Featured Application
Adding plant-origin ingredients, which are rich in pro-healthy compounds, such as polyphenolics, is one of the most important ways to develop functional foods. Since such foods should not differ from traditional foods in their quality characteristics, it is very important to evaluate the effects of new-type additives on the quality. Our study evaluated the application of strawberry leaf extract in dry fermented sausages, determining their quality and oxidative characteristics.
Abstract
Strawberry leaf extract (SLE) was used in dry fermented sausages, “Salchichón”, to enrich them with antioxidants. The effect of SLE on various characteristics was monitored during ripening and storage. SLE had a slight effect on microbiological characteristics; however, the pH after 3, 14, and 21 days was slightly lower (4.51–4.55) in the samples with higher SLE concentration (0.5% + 1% dextrose). Peroxide value (PV) and thiobarbituric acid reactive substances (TBARS) values of sausages with SLE and with ascorbic acid (reference antioxidant), at the end of ripening, were similar. SLE acted as a pro-oxidant when the sausage was stored in the light; however, it showed antioxidant activity in the dark and at 50 °C storage conditions. Higher extract concentration reduced redness a* value and increased yellowness b* value in the CIELab colour system. Addition of SLE to dry fermented sausages has no negative effect on the ripening process; however, storage conditions of the final product should be carefully controlled. Sensory analysis of the final product showed that SLE imparts a recognisable herbal odour; however, it did not reduce the overall product acceptability. It may be concluded that SLE may be a promising ingredient for increasing the nutritional quality of fermented sausages.
1. Introduction
Natural food additives derived from herbs and spices may confer several benefits to foods; they can provide desirable flavour properties, improve the stability of foods by antioxidant and/or antimicrobial activity and increase their health benefits by enriching them with bioactive plant phytochemicals. In addition, natural additives may be a substitute for synthetic ones, which are rather unwanted by consumers. All these aspects have been important for the increasing interest in the search for new sources of natural additives and the wide testing of their applications in various foods.
Meat products containing lipids and proteins, as well as high concentrations of pro-oxidants as metallo-proteins, are susceptible to oxidation [1,2]. During the manufacture of dry fermented sausages, apart from microbiological changes and various physicochemical modifications (including dehydration, fermentation, colour changes, proteolysis and lipolysis), lipid oxidation is one of the major deteriorative processes occurring during sausage ripening [3]. Lipid oxidation is considered the main factor responsible for the reduced quality and acceptability of meat products, due to the development of off-taste and off-odour, and the formation of potentially toxic compounds [4]. The use of natural antioxidants to increase the shelf-life of meat products is a promising technology since many plant-derived substances exhibit antioxidant and antimicrobial properties. Therefore, functional properties of numerous plant extracts such as rosemary [5], thyme [6], borage [7], stinging nettle, roselle [8], mate [9], green tea [10] and rose polyphenols [11] were investigated and proven to possess high antioxidant activity concerning their lipid oxidation inhibitory properties in dry fermented sausages. In addition, recent studies have demonstrated that plant extracts exhibited antiochratoxigenic effects against Penicillium nordicum in dry-cured sausages [12,13] and improved the safety of fermented sausage by inhibiting biogenic amine formation and spoilage bacterial growth [14].
Strawberry (Fragaria × ananassa) belongs to the subfamily Rosoideae of the Rosaceae family. Strawberry fruits and juice press-cake are rich in secondary metabolites such as phenolic acids, flavonols, anthocyanins, proanthocyanidins, galloylglucoses and ellagitannins [15]. They were reported to possess in vitro and in vivo antioxidant properties as well as various other beneficial biological effects [16,17]. However, the fruits are consumed fresh or processed into various confectionery products, while the leaves were also reported to contain valuable phytoconstituents, including phenolic acids (p-coumaric, chlorogenic, ellagic), flavonols (kaempferol, quercetin and catechin derivatives), proanthocyanidins, galloylglucoses and ellagitannins [18,19,20,21,22]. Hanhineva and others [23] first reported phenylethanol derivatives of phenylpropanoid glycosides in the leaves of strawberries, while another study determined that the most predominant phenolic group in strawberry leaves comprises quercetin derivatives, quercetin-3-O-rutinoside and quercetin-3-O-glucuronide, being the most abundant flavonol glycosydes [24].
In our previous study, we showed that ethanolic strawberry leaf extract (SLE) at the doses of 1, 2, and 5% retarded fish oil oxidation during its storage [25]; however, such extracts have not been tested in a system containing protein–lipids such as meat products. To fill this gap, this work aimed to evaluate the applicability of SLE in the production of fermented sausages. For this purpose, the effect of SLE on the following quality characteristics was investigated: oxidative stability (PV and TBARS values), some important quality parameters (pH, moisture, water activity, fatty acids composition), microbiological characteristics (lactic acid bacteria (LAB), Micrococcaceae, Enterobacteria) and sensorial attributes (colour and odour) of dry fermented sausage “Salchichón” formulated with different dextrose levels (0.3 and 1%) during ripening (28 days) and storage under different conditions (light, dark, 50 °C). The development of natural additives with antioxidant potential and their application in meat products may increase functional properties (health benefits) of such products and prolong their storage shelf life. In addition, the use of natural additives may improve consumers’ opinion about the processed meat products.
2. Materials and Methods
2.1. Preparation of Strawberry Leaf Extract (SLE)
Garden strawberry leaves were obtained from Lithuanian Institute of Horticulture; they were collected after the final harvest of berries, on 25–28 July; the combined sample consisted of cultivars ‘Elkat’, ‘Venta’, ‘Honeoye’, ‘Bogota’, ‘Pandora’, ‘Dukat’ and ‘Pegasus’. The leaves were dried in an oven SCC061E (Baltic Master, Vilnius, Lithuania) at 40 °C and milled in an ultra-Centrifugal Mill ZM 200 (Retsch GmbH, Haan, Germany). Dry plant powder (200 g) was extracted with 3 L of 70% ethanol for 8 h in a multipurpose apparatus UMC12 (Stephan Machinery GmbH, Hameln, Germany). The extract was centrifuged, and the residue was re-extracted twice, each time with 3 L of solvent. Supernatant fluids from 3 extractions were combined and dried in a spray dryer B-290 equipped with a refrigeration unit for collecting organic solvents, Inert Loop B-295 (Büchi, Flawil, Switzerland). Dried extract was stored at −20 °C until used.
