Abstract
The pet food industry faces significant sustainability challenges, including reducing energy consumption, lowering emissions, and adopting circular economy practices. This study aimed to assess and propose energy efficiency measures to enhance sustainability within the sector. The research evaluated the use of unapproved food as biomass for boiler combustion. It analyzed its chemical composition, energy impact, and emissions of volatile organic compounds (VOCs) through TD-GC/MS, as well as the corrosion effects on boiler metals. An energy assessment of the production process and a combustion characterization of the waste were conducted to identify opportunities for improving energy efficiency and sustainability. The results demonstrated that the chemical composition of the waste and other biomass-related parameters were within acceptable economic and environmental ranges. A reduction of 0.015 Mg of CO2eq per Mg of produced pet food was achieved. Regarding VOCs, their environmental impact was minimal due to the molecular structure of the compounds. Additionally, the corrosion rate caused by waste incineration was comparable to that of domestic gas in the case of cat food, with a rate of 214.74 mpy, while the dog food yielded 55.42 mpy, which is near that of other types of biomass, such as wood chips and pellets. The use of residual biomass in pet food production is a viable alternative for reducing carbon footprint, promoting a circular economy, and improving the industry’s sustainability.
1. Introduction
Environmental concerns, primarily driven by modern economic activity, have compelled countries and political communities to incentivize and develop policies aimed at reducing the environmental impact of industries and their production processes. These efforts have increasingly focused on lowering greenhouse gas (GHG) emissions and fostering discussions around energy efficiency [1,2], particularly in developing countries.
In Chile, where this study was conducted, several initiatives have emerged as regulatory frameworks to promote the adoption of sustainable industrial practices and reduce the environmental footprint of production processes. Notable examples include the Energy Policy 2050 [3], the Energy Efficiency Law [4] (Law 21.305), and the Framework Law on Waste Management, Extended Producer Responsibility, and Promotion of Recycling [5] (Law 20.920). These regulations have encouraged local industries to intensify their efforts to combat climate change by optimizing resource consumption to satisfy demand while maintaining a controlled or acceptable environmental impact. Additionally, consumer preferences are increasingly shifting toward products with lower carbon footprints [6].
The pet food industry is not exempt from this environmental challenge. It is a rapidly growing sector with significant environmental impacts. In the United States, pets are responsible for 25–30% of the environmental burden associated with animal production, including land, water, fossil fuel, phosphate, and biocide use. Pet consumption also contributes approximately 64 million Mg of CO2eq emissions annually [7,8].
The pet care market has significantly expanded in the country, reaching a value of USD 1.83 billion in 2024, and it is projected to grow to USD 3.25 billion by 2032 [9]. However, this growth also generates a considerable amount of solid waste, including pet food batches that do not meet commercial quality standards and are typically discarded or treated as waste.
Moreover, manufacturing processes in the pet food industry are characterized by high energy consumption due to the complexity and intensity of production activities [10]. Electrical energy is extensively used to operate machinery involved in grinding, mixing, extrusion, drying, and packaging [10]. Simultaneously, thermal energy, primarily from natural gas, is essential for cooking, extrusion heating, and drying processes that require precise temperature control to ensure product quality and food safety [11]. Peesel et al. [12] evaluated the technological feasibility of transitioning from fossil fuels to renewable alternatives for fueling boilers in the wet pet food processing industry, aiming to reduce emissions. In that study, the specific type of biomass used was not analyzed, as the focus was on commercialized and standardized biomass products.
The study highlighted that transitioning to a solid biomass-fueled boiler system in the case-study could cut CO2eq emissions by up to 42%, while a biomethane-fueled solid oxide fuel cell achieved up to a 27% reduction. Both methods increase operating costs, but the biomass system costs EUR 0.05–0.11 per kg CO2eq reduced annually, significantly less than the fuel cell’s EUR 0.69–1.14. As Peesel’s research was conducted with a primary focus on economic viability and emission reduction, insights about expected corrosion values or VOCs analysis remain elusive in the existing literature [12].
Many authors have researched the potential of converting waste into energy as a viable source for industrial boilers. Wang et al. [13] investigated the use of shoe manufacturing waste as a fuel for a CFB boiler system; however, high chlorine contents can cause severe deposition and corrosion issues. Güler [14] investigated the calorific value and emissions profile of tea waste for steam generation, revealing a promising energy potential. Nazar et al. [15] investigated the potential of rice husk as a boiler fuel from both environmental and techno-economic perspectives, concluding that rice husk can be utilized as a primary fuel source alongside coal. Bareschino et al. [16] investigated the use of dry tobacco stalks, a waste generated during cigarette manufacturing, as an alternative fuel to feed a biomass boiler. The results of that study concluded that the waste presented environmental and economic advantages compared to traditional systems. Animal by-products, including those from the pet food industry, have been identified as viable renewable energy sources, showing promising potential for energy recovery through thermal and biological conversion processes [17]. Recent studies have demonstrated that dog and cat food waste can be effectively used in anaerobic digestion, yielding high methane content and enabling energy recovery from non-commercial pet food residues [18].
As previously mentioned, the pet food industry generates batches that fail to meet commercial quality standards and are consequently discarded as waste. To the best of the authors’ knowledge, no prior research has explored the potential use of this pet food waste in pellet form as an alternative energy source for industrial or domestic boilers, particularly from a technical and environmental perspective. This study investigated the feasibility of utilizing discarded pet food products as a biofuel within the energy system of a pet food production facility. Emphasizing energy conservation, GHG emissions reduction, and circularity, the research explicitly investigated whether these materials could be used as biomass to power the industrial boiler of the manufacturing facility, which currently runs on natural gas. The analysis involved determining the calorific value of the samples to evaluate the energy potential of the residues. Additionally, it included the assessment of ash content, the presence of heavy metals, emissions of volatile organic compounds (VOCs), and potential corrosion effects on boiler chimneys. Furthermore, GHG emissions resulting from the combustion of these pellets were estimated to assess their environmental implications.
This approach aligns with global sustainability trends, such as waste-to-energy and circular economy practices, aiming to reduce fossil fuel dependency, mitigate emissions, and valorize industrial residues. By transforming production waste into a local energy source, the pet food industry could enhance both its environmental and economic performance, contributing to a more sustainable manufacturing model.
