Abstract
The gut microbiota consists of trillions of microorganisms, mostly bacteria, which establish a symbiotic relationship with the host. The host provides a favourable environment and the essential nutrients for their proliferation, while the gut microbiota plays a key role in maintaining the host’s health. Therefore, imbalances in its composition, a state known as dysbiosis, can contribute to the onset or progression of various pathological conditions, including atherosclerosis. Atherosclerosis is a chronic, slow-progressing inflammatory disease characterised by the formation and potential rupture of atheromatous plaques in medium- and large-calibre arteries. It underlies major cardiovascular events, such as stroke and myocardial infarction, and remains a leading cause of global morbidity and mortality. The modulation of the gut microbiota using prebiotics, probiotics, and faecal microbiota transplantation (FMT) has emerged as a promising approach for preventing and managing atherosclerosis. Although numerous studies have explored these strategies, further research is needed to establish their efficacy and mechanisms. This review explores the pathophysiology of atherosclerosis, its main risk factors, and the interplay between the gut microbiota and atherosclerosis, with a particular focus on the mechanisms by which microbiota-targeted interventions, including prebiotics, probiotics, and FMT, may serve as therapeutic adjuvants in the prevention and treatment of atherosclerosis.
1. Introduction
Atherosclerosis is a chronic inflammatory disease of the arteries characterised by alterations in lipid profile and other metabolic parameters, including blood glucose levels, insulin sensitivity, body weight, and markers of systemic inflammation, and it is the main risk factor associated with cardiovascular diseases (CVD) with complex underlying pathophysiological mechanisms [1,2].
Recently, the gut microbiota has been identified as a relevant factor in the progression of atherosclerosis [3,4]. Therefore, changes in its composition and function appear to influence cardiovascular risk through the modulation of inflammatory and metabolic processes [5].
The human microbiota consists of trillions of microorganisms that inhabit the human body, organised into complex and specific ecosystems depending on the different body regions, such as the mouth, skin, vagina, and, in particular, the gastrointestinal tract (GIT) [6]. The GIT harbours the largest, most complex, and diverse bacterial community that maintains a symbiotic and mutualistic relationship with the host’s cells. Beyond their immune and homeostatic roles, commensal gut bacteria are key regulators of digestion, being essential for nutrient extraction, synthesis, and absorption, as well as contributing to the production and modulation of some metabolites [7].
As a whole, the gut microbiota is essential for the proper functioning of the body. Disruptions in its composition and, consequently, in its function—a condition known as dysbiosis—may impair the host’s health status, and it has been linked to various pathological conditions, including atherosclerosis [8]. Strategies such as the use of prebiotics, probiotics, and faecal microbiota transplantation (FMT) have been investigated for their potential to modulate this ecosystem, contributing to the prevention and regression of atherosclerotic lesions [9].
This review aims to explore the interplay between the gut microbiota and atherosclerosis, as well as to highlight the therapeutic targets and potential mechanisms that may contribute to the prevention and treatment of this disease. To achieve this objective, this review provides a concise overview of the gut microbiota and the pathophysiology of atherosclerosis, as well as the link between them both. Furthermore, this review summarises the latest evidence on the use of prebiotics, probiotics, and FMT in the prevention and management of atherosclerosis.
2. Overview of the Human Gut Microbiota
The human gut microbiota comprises over 10 trillion microorganisms, including bacteria, archaea, viruses, protozoa, and fungi [10]. These microorganisms form a highly complex ecosystem, engaging in interactions both among themselves, as well as with the host, in a symbiotic relationship that is essential for metabolic and immune system development [11].
A balanced microbiota is composed mainly of bacteria from four major phyla that adapt to constant changes in lifestyle, Actinomycetota, Pseudomonadota, Bacillota, and Bacteroidota, with the latter two representing approximately 90% of the total composition [12].
While most microbiota studies focus on the bacterial component, the human gut is also colonised by archaea, viruses (including bacteriophages), unicellular eukaryotes, and fungi, which, despite their lower abundance, play important functional roles in maintaining microbial homeostasis [13]. Bacteriophages, in particular, exert a strong influence on bacterial diversity, survival, and activity, and altered virome profiles have been associated with conditions such as inflammatory bowel disease [14,15]. Furthermore, certain gut viruses, such as Siphoviridae infecting Streptococcaceae and Enterobacteriaceae, have been implicated in systemic disorders like atherosclerosis, where viral infections may promote vascular inflammation and plaque formation [16,17].
The fungal fraction of the gut microbiota, known as the mycobiota, accounts for approximately 0.1–0.3% of the total microbial community and includes genera such as Aspergillus, Candida, Cryptococcus, Saccharomyces, Malassezia, and Penicillium [18]. Fungal biodiversity appears to be diet-dependent, with Candida spp. being positively associated with carbohydrate intake and Aspergillus spp. being negatively associated with levels of short-chain fatty acids (SCFAs) [19]. Altered fungal profiles have been reported in various disease conditions, including GIT, neurological, and liver diseases, as well as in atherosclerosis [18,20,21].
This delicate and dynamic equilibrium is often referred to as eubiosis, a state in which microbial diversity and function support the host’s health. Eubiosis is not defined by a fixed composition, but by the maintenance of beneficial host–microbe interactions and essential physiological functions. Disruption of this balance, known as dysbiosis, may result from antibiotic use, infections, dietary shifts, or chronic inflammation. Dysbiosis is typically associated with reduced microbial diversity, the loss of beneficial species, and the expansion of opportunistic taxa. Functionally, it can impair intestinal barrier integrity and trigger inappropriate immune responses, contributing to gastrointestinal and systemic disorders [22,23,24,25,26,27].
Beyond taxonomic and functional shifts, it is also important to distinguish between the related concepts of the microbiota and the microbiome, sometimes misused as synonyms. While the microbiota refers exclusively to the community of microorganisms, the microbiome encompasses these microorganisms along with their collective genetic material [7]. The human microbiome is estimated to harbour approximately 3.3 million active genes, in contrast to the 22,000 active human genes [28].
2.1. Development and Maturation of the Gut Microbiota
The composition of the gut microbiota in adulthood is the result of a complex developmental trajectory that begins at birth. Early-life microbial colonisation is increasingly recognised as a key determinant of immune and metabolic programming, both of which are closely linked to cardiovascular health [29]. During gestation, while the amniotic fluid contains trace amounts of bacteria, it seems insufficient to influence the colonisation process of the foetus [30].
The initial composition of the neonatal gut microbiota is primarily determined by the mode of delivery. In vaginal delivery, the newborn is predominantly colonised by Lactobacillus spp., Sneathia spp., and Prevotella spp. through contact with the mother’s vaginal and faecal microbiota. Conversely, infants delivered by caesarean section tend to be colonised by microorganisms present on the mother’s skin, on surfaces of the hospital environment, and possibly in the mother’s respiratory tract. As a result, the initial colonisation is predominantly by bacteria belonging to the genera Corynebacterium spp., Staphylococcus spp., and Propionibacterium spp. [30,31]. Other important factors include the introduction of either breastfeeding or formula feeding. Breastfeeding promotes the proliferation of bacteria from the Bifidobacterium spp., which are capable of degrading particular oligosaccharides in breast milk and are known to have several benefits, including the modulation of the immune system and the production of vitamins [32]. Conversely, formula feeding tends to stimulate a more diverse microbiota, with a higher prevalence of opportunistic pathogenic bacteria belonging to the genera Enterococcus spp., Bacteroides spp., and Clostridium spp. [32,33].
The introduction and diversification of food is an important dietary event in childhood that causes profound shifts in the gut microbiota. This period is characterised by the diversification of the gut microbiota and by the dominance of bacterial species belonging to the phyla Bacteroidota and Bacillota [33,34,35,36].
The gut microbiota undergoes significant development until approximately three years of age, as this is the stage when food introduction is completed and weaning from breast or formula milk occurs. The microbial community evolves from a simple community with low richness and diversity to one that progressively acquires the composition of the adult gut microbiota [37]. Understanding how early microbial exposures shape the long-term composition and function of the gut microbiota provides valuable context for designing effective microbiota-targeted interventions aimed at preventing or mitigating atherosclerosis later in life [29,38].
2.2. Composition and Functional Organisation of the Human Gut Microbiota
The GIT is anatomically and functionally divided into the stomach, the small intestine, and the large intestine. The physiological characteristics of each region shape the growth of distinct microbial communities, resulting in variations in both microbiota density and diversity throughout the GIT (Figure 1) [39].
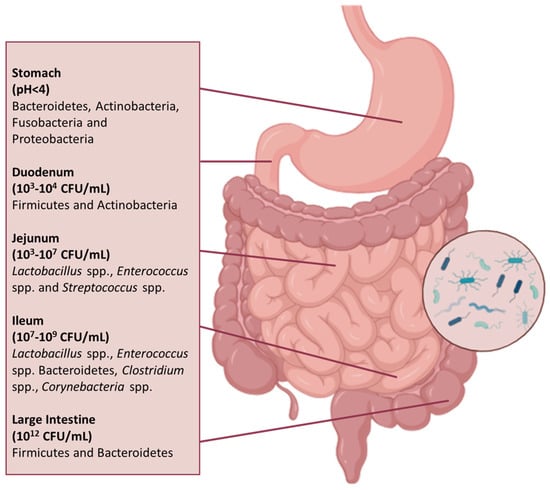
Figure 1.
Composition and distribution of the microbiota throughout the GIT. The composition of the microbiota differs throughout the GIT, reflecting the distinct environmental conditions and physiological functions of each segment. Anatomically, the GIT is divided into the stomach, small intestine (duodenum, jejunum, and ileum), and large intestine (caecum and colon). Microbial density and diversity increase along the tract, reaching their peak in the colon.
The stomach contains a relatively low number of microorganisms (<103 CFU/mL) compared to the distal regions of the GIT, due to its motile activity, gastric acid secretion, and low pH (<4). Gastric lumen harbours bacteria from the phyla Bacteroidota, Actinomycetota, Fusobacteria, and Pseudomonadota, while the mucosa is colonised primarily by Bacillota and Pseudomonadota [40]. Despite the hostile environment, acid-tolerant or transient species belonging to the genera Neisseria, Haemophilus, Fusobacterium, Prevotella, Veillonella, and Streptococcus have been detected in the gastric microbiota of healthy individuals. This microbial presence is likely due to the constant influx of bacteria originating from the oral cavity via swallowing, as well as reflux from the duodenum. Indeed, more than 65% of bacterial phylotypes identified in the stomach correspond to those found in the oral microbiota, suggesting that species, such as Veillonella, Lactobacillus, and Clostridium, detected in gastric samples may largely represent transient populations rather than established residents [41,42].
The duodenum, the first segment of the small intestine, is characterised by the presence of bile acids, pancreatic secretions, and antimicrobial agents, including bile salts (which can reach concentrations up to 11.2 mM postprandially); digestive enzymes, such as proteases and lipases, which can generate antimicrobial compounds; and innate immune molecules like defensins and lysozyme [43]. The rapid transit and the presence of an oxygen gradient along the GIT, with pO2 levels in the duodenum estimated between 10 and 30 mmHg and progressively decreasing to near-anoxic conditions (<3 mmHg) in the colon, limit both bacterial density (103–104 CFU/mL) and biodiversity. The microbial community is mainly composed of facultative anaerobes and transient oral-derived species, mainly from the Bacillota and Actinomycetota phyla, including species such as Streptococcus salivarius, Lactobacillus rhamnosus, Lactobacillus plantarum, Enterococcus faecalis, and Enterococcus faecium [44,45,46].
In contrast, the jejunum provides a more suitable environment for the growth of predominantly aerobic Gram-positive and facultative anaerobic and aerotolerant bacteria, with bacterial counts ranging from 103 to 107 CFU/mL. The most represented genera include Lactobacillus, Enterococcus, and Streptococcus. The most frequently detected species are oral-associated taxa, including Streptococcus spp. (particularly from the mitis and sanguinis groups), Granulicatella adiacens/para-adiacens, Gemella haemolysans/taiwanensis, and Schaalia odontolytica complex, suggesting that the jejunal microbiota may largely reflect transient colonisation [44,47].
In the transition to the ileum, there is a higher population of bacteria, around 109 CFU/mL, and the microbiota of this region is dominated by aerobic species, whereas the more distal part shows a distinctive microbial profile that resembles the oral microbiota. The most frequently detected genera include Streptococcus, Granulicatella, Actinomyces, Solobacterium, Rothia, Gemella, and members of the candidate phylum TM7 (G-1). Dominant species comprise streptococci from the mitis and sanguinis groups, Streptococcus salivarius, Rothia mucilaginosa, and Actinomyces spp., particularly from the A. meyeri/odontolyticus group. In contrast, strict anaerobes and Pseudomonadota are present in low abundance. This composition reflects a microbial continuity between the oral cavity and distal small intestine, which markedly differs from the colonic microbiota [48,49].
The large intestine is distinguished by a substantial bacterial density, reaching up to 1012 CFU/mL, with a preponderance of the Bacillota and Bacteroidota phyla [39]. The most abundant bacterial families include Bacteroidaceae, Prevotellaceae, and Rikenellaceae from Bacteroidota, as well as Lachnospiraceae and Ruminococcaceae from Bacillota. These families play key roles in the fermentation of complex polysaccharides and the production of SCFAs, which are essential for colonic health and host metabolism [7,50].
Understanding this close relationship and microbial transition from the oral cavity to the gut is essential, as disturbances in the oral microbiota may influence gastrointestinal health and systemic diseases through their impact on the gut microbiome [4].
Despite its complexity and inter-individual variability, the gut microbiota exhibits well-conserved functional capacities, referred to as core functions, which are consistently present in the host. These include metabolic processes, the modulation of the immune system, and protection against pathogens [51,52].
2.2.1. Metabolic Functions
The gut microbiota regulates numerous metabolic processes, including the glucose and lipid metabolisms, and contributes additional functions to the host’s metabolism, such as the fermentation of non-digestible substrates (e.g., dietary fibres) and vitamin synthesis. Consequently, by participating in the digestion and fermentation processes that produce energy, the microbiota also ensures energy homeostasis [53,54]. These processes lead to the production or modulation of metabolites by the microbiota, which act as metabolic substrates and signalling molecules, with important implications for the host health. These include trimethylamine N-oxide (TMAO) and SCFAs, such as acetate, butyrate, and propionate, derived from the fermentation of dietary fibres in the colon [53,55].
Several bacterial genera are involved in SCFA production. For instance, Faecalibacterium prausnitzii, Roseburia spp., and Eubacterium rectale are prominent butyrate producers, whereas Bacteroides spp., Prevotella spp., Bifidobacterium spp., and Ruminococcus spp. are key contributors to acetate and propionate synthesis [56,57]. Butyrate and propionate are particularly vital in the regulation of glucose metabolism. They have been shown to stimulate the release of gut hormones, such as glucagon-like peptide (GLP-1) and peptide YY (PYY), which, in turn, increase satiety via the gut–brain axis and regulate blood glucose levels by increasing pancreatic insulin secretion and inhibiting glucagon production. Additionally, these SCFAs enhance glucose uptake in skeletal muscle and adipose tissue by upregulating GLUT-4 expression through AMP-activated protein kinase (AMPK) activation [58].
The gut microbiota is also involved in the synthesis of trimethylamine (TMA) from dietary choline and L-carnitine, lecithin, phosphatidylcholine, and betaine. TMA production has been associated with species such as Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Edwardsiella tarda, Escherichia fergusonii, Proteus penneri, and Providencia rettgeri [59,60]. Once absorbed, TMA is transported to the liver, where it is oxidised into TMAO by hepatic flavin-containing monooxygenases, particularly FMO3. TMAO has been shown to modulate the cholesterol and bile acid metabolisms by inhibiting reverse cholesterol transport and altering bile acid synthesis and composition. These changes may promote lipid accumulation in macrophages and vascular inflammation, thereby contributing to atherosclerosis development [57,59,61,62].
