Stabilized Sewage Sludge as Fertilizer: Risks Related to the Presence of Microplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Separation and Digestion of Organic Matter
2.2.1. Density Separation
2.2.2. Quality Control Measures
2.3. Sample Processing
2.3.1. Confocal Microscope
2.3.2. Quality Control and Assurance
2.4. Identification of Microplastic Polymers via Raman Spectroscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mass
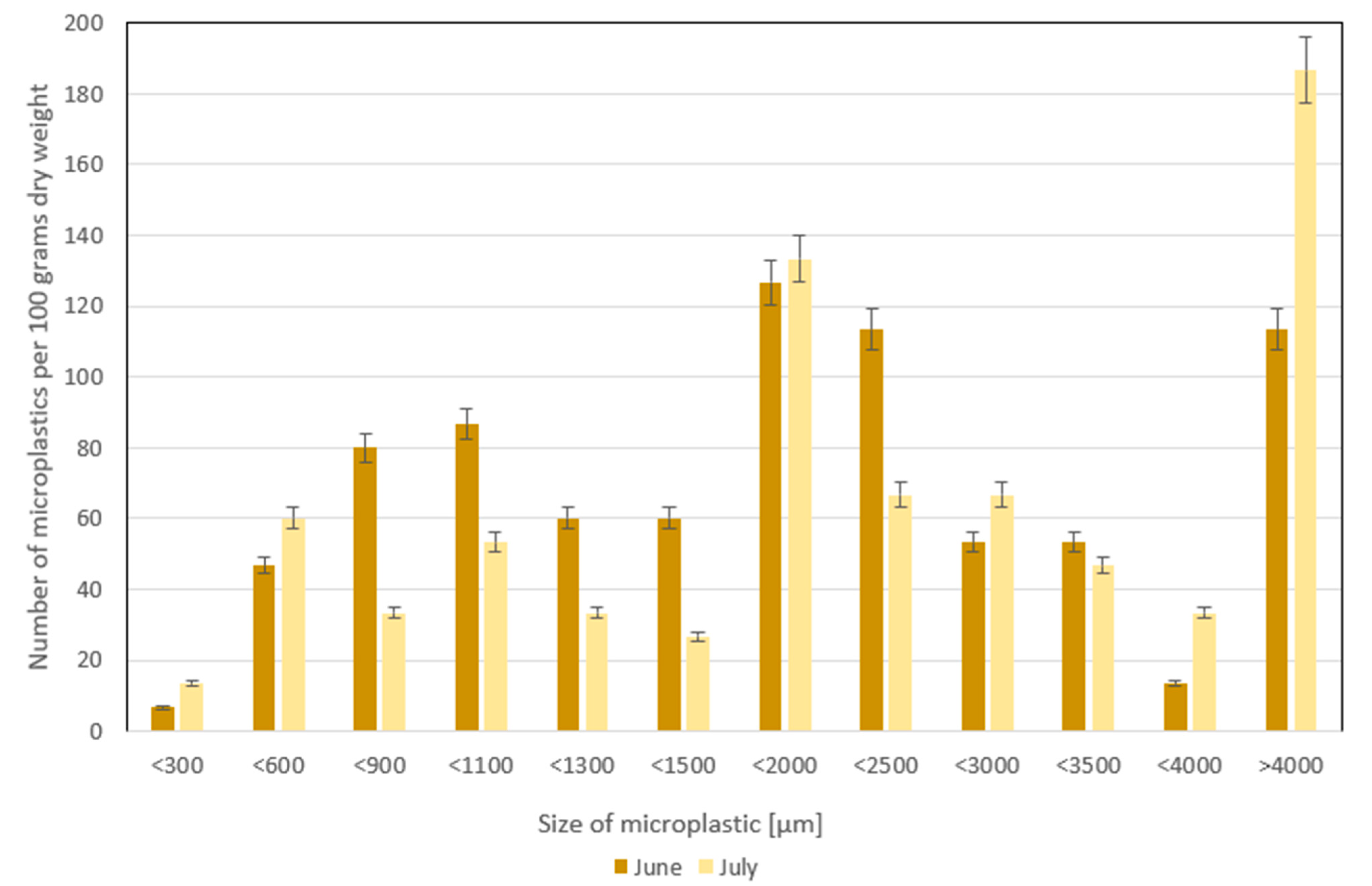
3.2. The Number of Microplastics
3.3. The Qualitative Analysis of Microplastics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| SSS | Stabilized Sewage Sludge |
| LDPE | Low-Density Polyethylene |
| PE | Polyethylene |
| PA | Polyamide |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| PVC | Polyvinyl chloride |
| PC | Polycarbonate |
References
- Worek, J.; Styszko, K. Comparative Study of Matrix Etching Methods for the Separation of Microplastics from Environmental Samples. Desalination Water Treat. 2025, 322, 101140. [Google Scholar] [CrossRef]
- Worek, J.; Badura, X.; Białas, A.; Chwiej, J.; Kawoń, K.; Styszko, K. Pollution from Transport: Detection of Tyre Particles in Environmental Samples. Energies 2022, 15, 2816. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the Human Body: A Comprehensive Review of Exposure, Distribution, Migration Mechanisms, and Toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, T.; Higashi, H.; Izumi, H.; Nishida, C.; Kawai, N.; Sato, K.; Morimoto, T.; Higashi, Y.; Yatera, K.; Morimoto, Y. Investigation of Pulmonary Inflammatory Responses Following Intratracheal Instillation of and Inhalation Exposure to Polypropylene Microplastics. Part. Fibre Toxicol. 2024, 21, 29. [Google Scholar] [CrossRef]
- Xu, J.-L.; Lin, X.; Wang, J.J.; Gowen, A.A. A Review of Potential Human Health Impacts of Micro- and Nanoplastics Exposure. Sci. Total Environ. 2022, 851, 158111. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci Technol 2016, 50, 2685–2691. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Shi, Z.; He, M.; Ma, D.; Liu, J. Effects of Polypropylene Micro(Nano)Plastics on Soil Bacterial and Fungal Community Assembly in Saline-Alkaline Wetlands. Sci. Total Environ. 