Implantable Medical Electronic Devices: Sensing Mechanisms, Communication Methods, and the Biodegradable Future
Abstract
1. Introduction
Research Method
2. Sensing Method
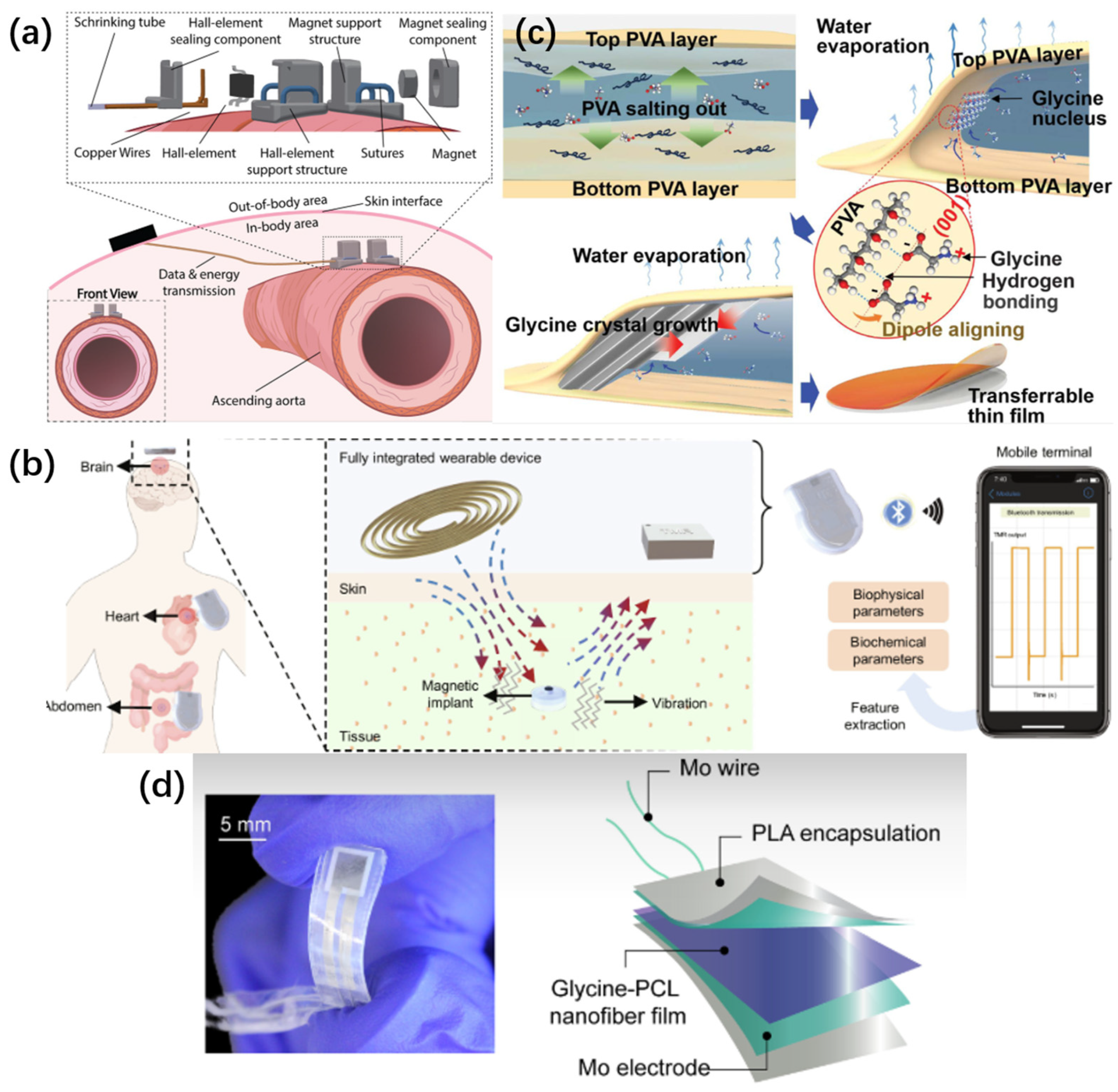
2.1. Magnetic Field Sensing
2.1.1. Applied Technologies
2.1.2. Developing Technologies
- ●
- Superconducting Quantum Interference Devices (SQUIDs) [21] operate based on the principle of Josephson junctions. This technology is capable of detecting magnetic fields within the range of pT to fT [22,23]. Nevertheless, it has certain limitations. The most notable drawbacks include high costs, large size, and the necessity for cooling equipment and a shielding room [21,24,25]. Prior to system simplification and miniaturization, the application of this technology in implantable devices remains challenging. Despite these challenges, current research indicates that this technology holds great promise.
- ●
- Atomic magnetometers (AM): These devices heat atoms to elevated temperatures and utilize the pumping and detection mechanisms of an optical system to detect the magnetic decay of atomic spins [26,27]. Atomic magnetometers are further classified into two distinct types: optically pumped magnetometers (OPM) and spin-exchange relaxation-free magnetometers (SERF). Under specific conditions, the performance of atomic magnetometers can be on par with that of Superconducting Quantum Interference Device (SQUID) sensors. In particular, the atomic magnetometer operating on the SERF principle can attain a sensitivity of 10 fT/√Hz at 10 Hz [28,29]. One of the significant advantages of this sensing approach is that it does not necessitate any cooling structure. Consequently, its volume is substantially smaller than that of SQUIDs and can be fabricated to be just a few centimeters in size [30,31]. It holds the potential for transformation into an implantable device. Nevertheless, atomic magnetometers are not without their limitations. A fundamental drawback is that they can only function in an environment characterized by a near-zero magnetic field [27]. All varieties of atomic magnetometers necessitate magnetic shielding to maintain a near-zero ambient noise level in order to attain ultra-high sensitivity. Another shortcoming is that, owing to theoretical constraints, the bandwidth of atomic magnetometers is extremely narrow [32], which restricts the frequency range of bio-magnetic signals that can be detected. Consequently, there remains a substantial distance to cover before atomic magnetometers can be put into practical use.
- ●
- Magnetic field shielding technologies (MSRs): Magnetic field shielding technologies play a crucial role in the magnetic field sensing system. The magnetic shielding room, as an essential component thereof, aims to mitigate background noise during the monitoring of bio magnetic fields. It can be classified into two categories: active and passive [30]. At present, research regarding magnetic shielding materials predominantly centers on the selection of materials for shielding rooms. A representative shielding material is the Mu material. This is a shielding material derived by incorporating various other components into a nickel-iron alloy as the base [33,34,35]. Moreover, novel materials such as manganese-zinc ferrite are under investigation as promising alternatives [36].
2.2. Piezoelectric Sensing
2.2.1. Applied Technologies
2.2.2. Developing Technologies
- ●
- Novel Forms of Piezoelectric Materials: Piezoelectric materials serve as the core constituents of piezoelectric sensors. The commonly utilized piezoelectric materials are classified into organic and inorganic piezoelectric materials. Organic materials typically exhibit excellent flexibility; however, their piezoelectric properties are generally inferior to those of inorganic piezoelectric materials. Researchers are delving into the manufacturing techniques of piezoelectric materials. By means of additive manufacturing and 3D printing technologies, piezoelectric ceramics, piezoelectric polymers, etc., can be fabricated into sensor components of appropriate geometries. For instance, piezoelectric thin films can be adhered to the surface or embedded within composite structures, departing from the conventional stacked wafer configuration [44].
- ●
- Distinct from the piezoelectric effect, which is exclusive to non-centrosymmetric materials, the flexoelectric effect represents an emerging area of research. It characterizes the coupling relationship between mechanical strain gradients and electrode polarization. The flexoelectric effect exhibits storage dependence, and this effect becomes more pronounced as the system size diminishes [45,46]. At present, investigations into flexoelectricity predominantly concentrate on principle exploration and the observation of the effect in crystals [47]. Researchers have developed curvature and torque sensors leveraging the flexoelectric effect [48,49]. However, there are no specific instances of its applications in the human body. There remains a vast expanse of research potential for flexoelectric effect sensors in biological tissues. Potential application scenarios might encompass mass sensing, drug delivery, implantable micro energy storage devices, etc. [50,51,52].
- ●
- Piezoelectric Power Supply Network: Piezoelectric sensors, serving as an intermediary in the conversion of mechanical energy into electrical energy, hold the potential to power other implanted electronic devices. Nevertheless, currently, piezoelectric nanogenerators (PENG) are still confronted with issues such as low output power, unstable output, and a narrow frequency spectrum range. Ye enhanced the driving capacity of PENG via an improved LC matching network for frequency tracking and power regulation [53]. However, there remains a significant distance to cover before its practical application. Research indicates that resonance-based piezoelectric energy harvesters (PEHs) can amplify the resonance frequency. Nevertheless, this inevitably introduces the drawback of a narrow bandwidth [54], and simultaneously restricts the miniaturization of PEHs [55]. At present, no practical piezoelectric energy harvesting devices are available.