2.2. Determination of Chlorophyll Content in Strawberry Leaf Extract
The contents of chlorophylls a (Chl a) and b (Chl b), as well as total chlorophylls, were determined spectrophotometrically [26]. Dried extract (0.25 g) was dissolved in 5 mL of 80% (v/v) acetone solution in water. After filtration through a Whatman No. 4 filter, the absorbance was read at 663 and 646 nm to measure the content of Chl a and Chl b, respectively. The total chlorophyll content was calculated as the sum of Chl a and Chl b. Three replicate analyses were performed, and the results were expressed in mg/100 g of SLE on a dry weight basis.
2.3. Production of Fermented Sausage
The mixture for dry fermented sausage “Salchichón” was prepared using the following formula (w/w): 60% lean pork meat, 40% pork back fat, 2.2% salt, 2% ground black pepper, 0.9% red vine, 0.6% ground nutmeg, 0.1% whole black pepper, 0.1% ground white pepper, 0.1% phosphate, 0.03% starter culture, 0.015% nitrite, and 0.01% nitrate. Meat was ground in a Cato mincer (Sabadell, Spain) to a particle size of approximately 8 mm at 0 °C. Then, it was mixed in an AV-30 Talleres Cato mixer (Sabadell, Spain) with other ingredients. Lactobacillus sakei, Staphylococcus xylosus, and Staphylococcus carnosus were used as starter cultures. The final mixture was divided into 5 parts, which were used to prepare 5 different batches of dry fermented sausages with the following ingredients: batch A—control with 0.3% ascorbic acid (reference antioxidant) and 0.3% dextrose; batch B1—with 0.3% dextrose and 0.1% SLE; batch B2—with 0.3% dextrose and 0.5% SLE; batch C1—with 1% dextrose and 0.1% SLE; batch C2—with 1% dextrose and 0.5% SLE. The content of the added SLE was selected based on a preliminary evaluation of the extract’s effect on sensory quality; higher concentrations resulted in an undesirable increase in herbal and acid notes. The mixtures were stuffed into 47 mm diameter collagen sausage casings (Viscofan, Navarre, Spain). The sausages were fermented in a ripening chamber at 15 °C and 95% relative humidity (RH) for 18 h. After this, the temperature was increased to 23 °C, and the RH was reduced to 90% for 48 h. For the next 10 days, temperature was reduced to 14–15 °C, and the RH to 85%. Finally, the ripening was completed at 75% RH in 28 days. The whole experiment was replicated twice, and three measurements were carried out for each parameter studied in each replica, unless otherwise indicated.
2.4. Microbiological Analysis
The changes in lactic acid bacteria (LAB), Enterobacteria and Staphylococcus spp. in sausages were determined during the first days of ripening [27]. A slice of 25 g of sausage without casing was aseptically transferred to a sterile plastic bag and pummelled for 120 s in a laboratory blender (Stomacher 400, Seward, London, UK) with 225 mL of Ringer solution (Oxoid, Basingstoke, UK). Appropriate decimal dilutions of the samples were prepared using the same solution, and 0.1 mL of each dilution was plated in duplicate on the following media: (1) Violet Red Bile agar with Glucose (VRBG, Oxoid), incubated at 37 °C for 24 h in anaerobic conditions, for Enterobacteriaceae counts; (2) De Man, Rogasa and Sharpe agar (MRS, Oxoid) incubated at 30 °C for 48 h, for LAB counts; (3) Mannitol Salt Agar (MSA, Oxoid) incubated at 30 °C for 48 h in anaerobic conditions for Staphylococci counts. Two replicate samples were taken for each sample on day 1 (meat mixture prior to stuffing), day 3 (end of fermentation) and day 7 (end of ripening) for microbiological analysis.
2.5. Determination of pH, Water Activity and Humidity
The humidity, water activity and pH were observed in dry fermented sausages during storage. Humidity was determined by drying at 100 °C to a constant weight [28]. Water activity (aw) was determined using Aqua Lab CX-2 equipment (Decagon, Washington, DC, USA). The pH was measured using a pH meter equipped with a glass electrode.
2.6. Determination of Fatty Acid Composition
Fatty acid composition was determined during ripening. Fatty acid methyl esters (FAMEs) were prepared using 14% boron trifluoride-methanol according to the AOCS official method [29]. Lipid extract (25 mg) was weighed into a glass tube containing 1 mL of internal standard (IS). Then, 1.5 mL of 0.5 mol/L methanolic NaOH solution was added, and the tube was sealed with a cap, followed by mixing, heating for 5 min at 100 °C and cooling. Two millilitres of 14% BH3 solution was added, and boiling was continued for 30 min. Afterwards, the mixture was cooled to 30–40 °C, and 1 mL of isooctane (Sigma-Aldrich, Buchs, Switzerland) was added. The tube was sealed with a cap and shaken vigorously for 30 s while still warm. Immediately, 5 mL of saturated salt solution was added, and the tube was closed with a cap and agitated thoroughly. One millilitre of the isooctane layer was transferred to a sample vial for the analysis of FAMEs by gas chromatography (GC). A gas chromatograph (Agilent 6890N Series, Hewlett-Packard Co., Avondale, PA, USA) equipped with a 7683B series auto-sampler and flame ionisation detector was used for FAMEs analysis. Separation was performed on a fused silica capillary column Omegawax TM-320 (30 m × 0.32 mm, 0.25 μm film thickness, Supelco, Bellefonte, PA, USA) by using helium as a carrier gas at a flow rate of 1.8 mL/min. Injection volume was 1 μL at a split ratio of 1:50. The temperature was held constant at 180 °C for 20 min, increased to 200 °C at 1 °C/min, held for 1 min, again increased to 220 °C at 5 °C/min and finally held constant for 20 min. The compounds were identified by comparing their retention indexes with those of FAME reference compounds (Sigma Chemical Co., St. Louis, MO, USA). The concentration was calculated by using methyl tricosanoate as an IS. Two replicate analyses were performed for each sample. Fatty acid composition was calculated as a percentage of the total fatty acids present in the extracted fat.
