Abstract
Introduction: Diabetes mellitus negatively influences oral health, significantly contributing to the worsening of odonto-periodontal pathology. Accurate diagnosis and monitoring of lesions are essential to prevent complications. CBCT (cone beam computed tomography) provides a detailed image of bone structures, being a valuable tool in this context. Materials and methods: Thisprospective observational comparative study included 120 patients with chronic periodontitis, divided into two equal groups: 60 with type 2 diabetes mellitus and 60 without systemic diseases. All were examined clinically and radiologically using high-resolution CBCT. Results: Diabetic patients presented a significantly higher mean bone loss (4.7 mm) compared to the non-diabetic patients (3.0 mm). Periodontal pockets >5 mm were more frequent in the diabetic group (75% vs. 50%). CBCT revealed vertical defects and extensive periapical lesions especially in patients with diabetes. Three-dimensional imaging allowed for detailed assessment and clear differentiation between the severity of cases. Conclusions: CBCT is essential in the early identification and evaluation of periodontal disease in diabetic patients. The use of this method significantly contributes to the correlation of clinical and imaging data, optimizing the diagnosis and treatment plan.
1. Introduction
Diabetes mellitus is a metabolic disorder that leads to irreversible degenerative lesions throughout the body, affecting various systems, including the odonto-periodontal unit. This area is one of the most affected by diabetes, which aggravates dental caries and periodontal disease, often leading to tooth loss, arch displacement, and various forms of edentulism [1]. Although diabetes can occur at any age, its prevalence has increased significantly in recent decades [2]. The diagnosis of diabetes is based on the identification of a combination of systemic and oral symptoms, such as gingivitis, periodontitis, recurrent fungal infections of the oral cavity, and delayed wound healing [2,3]. Although there are several general diseases that can influence the occurrence of edentulism, diabetes predominantly affects the rate of loss of odonto-periodontal units and deterioration of the prosthetic field. Understanding these changes is critical, as they have a direct impact on the prognosis of prosthetic treatments [4]. Patients with diabetes require careful preparation to minimize postoperative complications and maintain optimal metabolic balance. The goal of therapy is to promote oral health, as well as to help detect and prevent diabetes early through routine dental care, and to improve the quality of life of people affected by this chronic disease. To prevent serious oral complications caused by diabetes, early diagnosis and appropriate treatment of both diabetes and associated dental problems are essentialwhich plays a key role in establishing a correct diagnosis. It allows the dentist to observe details that cannot be revealed by a clinical examination and plays a critical role in identifying conditions, lesions, or other problems that are not directly visible. Interpretation refers to the analysis of the radiographic image, while diagnosis refers to the identification of a disease based on this examination and other examinations [5]. Periodontal disease encompasses several conditions that affect the health of the tissues surrounding the teeth [6,7]. Both detailed clinical and radiographic investigations are necessary to correctly diagnose and evaluate periodontal disease [8,9]. X-rays help the dentist determine the extent of alveolar bone loss, a common complication of periodontal disease. Today, modern dental radiodiagnostic techniques have evolved significantly, improving the accuracy of diagnosis and treatment of dental conditions. In addition to traditional methods, such as intraoral and extraoral radiographs, advanced techniques are used to provide a detailed three-dimensional visualization of dental and jaw structures [8,9,10]. One of these techniques is cone beam computed tomography (CBCT), which provides high-resolution 3D images of the bones and tissues around the teeth. CBCT provides more detailed images than panoramic radiographs, with the advantage of being able to visualize bone abnormalities and internal structures of the jaw in detail. This technique is useful in planning orthodontic and implant treatment and in evaluating more complex conditions, such as tumors or cysts [11]. Another advanced technique is magnetic resonance imaging (MRI), which provides a precise assessment of soft tissues, such as gums and ligaments. MRI does not use ionizing radiation, making it a safer option for patients, especially in cases that require continuous monitoring of soft tissue conditions, such as advanced periodontal disease or temporomandibular joint disorders Another important step in dental radiology is the integration of artificial intelligence and machine learning for the processing and analysis of radiographic images [12,13,14]. These technologies enable automated diagnosis and detection of subtle abnormalities that might be missed by the human eye. Artificial intelligence algorithms are used to analyze dental radiographs and quickly detect cavities, fractures, or signs of periodontal disease, increasing the efficiency and accuracy of diagnosis. In addition, traditional film radiography has gradually been replaced by digital radiography technology [15]. Digital radiographs are faster and require much less radiation, and the images can be viewed immediately on a screen, facilitating diagnosis and treatment planning. These digital images can also be easily stored and shared between specialists. Although dental radiographs are commonly used in dental practice, they can no longer be considered routine or screening investigations. Any radiation exposure, even at low doses of ionizing radiation, must be carefully justified and optimized, and radiological imaging data must be carefully evaluated to ensure a correct diagnosis. All these modern techniques contribute significantly to improving the accuracy of dental diagnosis, reducing the risks associated with radiation exposure, and allowing for faster and more effective treatment of patients. Their integrated use in dental practice facilitates a complete diagnosis and personalization of treatments for each individual patient [16,17]. Clinical signs of periodontal damage can occur at any age, but it is extremely rare to find evidence of a periodontitis-resistant population. Periodontal disease is a destructive inflammatory condition whose primary etiologic factor, in addition to numerous other local and general contributing causes, is bacterial plaque. It can occur in various forms, ranging from tissue damage to destruction of the periodontium, which in some cases can even lead to tooth loss. Periodontal pathology is characterized by gingivitis, periodontitis, and periodontal manifestations caused by various systemic problems. Its course and manifestations vary according to each form [18]. Irritating and functional factors influence the general state of the organism, modify its defensive capacity, and even if they do not initiate the destructive process, they accelerate its progress and the rate of tissue destruction. Bacterial plaque is the main etiologic agent, but systemic and local factors that may modify the response of periodontal tissues to plaque accumulation can be identified by anamnesis and rigorous clinical examination [19]. Numerous systemic risk factors can influence the effect of the bacterial plaque on the host, and the balance between the micro-organisms and the organism is influenced by environmental and genetic factors. Systemic factors that can have an impact on periodontal disease include cardiovascular disease, diabetes, endocrine disorders, obesity, metabolic diseases, as well as some digestive and kidney diseases. Diabetes mellitus has a significant impact on periodontal disease and is considered one of the most important systemic risk factors. Patients with diabetes are at increased risk of developing periodontitis, and this relationship is bidirectional: diabetes favors the progression of periodontal disease, and periodontal inflammation can worsen glycemic control [20]. Patients with poorly controlled diabetes have more severe forms of periodontitis, and chronic periodontal inflammation can increase insulin resistance, complicating diabetes management. Therefore, interdisciplinary collaboration between dentists and diabetologists is essential for effective prevention and treatment of both conditions. Studies show that patients with diabetes who receive periodontal therapy have a reduction in glycosylated hemoglobin (HbA1c) of up to 0.4–0.6%, similar to the effect of some antidiabetic medications [21,22]. In the evaluation and diagnosis of periodontal disease in patients with diabetes mellitus, the use of modern radiodiagnostic techniques is essential to determine the degree of damage to the periodontal structures and to establish an appropriate therapeutic plan [23]. Today, multiple intraoral and extraoral imaging methods are available, each with specific advantages and limitations. Two-dimensional intraoral radiography is the gold standard for detailed visualization of periodontal structures, as it provides high-resolution images of bone levels and of any morphologic changes. In certain situations, extraoral panoramic radiography may be used as an alternative for a general assessment of the dental arches and jaw structures. However, this method has significant drawbacks, including image distortion and overlapping anatomical structures, which limits the accuracy of diagnosis in advanced periodontal diseases. An essential aspect of periodontal imaging is the accurate representation of bone loss and resorption defects. Traditionally, accurate detection of bone defects is only possible by direct examination during surgery. This is due to the limitations of conventional radiographs, which transform the three-dimensional information of the bone into a two-dimensional projection, thus reducing the diagnostic value and potential for therapeutic planning. In this context, modern radiodiagnostic techniques, such as conebeam computed tomography (CBCT), play a crucial role in the diagnosis of diabetic patients by providing an accurate spatial representation of the alveolar bone [24,25,26]. This allows a detailed and with no distortion visualization of periodontal structures, thus facilitating therapeutic decisions and improving the accuracy of long-term prognostic estimates of affected teeth. Therefore, CBCT is an essential step in the management of patients with diabetes and periodontal disease [27]. It provides a detailed picture of bone status, helps detect complications early, and allows for more effective and personalized treatment. Several studies have shown that CBCT allows a more accurate assessment of bone loss and complex periodontal defects [28,29]. For example, a study by Walter et al. (2016) showed that CBCT has higher sensitivity and specificity in detecting vertical bone defects and bone resorption compared to periapical radiographs [30]. Also, a systematic review by Qian et al. (2022) highlighted that CBCT images significantly improve the accuracy of measuring interproximal bone loss and intra-osseous defects, providing relevant information for therapeutic decisions [31]. However, the use of CBCT in the assessment of periodontal disease is not without controversy. Increased radiation exposure is a major concern, especially compared to conventional intraoral X-rays, which require a lower dose of ionizing radiation. Therefore, the clinical benefits must be carefully weighed against the associated risks before its use in routine practice [32].
