in upper limb muscles post-stroke. Front. Neurol. 2023, 14, 1135564. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, L.; Jin, M.; Liao, D.; Privitera, A.J.; Wong, A.Y.; Fong, G.C.H.; Bao, S.C.; Sun, R. Assessing stroke-induced abnormal muscle coactivation in the upper limb using the surface EMG co-contraction Index: A systematic review. J. Electromyogr. Kinesiol. 2025, 81, 102985. [Google Scholar] [CrossRef]
- Clancy, E.A.; Morin, E.L.; Merletti, R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J. Electromyogr. Kinesiol. 2002, 12, 1–16. [Google Scholar] [CrossRef]
- Favata, A.; Gallart-Agut, R.; Pàmies-Vilà, R.; Torras, C.; Font-Llagunes, J.M. IMU-based systems for upper limb kinematic analysis in clinical applications: A systematic review. IEEE Sens. J. 2024, 24, 28576–28594. [Google Scholar] [CrossRef]
- Fang, Z.; Woodford, S.; Senanayake, D.; Ackland, D. Conversion of upper-limb inertial measurement unit data to joint angles: A systematic review. Sensors 2023, 23, 6535. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Yao, J.; Dewald, J.P. The impact of shoulder abduction loading on volitional hand opening and grasping in chronic hemiparetic stroke. Neurorehabilit. Neural Repair 2017, 31, 521–529. [Google Scholar] [CrossRef]
- Coscia, M.; Cheung, V.C.; Tropea, P.; Koenig, A.; Monaco, V.; Bennis, C.; Micera, S.; Bonato, P. The effect of arm weight support on upper limb muscle synergies during reaching movements. J. Neuroeng. Rehabil. 2014, 11, 22. [Google Scholar] [CrossRef]
- Leib, R.; Karniel, A.; Nisky, I. The effect of force feedback delay on stiffness perception and grip force modulation during tool-mediated interaction with elastic force fields. J. Neurophysiol. 2015, 113, 3076–3089. [Google Scholar] [CrossRef]
- Levin, M.F.; Liebermann, D.G.; Parmet, Y.; Berman, S. Compensatory versus noncompensatory shoulder movements used for reaching in stroke. Neurorehabilit. Neural Repair 2016, 30, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.D.; Acosta, A.M.; Yao, J.; Dewald, J.P. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp. Brain Res. 2007, 176, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, P. Myoelectric pattern identification of stroke survivors using multivariate empirical mode decomposition. J. Healthc. Eng. 2014, 5, 261–274. [Google Scholar] [CrossRef]
- Xie, Q.; Sheng, B.; Huang, J.; Zhang, Q.; Zhang, Y. A pilot study of compensatory strategies for reach-to-grasp-pen in patients with stroke. Appl. Bionics Biomech. 2022, 2022, 6933043. [Google Scholar] [CrossRef]
- Pregnolato, G.; Severini, G.; Maistrello, L.; Rimini, D.; Lencioni, T.; Carpinella, I.; Ferrarin, M.; Jonsdottir, J.; Cheung, V.C.; Turolla, A. Muscle synergy analysis for clinical characterization of upper limb motor recovery after stroke. Arch. Phys. Med. Rehabil. 2025. [CrossRef]