2.3. Capacitive Sensing and Inductive Sensing
2.3.1. Applied Technologies
2.3.2. Developing Technologies
- ●
- Employing differential sensors to mitigate the environmental influence on the sensors via the superposition principle [71,72]. Nevertheless, this method imposes extremely high demands on the symmetry between the two resonators, posing a challenge during the sensor design and manufacturing processes [73].
- ●
- Augmenting the electric field surrounding the resonator serves to enhance the sensor’s capacity to polarize molecules within the measured material, thereby boosting the sensor’s sensitivity. This approach has been implemented in certain passive resonant sensors [74,75,76]. Nevertheless, in specific scenarios, noise sources in the environmental space can obscure readings featuring relatively minor frequency shifts. Consequently, the key challenge confronting this type of device remains how to mitigate environmental noise and further elevate the sensor’s sensitivity [77]. There is an urgent need for the emergence of novel technologies.
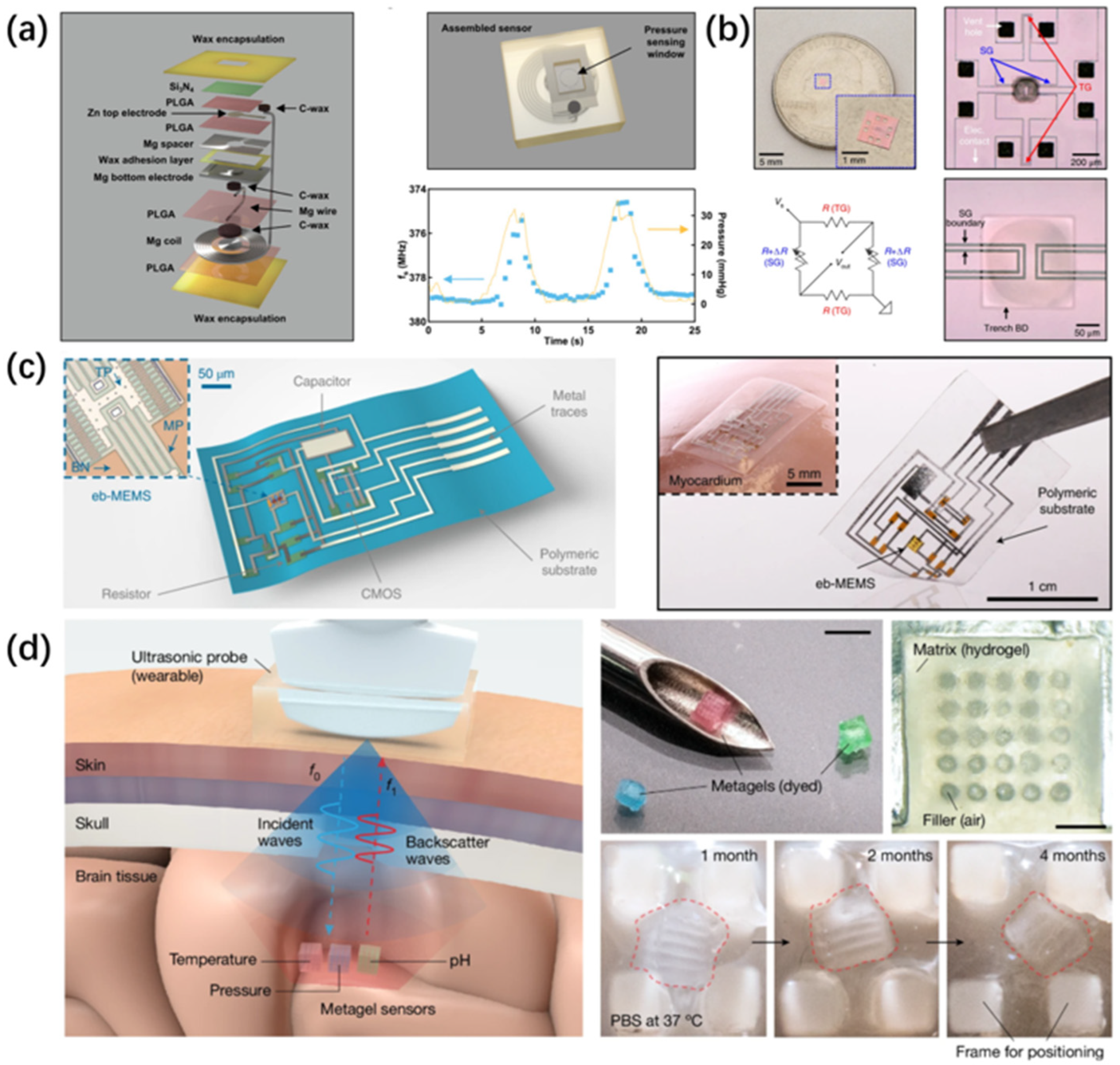
2.4. Ultrasonic Sensing
2.4.1. Applied Technologies
2.4.2. Developing Technologies
3. Wireless Communication and Power Transmission
3.1. Wireless Information Transmission
3.2. Wireless Charge
3.3. Antenna Design
4. Degradable Sensor Materials
4.1. Degradable Metallic Materials
| Metal | Degradation Rate | Degradation Product |
|---|---|---|
| Mg | In Hank’s solution, the degradation rate of magnesium is approximately 0.35 mm/year, as reported in reference [132]. | Mg (OH)2, H2 |
| Mo | In the simulated physiological solution (c-sbf-ca, pH = 7.4), the corrosion rate of pure molybdenum is approximately 10 μm/year, as presented in reference [133]. | H2MoO4 |
| Fe | Pure iron was immersed in the simulated body fluid (SBF) solution, and the mass loss was measured. The response of human endothelial cells (EC) to various concentrations of ferrous ions was investigated via the WST-8 assay. The results indicated that the average in vitro degradation rate of iron is approximately 20.4 μg/(cm2·h), as described in reference [134]. | Fe2O3, Fe3O4 |
| Zn | The corrosion rate of pure zinc in the simulated body fluid is approximately 0.02 mm/year, as shown in reference [135]. | Zn (OH)2, ZnO, ZnCO3 |
4.2. Silicon-Based Materials
4.3. Degradable Polymeric Materials
- ●
- Polymers and copolymers:
- ●
- Polysaccharides:
- ●
- Biomass Materials and Molecular Ferroelectric Materials:
5. Degradable Devices
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Clausen, I.; Glott, T. Development of Clinically Relevant Implantable Pressure Sensors: Perspectives and Challenges. Sensors 2014, 14, 17686–17702. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Kim, B.J.; Meng, E. Chronically Implanted Pressure Sensors: Challenges and State of the Field. Sensors 2014, 14, 20620–20644. [Google Scholar] [CrossRef] [PubMed]
- Ollmar, S.; Fernandez Schrunder, A.; Birgersson, U.; Kristoffersson, T.; Rusu, A.; Thorsson, E.; Hedenqvist, P.; Manell, E.; Rydén, A.; Jensen-Waern, M.; et al. A battery-less implantable glucose sensor based on electrical impedance spectroscopy. Sci. Rep. 2023, 13, 18122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Malik, J.; Seo, J.M.; Cho, Y.M.; Bien, F. Subcutaneously implantable electromagnetic biosensor system for continuous glucose monitoring. Sci. Rep. 2022, 12, 17395. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Shokoueinejad, M.; Iskandar, B.J.; Medow, J.E.; Webster, J.G. A Novel Intracranial Pressure Readout Circuit for Passive Wireless LC Sensor. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1123–1132. [Google Scholar] [CrossRef]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y.; et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.U.; Won, S.M.; Zhao, J.; Avila, R.; Wang, H.; Chun, K.S.; Jang, H.; Lee, K.H.; Kim, J.-H.; et al. A battery-less wireless implant for the continuous monitoring of vascular pressure, flow rate and temperature. Nat. Biomed. Eng. 2023, 7, 1215–1228. [Google Scholar] [CrossRef]
- Jang, J.; Habibagahi, I.; Mathews, R.P.; Gwak, W.; Rahmani, H.; Babakhani, A. A Wirelessly Powered, Battery-Less, and Miniaturized Microchip for Implantable Biopotential-Monitoring Applications. IEEE Sens. J. 2025, 25, 19545–19554. [Google Scholar] [CrossRef]
- Yang, M.; Ye, Z.; Alsaab, N.; Farhat, M.; Chen, P.Y. In-Vitro Demonstration of Ultra-Reliable, Wireless and Batteryless Implanted Intracranial Sensors Operated on Loci of Exceptional Points. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 287–295. [Google Scholar] [CrossRef]
- Herbert, R.; Mishra, S.; Lim, H.-R.; Yoo, H.; Yeo, W.-H. Fully Printed, Wireless, Stretchable Implantable Biosystem toward Batteryless, Real-Time Monitoring of Cerebral Aneurysm Hemodynamics. Adv. Sci. 2019, 6, 1901034. [Google Scholar] [CrossRef]
- Shi, B.; Liu, Z.; Zheng, Q.; Meng, J.; Ouyang, H.; Zou, Y.; Jiang, D.; Qu, X.; Yu, M.; Zhao, L.; et al. Body-Integrated Self-Powered System for Wearable and Implantable Applications. ACS Nano 2019, 13, 6017–6024. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Q.; Wang, Z.L.; Li, Z. Nanogenerator-Based Self-Powered Sensors for Wearable and Implantable Electronics. Research 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Kiran, N.; Wang, R.; Gummeson, J.; Lee, S.I. SkinnyPower: Enabling batteryless wearable sensors via intra-body power transfer. In Proceedings of the 17th Conference on Embedded Networked Sensor Systems, New York, NY, USA, 10–13 November 2019; pp. 68–82. [Google Scholar]
- Herrera-May, A.L.L.; Aguilera-Cortés, L.A.; García-Ramírez, P.J.; Mota-Carrillo, N.B.; Padrón-Hernández, W.Y.; Figueras, E.; Mota-Carrillo, N.B.; Padrón-Hernández, W.Y. Development of Resonant Magnetic Field Microsensors: Challenges and Future Applications. In Microsensors; Minin, O., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar][Green Version]