2. Case Study
The factory under study operates a single production line with a maximum annual capacity of 77,000 Mg of finished product. The plant holds the following certifications: ISO 9001 for Quality Management [19], ISO 14001 for Environmental Management [20], ISO 45001 for Occupational Health and Safety Management [21], and ISO 22000 for Food Safety Management [22]. These certifications attest to the implementation of an integrated management system, which is regularly audited both internally and externally.
Natural gas is the primary fuel used in the facility, mainly for boilers and dryers. The primary function of the boiler is to generate steam for the extrusion process. It has a maximum capacity of 6500 kg h−1 of steam at a maximum operating pressure of 10 bar. Under current conditions, it operates at 1800 kg h−1, consuming approximately 100 m3 h−1 of natural gas. The dryer used in the pet food processing line includes three burners: two rated at 5802.8 W each and a smaller burner rated at 3798.2 W. It consumes approximately 200 m3 h−1 of natural gas.
These stages involve the use of the boiler, which generates the steam required to cook ingredients during extrusion, and a dryer, which ensures a product shelf life of up to 18 months by reducing water activity and free moisture content, inhibiting microbial growth [23,24]. Unlike electrically powered equipment, it is important to highlight that both the boiler and the dryer operate on natural gas. As a result, they are not only the largest consumers of thermal energy but also the main contributors to GHG emissions during production. In terms of electrical energy, the plant has implemented several energy efficiency initiatives, including 100% LED lighting, motion-sensor lighting, and the stabilization of production line speeds. Additionally, the facility has installed a reverse osmosis system, which reduces calcium buildup in the water before it enters the boiler, thereby ensuring consistent water quality and lowering the energy required for heating. Furthermore, the plant recovers water from the production process to preheat water extracted from the well. This recovered water raises the temperature of incoming water from 5–10 °C to approximately 30 °C, improving overall thermal efficiency.
In 2020, the total natural gas consumption reached 46,635 GJ. Electricity consumption includes lighting, water heating for showers and restrooms, water pumps, space heating in offices, computer systems, and other auxiliary uses. Total electricity consumption during the same year amounted to 34,312 GJ.
Figure 1 presents the relationship between thermal energy consumption and pet food production. A clear correlation can be observed, as approximately 1 GJ of thermal energy is required to produce one Mg of pet food. In contrast, electricity consumption remains relatively stable regardless of production volume, averaging around 0.6 GJ of electricity per Mg produced.
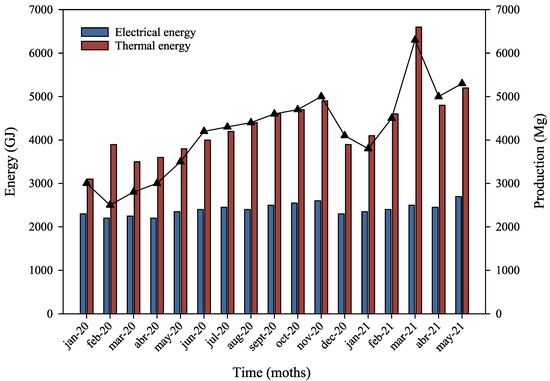
Figure 1.
Trends in pet food production and energy usage from January 2020 to May 2021. The blue bar corresponds to electrical energy consumed by the factory. The red bar corresponds to thermal energy consumed by the factory. The triangles corresponding to the pet food production in Mg.
Figure 1 also illustrates that, since December 2020, there has been a decrease in electricity consumption per Mg produced. This improvement is attributed to increased production run speeds, which allow for a greater output in less time without compromising product quality, thereby enhancing energy efficiency.
By comparing the types and quantities of energy used in the production process, as shown in Figure 1, it can be concluded that 62.3% of the total energy consumption is thermal, while electrical energy accounts for 37.7%. Notably, the electricity used is sourced from renewable energy, as established through agreements with the local utility provider. The above results in a lower environmental impact compared to thermal energy, which relies entirely on natural gas. Therefore, energy efficiency management is critical in the stages that require thermal input, specifically stages 3 (extrusion) and 4 (drying). During these stages, the boiler, which is used to generate steam for extrusion, and the dryer are the primary sources of thermal energy consumption. As such, these processes constitute the primary focus of the energy performance assessment. The final product of the pet food production process consists of croquettes in various shapes and diameters tailored to the nutritional needs of the target animal species, its life stage, and dietary formulation. However, a small but notable portion of the pet food produced does not meet the quality standards required for commercialization. Part of this off-spec product is reintegrated into the production process, while the remainder is sent for composting by an external contractor. Additionally, throughout the year, company personnel collect expired products from distribution centers and retail outlets. These products are destroyed under the supervision of a notary. In 2020, the total amount of waste generated by the production line was 906.43 Mg per year, representing approximately 1.2% of the total input classified as non-commercial waste on an annual basis.
3. Analytical Methods for Characterizing Production Waste
The objective of this research is to promote sustainability in the industry by utilizing waste from the production process as biomass, thereby enabling both waste reduction and the mitigation of GHG emissions. The analysis of production waste was conducted in the Environmental Gas and Biofuel Laboratory at the School of Engineering, University of Talca.
Six 20 kg samples were collected in the facilities in plastic containers filled with non-commercial-grade pet food: three for dogs and three for cats. The samples were ground into fragments smaller than 5 mm using an IKA-WERKE mill (model M20, serie S/N, Staufen, Alemania).
They were then dried to a 10% wt % moisture content using a Memmert oven (model UM500, serie S/N, Shangai, China) until a constant weight was achieved. Precision weighing (0.001 g) was performed using a Mettler Toledo balance (model AB 304, Santiago, Chile). Nitrogen determination was performed by weighing 1 g of the dry sample (Kjeldahl method—AOAC 979.09 [25]—Equip Velp Scientifica, DKL Heating Digester, Europe).
Following the ISO 18125 methodology [26], the higher and lower heating values (HHV and LHV) were determined by weighing 1 g of the dry sample and analyzing it with a Cal2k ECO bomb calorimeter (DDS Calorimeters, Randburg, South Africa), calibrated using certified benzoic acid (Merck, Saint Louis, MI, USA).
The determination of ash content was performed in a Vulcan furnace (model A-550, serie S/N, New York, NY, USA) following the ISO 18122:2015 methodology [27]. The ash content was calculated as the proportion of ash in the sample relative to the original sample weight, expressed as a percentage.
