The Importance of Gram-Negative Rods in Chronic Rhinosinusitis
Abstract
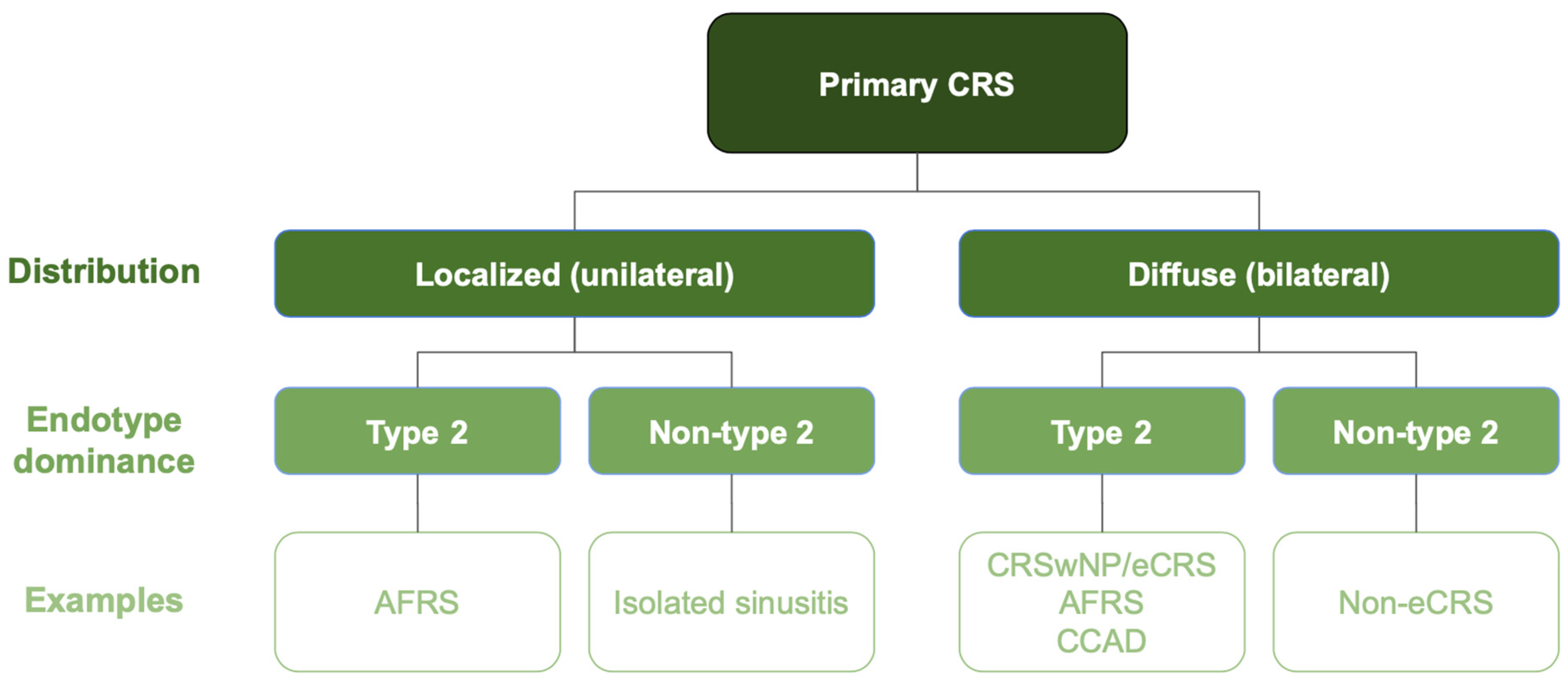
1. Introduction
2. Materials and Methods
2.1. Patients and Specimen Collection
2.2. Isolation and Identification of Sinonasal Microbiota
2.3. Antimicrobial Susceptibility Testing
2.4. Biofilm Formation
- Non-biofilm producer (N): OD ≤ ODc;
- Weak-biofilm producer (W): ODc < OD ≤ 2 × ODc;
- Moderate-biofilm producer (M): 2 × ODc < OD ≤ 4 × ODc;
- Strong-biofilm producer (S): OD > 4 × ODc.