- Phan, T.; Nguyen, H.; Vermillion, B.C.; Kamper, D.G.; Lee, S.W. Abnormal proximal-distal interactions in upper-limb of stroke survivors during object manipulation: A pilot study. Front. Hum. Neurosci. 2022, 16, 1022516. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscles synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Varghese, R.; Bhatt, T. Influence of chronic stroke on functional arm reaching: Quantifying deficits in the ipsilesional upper extremity. Rehabil. Res. Pract. 2019, 2019, 5182310. [Google Scholar] [CrossRef]
- Vieira, P.; Oliveira, R.; Fernandes, L.; Pereira, K.; Souza, L. Augmented muscle activation in reaching movements after stroke. Hum. Mov. 2020, 21, 39–46. [Google Scholar] [CrossRef]
- Silva, C.C.; Silva, A.; Sousa, A.; Pinheiro, A.R.; Bourlinova, C.; Silva, A.; Salazar, A.; Borges, C.; Crasto, C.; Correia, M.V.; et al. Co-activation of upper limb muscles during reaching in post-stroke subjects: An analysis of the contralesional and ipsilesional limbs. J. Electromyogr. Kinesiol. 2014, 24, 731–738. [Google Scholar] [CrossRef]
- Houston, M.; Li, X.; Zohu, P.; Li, S.; Roh, J.; Zhang, Y. Alterations in muscle networks in the upper extremity of chronic stroke survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1026–1034. [Google Scholar] [CrossRef]
- Tater, P.; Pandey, S. Post-stroke movement disorders: Clinical spectrum, pathogenesis, and management. Neurol. India 2021, 69, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Raffin, E.; Hummel, F.C. Restoring motor functions after stroke: Multiple approaches and opportunities. Neuroscientist 2018, 24, 400–416. [Google Scholar] [CrossRef]
- Ibrahim, R.U.; Abdullahi, A.; Salihu, A.T.; Lawal, I.U. Intensity of task-specific training for functional ability post-stroke: Systematic review and meta-analysis. Clin. Rehabil. 2025, 39, 1133–1155. [Google Scholar] [CrossRef]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The use of wearable sensors to assess and treat the upper extremity after stroke: A scoping review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef]
- Coscia, M.; Wessel, M.J.; Chaudary, U.; Millán, J.D.R.; Micera, S.; Guggisberg, A.; Vuadens, P.; Donoghue, J.; Birbaumer, N.; Hummel, F.C. Neurotechnology-aided interventions fur upper limb motor rehabilitation in severe chronic stroke. Brain 2019, 142, 2182–2197. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Y.; Ye, B.; Babineau, J.; Ding, Y.; Mihailidis, A. Technology-based compensation assessment and detection of upper extremity activities of stroke survivors: Systematic review. J. Med. Internet Res. 2022, 24, e34307. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Morone, G.; Palomba, A.; De Angelis, S.; Mercuro, A.; Caltagirone, C.; Grasso, M.G. Effectiveness of a sensor-based technology in upper limb motor recovery in post-acute stroke neurorehabilitation: A randomized controlled trial. J. Biol. Regul. Homeost. Agents 2020, 34, 165–174. [Google Scholar]
| Functional Domain | Task Name |
|---|---|