X-ray fluorescence (XRF) was employed to determine the qualitative elemental composition of the pet food samples. A sample capsule was prepared for each type of ash in triplicate using 2 g of dry, ground, and sieved sample (mesh size 1.6 mm). The equipment used was a Thermo Scientific Scientific (model Niton XL3t GOLDD+, Billerica, MA, USA) analyzer, which provided real-time measurements processed in an MS Excel spreadsheet.
To determine the quantitative elemental composition, an Atomic Absorption Spectrometer (AAS) by Analytik Jena (model NovAA 800, serie S/N, Jena, Germany) was used. It was equipped with a flame, graphite furnace, and hydride generation systems (HS 60). For each ash type, 2 g of sample was analyzed in triplicate. Samples were dry, ground, and sieved (mesh size 1.6 mm). Digestion was carried out using 7 mL of nitric acid (65% v%) (Winkler, Santiago, Chile) and 1 mL of hydrogen peroxide peroxide (30% v%) (Winkler, Santiago, Chile) at 60 °C under a gas extraction hood using a Labtech EH hot plate for 8 h. After digestion, samples were dried and reconstituted in HPLC-grade water (Winkler, Santiago, Chile) to a final volume of 50 mL. Metal standards supplied by Merck and analytical-grade reagents were used. Concentrations of metals were determined using flame and graphite furnace techniques, while arsenic, selenium, and mercury were analyzed using a hydride generator with a 4% NaBH4 modifier.
To determine volatile organic compounds (VOCs), a thermal desorber coupled with a gas chromatography–mass spectrometry system (TD-GC–MS, Milan, Italy) was used. For this analysis, 2 g of each sample was placed on a burner. Using a Markes Easy VOC LP-1200 suction pump, LLantrisant, UK 10 mL of gas-phase sample was collected in adsorption tubes (Markes C2-BAXX-5315 for odor and sulfur; C6/7-C30 for thiols and mercaptans, LLantrisant, United Kingdom). These were introduced into a cold trap thermal desorption injector (Markes Unity-xr, LLantrisant, United Kingdom). Gases were extracted with helium (Air Products Indura, Talca, Chile) and passed through a thermal trap set at 300 °C for one minute. The gases were then cooled to 20 °C and reheated to 300 °C for an additional 5 min. VOCs were transferred to a gas chromatography–mass spectrometer (Thermo Fisher Scientific, Milan, Italy, Trace 1300/ISQELTL) via a 200 °C transfer line, operating in split mode. The oven was programmed to increase from 40 to 220 °C at a rate of 10 °C min−1, with a flow rate of 1.2 mL min−1. The mass spectrometer detector line was set to 200 °C, and the ion source to 250 °C. The qualitative identification of VOCs was performed using Chromeleon V. 7.3.2 software, which utilized the NIST library based on standard retention times. The results were compared with odor thresholds described in the literature. Figure 2 presents the experimental setup scheme for the combustion analysis of cat and dog food, in addition to other biofuels.
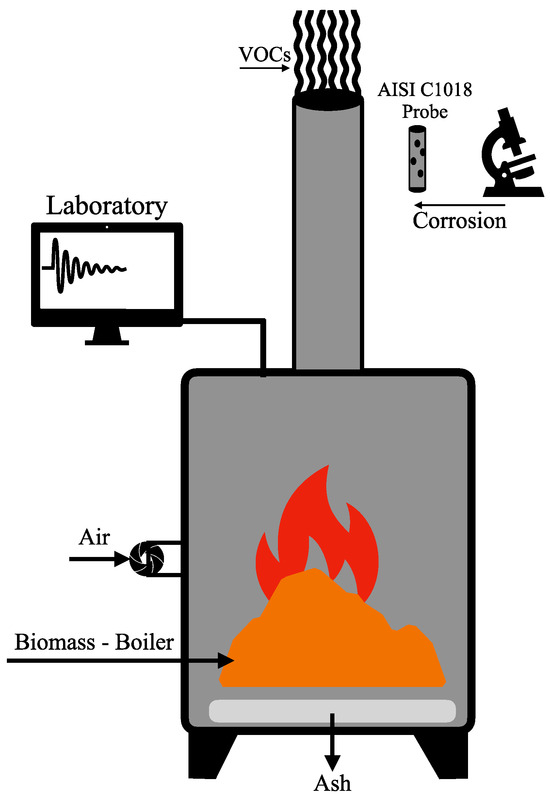
Figure 2.
Experimental setup diagram for the analysis of VOC material combustion and corrosion in test probe.
4. Use of Pet Food Waste for Energy Production
Metals and alloys are susceptible to corrosion when frequently exposed to corrosive gases such as water vapor, CO2, and H2S. The corrosion index of gases generated during the combustion of production waste was determined, as these gases can corrode the metallic pipes in the exhaust ducts. Mass loss, either in pipe wall thickness or surface area, is used as an indicator of the corrosion penetration rate. The most common unit used to quantify physical corrosion progression is mils per year (mpy) [28,29]. The corrosion rate, , expressed in mils per year (mpy), can be calculated from mass or thickness loss as
where m is the mass loss in grams, A is the exposed surface area in cm2, is the material density in g cm−3, t is the exposure time in hours, and is the conversion factor of 3.44882 × 106 mils h cm−1 a−1 (this correction factor results from converting the units used in this study (g, cm, h) to the original units (lb, in, year)).
Carbon steel AISI C1018 materials, each weighing approximately 60 g, with an average density of 9.3 g cm−3 and a surface area of 24.11 cm2, were used as corrosion samples. The experimental procedure utilized waste samples derived from dog and cat pet foods, along with three control fuels: Radiata pine sawdust, natural gas, and residential stove pellets. The tests were conducted over a five-hour period in a laboratory furnace (Memmert UM-500, Schwabach, Germany), which was specially modified for this purpose at temperatures ranging from 800 to 900 °C. An excess air ratio between 3 and 5 was maintained throughout the experiments. The pre-weighed metal specimens were exposed to the combustion exhaust gases for a defined duration. After exposure, they were reweighed to determine the mass loss, m, which served as a measure of corrosion. GHG emissions were estimated in accordance with the GHG Protocol, the most widely adopted global framework and the standard applied by the company under study across its industrial operations. This protocol classifies emissions into three scopes:
- Scope 1—Direct GHG Emissions: Emissions from sources owned or controlled by the company, such as boilers, furnaces, and company vehicles.