Simultaneously, the gut microbiota constitutes an intrinsic source of micronutrients, including vitamins. Vitamin B biosynthesis is attributed to bacteria such as Lactobacillus spp., Bifidobacterium spp., Bacteroides fragilis, and Escherichia coli, which are responsible for the synthesis of water-soluble B vitamins such as biotin, riboflavin, folate, thiamine, and pantothenic acid. These contribute to microbial stability and energy metabolism by acting as coenzymes in redox reactions [63,64,65]. Furthermore, the gut microbiota is responsible for the synthesis of the fat-soluble vitamin K, primarily menaquinones (vitamin K2), by species such as Enterococcus, Enterobacter, Eubacterium lentum, Veillonella, and Bacteroides, which is essential for the activation of clotting factors and free radical neutralisation, thereby protecting cells against oxidative damage [11,63,66].
2.2.2. Regulation of Immune Response and Protection Against Pathogenic Organisms
The microbiota also plays a crucial role in the induction, formation, and function of the host’s immune system. In turn, the immune system, composed of innate and adaptive components, develops mechanisms to maintain its symbiotic interaction with the microbiota, promoting its tolerance to beneficial commensal microorganisms and generating protective responses against pathogens [67].
Considering that the intestine is the most extensive interface between the external environment and the human body, the strategy adopted to ensure the continuity of this interaction is the maintenance of a stable and semi-permeable physical barrier that regulates host–microbe interactions [22].
Additionally, the intestinal epithelial barrier must maintain a balance between selective permeability for the passage of nutrients, electrolytes, and water from the intestinal lumen to the systemic circulation via intercellular or transcellular transport and protection against harmful components from the external environment, which are ultimately eliminated in faeces [22,68]. This barrier comprises multiple layers, including the mucus layer (which traps pathogens), the epithelial cell monolayer (sealed by tight junctions regulating paracellular permeability, which allow for the selective absorption of nutrients, electrolytes, and water, while preventing the translocation of both commensal and pathogenic microorganisms and harmful antigens), and the underlying immune elements (such as secretory immunoglobulin A (IgA), dendritic cells, and macrophages) that coordinate immune defence while maintaining immune homeostasis [69,70,71]. Various stimuli, such as microbial metabolites (e.g., SCFAs), dietary components, cytokines, and pattern recognition receptor signalling, modulate the function of this barrier. These stimuli contribute to the maintenance of barrier integrity, defined as the structural and functional cohesion of the epithelial and mucosal interface, by promoting epithelial cell renewal, upregulating the expression of tight-junction proteins, stimulating mucus secretion, and modulating immune tolerance, without compromising its semi-permeable nature, which refers to selective molecular trafficking while preventing microbial translocation [22,68,72,73].
The mucus layer, secreted primarily by Globet cells, consists of a physical barrier that harbours beneficial commensal microorganisms, such as Bacteroides spp., Faecalibacterium prausnitzii, and Akkermansia muciniphila, and contains defence proteins with immune-regulatory functions, including antimicrobial peptides (AMPs) and secretory IgA, which are secreted primarily by Paneth cells and plasma cells, respectively [74,75]. Beneath this layer lies a continuous and polarised monolayer of intestinal epithelial cells, under which the lamina propria contains immune cells such as T cells, B cells, macrophages, and dendritic cells, which provide both innate and adaptive immune responses that reinforce intestinal defence [68,76]. A schematic representation of the intestinal barrier, including the mucus layer, epithelial cell monolayer, and immune cell populations in the lamina propria, is provided in Figure 2 to illustrate these components and their spatial organisation.
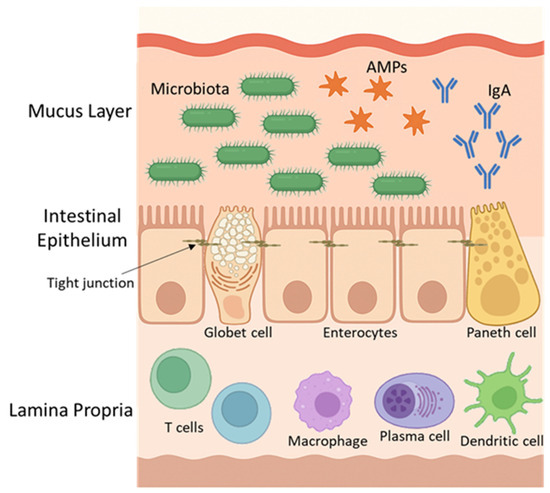
Figure 2.
Intestinal barrier structure. The barrier includes a mucus layer, with commensal bacteria, immunoglobulin A antibodies (IgA) and antimicrobial peptides (AMPs), epithelial cells with tight junctions, and immune cells in the lamina propria. Goblet cells secrete mucus; Paneth and plasma cells produce AMPs and IgA, respectively.
On the other hand, microbiota-derived SCFAs regulate immune cells by binding to G-protein-coupled receptors (GPCRs) and by altering gene expression through the reduction in histone deacetylase activity. Although the intestinal epithelial barrier separates microbes from the immune cells in the lamina propria, SCFAs, being small and soluble, can diffuse through epithelial cells via passive diffusion or be transported by specific monocarboxylate transporters (e.g., MCT1, SMCT1). Once in the lamina propria, they act on various immune cell populations. This results in decreased local inflammation, protection against pathogen infiltration, and the maintenance of the integrity of the intestinal barrier [8,77]. This process is illustrated in Figure 3.
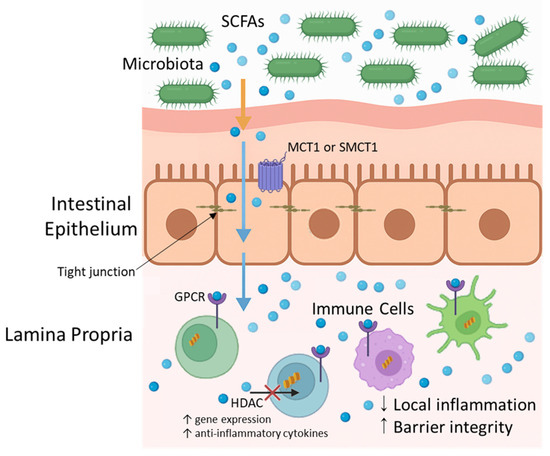
Figure 3.
Short-chain fatty acids (SCFAs) transport and immunomodulatory pathways in the intestine. SCFAs, produced by the gut microbiota through the fermentation of dietary fibres, diffuse or are transported across the intestinal epithelium via monocarboxylate transporters (e.g., MCT1, SMCT1). In the lamina propria, SCFAs interact with immune cells by binding to G-protein-coupled receptors (e.g., GPR43) or through histone deacetylase (HDAC) inhibition, promoting anti-inflammatory responses and the maintenance of the intestinal barrier.
Thus, the gut microbiota’s influence on the host’s immune system is evident, playing a critical role in maintaining immune homeostasis through the modulation of immune cell function and inflammatory responses. Disturbances in this symbiotic relationship, defined as dysbiosis, can disrupt immune tolerance and trigger chronic inflammation, which has been linked to the development of various autoimmune diseases. Understanding these mechanisms is essential to unravelling how microbiota imbalances contribute to immune dysregulation and disease pathogenesis [50,78].
2.3. Factors Influencing the Gut Microbiota
Gut microbiota composition is dynamic and can be shaped by various intrinsic and extrinsic factors, including exposure to antibiotics, dietary patterns, genetics, smoking, and physical activity, ultimately influencing the host’s health status [79].
2.3.1. Exposure to Antibiotics
The effects of antibiotic use depend on their spectrum of activity, pharmacokinetics, and pharmacodynamics, as well as the route of administration, dosage, and duration of treatment. The oral administration of antibiotics is often the preferred route, directly exposing the intestinal lumen and microbiota to their bactericidal and/or bacteriostatic action [80]. Antibiotics’ effects include alterations in the composition and function of the gut microbiota, increased susceptibility to infections, and the development of antibiotic resistance [51].
Disturbances in the composition and functions of the microbiota are often characterised by an imbalance, typically associated with a reduction in microbial diversity. This does not necessarily imply a decrease in the total number of microorganisms; in fact, microbial load may increase as antibiotic-susceptible bacteria, such as members of the Bacillota phylum, are eliminated, providing an opportunity for resistant bacteria, including some Pseudomonadota species, to proliferate. Additionally, the administration of a particular antibiotic can impair the metabolic functions of the microbiota by reducing the production of key microbial metabolites, such as SCFAs, which play a role in maintaining gut homeostasis. This disruption can lead to immune dysregulation, characterised by a decreased frequency and function of regulatory T cells (Tregs) and an increased differentiation of pro-inflammatory effector T cells, such as T helper 17 (Th17) cells, tipping the balance towards inflammation and impaired immune tolerance [81,82,83].
In terms of susceptibility to new infections, antibiotic use can lead to a reduction in the thickness of the mucus layer, facilitating bacterial invasion by newly acquired pathogens, or from the overgrowth and pathogenic behaviour of opportunistic organisms present, as observed in antibiotic-associated diarrhoea, the most severe form caused by Clostridioides difficile [81,84].
2.3.2. Diet
The composition and diversity of the gut microbiota, as well as the relative abundance of microbial metabolites, are profoundly shaped by diet, as specific nutrients exert selective pressures that favour bacterial species capable of metabolise them. As a result, changes in eating habits, as well as food scarcity or excess, can lead to transient alterations of the microbiota [85].
A diet rich in animal proteins and amino acids, such as Western diets, has pronounced effects at the level of microbial metabolism, leading to the increased production of branched-chain fatty acids and potentially toxic substances such as sulphides, ammonia, and N-nitroso compounds [86]. This type of diet also increases luminal bile acid content, favouring bile-tolerant anaerobic bacteria such as Bacteroides spp., Alistipes spp., and Bilophila spp., leading to an increase in TMAO. Additionally, this intake stimulates the synthesis of nitric oxide (•NO), a free radical with antimicrobial properties that can influence gut microbiota composition [87]. Gut bacteria, such as Lactobacillus and Bifidobacterium species, are generally susceptible to the antimicrobial effects of •NO, resulting in their decreased abundance under high-•NO conditions. Conversely, certain facultative anaerobes and nitrate-reducing bacteria, including Enterobacteriaceae family members like E. coli, are more resistant to •NO and can survive or even flourish, potentially contributing to dysbiosis [87,88,89]. Moreover, •NO in excessive concentrations may induce oxidative stress and inflammation, further impacting the gut microbial balance [90].
In contrast, the intake of plant-based proteins has been associated with an increase in beneficial bacteria such as Bifidobacterium spp. and Lactobacillus spp., along with a decrease in potentially pathogenic species such as Bacteroides fragilis and Clostridium perfringens [91].
Similarly, microbial composition and diversity are significantly affected by increased fat intake, which alters the Bacillota/Bacteroidota ratio by increasing the relative abundance of the former while reducing the latter [92]. It should also be noted that a high-fat diet indirectly influences microbiota composition by promoting an increase in bile acid secretion [93,94]. In the distal small intestine, luminal pH rises progressively from approximately 6.5 in the proximal segments to around 7.5 near the ileum. Upon reaching the large intestine, pH temporarily drops to approximately 6.0 near the caecum and then increases again toward the rectum, approaching pH 7.0 [95]. These pH shifts, along with the antimicrobial properties of unabsorbed bile acids, contribute to an inhospitable environment for acid-sensitive commensals such as Bifidobacterium spp. and Lactobacillus spp. Conversely, they favour the expansion of bile- and acid-resistant taxa, including Bilophila wadsworthia, Bacteroides spp., and Alistipes spp., which are frequently associated with pro-inflammatory profiles and metabolic dysregulation [96,97].
Conversely, a fibre-rich diet, particularly one high in plant polysaccharides, promotes the growth of Bacteroidota at the expense of Bacillota [98,99]. Bacteroidota, especially Bacteroides spp., are known for producing a variety of carbohydrate-active enzymes (CAZymes), including xylanases and pectinases, which degrade complex plant fibres. In contrast, members of the Bacillota phylum, such as Clostridium spp. and Ruminococcus spp., produce enzymes like cellulases and amylases, which also contribute to polysaccharide breakdown [100]. A higher prevalence of Bacillota compared to Bacteroidota ensures a the presence of a greater number of enzymes capable of breaking down dietary polysaccharides, leading to increased monosaccharide absorption and, consequently, greater energy extraction from the diet [87].
2.3.3. Other Factors
Other factors that have been reported to also influence the gut microbiota include genetic factors, smoking, and physical exercise [101,102,103].
The host’s genetic composition is a determining factor in how certain bacteria influence the metabolism and functions of the microbiota. Members of the same family, by sharing similar genetics, tend to have a more similar microbial community compared to unrelated individuals. For example, hosts carrying the rs651821 variant of the APOA5 gene are more likely to be colonised by the genera Lactobacillus, Sutterella, and Methanobrevibacter, which haves been associated with a higher risk of developing metabolic diseases [101].
Smoking is another relevant factor. Cigarette smoke is a complex chemical mixture, with nicotine as its main active substance. Nicotine is inhaled into the lungs, primarily absorbed by the pulmonary alveoli, but also by the GIT. Consequently, it can influence the composition of the gut microbiota by increasing the abundance of Bacteroidota while decreasing Bacillota and Pseudomonadota. On the other hand, it increases intestinal pH, which promotes the development of some pathogenic microorganisms, such as Peptococcaceae [102].
Finally, regular physical exercise, particularly moderate-intensity aerobic activities performed for 30–90 min 3–5 times per week, is also positively correlated with the composition and functions of the gut microbiota. Such exercise regimens promote increased microbial diversity and a balance between beneficial commensal microorganisms and pathogens, including the growth of beneficial bacteria such as A. muciniphila, Oscillospira, Faecalibacterium prausnitzii, Methanobrevibacter, Veillonella atypica, and Prevotella spp. Mechanistically, physical exercise enhances the production of SCFAs like butyrate, modulates immune responses by increasing IgA levels and reducing Toll-like receptor signalling, and decreases intestinal transit time, collectively contributing to improved intestinal barrier function and reduced inflammation [103,104,105].
3. Cardiovascular Diseases
The term CVD is a collective term that encompasses all types of conditions that affect the cardiovascular system, namely the heart and blood vessels [106]. CVDs remain the leading cause of mortality and morbidity worldwide. In 2019, they were responsible for an estimated 17.9 million deaths, representing 32% of global deaths, making them a major public health concern [107].
In this complex context, it is important to highlight that atherosclerosis, an often asymptomatic condition, is the underlying pathological process in most CVDs [2].
3.1. Atherosclerosis
Atherosclerosis is a chronic, slow-progressing, immune-inflammatory, and fibroproliferative disease that begins with the activation of the endothelium, a process in which endothelial cells (ECs) begin to express adhesion molecules and pro-inflammatory cytokines, facilitating the adhesion and migration of leukocytes. A cascade of events follows, resulting in the narrowing of vessels and the activation of inflammatory pathways, ultimately resulting in the formation of atherosclerotic plaques. These plaques are characterised by the accumulation of lipids, fibrous elements, and calcification within the walls of medium and large arteries, which can lead to further cardiovascular complications [2,108].