| Proximal Reaching and Transport (PRT) | Forearm to Table (side) |
| Forearm to Box (side) | |
| Extend Elbow (side) | |
| Extend Elbow (with weight) | |
| Hand to Table (front) | |
| Hand to Box (front) | |
| Reach and Retrieve | |
| Lift Basket | |
| Weight to Box | |
| Fine Motor Manipulation (FMM) | Lift Pencil |
| Lift Paper Clip | |
| Stack Checkers | |
| Flip Cards | |
| Turn Key in Lock | |
| Gross Motor Functional Control (GMFC) | Lift Can |
| Fold Towel |
| Muscle | PS (95% CI) | NPS (95% CI) | p-Value | Adjusted p (Bonferroni) | r | |
|---|---|---|---|---|---|---|
| PRT | UT | 38.06 ± 9.03 (35.45–40.19) | 32.25 ± 8.50 (29.79–34.35) | <0.001 | <0.001 *** | 0.553 |
| AD | 31.57 ± 8.96 (29.18–33.73) | 30.20 ± 7.61 (28.04–31.94) | 0.556 | 1.000 | 0.070 | |
| TB | 19.33 ± 4.88 (17.97–20.52) | 20.95 ± 5.25 (19.52–22.14) | 0.217 | 1.000 | 0.148 | |
| BB | 26.50 ± 8.13 (24.49–28.51) | 26.49 ± 6.65 (24.63–28.07) | 0.602 | 1.000 | 0.062 | |
| ECRL | 28.07 ± 7.73 (25.97–29.99) | 37.55 ± 5.36 (35.79–38.73) | <0.001 | <0.001 *** | 0.878 | |
| PQ | 19.20 ± 5.80 (17.62–20.60) | 27.38 ± 6.44 (25.66–28.95) | <0.001 | <0.001 *** | 0.943 | |
| FCU | 24.84 ± 6.37 (23.10–26.38) | 23.94 ± 6.45 (22.17–25.50) | 0.367 | 1.000 | 0.108 | |
| FCR | 19.32 ± 5.90 (17.67–20.71) | 22.86 ± 5.65 (21.35–24.23) | <0.001 | 0.017 * | 0.404 | |
| FMM | UT | 48.51 ± 7.27 (45.61–51.34) | 38.12 ± 8.62 (34.82–41.40) | <0.001 | 0.002 ** | 0.509 |
| AD | 35.81 ± 8.82 (32.30–39.14) | 44.78 ± 6.57 (42.22–47.34) | <0.001 | 0.021 * | 0.429 | |
| TB | 21.21 ± 7.41 (18.42–24.17) | 21.29 ± 5.73 (19.09–23.55) | 0.780 | 1.000 | 0.036 | |
| BB | 31.76 ± 8.13 (28.59–34.98) | 32.24 ± 5.61 (30.1–34.42) | 0.447 | 1.000 | 0.098 | |
| ECRL | 37.10 ± 8.46 (33.85–40.34) | 42.03 ± 6.02 (39.68–44.39) | 0.004 | 0.099 | 0.370 | |
| PQ | 26.52 ± 6.70 (23.95–29.26) | 37.25 ± 5.99 (34.89–39.57) | <0.001 | <0.001 *** | 0.704 | |
| FCU | 24.44 ± 8.15 (21.36–27.62) | 27.67 ± 6.35 (25.23–30.17) | 0.062 | 1.000 | 0.241 | |
| FCR | 20.37 ± 6.36 (17.98–22.89) | 22.54 ± 4.51 (20.83–24.32) | 0.066 | 1.000 | 0.238 | |
| GMFC | UT | 47.57 ± 8.06 (42.29–52.56) | 38.08 ± 8.62 (32.70–43.59) | 0.051 | 1.000 | 0.564 |
| AD | 35.83 ± 8.56 (30.19–41.21) | 38.76 ± 6.83 (34.40–43.02) | 0.377 | 1.000 | 0.255 | |
| TB | 21.76 ± 6.19 (18.02–25.87) | 20.20 ± 5.83 (16.67–24.07) | 0.700 | 1.000 | 0.111 | |
| BB | 31.84 ± 6.14 (27.97–35.79) | 38.02 ± 5.79 (34.21–41.52) | 0.004 | 0.093 | 0.834 | |
| ECRL | 36.35 ± 7.73 (31.45–41.22) | 39.66 ± 5.04 (36.49–42.86) | 0.202 | 1.000 | 0.368 | |
| PQ | 29.51 ± 6.23 (25.54–33.61) | 36.93 ± 4.87 (33.89–40.06) | <0.001 | 0.031 * | 0.930 | |
| FCU | 21.45 ± 5.36 (18.10–24.87) | 25.61 ± 5.47 (22.26–29.08) | 0.133 | 1.000 | 0.433 | |
| FCR | 18.75 ± 4.97 (15.65–21.91) | 20.88 ± 2.60 (19.26–22.56) | 0.301 | 1.000 | 0.298 | |
| Range of Motion | PS (95% CI) | NPS (95% CI) | p-Value | Adjusted p (Bonferroni) | r | |
|---|---|---|---|---|---|---|