2. Materials and Methods
2.1. Subject Recruitment
The subjects included in this research were individuals selected to participate in a clinical study that aimed to evaluate the impact of diabetes mellitus on periodontal pathology, with an emphasis on the importance of radiological examination in this category of patients.
In the initial selection phase, 275 patients were evaluated, of which only 120 were considered eligible to participate in the study. The selection was based on the application of strict inclusion and exclusion criteria, to ensure the homogeneity and relevance of the sample.
Inclusion criteria:
- Age between 26 and 70 years;
- Presence of type 2 diabetes mellitus, clinically diagnosed for at least 2 years (for the test group);
- Patients without diabetes mellitus, with good general status (for the non-diabetic group);
- Presence of at least 20 teeth per arch;
- Availability to participate in periodic check-ups and radiological investigations;
- Signing the informed consent for participation in the study.
Exclusion criteria:
- Type 1 diabetes or other rare forms of diabetes;
- Patients with serious systemic diseases;
- Recent treatment with antibiotics, anti-inflammatories, or corticosteroids (in the last 3 months);
- Active smoking or excessive alcohol consumption;
- Patients who have recently received periodontal treatment (in the last 6 months);
- Pregnancy or breastfeeding;
- Refusal to participate or inability to sign the informed consent.
Based on these criteria, eligible patients were divided into two distinct groups:
- Diabetic group: patients clinically diagnosed with type 2 diabetes mellitus, with a disease course of at least two years, without other severe systemic diseases;
- Non-diabetic group: patients without diabetes mellitus, with a good general health status and without major systemic diseases.
This division was made to allow the comparative evaluation of periodontal pathology in the presence and absence of diabetes mellitus, as well as to investigate the influence of this metabolic disease on the alveolar bone structure.
The study aimed to identify and analyzeodonto-periodontal pathology in diabetic patients, emphasizing the value of CBCT (Cone Beam Computed Tomography) technology in the diagnosis of this condition. Participants underwent a detailed clinical examination using standardized periodontal indices, as well as high-resolution three-dimensional imaging investigations by CBCT. This technology allowed the assessment of alveolar bone height and thickness, essential in identifying periodontal defects and planning treatment.
2.2. Clinical and Radiological Analysis
To assess the general and oral health of participants in the two research groups, they completed a personalized questionnaire divided into two sections: general health and oral health. A comprehensive diagnosis included a detailed clinical examination documented with a personalized form numerically correlated to the questionnaire responses, facilitating statistical analysis. CBCT images and clinical results were analyzed by a multidisciplinary team of two dentists and a radiologist. Each CBCT scan was independently reviewed by the dentists, and discrepancies were resolved by consensus to ensure diagnostic accuracy. All patients underwent CBCT imaging using aPaX-i3D device, which provides high-quality 3D images with four selectable fields of view (FOVs), ranging from 5 × 5 cm to 12 × 9 cm. For this study, the 12 × 9 cm FOV highlighted in Figure 1 was used, offering comprehensive coverage of maxillary and mandibular structures, including the wisdom tooth area, ideal for detailed assessment of bone and periodontal lesions. The equipment was provided by Samsung, headquartered at 13, Samsung 1-ro 2-gil, Hwaseong-si, Gyeonggi-do, 18449, Korea
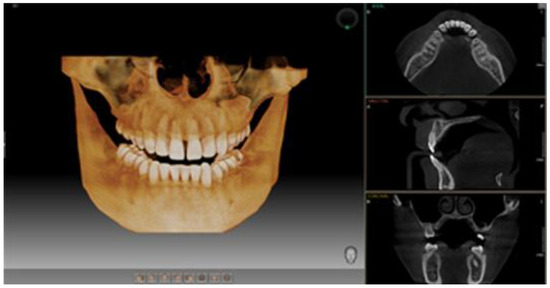
Figure 1.
Representative image of the 12 × 9 cm field of view (FOV) obtained through CBCT.
The estimated effective radiation dose ranged from 150 to 250 µSv, higher than conventional radiographs but significantly lower than medical CT scans. This FOV choice was justified by the need for detailed evaluation of bone structures in the context of diabetes mellitus. While traditional periodontal probing is useful, it lacks three-dimensional information critical for a complete diagnosis of bone defects. CBCT enabled establishing a detailed baseline for long-term monitoring and optimized treatment planning, particularly important for diabetic patients who often present with more severe and complex pathology.
Thus, CBCT provided a comprehensive view of bone status and disease progression, significantly contributing to therapeutic decisions and patient monitoring.
Findings were meticulously recorded and correlated with each patient’s questionnaire and clinical examination form.
2.3. Statistical Analyses
The data arepresented as frequencies and percentages for categorical variables, while mean (M), standard deviation (SD), and minimum/maximum values are provided for continuous variables. A p-value < 0.05 was deemed statistically significant. All analyses were performed using GraphPad Prism version 10.
3. Results
3.1. Patients’ Profile
The study included 120 subjects, organized according to several criteria. In the diabetic group we have 60 subjects and the gender distribution was equal, with 50% women and 50% men.
In terms of origin, the majority of subjects (60%) came from urban areas, and the remaining 40% from rural areas. The distribution by age group was varied, with the largest category being those aged between 51 and 60 years, representing 43.33% of the total, followed by the 41–50 group with 21.66%, and the over-61 category with 20%. The other age groups were less represented.
In the non-diabetic group, also composed of 60 people, the gender distribution was balanced, with 50% women and 50% men. Unlike the diabetic group, the majority of subjects came from rural areas, representing 70%, and 30% were from urban areas. In terms of age, the highest proportion was recorded in the 51–60 years category (40%), followed by the 41–50 years group (21.66%) and the over-61-years group (18.33%). The 31–40 and 26–30 years categories were represented in smaller proportions, 13.33% and 6.66%, respectively.
This structure provides a clear perspective on the distribution of subjects according to sex, origin, and age, thus allowing a detailed and comparative analysis between the diabetic and non-diabetic groups.