2.7. Determination of Lipid Oxidation Under Different Storage Conditions
Lipid oxidation parameters were determined in fermented sausages during their ripening (0, 7, 21 and 28 days) and under three different storage conditions. After ripening (28 days), the sausages were cut into 1 cm slices, put on trays and placed in the light (1st batch) and dark (2nd batch covered with aluminium foil). These two trays were kept for 9 days at 26 °C in the same room, and measurements were performed after 3, 6 and 9 days. The third tray was placed in the oven at 50 °C for 6 h. The lipid oxidation level was evaluated by TBARS values for the light, dark and 50 °C storage conditions.
2.8. Lipid Extraction
Lipids were extracted with 60 mL of petroleum ether at 60 °C in a Büchi Soxhlet extraction system (model B-811) for 4 h. The extracted lipids were dried in an oven at 60 °C for 10 min and were stored at 4 °C until further analysis.
2.9. Measurement of Peroxide Value (PV)
The PV of the extracted lipids was measured in sausages during the ripening time, using the AOCS Cd 8-53 official method [30]. Lipid extract (0.5 g) was dissolved in 10 mL of chloroform and 15 mL of acetic acid. Then, 1 mL saturated solution of potassium iodide was added, and the mixture was shaken by hand for 1 min and kept in the dark for 5 min. After stabilisation, 75 mL of distilled water and 0.5 mL of starch indicator (1%) solution were added. Then, the mixture was titrated with 0.002 M Na2S2O3 until the colour disappeared. PV was expressed in milliequivalents (meq) of active oxygen per kg of lipids.
2.10. Measurement of 2-Thiobarbituric Acid Reactive Substances (TBARS)
TBARS values of sausages were determined by the spectrophotometric method [31]. Two grams of sausage was transferred into a 25 mL centrifuge tube and homogenised with 8 mL of 5% aqueous trichloroacetic acid (TCA) and 5 mL of 0.8% butylated hydroxytoluene (BHT) in hexane. The content of the tube was centrifuged (3000× g, 3 min), the upper hexane layer was removed, and the lower layer was filtered through a Whatman No. 54 filter. An aliquot of 2.5 mL was mixed with 1.5 mL of 0.8% aqueous TBA and heated at 70 °C for 30 min. Finally, the absorbance was measured at 532 nm. The results were expressed in mg of malondialdehyde (MDA)/kg of sample.
2.11. Colour Measurement
Colour measurements of fermented sausages were performed using a Hunter spectrophotometer (Minolta CM-2600D, Osaka, Japan). Hunter values were obtained using a setting of D 65 (daylight, 65° light angle). The L* (lightness), a* (redness) and b* (yellowness) values were measured on the surface of sliced sausages.
2.12. Instrumental Analysis by Electronic Nose
An electronic nose (e-nose) α Fox 4000 (Alpha M.O.S., Toulouse, France) equipped with an 18-sensor array and controlled by AlphaSoft software version 9.1 was used to measure the responses generated by the emitted sausage volatile compounds, which might have an effect on product odour. Analyses were performed by an HS method using 6 g of ground sausage, which was placed in a 20 mL capacity vial and incubated in the oven at 50 °C for 5 min with intermittent shaking. Other parameters were as follows: acquisition time 120 s, acquisition period 0.5 s, acquisition delay 600 s, flow 150 mL/min, injection volume 1500 µL and injection speed 1.5 mL/s. Five replicates for each sample were prepared for measurements.
2.13. Sensory Evaluation
A sensory evaluation of sausages was performed at the end of the ripening process (at day 28). A total of 10 experienced panellists, including graduate students and staff members of the Department of Biotechnology and Food Science of the University of Burgos, who have experience in evaluating fermented meat products, were chosen to perform a sensory characterisation of sausages. Sausages were prepared for analysis as follows: casings were removed, cut into small pieces, and approximately 5 g was placed in headspace vials (covered with aluminium foil and identified by random 3-digit numbers) and closed with a rubber stopper. A preparatory session was held before testing, so that each panellist could thoroughly discuss and understand each attribute of the sausage to be evaluated. Also, panellists before the evaluation were familiarised with the strawberry leaf extract odour, which was described as an herbal odour. Evaluations were performed in a sensory room at ambient temperature under white fluorescent lighting in the individual booths. The panel of judges used quantitative descriptive analysis to evaluate differences in aroma: odour intensity, cured-meat aroma, acidity, rancidity, herbal and overall acceptability [32]. The intensity of every attribute was expressed on a structured scale from 1 (sensation not perceived) to 5 (maximum sensation). Panellists evaluated all five batches in one session.
2.14. Statistical Data Assessment
The results are presented as a mean ± SD (standard deviation). Statistical analysis and comparisons among means were carried out using the statistical package Statgraphics Centurion (V16.2.04 for Windows 7). All data were analysed by ANOVA at a significance level of 5%, followed by Fisher’s test. Principal component analysis (PC1–PC2) was applied to the results obtained by the electronic nose.
3. Results and Discussion
3.1. Strawberry Leaf Extract
The antioxidant potential of plant-origin extracts to inhibit lipid oxidation in food products usually depends on the content and composition of phenolic compounds. Generally, the antioxidant activity of phenolic compounds is defined by their radical scavenging effects. The antioxidant properties and phytochemical composition of SLE were reported in our previous work: for instance, the content of total phenolics was 257 mg gallic acid equivalents in g of dry extract, while Trolox equivalent radical scavenging capacity in the ABTS•+ scavenging assay was 1579 µmol TE/g [25]. The extract was rich in phenolic acids and their derivatives and flavonoids, particularly rutin (18.22 mg/g), kaempferol glucoside (10.88 mg/g), isorhamnetin glucoside (13.22 mg/g), proanthocyanidin B1 (6.24 mg/g) and (+)-catechin (24.10 mg/g). Consequently, SLE may be regarded as a potential natural antioxidant and a source of various bioactive phytochemicals.