| PRT | SF | 32.76 ± 11.80 (27.95–37.78) | 36.93 ± 13.08 (31.47–42.73) | 0.008 | 0.203 | 0.760 |
| SA | 44.19 ± 24.73 (34.85–55.56) | 41.94 ± 22.24 (33.64–52.67) | 0.510 | 1.000 | 0.190 | |
| SER | 61.22 ± 38.66 (46.23–79.16) | 42.46 ± 13.07 (36.99–47.90) | 0.703 | 1.000 | 0.110 | |
| EF | 34.73 ± 11.92 (29.79–39.99) | 44.53 ± 12.68 (39.22–49.95) | 0.001 | 0.025 * | 0.946 | |
| WF | 24.29 ± 9.37 (20.45–28.45) | 25.94 ± 9.16 (22.08–29.95) | 0.457 | 1.000 | 0.215 | |
| WRD | 14.26 ± 4.19 (12.50–16.08) | 16.44 ± 5.55 (14.11–18.83) | 0.163 | 1.000 | 0.402 | |
| WS | 77.28 ± 45.66 (59.08–97.21) | 47.68 ± 24.85 (38.16–59.22) | 0.013 | 0.301 | 0.721 | |
| UAP | 31.72 ± 11.11 (27.18–36.67) | 30.81 ± 8.87 (27.10–34.60) | 0.465 | 1.000 | 0.211 | |
| UAR | 43.88 ± 14.42 (37.77–50.19) | 40.78 ± 12.21 (35.49–46.05) | 0.666 | 1.000 | 0.125 | |
| FER | 68.39 ± 38.59 (52.97–86.07) | 68.61 ± 41.35 (52.34–87.20) | 0.573 | 1.000 | 0.163 | |
| FMM | SF | 52.36 ± 16.52 (44.08–63.60) | 52.25 ± 8.32 (47.36–57.28) | 0.142 | 1.000 | 0.329 |
| SA | 49.98 ± 17.30 (40.27–61.33) | 44.50 ± 12.93 (37.20–52.61) | 0.378 | 1.000 | 0.197 | |
| SER | 62.93 ± 41.02 (41.17–90.02) | 42.94 ± 9.02 (37.54–48.37) | 0.995 | 1.000 | 0.001 | |
| EF | 39.71 ± 8.00 (35.04–44.49) | 55.28 ± 7.34 (51.14–59.82) | <0.001 | 0.003 ** | 0.865 | |
| WF | 44.19 ± 10.09 (38.35–50.40) | 47.52 ± 8.00 (42.81–52.26) | 0.371 | 1.000 | 0.199 | |
| WRD | 30.60 ± 7.13 (26.35–34.89) | 31.80 ± 6.86 (27.80–36.02) | 0.827 | 1.000 | 0.049 | |
| WS | 77.83 ± 18.53 (67.17–89.09) | 73.40 ± 26.81 (60.09–91.37) | 0.138 | 1.000 | 0.331 | |
| UAP | 41.68 ± 11.13 (35.55–48.76) | 38.11 ± 7.36 (33.87–42.66) | 0.965 | 1.000 | 0.010 | |
| UAR | 52.48 ± 11.79 (45.77–60.04) | 45.43 ± 8.66 (40.52–50.66) | 0.156 | 1.000 | 0.317 | |
| FER | 98.45 ± 45.38 (73.07–126.90) | 106.37 ± 56.32 (74.95–142.60) | 0.886 | 1.000 | 0.032 | |
| GMFC | SF | 51.53 ± 7.89 (44.11–58.90) | 57.16 ± 10.12 (47.02–66.27) | 0.234 | 1.000 | 0.420 |
| SA | 59.77 ± 26.59 (38.70–88.08) | 71.76 ± 31.81 (43.87–95.80) | 0.379 | 1.000 | 0.311 | |
| SER | 50.08 ± 13.61 (38.11–63.56) | 62.14 ± 13.86 (48.55–75.33) | 0.134 | 1.000 | 0.530 | |
| EF | 54.08 ± 9.79 (44.67–63.17) | 67.08 ± 10.04 (57.45–76.60) | 0.070 | 1.000 | 0.640 | |
| WF | 46.54 ± 12.29 (35.31–58.47) | 56.87 ± 10.38 (46.76–66.64) | 0.278 | 1.000 | 0.384 | |
| WRD | 33.43 ± 6.52 (27.45–39.75) | 35.57 ± 6.89 (29.34–42.44) | 0.836 | 1.000 | 0.073 | |
| WS | 70.91 ± 13.49 (58.57–83.78) | 84.41 ± 12.28 (72.77–95.79) | 0.179 | 1.000 | 0.475 | |
| UAP | 36.81 ± 6.54 (30.53–43.17) | 43.13 ± 7.89 (35.28–50.35) | 0.163 | 1.000 | 0.494 | |
| UAR | 44.37 ± 8.43 (36.51–52.35) | 47.90 ± 11.55 (38.76–60.16) | 0.836 | 1.000 | 0.073 | |
| FER | 67.33 ± 19.30 (50.46–87.31) | 100.87 ± 49.07 (59.55–152.38) | 0.379 | 1.000 | 0.311 | |
| Root Mean Square | PS | NPS | p-Value | Adjusted p (Bonferroni) | r | |
|---|---|---|---|---|---|---|