- Magkoutas, K.; Weisskopf, M.; Falk, V.; Emmert, M.Y.; Meboldt, M.; Cesarovic, N.; Schmid Daners, M. Continuous Monitoring of Blood Pressure and Vascular Hemodynamic Properties With Miniature Extravascular Hall-Based Magnetic Sensor. JACC Basic Transl. Sci. 2023, 8, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Nie, Z.; Xu, J.; Zhang, Z.; Yao, S.; Xiang, Z.; Lin, X.; Lu, Y.; Xu, C.; Zhao, P.; et al. Millimeter-scale magnetic implants paired with a fully integrated wearable device for wireless biophysical and biochemical sensing. Sci. Adv. 2024, 10, eadm9314. [Google Scholar] [CrossRef]
- Zuo, S.; Heidari, H.; Farina, D.; Nazarpour, K. Miniaturized Magnetic Sensors for Implantable Magnetomyography. Adv. Mater. Technol. 2020, 5, 2000185. [Google Scholar] [CrossRef]
- Tan, E.L.; DeRouin, A.J.; Pereles, B.D.; Ong, K.G. Design, Fabrication, and Implementation of a Wireless, Passive Implantable Pressure Sensor Based on Magnetic Higher-Order Harmonic Fields. Biosensors 2011, 1, 134–152. [Google Scholar] [CrossRef]
- Pan, L.; Xie, Y.; Yang, H.; Li, M.; Bao, X.; Shang, J.; Li, R.-W. Flexible Magnetic Sensors. Sensors 2023, 23, 4083. [Google Scholar] [CrossRef]
- Zhu, K.; Kiourti, A. A Review of Magnetic Field Emissions From the Human Body: Sources, Sensors, and Uses. IEEE Open J. Antennas Propag. 2022, 3, 732–744. [Google Scholar] [CrossRef]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef]
- Kang, C.S.; Lee, Y.H.; Yu, K.K.; Kwon, H.; Kim, J.M.; Kim, K.; Lim, H.K.; Park, Y.K.; Lee, S.G. Measurement of MCG in Unshielded Environment Using a Second-Order SQUID Gradiometer. IEEE Trans. Magn. 2009, 45, 2882–2885. [Google Scholar] [CrossRef]
- Klein, A.; van Leeuwen, P.; Hoormann, J.; Grönemeyer, D. Magnetoneurographic registration of propagating magnetic fields in the lumbar spine after stimulation of the posterior tibial nerve. J. Neural Eng. 2006, 3, 125. [Google Scholar] [CrossRef] [PubMed]
- ter Brake, H.J.M.; Rijpma, A.P.; Stinstra, J.G.; Borgmann, J.; Holland, H.J.; Krooshoop, H.J.G.; Peters, M.J.; Flokstra, J.; Quartero, H.W.P.; Rogalla, H. Fetal magnetocardiography: Clinical relevance and feasibility. Phys. C Supercond. 2002, 368, 10–17. [Google Scholar] [CrossRef]
- Adachi, Y.; Miyamoto, M.; Kawai, J.; Uehara, G.; Ogata, H.; Kawabata, S.; Sekihara, K.; Kado, H. Improvement of SQUID Magnetometer System for Extending Application of Spinal Cord Evoked Magnetic Field Measurement. IEEE Trans. Appl. Supercond. 2011, 21, 485–488. [Google Scholar] [CrossRef]
- Li, J.; Quan, W.; Zhou, B.; Wang, Z.; Lu, J.; Hu, Z.; Liu, G.; Fang, J. SERF Atomic Magnetometer–Recent Advances and Applications: A Review. IEEE Sens. J. 2018, 18, 8198–8207. [Google Scholar] [CrossRef]
- Budker, D.; Romalis, M. Optical magnetometry. Nat. Phys. 2007, 3, 227–234. [Google Scholar] [CrossRef]
- Xia, H.; Ben-Amar Baranga, A.; Hoffman, D.; Romalis, M.V. Magnetoencephalography with an atomic magnetometer. Appl. Phys. Lett. 2006, 89, 211104. [Google Scholar] [CrossRef]
- Li, J.-J.; Du, P.-C.; Fu, J.-Q.; Wang, X.-T.; Zhou, Q.; Wang, R.-Q. Miniature quad-channel spin-exchange relaxation-free magnetometer for magnetoencephalography*. Chin. Phys. B 2019, 28, 040703. [Google Scholar] [CrossRef]
- Broser, P.J.; Knappe, S.; Kajal, D.S.; Noury, N.; Alem, O.; Shah, V.; Braun, C. Optically Pumped Magnetometers for Magneto-Myography to Study the Innervation of the Hand. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2226–2230. [Google Scholar] [CrossRef]
- Morales, S.; Corsi, M.C.; Fourcault, W.; Bertrand, F.; Cauffet, G.; Gobbo, C.; Alcouffe, F.; Lenouvel, F.; Le Prado, M.; Berger, F.; et al. Magnetocardiography measurements with 4He vector optically pumped magnetometers at room temperature. Phys. Med. Biol. 2017, 62, 7267. [Google Scholar] [CrossRef]
- Allred, J.C.; Lyman, R.N.; Kornack, T.W.; Romalis, M.V. High-Sensitivity Atomic Magnetometer Unaffected by Spin-Exchange Relaxation. Phys. Rev. Lett. 2002, 89, 130801. [Google Scholar] [CrossRef]
- Okada, Y.C.; Shah, B.; Jin-Chu, H. Ferromagnetic high-permeability alloy alone can provide sufficient low-frequency and eddy-current shieldings for biomagnetic measurements. IEEE Trans. Biomed. Eng. 1994, 41, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, M.; Uehara, G.; Adachi, Y. Prediction of Cylindrical Magnetic Shielding Performance by Considering the Magnetic Field Strength Inside the Material. IEEE Trans. Magn. 2022, 58, 6500404. [Google Scholar] [CrossRef]
- Sakakibara, M.; Uehara, G.; Adachi, Y.; Meguro, T. Evaluation of Heat Treatment of Mu-Metal Based on Permeability Under Very-Low-Frequency Micromagnetic Fields. IEEE Trans. Magn. 2021, 57, 2000204. [Google Scholar] [CrossRef]
- Kornack, T.W.; Smullin, S.J.; Lee, S.-K.; Romalis, M.V. A low-noise ferrite magnetic shield. Appl. Phys. Lett. 2007, 90, 223501. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.; Zhao, Y.; Jin, Z.; Wang, G.; Gao, S. Piezoelectric-Based Smart Bone Plate for Fracture Healing Progress Monitoring. J. Shanghai Jiaotong Univ. (Sci.) 2022, 27, 561–569. [Google Scholar] [CrossRef]
- Ali, M.; Hoseyni, S.M.; Das, R.; Awais, M.; Basdogan, I.; Beker, L. A Flexible and Biodegradable Piezoelectric-Based Wearable Sensor for Non-Invasive Monitoring of Dynamic Human Motions and Physiological Signals. Adv. Mater. Technol. 2023, 8, 2300347. [Google Scholar] [CrossRef]
- Owida, H.A. Biomechanical Sensing Systems for Cardiac Activity Monitoring. Int. J. Biomater. 2022, 2022, 8312564. [Google Scholar] [CrossRef]
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable nanofiber-based piezoelectric transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Long, Y.; Zhang, Z.; Wang, L.; Sui, J.; Dong, Y.; Wang, Y.; Taylor, R.; Ni, D.; et al. Wafer-scale heterostructured piezoelectric bio-organic thin films. Science 2021, 373, 337–342. [Google Scholar] [CrossRef]
- Chorsi, M.T.; Le, T.T.; Lin, F.; Vinikoor, T.; Das, R.; Stevens, J.F.; Mundrane, C.; Park, J.; Tran, K.T.M.; Liu, Y.; et al. Highly piezoelectric, biodegradable, and flexible amino acid nanofibers for medical applications. Sci. Adv. 2023, 9, eadg6075. [Google Scholar] [CrossRef]