- Scope 2—Indirect GHG Emissions from Electricity: Emissions from the generation of purchased electricity consumed by the company.
- Scope 3—Other Indirect Emissions: Emissions arising from company-related activities, including those from raw material procurement [30].
In this study, the focus was placed on Scope 1 emissions, specifically those stemming from the combustion of natural gas used in the facility’s boiler and dryer during the production process.
To quantify emissions, the GHG Emissions Calculation Tool available on the GHG Protocol Initiative’s website [31] was employed. Specifically, the stationary combustion worksheet provided within the tool was used for this purpose, as
where represents the fuel consumption, and denotes the fuel emission factor. The tool applies the default emission factors from the 2006 IPCC Guidelines for National Greenhouse Gas Inventories [31]. For natural gas, the emission factors are 5.049 × 107 kg GJ−1, 900 kg CH4 GJ−1, and 90 kg GJ−1 for CO2, CH4, and N2O emissions, respectively.
5. Results and Discussion
An evaluation of thermal energy consumption for the factory’s two main systems revealed that the dryer accounts for an average of 68.8% of monthly energy use, while the boiler accounts for 30.9%, as shown in Figure 3. The remaining thermal energy is consumed by auxiliary systems, including space heating and the personnel food preparation area. Periodic monitoring and internal records indicate that energy consumption for both systems remains relatively stable throughout the year, with minimal variation between the hottest and coldest months. Regarding the characterization of pet food waste for use as biomass, the calorific value (expressed in GJ kg−1) and ash content of the analyzed material were determined, with the results shown in Table 1.
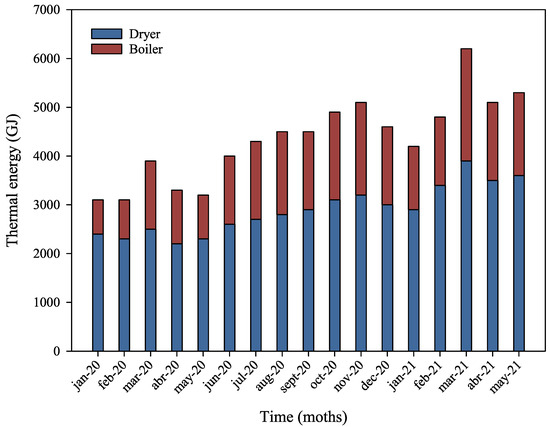
Figure 3.
Monthly distribution of thermal energy consumption (GJ) by the two main systems of the plant.

Table 1.
Calorific value and ash content of analyzed pet food samples.
These results are energetically favorable, considering that eucalyptus firewood has a higher heating value (HHV) ranging from 0.017176 to 0.017986 GJ kg−1 and an ash content of 0.69%, as reported by Lins et al. [32]. Therefore, the analyzed residues exhibit a high energy potential. Moreover, when compared to other agro-industrial byproducts commonly used as biofuels, these residues show superior energy content, with values comparable to those of corn cobs [33,34]. In addition to calorific value, ash content and elemental composition are critical parameters for evaluating the potential of a biomass fuel to form deposits in the boiler and to cause corrosion, erosion, or abrasion to heat exchange surfaces and other components [35].
The presence of metals and non-metals, as determined via X-ray Fluorescence (XRF) and Atomic Absorption Spectrometry (AAS), is presented in Table 2. These results enable the estimation of trace metal emissions that may be released during the combustion of food waste. Such emissions can occur in non-volatile forms that remain in the ash or in volatile forms that vaporize at high combustion temperatures. Trace metals with notable environmental relevance include arsenic (As), cadmium (Cd), and lead (Pb). Additionally, highly volatile elements such as mercury (Hg) and selenium (Se) were also detected. However, copper (Cu), nickel (Ni), thallium (Tl), and antimony (Sb) were not detected by any of the analytical methods.

Table 2.
Relevant chemical elements. Measurements were performed by two methods. AAS: Atomic Absorption Spectrophotometry (measured in mg kg−1). XRF: X-Ray Fluorescence (measured in wt %).
Although some of the metals identified may be toxic to pets, their mere presence does not necessarily indicate a health hazard. Heavy metals occur naturally in many ecosystems and can be absorbed by plants during growth, which explains their trace presence in raw materials used for pet food production, even at extremely low concentrations [36]. The plant enforces strict quality control procedures upon the arrival of raw materials to ensure compliance with established safety thresholds for animal health. Sulfur was also detected in the analyzed pet food samples. According to the literature, biomass typically contains low levels of sulfur, with 40–90% retained in the ash after combustion. However, the presence of volatile sulfur released during combustion is particularly relevant for estimating corrosion potential, as it serves as a precursor to hydrogen sulfide (H2S) and sulfuric acid (H2SO4) through direct or sequential reactions with emitted steam. These compounds are highly corrosive and can significantly damage machinery and infrastructure [37]. Therefore, quantifying sulfur content is essential for predicting corrosion risk and informing the implementation of gas cleaning systems. These may include filtration using quicklime or slaked lime or the injection of pure oxygen or air (2–6 % vol) at the reactor outlet to reduce H2S formation [38]. Future studies should quantify sulfur (S) concentrations more precisely to support these assessments.
The results obtained from the TD-GC/MS analysis of VOCs were categorized by compound families and compared with literature data, as shown in Figure 4. The exhaust gases from the combustion of cat food samples had higher concentrations of alcohols and aldehydes, while those from dog food contained more hydrocarbons, as illustrated in Figure 4. Among the hazardous VOCs, due to their potential to form ozone and secondary particulate matter, benzene and toluene were detected in dog food samples. In contrast, only benzene was found in cat food [39].
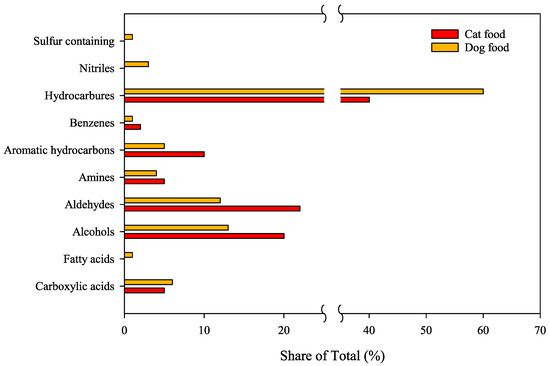
Figure 4.