| PRT | UAX | 671.82 ± 108.04 (625.34–718.31) | 727.59 ± 102.48 (684.50–771.68) | 0.002 | 0.044 * | 0.900 |
| UAY | 283.36 ± 65.78 (255.05–311.66) | 229.90 ± 51.58 (207.71–252.09) | 0.007 | 0.177 | 0.773 | |
| UAZ | 624.22 ± 94.62 (583.51–664.94) | 588.50 ± 93.36 (548.33–628.67) | 0.092 | 1.000 | 0.486 | |
| FAX | 301.41 ± 84.01 (265.26–337.55) | 290.50 ± 78.00 (256.94–324.06) | 0.975 | 1.000 | 0.009 | |
| FAY | 409.01 ± 70.28 (378.77–439.25) | 432.65 ± 73.80 (400.90–464.41) | 0.398 | 1.000 | 0.244 | |
| FAZ | 858.92 ± 61.23 (832.58–885.27) | 858.26 ± 64.72 (830.41–886.11) | 0.771 | 1.000 | 0.084 | |
| HX | 337.53 ± 85.91 (300.57–374.49) | 322.36 ± 95.71 (281.18–363.55) | 0.928 | 1.000 | 0.026 | |
| HY | 310.57 ± 72.71 (279.29–341.86) | 287.67 ± 80.70 (252.94–322.39) | 0.005 | 0.117 | 0.813 | |
| HZ | 915.97 ± 46.07 (896.15–935.80) | 940.29 ± 66.99 (911.47–969.11) | 0.001 | 0.015 * | 0.987 | |
| FMM | UAX | 700.22 ± 81.79 (650.75–749.70) | 779.48 ± 66.94 (738.99–819.97) | 0.029 | 0.699 | 0.488 |
| UAY | 341.76 ± 64.90 (302.50–381.01) | 264.13 ± 62.14 (226.54–301.71) | <0.001 | 0.006 ** | 0.815 | |
| UAZ | 573.74 ± 94.72 (516.44–631.03) | 535.66 ± 68.76 (494.07–577.25) | 0.291 | 1.000 | 0.236 | |
| FAX | 322.18 ± 76.30 (276.03–368.33) | 344.07 ± 50.10 (313.77–374.38) | 0.237 | 1.000 | 0.264 | |
| FAY | 457.30 ± 68.97 (415.58–499.02) | 513.46 ± 82.57 (463.51–563.41) | 0.047 | 1.000 | 0.443 | |
| FAZ | 814.13 ± 62.37 (776.41–851.86) | 787.90 ± 82.65 (737.91–837.89) | 0.385 | 1.000 | 0.194 | |
| HX | 368.15 ± 68.20 (326.89–409.40) | 352.50 ± 60.21 (316.08–388.92) | 0.472 | 1.000 | 0.161 | |
| HY | 407.01 ± 53.62 (374.58–439.44) | 396.72 ± 78.19 (349.42–444.01) | 0.345 | 1.000 | 0.211 | |
| HZ | 841.13 ± 50.85 (810.38–871.89) | 879.06 ± 59.62 (842.99–915.12) | 0.027 | 0.656 | 0.493 | |
| GMFC | UAX | 696.46 ± 67.38 (630.43–762.49) | 732.98 ± 64.32 (669.94–796.01) | 0.098 | 1.000 | 0.414 |
| UAY | 323.61 ± 58.26 (266.51–380.71) | 288.58 ± 44.78 (244.69–332.46) | 0.379 | 1.000 | 0.220 | |
| UAZ | 600.06 ± 97.44 (504.56–695.55) | 588.65 ± 85.70 (504.67–672.63) | 0.756 | 1.000 | 0.078 | |
| FAX | 360.11 ± 73.04 (288.53–431.69) | 359.42 ± 71.89 (288.96–429.87) | 0.408 | 1.000 | 0.207 | |
| FAY | 547.87 ± 71.77 (477.53–618.20) | 602.25 ± 83.21 (520.71–683.80) | 0.234 | 1.000 | 0.297 | |
| FAZ | 744.33 ± 86.93 (659.14–829.52) | 714.08 ± 86.71 (629.11–799.05) | 0.605 | 1.000 | 0.129 | |
| HX | 414.50 ± 75.89 (340.13–488.88) | 404.83 ± 67.35 (338.82–470.83) | 0.535 | 1.000 | 0.401 | |
| HY | 526.80 ± 56.76 (471.17–582.42) | 499.32 ± 73.70 (427.10–571.55) | 0.326 | 1.000 | 0.246 | |
| HZ | 759.44 ± 74.08 (686.84–832.04) | 812.55 ± 58.32 (755.39–869.70) | 0.044 | 1.000 | 0.504 | |
| Functional Domain | Variable 1 | Variable 2 | r | p-Value | |
|---|---|---|---|---|---|
| PRT | PS | AD | FCU | 0.685 | 0.029 * |
| ECRL | PQ | 0.830 | 0.003 ** | ||
| NPS | BB | PQ | 0.721 | 0.019 * | |
| ECRL | PQ | 0.818 | 0.004 ** | ||