Hypertension, obesity, smoking, age, gender, family history, and dyslipidaemia are some of the risk factors for atherosclerosis. Among these, dyslipidaemia plays a particularly important role in the incidence and progression of atherosclerosis [109]. It is characterised by high plasma levels of cholesterol, triglycerides, or both, which are transported by lipoproteins. Based on their density and size, lipoproteins are classified into five categories: very-low-density lipoproteins (VLDLs), low-density lipoproteins (LDLs), intermediate-density lipoproteins (IDLs), high-density lipoproteins (HDLs), and chylomicrons. Each category has specific functions in the lipid metabolism, and together they contribute to maintaining a healthy vasculature [110]. In this context, LDLs are considered to be the most atherogenic lipoproteins, as they alter the properties of the endothelium, upregulate adhesion molecules, promote monocyte recruitment and differentiation into macrophages, and induce the proliferation of smooth muscle cells [2]. To provide a better understanding of the role of circulating lipoproteins in atherogenesis, Figure 4 summarises the main metabolic pathways of lipoproteins, including the exogenous, endogenous, and reverse cholesterol transport pathways.
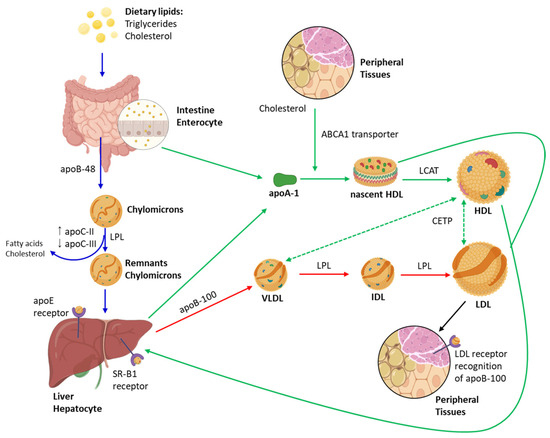
Figure 4.
Overview of lipoprotein metabolism: exogenous pathway, endogenous pathway, and reverse cholesterol transport. This schematic illustrates the major pathways of the human lipoprotein metabolism, encompassing its synthesis, transformation, clearance, and the key proteins involved in lipid transport and regulation. Blue arrows represent the exogenous pathway, which begins with the absorption of dietary lipids in the small intestine and the assembly of chylomicrons, rich in triglycerides and apolipoprotein B-48 (ApoB-48). Chylomicrons deliver triglycerides to peripheral tissues, such as adipose and muscle, through the action of lipoprotein lipase (LPL), which is activated by apolipoprotein C-II (ApoC-II). Apolipoprotein C-III (ApoC-III) acts as an inhibitor of LPL activity and the hepatic clearance of triglyceride-rich particles, thereby modulating plasma triglyceride levels. After triglyceride depletion, chylomicron remnants enriched in apolipoprotein E (ApoE) are taken up by the liver. Red arrows represent the endogenous pathway, in which the liver synthesises and secretes very-low-density lipoproteins (VLDL), containing ApoB-100 and triglycerides. As VLDL particles are hydrolysed by LPL, they are progressively transformed into intermediate-density lipoproteins (IDL) and, subsequently, into low-density lipoproteins (LDL). LDL particles, enriched in cholesterol, deliver cholesterol to peripheral tissues via LDL receptors that specifically recognise ApoB-100. Green arrows indicate the reverse cholesterol transport pathway, a key mechanism for maintaining cholesterol homeostasis. High-density lipoprotein (HDL) is synthesised in a nascent, discoidal form (pre-β HDL) by the liver and intestine, incorporating ApoA-I. HDL acquires cholesterol from peripheral tissues via the ATP-binding cassette transporter A1 (ABCA1). Cholesterol is esterified by lecithin–cholesterol acyltransferase (LCAT), leading to the formation of mature, spherical HDL. Cholesterol is returned to the liver via interactions with the scavenger receptor class B type I (SR-B1). The cholesteryl ester transfer protein (CETP) mediates the bidirectional exchange of cholesteryl esters and triglycerides between HDL and ApoB-containing lipoproteins (VLDL and LDL), contributing to lipid redistribution among lipoprotein classes.
The progression of atherosclerosis can be divided into four distinct phases, namely endothelial dysfunction, the formation of fatty streaks, lesion progression, and, finally, plaque rupture and thrombus formation, which can lead to acute cardiovascular events such as myocardial infarction and stroke (Figure 5) [110].
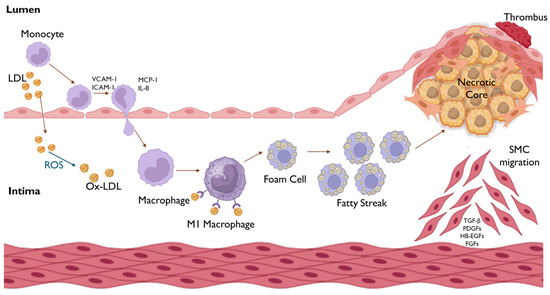
Figure 5.
Formation of the atherosclerotic plaque and progression of atherosclerosis. The process begins with endothelial dysfunction, associated with increased permeability, that allows for the accumulation of LDLs in the subendothelial space, where they undergo oxidative modification by reactive oxygen species (ROS) to form Ox-LDL. These modified LDLs further exacerbate endothelial activation, inducing the expression of adhesion molecules (VCAM-1, ICAM-1) that promote the tight adhesion of circulating monocytes to the endothelium. Subsequently, chemokines, such as MCP-1 and IL-8, mediate the transmigration of monocytes into the tunica intima, where they differentiate into pro-inflammatory M1 macrophages, which internalise Ox-LDL, transforming into foam cells. The accumulation of these foam cells leads to the formation of fatty streaks, which develop into a necrotic core, contributing to the development of the atherosclerotic plaque. Eventually, plaque rupture may result in thrombus formation, obstructing blood flow.
3.1.1. Endothelium
The vascular endothelium consists of a monolayer of specialised cells, known as ECs, connected by tight junctions. These ECs are covered by a dense layer of glycoproteins and/or glycolipids, known as the glycocalyx, forming a barrier that lines the lumen of blood vessels. This barrier serves as the first line of defence against circulating molecules, cells, and pathogens in the bloodstream. In the large vessels, the endothelium, together with collagen and elastic fibres, forms the tunica intima. The tunica media lies beneath, and it is predominantly composed of vascular smooth muscle cells (VSMCs), collagen, and elastic tissue, providing elasticity and contractility to the vessels. Surrounding this layer is the tunica adventitia, which is mainly composed of a dense connective tissue matrix, offering structural support and protection to the blood vessel [108,111,112].
Given its location, the endothelium, specifically the ECs, continuously senses hemodynamic and biochemical changes in the bloodstream and transmits signals to the underlying layers of the vascular wall. Through their ability to produce effector molecules that regulate thrombosis, vascular tone, vascular remodelling, and inflammatory response, ECs act as key regulators of vascular homeostasis [112,113]. A compromise of this regulatory capacity, triggered by several factors, including turbulent and oscillatory blood flow (low shear stress), oxidised LDL (ox-LDL), and oxidative stress, results in endothelial dysfunction, a crucial event in the pathogenesis of atherosclerosis [112,114].
3.1.2. Endothelial Dysfunction
Among the several factors implicated in endothelial dysfunction, low shear stress has particular relevance. In fact, the atherosclerotic lesions mainly develop in regions of arterial branching or curvature, where blood flow is reduced, being turbulent and oscillatory with low shear stress [115,116,117]. In these regions, ECs adopt a polygonal shape, lose their typical alignment with the direction of flow, and undergo an increase in apoptosis rate. This leads to an increase in the ECs’ turnover and promotes the accumulation of senescent cells, which are more adhesive to monocytes, produce less •NO, and generate more anion superoxide (O2•−). Together, these alterations compromise the functionality and integrity of the endothelial barrier [116]. Furthermore, the haemodynamic conditions in these regions of the vasculature increase the residence time of the circulating molecules, LDL and blood cells, favouring their interaction with the endothelium. This fact, combined with the increased permeability of endothelium, promotes the infiltration and accumulation of LDLs in the subendothelial matrix, where they form insoluble complexes with proteoglycans and are oxidised by reactive oxygen species (ROS) to ox-LDL [118]. The accumulation of LDL is greater the higher its plasma levels are. Ox-LDL further aggravates endothelial dysfunction [117].
3.1.3. Formation of Fatty Streaks
Endothelial stimulation, also known as type II endothelial activation, occurs in response to ox-LDL and involves the overactivation of the NF-κB inflammatory pathway within ECs. This leads to the increased expression of pro-inflammatory cytokines and chemokines (MCP-1 and IL-8), vascular (VCAM-1) and intercellular (ICAM-1) adhesion molecules for monocytes, and E-selectin and P-selectin [108,119].
The recruitment of monocytes from the bloodstream to the affected area of the endothelium is the first step in a cascade of subsequent events. Initially, monocytes are captured and roll along the endothelium surface, a process mediated by P-selectin, followed by their tight adhesion through their interactions with the endothelial VCAM-1 and ICAM-1. Chemokines, including CXCL1, CXCL2, CXCL4, and CCL5, present in the endothelial surface, activate monocytes as they roll, increasing their adhesiveness [108]. Subsequently, MCP-1 and IL-8 induce the transmigration of monocytes to the subendothelial space, where they mature and differentiate into macrophages, which can polarise into M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotypes, with the M1 predominating when the disease progresses. However, due to their sensitivity and plasticity, these macrophages can switch between M1 and M2 phenotypes in response to new signals [120].
M1 macrophages produce huge amounts of pro-inflammatory cytokines and chemokines, as well as reactive oxygen and nitrogen species (RONS) such as •NO and O2•−, contributing to an oxidative and inflammatory environment. They also express scavenger receptors, including CD36, SR-AI, and LOX-1, which recognise and internalise the modified forms of LDL, particularly ox-LDL. It is important to note that the activity of these receptors is not negatively regulated by the intracellular concentration of cholesterol, thus leading to a deregulated and exacerbated uptake of ox-LDL. Once internalised, ox-LDLs are degraded in lysosomes, and excess free cholesterol is transported to the endoplasmic reticulum (ER), where it is esterified by acyl-CoA acyltransferase (ACAT) into cholesterol esters, which are stored in lipid droplets in the cytoplasm or which are associated with the ER, a characteristic feature of foam cells [119,121]. Cholesterol esters are hydrolysed, and the resulting free cholesterol suffer efflux from macrophages to HDL via transporters like ABCA1. However, in the atherosclerotic lesions, the pro-inflammatory environment impairs these efflux systems. Therefore, the deregulated uptake of ox-LDL by macrophages, associated with the impaired efflux of cholesterol, promotes the formation and accumulation of foam cells, a key feature in atherosclerosis [122,123].
The excessive lipid uptake by macrophages sustains the inflammatory response, which is further exacerbated by the continuous activation of NF-κB signalling pathways by ox-LDL. This vicious cycle sustains EC activation, monocyte recruitment, and foam cell accumulation, ultimately leading to the development of fatty streaks [124]. In addition, VSMCs in the tunica intima can also internalise ox-LDL through their scavenger receptors (SR-A, CD36, LOX-1), further contributing to the growth of fatty streaks [125].
Fatty streaks are an important precursor; however, they generally do not cause immediate clinical complications and may even spontaneously regress [119].
3.1.4. Lesion Progression
The progression of fatty streaks into more advanced fibrolipidic lesions, whose regression is less likely, occurs through the infiltration and proliferation of VSMCs from the tunica media into the tunica intima. This migration is induced by growth factors secreted by foam cells derived from macrophages, as well as by the activated VSMCs themselves. These include platelet-derived growth factors, fibroblast growth factors, heparin-binding epidermal growth factors, and matrix metalloproteinases (MMPs) [121].
Within the tunica intima, VSMCs proliferate and synthesise an extracellular matrix composed of collagen, proteoglycans, and elastin, which forms a fibrous cap over the foam cells. This cap provides structural support to the plaque and separates the necrotic core, a hypocellular and lipid-rich region, from the vessel lumen, where coagulation factors and circulating platelets are present. The thickness of this fibrous cap is directly correlated with plaque vulnerability [108,126].
As the plaque develops, there is an increase in apoptosis among both macrophages and VSMCs in response to the oxidative and inflammatory microenvironment and activation of death-signalling receptors [127]. Due to the excessive apoptosis, the clearance process (efferocytosis) becomes insufficient, leading to secondary necrosis. The necrotic cells release intracellular inflammatory and oxidative contents, contributing to the expansion of the necrotic core. In addition, apoptotic and necrotic cells release tissue factor, which, together with oxidised lipids, increases the thrombogenicity of the necrotic core [108,127].
3.1.5. Plaque Rupture and Thrombus Formation
Inflammation plays a critical role throughout all stages of atherosclerotic plaque development, and plaque rupture is no exception. Inflammatory processes promote the instability of the fibrous cap that overlays the foam cells [128].
Some pro-inflammatory cytokines, such as interferon-gamma, inhibit collagen synthesis. In addition, the apoptosis of VSMCs reduces the production of the extracellular matrix, further decreasing collagen levels while simultaneously increasing the expression of MMPs. These combined factors contribute to plaque vulnerability. Consequently, a vulnerable plaque is more prone to rupture due to the action of ROS, which promote MMP release and the subsequent degradation of the fibrous cap. When the cap fissures or ruptures, the subendothelial space is exposed to circulating blood, triggering a coagulation process [129].
Initially, platelets adhere to exposed subendothelial collagen and promote the recruitment of additional platelets to this area to facilitate healing. Simultaneously, pro-thrombotic components within the plaque core, particularly tissue factor, come into contact with plasma coagulation factor VII, activating the coagulation cascade. This cascade results in the formation of the intermediate product, thrombin, and ultimately leads to the production of fibrin [130].
Fibrin is an insoluble protein that forms a meshwork that binds to platelets, creating a thrombus, which consists of a stable and organised structure whose purpose is to protect the site of injury. However, it is important to note that if the thrombus detaches from the arterial wall, it can lead to serious cardiovascular complications [128,130]. These include myocardial infarction, stroke, and peripheral artery disease, all of which highlight the clinical significance of atherosclerosis and the importance of identifying modifiable risk factors, such as those related to gut microbiota [108,131,132,133,134].
4. Interaction Between the Gut Microbiota and Cardiovascular Diseases
The gastrointestinal system is considered to be the largest endocrine organ in the human body due to its ability to produce a wide range of bioactive metabolites, such as SCFAs, TMA—which is converted by hepatic FMO3 into TMAO—secondary bile acids (e.g., deoxycholic acid, lithocholic acid), and tryptophan-derived indoles. These compounds can either be metabolised by the host’s enzymes, such as sulfotransferases, monooxigenases, or conjugating enzymes, into signalling molecules, or directly act as such [135]. For instance, SCFAs act via G protein-coupled receptors (e.g., GPR41 and GPR43), while indole derivatives modulate the aryl hydrocarbon receptor (AhR) pathway [135,136,137,138]. These molecules are absorbed by the intestinal epithelium through various mechanisms, including passive diffusion (e.g., for lipophilic bile acids), active transport (e.g., via monocarboxylate transporters for SCFAs), and carrier-mediated uptake. Upon entering the bloodstream, they can exert effects on various organs and systems, thereby influence multiple host metabolic pathways [135].