Green colour plant leaves also contain chlorophyll pigments, mainly consisting of Chl a and Chl b. It is known that chlorophyll acts as an antioxidant under dark conditions, while under light, it may possess pro-oxidant activity. Since chlorophylls were not measured in our previous study, the characterisation of SLE was extended by determining these pigments. Thus, the content of Chl a and Chl b in SLE was 10.35 ± 0.30 and 6.73 ± 0.13 mg/100 g, respectively, while the total chlorophyll content was 17.08 ± 0.17 mg/100 g. To determine the effect of light on chlorophyll content, the SLE was subjected to the same light as fermented sausages under the light storage conditions. The total chlorophyll and Chl a content significantly decreased in SLE after 24 h storage (11.35 ± 0.32 mg/100 g and 4.89 ± 0.16 mg/100 g, respectively), concerning Chl b (6.45 ± 0.26 mg/100 g), thus demonstrating lower stability, which is typical for Chl a [33].
3.2. Changes in Sausage Microbiota
Salchichón, the most popular Spanish dry fermented sausage, is typically fermented for 1 or 2 days. The most important quality parameters change during fermentation: the number of LAB increases, water activity and pH rapidly decrease, while myofibrillar and sarcoplasmic proteins solubilise [34]. The changes in microbial counts in the products sampled on the 1st, 3rd and 7th day of fermentation are presented in Figure 1. It may be observed that the initial flora of the fermented sausages was dominated by LAB, with the lowest counts of Enterobacteria. The MRS counts increased from 5 log CFU/g to a maximum level above 9 log CFU/g within 3 days and remained similar until the 7th day. These results are in agreement with the data of Rantsiou and Cocolin [35], who reported that after 3 days of fermentation, the number of LAB reached 7–8 log CFU/g, which remained unchanged during the entire maturation period. MRS counts were not significantly different (p < 0.05) in the different sausage samples produced in our study.
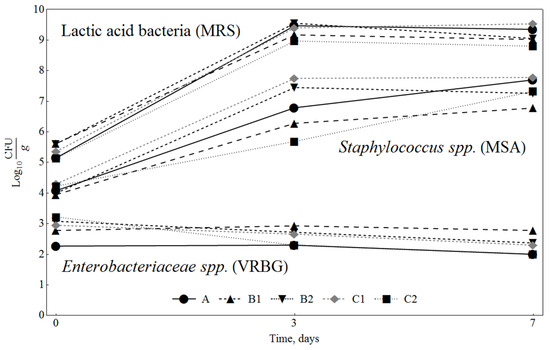
Figure 1.
Changes in microbiota in sausages during the first days of fermentation. A: 0.3% ascorbic acid + 0.3% dextrose; B1: 0.5% SLE + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C1: 0.5% SLE + 1% dextrose; C2: 0.1% SLE + 1% dextrose.
Regarding MSA counts, a significant increase (p < 0.05) of Staphylococci levels could be observed, without any significant differences concerning the addition of SLE, ranging from about 4 log CFU/g in the mixture before stuffing to counts above 7 log CFU/g in the samples collected after 7 days. Similar MSA counts were reported by Fonseca and others [36], in “chorizo” dry fermented sausages. Additionally, the Enterobacteriaceae population was measured to evaluate the contamination level throughout the fermentation process. Initial values of Enterobacteriaceae were quite low, approximately 2 log CFU/g in the control sample, indicating good hygienic quality of the raw materials. The counts of Enterobacteriaceae were significantly higher (p < 0.05) when the control batch was compared with the batches containing SLE addition (3 log CFU/g) at day 0; however, the counts later decreased, and no differences were observed. When Lactobacilli are added as a starter culture, it is common that Enterobacteriaceae are reduced, and in some cases disappear during the ripening process [37]. This is due to the formation of lactic and acetic acid by LAB, resulting in a remarkable lowering of pH, which may be sufficient to eliminate Enterobacteriaceae. Also, LAB produce bacteriocins, which inhibit the growth of pathogenic microorganisms, especially Staphylococcus aureus [38].
3.3. Humidity, Water Activity and pH
The levels of water activity (Figure 2) and humidity content (Figure 3) decreased during ripening; however, there were no significant differences between the analysed samples throughout the whole ripening time. The pH values decreased during the ripening period in all samples; these changes were influenced by the addition of SLE and dextrose (Figure 2). The samples with 1% dextrose had lower pH values (C1 and C2) than the samples with 0.3% dextrose (B1 and B2). During fermentation and ripening, LAB convert sugars to lactic acid, which is the main component responsible for the pH decrease. The lowering of pH has a preservation effect, due to inhibition of pathogenic and spoilage bacteria growth, and contributes to the typical organoleptic properties of the fermented sausages. In general, fermented sausages with a higher concentration of sugars have lower pH [39]. Also, the samples with 0.5% SLE (B1 and C1) had lower pH values than the samples with 0.1% SLE (B2 and C2). At the end of the ripening (day 28), there were no significant differences (p < 0.05) between the batches C1, C2 and B1 and also between the batches A and B2, while the pH of these two groups was significantly different.
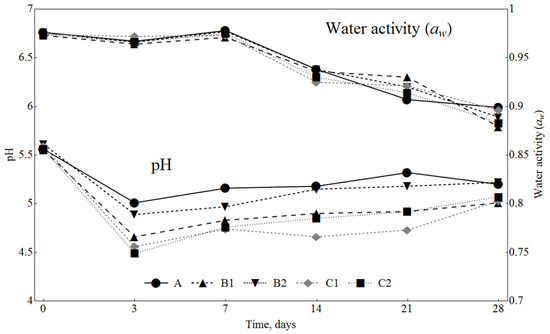
Figure 2.
Changes in water activity (aw) and pH value in sausages during ripening. A: 0.3% ascorbic acid + 0.3% dextrose; B1: 0.5% SLE + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C1: 0.5% SLE + 1% dextrose; C2: 0.1% SLE + 1% dextrose.
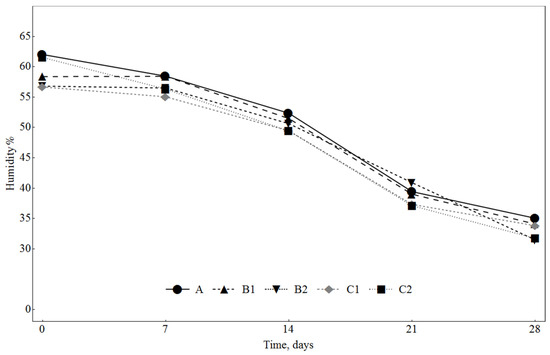
Figure 3.