- Bhamra, H.; Huang, Y.W.; Yuan, Q.; Irazoqui, P. An Ultra-Low Power 2.4 GHz Transmitter for Energy Harvested Wireless Sensor Nodes and Biomedical Devices. IEEE Trans. Circuits Syst. II Express Briefs 2021, 68, 206–210. [Google Scholar] [CrossRef]
- Cui, H.; Yao, D.; Hensleigh, R.; Lu, H.; Calderon, A.; Xu, Z.; Davaria, S.; Wang, Z.; Mercier, P.; Tarazaga, P.; et al. Design and printing of proprioceptive three-dimensional architected robotic metamaterials. Science 2022, 376, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Chen, W.J.; Zheng, Y. Flexoelectric Effect at the Nanoscale. In Handbook of Mechanics of Materials; Schmauder, S., Chen, C.-S., Chawla, K.K., Chawla, N., Chen, W., Kagawa, Y., Eds.; Springer: Singapore, 2018; pp. 549–589. [Google Scholar]
- Glinchuk, M.D.; Eliseev, E.A.; Morozovska, A.N. Spontaneous flexoelectric effect in nanosystems (topical review). Ferroelectrics 2016, 500, 90–98. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Zhang, S.; Chen, L.-Q. Flexoelectricity in solids: Progress, challenges, and perspectives. Prog. Mater. Sci. 2019, 106, 100570. [Google Scholar] [CrossRef]
- Yan, X.; Huang, W.; Ryung Kwon, S.; Yang, S.; Jiang, X.; Yuan, F.-G. A sensor for the direct measurement of curvature based on flexoelectricity. Smart Mater. Struct. 2013, 22, 085016. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Liu, K.; Shen, S. A flexoelectricity effect-based sensor for direct torque measurement. J. Phys. D Appl. Phys. 2015, 48, 485502. [Google Scholar] [CrossRef]
- Jia, L.; Li, L.; Guo, Z.H.; Sun, H.; Huang, H.; Sun, F.; Wang, Z.L.; Pu, X. Giant Iontronic Flexoelectricity in Soft Hydrogels Induced by Tunable Biomimetic Ion Polarization. Adv. Mater. 2024, 36, 2403830. [Google Scholar] [CrossRef]
- Mathew, A.; Kulkarni, Y. An Electro-Chemo-Mechanical Theory With Flexoelectricity: Application to Ionic Conductivity of Soft Solid Electrolytes. J. Appl. Mech. 2023, 91, 041001. [Google Scholar] [CrossRef]
- Sadeghi-Goughari, M.; Jeon, S.; Kwon, H.-J. Flutter instability of cantilevered carbon nanotubes caused by magnetic fluid flow subjected to a longitudinal magnetic field. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 98, 184–190. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Yan, H.; Cai, H. A Driving Power Supply for Piezoelectric Transducers Based on an Improved LC Matching Network. Sensors 2023, 23, 5745. [Google Scholar] [CrossRef]
- Liang, H.; Hao, G.; Olszewski, O.Z. A review on vibration-based piezoelectric energy harvesting from the aspect of compliant mechanisms. Sens. Actuators A Phys. 2021, 331, 112743. [Google Scholar] [CrossRef]
- Deng, J.; Rorschach, K.; Baker, E.; Sun, C.; Chen, W. Topology optimization and fabrication of low frequency vibration energy harvesting microdevices. Smart Mater. Struct. 2015, 24, 025005. [Google Scholar] [CrossRef]
- Farooq, M.; Amin, B.; Kraśny, M.J.; Elahi, A.; Rehman, M.R.U.; Wijns, W.; Shahzad, A. An Ex Vivo Study of Wireless Linkage Distance between Implantable LC Resonance Sensor and External Readout Coil. Sensors 2022, 22, 8402. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.J. A experimental method to the study of wireless passive LC sensors. Int. J. Biosens. Bioelectron. 2018, 4, 175–177. [Google Scholar] [CrossRef]
- Malik, N.A.; Sant, P.; Ajmal, T.; Ur-Rehman, M. Implantable Antennas for Bio-Medical Applications. IEEE J. Electromagn. RF Microw. Med. Biol. 2021, 5, 84–96. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Dong, L.; Huang, Q.-A. Inductor-capacitor passive wireless sensors using nonlinear parity-time symmetric configurations. Nat. Commun. 2024, 15, 9312. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Hsu, W. Design and characterization of LC strain sensors with novel inductor for sensitivity enhancement. Smart Mater. Struct. 2013, 22, 105015. [Google Scholar] [CrossRef]
- Herbert, R.; Lim, H.-R.; Rigo, B.; Yeo, W.-H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 2022, 8, eabm1175. [Google Scholar] [CrossRef]
- Silva, N.P.; Elahi, A.; Dunne, E.; O’Halloran, M.; Amin, B. Design and Characterisation of a Read-Out System for Wireless Monitoring of a Novel Implantable Sensor for Abdominal Aortic Aneurysm Monitoring. Sensors 2024, 24, 3195. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, Y.; Li, M.; Zhou, Z.; Chen, Y.; Liu, Q.; Yang, X. A paper-based self-inductive folding displacement sensor for human respiration and motion signals measurement. Npj Flex. Electron. 2022, 6, 67. [Google Scholar] [CrossRef]
- Dong, L.; Wang, L.F.; Huang, Q.A. A Passive Wireless Adaptive Repeater for Enhancing the Readout of LC Passive Wireless Sensors. IEEE Microw. Wirel. Compon. Lett. 2016, 26, 543–545. [Google Scholar] [CrossRef]
- Xie, M.Z.; Wang, L.F.; Zhou, B.B.; Huang, Q.A. An Impedance Matching Method for LC Passive Wireless Sensors. IEEE Sens. J. 2020, 20, 13833–13841. [Google Scholar] [CrossRef]
- Zhou, B.-B.; Deng, W.-J.; Wang, L.-F.; Dong, L.; Huang, Q.-A. Enhancing the Remote Distance of LC Passive Wireless Sensors by Parity-Time Symmetry Breaking. Phys. Rev. Appl. 2020, 13, 064022. [Google Scholar] [CrossRef]
- Sakhdari, M.; Hajizadegan, M.; Li, Y.; Cheng, M.M.C.; Hung, J.C.H.; Chen, P.Y. Ultrasensitive, Parity–Time-Symmetric Wireless Reactive and Resistive Sensors. IEEE Sens. J. 2018, 18, 9548–9555. [Google Scholar] [CrossRef]
- Ren, Q.Y.; Wang, L.F.; Huang, J.Q.; Zhang, C.; Huang, Q.A. Simultaneous Remote Sensing of Temperature and Humidity by LC-Type Passive Wireless Sensors. J. Microelectromechanical Syst. 2015, 24, 1117–1123. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Dong, L.; Huang, Q.-A. PT-Symmetric LC Passive Wireless Sensing. Sensors 2023, 23, 5191. [Google Scholar] [CrossRef]
- Lin, M.; Hu, H.; Zhou, S.; Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 2022, 7, 850–869. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Transmission Lines Terminated with LC Resonators for Differential Permittivity Sensing. IEEE Microw. Wirel. Compon. Lett. 2018, 28, 1149–1151. [Google Scholar] [CrossRef]