Shifts in microbial composition, characterised by an increased abundance of pro-inflammatory taxa, such as members of the Enterobacteriaceae family (including Escherichia-Shigella), Desulfovibrio spp., Collinsella spp., Eggerthella spp., and Streptococcus spp., along with a depletion of beneficial butyrate-producing genera, such as Faecalibacterium prausnitzii, Roseburia spp., Subdoligranulum spp., and Eubacterium rectale, have been strongly associated with the pathogenesis of CVDs. These alterations are frequently accompanied by reduced microbial diversity and changes in the relative proportions of dominant bacterial phyla. In particular, shifts in the Bacillota/Bacteroidota ratio have been linked to metabolic disturbances, such as increased energy harvest, low-grade inflammation, and adiposity, which collectively contribute to cardiovascular risk. Although some studies report a higher Bacillota/Bacteroidota ratio in obese and hypertensive individuals, findings are not universally consistent, highlighting the complexity of microbiota–host interactions in CVD development. Conversely, protective genera, such as Akkermansia, Blautia, and Lactobacillus, are often depleted in individuals with CVD. Such compositional shifts may modulate cardiovascular risk by influencing lipid metabolism, gut barrier integrity, immune activation, and the production of bioactive metabolites like TMAO, ultimately contributing to vascular dysfunction and atherogenesis [90]. Furthermore, bacterial components, such as lipopolysaccharide (LPS), a constituent of the outer membrane of Gram-negative bacteria, can trigger immune activation via the NF-κB pathway, promoting foam cell formation and atherogenesis [135,139]. The accompanying endothelial dysfunction, aggravated by elevated levels of ROS, further amplifies vascular inflammation, contributing to plaque formation and thrombosis [135].
While bacterial-derived metabolites have been extensively studied in the context of atherosclerosis, emerging evidence suggests that other non-bacterial components of the gut microbiota, including fungi, viruses, and bacteriophages, may also contribute to cardiovascular pathology, albeit through distinct mechanisms [16,20,140,141,142].
4.1. Trimethylamine N-Oxide
TMAO is the main diet-derived metabolite, linked to an increased risk of developing atherosclerosis, thrombosis, and stroke. This compound is formed from TMA, a precursor produced in the colon by the action of bacteria from the phylum Bacillota, Pseudomonadota, and Actinomycetota, whose species have been previously mentioned [143]. These bacteria convert dietary quaternary amines, such as L-carnitine and choline, present in foods like red meat and eggs, into TMA. Once produced, TMA is absorbed and transported via the portal vein to the liver, where it is converted into TMAO by the FMO3 enzyme. In addition, this metabolite is found in fish and seafood, so their consumption represents an exogenous and direct source of TMAO [144,145]. Once in circulation, TMAO contributes to endothelial dysfunction, promotes foam cell formation, and enhances platelet activity, which, together, increase the risk of thrombus formation [143].
Endothelial dysfunction is an early and critical step in atherosclerosis. In this context, TMAO is known to activate the NF-κB signalling pathway, partly through a mechanism involving high-mobility group box 1 (HMGB1), a key inflammatory mediator [146,147]. HMGB1 can be released extracellularly and interacts with the receptor for advanced glycation end-products (RAGE), initiating downstream pro-inflammatory signalling cascades. This interaction promotes the activation of NF-κB, leading to the upregulation of pro-inflammatory genes that encode adhesion molecules, chemokines, and inflammatory cytokines. Additionally, HMGB1/RAGE signalling can activate the NLRP3 inflammasome, further exacerbating the inflammatory response. Thus, TMAO-induced HMGB1 release, and the subsequent RAGE activation, constitute an important pathway, contributing to endothelial inflammation and dysfunction in atherosclerosis [146,147,148].
This also decreases the expression of tight junction proteins, such as occludin and zonula occludens-2 (ZO-2), as well as vascular endothelial cadherin in ECs, which increases permeability to LDL, a particle formed in the bloodstream from the metabolic conversion of VLDL secreted by the liver, as shown in Figure 4. Additionally, TMAO upregulates VCAM-1 and ICAM-1 expression, promoting monocyte adhesion [146].
Concerning foam cell formation, macrophages play a central role by accumulating lipoproteins. In this context, several studies support a crucial role of TMAO in promoting foam cell development [149,150]. TMAO, which structurally resembles ox-LDL, promotes monocyte differentiation into macrophages and increases the expression of scavenger receptors like CD36 and SRA-1. More recently, Luo and colleagues demonstrated that TMAO downregulates the expression of Nrf2 and its downstream effectors, heme oxygenase-1 and glutathione peroxidase 4, leading to oxidative stress and the reduced expression of cholesterol efflux proteins, such as ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1), thereby promoting lipid accumulation in foam cells [151]. In addition, TMAO has been associated with an inhibition of the reverse cholesterol transport, a key protective mechanism against atherosclerosis, as depicted in Figure 4 [138,151,152,153]. Reverse cholesterol transport involves the efflux of excess cholesterol from peripheral cells, such as macrophages, to apolipoprotein A-I (ApoA-I), initiating the formation of nascent HDL particles via their interactions with ABCA1 and ABCG1. These HDL particles mature and deliver cholesterol to the liver through scavenger receptor class B type 1 (SR-B1), either directly or indirectly, via the cholesteryl ester transfer protein (CETP). TMAO interferes with this pathway by downregulating the expression of LXRα, a transcriptional regulator of ABCA1 and ABCG1, thereby impairing cholesterol efflux from macrophages and HDL biogenesis. This disruption compromises reverse cholesterol transporter efficiency and contributes to lipid accumulation and foam cell formation within atherosclerotic plaques [138,153,154].
Finally, platelet activation and aggregation precede thrombus formation. TMAO enhances platelet reactivity by increasing intracellular calcium release and platelet activation. Moreover, TMAO exerts a pro-thrombotic effect by activating the pathway that leads to the expression of endothelial tissue factor, the membrane receptor for factor VII, which initiates the extrinsic coagulation cascade [155,156].
4.2. Bile Acids
Bile acids are amphipathic molecules synthesised in the liver from cholesterol and which, subsequently, are modified by the gut microbiota into secondary bile acids. They are stored in the gallbladder and are released into the small intestine after food intake, with their main functions being the emulsification of dietary fats and the promotion of intestinal absorption of lipids and fat-soluble vitamins. Bile acids can be classified into primary and secondary acids based on their structure, with primary bile acids being metabolised into secondary bile acids by enzymes derived from the gut microbiota [157,158].
Primary bile acids, such as cholic acid and chenodeoxycholic acid, result from cholesterol oxidation by cholesterol 7α-hydroxylase (CYP7A1), followed by conjugation with glycine or taurine by bile acid-CoA:amino acid N-acyltransferase (BAAT). Subsequently, through the action of bile salt hydrolase (BSH), primary bile acids are hydrolysed and deconjugated, ready to be metabolised by the gut microbiota through various mechanisms, including 7α-dehydroxylation, dehydrogenation, and epimerisation, leading to the formation of secondary bile acids such as deoxycholic acid and lithocholic acid (Figure 6) [159,160].
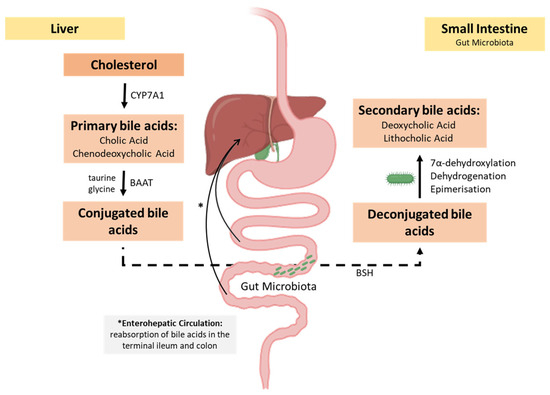
Figure 6.
Overview of bile acid metabolism and microbial transformation. In the liver, primary bile acids are synthesised from cholesterol through the action of cholesterol 7α-hydroxylase (CYP7A1) and are conjugated with glycine or taurine via bile acid-CoA:amino acid N-acyltransferase (BAAT). These conjugated bile acids are secreted into the intestine, where they may be hydrolysed by microbial bile salt hydrolase (BSH), resulting in unconjugated bile acids. The gut microbiota further convert these into secondary bile acids through 7α-dehydroxylation, dehydrogenation, and epimerisation. Conjugated bile acids are actively reabsorbed in the terminal ileum and return to the liver via the portal vein, completing the enterohepatic circulation. A smaller fraction of secondary bile acids is passively reabsorbed in the colon, while the remainder is excreted in faeces.
Bile acids play a protective role in the development of atherosclerosis, as they act as signalling molecules for a range of physiological processes mediated by the farnesoid X receptor (FXR) and the G-protein-coupled bile acid receptor (TGR5) [161]. FXR plays a central role in the regulation of the glucose, lipid, and bile acid metabolisms. Given its high expression in the GIT, it acts as a sensor of bile acid levels in hepatocytes and enterocytes, thus regulating the transcription of genes involved in bile acid synthesis, conjugation, and transport, including CYP7A1. The activation of FXR in ECs positively regulates endothelial nitric oxide synthase, enhancing nitric oxide production, which promotes vasodilation, reduces endothelin-1 expression, a potent vasoconstrictive peptide, and modulates angiotensin II receptors, inhibiting the inflammation and migration of VSMCs, a key factor limiting the progression of atherosclerotic lesions, as these cells in the intima produce the extracellular matrix (Figure 6) [137,160,162].
Additionally, FXR activation also increases faecal cholesterol excretion, thereby lowering plasma cholesterol levels. This contributes to reducing the amount of circulating LDL, limiting their retention in the endothelial barrier and, subsequently, decreasing ox-LDL formation, as shown in Figure 6 [163,164].
Furthermore, there is a reduction in pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which initiate monocyte recruitment, inhibiting the formation of fatty streaks [165,166,167]. On the other hand, as illustrated in Figure 6, FXR expression in hepatocytes, which can be upregulated by bile acids, regulates FMO3, the key enzyme in TMAO synthesis, the metabolite that actively contributes to the formation and development of atherosclerotic lesions, as previously explained [168].
The activation of the bile acid membrane receptor TGR5 also plays a protective role in atherosclerosis by interfering with the crucial mechanisms underlying the formation of fatty streaks. In this context, TGR5 receptor activation can counteract this process by acting in both ECs and macrophages. In ECs, it inhibits the expression of VCAM-1 and ICAM-2, thereby limiting the recruitment and adhesion of monocytes, while in macrophages, it inhibits the NF-κB inflammatory pathway, thus reducing the production of pro-inflammatory mediators (TNF-α, IL-1β, IL-6) and downregulates the expression of CD36 and SR-A receptors, which are involved in lipid uptake. As a result, macrophages take up fewer lipids, limiting their accumulation in lipid droplets and ultimately preventing foam cell formation and the development of fatty streaks, as represented in Figure 7 [159,169].
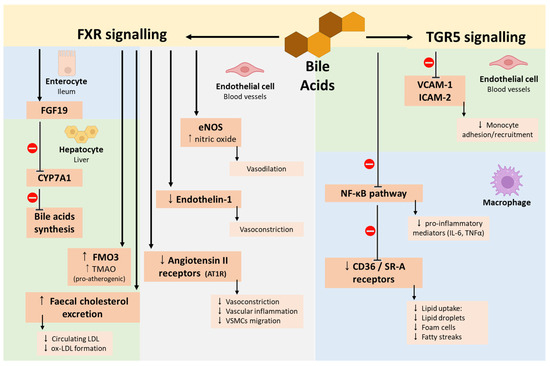
Figure 7.
Bile acid modulation of farnesoid X receptor (FXR) and G-protein-coupled bile acid receptor (TGR5) signalling pathways. FXR activation in enterocytes induces FGF19, which inhibits CYP7A1 expression and reduces bile acid synthesis. In hepatocytes, FXR promotes FMO3 expression, increasing TMAO production, upregulates bile acid transporters, and enhances faecal cholesterol excretion. In ECs, FXR activation upregulates eNOS, leading to NO-mediated vasodilation, and downregulates endothelin-1 and angiotensin II receptor expression, reducing vasoconstriction, vascular inflammation, and VSMC migration. TGR5 activation in ECs downregulates VCAM-1 and ICAM-2, reducing monocyte adhesion and recruitment. In macrophages, TGR5 activation inhibits the NF-κB pathway and downregulates CD36 and SR-A expression, decreasing lipid uptake, foam cell formation, and fatty streak development. Abbreviations: CD36/SR-A, scavenger receptors; CYP7A1, cholesterol 7α-hydroxylase; ECs, endothelial cells; eNOS, endothelial nitric oxide synthase; FGF19, fibroblast growth factor 19; FMO3, flavin-containing monooxygenase 3; FXR, farnesoid X receptor; ICAM-2, intercellular adhesion molecule-2; NF-κB, nuclear factor kappa B; NO, nitric oxide; TGR5, G protein-coupled bile acid receptor 1; TMAO, trimethylamine-N-oxide; VCAM-1, vascular cell adhesion molecule-1; VSMC, vascular smooth muscle cell.
4.3. Short-Chain Fatty Acids
The human digestive system cannot break down complex carbohydrates, particularly dietary fibres, which are not digested or absorbed in the small intestine. Thus, in the colon, the gut microbiota is responsible for fermenting these carbohydrates, resulting in the production of SCFAs. SCFAs consist of carboxylic acids with aliphatic chains ranging from one to six carbon atoms, with the three main ones being acetic acid (C2), propionic acid (C3), and butyric acid (C4), while valeric acid (C5) and hexanoic acid (C6) are less abundant. Bacteria belonging to the Bacteroidota phylum are primarily responsible for producing acetic acid and propionic acid, whereas butyric acid mainly arises from the metabolism of bacteria of the Bacillota phylum [170,171]. The main precursor of SCFAs is pyruvate, which is converted into acetyl-CoA. As illustrated in Figure 8, pyruvate is generated through the glycolytic pathway from hexoses and deoxy-hexoses, as well as through the pentose–phosphate pathway from pentoses. These monosaccharides are the products of the microbial hydrolysis of indigestible carbohydrates [172].
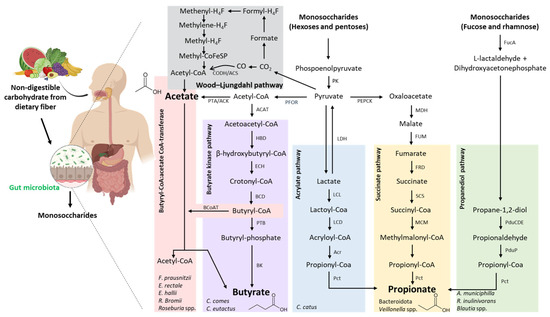
Figure 8.
Short-chain fatty acid biosynthesis by the gut microbiota: major pathways, the producing bacteria, and the enzymes involved. Non-digestible carbohydrates from the diet reach the colon, where they are metabolised by the gut microbiota into monosaccharides. These are further fermented into SCFAs, mainly acetate, butyrate, and propionate, through five major microbial pathways. The Wood–Ljungdahl pathway involves the fixation of CO2 into acetyl-CoA and, subsequently, acetate, via a series of reactions involving folate-bound intermediates. The butyrate synthesis pathway proceeds from acetyl-CoA through intermediates such as acetoacetyl-CoA, β-hydroxybutyryl-CoA, and butyryl-CoA, which is finally converted to butyrate either via the butyryl-CoA:acetate CoA-transferase route or the butyrate kinase route. The acrylate pathway converts lactate to propionate via lactoyl-CoA and acryloyl-CoA. The succinate pathway begins with the fermentation of hexoses to succinate, which is then converted to propionate through intermediates including succinyl-CoA and methylmalonyl-CoA. Finally, the propanediol pathway involves the conversion of deoxy sugars, such as fucose and rhamnose, into propionate via intermediate compounds like 1,2-propanediol and propionaldehyde. The representative microbial species or genera involved in each pathway are indicated, highlighting the functional diversity of the gut microbiota in SCFA production. Abbreviations: ACAT, Acetoacetil-CoA thiolase; ACK, acetate kinase; Acr, acryloyl-CoA reductase; BCD, butyryl-CoA dehydrogenase; BcoAT, butyryl-CoA:acetate CoA-transferase; BK, butyrate kinase; CODH/ACS, carbon monoxide dehydrogenase/acetyl-CoA synthase; ECH, enoyl-CoA hydratase (crotonase); FRD, fumarate reductase; FucA, L-fuculose-1-phosphate aldolase; FUM, fumarase; HBD, β-hydroxybutyryl-CoA dehydrogenase; LCD, lactoyl-CoA dehydratase; LCL, lactoyl-CoA ligase; LDH, lactate dehydrogenase; MCM, methylmalonyl-CoA mutase; MDH, malate dehydrogenase; Pct, propionate CoA-transferase; PduCDE, propanediol dehydratase complex; PduP, propionaldehyde dehydrogenase; PEPCK, phosphoenolpyruvate carboxykinase; PFOR, pyruvate:ferredoxin oxidoreductase; PK, pyruvate kinase; PTA, phosphate acetyltransferase; PTB, phosphotransbutyrylase; SCS, succinyl-CoA synthetase.