3.2. Data Analysis
In the context of this large study, an academic approach requires a reinterpretation of the results to draw a comprehensive picture. Given the significant volume of data and results obtained, we chose to highlight the most relevant findings, following a rigorous selection method. These key results are presented in detail, and their analysis and interpretation are supported by arguments and theoretical context. However, it is important to note that the study also includes other important findings, which are discussed in the Discussion Section. There, alternative approaches, methodological limitations, and implications of these less highlighted results are examined and analyzed in depth, contributing to a broader and more complete understanding of the study area.
There is a significant difference between the two groups in terms of the total number of teeth, with the non-diabetic group having a higher number of teeth present per arch (22.70 versus 18.25) as highlighted in Table 1. This difference is statistically significant, with a p-value < 0.0001. Regarding root remnants, there is also an important difference between the two groups. The non-diabetic group recorded a lower number of root remnants (2.383 versus 3.333), so although the difference is not too large, it is statistically significant (p = 0.0323). Regarding the number of intact teeth, the non-diabetic group had a significantly higher number of integrated teeth (12.12 versus 6.233), the difference being statistically significant (p < 0.0001). However, the differences in the number of impacted teeth were not statistically significant, with a p-value of 0.6108, indicating that this characteristic did not vary significantly between groups. Thus, the results suggest that the non-diabetic group has a higher number of total teeth, more intact teeth, and fewer root remnants compared to the diabetic group, but the differences in terms of impacted teeth are not significant.

Table 1.
Analysis of the teeth present on the arch.
When analyzing the total number of fillings, the diabetic group presents a significantly higher number of fillings compared to the non-diabetic group, the difference being 3933 versus 3000, with a p-value of 0.0088, indicating a statistically significant difference and suggesting that this difference cannot be attributed to chance and is highlighted in Table 2. Regarding the maxilla, the difference is significant, as the diabetic group presented a higher number of fillings (2167 vs. 1783). The p-value is 0.0355, indicating that the differences observed between the two groups are statistically significant. In the mandible, the difference is also considerable, with the diabetic group having 1767 fillings compared to 1217 in the non-diabetic group. The p-value is 0.0094, thus confirming that the difference is statistically significant. In terms of the total number of caries, the diabetic group presented a significantly higher number of caries compared to the non-diabetic group, 6167 versus 5433, and the difference was statistically significant with a p-value of 0.0376. This suggests that the observed difference was not random. The differences in the number of treated caries were also significant, with the diabetic group having 3933 treated caries compared to 2967 in the non-diabetic group, with a p-value of 0.0061, indicating that the second group had a significantly higher number of treated caries. In contrast, the differences in the number of untreated caries were not significant, with a p-value of 0.1137, suggesting that the differences between the two groups are not statistically significant. In conclusion, our results indicate that the diabetic group has a significantly higher number of fillings and a significantly higher number of treated caries compared to the non-diabetic group, both in the entire dental arch and in the maxilla and mandible. However, the differences in the number of untreated caries are not statistically significant. Despite a higher number of caries, these differences suggest a greater tendency to treat caries in the diabetic group.

Table 2.
Analysis of the number of fillings and carious processes.
Table 3 presents gingival recession data measured in millimeters comparing two specific tooth locations: incisors and molars. The results of the analysis highlight a statistically significant difference in the degree of gingival retraction between patients in the diabetic group and those in the non-diabetic group, both at the level of incisors and molars.

Table 3.
Gingival recession analysis.
At the level of incisors, diabetic patients presented a mean gingival retraction of 2250 mm, while non-diabetic patients had a significantly lower value of only 0.7333 mm. The difference is highly statistically significant, with a p-value < 0.0001, indicating that this variation cannot be attributed to chance and reflects a real influence of diabetes on periodontal condition.
Regarding molars, a similar trend was observed. The diabetic group recorded a mean gingival recession of 1950 mm, compared to only 0.4833 mm in the non-diabetic group, and in this case, the difference is very statistically significant, with p < 0.0001.
These results support the hypothesis that diabetes mellitus contributes to a more pronounced damage to the periodontium, favoring the appearance and progression of gingival retraction, both in the anterior (incisors) and posterior (molars) areas.
The values presented in Table 4 reflect the comparative analysis of the periodontal space at the maxilla and mandible level which highlighted statistically significant differences between the diabetic and non-diabetic groups. At the maxilla level, patients in the diabetic group presented a mean periodontal space widening of 1900 mm, while the non-diabetic group had a much lower mean value of 0.450 mm. The difference is highly statistically significant, with a p value < 0.0001, indicating a clear association between the presence of diabetes and periodontal structural changes. At the mandible level, the trend is maintained: the diabetic group had a mean widening of 1983 mm, compared to only 0.550 mm in the non-diabetic group. This difference is also highly statistically significant (p < 0.0001). These data suggest that diabetes mellitus contributes to radiological changes in the periodontium, reflected by periodontal space widening, a sign frequently associated with chronic inflammation and periodontal attachment loss. This widening may indicate deeper periodontal ligament damage and is a radiological indicator of periodontal disease progression in diabetic patients.

Table 4.
Periodontal space analysis.
Regarding the total periodontal pockets, Table 5 shows that the diabetic group has a mean value of 5.667 with a standard deviation of 2.152, while the non-diabetic group has a mean value of 1.017 with a standard deviation of 1.017. The difference between the two groups is statistically significant, with a p-value < 0.0001, suggesting a much higher prevalence of periodontal pockets in the diabetic group compared to the non-diabetic group.

Table 5.
Analysis of the average number of periodontal pockets.
At the maxillary level, the diabetic group has a mean value of 2.550 with a standard deviation of 1.096, and the non-diabetic group has a mean value of 0.433 with a standard deviation of 0.5928. The difference is statistically significant (p < 0.0001), indicating a much higher prevalence of maxillary periodontal pockets in the diabetic group. Regarding the mandible, the diabetic group has a mean of 3.20 and a standard deviation of 1.505, while the non-diabetic group has a mean of 0.5833 and a standard deviation of 0.6455. The difference is also statistically significant, with a p-value < 0.0001, indicating a significantly higher prevalence of periodontal pockets in the mandible in the diabetic group compared to the non-diabetic group.
Our results indicate that the diabetic group has a significantly higher number of periodontal pockets in both the total dental arch and the maxilla and mandible compared to the non-diabetic group. These statistically significant differences probably reflect poorer periodontal health in the diabetic group.
Analysis of the data in Table 6 and Table 7 highlights significant differences between the diabetic and non-diabetic groups in terms of periodontal pocket depth in the maxilla, mandible, incisors and molars. In the maxilla, the mean periodontal pocket depth was 3.433 mm in the diabetic group, compared to 0.4667 mm in the non-diabetic group (p < 0.0001), and in the mandible, the values were 3.417 mm and 0.6333 mm, respectively (p < 0.0001), indicating a much more severe periodontal involvement in diabetic patients.

Table 6.
Average depth analysis of maxillary and mandibular periodontal pockets.

Table 7.
Average depth analysis of periodontal pockets in incisors and molars.
At the level of the incisors, the diabetic group presented a mean depth of 2.433 mm, compared to 0.4833 mm in the non-diabetic group (p < 0.0001), and at the level of the molars, the differences were even more pronounced (3.750 mm vs. 0.6833 mm, p < 0.0001). These results confirm an increased prevalence and severity of periodontal disease among diabetic patients, suggesting a significant impact of diabetes on periodontal health. The statistically significant difference (p < 0.0001) confirms a significantly greater deepening of the periodontal pockets in the diabetic group compared to the non-diabetic group, indicating a significantly more severe periodontal involvement of the molars. The analyzed data, presented in Table 6 and Table 7, highlight the fact that the diabetic group presented significantly deeper periodontal pockets in the maxilla, mandible, incisors, and molars than the non-diabetic group. These statistically significant differences indicate a higher prevalence of periodontal disease in the diabetic group, with a more severe impact on periodontal health.