Changes in humidity in sausages during ripening. A: 0.3% ascorbic acid + 0.3% dextrose; B1: 0.5% SLE + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C1: 0.5% SLE + 1% dextrose; C2: 0.1% SLE + 1% dextrose.
3.4. Fatty Acid Composition
Nine fatty acids, represented by the saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) components in an approximate ratio of 4:4:1, were quantified during sausage ripening (Table 1). Palmitic (C16:0) and oleic (C18:1n-9) acids were major components in all sausages; however, the batches A and B2 may be characterised by the slightly lower amount of oleic acid (C18:1n-9) (p < 0.05) and higher content (p < 0.05) of linoleic acid (C18:2n-6) compared to the other batches of sausages. Lizaso and others [40] reported that during the fermentation of sausages, the content of SFA increased (palmitic and stearic acids), while the content of UFA decreased. PUFAs such as linoleic and linolenic acids are very susceptible to oxidation; therefore, they undergo a more remarkable decrease. In the present study, the content of palmitic and stearic acids increased, while that of oleic, linoleic and α-linolenic acids significantly decreased in the sausages after 7 days. However, it would be rather difficult to explain these very small differences by the effects of the added SLE and/or dextrose. Apart from the negligible chemical changes, one of the possible explanations might be the interference of these ingredients in the FA determination procedure, which consists of several steps.

Table 1.
Changes in fatty acids composition during sausage ripening.
3.5. Lipid Oxidation During Ripening and Storage
The PVs, showing primary oxidation level, were evaluated in dry fermented sausages during the whole ripening period (Figure 4). They were higher at the beginning of fermentation (0–7 days) than at the end of ripening (day 28) in all the sausage samples. It may be explained by the processes which might occur in the meat during mincing and mixing, e.g., due to the disruption of muscle structure and increase in surface exposed to oxygen and other oxidation catalysts [41]. It is well known that ascorbic acid and its salts improve colour stability and extend storage shelf-life by preventing fat oxidation in meat products [42]. Therefore, the control batch was produced with 0.3% of ascorbic acid as a reference antioxidant, while other batches were produced with plant-origin antioxidant SLE. So far as PVs were not significantly different in all fermented sausages, it may be assumed that the effects of both applied SLE concentrations were similar to those of ascorbic acid. Different dextrose concentrations did not affect PV, indicating that this ingredient does not interfere in the primary oxidation process.
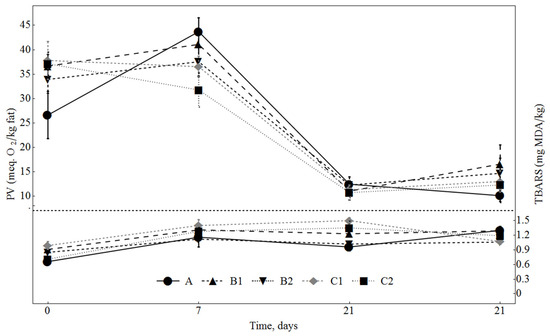
Figure 4.
Changes in PV (meq O2/kg oil) and TBARS (mg MDA/kg) values in sausages during ripening. A: 0.3% ascorbic acid + 0.3% dextrose; B1: 0.5% SLE + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C1: 0.5% SLE + 1% dextrose; C2: 0.1% SLE + 1% dextrose.
The oxidative stability of dry fermented sausages was also measured by TBARS values, which are linked to the formation of secondary oxidation products in meat and meat products; they show the amount of malondialdehyde formed during oxidation from PUFA [31]. The TBARS values were found to be higher in the sausages manufactured with the higher dextrose amount (C1 and C2) until the 21st day of ripening (Figure 4). The highest malondialdehyde amount was in the sausages containing 0.5% of SLE and 1% of dextrose (C1). This means that dextrose might interfere with the TBARS values in the dry fermented sausages. However, on the 28th day of ripening, TBARS values in all batches did not differ (p < 0.05).
The TBARS values were evaluated in the dry fermented sausages stored under different conditions (Table 2). They were about 2 times higher (p < 0.05) in the batches with SLE stored in light. This indicates that SLE in this case acted as a pro-oxidant, most likely due to the presence of chlorophyll in SLE acting as a photosensitizer. It was mentioned above that chlorophyll content decreased 2 times under light after 24 h. However, in the case of 6 h storage at 50 °C, the values of TBARS in the batches with SLE were significantly (p < 0.05) lower compared with the control sample prepared with ascorbic acid. This indicates the antioxidant action of SLE against lipid oxidation under the higher temperature storage conditions. Depending on the amount of dextrose, the TBARS values were different in sausages under dark storage conditions. The TBARS values were the same as in the control sample when 1% dextrose was added to sausages, while the sausages containing 0.3% dextrose had significantly (p < 0.05) higher TBARS values.

Table 2.
Changes in TBARS values (mg MDA/g) during storage of dry fermented sausages.
3.6. Determination of Colour Changes
The effects of the added SLE and dextrose, as well as ripening time, were determined for colour coordinates, namely lightness (L*), redness (a*), and yellowness (b*) (Figure 5). Lightness (L*) was not significantly affected by the SLE addition, while during ripening, its values significantly (p < 0.05) decreased in all samples. Hernández-Hernández and others [43] observed that in model raw pork batters, the lightness values correlated with the oxidation process (TBARS values); the lightness decreased when the oxidation increased. In this study, a relationship was also found between TBARS values and L* values, but the correlation coefficient was too low (0.21). The sausages with the added SLE had a lower a* value and higher b* value (p < 0.05) than the samples prepared without SLE. In the samples manufactured with 0.5% SLE, the redness was significantly lower than in the other samples (p < 0.05). This finding was expected because SLE powder containing high amounts of chlorophylls had a green colour. The effect on colour was dependent on the amount of dextrose added as well; the a* values were lower in the samples with 0.3% dextrose (p < 0.05) than in the samples with 1% dextrose. The samples manufactured with a lower concentration of SLE (0.1%) at the beginning and at the end of ripening were not statistically different compared with the control samples. The b* value was higher in the samples containing 0.5% SLE and 0.3% dextrose. Compared with the control, the samples manufactured with 0.5% SLE and 1% dextrose did not show significantly different yellowness at the beginning and at the end of ripening.