2024, 945, 173890. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, Q.; Yin, N.; Tu, C.; Luo, Y. Uptake and Accumulation of Microplastics in an Edible Plant. Chin. Sci. Bull. 2019, 64, 928–934. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the Soil Environment: A Critical Review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as Carriers of Toxic Pollutants: Source, Transport, and Toxicological Effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Styszko, K.; Bolesta, W.; Worek, J.; Prus, Z.; Cwynar, K.; Pyssa, J.; Uchmanowicz, D.; Frydel, L.; Daso, A.P.; Kasprzyk-Hordern, B. Tracking Nonregulated Micropollutants in Sewage Sludge: Antimicrobials, OH-PAHs, and Microplastics—Environmental Risks, Fertilizer Implications and Energy Considerations. Energy Rep. 2025, 13, 4756–4768. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic Fertilizer as a Vehicle for the Entry of Microplastic into the Environment. Sci. Adv. 2018, 4, aap8060. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The Fate of Microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Worek, J.; Kawoń, K.; Chwiej, J.; Berent, K.; Rego, R.; Styszko, K. Assessment of the Presence of Microplastics in Stabilized Sewage Sludge: Analysis Methods and Environmental Impact. Appl. Sci. 2024, 15, 1. [Google Scholar] [CrossRef]
- Głodniok, M.; Korol, J.; Zawartka, P.; Krawczyk, B.; Deska, M. Organic Fertilizer and Method for Obtaining It. Polish Patent 420420, 2017. [Google Scholar]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman Microspectroscopy as a Tool for Microplastic Particle Analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of Environmental Microplastics by Vibrational Microspectroscopy: FTIR, Raman or Both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Chen, X.; Jiang, X.; Li, J.; Yang, L.; Yin, X.; Zhang, X. Occurrence and Distribution of Microplastics in Organic Fertilizers in China. Sci. Total Environ. 2022, 844, 157061. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and Fate of Microplastics in Wastewater and Sludge Filter Cake from a Wastewater Treatment Plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics En Route: Field Measurements in the Dutch River Delta and Amsterdam Canals, Wastewater Treatment Plants, North Sea Sediments and Biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and Quantification of Macro- and Microplastics on an Agricultural Farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Y.; Liu, J.; Zhong, S.; Qian, Y.; Gao, P. An Overlooked Entry Pathway of Microplastics into Agricultural Soils from Application of Sludge-Based Fertilizers. Environ. Sci. Technol. 2020, 54, 4248–4255. [Google Scholar] [CrossRef]
- Karthika, S.; Sivasubramanian, K.; Dhevagi, P.; Thavamani, P.; Rajkishore, S.K.; Amrutha, V.M.; Sudhakaran, R.; Chitra, N.; Lakshmanan, A. Distribution Pattern and Risk Assessment of Microplastics Contamination in Different Agricultural Systems. Int. J. Environ. Anal. Chem. 2024, 1–20. [Google Scholar] [CrossRef]
- Kılıç, B.I.; Üstün, G.E.; Can, T. Characteristics, and Seasonal Change of Microplastics in Organized Industrial Zone Wastewater Treatment Plant. J. Environ. Chem. Eng. 2025, 13, 115516. [Google Scholar] [CrossRef]
- Militký, J.; Novotná, J.; Wiener, J.; Křemenáková, D.; Venkataraman, M. Microplastics and Fibrous Fragments Generated during the Production and Maintenance of Textiles. Fibers 2024, 12, 51. [Google Scholar] [CrossRef]
- Novotna, J.; Tunak, M.; Militky, J.; Kremenakova, D.; Wiener, J.; Novakova, J.; Sevcu, A. Release of Microplastic Fibers from Polyester Knit Fleece during Abrasion, Washing, and Drying. ACS Omega 2025, 10, 14241–14249. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of Microplastic Fibers from Microfiber Fleece during Domestic Washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.-K. Quantifying Shedding of Synthetic Fibers from Textiles: A Source of Microplastics Released into the Environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef]
- European Parliament, C. of the E.U. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products. Off. J. Eur. Union 2019, 25, 1–120. [Google Scholar]


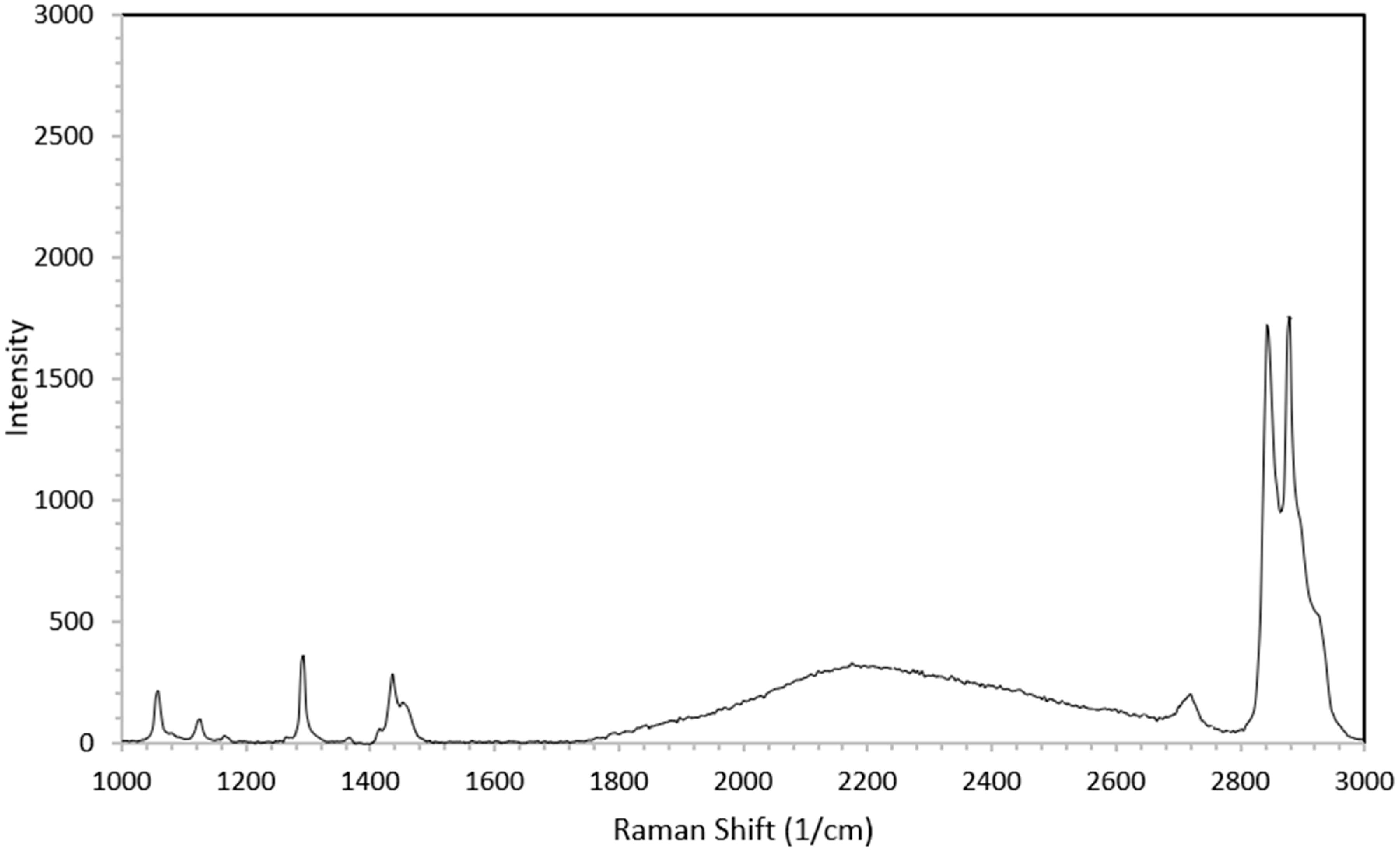