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Patients
3.2. Bacterial Microbiota
3.3. Characteristics of Gram-Negative Bacteria
3.3.1. Antimicrobial Susceptibility
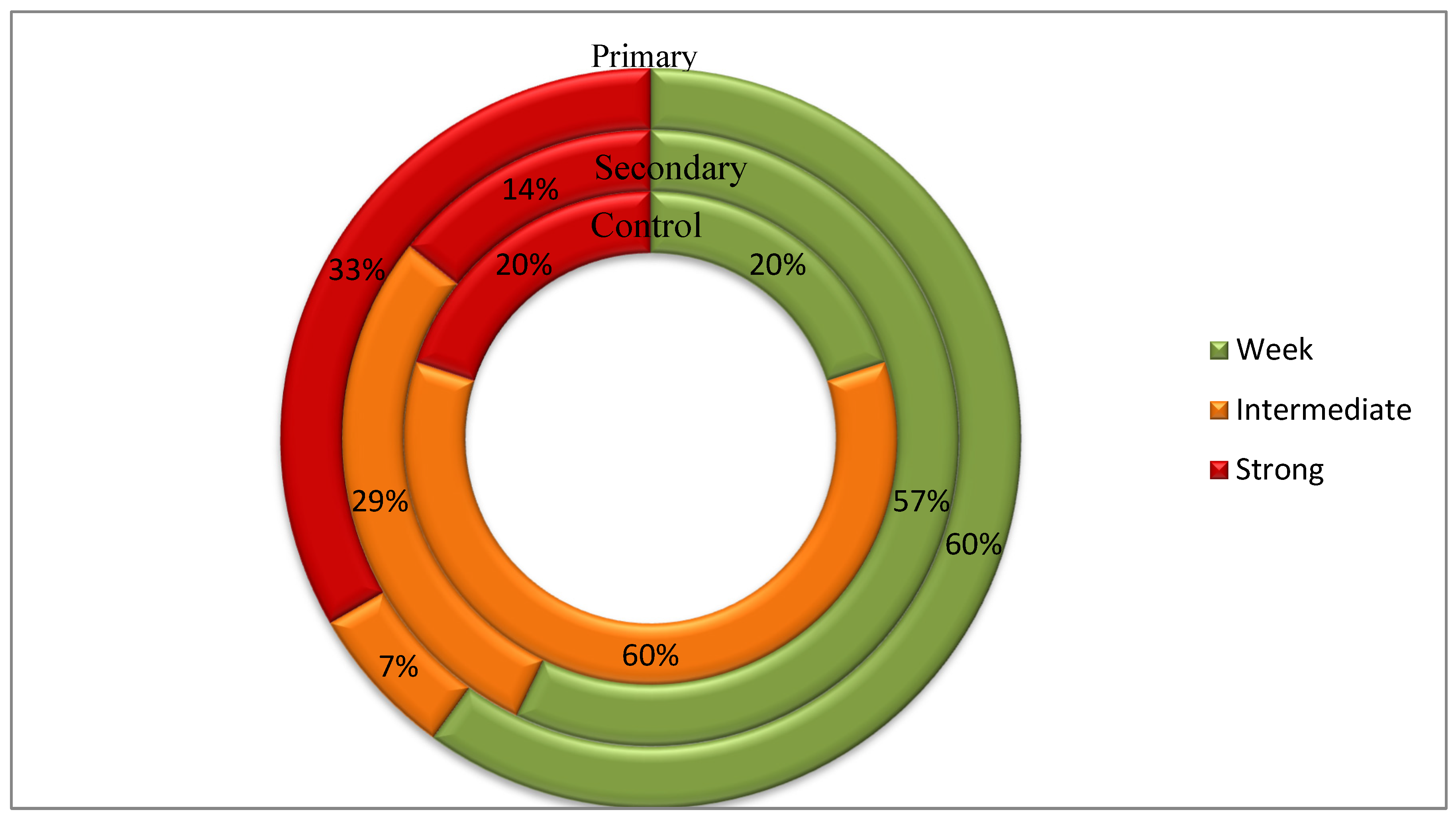
3.3.2. Biofilm Formation Rate
4. Discussion
- Future Perspectives
- Limitations
- The samples in our study were obtained from patients with CRS during ESS. The patients with acute rhinosinusitis or exacerbations of CRS were excluded.