The analysis of bone thickness in the maxilla and mandible provides a clear picture of the periodontal health status of the two groups and is an important indicator of bone loss associated with periodontal disease. The results analyzed in Table 8 suggest a significant impact on bone health in the diabetic group compared to the non-diabetic group, with clear and significant statistical differences (p < 0.0001). In the maxilla, the bone thickness in the region of the first molar, both on the right and left sides, is significantly lower in the diabetic group compared to the non-diabetic group. The analysis of the results shows, on the right side, that the bone thickness in the diabetic group is 9.8 mm, while in the non-diabetic group it is 18.67 mm. In the mandible, the differences are even more evident on the right side (diabetes: 17.43 mm, non-diabetes: 34.70 mm). This significant decrease in bone thickness is likely associated with the progression of severe periodontal disease, and diabetes may contribute to accelerated bone resorption through its impact on inflammation and tissue regeneration capacity.

Table 8.
Analysis of the first molar bone thickness.
We have highlighted in Figure 2A,B the thickness of the mandibular bone at the level of the first molar, both on the right side (A) and on the left side (B), comparing the diabetic group with the non-diabetic one. It is observed that the bone thickness is significantly lower in the diabetic group on both sides, indicating a pronounced bone loss in this group of patients, characteristic of periodontal damage associated with diabetes. Figure 3A,B shows the thickness of the maxillary bone at the level of the first molar on the right (A) and left (B) sides. As in the mandible, the diabetic group presented a reduced bone thickness compared to the non-diabetic group, suggesting a systemic impairment of bone density in patients with diabetes, regardless of the maxillofacial area analyzed. Figure 4 provides a general comparison between the bone thickness on both sides of the maxilla and mandible in the two groups. A significant reduction in bone thickness in the diabetic group is clearly evident in all regions analyzed, supporting the hypothesis that diabetes mellitus has a generalized negative impact on bone integrity in the oral cavity.
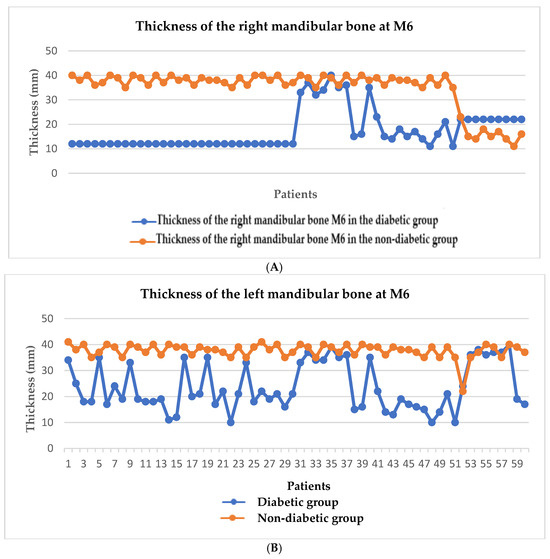
Figure 2.
Graphical representation of the mandibular bone thickness at the level of the first molar on the right (A) and left (B) sides.
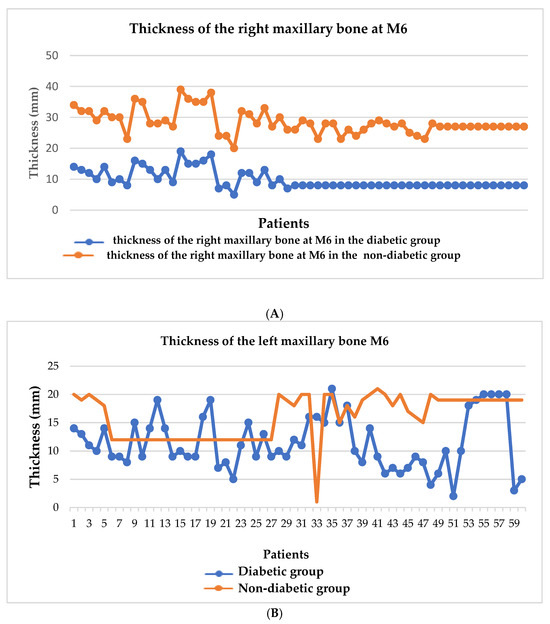
Figure 3.
Graphical representation of maxillary bone thickness at the level of the first molar on the right (A) and left (B) sides.
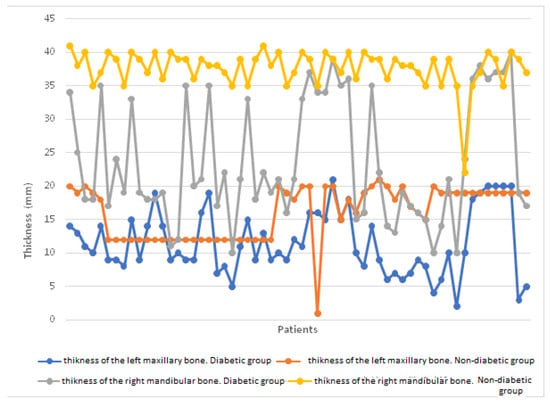
Figure 4.
Comparison of bone thickness on both sides of the maxilla and mandible in the two groups.
4. Discussion
The results of this study are consistent with the current level of research in periodontal imaging. The literature on the use of cone beam computed tomography (CBCT) in this context is limited, consisting mostly of in vitro studies and few in vivo studies [33]. The importance of using CBCT becomes even more evident in patients with diabetes mellitus, who are at increased risk of rapid periodontal disease progression and enhanced bone resorption. In these patients, poor glycemic control may worsen periodontal inflammation, accelerating the destruction of the supporting tissues of the tooth [34]. CBCT allows a detailed visualization of bone structures and the degree of periodontal damage, facilitating a more accurate diagnosis and a personalized treatment plan [35,36]. In addition, regular monitoring through CBCT of these patients can contribute to the early identification of pathological changes, thus improving the prognosis and the effectiveness of the applied treatments [37,38,39,40].
Existing studies confirm that diabetes mellitus and periodontal disease are major factors contributing to tooth loss, thus significantly affecting patients’ oral health. Diabetic patients have an increased risk of periodontal infections, leading to a reduction in the number of teeth present in the arch compared to people without diabetes [41].
The results of our study indicate significant differences between subjects in the diabetic group and those in the non-diabetic group. Diabetic patients have fewer teeth present per arch and more root remnants, suggesting a more advanced degree of dental decay. They also have significantly fewer intact teeth, reflecting the negative impact of diabetes on oral health. However, there were no significant differences in impacted teeth, suggesting that this characteristic is not directly influenced by diabetes or periodontal disease.
These findings are consistent with the literature, which emphasizes the impact of diabetes on oral health and the need for rigorous monitoring of these patients (Mealey & Ocampo, 2007) [42]. Tooth loss among diabetic patients may be associated with both chronic inflammatory processes and a reduced capacity for tissue regeneration (Preshaw et al., 2012) [43]. Also, recent studies suggest that patients with poorly controlled diabetes are at two to three times higher risk of developing severe periodontitis, implicitly leading to a higher rate of tooth loss (Chapple et al., 2014) [44].