- Varshney, P.K.; Kapoor, A.; Akhtar, M.J. Highly Sensitive ELC Resonator Based Differential Sensor. IEEE Trans. Instrum. Meas. 2021, 70, 8004710. [Google Scholar] [CrossRef]
- Naqui, J.; Damm, C.; Wiens, A.; Jakoby, R.; Su, L.; Mata-Contreras, J.; Martín, F. Transmission Lines Loaded With Pairs of Stepped Impedance Resonators: Modeling and Application to Differential Permittivity Measurements. IEEE Trans. Microw. Theory Tech. 2016, 64, 3864–3877. [Google Scholar] [CrossRef]
- Turgul, V.; Kale, I. Sensitivity of non-invasive RF/microwave glucose sensors and fundamental factors and challenges affecting measurement accuracy. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–5. [Google Scholar]
- Hosseini, N.; Baghelani, M. Selective real-time non-contact multi-variable water-alcohol-sugar concentration analysis during fermentation process using microwave split-ring resonator based sensor. Sens. Actuators A Phys. 2021, 325, 112695. [Google Scholar] [CrossRef]
- Sharafadinzadeh, N.; Abdolrazzaghi, M.; Daneshmand, M. Investigation on planar microwave sensors with enhanced sensitivity from microfluidic integration. Sens. Actuators A Phys. 2020, 301, 111752. [Google Scholar] [CrossRef]
- Carr, A.R.; Chan, Y.J.; Reuel, N.F. Contact-Free, Passive, Electromagnetic Resonant Sensors for Enclosed Biomedical Applications: A Perspective on Opportunities and Challenges. ACS Sens. 2023, 8, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Ouriel, K.; Silveira, P.G.; Katzen, B.; White, R.; Criado, F.; Diethrich, E. Initial results of wireless pressure sensing for endovascular aneurysm repair: The APEX Trial—Acute Pressure Measurement to Confirm Aneurysm Sac EXclusion. J. Vasc. Surg. 2007, 45, 236–242. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, Y.; Tang, H.; Wang, J.; Li, N.; Cheng, Y.; Kang, T.; Tang, J.; Zhou, M.; Chen, W.; et al. An implantable hydrogel-based phononic crystal for continuous and wireless monitoring of internal tissue strains. Nat. Biomed. Eng. 2025, 9, 1–14. [Google Scholar] [CrossRef]
- Shi, C.; Andino-Pavlovsky, V.; Lee, S.A.; Costa, T.; Elloian, J.; Konofagou, E.E.; Shepard, K.L. Application of a implantable mote for in vivo real-time wireless temperature sensing. Sci. Adv. 2021, 7, eabf6312. [Google Scholar] [CrossRef]
- Nam, J.; Byun, E.; Shim, H.; Kim, E.; Islam, S.; Park, M.; Kim, A.; Song, S.H. A Hydrogel-Based Ultrasonic Backscattering Wireless Biochemical Sensing. Front. Bioeng. Biotechnol. 2020, 8, 596370. [Google Scholar] [CrossRef]
- Farhoudi, N.; Laurentius, L.B.; Magda, J.J.; Reiche, C.F.; Solzbacher, F. In Vivo Monitoring of Glucose Using Ultrasound-Induced Resonance in Implantable Smart Hydrogel Microstructures. ACS Sens. 2021, 6, 3587–3595. [Google Scholar] [CrossRef]
- Huang, X.; Bell, M.A.L.; Ding, K. Deep Learning for Ultrasound Beamforming in Flexible Array Transducer. IEEE Trans. Med. Imaging 2021, 40, 3178–3189. [Google Scholar] [CrossRef]
- Yi, J.; Nguyen, K.T.; Wang, W.; Yang, W.; Pan, M.; Lou, E.; Major, P.W.; Le, L.H.; Zeng, H. Mussel-Inspired Adhesive Double-Network Hydrogel for Intraoral Ultrasound Imaging. ACS Appl. Bio. Mater. 2020, 3, 8943–8952. [Google Scholar] [CrossRef]
- Yi, J.; Nguyen, K.-C.T.; Wang, W.; Yang, W.; Pan, M.; Lou, E.; Major, P.W.; Le, L.H.; Zeng, H. Polyacrylamide/Alginate double-network tough hydrogels for intraoral ultrasound imaging. J. Colloid Interface Sci. 2020, 578, 598–607. [Google Scholar] [CrossRef] [PubMed]
- La, T.-G.; Le, L.H. Flexible and Wearable Ultrasound Device for Medical Applications: A Review on Materials, Structural Designs, and Current Challenges. Adv. Mater. Technol. 2022, 7, 2100798. [Google Scholar] [CrossRef]
- Wang, X.; Han, J.Q.; Li, G.X.; Xia, D.X.; Chang, M.Y.; Ma, X.J.; Xue, H.; Xu, P.; Li, R.J.; Zhang, K.Y.; et al. High-performance cost efficient simultaneous wireless information and power transfers deploying jointly modulated amplifying programmable metasurface. Nat. Commun. 2023, 14, 6002. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, M.; Liu, J.; Wang, R.; Wei, C.; Wang, R.; Zhou, Y.; Jiang, H.; Pan, J.; Guo, C. Noninvasive, wireless and real-time bladder pressure monitoring with biomimetic structured devices. Appl. Mater. Today 2022, 29, 101635. [Google Scholar] [CrossRef]
- Eversense E3 Continuous Glucose Montioring System. Available online: https://www.fda.gov/medical-devices/eversense-e3-continuous-glucose-monitoring-system-p160048s016 (accessed on 17 May 2025).
- Available online: https://nalumed.com/patients/peripheral-nerve-stimulator/ (accessed on 17 May 2025).
- Akin, T.; Najafi, K.; Bradley, R.M. A wireless implantable multichannel digital neural recording system for a micromachined sieve electrode. IEEE J. Solid-State Circuits 1998, 33, 109–118. [Google Scholar] [CrossRef]
- Fernandez, M.; Espinosa, H.G.; Guerra, D.; Peña, I.; Thiel, D.V.; Arrinda, A. RF Energy Absorption in Human Bodies Due to Wearable Antennas in the 2.4 GHz Frequency Band. Bioelectromagnetics 2020, 41, 73–79. [Google Scholar] [CrossRef]
- Available online: https://www.raumedic.com/application-areas/neuromonitoring/icp-measurement/telemetry (accessed on 18 May 2025).
- Available online: https://ichgcp.net/clinical-trials-registry/NCT00991120 (accessed on 18 May 2025).
- Kasper, K.A.; Romero, G.F.; Perez, D.L.; Miller, A.M.; Gonzales, D.A.; Siqueiros, J.; Margolis, D.S.; Gutruf, P. Continuous operation of battery-free implants enables advanced fracture recovery monitoring. Sci. Adv. 2025, 11, eadt7488. [Google Scholar] [CrossRef]
- Zhang, H.; Gutruf, P.; Meacham, K.; Montana, M.C.; Zhao, X.; Chiarelli, A.M.; Vázquez-Guardado, A.; Norris, A.; Lu, L.; Guo, Q.; et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 2019, 5, eaaw0873. [Google Scholar] [CrossRef]
- Available online: https://www.gentag.com/wireless-and-optical-skin-patches/ (accessed on 18 May 2025).
