Featured Application
The development of an integrated system using duckweed for the phytoremediation of zinc and ammonium from contaminated water, followed by converting the harvested biomass into biomethane via anaerobic digestion (AD).
Abstract
This study presents an integrated approach for the remediation of zinc- and ammonium-contaminated water using duckweed, followed by the valorization of the harvested biomass through anaerobic digestion for biogas production. Duckweed was cultured with various initial concentrations of zinc (Zn, 0 mg/L, 2.5 mg/L, and 5 mg/L) and ammonium (NH4+-N, 0 mg/L, 20 mg/L, and 40 mg/L). Subsequently, duckweed was subjected to chemical pretreatment with sulfuric acid and the obtained residual solid and liquid fractions were evaluated as substrates for methane production. The liquid fraction consistently yielded higher methane production compared to the solid fraction. However, when duckweed was grown in zinc- and ammonium-rich conditions (2.5 or 5.0 mg/L Zn and 20 mg/L NH4+-N), methane production from the liquid hydrolysate was significantly reduced (120.90 ± 12.03 mL/g COD and 129.82 ± 10.65 mL/g COD, respectively) compared to the control duckweed (201.67 ± 5.72 mL/g COD). The lowest methane yields were observed for duckweed grown solely in zinc (111.32 ± 5.72 and 99.88 ± 10.49 mL/g COD for 2.5 and 5.0 mg/L Zn, respectively), attributed to the inhibitory effect of high dissolved zinc concentrations in the liquid fraction. The applicability of this integrated system is particularly relevant for the agricultural and industrial sectors, where wastewater streams are often co-contaminated with nutrients and trace metals. By demonstrating that acid-pretreated, zinc-rich duckweed biomass can be used for biogas production—provided that process conditions are optimized to mitigate metal inhibition and acidification—this study provides actionable strategies for developing circular, sustainable wastewater treatment systems. The approach not only maximizes pollutant removal and resource recovery, but also addresses environmental safety concerns associated with residual metals in the digestate.
1. Introduction
The sustainable management of wastewater containing both nutrients and heavy metals remains a critical environmental challenge worldwide [1,2]. Livestock operations, particularly swine farming, generate large volumes of effluents rich in ammonium and phosphorus, as well as heavy metals such as zinc and copper, which are commonly added to animal feed but poorly assimilated by livestock [3,4,5]. Therefore, zinc appears at elevated concentrations in waste streams or the environment, such as in anaerobically digested swine wastewater (ADSW) (ca. 1.5–30 mg Zn/L) [6], in agricultural runoff, or in aquaculture wastewater (ca. 2–3 mg Zn/L) after the application of Zn minerals [7].
Zn-enriched wastewater not only affects the activities of aquatic organisms, but also results in excessive human Zn intake through the food chain. This can result in nonspecific gastrointestinal symptoms such as abdominal pain, diarrhea, nausea, and vomiting [8]. However, zinc is an essential trace element for all forms of life, playing a crucial role in a large number of enzyme functions, acting as a stabilizer of the molecular structures of subcellular constituents and membranes, and protecting biological structures from the damage caused by free radicals [9]. As an essential micronutrient for plant growth, Zn is an important cofactor for the enzyme carbonic anhydrase, which is essential to carbon uptake during photosynthesis and a component of many enzymes of cellular processes, including protein metabolism, gene expression, and chromosome construction, while also constructive to the synthesis of vitamin E [10,11,12]. However, excessive Zn concentrations not only have toxic effects in humans, but also in plants due to osmoregulatory disturbances and reduction in the metabolic activity of microorganisms in the plant rhizosphere [13,14]. On the other hand, Zn deficiency results in plant deficiency and affects plant yields and the activities of rhizosphere microorganisms [15]. Traditional physicochemical and biological treatment methods often struggle to simultaneously remove nutrients and heavy metals, and may incur high operational costs or generate secondary pollution. In this context, phytoremediation, which is a plant-based and environmentally sustainable approach, is used to eliminate, recover, extract, sequester, or detoxify pollutants [16]. It is extensively used for pollutant removal from wastewater due to low installation and operation costs [17,18]. As one of the smallest aquatic floating plants, duckweed—part of the Lemnaceae family—is commonly used for the phytoremediation of industrial and municipal wastewater due to its rapid growth, high nutrient uptake capacity, tolerance to polluted environments, removal of micropollutants, and ease of cultivation and harvesting [19,20].
Duckweed also has great potential as a feedstock to produce biofuel by fermentation and anaerobic digestion [21]. Gaur et al. reported high CH4 yields (60–468 mL CH4/g) from the anaerobic digestion (AD) of pretreated duckweed [22]. Some studies have reported that acid pretreatment is preferable for obtaining a larger biofuel yield and produces higher-grade fuels [23,24]. Some Lemna species can accumulate Zn concentrations 2700 times higher than in the medium [25]. Trace amounts of Zn in anaerobic digestion systems are necessary to maintain the cellular metabolism of methanogens, while high Zn concentrations inhibit the growth of methanogens [26]. Luna-delRisco et al. showed that the excess release of Zn ions inhibited biogas production in the anaerobic digestion of cattle manure [27]. Duckweed has also a high tolerance for NH4+-N and is an ideal plant for nutrient capture from NH4+-N-rich wastewater [28]. However, exposure to high NH4+-N concentrations is harmful to duckweed [29]. Although the presence of Zn is common in many NH4+-N-rich wastewater, very few studies have reported simultaneous bioremediation of Zn and NH4+-N using duckweed [14].
Despite advances in duckweed-based remediation, there is a critical knowledge gap regarding the integrated management of nutrient and metal removal, the impacts of metal accumulation on biogas production, and the environmental risks associated with residual metals in digestate. Addressing these gaps is essential for developing robust, circular wastewater treatment systems that maximize pollutant removal, energy recovery, and environmental safety. In this study, we investigate the simultaneous phytoremediation of zinc and ammonium from contaminated water using duckweed, followed by the anaerobic digestion of the harvested biomass for methane production. We systematically assess the effects of zinc and ammonium on duckweed growth and contaminant removal, evaluate the biochemical methane potential of acid-hydrolyzed biomass fractions, and examine the fate of zinc throughout the process. Our findings aim to provide actionable insights for optimizing integrated phytoremediation–biogas systems and inform strategies for safe, sustainable resource recovery from metal-laden wastewater. In particular, in this research duckweed was used to phytoremediate zinc- and ammonium-containing wastewater. Subsequently, the harvested duckweed was used as feedstock for anaerobic digestion, serving the role of a carbon source and providing Zn supplementation for the production of biogas to obtain the dual benefits of pollutant remediation and biofuel production. The purpose of this work was to (1) reveal the effects of Zn and NH4+-N on duckweed growth, (2) examine the ability of Zn bioaccumulation under different initial Zn and NH4+-N concentrations, (3) evaluate the biogas production and biomethane potential of Zn-enriched biomass, and (4) provide new insights into the formation of anaerobic digestion intermediates such as volatile fatty acids (VFAs) during the anaerobic digestion of duckweed.
This study represents a significant advancement in the field of integrated phytoremediation and resource recovery from contaminated wastewater, specifically through the use of duckweed. While numerous studies have established duckweed as a robust candidate for nutrient and heavy metal removal from polluted waters [18,30,31,32] and others have explored its potential for protein, pigment, or vitamin E production [14,33], the novelty of the current research lies in its comprehensive process integration and mechanistic insights. Unlike previous works [14], which have focused primarily on the effects of zinc ions on the nutrient removal efficiency and physiological responses of Lemna aequinoctialis, this work uniquely investigates the fate and impact of zinc and ammonium not only during phytoremediation, but also through the subsequent anaerobic digestion (AD) of the harvested biomass. This study is the first to systematically assess both the acid-hydrolyzed solid and liquid fractions of Zn/NH4⁺-rich duckweed as substrates for methane production, revealing the dual challenges of substrate degradability and zinc inhibition in AD, a dimension largely absent in the prior literature.
2. Materials and Methods
2.1. Experimental Design
The experiments were carried out in two stages. In the first stage, duckweed was grown in the presence of different Zn and NH4+-N concentrations to study their influence on duckweed growth. Duckweed grown in the absence of Zn and NH4+-N served as a control experiment. In the second stage, six growth conditions were chosen to study the biofuel production from duckweed cultures by AD after an acid pre-treatment. The pretreated solid fraction and liquid hydrolysate of the duckweed suspension were separated and then used as the substrates for biogas production by AD.
2.2. Duckweed Cultivation
Duckweed (a hybrid of Lemna minor and Lemna turionifera) was collected from the culture collection of IBBA (Istituto Di Biologia E Biotecnologia Agraria, Milan, Italy) and cultivated in a greenhouse in modified Steinberg medium at pH 5.5 to acclimatize for seven days [34,35]. The modified Steinberg medium contained 350 mg/L of KNO3, 90 mg/L of K2HPO4, 12.6 mg/L of KH2PO4, 100 mg/L of MgSO4, 295 mg/L of Ca(NO3)2·4H2O, 120 µg/L of H3BO3, 44 µg/L of Na2MoO4·2H2O, 180 µg/L of MnCl2·4H2O, 760 µg/L of FeCl3·6H2O, and 1500 µg/L of Na2EDTA·2H2O. Afterwards, 5 g (wet weight) of fresh duckweed was transplanted into 3 L of modified Steinberg medium in a PVC container (LxWxH, 0.26 × 0.15 × 0.18 m), with varied concentrations of ZnSO4·7H2O and NH4Cl. The temperature in the greenhouse was 18 °C ± 2 °C. Light was provided using spectron T8 LED 1.5 m GB tubes with a light intensity in the range of 130−180 µmol m−2 s−1 photons (16/8 h light/dark cycle).