- The sampling site for CRS and control patients was not the same. The anterior ethmoid sinus mucosa was collected from CRS patients, whereas the interior turbinate mucosa was collected from control patients.
- The study protocol did not include molecular methods, so the results refer only to cultivable bacteria.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRS | Chronic rhinosinusitis |
| ESS | Endoscopic sinus surgery |
| EPOS | European Position Paper on Rhinosinusitis and Nasal Polyps |
| CRSwNP | CRS with nasal polyps |
| CRSsNP | CRS without nasal polyps |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Lee, S.; Kim, S.; Son, Y.; Park, J.; Kim, H.J.; Lee, J.; Lee, H.; Smith, L.; Rahmati, M.; et al. Global Incidence and Prevalence of Chronic Rhinosinusitis: A Systematic Review. Clin. Exp. Allergy 2025, 55, 52–66. [Google Scholar] [CrossRef] [PubMed]
- de Loos, D.D.; Lourijsen, E.S.; Wildeman, M.A.M.; Freling, N.J.M.; Wolvers, M.D.J.; Reitsma, S.; Fokkes, W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019, 143, 1207–1214. [Google Scholar] [CrossRef]
- DeConde, A.S.; Bodner, T.E.; Mace, J.C.; Smith, T.L. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 712–719. [Google Scholar]
- Wahid, N.W.; Smith, R.; Clark, A.; Salam, M.; Philpott, C.M. The socioeconomic cost of chronic rhinosinusitis study. Rhinology 2020, 58, 112–125. [Google Scholar] [CrossRef]
- Rudmik, L. Economics of Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2017, 17, 20. [Google Scholar] [CrossRef]
- Bachert, C.; Marple, B.; Schlosser, R.J.; Hopkins, C.; Schleimer, R.P.; Lambrecht, B.N.; Bröker, B.M.; Laidlaw, T.; Song, W.J. Adult chronic rhinosinusitis. Nat. Rev. Dis. Primers 2020, 6, 86. [Google Scholar] [CrossRef]
- Kuan, E.C.; Suh, J.D. Systemic and Odontogenic Etiologies in Chronic Rhinosinusitis. Otolaryngol. Clin. N. Am. 2017, 50, 95–111. [Google Scholar] [CrossRef]
- Kato, A.; Schleimer, R.P.; Bleier, B.S. Mechanisms and pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1491–1503. [Google Scholar] [CrossRef]
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2022, 136, 1442–1453. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Hao, Y.; Wang, B.; Wang, Y.; Liu, Q.; Zhao, J.; Li, Y.; Wang, P.; Wang, X.; et al. Predicting the recurrence of chronic rhinosinusitis with nasal polyps using nasal microbiota. Allergy 2022, 77, 540–549. [Google Scholar] [CrossRef]
- Michalik, M.; Krawczyk, B. Chronic Rhinosinusitis—Microbiological Etiology, Potential Genetic Markers, and Diagnosis. Int. J. Mol. Sci. 2024, 25, 3201. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnana, V.R.; Hauser, L.J.; Frank, D.N. The sinonasal bacterial microbiome in health and disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 20–25. [Google Scholar] [CrossRef]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 211–739. [Google Scholar] [CrossRef]
- Sivasubramaniam, R.; Douglas, R. The microbiome and chronic rhinosinusitis. World J. Otorhinolaryngol. Head Neck Surg. 2018, 314, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The microbiome of the middle meatus in healthy adults. PLoS ONE 2013, 8, e85507. [Google Scholar] [CrossRef]