Butyric acid is the main energy source for colonocytes and has two endogenous metabolic production pathways. The first pathway involves the phosphorylation of butyryl-CoA, resulting from the reduction in acetoacetyl-CoA, a combination of two acetyl-CoA molecules, to butyryl-phosphate, which is then converted into butyrate by the action of butyrate kinase (BK), a pathway characteristic in certain Coprococcus species. In the second and main pathway, the CoA fraction of butyryl-CoA is transferred to exogenous acetate via butyryl-CoA:acetate CoA transferase (BCoAT), leading to the formation of butyrate and acetyl-CoA. This pathway is utilised by bacteria such as Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii, Ruminococcus bromii, and Roseburia spp. (Figure 8) [173,174].
Propionic acid, like butyric acid, can be synthesised via three main pathways (Figure 2). One is the propanediol pathway, which converts deoxy-hexoses into propionic acid through the action of propionaldehyde dehydrogenase (PduP)—a route commonly found in bacteria of the Lachnospiraceae family. The second is the succinate pathway, which is considered to be the predominant route for propionate production in the human colon. It is mainly carried out by bacteria from the Bacteroidota phylum and involves the conversion of phosphoenolpyruvate into succinate, which is then metabolised into propionate through intermediate steps involving methylmalonyl-CoA and propionyl-CoA [175]. The third is the acrylate pathway, in which lactate is converted into propionate through a series of steps involving lactoyl-CoA and acryloyl-CoA intermediates, catalysed by enzymes such as acryloyl-CoA reductase (Acr), a mechanism characteristic of Coprococcus catus [176].
Acetate production can also occur in two ways: through the Wood–Ljungdahl pathway, by which acetogenic bacteria synthesise it from hydrogen, carbon dioxide, or formic acid, or, more predominantly, through the decarboxylation of pyruvate into acetyl-CoA, which is then hydrolysed into acetate [177].
SCFAs, by binding to G-protein-coupled receptors such as free fatty acid receptors (FFAR) 2 and 3 (also known as GPR41 and GPR43, respectively), play regulatory functions in the cellular metabolism of fatty acids, glucose, and cholesterol. FFAR2 and FFAR3 are primarily expressed in intestinal epithelial cells, immune cells (such as neutrophils and macrophages), and adipose tissue, where they mediate anti-inflammatory and metabolic effects. Therefore, a dysbiotic gut microbiota, characterised by reduced SCFA production, is associated with impaired signalling through these receptors [135,178].
These carboxylic acids also play an important role in regulating immune cell activity within the intestinal mucosa and in preserving epithelial integrity. This is achieved by reducing the pH of the colon, which inhibits the growth of pathogenic bacteria, and by the expression of tight junction proteins. In this way, butyric acid is considered to be the primary positive regulator of genes encoding these proteins. It also stimulates the expression of mucin 2 (MUC2), which strengthens the mucus barrier against pathogenic agents, and enhances AMP production, both essential components of the host’s first defence line [179].
In the case of atherosclerosis, SCFAs, particularly butyrate and propionate, exhibit protective effects through multiple mechanisms, as demonstrated in both in vitro and in vivo studies. In vitro experiments using human umbilical vein endothelial cells (HUVECs) have shown that these SCFAs inhibit NF-κB activation and decrease the expression of pro-inflammatory cytokines such as TNF-α and IL-6, primarily through the inhibition of histone deacetylases (HDACs). This modulation leads to the expression of IL-10, an anti-inflammatory cytokine, and reduced expression of vascular adhesion molecules like VCAM-1 and ICAM-1, which are critical for monocyte adhesion and early plaque development. In addition to this HDAC-dependent mechanism, SCFAs, particularly butyrate, can also activate intracellular signalling pathways by binding to FFAR2 on leukocytes. This interaction leads to AMPK activation, which stimulates PGC-1α and its downstream effector PPAR-α, further contributing to NF-κB inhibition and the suppression of ICAM-1 and VCAM-1 expression. These converging anti-inflammatory pathways underscore the multifaceted protective role of SCFAs in atherosclerosis [159,178,180,181,182]. In vivo studies using apolipoprotein E-deficient (ApoE−/−) mice, a well-established model for atherosclerosis, have shown that dietary supplementation with butyrate attenuates atherosclerotic lesion development, decreases macrophage infiltration, and improves endothelial function. These outcomes are associated with the downregulation of oxidative stress pathways, including the suppression of NADPH oxidase activity and inhibition of the NLRP3 inflammasome [159,182,183,184,185].
Regarding cholesterol metabolism, propionic acid is a potent inhibitor of cholesterol synthesis, while the three main SCFAs increase hepatic cholesterol uptake from the blood and accelerate its excretion, contributing to a reduction in plasma cholesterol levels. Additionally, SCFAs can regulate lipolysis and adipogenesis. Specifically, acetic acid and propionic acid inhibit endogenous lipolysis, while propionic acid regulates extracellular lipolysis by promoting lipoprotein lipase expression, resulting in decreased plasma lipid levels. Both of these metabolic processes contribute to reducing the risk factors associated with the development and progression of atherosclerosis [186].
4.4. Tryptophan Metabolites
Tryptophan is an essential amino acid that cannot be synthesised endogenously and must, therefore, be acquired through dietary protein intake [187]. The conversion of tryptophan to indole is an important metabolic process, mediated mainly by the enzyme tryptophanase, which is expressed in many Gram-negative, as well as Gram-positive, bacterial species, including E. coli, Clostridium spp., and Bacteroides spp. [188].
While this section focuses on the microbiota-dependent tryptophan metabolism, it is important to acknowledge that host-mediated pathways, such as the kynurenine pathway, also contribute to the pathogenesis of atherosclerosis. The enzyme indoleamine 2,3-dioxygenase (IDO1), which catalyses the first step in this pathway, is induced by inflammation and has been implicated in both pro- and anti-atherogenic processes. Although the microbiota may influence systemic tryptophan availability and thus indirectly affect the kynurenine metabolism, the mechanistic interactions between these axes remain incompletely understood and lie beyond the scope of the present discussion [189]. In this context, several studies have demonstrated that, through several other metabolic pathways, intestinal microbial species produce not only indole, but also other tryptophan catabolites. For instance, Clostridium sporogenes converts tryptophan into tryptamine, indolelactic acid (ILA), and indolepropionic acid (IPA). Similarly, some species of the genus Peptostreptococcus, including P. russellii, P. anaerobius, and P. stomatis, are able to convert tryptophan into indoleacrylic acid (IA) and IPA. Additionally, Lactobacillus species convert tryptophan into indole-3-aldehyde (IAld) and ILA via the action of aromatic amino acid aminotransferase and indolelactic acid dehydrogenase. Ruminococcus gnavus also metabolises tryptophan, producing tryptamine through the enzyme tryptophan decarboxylase. Several Bacteroides species, along with Clostridium bartlettii, are capable of producing ILA and indole-3-acetic acid (IAA), whereas Bifidobacterium species primarily generate ILA. Moreover, the metabolite 3-methylindole (skatole), known for its strong odour, is produced by Bacteroides and Clostridium species via the decarboxylation of IAA [187,190]. These compounds have emerged as key modulators of vascular inflammation, oxidative stress, and endothelial function [189], primarily through their interaction with host receptors such as the AhR, by modulating the expression of genes involved in xenobiotic metabolism, inflammation, and barrier integrity [191].
AhR is a ligand-dependent transcription factor expressed in a variety of cell types, including endothelial cells, epithelial cells, and several subsets of immune cells, that binds a variety of endogenous and exogenous compounds, including indole metabolites produced by the gut microbiota. In the context of atherosclerosis, AhR activation by tryptophan metabolites can have both positive and negative effects, depending on the specific metabolite, its concentration, and the cells or tissue involved [136]. Therefore, tryptophan metabolites, such as IAld, ILA, and IPA, have been shown to have atheroprotective effects, contributing to the maintenance of vascular homeostasis, since they seem to promote anti-inflammatory responses, preserve endothelial barrier function, and supress the production of ROS [189,192,193].
Conversely, other compounds derived from tryptophan, namely indoxyl sulphate (IS), a hepatic metabolite derived from microbial indole, act as uremic toxins and have been associated with vascular calcification, endothelial dysfunction, and pro-inflammatory signalling through the AhR and other pathways [194]. Since its clearance is impaired in patients with chronic renal disease, IS accumulation is especially significant in these patients, aggravating atherosclerotic processes [195,196].
The dual role of AhR ligands derived from microbiota is supported by data from animal models of atherosclerosis. A study in Apoe-deficient mice show that the administration of beneficial AhR ligands, such as IPA or IAld, ameliorated vascular inflammation and reduced atherosclerotic lesion formation [192,193]. In contrast, exposure to IS aggravated vascular damage, promoted oxidative stress, and impaired cardiac function [194,197]. These findings suggest that AhR acts as a molecular sensor that combines microbial signals to modulate the cardiovascular physiology of the host, with the nature of the ligand and the inflammatory context influencing the outcome [136].
Altogether, these insights highlight the therapeutic potential of modulating the microbiota–tryptophan–AhR axis as a novel avenue for preventing or attenuating atherosclerosis.
4.5. Non-Bacterial Microbiota and Atherosclerosis
Recent research has begun to investigate how non-bacterial components of the gut microbiota, such as fungus, viruses, and bacteriophages, may affect CVD by indirect but biologically significant routes, in addition to the known functions of microbial metabolites. These organisms seem to modify the host’s physiology via modifying barrier integrity, microbial community structure, and mucosal immunity, all of which are increasingly acknowledged as significant factors in the pathophysiology of atherosclerosis, rather than acting through particular circulating metabolites [16,198].
Fungi represent a small but metabolically active component of the gut ecosystem, collectively known as the mycobiome. Although Candida albicans is the most frequently detected species among the fungal elements of the gut mycobiota, other genera, including Malassezia, Saccharomyces, and Mucor, have also been implicated in human disease and may play distinct roles in gut–immune–vascular interactions [199].
A preliminary study exploring the role of the gut mycobiota in subclinical atherosclerosis found that the relative abundance of Mucor racemosus in the gut was negatively associated with both carotid intima-media thickness (cIMT) and the Framingham Risk Score (FRS), a clinical estimate of 10-year cardiovascular risk based on traditional factors. The results of the study point to a potential preventive effect of some fungal species in cardiovascular health, even though the underlying processes are currently unknown [200]. While M. racemosus may exert a protective influence, other fungal taxa appear to have the opposite effect. A recent study suggests that C. albicans is implicated in promoting pro-inflammatory responses and exacerbating atherosclerotic processes. The study showed that, in patients with dyslipidaemia, elevated levels of C. albicans were positively correlated with total cholesterol and LDL cholesterol, and its colonisation in murine models aggravated atherosclerosis. From a mechanistic point of view, a metabolite of C. albicans, formyl-methionine, activates the intestinal HIF-2α–ceramide signalling pathway, leading to increased ceramide synthesis and accelerated plaque development, an effect that can be mitigated by the pharmacological inhibition of HIF-2α [20].
The composition of the gut mycobiota in patients with atherosclerotic cardiovascular disease (ACVD) differs markedly from that of healthy individuals, according to a recent study. Shotgun metagenomic analyses revealed an increase in opportunistic fungal pathogens, including C. albicans, Exophiala spinifera, and Malassezia restricta. Notably, network analysis revealed that the fungal–bacterial ecosystem in these individuals displayed greater dysregulation and lower interconnectivity, with species such as Malassezia globosa, Nakaseomyces glabratus, and Penicillium sumatraense emerging as central nodes [140]. Similarly, a cross-sectional study that examined the bacterial and fungal communities in patients with coronary artery disease found that the severity of the condition was correlated with increasing changes in gut fungal composition [141].
In addition to fungi, viruses and bacteriophages, collectively known as the gut virome, represent another important non-bacterial component of the intestinal microbiota with potential implications in cardiovascular health. The virome is mainly composed of bacteriophages, and has increasingly been recognised as a potential regulator of the bacterial community in the gut and can indirectly impact the cardiometabolic pathways that influence the development and progression of atherosclerosis. Phages can alter bacterial communities through lytic or lysogenic cycles, and this can result in the transmission of auxiliary metabolic genes by transduction, a horizontal gene transfer mechanism. These genes may modulate bacterial metabolism in mechanisms that ultimately influence the host’s immunity, intestinal permeability, and the production of inflammatory mediators. In fact, individuals with cardiometabolic diseases, including obesity, type 2 diabetes, and atherosclerosis, have been found to have altered gut phageome composition. For instance, enrichment in phages infecting Enterobacteriaceae and Streptococcus species has been reported among these individuals [17,142].
In parallel, although through different pathways, eukaryotic viruses have also been implicated in the pathogenesis of atherosclerosis. Various human viruses, including influenza virus, herpes simplex virus (HSV), hepatitis viruses (HAV, HBV, HCV), human cytomegalovirus (HCMV), human papillomavirus (HPV), human immunodeficiency virus (HIV), and SARS-CoV-2, have been associated with this disease through direct and indirect mechanisms, such as chronic systemic inflammation, immune activation, endothelial dysfunction, and oxidative stress. For example, influenza virus can increase LDL uptake by desialylation and induce endothelial permeability and thrombosis. In arterial walls, HCMV has been detected, and this virus has been implicated in the formation of foam cells, the proliferation of smooth muscle cells, and endothelial injury. Finally, cytokine storms and persistent inflammation can be triggered by both HIV and SARS-CoV-2, further contributing to vascular damage and plaque formation [16].
Collectively, these findings suggest that cardiovascular health, and, in particular, atherosclerosis, is actively influenced by the non-bacterial gut microbiota and may act synergistically with bacteria derived effects. To fully understand their involvement in atherogenesis and their potential as therapeutic targets, further studies are required.
4.6. Microbiota-Induced Immune and Inflammatory Pathways in Atherosclerosis
The continuous communication between the gut microbiota and the immune system is essential for maintaining systemic homeostasis. However, under conditions of intestinal dysbiosis, this balance is disrupted, triggering chronic low-grade inflammation, characterised by elevated levels of pro-inflammatory cytokines such as TNF-α and IL-6, implicated in the pathophysiology of atherosclerosis, since they promote an inflammatory state that damages the endothelium and facilitates the formation of atherosclerotic plaques [201,202].
In parallel, increased intestinal permeability, also known as “leaky gut”, another common alteration associated with dysbiosis, allows for the translocation of bacterial products, such as LPS, into the systemic circulation [203]. These components, in turn, activate Toll-like receptors, notably TLR4, present on endothelial and immune cells, thereby promoting a systemic inflammatory response. This process contributes to atherosclerosis progression by stimulating monocyte adhesion to the endothelium and promoting foam cell formation within atherosclerotic plaques [204,205]. Bacterial translocation is also associated with the activation of the NF-κB signalling pathway, which is crucial in regulating vascular inflammation [206].