2.3. Zn and NH4+-N Removal by Duckweed
Table 1 and Figure 1 overview the different growth conditions of the duckweed. Each treatment was performed in triplicate, with a total of 27 boxes for the 9 treatments. Plant biomass and water samples were collected for further experiments and analytical determinations.

Table 1.
Zn and NH4+-N concentrations applied in each duckweed treatment.
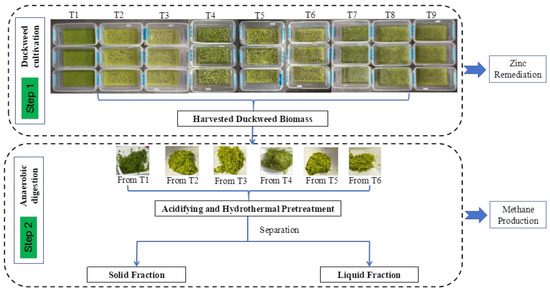
Figure 1.
Schematic diagram of the experimental design used in this study.
2.4. Duckweed Pretreatment
The acid pretreatment of solid substrates is a critical step in enhancing the efficiency of anaerobic digestion, particularly for lignocellulosic biomass [36,37,38]. Sulfuric acid is predominantly used for this purpose due to its effectiveness in hydrolyzing complex carbohydrates, thereby converting cellulose and hemicellulose into simpler sugars that are more amenable to fermentation by anaerobic microorganisms [37,39,40,41,42]. In contrast, nitric acid is less favored because its oxidative properties can lead to the formation of toxic byproducts, such as nitrogen oxides, which may inhibit microbial activity and compromise the anaerobic digestion process. Moreover, nitric acid can degrade organic matter, ultimately reducing biogas yields. Hydrochloric acid, while effective in hydrolysis, can lead to the formation of chloro–organic compounds that may inhibit microbial activity or result in corrosive chloride ions, which may corrode the equipment [38]. The cost-effectiveness and ease of handling of sulfuric acid further solidify its position as the preferred choice for large-scale operations. Additionally, research indicates that sulfuric acid not only enhances biogas production, but also minimizes operational challenges and the environmental impacts associated with the pretreatment process [42]. Thus, the application of sulfuric acid in acid pretreatment aligns with the overarching goal of maximizing biogas production while ensuring the sustainability of anaerobic digestion systems.
In this study, duckweed underwent acid pretreatment using the following optimized protocol described by Kotamraju et al. [35]: sulfuric acid (1% H2SO4) at a solid/liquid ratio (SLR) of 1:20 (w/v) for 60 min at 100 °C. The pretreated liquid and solid fraction of the duckweed suspension were separated by using a 100 µm pore size sieve.
2.5. Biochemical Methane Production Potential Measurements
The biochemical methane production potential (BMP) of the pretreated duckweed was determined in 250 mL borosilicate glass bottles with a working volume of 200 mL and head space volume of 50 mL for biogas accumulation, as described by Morais et al. [43]. Anaerobic granular sludge obtained from a 200 m3 upflow anaerobic sludge bed (UASB) reactor treating dairy wastewater (Kilconnel, Ireland) was collected and used as inoculum. The inoculum to substrate ratio was kept at 1:2 in terms of grams of chemical oxygen demand (COD).
The headspace of the bottles was flushed with N2, and the bottles were then sealed with caps and placed in a shaker incubator at 110 rpm and 37 °C. Each glass bottle had two ports in the lid, one for liquid sampling and the other for gas sampling. All experiments were carried out in triplicate and biogas production was recorded for 30 days. The results are reported as net cumulative methane production.
2.6. Analytical Methods
2.6.1. Analyses of Duckweed Samples
All duckweed samples were harvested after 7 days of growth and the biomass was dried at 60 °C until the weight of the plant samples remained constant. The dried duckweed was ground into a fine powder with a mortar and pestle for further analysis. The Zn content of the duckweed was determined, following microwave digestion as outlined by Kotamraju et al. [35], by inductively coupled plasma–optical emission spectroscopy (ICP-OES) 5110 synchronous vertical dual view (5110, Agilent Technologies, Santa Clara, CA, USA). The recovery of Zn in the duckweed samples was assessed using the reference material BCR-402 White Clover (certified value: 25.2 ± 0.6 mg/kg Zn).
The chemical composition of the duckweed surface was determined by Fourier transform infrared spectroscopy (Nicolet iS5, Thermo Fisher Scientific Inc., Waltham, MA, USA), described in detail by Hu et al. [44]. The external surface of the dried duckweed plants enriched with and without Zn was observed using scanning electron microscopy (SEM) (S2600N, Hitachi, Tokyo, Japan). The dried duckweed powder was made conductive by coating with gold using a sputter coater (SC500, EMSCOPE, Barcelona, Spain). The protocol followed for SEM preparation, fixation, and imaging was described in detail by Logan et al. [45].
2.6.2. Analyses of Liquid Samples
All digestion aqueous water samples were centrifuged for 15 min at 14,500 rpm and filtered through 0.2 μm polyethersulfone membranes (Filtropur S 0.2, Sarstedt, Germany), while water samples from the duckweed culturing were filtered with 0.2 μm polyethersulfone membranes. Total solids (TS), volatile solids (VS), and total COD were determined according to standard methods [46]. The pH of the liquid samples was measured with a pH meter (Cole-Parmer 300 pH/ORP, Cambridgeshire, UK). NH4+-N concentration, total hardness, and conductivity were determined by using Gallery Plus according to standard procedures [46]. Volatile fatty acids (VFAs) were determined using a high-performance liquid chromatograph (HPLC) (1260 Infinity II, Agilent, Santa Clara, CA, USA), as described by Kotamraju et al. [35]. The Zn concentration of the water samples was determined by using an ICP-OES 5110 (Agilent, Santa Clara, CA, USA), as described by Logan et al. [47].
2.6.3. Analysis of Biogas
Biogas accumulation in the headspace volume was monitored with a pressure meter (Leo 1, Keller, Winterthur, Switzerland) and calculated as pressure difference using the ideal gas law, as described by Logan et al. [45]. Methane was converted to its COD equivalent following the ideal gas law and the g COD/g CH4 conversion factor. The gas composition was determined with a gas chromatograph (7890B, Agilent, Santa Clara, CA, USA), equipped with a thermal conductivity detector (TCD) heated at 250 °C, able to detect CH4, CO2, N2, O2, and H2, as described by Logan et al. [45]. Helium was used as a carrier gas with a flow rate of 10 mL/min.
2.7. Calculations
2.7.1. Growth Parameters
Duckweed growth obeyed the classic growth model. The relative growth rate (RGR), with respect to changes in fresh weight from day 0 to day 7, was calculated using the following reference formula given by Wang et al. [48]:
where W7 and W0 are the initial and final fresh weights (g), respectively, and t is the incubation time (d).
The pollutant removal efficiency of the plant was determined as follows:
where Ci is the initial Zn concentration (mg/L) in the growth medium, Vi is the initial volume of the aquatic medium (3 L), Cf is the final Zn concentration (mg/L), and Vf is the final volume of the aquatic medium (L).
The tolerance index (TI), the bioconcentration factor (BCF), the Zn recovery efficiency, and the methane production from the BMP tests were calculated using the equations described by Kotamraju et al. [35].
2.7.2. Kinetic Models
Methane production from the BMP tests was used in non-linear curve fitting by using the modified Gompertz model, as described by Kotamraju et al. [35], as follows:
where M(t) is the cumulative methane yield (mL/g COD) at time t (days). M0 is the maximum methane yield (mL/g COD), Rm is the maximum methane production rate (mL/g COD d), λ is the lag phase (days), and e is Euler’s number.
2.7.3. Statistical Analysis
The SPSS 16.0 statistical package and Origin 8.6 software were used for statistical analysis and figure drawing, respectively. All data are presented as the mean values and standard deviation (SD) of three replicates. One-way analysis of variance (ANOVA) was performed to compare all data. Statistical significance was defined as p < 0.05. The kinetic fitting using the modified Gompertz model was conducted using Origin 8.6 software.
3. Results
3.1. Growth of Duckweed in the Presence of Zn and NH4+-N
The control duckweed (T1; 0 mg/L Zn and 0 mg/L NH4+-N) depicted the greatest biomass growth and the greatest RGR of 0.298 (±0.013) d−1 in comparison to duckweed grown in the presence of Zn and/or NH4+-N (T1 vs. T2–T9, Figure 2a). The presence of Zn at concentrations ranging from 2.5 to 5.0 mg/L reduced the duckweed relative growth rate (T1–T3) when NH4+-N was absent, indicating the toxic effect of Zn2+ at the concentrations tested. In the presence of NH4+-N, zinc toxicity was partly mediated (T2–T3 vs. T5–T6 vs. T8–T9), indicating that in the presence of NH4+, zinc toxicity is reduced, possibly due to the formation of zinc ammonium complexes, which are less bioavailable to duckweed. At the same Zn concentration, there was no significant statistical difference between the treatments with 20 mg/L NH4+-N and 40 mg/L NH4+-N (p > 0.5).