| FMM | PS | ECRL | PQ | 0.721 | 0.019 * |
| FCR | 0.673 | 0.033 * | |||
| PQ | FCR | 0.697 | 0.025 * | ||
| FCU | FCR | 0.758 | 0.011 * | ||
| NPS | AD | ECRL | −0.636 | 0.048 * | |
| PQ | FCU | 0.673 | 0.033 * | ||
| FCR | 0.685 | 0.029 * | |||
| FCU | FCR | 0.782 | 0.008 ** | ||
| GMFC | PS | UT | BB | 0.857 | 0.007 ** |
| ECRL | 0.762 | 0.028 * | |||
| BB | PQ | 0.810 | 0.015 * | ||
| ECRL | FCR | 0.762 | 0.028 * | ||
| PQ | FCR | 0.905 | 0.002 ** | ||
| FCU | FCR | 0.738 | 0.037 * | ||
| NPS | TB | ECRL | 0.929 | 0.001 ** | |
| BB | ECRL | 0.810 | 0.015 * | ||
| PQ | 0.857 | 0.007 ** | |||
| FCU | FCR | 0.905 | 0.002 ** | ||
| Functional Domain | Variable 1 | Variable 2 | r | p-Value | |
|---|---|---|---|---|---|
| PRT | PS | SF | WF | 0.673 | 0.033 * |
| UAP | 0.915 | <0.001 *** | |||
| UAR | 0.709 | 0.022 * | |||
| SER | WS | 0.758 | 0.011 * | ||
| UAP | 0.758 | 0.011 * | |||
| UAR | 0.697 | 0.025 * | |||
| FER | 0.952 | <0.001 *** | |||
| WF | UAP | 0.697 | 0.025 * | ||
| FER | 0.697 | 0.025 * | |||
| WS | UAP | 0.636 | 0.048 * | ||
| UAR | 0.745 | 0.013 * | |||
| FER | 0.830 | 0.003 ** | |||
| UAP | UAR | 0.830 | 0.003 ** | ||
| FER | 0.733 | 0.016 * | |||
| UAR | FER | 0.758 | 0.011 * | ||
| NPS | SF | SER | 0.697 | 0.025 * | |
| EF | 0.758 | 0.011 * | |||
| WF | 0.830 | 0.003 ** | |||
| UAP | 0.867 | 0.001 ** | |||
| UAR | 0.952 | <0.001 *** | |||
| SRE | EF | 0.939 | <0.001 *** | ||
| UAP | 0.733 | 0.016 * | |||
| UAR | 0.770 | 0.009 ** | |||
| EF | UAP | 0.733 | 0.016 * | ||
| UAR | 0.806 | 0.005 ** | |||
| WF | UAP | 0.636 | 0.048 * | ||
| UAR | 0.697 | 0.025 * | |||
| WR | FER | 0.733 | 0.016 * | ||
| WS | UAR | 0.685 | 0.029 * | ||
| UAP | UAR | 0.903 | <0.001 *** | ||
| FMM | PS | SF | WF | 0.636 | 0.048 * |
| UAR | 0.685 | 0.029 * | |||
| FER | 0.685 | 0.029 * | |||
| SRE | WS | 0.721 | 0.019 * | ||
| UAP | 0.648 | 0.043 * | |||
| UAP | UAR | 0.673 | 0.033 * | ||
| UAR | FER | 0.758 | 0.011 * | ||
| NPS | SF | SER | 0.745 | 0.013 * | |
| WF | 0.915 | <0.001 *** | |||
| UAP | 0.794 | 0.006 ** | |||
| SRE | UAP | 0.661 | 0.038 * | ||
| EF | FER | 0.830 | 0.003 ** | ||
| WF | UAP | 0.758 | 0.011 * | ||
| UAR | FER | 0.770 | 0.009 ** | ||
| GMFC | PS | SF | EF | 0.857 | 0.007 ** |
| UAP | 0.762 | 0.028 * | |||
| UAR | 0.810 | 0.015 * | |||
| FER | 0.714 | 0.047 * | |||
| SAB | SER | 0.786 | 0.021 * | ||
| WS | 0.786 | 0.021 * | |||
| SRE | EF | 0.905 | 0.002 ** | ||
| WS | 0.952 | <0.001 *** | |||
| UAR | 0.810 | 0.015 * | |||
| EF | WF | 0.738 | 0.037 * | ||
| WS | 0.905 | 0.002 ** | |||
| UAP | 0.714 | 0.047 * | |||
| UAR | 0.905 | 0.002 ** | |||
| FER | 0.714 | 0.047 * | |||
| WF | WS | 0.786 | 0.021 * | ||
| WS | UAR | 0.738 | 0.037 * | ||
| UAP | UAR | 0.786 | 0.021 * | ||
| UAR | FER | 0.833 | 0.010 * | ||
| NPS | SF | SER | 0.762 | 0.028 * | |
| EF | 0.905 | 0.002 ** | |||
| WF | 0.857 | 0.007 ** | |||
| UAR | 0.738 | 0.037 * | |||
| SRE | WR | 0.810 | 0.015 * | ||
| EF | WF | 0.762 | 0.028 * | ||