In addition, the activation of TLR4 and TLR2 receptors, which recognise pathogen-associated molecular patterns (PAMPs) such as LPS, peptidoglycans, and bacterial lipoproteins, leads to the activation of pro-inflammatory intracellular signalling pathways, including the NF-κB pathway. This promotes the expression of inflammatory cytokines and endothelial adhesion molecules, facilitating the infiltration of monocytes and T lymphocytes into atherosclerotic plaques, which aggravates inflammation and accelerates the progression of the disease. These alterations also contribute to endothelial dysfunction, a critical step in the pathophysiology of atherosclerosis, as previously discussed [201,207,208,209].
On the other hand, dysbiosis favours the proliferation of pathobionts, such as members of the Enterobacteriaceae family, which have the ability to proliferate in inflammatory intestinal environments [88]. These microorganisms utilise nitrates as final electron acceptors in anaerobic respiration, giving them a competitive advantage under inflammatory conditions [88,210]. This process is associated with increased production of TMA, as discussed in Section 4.1, and contributes to alterations in the lipid composition of endothelial cells, promoting the formation of atherosclerotic plaque [211].
Beyond the innate immune response mediated by TLRs, the gut microbiota can also influence the adaptive immune response, namely the expansion of Th17 lymphocytes, which play a central role in the chronic inflammation associated with atherosclerosis [212]. Studies have shown that intestinal dysbiosis can favour the differentiation of Th17 cells, which secrete cytokines such as IL-17 that promote vascular inflammation and plaque instability. This mechanism is particularly relevant, given that Th17 lymphocytes are associated with autoimmune and chronic inflammatory processes [212,213]. Nonetheless, a study has suggested that IL-17 might also exert stabilising effects on atherosclerotic plaques by downregulating VCAM-1 expression on endothelial cells [214], highlighting the complex and context-dependent role of IL-17 in atherosclerosis. The increase in Th17 cells in atherosclerosis enhances the activation of the NF-κB pathway and the production of matrix MMPs, which degrade the extracellular matrix and weaken plaque structure, increasing the risk of rupture and thrombosis [215,216].
5. Gut Microbiota-Targeted Interventions in the Prevention and Progression of Atherosclerosis
In recent years, the gut microbiota has attracted considerable attention, aimed at understanding how alterations in its composition, structure, and metabolism can influence host metabolic and physiological processes, particularly in relation to the cardiovascular system and, more specifically, atherosclerosis [217]. In this context, the critical and multifaceted role of the gut microbiota in atherosclerosis has been evidenced in small clinical studies and some preliminary reports, highlighting the potential of novel therapeutic approaches that modulate the gut microbiota and may exert a relevant cardioprotective effect on the development and progression of the disease [218]. Accordingly, emerging treatment strategies include the use of prebiotics and probiotics, as well as FMT. These interventions have been associated with improvements in lipid profiles, a reduction in inflammatory state, and the potential benefits provided by the previously discussed active metabolites [217].
Beyond targeted microbial interventions, commonly used pharmacological agents may also influence gut microbial composition. Statins, widely prescribed for their cholesterol-lowering effects, have recently been shown to modulate the gut microbiota. A recent review reported that statin therapy can alter the relative abundance of key microbial taxa and affect the production of microbial metabolites, such as short-chain fatty acids and bile acids, potentially contributing to their pleiotropic cardiovascular benefits beyond their lipid-lowering effects. These findings highlight the relevance of considering drug–microbiota interactions when evaluating the overall metabolic and vascular effects of lipid-lowering therapies [219].
5.1. Prebiotics
Currently, a prebiotic is defined as a substrate that is selectively utilised by host microorganisms conferring a health benefit [220,221].
This definition encompasses not only nondigestible carbohydrate-based compounds, such as fructooligosaccharides (FOS) and galactooligosaccharides (GOS), but also other compounds like certain polyphenols and polyunsaturated fatty acids, provided that there is robust evidence of their selective microbial utilisation and associated health benefits. Furthermore, prebiotic effects are no longer confined to the gut, but may also occur at other body locals colonised by microorganisms [220].
The key concept of prebiotics is selectivity, as they specifically promote the stimulation, growth, and proliferation of beneficial microorganisms, including, but not limited to, Lactobacillus spp. and Bifidobacterium spp., which inhibit the proliferation of pathogenic bacteria by reducing intestinal pH [222,223]. Additionally, prebiotics stimulate changes in bacterial metabolism, such as increased production of SCFAs, namely butyric and propionic acids, which, among the other benefits already discussed, improve epithelial integrity and exert anti-inflammatory effects [224]. Moreover, prebiotics may improve lipid metabolism through microbiota-mediated mechanisms, including bile acid metabolism and SCFA production. Taken together, and as previously discussed, prebiotics exert positive effects on the prevention and development of atherosclerosis [203].
Inulin or GOS supplementation has been associated with modest reductions in IL-6, TNF-α, high-sensitivity C-reactive protein (CRP), and circulating lipopolysaccharides in patients with coronary artery disease or elevated metabolic risk, according to recent clinical research. In addition, improvements in lipid profiles and endothelial function have also been reported. However, evidence for direct effects on atherosclerotic burden remains scarce, and no trials to date have demonstrated the clear regression of vascular lesions [225,226]. While preclinical findings in animal models have provided mechanistic support, the translation of these effects to clinical outcomes in humans remains to be firmly established [227].
5.2. Probiotics
The term “probiotic”, derived from the Greek meaning “promotion of life”, refers to “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”. Ideally, these microorganisms should be of human origin, safe, and free from genes conferring antibiotic resistance, virulence, pathogenicity, or toxicity, and should not exhibit haemolytic activity [228,229].
Functionally, probiotics can be divided into three main groups: lactic acid bacteria, with bacteria belonging to the genera Lactobacillus and Bifidobacterium; non-lactic acid bacteria, such as Bacillus spp., Escherichia spp., and Propionibacterium spp.; and yeasts and fungi, mainly Saccharomyces spp. and Aspergillus spp. Nevertheless, Lactobacillus spp. and Bifidobacterium spp. have been most extensively used as probiotics in recent decades [230].
To be suitable for human use, probiotics must tolerate gastric acid and bile salts to reach the intestine in a viable and stable form. Once this chemical barrier is overcome, they must adhere to the intestinal ECs, where they exert their functions. In addition, they should exhibit antimicrobial activity to inhibit the growth of pathogenic bacteria. Probiotics should also benefit the host by strengthening the immune system, preventing infections, promoting intestinal homeostasis, and reducing plasma cholesterol levels [230,231].
Considering the benefits associated with lowering plasma cholesterol in the context of atherosclerosis, the hypocholesterolaemic and anti-atherosclerotic effects of probiotics may be explained through different mechanisms. These include the activity of bile salt hydrolase (BSH), the production of SCFAs, the co-precipitation of cholesterol with deconjugated bile salts, and the assimilation of cholesterol during bacterial growth, with the subsequent incorporation into the cell membrane [232].
Several studies have shown that many Bifidobacterium and Lactobacillus species produce BSH, an enzyme whose activation may reduce cholesterol levels by increasing the excretion of free bile acids and, consequently, decreasing their intestinal reabsorption. In response, the liver increases cholesterol uptake to synthesise new bile acids, ultimately contributing to reduced circulating cholesterol levels. In addition, certain strains of these bacteria have the capacity to bind cholesterol and either incorporate it into their cell membranes or sequester it on their surface, further contributing to plasma cholesterol reduction. Another important metabolic pathway involves SCFAs, which lower cholesterol via two different mechanisms: the inhibition of the enzyme HMG-CoA reductase, the rate-limiting enzyme for hepatic cholesterol synthesis; and the stimulation of 7α-hydroxylase activity by propionic acid, thereby enhancing hepatic bile acid synthesis via cholesterol oxidation [217,233,234,235].
Probiotics also contribute to intestinal homeostasis by improving epithelial barrier integrity through the promotion and maintenance of tight junctions. This provides an additional mechanism of protection against atherosclerosis by preventing the absorption of microbial metabolites and components such as TMAO and LPS, which are key contributors to its pathogenesis [236]. In this context, Lactobacillus plantarum ZDY04 has been shown to reduce plasma TMAO levels through the modulation of the gut microbiota [237].
In addition, E. faecium GEFA01 shows potential as a probiotic for atherosclerosis by lowering cholesterol and modulating gut microbiota in hyperlipidaemic mice [154].
Finally, by inhibiting NF-κB activation, as observed in a study with Lactobacillus acidophilus ATCC 4356, probiotics have a proactive role in preventing atherosclerosis by promoting an anti-inflammatory action. This is demonstrated by their capacity to decrease levels of the pro-inflammatory cytokine TNF-α and increase expression of the anti-inflammatory cytokine IL-10 [238].
Recent clinical trials investigating the use of isolated probiotic strains in individuals with metabolic disorders or hypercholesterolaemia have demonstrated promising, albeit preliminary, cardiometabolic benefits. Daily supplementation with specific Lactobacillus and Bifidobacterium strains [239], such as L. plantarum ECGC 13110402 [240], L. reuteri V3401 [241] or NCIMB 30242 [242], and B. animalis ssp. lactis Lafti®B94 [239,243], over 8–12 weeks has been associated with reductions in LDL cholesterol, systemic inflammatory markers (e.g., hs-CRP, TNF-α, IL-6), and circulating endotoxins such as LPS. Notably, the PROMISE study was among the first to employ PET-CT imaging to assess vascular inflammation in response to a probiotic intervention, offering a mechanistic link between microbial modulation and vascular health. While these interventions did not report the structural regression of atherosclerotic lesions, the observed anti-inflammatory and lipid-lowering effects suggest that specific probiotics may contribute to a favourable shift in the systemic milieu that precedes atherogenesis. Nonetheless, larger and longer-term trials are required to validate these findings and clarify their potential in cardiovascular risk reduction [243].
5.3. Faecal Microbiota Transplantation
Although it is a concept that dates back to the late 1960s, FMT is currently an attractive method that is increasingly being used to alter the composition and restore the functions of the gut microbiota in pathological states associated with dysbiosis [244]. This procedure involves the collection of filtered faecal samples from carefully selected healthy donors, or from the recipient before the onset of the pathology, in a process known as autologous FMT, followed by their transplantation into the recipient’s GIT [57]. FMT has proven to be a highly effective method in treating C. difficile infection [245]. Furthermore, FMT is considered an emerging therapy in cases of extra-intestinal diseases, including CVDs, where a shift in the recipient’s bile acid composition has been observed, bringing it closer to that of the donor [246,247]. Additionally, FMT performed from lean individuals to obese recipients has shown improvement in the sensitivity of recipients to triglycerides, resulting in a decrease in their plasma concentration [248]. These observations suggest an anti-atherosclerotic effect, considering the previously mentioned benefits of bile acids, as well as a reduction in dyslipidaemia, one of the main risk factors for the disease. However, FMT remains a limited therapeutic approach due to various associated risks, including the transfer of endotoxins and infectious agents, and its long-term effects are still not fully understood [9,217].
Current evidence for FMT in atherosclerosis is limited, but evolving. Preclinical models consistently show that FMT from healthy donors can reduce plaque development, modulate systemic inflammation, and lower pro-atherogenic metabolites such as TMAO [249,250]. In humans, only one ongoing clinical trial (NCT04410003) is directly investigating FMT in severe unexplained atherosclerosis [251]. Other small-scale studies on metabolic syndrome, including one involving 32 women [252] and another with 20 men receiving FMT from vegan donors, have demonstrated shifts in microbiota composition, but no significant clinical improvement in TMAO levels, lipid profiles, or endothelial function [253]. Larger trials targeting atherosclerotic outcomes are needed to clarify FMT’s therapeutic potential.
6. Conclusions
The gut microbiota plays an essential role in maintaining human health, influencing processes ranging from digestion to immune regulation and metabolic functions. It is increasingly recognised as an important factor in cardiovascular health. However, several factors can alter its composition and functions, leading to a state of dysbiosis, which is associated with several pathological conditions, including atherosclerosis.
Atherosclerosis, a complex and multifactorial condition, is closely associated with CVDs and represents one of the leading causes of morbidity and mortality worldwide. Therefore, a detailed understanding of the risk factors and underlying mechanisms of atherosclerosis is essential for the development of effective prevention and treatment strategies. Thus, the study of the interaction between the gut microbiota and atherosclerosis has emerged as a promising research field, exploring metabolic products, the regulation of inflammatory processes, and how restoring the balance of the gut microbiota can positively impact this pathological state.
Therapeutic interventions that target not only the modulation of the gut microbiota, but that also influence associated metabolic and inflammatory processes, such as the consumption of prebiotics, probiotics, and FMT, have been explored and show promise for the prevention and treatment of atherosclerosis.
Although benefits have been observed with the use of prebiotics and FMT, including improvements in cholesterol and triglyceride profiles, increased production of SCFAs, and improved bile acid profiles, all of which play a protective role in atherosclerosis, probiotics stand out for their proven clinical efficacy. These act through hypocholesterolaemic mechanisms, maintaining intestinal barrier integrity, reducing plasma TMAO levels, and exhibiting anti-inflammatory effects, thereby reinforcing their therapeutic potential in the prevention and management of atherosclerosis.
Nonetheless, although these three therapeutic strategies have shown promising results, further clinical studies are required to determine their efficacy, establish optimal dosages, and elucidate the precise mechanisms underlying their cardiovascular benefits. Additionally, long-term clinical trials are crucial to ensure the safety and efficacy of these interventions in the context of atherosclerosis.