- Anderson, M.; Stokken, J.; Sanford, T.; Aurora, R.; Sindwani, R. A systematic review of the sinonasal microbiome in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 161–166. [Google Scholar] [CrossRef]
- Pattaroni, C.; Marsland, B.J.; Harris, N.L. Early-Life Host-Microbial Interactions and Asthma Development: A Lifelong Impact? Immunol. Rev. 2025, 330, e70019. [Google Scholar] [CrossRef]
- Kloepfer, K.M.; Kennedy, J.L. Childhood respiratory viral infections and the microbiome. J. Allergy Clin. Immunol. 2023, 152, 827–834. [Google Scholar] [CrossRef]
- Clark, S.E. Commensal bacteria in the upper respiratory tract regulate susceptibility to infection. Curr. Opin. Immunol. 2020, 66, 42–49. [Google Scholar] [CrossRef]
- Durack, J.; Christophersen, C.T. Human Respiratory and Gut Microbiomes—Do They Really Contribute to Respiratory Health? Front. Pediatr. 2020, 8, 528. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, R.; Xiong, X.; Hu, Z.; Mao, X.; Wang, X.; Zhang, J.; Sun, P.; Yue, Z.; Wang, W.; et al. Alterations of nasal microbiome in eosinophilic chronic rhinosinusitis. J. Allergy Clin. Immunol. 2023, 151, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.; Moreno-Jimenez, E.; Morgado, N.; García-Sánchez, A.; Gil-Melcón, M.; Pérez-Pazos, J.; Estravís, M.; Isidoro-García, M.; Dávila, I.; Sanz, C. The Role of the Gut and Airway Microbiota in Chronic Rhinosinusitis with Nasal Polyps: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8223. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Wang, S.H.; Liou, M.L.; Shen, T.A.; Lu, Y.C.; Hsin, C.H.; Yang, S.F.; Chen, Y.Y.; Chang, T.H. Microbiota Dysbiosis in Fungal Rhinosinusitis. J. Clin. Med. 2019, 8, 1973. [Google Scholar] [CrossRef]
- Ccami-Bernal, F.; Barriga-Chambi, F.; Ortiz-Benique, Z.N.; Ferrary, E.; Torres, R. Variability of the Microbiota in Chronic Rhinosinusitis: A Scoping Review. Am. Acad. Otolaryngol.–Head Neck Surg. Found. 2024, 8, 70029. [Google Scholar] [CrossRef]
- Koeller, K.; Herlemann, D.P.R.; Schuldt, T.; Ovari, A.; Guder, E.; Podbielski, A.; Kreikemeyer, B.; Olzowy, B. Microbiome and Culture Based Analysis of Chronic Rhinosinusitis Compared to Healthy Sinus Mucosa. Front. Microbiol. 2018, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Z.; Li, Y.C.; Wang, X.D.; Lu, X.X.; Hu, C.H.; He, S.; Liu, X. The microbiology of chronic rhinosinusitis with and without nasal polyps. Eur. Arch. Otorhinolaryngol. 2018, 275, 1439–1447. [Google Scholar] [CrossRef]
- Vanderpool, E.J.; Rumbaugh, K.P. Host-microbe interactions in chronic rhinosinusitis biofilms and models for investigation. Biofilm 2023, 29, 100160. [Google Scholar] [CrossRef]
- Szaleniec, J.; Gibała, A.; Hartwich, P.; Hydzik-Sobocińska, K.; Konior, M.; Gosiewski, T.; Szaleniec, M. Challenging the gold standard: Methods of sampling for microbial culture in patients with chronic rhinosinusitis. Eur. Arch. Otorhinolaryngol. 2021, 278, 4795–4803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vandepitte, J.; Verhaegen, J.; Engbaek, K.; Rohner, P.; Piot, P.; Heuck, C.C. Basic Laboratory Procedures in Clinical Bacteriology, 2nd ed.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 14 October 2024).