| WF | FER | 0.738 | 0.037 * | ||
| Functional Domain | Variable 1 | Variable 2 | r | p-Value | |
|---|---|---|---|---|---|
| PRT | PS | UAX | UAY | −0.939 | <0.001 *** |
| FAY | FAZ | −0.842 | 0.002 ** | ||
| NPS | UAX | UAY | −0.939 | <0.001 *** | |
| FAY | −0.782 | 0.008 ** | |||
| HY | −0.673 | 0.033 * | |||
| UAZ | FAY | 0.830 | 0.003 ** | ||
| HY | 0.733 | 0.016 * | |||
| FAX | HX | 0.745 | 0.013 * | ||
| FAY | HY | 0.818 | 0.004 ** | ||
| FAZ | HZ | 0.952 | <0.001 *** | ||
| FMM | PS | UAX | UAY | −0.855 | 0.002 ** |
| FAX | FAZ | −0.636 | 0.048 * | ||
| FAY | FAZ | −0.661 | 0.038 * | ||
| HY | 0.782 | 0.008 ** | |||
| HX | HZ | −0.721 | 0.019 * | ||
| NPS | UAX | UAZ | −0.782 | 0.008 ** | |
| FAZ | 0.648 | 0.043 * | |||
| UAZ | HX | 0.648 | 0.043 * | ||
| FAY | FAZ | −0.915 | <0.001 *** | ||
| HX | 0.733 | 0.016 * | |||
| HY | 0.939 | <0.001 *** | |||
| HZ | −0.709 | 0.022 * | |||
| FAZ | HX | −0.685 | 0.029 * | ||
| HY | −0.879 | 0.001 ** | |||
| HZ | 0.842 | 0.002 * | |||
| HX | HY | 0.648 | 0.043 * | ||
| HZ | −0.733 | 0.016 * | |||
| HY | HZ | −0.794 | 0.006 ** | ||
| GMFC | PS | UAX | UAZ | −0.928 | <0.001 *** |
| FAX | HX | 0.714 | 0.047 * | ||
| FAY | FAZ | −0.905 | 0.002 ** | ||
| NPS | UAX | UAZ | −0.976 | <0.001 *** | |
| FAX | 0.905 | 0.002 ** | |||
| UAZ | FAX | −0.857 | 0.007 ** | ||
| FAX | HX | 0.762 | 0.028 * | ||
| FAY | FAZ | −0.738 | 0.037 * | ||
| HY | 0.881 | 0.004 ** | |||
| FAZ | HZ | 0.952 | <0.001 *** | ||
| Functional Domain | Variable 1 | Variable 2 | r | p-Value | ||
|---|---|---|---|---|---|---|
| PRT | PS | EMG–ROM | UT | WR | −0.673 | 0.033 * |
| AD | SAB | 0.770 | 0.009 ** | |||
| WR | −0.770 | 0.009 ** | ||||
| FCU | SAB | 0.794 | 0.006 ** | |||
| FCR | SAB | 0.818 | 0.004 ** | |||
| EMG–RMS | AD | HZ | 0.673 | 0.033 * | ||
| FCU | HZ | 0.697 | 0.025 * | |||
| FCR | HZ | 0.721 | 0.019 * | |||
| ROM–RMS | SAB | HZ | 0.745 | 0.013 * | ||
| EF | HY | 0.794 | 0.006 ** | |||
| NPS | EMG–ROM | UT | WR | 0.648 | 0.043 * | |
| BB | WF | 0.709 | 0.022 * | |||
| EMG–RMS | BB | HZ | 0.636 | 0.048 * | ||
| ECRL | FAY | −0.697 | 0.025 * | |||
| PQ | HY | −0.636 | 0.048 * | |||
| ROM–RMS | SF | FAX | 0.648 | 0.043 * | ||
| SAB | UAZ | 0.721 | 0.019 * | |||
| HY | 0.648 | 0.043 * | ||||
| WF | FAX | 0.636 | 0.048 * | |||
| HZ | 0.685 | 0.029 * | ||||
| WS | FAX | 0.648 | 0.043 * | |||
| HZ | 0.673 | 0.033 * | ||||
| FRE | UAY | −0.721 | 0.019 * | |||
| HX | −0.685 | 0.029 * | ||||
| FMM | PS | EMG–ROM | UT | UAR | 0.733 | 0.016 * |
| FRE | 0.697 | 0.025 * | ||||
| AD | SF | 0.721 | 0.019 * | |||
| FRE | 0.818 | 0.004 ** | ||||
| ECRL | EF | 0.794 | 0.006 ** | |||
| PQ | EF | 0.745 | 0.013 * | |||
| FCR | EF | 0.709 | 0.022 * | |||
| EMG–RMS | AD | FAX | 0.636 | 0.048 * | ||
| BB | FAX | 0.685 | 0.029 * | |||
| ECRL | UAY | −0.818 | 0.004 ** | |||
| FCU | FAX | 0.685 | 0.029 * | |||
| ROM–RMS | EF | HZ | 0.685 | 0.029 * | ||
| WS | UAZ | 0.709 | 0.022 * | |||