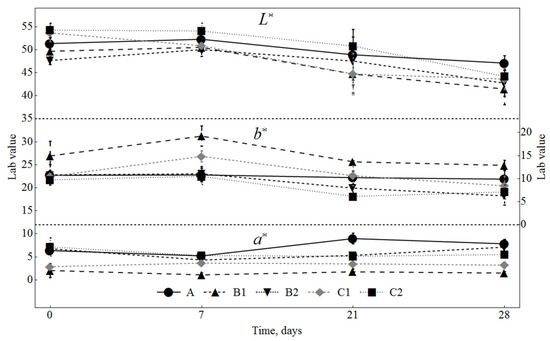
Figure 5.
Changes in CIElab coordinates (lightness (L*), redness (a*), yellowness (b*)) in sausages during ripening. A: 0.3% ascorbic acid + 0.3% dextrose; B1: 0.5% SLE + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C1: 0.5% SLE + 1% dextrose; C2: 0.1% SLE + 1% dextrose.
3.7. Assessment of Released Volatile Compounds
The overall profile of volatile compounds released from sausages was assessed by the electronic nose (Figure 6). The electronic nose was applied to observe the differences between the batches due to the addition of SLE and dextrose, and due to the sausage ripening time. At the beginning of the ripening (day 0), there were no differences between the batches. It was observed that dextrose had the highest impact on the response from the volatiles emitted by the fermented sausages. The signal dots were in different plots starting after the 7th day in the batches with different dextrose concentrations. The overall e-nose profiles of sausages with 0.5 and 1% of SLE were not significantly different. This suggests that the addition of SLE to sausages may have only a slight effect on the total volatiles. Moreover, the signal dots of sausages with both SLE concentrations and higher dextrose content (C1, C2) were less scattered than sausages manufactured with lower dextrose (0.3%) content (B1, B2). The overall aroma profile of sausages only slightly changed from the 7th to the 28th day of ripening time.
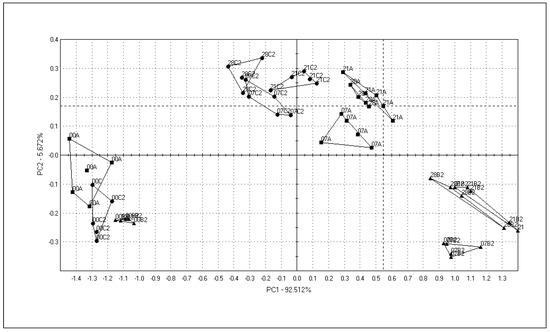
Figure 6.
Changes in olfactory profile of sausages manufactured with 0.1% SLE and different dextrose amounts, during ripening. A: 0.3% ascorbic acid + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C2: 0.1% SLE + 1% dextrose.
3.8. Sensory Evaluation of Final Products
To assess the acceptability of SLE extract in dry fermented sausages, a quantitative odour descriptive test was carried out. Mean scores given by the panellists for the five manufactured batches are shown in Figure 7. No significant differences (p > 0.05) were observed for cured-meat aroma compared to the control batch. It was observed that acid and rancid odours were higher (p < 0.05) in sausages with higher extract and dextrose concentration (C1). Odour intensity was lower (p > 0.05) for sausages with 0.3% dextrose than for the control sausages and sausages with 1% dextrose. Herbal odour was noticeable in sausage samples with added SLE; the higher SLE concentration, the stronger the herbal odour (p < 0.05). Sausage samples with SLE and 1% dextrose, as well as the control batch, were more acceptable overall (p < 0.05) than the samples with added SLE and 0.3% dextrose. The sausages with higher dextrose amounts and added SLE were acceptable independently of the added SLE amount.
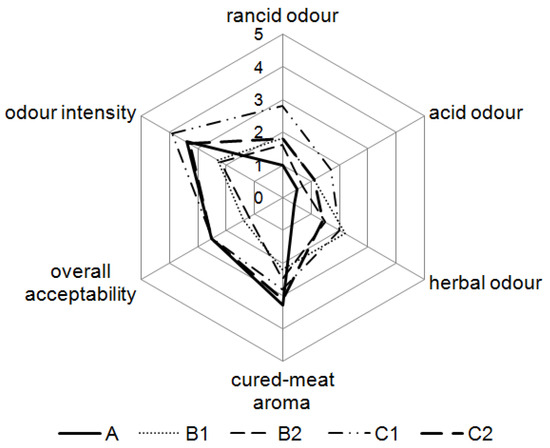
Figure 7.
Sensory evaluation of dry fermented sausages at the 28th day of ripening. A: 0.3% ascorbic acid + 0.3% dextrose; B1: 0.5% SLE + 0.3% dextrose; B2: 0.1% SLE + 0.3% dextrose; C1: 0.5% SLE + 1% dextrose; C2: 0.1% SLE + 1% dextrose.
4. Conclusions
The application of SLE to dry fermented sausages does not negatively affect the quality parameters during the ripening process. However, the sensorial properties such as colour and odour were affected by the addition of SLE. The sausages with higher dextrose amounts were more acceptable, regardless of what extract concentration was used. This might be associated with the higher intensity of total volatiles, which increased in the sausages with higher dextrose amounts compared to those with the lower content of dextrose, as e-nose analysis showed. The effect of SLE on the stability of sausages was dependent on storage conditions: under the light, the extract acted as a pro-oxidant, while under the high temperature and dark storage conditions, it acted as an antioxidant. Strawberry leaf extract is a cheap source of bioactive constituents exhibiting various health benefits; therefore, its application in meat products may be a promising way for increasing their functionality. However, additional in vivo studies should be performed to obtain proof of such benefits.