Author Contributions
Conceptualization, T.L. and V.C.; writing—original draft preparation, T.L. and V.C.; figure conceptualization, T.L. and V.C.; writing—review and editing, T.L., C.N., S.D. and G.J.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Faculty of Pharmacy of the University of Coimbra, by Fundação para a Ciência e Tecnologia, under the project 2023.12972.PEX (http://doi.org/10.54499/2023.12972.PEX), and by the COMPETE 2020-Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT–Fundação para a Ciência e a Tecnologia, under projects UIDB/04539/2020, UIDP/04539/2020, and LA/P/0058/2020.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barquera, S.; Pedroza-Tobias, A.; Medina, C.; Hernandez-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.C.; David, S.G.; David, A.G.; Tarca, V.; Paduret, I.A.; Mindru, D.E.; Rosu, S.T.; Rosu, E.V.; Adumitrachioaiei, H.; Bernic, J.; et al. Atherosclerosis from newborn to adult-epidemiology, pathological aspects, and risk factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef]
- Papadopoulos, P.D.; Tsigalou, C.; Valsamaki, P.N.; Konstantinidis, T.G.; Voidarou, C.; Bezirtzoglou, E. The emerging role of the gut microbiome in cardiovascular disease: Current knowledge and perspectives. Biomedicines 2022, 10, 948. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fak, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Del Campo-Moreno, R.; Alarcon-Cavero, T.; D’Auria, G.; Delgado-Palacio, S.; Ferrer-Martinez, M. Microbiota and human health: Characterization techniques and transference. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Sniffen, S.; McGill Percy, K.C.; Pallaval, V.B.; Chidipi, B. Gut dysbiosis and immune system in atherosclerotic cardiovascular disease (ACVD). Microorganisms 2022, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut microbiota in hypertension and atherosclerosis: A review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Almeida, C.; Barata, P.; Fernandes, R. The influence of gut microbiota in cardiovascular diseases-a brief review. Porto Biomed. J. 2021, 6, e106. [Google Scholar] [CrossRef]
- Dimri, S.; Rizzo, A. The Gut Microbiome: An overview. J. Stud. Res. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; Harun-Or-Rashid, M.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Matijasic, M.; Mestrovic, T.; Paljetak, H.C.; Peric, M.; Baresic, A.; Verbanac, D. Gut microbiota beyond bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Lee, K.T. Atherosclerosis by virus infection-A short review. Biomedicines 2022, 10, 2634. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, J.; Meng, J.; Li, S.; Zhang, Y.; You, W.; Sai, X.; Yang, J.; Zhang, S.; Sun, W. Structural changes in the gut virome of patients with atherosclerotic cardiovascular disease. Microbiol. Spectr. 2024, 12, e0105023. [Google Scholar] [CrossRef]
- El Jaddaoui, I.; Sehli, S.; Al Idrissi, N.; Bakri, Y.; Belyamani, L.; Ghazal, H. The gut mycobiome for precision medicine. J. Fungi 2025, 11, 279. [Google Scholar] [CrossRef]
- Buttar, J.; Kon, E.; Lee, A.; Kaur, G.; Lunken, G. Effect of diet on the gut mycobiome and potential implications in inflammatory bowel disease. Gut Microbes 2024, 16, 2399360. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Hu, X.; Ye, C.; Nie, Q.; Wang, K.; Yan, S.; Lin, J.; Xu, F.; Li, M.; et al. Candida albicans accelerates atherosclerosis by activating intestinal hypoxia-inducible factor 2-alpha signaling. Cell Host Microbe 2024, 32, 964–979.e7. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the gastrointestinal tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Fernandez Real, J.M.; Guarner, F.; Gueimonde, M.; Rodriguez, J.M.; Saenz de Pipaon, M.; Sanz, Y. Gut microbes and health. Gastroenterol. Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut microbiota dysbiosis: Triggers, consequences, diagnostic and therapeutic options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Singh, R.; Ro, S.; Ghoshal, U.C. Gut microbiota dysbiosis in functional gastrointestinal disorders: Underpinning the symptoms and pathophysiology. JGH Open 2021, 5, 976–987. [Google Scholar] [CrossRef]
- Saeed, N.K.; Al-Beltagi, M.; Bediwy, A.S.; El-Sawaf, Y.; Toema, O. Gut microbiota in various childhood disorders: Implication and indications. World J. Gastroenterol. 2022, 28, 1875–1901. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut microbial changes, interactions, and their implications on human lifecycle: An ageing perspective. Biomed. Res. Int. 2018, 2018, 4178607. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Homann, C.M.; Rossel, C.A.J.; Dizzell, S.; Bervoets, L.; Simioni, J.; Li, J.; Gunn, E.; Surette, M.G.; de Souza, R.J.; Mommers, M.; et al. Infants’ first solid foods: Impact on gut microbiota development in two intercontinental cohorts. Nutrients 2021, 13, 2639. [Google Scholar] [CrossRef]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Ostbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Wu, W.M.; Yang, Y.S.; Peng, L.H. Microbiota in the stomach: New insights. J. Dig. Dis. 2014, 15, 54–61. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Compare, D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.A.H.; Heyndrickx, M.; Jonkers, D.; Mackie, A.; Millet, S.; Naghibi, M.; Paerregaard, S.I.; Pot, B.; Saulnier, D.; Sina, C.; et al. Small intestine vs. colon ecology and physiology: Why it matters in probiotic administration. Cell Rep. Med. 2023, 4, 101190. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; van den Bogert, B.; Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 2015, 32, 14–20. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef]
- Villmones, H.C.; Svanevik, M.; Ulvestad, E.; Stenstad, T.; Anthonisen, I.L.; Nygaard, R.M.; Dyrhovden, R.; Kommedal, O. Investigating the human jejunal microbiota. Sci. Rep. 2022, 12, 1682. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Villmones, H.C.; Haug, E.S.; Ulvestad, E.; Grude, N.; Stenstad, T.; Halland, A.; Kommedal, O. Species level description of the human ileal bacterial microbiota. Sci. Rep. 2018, 8, 4736. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Matzaras, R.; Nikopoulou, A.; Protonotariou, E.; Christaki, E. Gut microbiota modulation and prevention of dysbiosis as an alternative approach to antimicrobial resistance: A narrative review. Yale J. Biol. Med. 2022, 95, 479–494. [Google Scholar] [PubMed]
- İlhan, N. Gut microbiota and metabolism. Int. J. Med. Biochem. 2018, 1, 115–128. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Suceveanu, A.P.; Stanigut, A.M.; Tofolean, D.E.; Axelerad, A.D.; Iordache, I.E.; Herlo, A.; Nelson Twakor, A.; Nicoara, A.D.; Tocia, C.; et al. Intestinal insights: The gut microbiome’s role in atherosclerotic disease: A narrative review. Microorganisms 2024, 12, 2341. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-derived metabolite, trimethylamine-N-oxide (TMAO) in cardio-metabolic diseases: Detection, mechanism, and potential therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Ghazalpour, A.; Cespedes, I.; Bennett, B.J.; Allayee, H. Expanding role of gut microbiota in lipid metabolism. Curr. Opin. Lipidol. 2016, 27, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, L.; Chen, C.; Li, P.; Lu, B. The gut microbiota-artery axis: A bridge between dietary lipids and atherosclerosis? Prog. Lipid Res. 2023, 89, 101209. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, N.; Berweiler, V.; Wang, H.; Trajkovski, M. Intestinal microbiota as a route for micronutrient bioavailability. Curr. Opin. Endocr. Metab. Res. 2021, 20, 100285. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef]
- Hertli, S.; Zimmermann, P. Molecular interactions between the intestinal microbiota and the host. Mol. Microbiol. 2022, 117, 1297–1307. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic immunity and the microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Choden, T.; Cohen, N.A. The gut microbiome and the immune system. Explor. Med. 2022, 3, 219–233. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Cruz Neto, J.P.R.; de Luna Freire, M.O.; de Albuquerque Lemos, D.E.; Ribeiro Alves, R.M.F.; de Farias Cardoso, E.F.; de Moura Balarini, C.; Duman, H.; Karav, S.; de Souza, E.L.; de Brito Alves, J.L. Targeting gut microbiota with probiotics and phenolic compounds in the treatment of atherosclerosis: A comprehensive review. Foods 2024, 13, 2886. [Google Scholar] [CrossRef]
- Jergens, A.E.; Parvinroo, S.; Kopper, J.; Wannemuehler, M.J. Rules of engagement: Epithelial-microbe interactions and inflammatory bowel disease. Front. Med. 2021, 8, 669913. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.K.; Azmal, M.; Haque, A.; Meem, M.; Talukder, O.F.; Ghosh, A. Unlocking the secrets of the human gut microbiota: Comprehensive review on its role in different diseases. World J. Gastroenterol. 2025, 31, 99913. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Mendez-Garcia, C.; Rojo, D.; Barbas, C.; Moya, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 2017, 134, 114–126. [Google Scholar] [CrossRef]
- Omenetti, S.; Pizarro, T.T. The Treg/Th17 axis: A dynamic balance regulated by the gut microbiome. Front. Immunol. 2015, 6, 639. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Demehri, F.R.; Barrett, M.; Teitelbaum, D.H. Changes to the intestinal microbiome with parenteral nutrition: Review of a murine model and potential clinical implications. Nutr. Clin. Pract. 2015, 30, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Moreira de Gouveia, M.I.; Bernalier-Donadille, A.; Jubelin, G. Enterobacteriaceae in the human gut: Dynamics and ecological roles in health and disease. Biology 2024, 13, 142. [Google Scholar] [CrossRef]
- Gusarov, I.; Shatalin, K.; Starodubtseva, M.; Nudler, E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 2009, 325, 1380–1384. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Swiatecka, D.; Narbad, A.; Ridgway, K.P.; Kostyra, H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Chen, S.; Lin, L. Research progress of gut microbiota and obesity caused by high-fat diet. Front. Cell Infect. Microbiol. 2023, 13, 1139800. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S. Changes seen in gut bacteria content and distribution with obesity: Causation or association? Postgrad. Med. 2015, 127, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Fukiya, S.; Islam, K.B.; Ooka, T.; Ogura, Y.; Hayashi, T.; Hagio, M.; Ishizuka, S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012, 3, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, R.; Inoue, K.Y.; Nishino, K.; Yamasaki, S. Intestinal and fecal pH in human health. Front. Microbiomes 2023, 2, 1192316. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, T.; Gu, Y.; Wang, X.; Xie, R.; Sun, Y.; Wang, B.; Cao, H. Regulation of gut microbiota-bile acids axis by probiotics in inflammatory bowel disease. Front. Immunol. 2022, 13, 974305. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public. Health 2020, 17, 7681. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Yang, Z.; Li, M.D. Effect of cigarette Smoke on gut microbiota: State of knowledge. Front. Physiol. 2021, 12, 673341. [Google Scholar] [CrossRef]
- Rojas-Valverde, D.; Bonilla, D.A.; Gomez-Miranda, L.M.; Calleja-Nunez, J.J.; Arias, N.; Martinez-Guardado, I. Examining the interaction between exercise, gut microbiota, and neurodegeneration: Future research directions. Biomedicines 2023, 11, 2267. [Google Scholar] [CrossRef] [PubMed]
- Boytar, A.N.; Skinner, T.L.; Wallen, R.E.; Jenkins, D.G.; Dekker Nitert, M. The effect of exercise prescription on the human gut microbiota and comparison between clinical and apparently healthy populations: A systematic review. Nutrients 2023, 15, 1534. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichon, J.; Skarpanska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, W.; Liu, W.; Zhou, M. Roles and mechanisms of renalase in cardiovascular disease: A promising therapeutic target. Biomed. Pharmacother. 2020, 131, 110712. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 12 August 2024).
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Makaryus, A.N.; Wengrofsky, P.; Lee, J. Dyslipidemia and its role in the pathogenesis of atherosclerotic cardiovascular disease: Implications for evaluation and targets for treatment of dyslipidemia based on recent guidelines. In Dyslipidemia; McFarlane, S.I., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Lu, Y.; Cui, X.; Zhang, L.; Wang, X.; Xu, Y.; Qin, Z.; Liu, G.; Wang, Q.; Tian, K.; Lim, K.S.; et al. The functional role of lipoproteins in atherosclerosis: Novel directions for diagnosis and targeting therapy. Aging Dis. 2022, 13, 491–520. [Google Scholar] [CrossRef]
- Bjorkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksass, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Back, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lv, Y.; Bai, X.; Qi, J.; Weng, X.; Liu, S.; Bao, X.; Jia, H.; Yu, B. Plaque erosion: A distinctive pathological mechanism of acute coronary syndrome. Front. Cardiovasc. Med. 2021, 8, 711453. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, S.; Li, J.; Wang, L.; Zhang, Q.; Hou, L.; Yu, X.; Liu, Z.; Lv, T.; Shang, L. Low shear stress induces vascular endothelial cells apoptosis via miR-330 /SOD2 /HSP70 signaling pathway. Exp. Cell Res. 2025, 445, 114410. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Higashi, Y. Endothelial function in dyslipidemia: Roles of LDL-cholesterol, HDL-cholesterol and triglycerides. Cells 2023, 12, 1293. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and their role in atherosclerosis: Pathophysiology and transcriptome analysis. Biomed. Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam cells in atherosclerosis: Novel insights into its origins, consequences, and molecular mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arter. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef]
- Peled, M.; Fisher, E.A. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front. Immunol. 2014, 5, 579. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Ortega, C.; Francis, G.A. Smooth Muscle Cell-Proteoglycan-Lipoprotein Interactions as Drivers of Atherosclerosis. In Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; von Eckardstein, A., Binder, C.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 335–358. [Google Scholar]
- Beck-Joseph, J.; Tabrizian, M.; Lehoux, S. Molecular Interactions Between Vascular Smooth Muscle Cells and Macrophages in Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 737934. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Vergallo, R.; Crea, F. Atherosclerotic plaque healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arter. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef]
- Monaji, S.R. Steps of atherosclerosis and its complication. J. Med. Pharm. Allied Sci. 2020, 9, 2577–2579. [Google Scholar] [CrossRef]
- Hermiz, C.; Sedhai, Y.R. Angina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Takahashi, J.; Onuma, S.; Hao, K.; Godo, S.; Shiroto, T.; Yasuda, S. Pathophysiology and diagnostic pathway of myocardial infarction with non-obstructive coronary arteries. J. Cardiol. 2024, 83, 17–24. [Google Scholar] [CrossRef]
- Salaudeen, M.A.; Bello, N.; Danraka, R.N.; Ammani, M.L. Understanding the pathophysiology of ischemic stroke: The basis of current therapies and opportunity for new ones. Biomolecules 2024, 14, 305. [Google Scholar] [CrossRef]
- Murphy, K.; O’Donovan, A.N.; Caplice, N.M.; Ross, R.P.; Stanton, C. Exploring the gut microbiota and cardiovascular disease. Metabolites 2021, 11, 493. [Google Scholar] [CrossRef]
- Eshghjoo, S.; Jayaraman, A.; Sun, Y.; Alaniz, R.C. Microbiota-mediated immune regulation in atherosclerosis. Molecules 2021, 26, 179. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: A review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Srivatsav, V.; Rizwan, A.; Nashed, A.; Liu, R.; Shen, R.; Akhtar, M. Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients 2017, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Huang, P.; Liu, D.; Xing, G.; Guo, R.; Li, S.; Fan, S.; Cheng, L.; Yan, Q.; Yang, W. Gut mycobiome alterations and network interactions with the bacteriome in patients with atherosclerotic cardiovascular disease. Microbiol. Spectr. 2025, 13, e0218224. [Google Scholar] [CrossRef]
- An, K.; Jia, Y.; Xie, B.; Gao, J.; Chen, Y.; Yuan, W.; Zhong, J.; Su, P.; Liu, X. Alterations in the gut mycobiome with coronary artery disease severity. EBioMedicine 2024, 103, 105137. [Google Scholar] [CrossRef] [PubMed]
- Kirk, D.; Costeira, R.; Visconti, A.; Khan Mirzaei, M.; Deng, L.; Valdes, A.M.; Menni, C. Bacteriophages, gut bacteria, and microbial pathways interplay in cardiometabolic health. Cell Rep. 2024, 43, 113728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Jiang, H. Gut microbiota in atherosclerosis: Focus on trimethylamine N-oxide. APMIS 2020, 128, 353–366. [Google Scholar] [CrossRef]