Distribution of VOC families in pet food used as biomass source. The percentage corresponds to the percentage of VOC families.
Respecting odor thresholds, around 17.8% of the compounds found in dog food and 20.0% present in cat food have been associated with known odor characteristics [40]. Based on these results, it is reasonable to anticipate that the use of pet food waste as a biofuel could lead to the generation of odorous emissions following combustion. However, the degree of discomfort or nuisance caused by these odors cannot be determined at this stage. Further analysis is required to quantify the concentrations of VOCs relative to odor thresholds. Quantifying these concentrations will be essential for implementing mitigation strategies to manage potential odor impacts associated with biomass combustion. It is also noteworthy that many of the VOCs identified in both pet food samples, particularly those with known odor thresholds, are also commonly emitted during the combustion of Radiata pine and Eucalyptus wood [41].
5.1. Definition and Evaluation of Energy Efficiency Opportunities
In terms of energy efficiency opportunities identified within the production process, it is essential to highlight the strong dependence on thermal energy of the two key systems involved. As previously discussed, replacing the dryer or its associated fuel is currently not technically feasible. Therefore, the energy efficiency measures proposed in this study focus on optimizing the boiler’s performance. The primary opportunity evaluated was the use of food waste generated during the production process as fuel. This strategy would not only generate thermal energy through combustion but also support carbon neutrality goals and promote circular economy practices within the facility. To estimate the biomass requirement, the following parameters were considered: a boiler thermal demand of 1256 kW, an average lower heating value (LHV) of 0.01958 GJ kg−1 (based on sample analysis), a boiler efficiency of 85%, and 8040 operating hours per year (accounting for scheduled maintenance). Based on these assumptions, the total annual biomass requirement yields 2183.8 Mg. Considering that the waste generated from the production line (including expired and off-spec products) amounts to 906 Mg per year, this internal biomass could meet approximately 41.5% of the boiler’s demand, equivalent to 521.3 kW of thermal output. The remaining 58.5% would need to be supplied from external biomass sources, such as wood chips procured from local suppliers. Given the lower LHV of wood chips (0.0165 GJ kg−1), the required quantity was estimated at 1516.2 Mg per year, corresponding to 5415.12 m3 (assuming a bulk density of 280 kg m−3). This combination of internal and external biomass would meet the boiler’s energy demand for producing 1800 kg h−1 of steam required by the extrusion process.
5.2. Corrosion Index
Corrosion tests conducted on carbon steel specimens revealed that exposure to exhaust gases from cat food combustion resulted in the highest corrosion rate, with a measured value of 214.74 ± 53 mpy. This rate is comparable to the 174.82 ± 6.89 mpy observed during exposure to domestic propane–butane gas. For reference, similar corrosion levels have been reported for AISI C1018 steel immersed in seawater for 24 h, reaching up to 228.70 mpy [42]. In contrast, the combustion of dog food resulted in a significantly lower corrosion rate of 55.42 ± 1.22 mpy, aligning more closely with values typically reported for biomass combustion. This value is comparable to the 50 mpy reported for the Superni 718 alloy during the combustion of medical waste over a 1000 h period, as reported by Mudgal et al. [29]. Among the fuels analyzed, wood chips and residential pellets exhibited the lowest corrosion rates, with values of 34.69 ± 2.69 mpy and 37.18 ± 1.88 mpy, respectively. These levels are consistent with those reported for T91 steel used in boiler systems, where after 1500 h of biomass combustion, corrosion rates reached approximately 25 mpy [43]. It is important to note that the temperature of the exhaust gases has a significant impact on the corrosion rate: lower post-combustion temperatures (30 °C to 150 °C), typically found in the exhaust ducts, tend to accelerate corrosion due to the condensation of corrosive compounds, whereas higher combustion temperatures (350 °C to 900 °C) are generally associated with reduced corrosion rates due to the formation of more stable oxide layers [44]. Furthermore, mass loss due to corrosion typically follows a logarithmic trend, characterized by a rapid initial rate followed by a slower increase over time [45]. This behavior suggests that short-duration tests may yield higher apparent mpy values compared to those observed over longer exposure periods [46], which may explain discrepancies between this study and previous reports [46,47,48]. The physical effects of corrosion are illustrated in Figure 5.
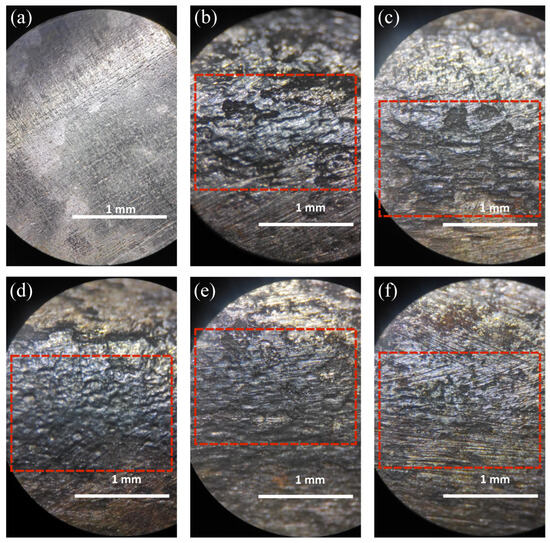
Figure 5.
Photographs of carbon steel specimens before and after exposure to combustion gases. (a) Unexposed reference specimen; (b) exposed to combustion gases from cat food; (c) exposed to combustion gases from dog food; (d) exposed to propane–butane domestic gas; (e) exposed to combustion gases from residential pellets; (f) exposed to combustion gases from wood chips. The red rectangle represents the focus of corrosion and pitting on the sample.
5.3. Greenhouse Gas Measurement Results and Mitigation Proposal
As previously noted, GHG emission estimates focus exclusively on processes that use natural gas as a fuel, since the facility’s electricity supply is certified as 100% renewable. As a result, there are no emissions attributed to the generation of electricity consumed by the company.