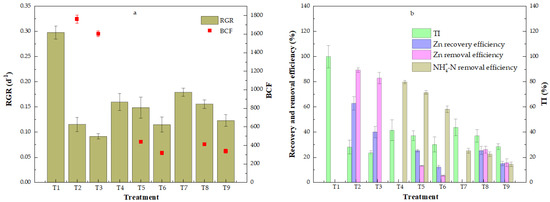
Figure 2.
(a) Duckweed relative growth rate (RGR) and Zn bioconcentration factor (BCF) and (b) tolerance index (TI), Zn recovery efficiency, Zn and NH4+-N removal efficiency under the different applied growth conditions. Error bars show standard deviation.
The BCF values in all treatments were greater than one (Figure 2a), indicating the potential of duckweed for the phytoremediation of Zn-contaminated waters. The greatest BCF was found in T2 (2.5 mg/L Zn and 0 mg/L NH4+-N), indicating that in absence of NH4+, zinc is fully bioavailable for uptake from duckweed. The BCF decreased with increasing Zn concentrations in all treatments, indicating that high Zn concentrations alleviated Zn accumulation. In the presence of NH4+-N, the BCF significantly decreased, indicating that the presence of NH4+-N suppressed the accumulation of Zn in duckweed, possibly due to the formation of less bioavailable zinc–ammonium complexes. However, no significant difference was found between the BCF of duckweed grown in the presence of 20 mg/L NH4+-N or 40 mg/L NH4+-N.
3.2. Zn Uptake and Zn/NH4+-N Removal by Duckweed
Figure 2b shows the ability of duckweed to take up both Zn and NH4+-N. The tolerance index (TI) was well correlated with the Zn removal efficiency, while no obvious correlation was found between the TI and the NH4+-N removal efficiency. Lower TI values were found in the treatments with Zn and without NH4+-N, indicating the toxicity of Zn (T1 through T3, T4 through T6, and T7 through T9). The TI decreased with increasing Zn concentrations, however, the TI increased when NH4+-N was also present. This finding indicates that the presence of ammonium partly reduced the toxicity of zinc to duckweed growth. Duckweed in the treatments with Zn and without NH4+-N showed a good Zn recovery and removal efficiency. The Zn recovery efficiency of T2 was significantly different from that of T3 (p < 0.05), while there was no difference in the Zn removal efficiency. The Zn recovery efficiency was not significantly different between the treatment with 20 mg/L NH4+-N or 40 mg/L NH4+-N (T5 vs. T8 and T6 vs. T9) at the same Zn concentration, while the Zn recovery efficiency decreased with an increasing Zn concentration. The Zn removal efficiency decreased with an increasing NH4+-N concentration (T2 vs. T3, T5 vs. T6 and T8 vs. T9). Minimal Zn removal efficiency was found in the presence of 20 mg/L NH4+-N (T6). NH4+-N removal also decreased with an increasing Zn concentration (T4 through T6 and T7 through T9), whereas the NH4+-N removal increased with an increasing NH4+-N concentration (T4–T6 vs. T7–T9), indicating that duckweed preferably took up more NH4+-N at higher ammonium concentrations.
The uptake of Zn by the duckweed was further analyzed with SEM-EDX analysis (Figure 3a,b). EDX analysis confirmed the uptake of Zn by duckweed in treatments with Zn, while, as expected, no Zn was detected in duckweed grown in the absence of Zn. A higher percentage of Zn was detected in duckweed from treatments with Zn and without NH4+-N (T2 and T3), while a lower percentage of Zn was found in duckweed from treatment with NH4+-N (T5, T6, T8, and T9). EDX analysis further confirmed that the zinc accumulation ability of duckweed was weakened by the presence of NH4+-N, possibly due to the less bioavailable forms of zinc species.
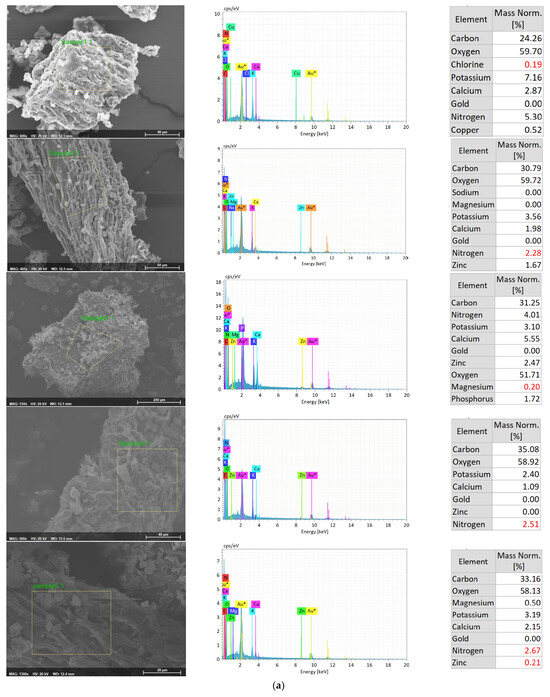
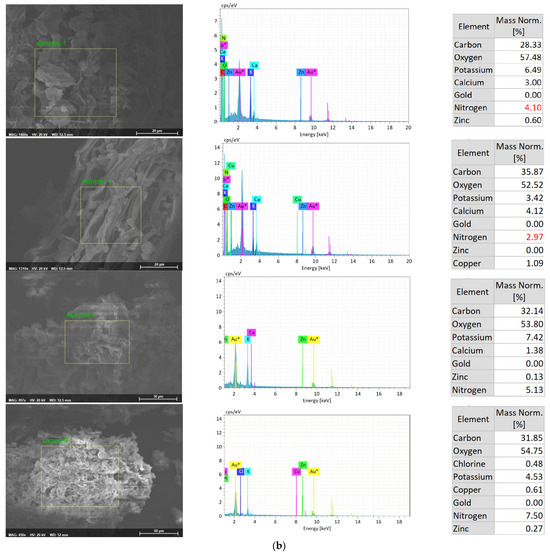
Figure 3.
(a) SEM-EDX analysis of Zn-enriched duckweed from T1 (sample 1), T2 (sample 2), T3 (sample 3), T4 (sample 4), and T5 (sample 5). (b) SEM-EDX analysis of Zn-enriched duckweed from T6 (sample 6), T7 (sample 7), T8 (sample 8), and T9 (sample 9).
FTIR spectra of the duckweed biomass with and without Zn exposure were obtained to investigate the roles of various functional groups in Zn absorption (Figure 4). Similar FTIR spectra with slight peak/band shifts were found among the control and other treatments. Peaks at approximately 3279, 2926, 1622, 1545, 1387, 1317, and 1019 cm−1 were observed in the spectrum of the control duckweed (Figure 4). These peaks correspond to the hydroxyl, hydrocarbons, carboxyl, and amide stretching of the C–H, O–H, C=O, and –NH functional groups [49]. Some of these are involved in zinc binding [50], and the strong peak value at 1019 cm−1 represents the symmetric stretching vibration of –SO3. The intensity values of the peaks increased in the presence of 2.5 mg/L Zn. An increase in the peak intensity usually means an increase in the amount (per unit volume) of the functional group associated with the molecular bond. The maximum intensity values were found in the duckweed samples from T5, while the peak intensity values decreased with an increasing NH4+-N concentration, especially in the adsorption intensity of the amide group at 1622 and 1545 cm−1, illustrating that the amide group played a main role in binding Zn under this condition. The variation in the peak position and intensity indicated that the functional groups, such as the hydroxyl and carbonyl groups, may be involved in the absorption of Zn and NH4+.
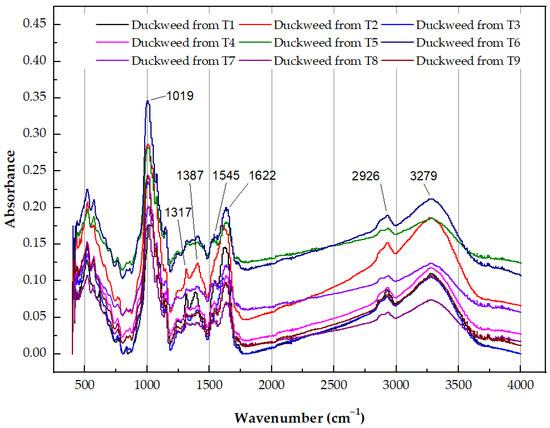
Figure 4.
FTIR spectra of dried Zn-enriched duckweed grown in the different treatments.