| UAP | FAY | −0.697 | 0.025 * | |||
| FRE | HY | −0.661 | 0.038 * | |||
| NPS | EMG–ROM | UT | WR | 0.794 | 0.006 ** | |
| BB | SRE | 0.636 | 0.048 * | |||
| UAR | 0.636 | 0.048 * | ||||
| EMG–RMS | UT | UAX | 0.685 | 0.029 * | ||
| AD | HX | −0.648 | 0.043 * | |||
| HZ | 0.697 | 0.025 * | ||||
| TB | UAX | 0.770 | 0.009 ** | |||
| ECRL | HX | 0.721 | 0.019 * | |||
| PQ | HX | 0.648 | 0.043 * | |||
| ROM–RMS | SRE | UAY | −0.721 | 0.019 * | ||
| SAB | UAZ | 0.782 | 0.008 ** | |||
| WS | FAY | 0.818 | 0.004 ** | |||
| FAZ | −0.685 | 0.029 * | ||||
| HY | 0.745 | 0.013 * | ||||
| FRE | HX | 0.636 | 0.048 * | |||
| GMFC | PS | EMG–ROM | UT | WF | 0.762 | 0.028 * |
| AD | WF | 0.714 | 0.047 * | |||
| TB | SAB | −0.810 | 0.015 * | |||
| WF | −0.881 | 0.004 ** | ||||
| WS | −0.762 | 0.028 * | ||||
| BB | SF | 0.857 | 0.007 ** | |||
| EF | 0.762 | 0.028 * | ||||
| WF | 0.762 | 0.028 * | ||||
| PQ | SF | 0.857 | 0.007 ** | |||
| SRE | 0.714 | 0.047 * | ||||
| EF | 0.905 | 0.002 ** | ||||
| UAR | 0.810 | 0.015 * | ||||
| FCU | SRE | 0.714 | 0.047 * | |||
| WR | 0.881 | 0.004 ** | ||||
| FRE | 0.714 | 0.047 * | ||||
| FCR | SF | 0.762 | 0.028 * | |||
| EF | 0.857 | 0.007 ** | ||||
| WR | 0.810 | 0.015 * | ||||
| UAR | 0.714 | 0.047 * | ||||
| ROM–RMS | SAB | HZ | 0.738 | 0.037 * | ||
| NPS | EMG–ROM | BB | FRE | 0.929 | 0.001 ** | |
| ECRL | FRE | 0.810 | 0.015 * | |||
| EMG–RMS | AD | FAZ | 0.738 | 0.037 * | ||
| TB | UAY | −0.738 | 0.037 * | |||
| ROM–RMS | SF | FAZ | 0.714 | 0.047 * | ||
| HZ | 0.738 | 0.037 * | ||||
| SAB | HZ | 0.738 | 0.037 * | |||
| SRE | FAY | −0.786 | 0.021 * | |||
| FAZ | 0.833 | 0.010 * | ||||
| HY | −0.714 | 0.047 * | ||||
| HZ | 0.714 | 0.047 * | ||||
| WF | FAZ | 0.738 | 0.037 * | |||
| HZ | 0.738 | 0.037 * | ||||
| WS | HY | −0.881 | 0.004 ** | |||
| UAP | HY | −0.738 | 0.037 * | |||
| UAR | HX | 0.786 | 0.021 * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-Y.; Kim, J.-J. Sensor-Based Analysis of Upper Limb Motor Coordination After Stroke: Insights from EMG, ROM, and Motion Data During the Wolf Motor Function Test. Appl. Sci. 2025, 15, 9836. https://doi.org/10.3390/app15179836
Jung J-Y, Kim J-J. Sensor-Based Analysis of Upper Limb Motor Coordination After Stroke: Insights from EMG, ROM, and Motion Data During the Wolf Motor Function Test. Applied Sciences. 2025; 15(17):9836. https://doi.org/10.3390/app15179836
Chicago/Turabian StyleJung, Ji-Yong, and Jung-Ja Kim. 2025. "Sensor-Based Analysis of Upper Limb Motor Coordination After Stroke: Insights from EMG, ROM, and Motion Data During the Wolf Motor Function Test" Applied Sciences 15, no. 17: 9836. https://doi.org/10.3390/app15179836
APA StyleJung, J.-Y., & Kim, J.-J. (2025). Sensor-Based Analysis of Upper Limb Motor Coordination After Stroke: Insights from EMG, ROM, and Motion Data During the Wolf Motor Function Test. Applied Sciences, 15(17), 9836. https://doi.org/10.3390/app15179836