Author Contributions
Conceptualization, P.R.V. and J.R.; methodology, J.R., I.J. and M.L.G.-S.J.; software, I.J.; validation, P.R.V., J.R. and I.J.; formal analysis, I.R. and I.J.; investigation, I.R., I.J. and M.L.G.-S.J.; resources, P.R.V. and J.R.; data curation, I.R. and I.J.; writing—original draft preparation, I.R. and I.J.; writing—review and editing, P.R.V. and J.R.; visualization, I.R. and M.L.G.-S.J.; supervision, P.R.V. and J.R.; project administration, P.R.V. and J.R.; funding acquisition, P.R.V. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Verbal informed consent was obtained from the participants. Verbal consent was obtained rather than written because sensory evaluation in this study was conducted by 10 experienced judges (including graduate students and staff members of the Department of Biotechnology and Food Science at Burgos University).
Data Availability Statement
Data are available upon request.
Acknowledgments
Author wants to thank Maria Elena Corcuera for providing valuable technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Yılmaz, H.; Gultekin Subasi, B.; Capanoglu, E. Protein Oxidation: The Effect of Different Preservation Methods or Phenolic Additives During Chilled and Frozen Storage of meat/Meat Products. Food Res. Int. 2025, 200, 115378. [Google Scholar] [CrossRef]
- Cava, R.; Ladero, L. Pomegranate Peel as a Source of Antioxidants for the Control of Lipid and Protein Oxidation During the Ripening of Iberian Dry Uncured Sausages. Meat Sci. 2023, 202, 109198. [Google Scholar] [CrossRef]
- Rubio, B.; Martínez, B.; García-Cachán, M.D.; Rovira, J.; Jaime, I. Effect of the Packaging Method and the Storage Time on Lipid Oxidation and Colour Stability on Dry Fermented Sausage Salchichón Manufactured with Raw Material with a High Level of Mono and Polyunsaturated Fatty Acids. Meat Sci. 2008, 80, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Nassu, R.; Goncalves, L.; da Silva, M.; Beserra, F. Oxidative Stability of Fermented Goat Meat Sausage with Different Levels of Natural Antioxidant. Meat Sci. 2003, 63, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kaya, M.; Aksu, M.I. Utilization of Thymus Vulgaris, L. in the Production of Sucuk. J. Food Process. Preserv. 2011, 35, 483–487. [Google Scholar] [CrossRef]
- Ciriano, M.G.; García-Herreros, C.; Larequi, E.; Valencia, I.; Ansorena, D.; Astiasarán, I. Use of Natural Antioxidants from Lyophilized Water Extracts of Borago officinalis in Dry Fermented Sausages Enriched in ω-3 PUFA. Meat Sci. 2009, 83, 271–277. [Google Scholar] [CrossRef]
- Karabacak, S.; Bozkurt, H. Effects of Urtica dioica and Hibiscus sabdariffa on the Quality and Safety of Sucuk (Turkish Dry-Fermented Sausage). Meat Sci. 2008, 78, 288–296. [Google Scholar] [CrossRef]
- Beal, P.; Faion, A.M.; Cichoski, A.J.; Cansian, R.L.; Valduga, A.T.; de Oliveira, D.; Valduga, E. Oxidative Stability of Fermented Italian-Type Sausages Using Mate Leaves (Ilex paraguariensis St. Hil) Extract as Natural Antioxidant. Int. J. Food Sci. Nutr. 2011, 62, 703–710. [Google Scholar] [CrossRef]
- Bozkurt, H. Utilization of natural antioxidants: Green Tea Extract and Thymbra spicata Oil in Turkish Dry-Fermented Sausage. Meat Sci. 2006, 73, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Jiang, M.; Rui, X.; Li, W.; Chen, X.H.; Dong, M.S. Effect of Rose Polyphenols on Oxidation, Biogenic Amines and Microbial Diversity in Naturally Dry Fermented Sausages. Food Control 2017, 78, 324–330. [Google Scholar] [CrossRef]
- Roncero, E.; Alvarez, M.; Delgado, J.; Cebrián, E.; Andrade, M.J. Mechanisms of Action of Bioprotective Plant Extracts Against the Ochratoxigenic Penicillium nordicum in Dry-Cured Sausages. Int. J. Food Microbiol. 2025, 434, 111133. [Google Scholar] [CrossRef] [PubMed]
- Roncero, E.; Delgado, J.; Morcuende, D.; Silva, A.; Andrade, M.J. Plant extracts as biopreservatives against Penicillium nordicum in dry-cured sausages. Food Control 2023, 153, 109972. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, X.H.; Yu, Z.H.; Hu, J.R.; Zhu, Y.C. Effects of Plant Extracts on Biogenic Amine Accumulation, Bacterial Abundance and Diversity in Fermented Sausage. CYTA-J. Food 2021, 19, 771–781. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, L.; Xu, J.; Tian, X.; Zhao, L.; Liao, X. In-Depth Profiling of Phenolics in Strawberries and their Potential Modulatory Effects on the Oxidative Stress-Disease Axis: Insights from UHPLC-QTOF-MS-Based Untargeted Metabolomics, Network Pharmacology, and Molecular Docking. Food Chem. 2025, 489, 145044. [Google Scholar] [CrossRef]
- Charoenwoodhipong, P.; Zuelch, M.L.; Keen, C.L.; Hackman, R.M.; Holt, R.R. Strawberry (Fragaria × Ananassa) Intake on Human Health and Disease Outcomes: A Comprehensive Literature Review. Crit. Rev. Food Sci. Nutr. 2024, 1–31. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of Bioactive Compounds from Strawberry (Fragaria × ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef]
- Hanhineva, K.; Soininen, P.; Anttonen, M.J.; Kokko, H.; Rogachev, I.; Aharoni, A.; Laatikainen, R.; Kärenlampi, S. NMR and UPLC-qTOF-MS/MS Characterisation of Novel Phenylethanol Derivatives of Phenylpropanoid Glucosides from the Leaves of Strawberry (Fragaria × ananassa cv. Jonsok). Phytochem. Anal. 2009, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, A.T.; Kokko, H.I.; Buchala, A.J.; McDougall, G.J.; Stewart, D.; Kärenlampi, S.O.; Karjalainen, R.O. Benzothiadiazole Induces the Accumulation of Phenolics and Improves Resistance to Powdery Mildew in Strawberries. J. Agric. Food Chem. 2007, 55, 1862–1870. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Tomise, K.; Aburatani, M.; Onuki, H.; Hirorta, H.; Ishiharajima, E.; Ohta, T. Isolation of Cytochrome P450 Inhibitors from Strawberry Fruit, Fragaria ananassa. J. Nat. Prod. 2004, 67, 1839–1841. [Google Scholar] [CrossRef]