The results of GHG emissions, based on the total natural gas consumption for the dryer and boiler systems, are shown in Figure 6. Greenhouse gas emissions, in CO2eq, were found to be directly proportional to production levels, reflecting the thermal energy demands of the manufacturing process. In 2020, the plant emitted a total of 2347.8 Mg of CO2eq from natural gas use, corresponding to 0.048 Mg of CO2eq per Mg of pet food produced.
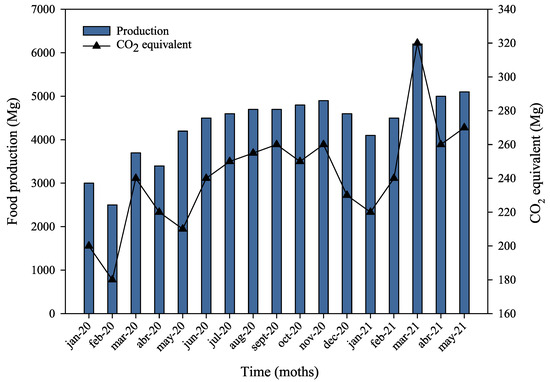
Figure 6.
Pet food production versus GHG emissions associated with thermal energy use.
Figure 7 shows that the dryer is the largest contributor to GHG emissions, accounting for 68.76% of the total. This result is expected, as the dryer is the system with the highest natural gas consumption. The boiler contributes an additional 30.87% of the plant’s GHG emissions. Accordingly, for every Mg of pet food produced, the dryer is responsible for 0.033 Mg of CO2eq, while the boiler accounts for 0.015 Mg.
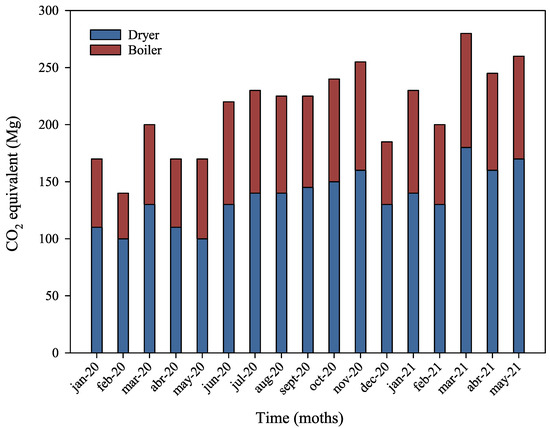
Figure 7.
GHG emission distribution by equipment.
The proposed GHG mitigation strategy involves replacing natural gas with biomass as the fuel source for the boiler, as previously discussed. Biomass is considered carbon-neutral in terms of CO2 emission, thereby eliminating all GHG emissions currently attributed to the boiler’s natural gas consumption. If biomass had been used in 2020, during the production of 48,319 Mg of pet food, the boiler’s annual emissions would have been reduced by approximately 709.34 Mg of CO2eq. This substitution would correspond to a reduction of 0.015 Mg of CO2eq per Mg of pet food produced. Given the rapid growth of the pet food industry in Chile and worldwide, adopting biomass as a boiler fuel would have a significant long-term impact on the company under study. While this change alone would reduce the plant’s current GHG emissions by 31%, the company’s broader sustainability goals, together with increasingly stringent environmental regulations, are expected to drive the future adoption of technologies that could eventually decarbonize the drying process as well. Although Scope 3 GHG emissions were not assessed in this study, the proposed measure would likely contribute to additional emission reductions by lowering the frequency of natural gas deliveries to the facility and decreasing the volume of food waste transported for composting.
The results of this study provide valuable insights into the viability of using sustainable biomass to improve energy efficiency in boiler systems. From an energy perspective, both cat and dog food samples demonstrated potential as standalone fuels or co-firing alternatives, with higher heating values (HHVs) of 0.0185 GJ kg−1 0.0207 GJ kg−1 for dog and cat food samples, which is comparable to other wastes researched as biocombustible in boiler systems, such as tea waste pellets (0.0182 GJ kg−1), shoe manufacturing waste (0.0235 GJ kg−1), rice husk (0.015 GJ kg−1), and Tobaco stalks (0.0174 GJ kg−1) [13,14,15].
In terms of greenhouse gas (GHG) emissions, substituting natural gas with pet food biomass could potentially reduce CO2eq emissions by 0.015 Mg GJ−1 or, equivalently, 0.015 Mg per Mg of pet food produced. Comparatively, [15] reported that the use of rice husk biomass could reduce CO2eq emissions to 0.0361 Mg GJ−1, while Bareschino et al. [16] found that tobacco stalk biomass could reduce emissions to 0.0618 and 0.0598 Mg GJ−1, respectively, depending on the combustion conditions.
However, the corrosive potential of waste-derived biomass remains a critical concern, as also noted in previous studies [44]. In our analysis, the presence of chlorine in cat and dog food samples suggests a possible risk of corrosion during combustion. A similar issue was observed by Wang et al. [13], who investigated shoe manufacturing waste as a promising fuel (HHV of 0.0235 GJ kg−1) for circulating fluidized bed technology (CBT) boilers. Despite its energetic viability, the high chlorine content posed a serious threat to boiler integrity due to corrosion.
6. Concluding Remarks
This study confirmed the high energy dependency of pet food production processes, with thermal energy accounting for 62.3% and electrical energy for 37.7% of the company’s total energy demand. At the product level, this corresponds to approximately 1 GJ of thermal energy and 0.6 GJ of electrical energy per Mg of pet food produced. While the company benefits from a supply contract with the local electricity distributor for non-conventional renewable energy, its dependence on natural gas for thermal processes remains a significant obstacle to achieving carbon neutrality by 2050. Drying and extrusion were identified as the most energy-intensive processes, with the dryer accounting for 68.8% and the boiler for 30.9% of the natural gas used for steam generation.
These two systems were also the main contributors to GHG emissions in proportion to their natural gas consumption. The production of one Mg of pet food results in 0.048 Mg of CO2eq emission, of which 68.8% is attributed to the dryer and 30.9% to the boiler. These findings underscore the need for targeted energy efficiency and mitigation strategies that specifically address these units.