- He, D.; Cui, Y.; Ming, F.; Wu, W. Advancements in Passive Wireless Sensors, Materials, Devices, and Applications. Sensors 2023, 23, 8200. [Google Scholar] [CrossRef]
- Mohammad, I.; Huang, H. Shear sensing based on a microstrip patch antenna. Meas. Sci. Technol. 2012, 23, 105705. [Google Scholar] [CrossRef]
- Keat Ghee, O.; Craig, A.G. A resonant printed-circuit sensor for remote query monitoring of environmental parameters. Smart Mater. Struct. 2000, 9, 421. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Pan, B.; Kim, S.-H.; Liu, Z.; Tentzeris, M.M.; Papapolymerou, J.; Allen, M.G. RF evanescent-mode cavity resonator for passive wireless sensor applications. Sens. Actuators A Phys. 2010, 161, 322–328. [Google Scholar] [CrossRef]
- Yue, W.; Guo, Y.; Lee, J.C.; Ganbold, E.; Wu, J.-K.; Li, Y.; Wang, C.; Kim, H.S.; Shin, Y.-K.; Liang, J.-G.; et al. Advancements in Passive Wireless Sensing Systems in Monitoring Harsh Environment and Healthcare Applications. Nano-Micro Lett. 2025, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Chen, C.; Li, S.; Shi, Y.; Wang, H.; Guo, W.; Liu, X. Array Integration and Far-Field Detection of Biocompatible Wireless LC Pressure Sensors. Small Methods 2021, 5, 2001055. [Google Scholar] [CrossRef]
- Kalra, P.R.; Gogorishvili, I.; Khabeishvili, G.; Málek, F.; Toman, O.; Critoph, C.; Flett, A.S.; Cowburn, P.J.; Mehra, M.R.; Sheridan, W.S.; et al. First-in-Human Implantable Inferior Vena Cava Sensor for Remote Care in Heart Failure. JACC Heart Fail. 2025, 13, 1000–1010. [Google Scholar] [CrossRef]
- Lyu, H.; Wang, J.; La, J.H.; Chung, J.M.; Babakhani, A. An Energy-Efficient Wirelessly Powered Millimeter-Scale Neurostimulator Implant Based on Systematic Codesign of an Inductive Loop Antenna and a Custom Rectifier. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1131–1143. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Peng, F.; Li, Y.; Yang, T.; Wang, B.; Fang, D. A Wireless Magnetic Resonance Energy Transfer System for Micro Implantable Medical Sensors. Sensors 2012, 12, 10292–10308. [Google Scholar] [CrossRef]
- Kim, A.; Powell, C.R.; Ziaie, B. An Implantable Pressure Sensing System With Electromechanical Interrogation Scheme. IEEE Trans. Biomed. Eng. 2014, 61, 2209–2217. [Google Scholar] [CrossRef]
- Sheng, F.; Zhang, B.; Zhang, Y.; Li, Y.; Cheng, R.; Wei, C.; Ning, C.; Dong, K.; Wang, Z.L. Ultrastretchable Organogel/Silicone Fiber-Helical Sensors for Self-Powered Implantable Ligament Strain Monitoring. ACS Nano 2022, 16, 10958–10967. [Google Scholar] [CrossRef]
- Mohan, A.; Kumar, N. Implantable antennas for biomedical applications: A systematic review. Biomed. Eng. OnLine 2024, 23, 87. [Google Scholar] [CrossRef]
- Karacolak, T.; Hood, A.Z.; Topsakal, E. Design of a Dual-Band Implantable Antenna and Development of Skin Mimicking Gels for Continuous Glucose Monitoring. IEEE Trans. Microw. Theory Tech. 2008, 56, 1001–1008. [Google Scholar] [CrossRef]
- Tawk, Y. A Dynamic Dual Tapered 3-D Printed Nested Helical Antenna. IEEE Trans. Antennas Propag. 2020, 68, 697–702. [Google Scholar] [CrossRef]
- Lamkaddem, A.; Yousfi, A.E.; Abdalmalak, K.A.; Posadas, V.G.; Segovia-Vargas, D. Circularly Polarized Miniaturized Implantable Antenna for Leadless Pacemaker Devices. IEEE Trans. Antennas Propag. 2022, 70, 6423–6432. [Google Scholar] [CrossRef]
- Yang, X.T.; Wong, H.; Xiang, J. Polarization Reconfigurable Planar Inverted-F Antenna for Implantable Telemetry Applications. IEEE Access 2019, 7, 141900–141909. [Google Scholar] [CrossRef]
- Permana, H.; Fang, Q.; Cosic, I. 3-layer implantable microstrip antenna optimised for retinal prosthesis system in MICS band. In Proceedings of the International Symposium on Bioelectronics and Bioinformations 2011, Suzhou, China, 3–5 November 2011; pp. 65–68. [Google Scholar]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Moradi, E.; Sydänheimo, L.; Björninen, T.; Rahmat-Samii, Y.; Ukkonen, L. Miniature Coplanar Implantable Antenna on Thin and Flexible Platform for Fully Wireless Intracranial Pressure Monitoring System. Int. J. Antennas Propag. 2017, 2017, 9161083. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Z.; Wu, J.; Bao, J.; Liu, J.; Cai, L.; Dang, T.; Zheng, H.; Li, E. Investigation of SAR Reduction Using Flexible Antenna With Metamaterial Structure in Wireless Body Area Network. IEEE Trans. Antennas Propag. 2018, 66, 3076–3086. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Quality Management Systems—Requirements. ISO: Geneva, Switzerland, 2018.
- Xu, K.; Li, S.; Dong, S.; Zhang, S.; Pan, G.; Wang, G.; Shi, L.; Guo, W.; Yu, C.; Luo, J. Bioresorbable Electrode Array for Electrophysiological and Pressure Signal Recording in the Brain. Adv. Healthc. Mater. 2019, 8, 1801649. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, T.H.; Razak, K.A.; Lin, J.; Villafana, E.; Jimenez, H.; Liu, H. Fabrication and Characterization of Biodegradable Metal Based Microelectrodes for In Vivo Neural Recording. MRS Adv. 2019, 4, 2471–2477. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zhang, Y.; Wu, H.; Wei, L.; Zhou, G.; Zhang, Y.; Deng, L.; Cheng, Y.; Li, M.; et al. Endovascular Metal Devices for the Treatment of Cerebrovascular Diseases. Adv. Mater. 2019, 31, 1805452. [Google Scholar] [CrossRef]
- Toong, D.W.Y.; Ng, J.C.K.; Huang, Y.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.S.; Ang, H.Y. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia 2020, 12, 100727. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Jun, I.; Seok, H.-K.; Lee, K.-S.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.-C.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, M.; Blum, R.; Boero, G.; Brugger, J. Biodegradable Frequency-Selective Magnesium Radio-Frequency Microresonators for Transient Biomedical Implants. Adv. Funct. Mater. 2019, 29, 1903051. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Deng, Y.; Yang, Q.; Zhao, J.; Seo, M.-H.; Bai, W.; MacEwan, M.R.; Huang, Y.; Ray, W.Z.; et al. Bioresorbable Wireless Sensors as Temporary Implants for In Vivo Measurements of Pressure. Adv. Funct. Mater. 2020, 30, 2003754. [Google Scholar] [CrossRef]
- Wu, C.; Lin, F.; Liu, H.; Pelletier, M.H.; Lloyd, M.; Walsh, W.R.; Nie, J.-F. Stronger and coarser-grained biodegradable zinc alloys. Nature 2025, 638, 684–689. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, Y.; Wang, L.; Kang, Y.; Dong, H.; Li, H.; Zhao, X.; Li, B.; Chen, H.; Qiu, J.; et al. Implantable Zinc Ion Battery and Osteogenesis-Immunoregulation Bifunction of Its Catabolite. ACS Nano 2024, 18, 21246–21257. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Tang, Y.-Y.; Gu, Z.-X.; Wang, P.; Chen, X.-G.; Lv, H.-P.; Li, P.-F.; Jiang, Q.; Gu, N.; Ren, S.; et al. Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science 2024, 383, 1492–1498. [Google Scholar] [CrossRef]
- Sheng, H.; Zhou, J.; Li, B.; He, Y.; Zhang, X.; Liang, J.; Zhou, J.; Su, Q.; Xie, E.; Lan, W.; et al. A thin, deformable, high-performance supercapacitor implant that can be biodegraded and bioabsorbed within an animal body. Sci. Adv. 2021, 7, eabe3097. [Google Scholar] [CrossRef]
- Shao, M.; Sheng, H.; Lin, L.; Ma, H.; Wang, Q.; Yuan, J.; Zhang, X.; Chen, G.; Li, W.; Su, Q.; et al. High-Performance Biodegradable Energy Storage Devices Enabled by Heterostructured MoO3–MoS2 Composites. Small 2023, 19, 2205529. [Google Scholar] [CrossRef]
- Mohamed, A.; El-Aziz, A.M.; Breitinger, H.-G. Study of the degradation behavior and the biocompatibility of Mg–0.8Ca alloy for orthopedic implant applications. J. Magnes. Alloys 2019, 7, 249–257. [Google Scholar] [CrossRef]
- Redlich, C.; Schauer, A.; Scheibler, J.; Poehle, G.; Barthel, P.; Maennel, A.; Adams, V.; Weissgaerber, T.; Linke, A.; Quadbeck, P. In Vitro Degradation Behavior and Biocompatibility of Bioresorbable Molybdenum. Metals 2021, 11, 761. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1141–1151. [Google Scholar] [CrossRef]
- Yin, L.; Bozler, C.; Harburg, D.V.; Omenetto, F.; Rogers, J.A. Materials and fabrication sequences for water soluble silicon integrated circuits at the 90 nm node. Appl. Phys. Lett. 2015, 106, 014105. [Google Scholar] [CrossRef]