- Peña-Romero, I.; Céspedes-Camacho, I.F.; Ramírez-Gualito, K.; Salas-Arias, K.; Villalta-Romero, F. Comparative NMR-based profiling of polyphenols in Fragaria × ananassa cv. Marisol and Festival strawberry leaves. Pure Appl. Chem. 2025. [Google Scholar] [CrossRef]
- Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria × ananassa cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules 2023, 28, 1865. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Kokko, H.; Mintz-Oron, S.; Venger, I.; Kärenlampi, S.; Aharoni, A. Non-targeted Analysis of Spatial Metabolite Composition in Strawberry (Fragaria × ananassa) Flowers. Phytochemistry 2008, 69, 2463–2481. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and Characterization of Low Molecular Weight Polyphenols in Berry Leaf Extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef]
- Raudoniūtė, I.; Rovira., J.; Venskutonis, P.R.; Damašius, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L. Antioxidant Properties of Garden Strawberry Leaf Extract and Its Effect on Fish Oil Oxidation. Int. J. Food Sci. Technol. 2011, 46, 935–943. [Google Scholar] [CrossRef]
- Yang, C.M.; Chang, K.W.; Yin, M.H.; Huang, H.M. Methods for the Determination of the Chlorophylls and dheir Derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- González-Fernández, C.; Santos, E.M.; Jaime, I.; Rovira, J. Influence of Starter Cultures and Sugar Concentrations on Biogenic Amine Contents in Chorizo Dry Sausage. Food Microbiol. 2003, 20, 275–289. [Google Scholar] [CrossRef]
- ISO 1442:2023; Meat and Meat Products—Determination of Moisture Content—Reference Method, Edition 3. The International Organization for Standardization: Genf, Switzerland, 2023.
- AOAC. Official Methods of Analysis, 991.39. Fatty Acids in Encapsulated Fish Oils and Fish Oil Methyl and Ethyl Esters; Association of Official Analytical Chemists: Rockville, MD, USA, 1995. [Google Scholar]
- AOCS. Official Methods and Recommended Practices, Cd 8–53. Determination of Peroxide Value; American Oil Chemists Society: Champaign, IL, USA, 1993. [Google Scholar]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, Sensitive, and Specific Thiobarbituric Acid Method for Measuring Lipid Peroxidation in Animal Tissue, Food, and Feedstuff Samples. J. Agric. Food. Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Benito, M.J.; Rodríguez, M.; Martín, A.; Aranda, E.; Córdoba, J.J. Effect of the Fungal Protease Epg222 on the Sensory Characteristics of Dry Fermented Sausage “Salchichón” Ripened with Commercial Starter Cultures. Meat Sci. 2004, 67, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Nabechima, G.H.; Provesi, J.G.; Henriquez Mantelli, M.B.; Vieira, M.A.; De Mello Castanho Amboni, R.D.; Amante, E.R. Effect of the Mild Temperature and Traditional Treatments on Residual Peroxidase Activity, Color, and Chlorophyll Content on Storage of Mate (Ilex paraguariensis) tea. J. Food Sci. 2014, 79, C163–C168. [Google Scholar] [CrossRef]
- Rivas-Cañedo, A.; Nuñez, M.; Fernández-García, E. Volatile Compounds in Spanish Dry-Fermented Sausage ‘Salchichón’ Subjected to High Pressure Processing. Effect of the Packaging Material. Meat Sci. 2009, 83, 620–626. [Google Scholar] [CrossRef]
- Rantsiou, K.; Cocolin, L. New Developments in the Study of the Microbiota of Naturally Fermented Sausages as Determined by Molecular Methods: A Review. Int. J. Food Microbiol. 2006, 108, 255–267. [Google Scholar] [CrossRef]
- Fonseca, S.; Cachaldora, A.; Gómez, M.; Franco, I.; Carballo, J. Effect of Different Autochthonous Starter Cultures on the Volatile Compounds Profile and Sensory Properties of Galician Chorizo, a Traditional Spanish Dry Fermented Sausage. Food Control 2013, 33, 6–14. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Martín, A.; Ruiz-Moyano, S.; Aranda, E.; Córdoba, M.G. Microbiological Quality of Salchichón and Chorizo, Traditional Iberian Dry-Fermented Sausages from Two Different Industries, Inoculated with Autochthonous Starter Cultures. Food Control 2012, 24, 191–198. [Google Scholar] [CrossRef]
- Lücke, F. Utilization of Microbes to Process and Preserve Meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Santos, E.M.; Rovira, J.; Jaime, I. The Effect of Sugar Concentration and Starter Culture on Instrumental and Sensory Textural Properties of Chorizo-Spanish Dry-Cured Sausage. Meat Sci. 2006, 74, 467–475. [Google Scholar] [CrossRef]
- Lizaso, G.; Chasco, J.; Beriain, M.J. Microbiological and Biochemical Changes During Ripening of Salchichón, a Spanish Dry Cured Sausage. Food Microbiol. 1999, 16, 219–228. [Google Scholar] [CrossRef]
- Chizzolini, R.; Novelli, E.; Zanardi, E. Oxidation in Traditional Mediterranean Meat Products. Meat Sci. 1998, 49, S87–S99. [Google Scholar] [CrossRef]
- Wu, H.; Richards, M.P.; Undeland, I. Lipid Oxidation and Antioxidant Delivery Systems in Muscle Food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1275–1299. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Ponce-Alquicira, E.; Jaramillo-Flores, M.E.; Legarreta, I.G. Antioxidant Effect Rosemary (Rosmarinus officinalis L.) and Oregano (Origanum vulgare L.) Extracts on TBARS and Colour of Model Raw Pork Batters. Meat Sci. 2009, 81, 410–417. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).