A meta-analysis by Borgnakke (2019) demonstrated that diabetic patients are significantly more likely to have fewer teeth present in the arch and periodontal disease progression is faster in these patients [45]. Other research emphasizes that intensive periodontal treatment may improve glycemic control and thus contribute to the maintenance of more teeth (Simpson et al., 2015) [46].
Existing studies show a strong link between diabetes and oral health, particularly in terms of the incidence of tooth decay and the need for fillings. Patients with type 2 diabetes often have an increased risk of oral diseases, including dental caries and periodontal disease, compared to people without diabetes. A study by Wagner (2020) included patients with type 2 diabetes mellitus and non-diabetic patients, all with partial or total edentulism [47]. The results showed that diabetic patients had mean blood glucose values twice as high as those of non-diabetic patients, and that longer disease duration was associated with a greater degree of edentulism. Although the study did not provide specific data on the number of cavities or fillings, the presence of edentulism and high glycemic values suggests a link between diabetes and compromised oral health (Wagner, 2020) [47].
In terms of the total number of fillings, the diabetic group had significantly more fillings compared to the non-diabetic group, the difference being statistically significant (p < 0.05). This is consistent with the findings of Chapple and Genco (2013) [48], who highlighted that patients with diabetes are at increased risk of tooth loss and require more restorative interventions. In the maxilla and mandible, the significant differences observed between the two groups may be explained by the systemic effects of diabetes on oral tissues. However, the differences in the number of untreated caries were not statistically significant, suggesting that access to treatment and oral care behaviors may have a major impact on dental health in these patients [48].
Another study analyzed the effect of age and menopause on oral health in women and found that the number of caries increased with age, and after the onset of menopause, the number of missing teeth and periodontal pockets greater than 5 mm increased (Blidaru et al., 2021 [49]). Although this study did not directly analyze diabetic patients, hormonal changes and aging may affect oral health in a manner similar to the effects observed in patients with diabetes [49].
In conclusion, our results indicate that the diabetic group has a significantly higher number of fillings and a significantly higher number of treated caries compared to the non-diabetic group, both across the entire dental arch and specifically in the maxilla and mandible. However, the differences in the number of untreated caries are not statistically significant. These differences suggest a greater tendency toward caries treatment in the diabetic group, despite a higher overall number of caries, supporting previous findings that glycemic control and oral hygiene are essential for preventing dental complications in patients with diabetes mellitus.
Gingival recession is an important indicator of periodontal health. Its analysis can provide valuable information about the impact of periodontal disease, especially in patients with diabetes. Studies show that patients with diabetes have a higher prevalence of gingival recession, which may be explained by chronic inflammation and microvascular changes specific to this condition.
In our analysis, we compared gingival recession in two specific tooth locations: incisors and molars. The results presented in Table 3 show that patients in the diabetic group have significantly higher values of gingival retraction than the non-diabetic group, the difference being statistically significant. These findings are consistent with the results of other studies, which have demonstrated a direct correlation between diabetes mellitus and the severity of periodontal disease, including gingival recession.
We can conclude that patients in the diabetic group have more significant gingival recession in both incisors and molars, compared to the non-diabetic group. All the differences observed are statistically significant, suggesting that these variations are not random, but may reflect a real impact of diabetes on gingival health. These results support the importance of interdisciplinary management of patients with diabetes mellitus and periodontal disease, with collaboration between dentists and diabetologists for the effective prevention and control of periodontal disease.
Previous studies show a significant link between diabetes and periodontal health, and recent research data are relevant for the interpretation of our results. For example, the study by Sánchez and de Oliveira (2020) [50] emphasizes that glycemic control plays a crucial role in periodontal disease progression. According to them, patients with poorly controlled diabetes are predisposed to a more severe form of periodontitis and may show widening of the periodontal space as a sign of disease progression. This observation is consistent with the results of our study, in which we identified a significant widening of the periodontal space in the diabetic group compared to the non-diabetic group, indicating a direct link between diabetes and periodontal changes [50].
In addition, Sun et al. (2014) [51] conducted a systematic review emphasizing that periodontal treatments can improve glycemic control in patients with type 2 diabetes. These results suggest that periodontal interventions may not only contribute to improving gingival health, but also to reducing HbA1c levels. Our data support this theory, as widening of the periodontal space is an indicator of periodontal disease progression and may indirectly influence glycemic control in patients. Therefore, effective periodontal treatments may have a significant role in the prevention and treatment of gingival complications and may contribute to improve diabetes management [51].
The study by Nervi, Lancellotti, and Di Carlo (2019) [52] emphasizes that periodontal disease may be a risk factor for diabetic complications and periodontal changes may worsen the overall condition of diabetic patients. This is an important aspect that correlates with our observations on periodontal space widening and its impact on gingival health in patients with diabetes. Widening of the periodontal space may signal chronic inflammation, which in patients with diabetes can worsen glycemic control and increase the risk of complications [52].
In another study by Teshome and Yitayeh periodontal treatment was found to have a positive effect on glycemic control in patients with diabetes. They observed a significant reduction in HbA1c levels after periodontal treatments. These findings are important in the context of our study because they suggest that periodontal interventions not only improve gingival health, but may also influence glycemic control, which could help prevent complications of diabetes [53].
Our data, presented in Table 4, are consistent with these studies showing a strong association between periodontal health and diabetes. Periodontal space widening, which may be a sign of periodontal disease progression, is more pronounced in patients with diabetes. Poor glycemic control can also exacerbate periodontal changes and contribute to their severity. Therefore, it is essential that periodontal treatment be integrated into the management of patients with diabetes, as it has a significant impact on gingival health.
These studies suggest that appropriate monitoring and treatment of periodontal disease is essential in the prevention and management of diabetes, and that optimal glycemic control may help prevent the progression of periodontal disease and improve the patient’s overall health.
Our study investigated the prevalence and severity of periodontal pockets in patients with diabetes compared to a non-diabetic group. The results presented in Table 5 revealed significant differences between the two groups, indicating a much poorer periodontal health status in the diabetic patient group. In particular, the diabetic group presented significantly more periodontal pockets both throughout the dental arch and at the maxilla and mandible level, compared to the non-diabetic group. The differences observed were statistically significant, with p-values < 0.0001, suggesting that periodontal disease is more prevalent and severe in patients with diabetes. These findings align with numerous studies in the literature emphasizing the close link between diabetes and periodontal disease. For example, a study by Preshaw etal. (2012) highlighted the fact that patients with diabetes, particularly those with poor glycemic control, are more prone to the development of periodontal disease due to the immune and inflammatory changes associated with this condition [54]. In addition, Stöhr et al. (2021) [55] showed that patients with type 2 diabetes may experience rapid progression of periodontal disease and that periodontal treatments may contribute to better glycemic control. In this context, our study suggests that poor periodontal status in patients with diabetes may be an indicator of inadequate glycemic control, and integrated management of these conditions is needed [55].
The results of our study are also supported by the research of Löe (2000) [56], who showed that diabetes promotes gingival inflammation and increases the risk of periodontitis, which can lead to periodontal pockets. In addition, our study underscores the importance of regular monitoring of periodontal health in patients with diabetes. Deteriorating periodontal health may contribute to worsening diabetes complications [56].
A key aspect of our study was the use of conebeam computed tomography (CBCT) to evaluate periodontal structures in more detail. CBCT is proving to be an extremely useful technique for the diagnosis and monitoring of periodontal disease, providing detailed three-dimensional images of periodontal bone and tissues that cannot be obtained with conventional two-dimensional radiography. This allows a more accurate assessment of the depth of periodontal pockets and bone loss, which are essential in the diagnosis and treatment of periodontal disease. In particular, in patients with diabetes mellitus, the use of CBCT can help to monitor the evolution of bone loss and to assess the effectiveness of therapeutic interventions such as periodontal treatments and glycemic management.