- Dlugaszewska, J.; Leszczynska, M.; Lenkowski, M.; Tatarska, A.; Pastusiak, T.; Szyfter, W. The pathophysiological role of bacterial biofilms in chronic sinusitis. Eur. Arch. Otorhinolaryngol. 2016, 273, 61989–61994. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćwirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Psaltis, A.P.; Mackenzie, B.W.; Cope, E.K.; Ramakrishnan, V.R. Unraveling the role of the microbiome in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1513–1521. [Google Scholar] [CrossRef]
- Chegini, Z.; Shariati, A.; Asghari, A.; Rajaeih, S.; Ghorbani, M.; Jalessi, M.; Mirshekar, M.; Razavi, S. Molecular analysis of dominant paranasal sinus bacteria in patients with and without chronic rhinosinusitis. Arch. Microbiol. 2022, 204, 327. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.K.; Feddema, E.; Boyer, H.C.; Hunter, R.C. Diversity of cystic fibrosis chronic rhinosinusitis microbiota correlates with different pathogen dominance. J. Cyst. Fibros. 2021, 20, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Hoggard, M.; Wagner Mackenzie, B.; Jain, R.; Taylor, M.W.; Biswas, K.; Douglas, R.G. Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease. Clin. Microbiol. Rev. 2017, 30, 321–348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rom, D.; Bassiouni, A.; Eykman, E.; Liu, Z.; Paramasivan, S.; Alvarado, R.; Earls, P.; Psaltis, A.J.; Harvey, R.J. The Association Between Disease Severity and Microbiome in Chronic Rhinosinusitis. Laryngoscope 2019, 129, 1265–1273. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, D.Y.; Choi, S.; Won, S.; Kang, H.R.; Yi, H. Microbiome profiling of uncinate tissue and nasal polyps in patients with chronic rhinosinusitis using swab and tissue biopsy. PLoS ONE 2021, 16, e0249688. [Google Scholar] [CrossRef]
- Biswas, K.; Hoggard, M.; Jain, R.; Taylor, M.W.; Douglas, R.G. The nasal microbiota in health and disease: Variation within and between subjects. Front. Microbiol. 2015, 9, 134. [Google Scholar] [CrossRef]
- Hoggard, M.; Biswas, K.; Zoing, M.; Wagner Mackenzie, B.; Taylor, M.W.; Douglas, R.G. Evidence of microbiota dysbiosis in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 230–239. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Wormald, P.J. Therapy of Sinonasal Microbiome in CRS: A Critical Approach. Curr. Allergy Asthma Rep. 2017, 17, 59. [Google Scholar] [CrossRef]
- Boase, S.; Foreman, A.; Cleland, E.; Tan, L.; Melton-Kreft, R.; Pant, H.; Hu, F.Z.; Ehrlich, G.D.; Wormald, P.J. The microbiome of chronic rhinosinusitis: Culture, molecular diagnostics and biofilm detection. BMC Infect. Dis. 2013, 8, 210. [Google Scholar] [CrossRef]
- Morawska-Kochman, M.; Jermakow, K.; Nelke, K.; Zub, K.; Pawlak, W.; Dudek, K.; Bochnia, M. The pH Value as a Factor Modifying Bacterial Colonization of Sinonasal Mucosa in Healthy Persons. Ann. Otol. Rhinol. Laryngol. 2019, 128, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Jeican, I.I.; Barbu Tudoran, L.; Florea, A.; Flonta, M.; Trombitas, V.; Apostol, A.; Dumitru, M.; Aluaș, M.; Junie, L.M.; Albu, S. Chronic Rhinosinusitis: MALDI-TOF Mass Spectrometry Microbiological Diagnosis and Electron Microscopy Analysis; Experience of the 2nd Otorhinolaryngology Clinic of Cluj-Napoca, Romania. J. Clin. Med. 2020, 9, 3973. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Hauser, L.J.; Feazel, L.M.; Ir, D.; Robertson, C.E.; Frank, D.N. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J. Allergy Clin. Immunol. 2015, 136, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Pletcher, S.D.; Lynch, S.V.; Goldberg, A.N.; Cope, E.K. Heterogeneity of microbiota dysbiosis in chronic rhinosinusitis: Potential clinical implications and microbial community mechanisms contributing to sinonasal inflammation. Front. Cell. Infect. Microbiol. 2018, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Frank, D.N.; Ramakrishnan, V. Microbiome of the paranasal sinuses: Update and literature review. Am. J. Rhinol. Allergy 2016, 30, 3–16. [Google Scholar] [CrossRef]
- Tabet, P.; Endam, L.M.; Boisvert, P.; Boulet, L.P.; Desrosiers, M. Gram-negative bacterial carriage in chronic rhinosinusitis with nasal polyposis is not associated with more severe inflammation. Int. Forum Allergy Rhinol. 2015, 5, 289–293. [Google Scholar] [CrossRef]
- Stern, S.; Hadar, T.; Nachalon, Y.; Ben-Zvi, H.; Soudry, E.; Yaniv, E. Bacteriology of the Middle Meatus in Chronic Rhinosinusitis with and without Polyposis. ORL 2016, 78, 223–231. [Google Scholar] [CrossRef]
- Saibene, A.M.; Vassena, C.; Pipolo, C.; Trimboli, M.; De Vecchi, E.; Felisati, G.; Drago, L. Odontogenic and rhinogenic chronic sinusitis: A modern microbiological comparison. Int. Forum Allergy Rhinol. 2016, 6, 41–45. [Google Scholar] [CrossRef]
- Bassiouni, A.; Paramasivan, S.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.S.; et al. Microbiotyping the sinonasal microbiome. Front. Cell. Infect. Microbiol. 2020, 10, 137. [Google Scholar] [CrossRef]
- Lam, K.; Schleimer, R.; Kern, R.C. The Etiology and Pathogenesis of Chronic Rhinosinusitis: A Review of Current Hypotheses. Curr. Allergy Asthma Rep. 2015, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, J.; Stryjewska-Makuch, G.; Ścierski, W.; Lisowska, G. Bacterial Flora of the Nose and Paranasal Sinuses Among Patients Over 65 Years Old with Chronic Rhinosinusitis Who Underwent Endoscopic Sinus Surgery. Clin. Interv. Aging 2020, 14, 207–215. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huntley, K.S.; Raber, J.; Fine, L.; Bernstein, J.A. Influence of the Microbiome on Chronic Rhinosinusitis with and Without Polyps: An Evolving Discussion. Front. Allergy 2021, 1, 737086. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Huang, S.S.; Ma, J.H.; Hsieh, B.H.; Tsou, Y.A.; Lin, C.D.; Tai, C.J.; Shih, L.C. Bacteriology of Different Phenotypes of Chronic Rhinosinusitis. Laryngoscope 2024, 134, 1071–1076. [Google Scholar] [CrossRef]
- Wagner Mackenzie, B.; Dassi, C.; Vivekanandan, A.; Zoing, M.; Douglas, R.D.; Biswas, K. Longitudinal analysis of sinus microbiota post endoscopic surgery in patients with cystic fibrosis and chronic rhinosinusitis: A pilot study. Respir. Res. 2021, 22, 106. [Google Scholar] [CrossRef]
- Gan, W.; Zhang, H.; Yang, F.; Liu, S.; Liu, F.; Meng, J. The influence of nasal bacterial microbiome diversity on the pathogenesis and prognosis of chronic rhinosinusitis patients with polyps. Eur. Arch. Otorhinolaryngol. 2021, 278, 1075–1088. [Google Scholar] [CrossRef]
- Kim, D.; Assiri, A.M.; Kim, J.H. Recent Trends in Bacteriology of Adult Patients with Chronic Rhinosinusitis. J. Clin. Med. 2019, 8, 1889. [Google Scholar] [CrossRef]