- Lee, Y.K.; Yu, K.J.; Song, E.; Barati Farimani, A.; Vitale, F.; Xie, Z.; Yoon, Y.; Kim, Y.; Richardson, A.; Luan, H.; et al. Dissolution of Monocrystalline Silicon Nanomembranes and Their Use as Encapsulation Layers and Electrical Interfaces in Water-Soluble Electronics. ACS Nano 2017, 11, 12562–12572. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, B.; Liu, X.; Ni, Z.; Shu, Y.; Zhang, X.; Shen, J.; Liang, L.; Zhou, L.; Liu, J.; et al. Multi-functional, conformal systems with ultrathin crystalline-silicon-based bioelectronics for characterization of intraocular pressure and ocular surface temperature. Biosens. Bioelectron. 2025, 267, 116786. [Google Scholar] [CrossRef]
- Wu, M.; Yao, K.; Huang, N.; Li, H.; Zhou, J.; Shi, R.; Li, J.; Huang, X.; Li, J.; Jia, H.; et al. Ultrathin, Soft, Bioresorbable Organic Electrochemical Transistors for Transient Spatiotemporal Mapping of Brain Activity. Adv. Sci. 2023, 10, 2300504. [Google Scholar] [CrossRef]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of Silicone Breast Implants: Anaplastic Large Cell Lymphoma, Biofilm and Capsular Contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef]
- Zappi, E.; Barnett, J.G.; Zappi, M.; Barnett, C.R. The Long-Term Host Response to Liquid Silicone Injected during Soft Tissue Augmentation Procedures: A Microscopic Appraisal. Dermatol. Surg. 2007, 33, S186–S192. [Google Scholar] [CrossRef]
- Lee, J.C.; Heo, C.Y. Implementing Tissue Engineering and Regenerative Medicine Solutions in Silicone Implants. In Regenerative Medicine and Plastic Surgery: Elements, Research Concepts and Emerging Technologies; Duscher, D., Shiffman, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 303–318. [Google Scholar]
- Li, M.; Neoh, K.G.; Xu, L.Q.; Wang, R.; Kang, E.-T.; Lau, T.; Olszyna, D.P.; Chiong, E. Surface Modification of Silicone for Biomedical Applications Requiring Long-Term Antibacterial, Antifouling, and Hemocompatible Properties. Langmuir 2012, 28, 16408–16422. [Google Scholar] [CrossRef] [PubMed]
- Yesilirmak, N.; Altınors, D.D. A silicone hydrogel contact lens after 7 years of continuous wear. Contact Lens Anterior Eye 2013, 36, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef]
- Tao, H.; Hwang, S.-W.; Marelli, B.; An, B.; Moreau, J.E.; Yang, M.; Brenckle, M.A.; Kim, S.; Kaplan, D.L.; Rogers, J.A.; et al. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl. Acad. Sci. USA 2014, 111, 17385–17389. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R.; Altstädt, V. Poly(lactic acid)/coplasticized thermoplastic starch blend: Effect of plasticizer migration on rheological and mechanical properties. Polym. Adv. Technol. 2019, 30, 839–851. [Google Scholar] [CrossRef]
- Sickles, C.K.; Nassereddin, A.; Patel, P.; Gross, G.P. Poly-L-Lactic Acid; StatPearls: Tampa/St. Petersburg, FL, USA, 2024. [Google Scholar]
- Valencia, C.H. Hydrolytic degradation and in vivo resorption of poly-<l-lactic acid-chitosan biomedical devices in the parietal bones of Wistar rats. J. Int. Med. Res. 2019, 47, 1705–1716. [Google Scholar] [CrossRef]
- Yoon, S.-K.; Chung, D.-J. In Vivo Degradation Studies of PGA-PLA Block Copolymer and Their Histochemical Analysis for Spinal-Fixing Application. Polymers 2022, 14, 3322. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Shariatinia, Z. Chapter 2-Pharmaceutical applications of natural polysaccharides. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 15–57. [Google Scholar]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [PubMed]
- Saidin, N.M.; Anuar, N.K.; Affandi, M.M. Roles of Polysaccharides in Transdermal Drug Delivery System and Future Prospects. J. Appl. Pharm. Sci. 2018, 8, 141–157. [Google Scholar] [CrossRef]
- van Dam, J.E.G.; van den Broek, L.A.M.; Boeriu, C.G. Polysaccharides in Human Health Care. Nat. Prod. Commun. 2017, 12, 1934578X1701200604. [Google Scholar] [CrossRef]
- Moraïs, S.; Winkler, S.; Zorea, A.; Levin, L.; Nagies, F.S.P.; Kapust, N.; Lamed, E.; Artan-Furman, A.; Bolam, D.N.; Yadav, M.P.; et al. Cryptic diversity of cellulose-degrading gut bacteria in industrialized humans. Science 2024, 383, eadj9223. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, W. Preparation, characterization and application in environmental protection of low-molecular-weight chitosan: A review. Sustain. Environ. Res. 2024, 34, 29. [Google Scholar] [CrossRef]
- Rivas-Fernández, J.P.; Vuillemin, M.; Pilgaard, B.; Klau, L.J.; Fredslund, F.; Lund-Hanssen, C.; Welner, D.H.; Meyer, A.S.; Morth, J.P.; Meilleur, F.; et al. Unraveling the molecular mechanism of polysaccharide lyases for efficient alginate degradation. Nat. Commun. 2025, 16, 2670. [Google Scholar] [CrossRef]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A comprehensive review of natural fibers and their composites: An eco-friendly alternative to conventional materials. Results Eng. 2023, 19, 101271. [Google Scholar] [CrossRef]
- Zwawi, M. A Review on Natural Fiber Bio-Composites, Surface Modifications and Applications. Molecules 2021, 26, 404. [Google Scholar] [CrossRef]
- Nair, R.R.; Wolansky, J.; Uhlig, K.; Solgi, A.; Teuerle, L.; Zhang, T.; Schröder, J.; Antrack, T.; Benduhn, J.; Kleemann, H.; et al. Leaftronics: Natural lignocellulose scaffolds for sustainable electronics. Sci. Adv. 2024, 10, eadq3276. [Google Scholar] [CrossRef]
- Sheng, H.; Jiang, L.; Wang, Q.; Zhang, Z.; Lv, Y.; Ma, H.; Bi, H.; Yuan, J.; Shao, M.; Li, F.; et al. A soft implantable energy supply system that integrates wireless charging and biodegradable Zn-ion hybrid supercapacitors. Sci. Adv. 2023, 9, eadh8083. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, C.; Wang, X.; Meng, J.; Zou, Y.; Noreen, S.; Zhao, L.; Liu, Z.; Ouyang, H.; Tan, P.; et al. Fully Bioabsorbable Capacitor as an Energy Storage Unit for Implantable Medical Electronics. Adv. Sci. 2019, 6, 1801625. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zou, Y.; Zhang, Y.; Liu, Z.; Shi, B.; Wang, X.; Jin, Y.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, e1501478. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Yan, Y.; Bai, W.; Xue, Y.; Gamble, P.; Tian, L.; Kandela, I.; Haney, C.R.; Spees, W.; Lee, Y.; et al. Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat. Biomed. Eng. 2019, 3, 37–46. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, T.-L.; Xue, Y.; Wang, H.; Xu, Y.; Emon, B.; Wu, M.; Rountree, C.; Wei, T.; Kandela, I.; et al. Ecoresorbable and bioresorbable microelectromechanical systems. Nat. Electron. 2022, 5, 526–538. [Google Scholar] [CrossRef]
- Tang, H.; Yang, Y.; Liu, Z.; Li, W.; Zhang, Y.; Huang, Y.; Kang, T.; Yu, Y.; Li, N.; Tian, Y.; et al. Injectable ultrasonic sensor for wireless monitoring of intracranial signals. Nature 2024, 630, 84–90. [Google Scholar] [CrossRef]
- Han, Y.; Xu, B.; Fu, G.; Wang, X.; Xu, K.; Jin, C.; Tao, L.; Li, L.; Hou, Y.; Su, X.; et al. A Randomized Trial Comparing the NeoVas Sirolimus-Eluting Bioresorbable Scaffold and Metallic Everolimus-Eluting Stents. JACC Cardiovasc. Interv. 2018, 11, 260–272. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, J.; Chen, J.; Yao, Z.; Qian, J.; Shen, L.; Ge, L.; Ge, J. Final report of the 5-year clinical outcomes of the XINSORB bioresorbable sirolimus-eluting scaffold in the treatment of single de novo coronary lesions in a first-in-human study. Ann. Transl. Med. 2020, 8, 1162. [Google Scholar] [CrossRef]


| Reference | Sensing Parameters | Sensing Performance | |
|---|---|---|---|
| Magnetic field sensor | Miniature extravascular hall-based magnetic sensor [15] | Mean arterial pressure (ABP) | MAE less than 5 mmHg |
| Millimeter-level magnetic implants [16] | Cerebrospinal fluid (CSF) viscosity, intracranial pressure and cerebrospinal fluid glucose level | Accuracy exceeds 95% | |
| Piezoelectric sensor | Polylactic acid nanofiber film [40] | d33 | 19 pC/N |
| Glycine-PVA film [41] | d33 | 6 pC/N | |
| Glycine-PCL nanofibers [42] | d33 | 18.91 pC/N | |
| Passive LC sensor | Nonlinear parity-time symmetric structure [59] | Temperature | resolution: 0.027 °C |
| LC sensor with a new type of encapsulated helical coil [60] | Strain | MAE: 5.57% | |
| Wireless printing soft sensor [61] | Pressure | MAE < 1% Effective reading distances are 5.5 cm (in air) and 3.5 cm (in blood) | |
| Implantable sensor for abdominal aortic aneurysm [62] | Pressure | MAE < 10% Effective reading distance is 10 cm | |