Previous studies have demonstrated that CBCT is an essential tool in assessing the severity of periodontal disease in patients with diabetes, as it allows precise observations of structural alterations that may not be visible with other imaging techniques. CBCT can provide 3D images of periodontal pockets and bone loss, allowing for a more complete assessment of periodontal status. In patients with diabetes, who are at higher risk of developing severe periodontal complications, CBCT becomes a valuable method for continuously monitoring disease progression and adjusting treatments according to the patient’s progress.
Therefore, a much higher prevalence of periodontal pockets in patients with diabetes, reflecting poorer overall periodontal health, is the main conclusion of our study. These findings underscore the need for appropriate periodontal treatment and strict glycemic control to prevent worsening of diabetes and periodontal complications. The use of modern radiodiagnostic techniques in the evaluation of these patients is an important step towards a more accurate and effective management of periodontal disease in diabetic patients [57].
The analysis of the data presented in Table 6 and Table 7 compares the depth of periodontal pockets between the diabetic and non-diabetic groups at the level of the maxilla and mandible, as well as at their different locations. These data are essential for assessing periodontal health and the severity of periodontal disease in the two groups. According to a study conducted by researchers, the depth of periodontal pockets is an important indicator of the progression of periodontal disease and can help in the correct diagnosis of periodontal disease, especially in patients with systemic diseases. In the analysis of the presented data, the diabetic group presented a significantly greater depth of periodontal pockets compared to the non-diabetic group, reflecting a significantly higher prevalence of periodontal disease. Specifically, in the diabetic group, the depth of periodontal pockets is much greater at the level of the maxilla, mandible, incisors, and molars. These statistically significant differences suggest a significantly more severe periodontal disease in the diabetic group, and the data indicate a significantly worse periodontal health status than in the non-diabetic group, which has significantly lower values of periodontal pocket depth. This is similar to previous research that emphasizes the close relationship between periodontal disease and systemic conditions such as diabetes, and demonstrates the importance of monitoring periodontal pocket depth as a predictor of long-term periodontal health.
CBCT (Cone Beam Computed Tomography) plays a crucial role in the management of patients with diabetes mellitus and periodontal disease, making a significant contribution to the diagnosis, monitoring, and treatment of this complex condition. By providing detailed three-dimensional images, CBCT allows a much more accurate assessment of periodontal status and bone structure than conventional radiographs. These images help the dentist to detect bone loss at an earlier stage, allowing for more prompt and effective treatment. Early detection of bone loss can prevent the progression of periodontal disease, thereby reducing the risks associated with tooth loss [58].
In addition, CBCT allows accurate monitoring of the progression of periodontal disease in patients with diabetes, who are prone to rapid development of periodontal disease. Comparing images from different points in the treatment can show exactly how the disease is progressing, and the doctor can adjust interventions based on the results. CBCT is also essential for personalizing treatment. For example, the degree of bone resorption visible on CBCT imaging can be used to determine whether conservative treatments are sufficient or whether surgical or other procedures are needed to regenerate bone.
Monitoring the effectiveness of periodontal treatments is another advantage of using CBCT. After periodontal interventions, CBCT can be used to assess whether the treatments have been successful by comparing the bone status before and after treatment. Thesedataareessential for adjusting treatment plans and ensuring that the patient receives the best possible care. Moreover, because diabetes can complicate tissue healing, CBCT helps prevent further complications and manage postoperative risks.
The detailed images generated by CBCT can also be used for patient education. By visually explaining how periodontal disease affects the supporting structures of the teeth, the patient better understands the importance of treatment and the need for proper glycemic control. These images can significantly contribute to the patient’s motivation to adhere to the proposed treatments and improve long-term oral health [58,59].
Several studies in the literature have shown that type 2 diabetes mellitus is a major risk factor for the development of periodontal disease, which can lead to significant bone loss in the maxilla and mandible. They have also identified that insulin resistance correlates with the severity of bone loss in patients with diabetes, and periodontal treatments can help to stop this process. Other studies have also investigated the effect of full-thickness diabetes on periodontal regions and concluded that patients with diabetes mellitus present a significant loss of bone density in the molar area, especially in cases of long-standing and poorly controlled diabetes. This loss of bone density may explain the decrease in bone thickness observed in the diabetic group in our study. Another important study has demonstrated that patients with diabetes, especially those with poor glycemic control, present an accelerated bone loss and a higher incidence of periodontal disease. This research confirms and supports our results, indicating a significant decrease in bone thickness in the maxillary and mandibular regions in patients with diabetes. This visible difference between the two groups was highlighted in Figure 2, Figure 3 and Figure 4 and represents a significant reduction in bone thickness in diabetic patients, which may reflect a higher degree of bone resorption associated with periodontal disease and metabolic disorders related to diabetes [59].
The aforementioned studies and our research are consistent and suggest that the significant bone loss observed in the diabetic group of our study is the result of the interaction between chronic inflammation caused by periodontal disease and systemic factors such as diabetes mellitus. These findings highlight the importance of adequate glycemic control and periodontal interventions to prevent the progression of bone loss in such cases.
In the context of our study, CBCT was essential in analyzing bone thickness in the maxilla and mandible, thus helping to detect and quantify bone loss in more detail. A study by Zhao showed that the use of CBCT in the assessment of periodontal disease allows for more accurate detection of vertical and horizontal bone loss, which helps in the effective monitoring of patients with diabetes and periodontitis. Research also suggests that CBCT can help in the early diagnosis of periodontal disease so that therapeutic interventions can be implemented before bone loss becomes significant. In addition, CBCT can be useful in assessing the effectiveness of periodontal treatment. Studies suggest that the use of CBCT to monitor post-treatment bone changes helps in assessing the success of interventions and adjusting the treatment plan based on progress.
With technological advances, significant improvements in CBCT imaging are anticipated, including a significant reduction in radiation dose, the development of faster detection panels that will shorten image acquisition time, and the optimization of reconstruction algorithms to minimize artifacts. New generations of CBCT devices are already capable of providing higher-resolution images while maintaining a minimal dose of radiation exposure, which is especially essential for patients who require frequent monitoring, such as those with systemic conditions, including diabetes. For diabetic patients, who are at increased risk of bone resorption and periodontal infections, CBCT plays a crucial role in diagnosis. Advanced imaging allows the identification of areas of low bone density so that treatment can be adjusted to increase the success rate. In addition, the use of emerging technologies, such as artificial intelligence integrated into CBCT analysis software, can contribute to faster and more accurate interpretation of data, facilitating personalized clinical decisions for each patient [59,60].
However, although CBCT has established itself as a valuable tool in the diagnosis and monitoring of periodontal disease, especially in patients with diabetes, its use in routine dental practice presents multiple limitations that deserve critical discussion. First, the high costs associated with the acquisition and operation of the equipment may restrict the accessibility of this technology, especially in the context of clinics with limited financial resources, thus raising important questions regarding the economic profitability of its integration into the usual treatment protocols. Second, although CBCT provides high-resolution three-dimensional images, they may be affected by artifacts generated by metallic dental restorations or patient movement, aspects that may compromise the accuracy of structural assessments. Furthermore, bone density derived from CBCT does not benefit from a standardization equivalent to that offered by conventional computed tomography, which limits the quantitative interpretation of the results. Regarding safety, although the radiation dose emitted is much lower compared to medical computed tomography, it remains higher than that associated with conventional radiographs, requiring a rigorous justification of the indication.