- Canyelles, M.; Borras, C.; Rotllan, N.; Tondo, M.; Escola-Gil, J.C.; Blanco-Vaca, F. Gut microbiota-derived TMAO: A causal factor promoting atherosclerotic cardiovascular disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Papandreou, C.; More, M.; Bellamine, A. Trimethylamine N-oxide in relation to cardiometabolic health-cause or effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef]
- Zhen, J.; Zhou, Z.; He, M.; Han, H.X.; Lv, E.H.; Wen, P.B.; Liu, X.; Wang, Y.T.; Cai, X.C.; Tian, J.Q.; et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. 2023, 14, 1085041. [Google Scholar] [CrossRef] [PubMed]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Modulation of endothelial function by TMAO, a gut microbiota-derived metabolite. Int. J. Mol. Sci. 2023, 24, 5806. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.B.; Zhang, Y.; Boini, K.M.; Koka, S. High mobility group box 1 mediates TMAO-induced endothelial dysfunction. Int. J. Mol. Sci. 2019, 20, 3570. [Google Scholar] [CrossRef]
- Holme, S.A.N.; Sigsgaard, T.; Holme, J.A.; Holst, G.J. Effects of particulate matter on atherosclerosis: A link via high-density lipoprotein (HDL) functionality? Part. Fibre Toxicol. 2020, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yu, X.; Wang, C.; Zhao, H.; Wang, X.; Guan, X. Trimethylamine N-oxide promotes oxidative stress and lipid accumulation in macrophage foam cells via the Nrf2/ABCA1 pathway. J. Physiol. Biochem. 2024, 80, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, H.; Xiang, Q.; Shen, L.; Guo, X.; Zhai, C.; Hu, H. Targeting trimethylamine N-Oxide: A new therapeutic strategy for alleviating atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 864600. [Google Scholar] [CrossRef]
- Vourakis, M.; Mayer, G.; Rousseau, G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef]
- Xu, W.; Zou, K.; Zhan, Y.; Cai, Y.; Zhang, Z.; Tao, X.; Qiu, L.; Wei, H. Enterococcus faecium GEFA01 alleviates hypercholesterolemia by promoting reverse cholesterol transportation via modulating the gut microbiota-SCFA axis. Front. Nutr. 2022, 9, 1020734. [Google Scholar] [CrossRef]
- Witkowski, M.; Witkowski, M.; Friebel, J.; Buffa, J.A.; Li, X.S.; Wang, Z.; Sangwan, N.; Li, L.; DiDonato, J.A.; Tizian, C.; et al. Vascular endothelial tissue factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc. Res. 2022, 118, 2367–2384. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, J.; Lian, J.; Yang, X.; Zhou, J. Gut metabolite trimethylamine-N-oxide in atherosclerosis: From mechanism to therapy. Front. Cardiovasc. Med. 2021, 8, 723886. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The role of the gut microbiota in bile acid metabolism. Ann. Hepatol. 2017, 16 (Suppl. S1), S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Majait, S.; Nieuwdorp, M.; Kemper, M.; Soeters, M. The black box orchestra of gut bacteria and bile acids: Who is the conductor? Int. J. Mol. Sci. 2023, 24, 1816. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Morillo, V.; Suarez, M.K.; Castro, A.; Ramirez, P.; Rojas, M.; Anez, R.; D’Marco, L.; Chacin-Gonzalez, M.; Bermudez, V. Role of gut microbiome in atherosclerosis: Molecular and therapeutic aspects. Curr. Cardiol. Rev. 2023, 19, e020223213408. [Google Scholar] [CrossRef]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond intestinal soap-bile acids in metabolic control. Nat. Rev. Endocrinol. 2014, 10, 488–498. [Google Scholar] [CrossRef]
- Yntema, T.; Koonen, D.P.Y.; Kuipers, F. Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases. Nutrients 2023, 15, 1850. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yi, Q.; Luo, L.; Xiong, Y. Regulation of bile acids and their receptor FXR in metabolic diseases. Front. Nutr. 2024, 11, 1447878. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.K.; Yin, L.; Zhang, Y. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef]
- Singh, A.B.; Dong, B.; Kraemer, F.B.; Xu, Y.; Zhang, Y.; Liu, J. Farnesoid X receptor activation by obeticholic acid elevates liver low-density lipoprotein receptor expression by mRNA stabilization and reduces plasma low-density lipoprotein cholesterol in mice. Arter. Thromb. Vasc. Biol. 2018, 38, 2448–2459. [Google Scholar] [CrossRef]
- Ma, J.; Li, H. The role of gut microbiota in atherosclerosis and hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Wu, W.; Zhu, Y.; Liu, X. The role of bile acids in cardiovascular diseases: From mechanisms to clinical implications. Aging Dis. 2023, 14, 261–282. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M.; Wu, Y.; Boehme, S. Bile acid and cholesterol metabolism in atherosclerotic cardiovascular disease and therapy. Cardiol. Plus 2020, 5, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.P.; Chapman, J.A.; Stewart, C.J. Gut microbiome derived short chain fatty acids: Promising strategies in necrotising enterocolitis. Curr. Res. Microb. Sci. 2024, 6, 100219. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Richards, L.B.; Li, M.; van Esch, B.C.A.M.; Garssen, J.; Folkerts, G. The effects of short-chain fatty acids on the cardiovascular system. PharmaNutrition 2016, 4, 68–111. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kolodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-kappaB and PPARalpha. J. Nutr. Biochem. 2004, 15, 220–228. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide or tumor necrosis factor alpha-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, D. Short-chain fatty acids are potential goalkeepers of atherosclerosis. Front. Pharmacol. 2023, 14, 1271001. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.; Capettini, L.S.; Lemos, V.S.; Santos, R.A.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFkappaB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Z.; Li, Z.; Ling, H.; Song, C. Role and mechanism of action of butyrate in atherosclerotic diseases: A review. J. Appl. Microbiol. 2021, 131, 543–552. [Google Scholar] [CrossRef]
- Mao, Y.; Kong, C.; Zang, T.; You, L.; Wang, L.S.; Shen, L.; Ge, J.B. Impact of the gut microbiome on atherosclerosis. mLife 2024, 3, 167–175. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Nishimura, N.; Kuo, V.; Fiehn, O.; Shahbaz, S.; Van Winkle, L.; Matsumura, F.; Vogel, C.F. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arter. Thromb. Vasc. Biol. 2011, 31, 1260–1267. [Google Scholar] [CrossRef]
- Xue, H.; Chen, X.; Yu, C.; Deng, Y.; Zhang, Y.; Chen, S.; Chen, X.; Chen, K.; Yang, Y.; Ling, W. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Circ. Res. 2022, 131, 404–420. [Google Scholar] [CrossRef]
- Chuang, H.L.; Chiu, C.C.; Lo, C.; Hsu, C.C.; Liu, J.Y.; Hung, S.W.; Tsai, S.C.; Sung, H.H.; Wang, C.L.; Huang, Y.T. Circulating gut microbiota-related metabolites influence endothelium plaque lesion formation in ApoE knockout rats. PLoS ONE 2022, 17, e0264934. [Google Scholar] [CrossRef]
- Kaminski, T.W.; Pawlak, K.; Karbowska, M.; Mysliwiec, M.; Pawlak, D. Indoxyl sulfate—The uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017, 18, 35. [Google Scholar] [CrossRef]
- Hung, S.C.; Kuo, K.L.; Wu, C.C.; Tarng, D.C. Indoxyl sulfate: A novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 2017, 6, e005022. [Google Scholar] [CrossRef]
- Skye, S.M.; Hazen, S.L. Microbial modulation of a uremic toxin. Cell Host Microbe 2016, 20, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Lano, G.; Burtey, S.; Sallee, M. Indoxyl sulfate, a uremic endotheliotoxin. Toxins 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, B.S.; Rosu, O.A.; Enache, R.M.; Profir, M.M.; Pavelescu, L.A.; Cretoiu, S.M. Gut mycobiome: Latest findings and current knowledge regarding its significance in human health and disease. J. Fungi 2025, 11, 333. [Google Scholar] [CrossRef]
- Chacon, M.R.; Lozano-Bartolome, J.; Portero-Otin, M.; Rodriguez, M.M.; Xifra, G.; Puig, J.; Blasco, G.; Ricart, W.; Chaves, F.J.; Fernandez-Real, J.M. The gut mycobiome composition is linked to carotid atherosclerosis. Benef. Microbes 2018, 9, 185–198. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Shen, X.; Li, L.; Sun, Z.; Zang, G.; Zhang, L.; Shao, C.; Wang, Z. Gut microbiota and atherosclerosis-focusing on the plaque stability. Front. Cardiovasc. Med. 2021, 8, 668532. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Parantainen, J.; Barreto, G.; Strandberg, T.E.; Mars, N.; Nurmi, K.; Eklund, K.K. Increased intestinal mucosal permeability and metabolic endotoxemia predict the risk of cardiovascular mortality. Atherosclerosis 2025, 405, 119220. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Wick, G.; Jakic, B.; Buszko, M.; Wick, M.C.; Grundtman, C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Metzler, B.; Jahangiri, M.; Mandal, K. Molecular chaperones and heat shock proteins in atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H506–H514. [Google Scholar] [CrossRef] [PubMed]
- Falck-Hansen, M.; Kassiteridi, C.; Monaco, C. Toll-like receptors in atherosclerosis. Int. J. Mol. Sci. 2013, 14, 14008–14023. [Google Scholar] [CrossRef] [PubMed]
- Stewart, V. Regulation of nitrate and nitrite reductase synthesis in enterobacteria. Antonie Van Leeuwenhoek 1994, 66, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Xu, D. Research progress on Th17 and T regulatory cells and their cytokines in regulating atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 929078. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Frutos Rde, L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, J.; Liu, C.; Yan, Z.; Shi, D. Interleukin-17 links Inflammatory cross-talks between comorbid psoriasis and atherosclerosis. Front. Immunol. 2022, 13, 835671. [Google Scholar] [CrossRef]
- Erbel, C.; Akhavanpoor, M.; Okuyucu, D.; Wangler, S.; Dietz, A.; Zhao, L.; Stellos, K.; Little, K.M.; Lasitschka, F.; Doesch, A.; et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J. Immunol. 2014, 193, 4344–4355. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Liu, X.; Awar, L.; McMickle, A.; Bai, F.; Nagarajan, S.; Yu, S. IL-17 induces expression of vascular cell adhesion molecule through signalling pathway of NF-kappaB, but not Akt1 and TAK1 in vascular smooth muscle cells. Scand. J. Immunol. 2013, 77, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Diaz, J.; Aragon-Vela, J.; Munoz-Quezada, S.; Tercedor-Sanchez, L.; Abadia-Molina, F. The gut microbiota and its implication in the development of atherosclerosis and related cardiovascular diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Probiotics, prebiotics and synbiotics-A promising strategy in prevention and treatment of cardiovascular diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Sun, L.; Yu, Y.; Fan, H.; Li, X.; Zhang, X.; Zhuo, X.; Guo, M.; Liu, J.; Liu, P.; et al. A gut feeling of statin. Gut Microbes 2024, 16, 2415487. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Deehan, E.C.; Al Antwan, S.; Witwer, R.S.; Guerra, P.; John, T.; Monheit, L. Revisiting the concepts of prebiotic and prebiotic effect in light of scientific and regulatory progress-A consensus paper from the Global Prebiotic Association. Adv. Nutr. 2024, 15, 100329. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymanska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Wu, H.; Chiou, J. Potential benefits of probiotics and prebiotics for coronary heart disease and stroke. Nutrients 2021, 13, 2878. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martinez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Q.; Huangfu, N.; Cui, H. The effect and mechanism of inulin on atherosclerosis is mediated by the characteristic intestinal flora and metabolites. Coron. Artery Dis. 2024, 35, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Phumkhachorn, P.; Rattanachaikunsopon, P. Probiotics: Sources, selection and health benefits. Res. J. Biotechnol. 2023, 18, 102–113. [Google Scholar] [CrossRef]
- Din, A.U.; Hassan, A.; Zhu, Y.; Yin, T.; Gregersen, H.; Wang, G. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl. Microbiol. Biotechnol. 2019, 103, 9217–9228. [Google Scholar] [CrossRef]
- Reis, S.A.; Conceicao, L.L.; Rosa, D.D.; Siqueira, N.P.; Peluzio, M.C.G. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr. Res. Rev. 2017, 30, 36–49. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Gallardo, A.L.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020, 10, 1165. [Google Scholar] [CrossRef]
- Hou, G.; Peng, W.; Wei, L.; Li, R.; Yuan, Y.; Huang, X.; Yin, Y. Lactobacillus delbrueckii interfere with bile acid enterohepatic circulation to regulate cholesterol metabolism of growing-finishing pigs via its bile salt hydrolase activity. Front. Nutr. 2020, 7, 617676. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism In Vitro and In Vivo. Sci. World J. 2014, 2014, 690752. [Google Scholar] [CrossRef]
- El Hage, R.; Al-Arawe, N.; Hinterseher, I. The role of the gut microbiome and trimethylamine oxide in atherosclerosis and age-related disease. Int. J. Mol. Sci. 2023, 24, 2399. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, W.; Li, Y.; Luo, S.; Liu, Q.; Zhong, Y.; Jian, Z.; Bao, M. Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int. Immunopharmacol. 2013, 17, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Khongrum, J.; Yingthongchai, P.; Boonyapranai, K.; Wongtanasarasin, W.; Aobchecy, P.; Tateing, S.; Prachansuwan, A.; Sitdhipol, J.; Niwasabutra, K.; Thaveethaptaikul, P.; et al. Safety and effects of Lactobacillus paracasei TISTR 2593 supplementation on improving cholesterol metabolism and atherosclerosis-related parameters in subjects with hypercholesterolemia: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2023, 15, 661. [Google Scholar] [CrossRef]
- Keleszade, E.; Kolida, S.; Costabile, A. The cholesterol lowering efficacy of Lactobacillus plantarum ECGC 13110402 in hypercholesterolemic adults: A double-blind, randomized, placebo controlled, pilot human intervention study. J. Funct. Foods 2022, 89, 104939. [Google Scholar] [CrossRef]
- Tenorio-Jimenez, C.; Martinez-Ramirez, M.J.; Del Castillo-Codes, I.; Arraiza-Irigoyen, C.; Tercero-Lozano, M.; Camacho, J.; Chueca, N.; Garcia, F.; Olza, J.; Plaza-Diaz, J.; et al. Lactobacillus reuteri V3401 reduces inflammatory biomarkers and modifies the gastrointestinal microbiome in adults with metabolic syndrome: The PROSIR study. Nutrients 2019, 11, 1761. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012, 107, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Probiotics for Vacular Iflammation in Mtabolic Sndrome (PROMISE)—Clinical Trial NCT03719794. Available online: https://clinicaltrials.gov/study/NCT03719794 (accessed on 1 June 2025).
- Cha, R.R.; Sonu, I. Fecal microbiota transplantation: Present and future. Clin. Endosc. 2025, 58, 352–359. [Google Scholar] [CrossRef]
- Chen, J.H.; Chiu, C.H.; Chen, C.C.; Chen, Y.C.; Yeh, P.J.; Kuo, C.J.; Chiu, C.T.; Cheng, H.T.; Pan, Y.B.; Le, P.H. Comparative efficacy of fecal microbiota transplantation in treating refractory or recurrent Clostridioides difficile infection among patients with and without inflammatory bowel disease: A retrospective cohort study. Biomedicines 2024, 12, 1369. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863.e2. [Google Scholar] [CrossRef]
- Leshem, A.; Horesh, N.; Elinav, E. Fecal microbial transplantation and its potential application in cardiometabolic syndrome. Front. Immunol. 2019, 10, 1341. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Laurans, L.; Mouttoulingam, N.; Chajadine, M.; Lavelle, A.; Diedisheim, M.; Bacquer, E.; Creusot, L.; Suffee, N.; Esposito, B.; Melhem, N.J.; et al. An obesogenic diet increases atherosclerosis through promoting microbiota dysbiosis-induced gut lymphocyte trafficking into the periphery. Cell Rep. 2023, 42, 113350. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Yoon, B.H.; Lee, S.M.; Choi, M.; Kim, E.H.; Lee, B.W.; Kim, S.Y.; Pack, C.G.; Sung, Y.H.; Baek, I.J.; et al. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp. Mol. Med. 2022, 54, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Metabolic and Metagenomic Effects of Intestinal Microbiome Repopulation in Unexplained Atherosclerosis—Clinical Trial NCT04410003. Available online: https://clinicaltrials.gov/study/NCT04410003 (accessed on 1 June 2025).
- da Ponte Neto, A.M.; Clemente, A.C.O.; Rosa, P.W.; Ribeiro, I.B.; Funari, M.P.; Nunes, G.C.; Moreira, L.; Sparvoli, L.G.; Cortez, R.; Taddei, C.R.; et al. Fecal microbiota transplantation in patients with metabolic syndrome and obesity: A randomized controlled trial. World J. Clin. Cases 2023, 11, 4612–4624. [Google Scholar] [CrossRef]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J. Am. Heart Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).