- Copeland, E.; Leonard, K.; Carney, R.; Kong, J.; Forer, M.; Naidoo, Y.; Oliver, B.G.G.; Seymour, J.R.; Woodcock, S.; Burke, C.M.; et al. Chronic Rhinosinusitis: Potential Role of Microbial Dysbiosis and Recommendations for Sampling Sites. Front. Cell. Infect. Microbiol. 2018, 8, 57. [Google Scholar] [CrossRef]
- Krawczyk, B.; Michalik, M.; Fordon, M.; Wysocka, M.; Samet, A.; Nowicki, B. Escherichia coli Strains with Virulent Factors Typical for Uropathogens were Isolated from Sinuses from Patients with Chronic Rhinosinusitis-Case Report. Pathogens 2020, 25, 318. [Google Scholar] [CrossRef]
- Chalermwatanachai, T.; Vilchez-Vargas, R.; Holtappels, G.; Lacoere, T.; Jáuregui, R.; Kerckhof, F.M.; Pieper, D.H.; Van de Wiele, T.; Vaneechoutte, M.; Van Zele, T.; et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci. Rep. 2018, 8, 7926. [Google Scholar] [CrossRef]
- Feazel, L.M.; Robertson, C.E.; Ramakrishnan, V.R.; Frank, D.N. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope 2012, 122, 467–472. [Google Scholar] [CrossRef]
- Smith, S.S.; Kim, R.; Douglas, R. Is there a role for antibiotics in the treatment of chronic rhinosinusitis? J. Allergy Clin. Immunol. 2022, 149, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Kamińska, D.; Nowak-Malczewska, D.M.; Schneider, A.; Długaszewska, J. Relationship between antibiotic resistance, biofilm formation, genes coding virulence factors and source of origin of Pseudomonas aeruginosa clinical strains. Ann. Agric. Environ. Med. 2021, 28, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Sabino, H.A.C.; Valera, F.C.P.; Santos, D.V.; Fantucci, M.Z.; Titoneli, C.C.; Martinez, R.; Anselmo-Lima, W.T.; Tamashiro, E. Biofilm and Planktonic Antibiotic Resistance in Patients with Acute Exacerbation of Chronic Rhinosinusitis. Front. Cell. Infect. Microbiol. 2022, 17, 813076. [Google Scholar] [CrossRef] [PubMed]
- Manciula, L.G.; Jeican, I.I.; Tudoran, L.B.; Albu, S. Biofilms and inflammation in patients with chronic rhinosinusitis. Med. Pharm. Rep. 2020, 93, 374–383. [Google Scholar] [CrossRef]
- Vlad, D.; Dutu, A.; Apostol, A.; Trombitas, V.; Mihalca, A.; Albu, S. The Effect of Spray Cryotherapy on Microbial Biofilms in Chronic Rhinosinusitis. Curr. Infect. Dis. Rep. 2018, 13, 41. [Google Scholar] [CrossRef]
- Di Luca, M.; Navari, E.; Esin, S.; Menichini, M.; Barnini, S.; Trampuz, A.; Casani, A.; Batoni, G. Detection of Biofilms in Biopsies from Chronic Rhinosinusitis Patients: In Vitro Biofilm Forming Ability and Antimicrobial Susceptibility Testing in Biofilm Mode of Growth of Isolated Bacteria. Adv. Exp. Med. Biol. 2018, 1057, 1–27. [Google Scholar] [CrossRef]
- Foreman, A.; Boase, S.; Psaltis, A.; Wormald, P.J. Role of bacterial and fungal biofilms in chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2012, 12, 127–135. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Stolz, D.B. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am. J. Rhinol. 2005, 19, 452–457. [Google Scholar] [CrossRef]
- Bezerra, T.F.; Padua, F.G.; Gebrim, E.M.; Saldiva, P.H.; Voegels, R.L. Biofilms in chronic rhinosinusitis with nasal polyps. Otolaryngol. Head Neck Surg. 2011, 144, 612–616. [Google Scholar] [CrossRef] [PubMed]

| Control Group (n = 47) | Patients with Primary CRS (n = 52) | Patients with Secondary CRS (n = 22) | |
|---|---|---|---|
| Mean age | 38 | 45 | 43 |