3.3. Methane Production Using the Solid and Liquid Fractions of the Duckweed
3.3.1. Methane Production
The applicability of Zn-enriched duckweed biomass as a feedstock in anaerobic digestion (AD) was tested with duckweed from six different treatments (T1, T2, T3, T4, T5, and T6). The solid fractions of duckweed from all pretreatments had lower methane production compared to the liquid fractions (Figure 5a vs. Figure 5b). Methane production from the liquid hydrolysate of T1 was significantly higher than that from the liquid fractions from the other treatments (p < 0.05). Only a negligible increment in the methane production of the liquid fraction from T1 from the 24th day onwards was observed (Figure 5b), and the greatest value of methane production was 201.67 (±5.72) mL/g COD by the 30th day of incubation. A decrease in methane production from the pretreated duckweed liquid hydrolysate from treatments with Zn or NH4+ was observed. Among the different incubations, the duckweed liquid fraction from T4 had a higher methane production than that from Zn-enriched duckweed liquid fractions (T2, T3, T5, and T6), indicating that the presence of Zn suppressed methane production. The methane production from pretreated duckweed hydrolysate from T5 and T6 was higher than that from T2 and T3, indicating that the liquid fraction from duckweed grown with NH4+-N produced more methane under the experimental conditions tested. No statistical difference in methane production from the liquid fractions in T5 and T6 was found (p > 0.05), and there was also no significant difference between the duckweed liquid fractions from T2 and T3, indicating that the same inhibition level in methanogenesis was observed in both treatments, with zinc at 2.5 and 5.0 mg/L.
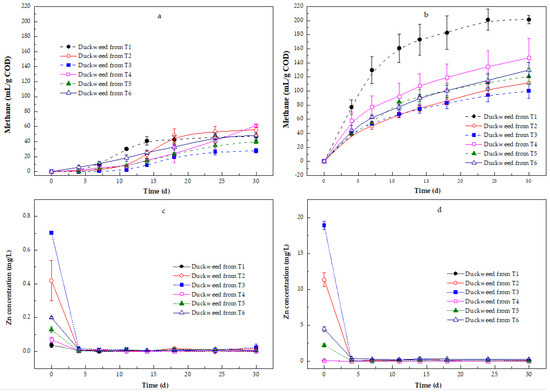
Figure 5.
Methane production from pre-treatment of different Zn-enriched duckweed samples and Zn concentration variation on the methane production using the solid (a,c) and liquid (b,d) fractions. Error bars show the standard deviation.
The duckweed liquid fractions from T2, T3, T5, and T6 on the 4th day depicted 38.41 (±0.06) mL/g COD, 41.43 (±1.62) mL/g COD, 39.32 (±0.45) mL/g COD, and 44.54 (±6.42) mL/g COD, respectively, which increased to 75.90 (±4.39) mL/g COD, 74.26 (±5.60) mL/g COD, 91.75 (±10.27) mL/g COD, and 90.29 (±4.91) mL/g COD by the 14th day, respectively. The methane production of duckweed liquid fractions from treatments with Zn increased with incubation time, and a maximum of 111.32 (±8.48) mL/g COD, 99.88 (±10.49) mL/g COD, 120.90 (±12.03) mL/g COD, and 129.82 (±10.65) mL/g COD was achieved in duckweed liquid fractions from T2, T3, T5, and T6 by the 30th day of incubation, respectively. This meant that prolonging the incubation time could increase the overall methane production.
The highest methane production achieved in pretreated duckweed solid fractions from T1, T2, T3, T4, T5, and T6 by the 30th day of incubation amounted to 46.52 (±4.74) mL/g COD, 55.91 (±6.94) mL/g COD, 27.85 (±2.93) mL/g COD, 61.45 (±1.69) mL/g COD, 40.051 (±2.52) mL/g COD, and 48.23 (±3.30) mL/g COD, respectively (Figure 5a). Methane production in the duckweed solid fractions from T1 was significantly higher than that from the other treatments before the 18th day of incubation (p < 0.05). Methane production in the duckweed solid fraction from T6 was significantly greater than that from T2, T3, T4, and T5 before the 14th day, while there was no difference in methane production between T2, T3, T4, and T5 during the initial 14 days (p > 0.05). Except for duckweed from T4, the methane production in duckweed solid fractions from all treatments showed only a negligible increment from the 24th day of incubation onwards. In the solid fraction of duckweed from T4, a higher increment in the methane production was observed from the 24th day of incubation onwards. The lowest methane production was noticed in the solid fraction of duckweed from T3, suggesting that a higher Zn-enriched duckweed solid fraction inhibited methanogenesis.
These data reveal that Zn contamination reduced the anaerobic digestibility of duckweed, particularly in the solid fractions, but NH4⁺-N could partially offset Zn’s negative effects. The liquid fractions provided superior methane yields, emphasizing the importance of effective pretreatment to solubilize organics. These findings suggest that Zn-enriched duckweed can still be utilized for methane production though the optimization of pretreatment, and nutrient balance is key for unlocking its full bioenergy potential.
Kinetic Parameters of Methane Production
Table 2 shows the kinetic parameters of methane production from the duckweed, derived by the modified Gompertz model. The modified Gompertz model fitted the methane production data of all the conditions tested very well (R2 > 0.94).

Table 2.
Estimated kinetic parameters from the modified Gompertz model simulating methane production in the solid and liquid fractions of cultured duckweeds.
The maximum methane yield and methane production rate for T5 and T6 were higher than those for duckweed from T2 and T3. The liquid fractions of duckweed from T1 had a maximum methane yield (Mmax) of 194.689 mL/g COD, a maximum methane production rate (Rm) of 18.936 mL/g COD d, a lag phase (λ) of 0.174 days, and a smaller peak time of 3.956 days. A maximum methane yield (Mmax) of 112.207 mL/g COD and maximum methane production rate (Rm) of 5.448 mL/g COD d were predicted for T2, and the lowest maximum methane yield (95.569 mL/g COD) and a maximum methane production rate of 6.097 mL/g COD d were calculated for the liquid fraction from T3. For duckweed from the treatments with NH4+-N (T4, T5, and T6), the liquid fraction from T4 had a maximum methane yield (Mmax) of 141.182 mL/g COD and maximum methane production rate (Rm) of 8.309 mL/g COD d, whereas maximum methane yields (Mmax) of 117.230 mL/g COD and 126.647 mL/g COD and maximum methane production rates (Rm) of 7.749 mL/g COD d and 6.584 mL/g COD d were estimated for the liquid fractions from T5 and T6, respectively.
Compared to the liquid fractions, the kinetics of the duckweed solid fractions from all treatments were slower. Among the solid fractions of all treatments, the lowest maximum methane yield (Mmax) of 28.130 mL/g COD, a maximum methane production rate (Rm) of 3.039 mL/g COD d, and a longer lag phase (λ) of 11.126 days were observed for duckweed from T3. The solid fraction of pretreated duckweed from T6 had the fastest kinetics, with a maximum methane yield (Mmax) of 56.395 mL/g COD, a maximum methane production rate (Rm) of 2.316 mL/g COD d, and a lag phase (λ) of 2.922 days. The highest maximum methane yield (Mmax) of 153.409 mL/g COD, a maximum methane production rate (Rm) of 3.364 mL/g COD d, and the longest lag phase (λ) of 11.763 days were calculated for the solid fraction of duckweed from T4.
The kinetic analysis reaffirms earlier trends: (a) Zn negatively affected methane production, with the liquid fraction of uncontaminated duckweed (T1) being the most efficient substrate for anaerobic digestion; (b) NH4⁺-N showed the partial mitigation of Zn’s effects, but not enough to match the control; (c) solid fractions showed significantly slower kinetics, limiting their practicality unless pretreatment is optimized. Overall, Gompertz modeling offers crucial insights into the bioconversion efficiency and system design needs for duckweed-based biogas systems.
3.3.2. Variation in Process Parameters
Zn Removal in the Methane Production Process
Figure 5c,d show the Zn concentrations in the solid and liquid fractions from different duckweed digestions. The highest Zn concentrations were found in the solid and liquid fractions of duckweed digestion from T3 (0.70 ± 0.01 mg/L and 18.89 ± 0.60 mg/L, respectively), followed by duckweed from T2 (0.42 ± 0.12 mg/L and 11.32 ± 0.95 mg/L, respectively) and duckweed from T6 (0.20 ± 0.0 mg/L and 4.46 ± 0.35 mg/L, respectively). This means that the adsorbed Zn content of duckweed was directly related to the zinc content of the solid and liquid fractions after pretreatment.
During anaerobic digestion, the Zn concentrations sharply dropped in the solid and the liquid fractions of duckweed grown in the presence of Zn. The Zn concentrations in the solid and liquid fractions of duckweed cultured with Zn and NH4+-N were lower than those of duckweed grown in the presence of Zn (Figure 5c,d). The Zn concentrations in the solid and liquid fractions from all treatments were lower than 0.02 mg/L and 0.22 mg/L until the end of the incubation, respectively. The removal efficiencies of Zn in the solid fractions of duckweed from T2, T3, T5, and T6 were 92.63% (±2.33%), 90.50% (±1.26%), 98.22% (±1.23%), and 96.59% (±3.13%) on the 4th day, respectively, and their removal efficiencies in the liquid fractions were 99.65% (±0.10%), 99.72% (±0.10%), 96.22% (±0.21%), and 91.15% (±1.77%) on the 4th day, respectively, indicating that Zn was likely removed from the incubation medium by sulfide precipitation [51]. A removal efficiency above 91% was achieved. However, the Zn concentrations fluctuated in the solid and liquid fractions throughout 30 days, indicating that Zn was released due to the microbial degradation of duckweed biomass in the solid fractions and the solubilization of Zn precipitates in the liquid fractions, and was later used by the anaerobic sludge towards the end of the incubation.