| Polymer | Main Raman Peaks (cm−1) | Vibrational Assignment | References |
|---|---|---|---|
| Polystyrene (PS) | ~620, ~1000, ~1030, ~1600, ~2900 | Aromatic ring deformation, ring breathing, C=C stretching, C–H stretching | Araujo et al. (2018) [16] |
| Polyethylene (PE) | ~1060, ~1130, ~1295, ~1440, 2880–2950 | C–C stretching, CH2 twisting, CH2 scissoring, C–H stretching | Anger et al. (2018) [17] |
| Polypropylene (PP) | ~840, ~973, ~1165, ~1330, ~1450, 2880–2950 | CH3 rocking, C–C stretching, CH bending, and stretching | Käppler et al. (2015); Araujo et al. (2018) [16,18] |
| Polyvinyl chloride (PVC) | ~638, ~695, ~830, ~1430, ~2900 | C–Cl stretching, C–H deformation, skeletal vibrations | Anger et al. [17] |
| Polyethylene terephthalate (PET) | ~860, ~1000, ~1240, ~1615, ~1725, ~2900 | C–C, C–O stretching, aromatic C=C, ester C=O stretching | Araujo et al. (2018) [16] |
| Polyamide (PA, e.g., Nylon) | ~935, ~1200, ~1440, ~1640, ~2900 | C–N stretching, CH2 deformation, amide C=O stretching | Käppler et al. (2015); Araujo et al. (2018) [16,18] |
| Polycarbonate (PC) | ~640, ~1130, ~1600, ~1770 | C–O stretching, aromatic C=C, carbonate C=O stretching | Anger et al. [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worek, J.; Kawoń, K.; Chwiej, J.; Styszko, K. Stabilized Sewage Sludge as Fertilizer: Risks Related to the Presence of Microplastics. Appl. Sci. 2025, 15, 7420. https://doi.org/10.3390/app15137420
Worek J, Kawoń K, Chwiej J, Styszko K. Stabilized Sewage Sludge as Fertilizer: Risks Related to the Presence of Microplastics. Applied Sciences. 2025; 15(13):7420. https://doi.org/10.3390/app15137420
Chicago/Turabian StyleWorek, Jagoda, Kamil Kawoń, Joanna Chwiej, and Katarzyna Styszko. 2025. "Stabilized Sewage Sludge as Fertilizer: Risks Related to the Presence of Microplastics" Applied Sciences 15, no. 13: 7420. https://doi.org/10.3390/app15137420
APA StyleWorek, J., Kawoń, K., Chwiej, J., & Styszko, K. (2025). Stabilized Sewage Sludge as Fertilizer: Risks Related to the Presence of Microplastics. Applied Sciences, 15(13), 7420. https://doi.org/10.3390/app15137420