| Paper-based self-sensing folding displacement sensor [63] | Displacement | Displacement resolution is 20 um Effective reading distance is 42.3 mm | |
| Ultrasonic sensor | Hydrogel particles used for temperature monitoring [80] | Temperature | MAE is 0.044/−0.040 °C |
| Hydrogel embedded with silica nanoparticles [81] | pH | The resolution is 0.2 pH unit variation, the effective reading distance is 10 cm | |
| Resonant hydrogel sensor [82] | Glucose | Accuracy ≥ 83% |
| Method | Range | Transfer Rate |
|---|---|---|
| Radio Frequency | 100 m | 1–10 kbps |
| Bluetooth | 10 m | 1 Mbps |
| Near Field Communication | 4–20 cm | 106–424 kbps |
| Reference | Transmission Power | Energy Transmission Distance | Size |
|---|---|---|---|
| Inductive coupled stimulator [105] | >2.7 μW | 14 cm | 5 mm × 7.5 mm |
| Wireless magnetic resonance energy transmission system [106] | At a distance of 2 cm, the received voltage signal is converted into a stable 3.3 V output voltage and a current of 10 mA. | 1–3 cm | The diameter of the receiving coil is 1.9 cm |
| Ultrasonic piezoelectric transducer [107] | 16 mW | 10 cm | 40 mm × 8 mm |
| Triboelectric nanogenerator [108] | Maximum 0.7 V (150% stretching) | N/A | 6 cm |
| Reference | Frequency Band | Energy/Bandwidth | Size |
|---|---|---|---|
| Dual-frequency implantable antenna [110] | 402–405 MHz/2.4–2.48 GHz | The bandwidth of the −10 dB bandwidth MICS band is 35.3%, and the bandwidth of the ISM band is 7.1% | 22.5 mm × 22.5 mm × 2.5 mm |
| Double-cone nested antenna [111] | 2.4 GHz (Outer arm) 5.4 GHz (Inner arm) | The peak gain of the external component is 9.8 decibels at 2.4 GHz, while that of the internal component is 7.8 decibels at 5.4 GHz. | Diameter: 4 cm |
| Circularly polarized small antenna [112] | 915 MHz | With an impedance bandwidth of 18.9% in the 810 to 980 MHz band and an axial ratio of 17.2% in the 850 to 1010 MHz band, the gain value is −23 dBi. | 5.2 mm × 5.6 mm × 0.25 mm |
| PIFA for polarization reconfigurability [113] | 2.45-GHz ISM band | The measured bandwidths were 11.3% (2.26–2.53 GHz) and 9.1% (2.3–2.52 GHz), respectively, and the measured gains were −24.5 and −24.2 dBi, respectively. | 13.5 mm × 11.5 mm × 1.905 mm |
| Microstrip technology PIFA [114] | 402 MHz | The bandwidth is 39 MHz, the gain is −24.31 decibels, and the radiation efficiency is 8.72% | 12 mm × 12 mm × 1.9 mm |
| Conformal antenna [115] | 2.45 GHz | With a bandwidth of −10 dB and 280 MHz, at 2.45 GHZ, the antenna reflection coefficient is −14 decibels and the peak gain is −19.6 decibels | 6 mm × 5 mm |
| Categories | Materials | Typical Properties | Application Scenarios |
|---|---|---|---|
| Polyester | PLA | High mechanical strength, moderate degradation cycle | Intracranial pressure monitoring, cardiovascular device encapsulation |
| PGA | High rigidity and rapid degradation | Orthopedic fixation monitoring, drug-controlled release system | |
| PLGA | Controlled degradation (weeks to years), adjustable flexibility | Tissue engineering scaffolds integrate sensors and multi-parameter biochemical monitoring | |
| PCL | Excellent flexibility and slow degradation (2–3 years) | Flexible hemodynamic sensors, self-powered ECG monitoring | |
| PHA | High biocompatibility and variable degradation cycles | Biodegradable electrode packaging, bio signal monitoring | |
| PHB | High crystallinity and long degradation cycle | Bone repair monitoring and implantable energy storage devices | |
| P4HB | High elasticity, moderate degradation cycle | Vascular stent sensor, soft tissue mechanical monitoring | |
| PLA-PCL | Balance strength and flexibility, adjustable degradation cycle | Multimodal sensor base, heart valve monitoring | |
| PLATMC | High flexibility and moderate degradation cycle | Nerve conduit pressure monitoring, soft tissue repair sensor | |
| Polyanhydrides | PSA | Rapid degradation (weeks), surface erosion release | Short-term controlled drug release sensor, postoperative inflammation monitoring |
| P(CPP-SA) | Linear drug release and controlled degradation cycle (1–6 months) | Targeted therapy sensors, tumor microenvironment monitoring | |
| P(FAD-SA) | Good thermal stability and non-toxic degradation products | Stress monitoring and drug release feedback of bone repair materials | |
| PTMC | Excellent flexibility and slow degradation (>2 years) | Vascular stent deformation monitoring, dynamic force sensor | |
| PPC | Good low temperature toughness, moderate degradation cycle (1–3 years) | Low temperature environment for implant device encapsulation and tissue engineering monitoring | |
| Chitosan | Antibacterial, rapid degradation (weeks to months) | Wound healing monitoring, infection warning sensors | |
| Hyaluronic Acid | High water retention, promote cell migration | Soft tissue repair monitoring, dynamic analysis of joint fluid | |
| Sodium Alginate | Ion-crosslinked gelation, rapid degradation (weeks) | Injectable sensor carrier, ph-responsive monitoring | |
| CMC | Good water solubility and high biocompatibility | Electrode paste substrate, degradable circuit board | |
| HPMC | Thermal gelability, moderate degradation cycle (months) | Drug sustained-release sensor, intestinal motility monitoring | |
| Methylcellulose | Strong film formation and rapid degradation (weeks) | Temporary encapsulation material, short-term physiological parameter monitoring | |
| PSA | Rapid degradation (weeks), surface erosion release | Short-term controlled drug release sensor, postoperative inflammation monitoring | |
| Proteins | Silk Fibroin | High mechanical strength and controlled degradation cycle (3–6 months) | Flexible electrode base, neural signal monitoring |
| Collagen | Promotes cell adhesion and rapid degradation (weeks) | Skin sensors, dynamic monitoring of tissue regeneration | |
| Gelatin | Temperature-sensitive gelation, rapid degradation (days to weeks) | Injectable sensor carrier, short-term drug release monitoring | |
| Other Synthetic Materials | Polydioxanone | Good flexibility and moderate degradation cycle (6–12 months) | Suture tension monitoring, soft tissue repair sensor |
| PGS | High elasticity, moderate degradation cycle (1–2 years) | Myocardial strain monitoring, dynamic tissue mechanics sensors | |
| PCLA | Balancing flexibility with degradation rate (1–3 years) | Multimodal sensor integration, organ transplant monitoring | |
| PLGA-PEG-PLGA | Thermosensitive gelation and degradation cycle are controllable | Injectable temperature response sensor and dynamic drug release monitoring | |
| (PLGA, variations in different proportions) | Adjustable degradation rate and mechanical properties (weeks to years) | Customized tissue engineering sensors, multi-parameter monitoring | |
| (PLGA, supplemental variant) | Rapid degradation (weeks to months), good flexibility | Short-term postoperative monitoring and inflammatory microenvironment feedback | |
| Natural derived materials | Starch | Fast degradation (about 90 days), low cost | Temporary sensor encapsulation, gut microbial monitoring |
| Lignocellulose | Natural porous structure, compostable degradation | Degradable circuit board base, environmentally responsive sensor | |
| Nitrocellulose | High mechanical strength, controlled degradation (months to years) | Microfluidic chip sensors, drug release monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Zhou, Y.; Li, S.; Xu, Q.; Pan, L. Implantable Medical Electronic Devices: Sensing Mechanisms, Communication Methods, and the Biodegradable Future. Appl. Sci. 2025, 15, 7599. https://doi.org/10.3390/app15137599
Chu Z, Zhou Y, Li S, Xu Q, Pan L. Implantable Medical Electronic Devices: Sensing Mechanisms, Communication Methods, and the Biodegradable Future. Applied Sciences. 2025; 15(13):7599. https://doi.org/10.3390/app15137599
Chicago/Turabian StyleChu, Zhengdao, Yukai Zhou, Saite Li, Qiaosheng Xu, and Lijia Pan. 2025. "Implantable Medical Electronic Devices: Sensing Mechanisms, Communication Methods, and the Biodegradable Future" Applied Sciences 15, no. 13: 7599. https://doi.org/10.3390/app15137599
APA StyleChu, Z., Zhou, Y., Li, S., Xu, Q., & Pan, L. (2025). Implantable Medical Electronic Devices: Sensing Mechanisms, Communication Methods, and the Biodegradable Future. Applied Sciences, 15(13), 7599. https://doi.org/10.3390/app15137599