This section highlights the strong Zn removal capability of anaerobic digestion systems using duckweed biomass, especially in the presence of sulfide-generating conditions. Zn is mostly removed in the early stages via chemical precipitation, but the biological degradation of Zn-loaded biomass can lead to temporary re-mobilization, underscoring the need for monitoring metal dynamics throughout the digestion process. These insights are crucial for scaling up AD systems where metal-laden biomass is used and for ensuring biosafety in digestate management.
COD Variation in the Methane Production Processes
Figure 6a,b present the COD concentration in the medium in both the solid and liquid fractions. For the liquid fractions, the COD concentrations of all treatments initially decreased rapidly and fluctuated until the end of the incubation (Figure 6b). The COD removal efficiencies in all liquid fractions amounted to 61.37–81.34%. The lowest COD concentrations of duckweed were found in T1 throughout the entire incubation.
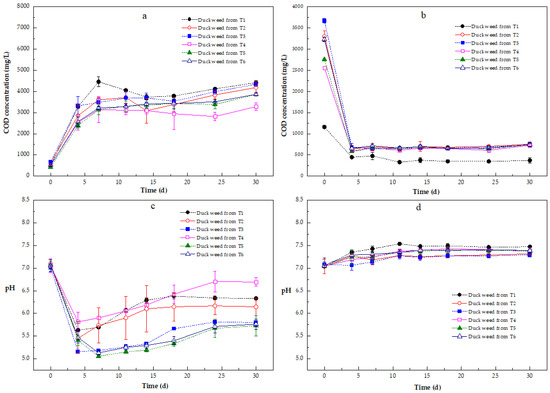
Figure 6.
Variations in COD and pH in the solid (a,c) and liquid (b,d) fractions of Zn-enriched duckweed used for methane production after acid hydrothermal pre-treatments. Error bars show the standard deviation.
The COD concentrations in the solid fractions of duckweed cultured with solely Zn were less than those in the solid fraction of control duckweed from the 4th day onwards, indicating that Zn affected the release of organic matter from the solid fraction of the pretreated duckweed biomass. The COD concentrations in the solid fractions of duckweed from T1, T2, and T3 were higher than those of duckweed from T4, T5, and T6 throughout the entire incubation period (Figure 6a). From the 7th day onwards, lower COD concentrations were observed in the solid fraction of duckweed from T4, followed by the solid fractions in the duckweed from T5 and T6. However, the COD concentrations in the solid fractions of duckweed from T5 and T6 were similar during the incubation period. The COD concentrations were more than 3270 mg/L in AD digestion tests with the solid fractions at the end of incubation (Figure 6a).
This section underscores the importance of monitoring COD dynamics in both phases during the anaerobic digestion of pretreated duckweed. While the system demonstrated a good performance in liquid-phase COD removal, especially under T1 and NH4⁺-N co-treatments, the persistent high COD in solids reflects limitations in hydrolysis and suggests that Zn, while partially mitigated by NH4⁺-N, still imposes constraints on biomass degradation. These findings pose the need for enhanced pretreatment or digester design when using metal-rich biomass.
pH Variation During Anaerobic Digestion
In all liquid fractions (Figure 6d), the pH increased with fluctuation during the entire incubation period, and the pH values were higher than 7 from the 4th day onwards. The pH in the liquid fraction of duckweed from T1 was higher than that of the other liquid fractions during the incubation. The pH in the liquid fraction of duckweed from T5 and T6 was similar, and was higher than that of duckweed from T2 and T3, whereas the pH in the liquid fractions of duckweed from T2 and T3 was also similar.
The pH fluctuated during the AD digestion tests with the solid fractions of duckweed from T1, T2, T3, and T4, whereas the pH values decreased initially and then increased in the solid fractions of duckweed from T5 and T6 during the AD tests (Figure 6c). The pH of the solid fractions of duckweed from T1, T2, and T4 was higher than that of duckweed from T3, T5, and T6 from the 4th day onwards, while a difference in pH between the incubations with duckweed from T3, T4, and T6 was noticed. The highest pH was found in the solid fraction of duckweed from T4 from the 18th day onwards, followed by duckweed from T1 and T2. Except the initial pH, the pH values were below 7 in all solid fractions. On the 4th day, the minimum pH values were observed in duckweed from T1 (5.63 ± 0.19), T2 (5.45 ± 0.33), T3 (5.16 ± 0.02), and T4 (5.81 ± 0.22), and on the 7th day in duckweed from T5 (5.15 ± 0.02) and T6 (5.13 ± 0.04). By the end of the incubation, the pH was 6.33 (±0.01) (duckweed from T1), 6.15 (±0.19) (duckweed from T2), 5.80 (±0.08) (duckweed from T3), 6.70 (±0.09) (duckweed from T4), 5.72 (±0.23) (duckweed from T5), and 5.76 (±0.11) (duckweed from T6).
This section effectively highlights the pH challenges posed by Zn contamination in duckweed and the potential of NH4⁺-N supplementation to stabilize digestion conditions. While liquid fractions generally maintained a favorable pH for methane production, solid fractions were acidified, pointing to incomplete or unbalanced digestion processes. These findings underscore the importance of pretreatment strategies and pH control in maximizing the efficiency of anaerobic digestion using metal-enriched biomass.
VFA Variation During Anaerobic Digestion
The variations in VFAs produced by the liquid (Figure 7) and solid fractions (Figure 8), which included formic acid (FA), acetic acid (AA), propionic acid (PA), isobutyric acid (IBA), butyric acid (BA), isovaleric acid (IVA), valeric acid (VA), isocaproic acid (ICA), and caproic acid (CA), were investigated during the entire AD incubation period.
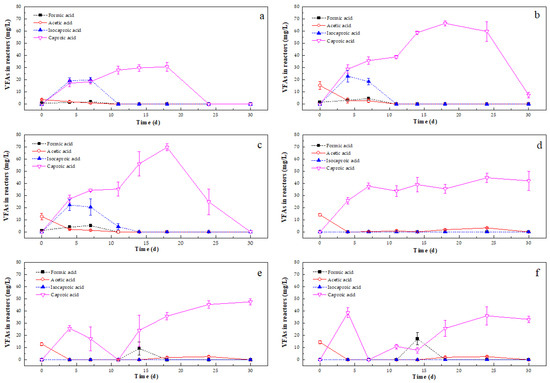
Figure 7.
Variations in volatile fatty acid (VFA) concentrations in different Zn-enriched duckweed liquid fractions from (a) T1, (b) T2, (c) T3, (d) T4, (e) T5, and (f) T6. Error bars show standard deviation.
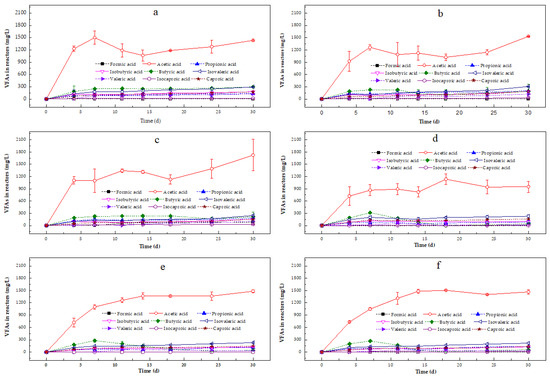
Figure 8.
Variations in the concentrations of volatile fatty acids (VFAs) in different Zn-enriched duckweed solid fractions from (a) T1, (b) T2, (c) T3, (d) T4, (e) T5, and (f) T6. Error bars show the standard deviation.
All VFAs in the liquid fractions of the AD tests were significantly lower than the AD tests with the solid fractions: (a) lower FA, AA, ICA, and CA concentrations were detected in the liquid fractions from the T1, T2, and T3 incubations; (b) lower AA and CA concentrations were detected in the liquid fractions from T4; and (c) lower FA, AA, and CA concentrations were detected in the liquid fractions from the T5 and T6 incubations, in which 9.28 (±5.48) mg/L and 17.18 (±4.94) mg/L FA was found on the 14th day (Figure 7). CA was present at the highest concentration among the VFAs in each reactor. No VFAs were detected in the liquid fraction of duckweed from T1 on the 24th day and T3 on the 30th day, respectively, and lower CA concentrations (8.55 ± 2.28 mg/L) were found in the liquid fraction of duckweed from T2 on the 30th day, indicating that the presence of Zn in the liquid fraction prolonged the VFAs’ degradation time. Compared with duckweed cultured without NH4+-N, larger variations in the types of VFAs produced were observed in the liquid fractions of duckweed from the T4, T5, and T6 incubations. Only traces of AA were found from the 4th day onwards, and AA disappeared in the liquid fractions of these three duckweed types by the 30th day. CA was observed from the 4th day onwards, and the CA concentrations in the AD tests with hydrolysates from T4, T5, and T6 duckweed reached 42.07 (±7.91) mg/L, 47.54 (±2.46) mg/L, and 33.11 (±2.75) mg/L, respectively, at the end of the incubation.