Last but not least, although the existing literature highlights the clinical benefits of CBCT, there is a significant lack of clinical-economic studies that analyze the cost-effectiveness of this method in current dental practice, an essential aspect for substantiating broad recommendations for use.
Consequently, although CBCT constitutes an advanced technological resource with considerable potential in the management of periodontal disease, its widespread adoption must be accompanied by a careful assessment of the cost–benefit ratio, technical limitations, and risks associated with radiological exposure. Especially for patients with diabetes mellitus, in whom the risk and severity of periodontal complications are significantly increased, the use of CBCT represents an indispensable tool, offering superior accuracy in diagnosis and monitoring, which can contribute to improving the prognosis and quality of life of these patients.
5. Conclusions
Our study supports that careful monitoring of periodontal health, including assessment of bone loss by CBCT and measurement of periodontal pocket depth, is essential for the effective management of patients with diabetes. We recommend the use of CBCT not only for diagnosis and treatment planning, but also for periodic monitoring of the disease, to adjust and enable early detection of periodontal changes, disease prevention, and complications. The integration of radiodiagnostic technologies, such as CBCT, into clinical practice is indispensable for creating modern therapeutic outcomes and quality of life for patients with diabetes and periodontal disease.
Author Contributions
Conceptualization, A.D. and E.A.; methodology, A.C. and G.B.; software, L.S. and O.-R.A.; validation, E.A., G.B., A.D. and A.C.; formal analysis, L.S.; investigation, A.D. and A.C.; resources, A.D.; data curation, E.A.; writing—original draft preparation, A.D. and O.-R.A.; writing—review and editing, E.A. and A.D.; visualization, E.A. and G.B.; supervision, L.S.; project administration, E.A. and A.D.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Lucian Blaga University of Sibiu through the research grant LUBS-IRG-2022-08, contract number 2876/18.07.2022.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of CMI DR. ALINA CRISTIAN (protocol code 030 and date of approval 2 May 2022).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Yameny, A.A. Diabetes Mellitus Overview 2024. J. Biosci. Appl. Res. 2024, 10, 641–645. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Schleicher, E.; Standl, E.; Häring, H.U. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Sinha, C. A Review on Diabetes Mellitus: Type1 & Type2. World J. Pharm. Pharm. Sci. 2020, 9, 838–850. [Google Scholar]
- Olisah, C.C.; Smith, L.; Smith, M. Diabetes Mellitus Prediction and Diagnosis from a Data Preprocessing and Machine Learning Perspective. Comput. Methods Programs Biomed. 2022, 220, 106773. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Lupsa, B. Clinical Presentation, Diagnosis, and Initial Evaluation of Diabetes Mellitus in Adults. UpToDate. 2021. Available online: https://www.uptodate.com/contents/clinical-presentation-diagnosis-and-initial-evaluation-of-diabetes-mellitus-in-adults (accessed on 10 August 2025).
- Hegde, S.; Gao, J.; Vasa, R.; Cox, S. Factors Affecting Interpretation of Dental Radiographs. Dentomaxillofac. Radiol. 2023, 52, 20220279. [Google Scholar] [CrossRef]
- Woodward, T.M. Interpretation of Dental Radiographs. Top. Companion Anim. Med. 2009, 24, 37–43. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, H.; Lee, J.S. Fundamentals of Radiographic Interpretation for the Dentist. Dent. Clin. N. Am. 2021, 65, 409–425. [Google Scholar] [CrossRef]
- Small, L. The Role of Clinical History in the Interpretation of Chest Radiographs. Radiography 2021, 27, 698–703. [Google Scholar] [CrossRef]
- Kiljunen, T.; Kaasalainen, T.; Suomalainen, A.K.; Kortesniemi, M. Dental cone beam CT: A review. Phys. Med. 2015, 31, 844–860. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Soltani, P. Magnetic Resonance Imaging in Digital Dentistry: The Start of a New Era. Prosthesis 2024, 6, 798–802. [Google Scholar] [CrossRef]
- Al-Haj Husain, A.; Oechslin, D.A.; Stadlinger, B.; Valdec, S. Magnetic resonance imaging in dental implant surgery: A systematic review. Int. J. Implant. Dent. 2024, 10, 14. [Google Scholar] [CrossRef]
- Panahi, O.; Jabbarzadeh, M. The Expanding Role of Artificial Intelligence in Modern Dentistry. J. Dent. Oral Health 2025, 8, 2025. [Google Scholar] [CrossRef]
- Ali, M.A. The Role of Artificial Intelligence in Modern Dentistry: Applications, Challenges, and Future Directions. Future Dent. Res. 2024, 2, 39–49. [Google Scholar] [CrossRef]
- Distefano, S.; Cannarozzo, M.G.; Spagnuolo, G.; Bucci, M.B.; Lo Giudice, R. The “dedicated” CBCT in dentistry. Int. J. Environ. Res. Public Health 2023, 20, 5954. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Saeidi, N.; Salehi, A.; Attari, K.; Irani, M.A.; Aryanezhad, S.S.; Malekzadeh, R.; Mahmoudi Anzabi, R. Advances in Radiation Protection in Oral and Dental Radiology: Pragmatic Approaches and Recent Innovations. Front. Biomed. Technol. 2025, 12, 166–178. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 2020, 82, 257–267. [Google Scholar] [CrossRef]
- Dinu, A.; Antonescu, O.R. Clinical Study on the Implications of Immunological Markers in the Diagnosis of Periodontitis in People with Diabetes Mellitus. Dent. J. 2024, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Z.; Yuan, Y.-H.; Liu, H.-H.; Li, S.-S.; Zhang, B.-W.; Chen, W.; An, Z.-J.; Chen, S.-Y.; Wu, Y.-Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Mehriz, B.M.; Atteya, M.A.; Skipina, T.M.; Mostafa, M.A.; Soliman, E.Z. Association between periodontitis and diabetes mellitus in the general population. J. Diabetes Metab. Disord. 2022, 21, 1249–1254. [Google Scholar] [CrossRef]
- Păunică, I.; Giurgiu, M.; Dumitriu, A.S.; Păunică, S.; Pantea Stoian, A.M.; Martu, M.-A.; Serafinceanu, C. The bidirectional relationship between periodontal disease and diabetes mellitus—A review. Diagnostics 2023, 13, 681. [Google Scholar] [CrossRef]
- Orstavik, D. (Ed.) Essential Endodontology: Prevention and Treatment of Apical Periodontitis; John Wiley & Sons: Chichester, UK, 2020. [Google Scholar]
- Tyndall, D.; Rathore SCone-beam, C.T. Diagnostic applications: Caries, periodontal bone assessment, and endodontic applications. Dent. Clin. N. Am. 2008, 52, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Jervøe-Storm, P.M.; Hagner, M.; Neugebauer, J.; Ritter, L.; Zöller, J.E.; Jepsen, S.; Frentzen, M. Comparison of Cone-Beam Computerized Tomography and Intraoral Radiographs for Determination of the Periodontal Ligament in a Variable Phantom. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Schmidt, J.C.; Rinne, C.A.; Mendes, S.; Dula, K.; Sculean, A. Cone beam computed tomography (CBCT) for diagnosis and treatment planning in periodontology: Systematic review update. Clin. Oral Investig. 2020, 24, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Assiri, H.; Dawasaz, A.A.; Alahmari, A.; Asiri, Z. Cone beam computed tomography (CBCT) in periodontal diseases: A systematic review based on the efficacy model. BMC Oral Health 2020, 20, 191. [Google Scholar] [CrossRef]