| Male/female | 30/17 | 29/23 | 12/10 |
| (63.8%/36.2%) | (55.8%/44.2%) | (54.5%/45.5%) | |
| Asthma | 4 (8.5%) | 16 (30.8%) | 1 (4.5%) |
| Inhalant allergy | 12 (25.5%) | 9 (17.3%) | 8 (35.5%) |
| Hypertension | 6 (12.8%) | 8 (15.4%) | 3 (13.6%) |
| Cancer | 1 (2.1%) | 1 (1.9%) | 0 (0.0%) |
| Diabetes | 3 (6.4%) | 1 (1.9%) | 6 (27.3%) |
| Steroid use | 6 (12.8%) | 13 (25%) | 9 (40.9%) |
| Smoking | 7 (14.9%) | 8 (15.45%) | 4 (18.2%) |
| Antibiotics | 6 (12.8%) | 7 (13.5%) | 3 (13.6%) |
| Sinus irrigation | 3 (6.4%) | 10 (19.2%) | 7 (31.8%) |
| Contact with | |||
| chemicals | 17 (36.2%) | 12 (23.1%) | 5 (22.7%) |
| Facial trauma | 6 (12.8%) | 2 (3.8%) | 1 (4.5%) |
| Sinus surgery | 1 (2.1%) | 16 (30.8%) | 6 (27.3%) |
| Nose surgery | 10 (21.3%) | 7 (13.5%) | 9 (40.9%) |
| Bacteria | Primary CRS (n = 90 Strains) | Secondary CRS (n = 35 Strains) | Control (n = 73 Strains) | p-Value * |
|---|---|---|---|---|
| Anaerobes (obligate) | ||||
| Cutibacterium acnes | 2 (2.2%) | 1 (2.9%) | 2 (2.7%) | 0.9690 |
| Peptostreptococcus spp. | 1 (1.1%) | - | 1 (1.4%) | 0.7942 |
| Fusobacterium spp. | 1 (1.1%) | - | - | 0.5471 |
| Mixed anaerobic component of the URT microbiota | 4 (4.4%) | 2 (5.7%) | 6 (8.2%) | 0.6012 |
| Gram-positive Aerobes | ||||
| Staphylococcus epidermidis | 33 (36.7%) | 13 (37.1%) | 16 (22.0%) | 0.0931 |
| Staphylococcus aureus | 14 (15.6%) | 4 (11.4%) | 6 (8.2%) | 0.3578 |
| CNS | 8 (8.9%) | 2 (5.7%) | 3 (4.1%) | 0.4605 |
| Corynebacterium spp. | 3 (3.3%) | - | 4 (5.5%) | 0.3495 |
| Streptococcus pneumoniae | 1 (1.1%) | 1 (2.9%) | - | 0.3775 |
| Streptococcus spp. | 3 (3.3%) | 2 (5.7%) | 1 (1.4%) | 0.4559 |
| Enterococcus faecalis | - | - | 1 (1.4%) | 0.4229 |
| Enterococcus spp. | 2 (2.2%) | - | - | 0.2975 |
| Gram-negative Aerobes | ||||
| Escherichia coli | 7 (7.8%) | 4 (11.4%) | 1 (1.4%) | 0.0797 |
| Klebsiella oxytoca | 2 (2.2%) | 1 (2.9%) | - | 0.3971 |
| Serratia marcescens | 1 (1.1%) | - | - | 0.5471 |
| Citrobacter koseri | - | 1 (2.9%) | 3 (4.1%) | 0.1662 |
| Citrobacter freundii | - | 1 (2.9%) | - | 0.0963 |
| Proteus mirabilis | 1 (1.1%) | - | 1 (1.4%) | 0.7942 |
| Enterobacter cloacae | 2 (2.2%) | - | - | 0.2975 |
| Morganella morgani | 2 (2.2%) | - | - | 0.2975 |
| Mixed aerobic component of the URT microbiota | 3 (3.3%) | 3 (8.5%) | 28 (38.4%) | 0.00000001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, M.; Fijalkowska-Ratajczak, T.; Kaminska, D.; Leszczyńska, M.; Dlugaszewska, J. The Importance of Gram-Negative Rods in Chronic Rhinosinusitis. Appl. Sci. 2025, 15, 6108. https://doi.org/10.3390/app15116108
Ratajczak M, Fijalkowska-Ratajczak T, Kaminska D, Leszczyńska M, Dlugaszewska J. The Importance of Gram-Negative Rods in Chronic Rhinosinusitis. Applied Sciences. 2025; 15(11):6108. https://doi.org/10.3390/app15116108
Chicago/Turabian StyleRatajczak, Magdalena, Tatiana Fijalkowska-Ratajczak, Dorota Kaminska, Małgorzata Leszczyńska, and Jolanta Dlugaszewska. 2025. "The Importance of Gram-Negative Rods in Chronic Rhinosinusitis" Applied Sciences 15, no. 11: 6108. https://doi.org/10.3390/app15116108
APA StyleRatajczak, M., Fijalkowska-Ratajczak, T., Kaminska, D., Leszczyńska, M., & Dlugaszewska, J. (2025). The Importance of Gram-Negative Rods in Chronic Rhinosinusitis. Applied Sciences, 15(11), 6108. https://doi.org/10.3390/app15116108