In all the solid fractions, the VFAs constituted only lower concentrations of AA at the start of the AD experiment, and the highest concentration among the VFAs was AA in each vial during the incubation period (Figure 8). The lowest AA concentrations were found in duckweed from T4 in the whole incubation. FA was only detected in duckweed from T2 and T3. The only trace of FA was observed in the solid fraction of duckweed from T2 on the 30th day, while FA in duckweed from T3 was noticed on the 11th day and increased until the end of the incubation (86.22 ± 9.66 mg/L). ICA was not detected in AD runs with the solid fraction of duckweed from T5, and only traces of ICA from T5 and T6 were observed during the incubation. AA, PA, IBA, BA, IVA, VA, ICA, and CA were detected throughout the 30 days of the operation (duckweed from T1, T2, and T3). The total VFA concentrations in the solid fraction from T1 were higher than those in duckweed from T2 and T3 during the initial 24 days, indicating that duckweed biomass with Zn inhibited VFAs’ release, however, the types of VFAs increased by the end of these three incubations. For duckweed cultured in the presence of NH4+-N, the total VFAs in the fractions of duckweed from T5 and T6 were higher than those from T4, meaning that the presence of Zn enhanced the total VFAs under the applied experimental conditions. However, no difference in the total VFAs between duckweed from T5 and T6 was noticed. This section effectively shows that Zn toxicity suppresses early fermentation, especially in solid fractions, but microbial communities may partially adapt over time. NH4⁺-N acts as a modulator, helping to buffer or recover microbial activity. VFA accumulation, particularly FA and CA, in Zn-rich treatments also implies the inhibition of methanogenesis, which could limit methane yield and cause system instability if not managed. To optimize AD with Zn-rich duckweed, the careful control of NH4⁺-N levels, monitoring of VFA profiles, and possibly targeted inoculation strategies are, thus, essential.
4. Discussion
4.1. Phytoremediation of Zn/NH4+-Polluted Water by Duckweed
4.1.1. Influence of Zn on Duckweed Growth and Zn Removal Efficiency
This study showed that duckweed growth was seriously affected by the presence of the pollutants Zn and NH4+ in the growth medium. Figure 2a shows that duckweed was able to grow at a Zn concentration of 5.0 mg/L, indicating that duckweed can be used for Zn accumulation; however, both Zn concentrations of 2.5 mg/L and 5.0 mg/L seriously decreased the growth rate compared to the control. Zn directly destroys the structure and function of the chloroplast and inhibits overall chlorophyll biosynthesis. Zn also enters the frond chloroplast and can damage the chloroplast membranes due to oxidative stress via peroxidation [52,53]. In addition, Zn may inhibit the uptake and transportation of manganese and iron by antagonistic effects [53], which inhibits duckweed growth.
Previous work showed that Zn concentrations of 4 mg/L inhibited Lemna gibba (L.) growth by 50% and 10 mg/L Zn reduced the specific biomass growth of Lemna gibba by 90%, while less than 1.82 mg/L Zn promoted the growth of Lemna minor (L.) [54]. The addition of Zn reduced the growth rate of Lemna minor by 15.5% and 56.6% when the Zn concentration increased from 0.205 mg/L to 0.819 mg/L [55]. Chen et al. also observed a reduction in the growth rate of Lemna minor by 70% when Zn concentrations increased from 0 to 10 mg/L [56]. Li et al. found a reduction in the dry weight of Lemna minor from 0.12 g to 0.05 g for 5.0 mg/L Zn [57]. Hence, duckweed has potential for the remediation of Zn-polluted water, while the growth rate parameter indicates that duckweed is sensitive to elevated Zn levels.
Zn can be removed from water by duckweed bioaccumulation or chemical precipitation (Table 3). At a Zn concentration of 5.0 mg/L, on the 7th day, precipitation could have played an important role in Zn removal by producing Zn3(PO4)2 [58], resulting in a low Zn recovery efficiency and greater removal efficiency by duckweed (Figure 2b). Meanwhile, a Zn concentration of 5.0 mg/L inhibited duckweed growth and reduced the fresh biomass production (Figure 2), resulting in a lower Zn recovery efficiency. Jayasri and Suthindhiran observed that Lemna minor showed the maximum accumulation of 10 mg/L of Zn after 4 days, and the Zn removal efficiency was approximately 70–80% [53]. Jafari and Akhavan found the highest removal efficiency for Lemna trisulca (97%) as compared to Lemna minuta (89%) and Lemna minor (83%) for 10 days of incubation with 1, 5, 10, 15, and 20 mg/L Zn [59]. Khellaf and Zerdaoui found that the duckweed Lemna gibba removed 61–71% of Zn after 7 days of exposure [58]. Figure 2 shows that a removal efficiency of more than 82% was achieved when the hybrid of Lemna minor and Lemna turionifera in this study was grown in the presence of solely Zn. This indicates that duckweed is a significant option for the phytoremediation of Zn-contaminated waters.

Table 3.
Zn removal efficiency of duckweed reported in different studies using synthetic and real wastewater.
4.1.2. Effect of NH4+ on Zn Phytoremediation and Duckweed Growth
The direct assimilation of NH4+ by duckweed and the biodegradation by its rhizosphere microorganisms synergistically purify wastewater. However, Zn recovery was inhibited in the presence of NH4+-N. The pH was very low at the end of incubation in the presence of NH4+-N, particularly in the incubations with 5.0 mg/L Zn and 40 mg/L NH4+-N (Figure 6c). This was likely because NH4+-N was absorbed onto the root cells, exchanging a hydrogen ion (H+) from the functional groups on duckweed, thus adding an acidic component to water. In addition, ammonium is converted to nitrate by the action of nitrifying microorganisms. In the process, protons are released to the medium, reducing pH. In this condition, Zn absorption onto plants decreases because of the competition between Zn and H+ ions for uptake sites on the root surface [60,61]. In addition, acid conditions are beneficial for Zn precipitation in the presence of NH4+-N [58], which led to a better Zn removal efficiency in the treatment with a NH4+-N concentration of 40 mg/L (T9) compared to the 20 mg/L NH4+-N incubation (T6).
Overall, the above results demonstrate a clear antagonism between Zn and NH4⁺-N in duckweed systems. While NH4⁺-N can stimulate growth and enhance stress tolerance, it compromises Zn uptake and phytoremediation efficiency. The balance between nutrient supplementation and metal stress is critical—optimal phytoremediation may require the careful tuning of nutrient inputs to avoid suppressing metal accumulation.
4.2. Anaerobic Digestion of Duckweed Used for Zn Phytoremediation
4.2.1. Effect of Zn on Methane Production Step
The presence of Zn can stimulate or inhibit the AD process, depending on its concentration. Zn is an essential trace element of various enzymes that catalyze anaerobic reactions and transformations in the AD process, and it is also part of enzymes such as formate dehydrogenase, superoxide dismutase (SOD), and hydrogenase [62]. Although high Zn concentrations (50–630 mg/L) can accumulate in methanogenic archaea, Zn has not yet been shown to be an essential element for methanogenesis [63]. However, Ram and Nikhil found that Zn was a necessary trace element to maintain the cell metabolism of methanogens, whereas more than 5 mg/L Zn inhibited the growth of methanogens [64].
Kumar et al. observed the highest biomethane production from activated sludge at a Zn concentration of 93 mg/L [51]. In another study, 4 mg/L Zn promoted the AD of dairy manure to obtain the highest biomethane production of 331.85 mL/g VS, which was 37.59% higher than that of the control [62]. Wu et al. obtained the highest methane yield using de-oiled grease trap waste at 2.1 mg/L Zn, a short lag time, and a decrease in maximum methane production with an increasing Zn concentration [65]. However, Alrawashdeh et al. reported a 40% reduction in biogas production from the AD of olive mill waste with respect to the base case, because the addition of Zn delayed the degradation during the acidogenic–anaerobic treatment [66]. Guo et al. reported that the maximum methanogen activity was obtained in a digestive system with 2 mg/L Zn, and the inhibition effect on methanogenic activity increased as Zn concentrations increased to more than 3 mg/L [26].
The liquid fraction of the acid-pretreated duckweed (Figure 6b) contained more available organic matter in comparison to the solid fractions (Figure 6a), thus giving higher methane production (Figure 5b vs. Figure 5a), as also reported by Kotamraju et al. [51]. However, higher initial Zn concentrations in liquid fractions (Figure 5d) exerted toxic effects and inhibited AD, which reduced the methane yield of the liquid fractions of duckweed cultured with Zn (T2, T3, T5, and T6 versus T1 in Figure 5b). Since the Zn concentrations in the liquid fractions of duckweed cultured with both Zn and NH4+-N were lower than those of duckweed grown in the presence of solely Zn, a higher methane production was observed in the liquid fractions of duckweed cultured with both Zn and NH4+-N. The lower biomethane production can be explained by the fact that a higher initial Zn concentration was inhibitory to methanogens in the liquid hydrolysate of duckweed cultured with solely Zn and affected the methane yield. Methanogens were not inhibited in the control incubation (T1), and the liquid fractions of duckweed cultured without Zn had higher methane production in comparison to the liquid fractions of duckweed cultured in the presence of Zn (T2 and T3).
In the solid fraction of the (acid) pretreated duckweed, the rapid acidification of the cellulose and hemicellulose resulted in a low pH (Figure 6c) and inhibited the hydrolysis process. Thus, the high biomass concentrations prolonged the lag phase of methane production from the solid fraction due to the absence of the enzymatic hydrolysis of non-soluble organic polymers to soluble forms available to microorganisms in the initial experiment, especially for duckweed cultured with Zn at 5.0 mg/L. Indeed, adjusting the pH to 7 did improve the methane production in AD tests with the solid fraction of pretreated duckweed.