To further enhance sustainability, the study investigated the valorization of unsellable pet food waste as a biomass fuel. The material exhibited a high calorific value of 0.0195 GJ kg−1, an ash content below 1%, and low levels of heavy metals, characteristics comparable to radiata pine wood. Combustion tests using TD-GC/MS indicated that emissions from dog food waste are not significant contributors to environmental odors. Corrosion studies revealed that the effect on boiler pipes was comparable to that of natural gas. Although cat food combustion proved to be approximately 30% more corrosive tham . Overall, these findings support the feasibility of fueling industrial boilers with a biomass blend of unsellable pet food waste and wood chips, enabling the generation of thermal energy for extrusion with a carbon-neutral footprint. This strategy not only advances circular economy principles but also significantly reduces GHG emissions, aligning with the company’s long-term commitment to achieving carbon neutrality by 2050.
Author Contributions
Conceptualization, D.H.; methodology, D.H. and J.A.-H.; validation, J.A.-H., F.d.G., F.A., and D.H.; formal analysis, J.G. and D.H.; investigation, J.A.-H., F.d.G., F.A., and D.H.; resources, J.G., H.Q.-L., and D.H.; writing—original draft preparation, J.D. and J.A.-H.; writing—review and editing, J.G. and H.Q.-L.; visualization, J.G. and H.Q.-L.; supervision, D.H. and H.P.-F.; project administration, H.Q.-L. and D.H.; funding acquisition, H.Q.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
D.H., J.G., and H.Q.-L. acknowledge funding from the National Fund for Scientific and Technological Development (FONDECYT), Chile, under Grant Nos. 1240819, 11250144, and 1240765. The authors also acknowledge the support from the Centro Tecnológico de Conversión de Energía (CTCE), Universidad de Talca.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAS | Atomic Absorption Spectrometer |
| GC | Gas chromatography |
| GHG | Greenhouse gas |
| HHV | Higher heating value |
| HS | Hydride generation system |
| LHV | Lower heating value |
| MS | Mass spectrometry |
| TD | Thermal desorber |
| VOC | Volatile organic compound |
| XRF | X-ray fluorescence |
References
- ISO 50001; Requisitos ISO 50001 Sistema de Gestión de la Energía. Agencia de Sostenibilidad Energética: Providencia, Chile, 2012. Available online: https://www.iso.org/iso-50001-energy-management.html (accessed on 10 March 2025).
- Birriel, A.; Romero, J.; Saavedra, N.; Quinteros-Lama, H.; González, J. Thermal and Exergetic Performance Assessment of an ORC Coupled with Thermal Energy Storage Using Thermal Oils for Low-Grade Heat Recovery. Appl. Sci. 2025, 15, 6153. [Google Scholar] [CrossRef]
- Ministerio de Energía, Chile. Energía 2050: Política energética de Chile. 2025. Available online: https://energia.gob.cl (accessed on 10 March 2025).
- Ministerio de Energía, Chile. Ley Número 21.305–Sobre Eficiencia Energética, 2021. Promulgada el 8 de Febrero de 2021; Publicada en el Diario Oficial el 13 de Febrero de 2021. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1155887 (accessed on 12 April 2025).
- Ministerio del Medio Ambiente, Chile. Ley Número 20.920–Establece Marco para la Gestión de Residuos, la Responsabilidad Extendida del Productor y Fomento al Reciclaje, 2016. Promulgada el 17 de Mayo de 2016; Publicada en el Diario Oficial el 1 de Junio de 2016. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1090894 (accessed on 10 March 2025).
- Pet Sustainability Coalition and World Pet Association. Ready for Business: Looking Towards a Sustainable Pet Industry–State of the Pet Industry Report. 2019. Available online: https://petsustainability.org (accessed on 14 December 2024).
- Okin, G. Environmental impacts of food consumption by dogs and cats. PLoS ONE 2017, 12, e0181301. [Google Scholar] [CrossRef] [PubMed]
- Vertakova, Y.; Plotnikov, V. Problems of sustainable development worldwide and public policies for green economy. Econ. Ann.-XXI 2017, 166, 4–10. [Google Scholar] [CrossRef]
- Research Expert Market. Chile Pet Care Market Report and Forecast 2024–2032. 2023. Available online: https://www.researchandmarkets.com/reports/5982179/pet-care-market-report-forecast?srsltid=AfmBOooHnqVXxnK4ftrinR9M_B7tRJLzAZ59qhDF3aA5h_Ghq7br7B_p (accessed on 10 March 2025).
- Costa, J.L.; Bánkuti, F.I.; Oiko, O.T.; Monti, M.; Loureiro, B.A.; Henríquez, L.B.; Florindo, T.J.; Vasconcellos, R.S. Life cycle assessment of the production of an extruded dog food in Brazil. J. Clean. Prod. 2024, 458, 142505. [Google Scholar] [CrossRef]
- Huss, A.; Cochrane, R.; Jones, C.; Atungulu, G.G. Physical and Chemical Methods for the Reduction of Biological Hazards in Animal Feeds. In Food and Feed Safety Systems and Analysis; Academic Press: Cambridge, MA, USA, 2020; pp. 83–95. [Google Scholar] [CrossRef]
- Peesel, R.H.; Otte, A.; Stark, M. Transition of Steam Utility Systems to Solid Biomass-Fuelled Boilers and Biomethane-Fuelled Fuel Cells in the Wet Pet Food Processing Industry. Chem. Eng. Trans. 2019, 76, 931–936. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, X.; Zhou, Z.; Duan, Y.; Li, L.; Dai, J.; Lin, H.; Luo, Y.; Sun, Z.; Duan, L. Ash deposition mechanism of shoe manufacturing waste combustion in a full-scale CFB boiler. Fuel Process. Technol. 2021, 221, 106948. [Google Scholar] [CrossRef]
- Güler, B. Biomass valorization: Comparative analysis of tea waste pellets and wood pellets for steam generation and emission profiles. Sustain. Energy Technol. Assessments 2024, 72, 104103. [Google Scholar] [CrossRef]
- Nazar, M.; Yasar, A.; Raza, S.A.; Ahmad, A.; Rasheed, R.; Shahbaz, M.; Tabinda, A.B. Techno-economic and environmental assessment of rice husk in comparison to coal and furnace oil as a boiler fuel. Biomass Convers. Biorefinery 2023, 13, 1671–1679. [Google Scholar] [CrossRef]
- Bareschino, P.; Marrasso, E.; Roselli, C. Tobacco stalks as a sustainable energy source in civil sector: Assessment of techno-economic and environmental potential. Renew. Energy 2021, 175, 373–390. [Google Scholar] [CrossRef]
- Moradian, F.; Pettersson, A.; Svärd, S.H.; Richards, T. Co-Combustion of Animal Waste in a Commercial Waste-to-Energy BFB Boiler. Energies 2013, 6, 6170–6187. [Google Scholar] [CrossRef]
- Gaydamaka, S.; Gladchenko, M.; Kornilov, I.; Ryazanov, M.; Gerasimov, M.; Kornilova, A. Anaerobic decomposition of substandard pet food as a raw material source for producing hydrogen from methane. Int. J. Hydrogen Energy 2024, 96, 803–810. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization: Geneva Switzerland, 2015.