- Kaasalainen, T.; Ekholm, M.; Siiskonen, T.; Kortesniemi, M. Dental cone beam CT: An updated review. Phys. Med. 2021, 88, 193–217. [Google Scholar] [CrossRef]
- Morar, L.; Băciuț, G.; Băciuț, M.; Bran, S.; Colosi, H.; Manea, A.; Almășan, O.; Dinu, C. Analysis of CBCT bone density using the Hounsfield scale. Prosthesis 2022, 4, 414–423. [Google Scholar] [CrossRef]
- Walter, C.; Weiger, R.; Zitzmann, N.U. Accuracy of three-dimensional imaging in assessing periodontal defects: A systematic review. J. Clin. Periodontol. 2016, 43, 313–323. [Google Scholar]
- Qian, Y.; Qiao, H.; Wang, X.; Zhan, Q.; Li, Y.; Zheng, W.; Li, Y. Comparison of the accuracy of 2D and 3D cephalometry: A systematic review and meta-analysis. Aust. Orthod. J. 2022, 38, 130–144. [Google Scholar] [CrossRef]
- Katlapa, A.; Kaartinen, S.M.; Koivisto, J.H.; Matikka, H. Radiation exposure to fetus from extremity CBCT examinations. Eur. J. Radiol. 2022, 156, 110548. [Google Scholar] [CrossRef]
- He, L.; Zhang, S.; Xie, Y.; Zhang, L. The Role of CBCT in the Diagnosis and Management of Periodontal Disease in Patients with Diabetes. J. Clin. Periodontol. 2022, 49, 617–625. [Google Scholar] [CrossRef]
- Tariq, M.A.; Shafique, M.; Ahmed, N. Use of Cone-Beam Computed Tomography (CBCT) in Periodontal Disease Diagnosis and Management in Diabetic Patients: A Systematic Review. J. Periodontol. 2020, 91, 345–354. [Google Scholar] [CrossRef]
- Mendes, J.J.; Viana, J.; Cruz, F.; Garrido, L.; Jessen, I.; Rodrigues, J.; Proença, L.; Delgado, A.S.; Machado, V.; Botelho, J. Radiographically Screened Periodontitis Is Associated with Deteriorated Oral-Health Quality of Life: A Cross-Sectional Study. PLoS ONE 2022, 17, e0269934. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Misra, D.; Misra, A. Cone-Beam Computed Tomography Assessment of Bone Using Grayscale Values in Patients with Diabetes Mellitus: A Case–Control Observational Study. J. Indian Soc. Periodontol. 2020, 24, 560–566. [Google Scholar] [CrossRef]
- Braun, X.; Ritter, L.; Jervøe-Storm, P.-M.; Frentzen, M. Diagnostic Accuracy of CBCT for Periodontal Lesions. Clin. Oral Investig. 2014, 18, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Valiyaparamabil, J.V.; Yamany, I.; Ortiz, D.; Shafer, D.M.; Pendrys, D.; Freilich, M.; Mallya, S.M. Bone Quality Evaluation: Comparison of Cone Beam Computed Tomography and Subjective Surgical Assessment. Int. J. Oral Maxillofac. Implants 2012, 27, 1271–1277. [Google Scholar]
- Haiderali, Z. The Role of CBCT in Implant Dentistry: Uses, Benefits and Limitations. Br. Dent. J. 2020, 228, 560–561. [Google Scholar] [CrossRef]
- Jacobs, R. Dental Cone Beam CT and Its Justified Use in Oral Health Care. J. Belg. Soc. Radiol. 2011, 94, 254–265. [Google Scholar] [CrossRef]
- Taylor, G.W. Bidirectional Interrelationships between Diabetes and Periodontal Diseases: An Epidemiologic Perspective. Ann. Periodontol. 2001, 6, 99–112. [Google Scholar] [CrossRef]
- Mealey, B.L.; Ocampo, G.L. Diabetes Mellitus and Periodontal Disease. Periodontology 2000 2007, 44, 127–153. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and Diabetes: A Two-Way Relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Borgnakke, W.S.; Genco, R.J. Hemoglobin A1c Levels among Patients with Diabetes Receiving Nonsurgical Periodontal Treatment. JAMA 2014, 311, 1919–1920. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, W.S. Does Treatment of Periodontal Disease Influence Systemic Disease? Dent. Clin. N. Am. 2019, 63, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.C.; Weldon, J.C.; Worthington, H.V.; Needleman, I.; Wild, S.H.; Moles, D.R.; Stevenson, B.; Glenny, A.-M. Treatment of Periodontal Disease for Glycaemic Control in People with Diabetes Mellitus. Cochrane Database Syst. Rev. 2015, 11, CD004714. [Google Scholar] [CrossRef]
- Wagner, N.A.G. Evaluarea Sănătății Orale la Pacienții cu Diabet Zaharat Tip 2. Bachelor’s Thesis, Universitatea de Medicină și Farmacie “Carol Davila”, Bucharest, Romania, 2020. [Google Scholar]
- Chapple, I.L.; Genco, R.; Working Group 2 of the Joint EFP/AAP Workshop. Diabetes and Periodontal Diseases: Consensus Report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S106–S112. [Google Scholar] [CrossRef]
- Blidaru, C.L.; Lescai, I.I.; Ioana-Mădălina. Impactul Vârstei și al Menopauzei Asupra Sănătății Orale. Bachelor’s Thesis, Universitatea de Medicină și Farmacie “Carol Davila”, Bucharest, Romania, 2021. [Google Scholar]
- Sánchez, G.A.; de Oliveira, L.F. Influence of Diabetes on Periodontal Health: A Review of the Relationship between Glycemic Control and Periodontal Disease Progression. J. Clin. Periodontol. 2020, 47, 723–730. [Google Scholar] [CrossRef]
- Sun, Q.-Y.; Feng, M.; Zhang, M.-Z.; Zhang, Y.-Q.; Cao, M.-F.; Bian, L.-X.; Guan, Q.-B.; Song, K.-L. Effects of periodontal treatment on glycemic control in type 2 diabetic patients: A meta-analysis of randomized controlled trials. Chin. J. Physiol. 2014, 57, 279–285. [Google Scholar] [CrossRef]
- Nervi, C.; Lancellotti, C.; Di Carlo, M. Periodontal Disease as a Risk Factor for Diabetes Complications: A Systematic Review. Diabetes Res. Clin. Pract. 2019, 153, 90–100. [Google Scholar] [CrossRef]
- Teshome, A.; Yitayeh, A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health 2016, 17, 31. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Taylor, J.J.; Thomas, S.R. Diabetes and Periodontal Disease: A Review of the Evidence. J. Clin. Periodontol. 2012, 39, 33–39. [Google Scholar] [CrossRef]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Löe, H. Periodontal Disease: The Sixth Complication of Diabetes Mellitus. Diabetes Care 2000, 23, 456–463. [Google Scholar] [CrossRef]
- Alshabab, M.A.; Al-Askar, M.; Al-Harbi, A.S. Influence of Diabetes on Bone Loss in the Periodontal Region: A Cross-Sectional Study. J. Diabetes Endocrinol. 2022, 8, 234–240. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Li, Y. Cone-Beam Computed Tomography in the Diagnosis and Evaluation of Periodontal Disease. J. Periodontal Res. 2019, 54, 242–251. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, C.; Li, P. The Role of CBCT in Monitoring Periodontal Bone Loss and Evaluating Treatment Outcomes in Patients with Periodontitis. J. Clin. Periodontol. 2020, 47, 848–857. [Google Scholar] [CrossRef]
- Borgnakke, W.S.; Poudel, P. Diabetes and Oral Health: Summary of Current Scientific Evidence for Why Transdisciplinary Collaboration Is Needed. Front. Dent. Med. 2021, 2, 709831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).