4.2.2. Effect of Zn on Acidification Step
Zn is also essential for hydrolysis and the acidogenesis process of AD [67]. The solid and liquid fractions with different background levels of Zn indeed produced different amounts of VFAs (Figure 7 and Figure 8). Higher VFA concentrations were related to the fast hydrolysis and acidification of the duckweed substrate, which slowed down the start of methanogenesis in all solid fractions (Figure 8). Indeed, the degradation of the substrate and production of VFAs changed the pH of the AD processes (Figure 6c), especially for duckweed cultured with Zn (pH of 5.13–5.80), resulting in the inhibition of methanogenic activity, methane production, and acido-/acetogenic bacterial activity. Moreover, Yu and Fang [68] found that low and high concentrations of Zn inhibited the production of acetic acid in AD of dairy wastewater, while the present study showed that Zn enhanced the production of AA in the solid fractions of duckweed cultured with NH4+-N (T5 and T6; Figure 8e,f). In contrast, in the liquid fractions of duckweed cultured with solely Zn, the presence of Zn did not significantly affect VFA production (T2 and T3; Figure 7b,c).
5. Conclusions
This research showed that duckweed can accumulate a high amount of Zn, indicating that phytoremediation using duckweed can be an effective method. However, the presence of NH4+-N inhibited Zn uptake. The study subsequently showed the feasibility of using acid-pretreated Zn-enriched duckweed biomass for biomethane production by anaerobic digestion. The acid pretreatment of the duckweed resulted in a liquid hydrolysate with high Zn concentrations that inhibited methane production. The residual solid fraction had low methane production because of the fast acidification of the incubation medium to about pH 5. Hence, liquid fractions of Zn-enriched pretreated duckweed could be used as substrates for methane production by AD, while the AD of solid fractions requires appropriate neutralization of the pH during anaerobic conversion.
This research offers a practical blueprint for advancing both wastewater treatment and renewable energy generation through an integrated duckweed-based system. By cultivating duckweed in zinc- and ammonium-contaminated wastewater, this process enables the simultaneous removal of heavy metals and nutrients–two major classes of pollutants that frequently co-occur in agricultural, industrial, and livestock effluents. This phytoremediation step not only purifies the water, but also results in a nutrient- and metal-rich biomass. Rather than discarding this biomass, our approach valorizes it through acid hydrolysis followed by anaerobic digestion (AD), producing methane-rich biogas that can be used as a renewable energy source. This integration transforms a treatment challenge into a circular system that recovers both purified water and energy. A key practical insight from this work is the need for the pH neutralization of the acid-hydrolyzed solids before AD, as residual acidity can inhibit methane production. The study also highlights that high zinc concentrations in the hydrolysate can suppress biogas yields, underlining the importance of monitoring and managing metal content throughout the process. Importantly, the research reveals that most zinc remains in the digestate after AD, raising environmental concerns if the digestate is to be used as fertilizer. This finding points to the necessity of additional steps for zinc recovery or stabilization to prevent secondary pollution. For practitioners, this research provides actionable recommendations for optimizing integrated phytoremediation–biogas systems, including process control for pH and metals and careful management of the digestate. For policy makers and engineers, it demonstrates a scalable, sustainable approach to circular wastewater management that aligns with environmental protection and resource recovery goals, while also identifying future research needs in digestate treatment and metal recovery technologies.
Author Contributions
Conceptualization, Y.Z., X.Z. and A.H.; methodology, Y.Z.; investigation Y.Z., formal analysis, Y.Z.; resources, Y.Z.; data curation, Y.Z. and A.H.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z., X.Z., A.H. and P.N.L.L.; supervision, A.H. and P.N.L.L.; project administration, P.N.L.L.; funding acquisition, Y.Z. and P.N.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Chinese Government Scholarship (CSC NO 202009370006), the Visiting Scholar Project of Shandong Academy of Agricultural Sciences, Agricultural Science and Technology Innovation project of Shandong Province (CXGC2023B01), Science Foundation Ireland (SFI) through the SFI Research Professorship Programme entitled Innovative Energy Technologies for Biofuels, Bioenergy and a Sustainable Irish Bioeconomy (IETSBIO3; grant number 15/RP/2763), the Research Infrastructure research grant Platform for Biofuel Analysis (Grant Number 16/RI/3401) and the Department of Foreign Affairs (DFA) under the SDG challenge project Floating Treatment Wetland, supporting teams to develop solutions addressing challenges under UN SDG3: Good Health and Well-being (grant No SFI/21/FIP/SDG/9933).
Data Availability Statement
The data that support this study are available by the authors upon request.
Acknowledgments
The authors thank Borja Khatabi Soliman Tamayo and Marlee Wasserman (University of Galway, Ireland) for their technical assistance as well as Amulya Kotamraju and Mohanakrishnan Logan (University of Galway, Ireland) for lab guidance and critical discussions during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Acetic Acid |
| AD | Anaerobic Digestion |
| BA | Butyric Acid |
| BCF | Bioconcentration Factor |
| BMP | Biomethane potential |
| CA | Caproic Acid |
| COD | Chemical Oxygen Demand |
| FA | Formic Acid |
| PA | Propionic Acid |
| IBA | Isobutyric Acid |
| ICA | Isocaproic Acid |
| IVA | Isovaleric Acid |
| RGR | Relative Growth Rate |
| TI | Tolerance Index |
| VA | Valeric Acid |
| VFAs | Volatile Fatty Acids |
| VS | Volatile Solids |
References
- Ulusoy, A.; Atılgan, A.; Rolbiecki, R.; Jagosz, B.; Rolbiecki, S. Innovative Approaches for Sustainable Wastewater Resource Management. Agriculture 2024, 14, 2111. [Google Scholar] [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative approaches to sustainable wastewater treatment: A comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Hejna, M.; Alessandra, M.; Elisabetta, O.; Antonella, B.; Salvatore, P.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, J.; Zou, P.; Zhou, Y.; Wang, B.; Yu, D.; Li, W.; Zhan, X. Effects of Dietary Supplementation of Humic Acid Sodium and Zinc Oxide on Growth Performance, Immune Status and Antioxidant Capacity of Weaned Piglets. Animals 2020, 10, 2104. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Alagawany, M.; Hashem, N.M.; Farag, M.R.; Alghamdi, E.S.; Hassan, F.U.; Bilal, R.M.; Elnesr, S.S.; Dawood, M.A.O.; Nagadi, S.A.; et al. Nanominerals: Fabrication Methods, Benefits and Hazards, and Their Applications in Ruminants with Special Reference to Selenium and Zinc Nanoparticles. Animals 2021, 11, 1916. [Google Scholar] [CrossRef]
- Daverey, A.; Chen, Y.-C.; Sung, S.; Lin, J.-G. Effect of zinc on anammox activity and performance of simultaneous partial nitrification, anammox and denitrification (SNAD) process. Bioresour. Technol. 2014, 165, 105–110. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Rouff, A.A.; Juarez, K.M. Zinc Interaction with Struvite During and After Mineral Formation. Environ. Sci. Technol. 2014, 48, 6342–6349. [Google Scholar] [CrossRef]
- Jain, D.; Kour, R.; Bhojiya, A.A.; Meena, R.H.; Singh, A.; Mohanty, S.R.; Rajpurohit, D.; Ameta, K.D. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci. Rep. 2020, 10, 13865. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Tsonev, T.; Lidon, F. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Zhou, Q.; Lin, Y.; Li, X.; Yang, C.; Han, Z.; Zeng, G.; Lu, L.; He, S. Effect of zinc ions on nutrient removal and growth of Lemna aequinoctialis from anaerobically digested swine wastewater. Bioresour. Technol. 2018, 249, 457–463. [Google Scholar] [CrossRef]
- Abadi, V.A.J.M.; Sepehri, M. Effect of Piriformospora indica and Azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.). Symbiosis 2016, 69, 9–19. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Ahern, R.; Burnell, G.; O’Mahoney, R.; Kuehnhold, H.; Jansen, M.A.K. Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants 2022, 11, 2170. [Google Scholar] [CrossRef]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef]
- Paolacci, S.; Stejskal, V.; Toner, D.; Jansen, M.A.K. Wastewater valorisation in an integrated multitrophic aquaculture system; assessing nutrient removal and biomass production by duckweed species. Environ. Pollut. 2022, 302, 119059. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Guo, B.; Liu, C.; Liu, J.; Qiu, G.; Fu, Q.; Li, H. Alleviation of aqueous nitrogen loss from paddy fields by growth and decomposition of duckweed (Lemna minor L.) after fertilization. Chemosphere 2023, 311, 137073. [Google Scholar] [CrossRef]