- ISO 14001:2015; Environmental Management Systems—Requirements with Guidance for Use. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 45001:2018; Occupational Health and Safety Management Systems—Requirements with Guidance for Use. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization: Geneva, Switzerland, 2018.
- Luna-Solano, G.; Viloria-Perez, D.G.; Villegas-Santiago, J.; Salgado-Cervantes, M.A.; Domínguez-Niño, A. Drying and extraction process of lemongrass (Cymbopogon citratus). Agrociencia 2019, 53, 447–464. [Google Scholar]
- Ayala, A. Estimación De Las Isotermas De Adsorción Y Del Calor Isostérico En Harina De Yuca. Biotecnol. Sect. Agropecu. Agroind. 2011, 9, 88–96. [Google Scholar]
- AOAC International. AOAC: Officials Methods of Analysis (Volume 1); Association of Official Analytical Chemists, Inc.: Rockville, MD, USA, 1990; Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 1 February 2024).
- ISO 18125:2017; Solid Biofuels—Determination of Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/71546.html (accessed on 1 March 2025).
- ISO 18122:2022; Solid Biofuels—Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/83190.html (accessed on 1 March 2025).
- Hassanzadeh, M.; Rahmani, K. Hydrostatic test of storage tanks using seawater and corrosion considerations. Eng. Fail. Anal. 2021, 122, 105267. [Google Scholar] [CrossRef]
- Mudgal, D.; Ahuja, L.; Bhatia, D.; Singh, S.; Prakash, S. High temperature corrosion behaviour of superalloys under actual waste incinerator environment. Eng. Fail. Anal. 2016, 63, 160–171. [Google Scholar] [CrossRef]
- WBCSD; WRI. The Greenhouse Gas Protocol. 2025. Available online: https://ghgprotocol.org/sites/default/files/standards/ghg-protocol-revised.pdf (accessed on 13 May 2025).
- WBCSD; WRI. The Greenhouse Gas Protocol—Calculation Tools and Guidance. 2025. Available online: https://ghgprotocol.org/calculation-tools-and-guidance (accessed on 13 May 2025).
- Lins, T.; Braz, R.; Silva, T.; Reis, C.; Silva, D.; Silva, J. Energetic potential of Eucalyptus sp. Wood cultivated in the Plaster’s Pole of Araripe, PE, Brazil. Rev. Bras. Cienc. Agrar. 2021, 16, e8961. [Google Scholar] [CrossRef]
- Gaur, S.; Reed, T.B. Thermal Data for Natural and Synthetic Fuels, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998; 280p. [Google Scholar] [CrossRef]
- Moliner-Estopiñan, C.E. Valorisation of Agricultural Residues. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2016. [Google Scholar] [CrossRef]
- Nunes, L.; Matias, J.; Catalão, J. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicicology Volume 3: Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 3, pp. 133–164. [Google Scholar] [CrossRef]
- Reumont, G.; Perrot, P.; Giuliana, N.; Foct, J. Thermochemical Study of High Temperature Corrosion of Iron in a Diluted HCl + SO2 Environment. Int. J. Mater. Res. 2001, 92, 361–366. [Google Scholar]
- Comision Nacional de Energia (CNE). Proyectos de Biomasa—Guia para Evaluacion Ambiental Energias Renovables No Convencionales. 2007. Available online: https://energia.gob.cl/documentos/proyectos-de-biomasa (accessed on 13 May 2025).
- U.S. EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2020. 2024. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2020 (accessed on 10 March 2025).
- Nagata, Y. Measurement of Odor Threshold by Triangle Odor Bag Method; Technical Report; Japan Ministry of the Environment: Tokyo, Japan, 2003.
- Wang, H.; Lou, S.; Huang, C.; Qiao, L.; Tang, X.; Chen, C.; Zeng, L.; Wang, Q.; Zhou, M.; Lu, S.; et al. Source profiles of volatile organic compounds from biomass burning in yangtze river Delta, China. Aerosol Air Qual. Res. 2014, 14, 818–828. [Google Scholar] [CrossRef]
- Yahya, S.; Kee, K.E.; Puad, M.J.M.; Ismail, M.C. Evaluation on steel corrosion in water-based drilling fluids: Inhibitors and scale involvement. J. Pet. Sci. Eng. 2022, 211, 110127. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. High-temperature behavior of a NiCr-coated T91 boiler steel in the platen superheater of coal-fired boiler. J. Therm. Spray Technol. 2013, 22, 838–847. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, V.; Mittal, S.; Pandey, G.; Mudgal, D.; Gupta, P. An overview of problems and solutions for components subjected to fireside of boilers. Int. J. Ind. Chem. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Cheshideh, H.; Nasirpouri, F.; Mardangahi, B.; Jabbarpour, A. Failure analysis and preventive recommendations against corrosion of steel tubes of gas risers in natural gas urban distribution lines. Eng. Fail. Anal. 2021, 122, 105240. [Google Scholar] [CrossRef]
- Sultan, J.; Abbas, M.; Ibrahim, M.; Karash, E.; Ali, A.; Ibrhim, H. Corrosion Behavior of Thermal Seamless Carbon Steel Boiler Pipes. Ann. Chim.-Sci. Matériaux 2021, 45, 399–405. [Google Scholar] [CrossRef]
- Bavarian, B.; Zeinali, Y.; Miksic, B. Developing Vapor Corrosion Inhibitor Powders to Protect the Above Ground Storage Tanks Bottoms. In Proceedings of the CONFERENCE 2025, Nashville, TN, USA, 6–10 April 2025; pp. 1–10. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Impact of metals on corrosive behavior of biodiesel-diesel-ethanol (BDE) alternative fuel. Renew. Energy 2016, 94, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).