- Amulya, K.; Morris, S.; Lens, P.N.L. Aquatic biomass as sustainable feedstock for biorefineries. Biofuels Bioprod. Biorefining 2023, 17, 1012–1029. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khan, A.A.; Suthar, S. Effect of thermal pre-treatment on co-digestion of duckweed (Lemna gibba) and waste activated sludge on biogas production. Chemosphere 2017, 174, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Liu, H.; Lin, W.; Shukla, P.; Luo, J. Simultaneous biohydrogen production from dark fermentation of duckweed and waste utilization for microalgal lipid production. Bioresour. Technol. 2020, 302, 122879. [Google Scholar] [CrossRef] [PubMed]
- Arefin, M.A.; Rashid, F.; Islam, A. A review of biofuel production from floating aquatic plants: An emerging source of bio-renewable energy. Biofuels Bioprod. Biorefining 2021, 15, 574–591. [Google Scholar] [CrossRef]
- Sharma, S.S.; Gaur, J.P. Potential of Lemna polyrrhiza for removal of heavy metals. Ecol. Eng. 1995, 4, 37–43. [Google Scholar] [CrossRef]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef]
- Luna-delRisco, M.; Orupõld, K.; Dubourguier, H.-C. Particle-size effect of CuO and ZnO on biogas and methane production during anaerobic digestion. J. Hazard. Mater. 2011, 189, 603–608. [Google Scholar] [CrossRef]
- Baek, G.; Saeed, M.; Choi, H.-K. Duckweeds: Their utilization, metabolites and cultivation. Appl. Biol. Chem. 2021, 64, 73. [Google Scholar] [CrossRef]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat. Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A state-of-the-art of phytoremediation approach for sustainable management of heavy metals recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Wang, X.; Xia, M.; Ba, S.; Lim, B.L.; Hou, H. Biomonitoring of heavy metals and their phytoremediation by duckweeds: Advances and prospects. Environ. Res. 2024, 245, 118015. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Kanwar, N. Duckweed: A model for phytoremediation technology. Holist. Approach Environ. 2022, 12, 39–58. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Xie, Y.; Li, J.; Li, J.; Ran, C. Optimal cultivation concentration of duckweed for pollutant removal from biogas slurry. Sci. Rep. 2025, 15, 5193. [Google Scholar] [CrossRef]
- Murillo, A.M.; Kotamraju, A.; Mulkeen, C.J.; Healy, M.G.; Sulpice, R.; Lens, P.N. Selenite (IV) and selenate (VI) uptake and accumulation capacity of Lemna minor L. from an aquatic medium. Environ. Technol. 2024, 45, 5630–5640. [Google Scholar] [CrossRef]
- Kotamraju, A.; Logan, M.; Lens, P.N.L. Integrated bioprocess for Se(VI) remediation using duckweed: Coupling selenate removal to biogas production. J. Hazard. Mater. 2023, 459, 132134. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production From Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, J.; Ji, Z.; Zhang, X.; Ramaswamy, S.; Xu, F.; Sun, R.-c. Dilute acid pretreatment differentially affects the compositional and architectural features of Pinus bungeana Zucc. compression and opposite wood tracheid walls. Ind. Crops Prod. 2014, 62, 196–203. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mohan, M.; Veeranki, V.D.; Goud, V.V.; Pinnamaneni, S.R.; Benarjee, T. Dilute acid pretreatment of sorghum biomass to maximize the hemicellulose hydrolysis with minimized levels of fermentative inhibitors for bioethanol production. 3 Biotech 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, M.J.; Mushtaq, M.; Tian, L.; Jiménez-Rodríguez, A.; Rincón, B.; Gilroyed, B.H.; Borja, R. Evaluation and modelling of methane production from corn stover pretreated with various physicochemical techniques. Waste Manag. Res. 2022, 40, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Morais, N.W.S.; Coelho, M.M.H.; de Oliveira, M.G.; Mourão, J.M.M.; Pereira, E.L.; dos Santos, A.B. Kinetic Study of Methanization Process Through Mathematical Modeling in Biochemical Methane Potential Assays from Four Different Inoculants. Water Air Soil Pollut. 2021, 232, 423. [Google Scholar] [CrossRef]
- Hu, L.X.; Ying, G.G.; Chen, X.W.; Huang, G.Y.; Liu, Y.S.; Jiang, Y.X.; Pan, C.G.; Tian, F.; Martin, F.L. Fourier-transform infrared spectroscopy as a novel approach to providing effect-based endpoints in duckweed toxicity testing. Environ. Toxicol. Chem. 2016, 36, 346–353. [Google Scholar] [CrossRef]
- Logan, M.; Tan, L.C.; Lens, P.N.L. Anaerobic co-digestion of dissolved air floatation slurry and selenium rich wastewater for simultaneous methane production and selenium bioremediation. Int. Biodeterior. Biodegrad. 2022, 172, 105425. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Logan, M.; Tan, L.C.; Nzeteu, C.O.; Lens, P.N.L. Effect of selenate on treatment of glycerol containing wastewater in UASB reactors. Renew. Energy 2023, 206, 97–110. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Wu, D.; Huang, T.; Zhang, B.; Cai, G.; Gao, G.; Liu, Z.; Huang, X.; Zhong, Z. Large-scale screening and characterization of Cd accumulation and ultrastructural deformation in duckweed. Sci. Total Environ. 2022, 832, 154948. [Google Scholar] [CrossRef]
- FTIR Functional Group Database Table with Search—InstaNANO. Available online: https://instanano.com/all/characterization/ftir/ftir-functional-group-search/ (accessed on 18 May 2025).
- Yang, C.; Wang, J.; Lei, M.; Xie, G.; Zeng, G.; Luo, S. Biosorption of zinc(II) from aqueous solution by dried activated sludge. J. Environ. Sci. 2010, 22, 675–680. [Google Scholar] [CrossRef]
- Kumar, M.; Matassa, S.; Bianco, F.; Oliva, A.; Papirio, S.; Pirozzi, F.; De Paola, F.; Esposito, G. Effect of Varying Zinc Concentrations on the Biomethane Potential of Sewage Sludge. Water 2023, 15, 729. [Google Scholar] [CrossRef]
- McGeer, J.C.; Szebedinszky, C.; McDonald, D.G.; Wood, C.M. Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout. 1: Iono-regulatory disturbance and metabolic costs. Aquat. Toxicol. 2000, 50, 231–243. [Google Scholar] [CrossRef]
- Jayasri, M.A.; Suthindhiran, K. Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef]
- Lanthemann, L.; van Moorsel, S.J. Species interactions in three Lemnaceae species growing along a gradient of zinc pollution. Ecol. Evol. 2022, 12, e8646. [Google Scholar] [CrossRef]
- Bodnar, I.S.; Cheban, E.V. Joint effects of gamma radiation and zinc on duckweed Lemna minor L. Aquat. Toxicol. 2023, 257, 106438. [Google Scholar] [CrossRef]
- Chen, X.; O’Halloran, J.; Jansen, M.A.K. The toxicity of zinc oxide nanoparticles to Lemna minor (L.) is predominantly caused by dissolved Zn. Aquat. Toxicol. 2016, 174, 46–53. [Google Scholar] [CrossRef]
- Li, J.; Lens, P.N.L.; Otero-Gonzalez, L.; Du Laing, G. Production of selenium- and zinc-enriched Lemna and Azolla as potential micronutrient-enriched bioproducts. Water Res. 2020, 172, 115522. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Phytoaccumulation of zinc by the aquatic plant, Lemna gibba L. Bioresour. Technol. 2009, 100, 6137–6140. [Google Scholar] [CrossRef]
- Jafari, N.; Akhavan, M. Effect of pH and heavy metal concentration on phytoaccumulation of zinc by three Duckweeds species. J. Agric. Environ. Sci. 2011, 10, 34–41. [Google Scholar]
- Chaudhry, F.M.; Loneragan, J.F. Zinc Absorption by Wheat Seedlings: I. Inhibition by Macronutrient Ions in Short-Term Experiments and its Relevance to Long-Term Zinc Nutrition. Soil Sci. Soc. Am. J. 1972, 36, 323–327. [Google Scholar] [CrossRef]
- Chairidchai, P.; Ritchie, G.S.P. The Effect of Citrate and pH on Zinc Uptake by Wheat. Agron. J. 1993, 85, 322–328. [Google Scholar] [CrossRef]
- Yu, L.; Kim, D.-G.; Ai, P.; Yuan, H.; Ma, J.; Zhao, Q.; Chen, S. Effects of Metal and Metal Ion on Biomethane Productivity during Anaerobic Digestion of Dairy Manure. Fermentation 2023, 9, 262. [Google Scholar] [CrossRef]
- Myszograj, S.; Stadnik, A.; Płuciennik-Koropczuk, E. The Influence of Trace Elements on Anaerobic Digestion Process. Civ. Environ. Eng. Rep. 2018, 28, 105–115. [Google Scholar] [CrossRef]
- Raja Ram, N.; Nikhil, G.N. A critical review on sustainable biogas production with focus on microbial-substrate interactions: Bottlenecks and breakthroughs. Bioresour. Technol. Rep. 2022, 19, 101170. [Google Scholar] [CrossRef]
- Wu, L.-J.; Kobayashi, T.; Kuramochi, H.; Li, Y.-Y.; Xu, K.-Q. Effects of Potassium, Magnesium, Zinc, and Manganese Addition on the Anaerobic Digestion of De-oiled Grease Trap Waste. Arab. J. Sci. Eng. 2016, 41, 2417–2427. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.b.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Huang, Z.; Niu, Q.; Nie, W.; Li, X.; Yang, C. Effects of heavy metals and antibiotics on performances and mechanisms of anaerobic digestion. Bioresour. Technol. 2022, 361, 127683. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Inhibition on Acidogenesis of Dairy Wastewater by Zinc and Copper. Environ. Technol. 2001, 22, 1459–1465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).