Investigation of the Antioxidant and Antimicrobial Properties of Ultrasound-Assisted Extracted Phenolics from Aronia melanocarpa Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BC Pomace
2.3. UAE
2.4. Total Phenolic Content (TPC)
2.5. Antioxidant Activity
2.6. Ultra-High-Performance Liquid Chromatography (UHPLC-HRMS-MS)
2.7. Antimicrobial Activity
2.8. Molecular Docking Tests
3. Results and Discussion
3.1. Effects of Ultrasound Amplitude on Bioactive Extraction in Different Solvents
3.2. Influence of Ultrasound Time on Bioactive Extraction in Different Solvents
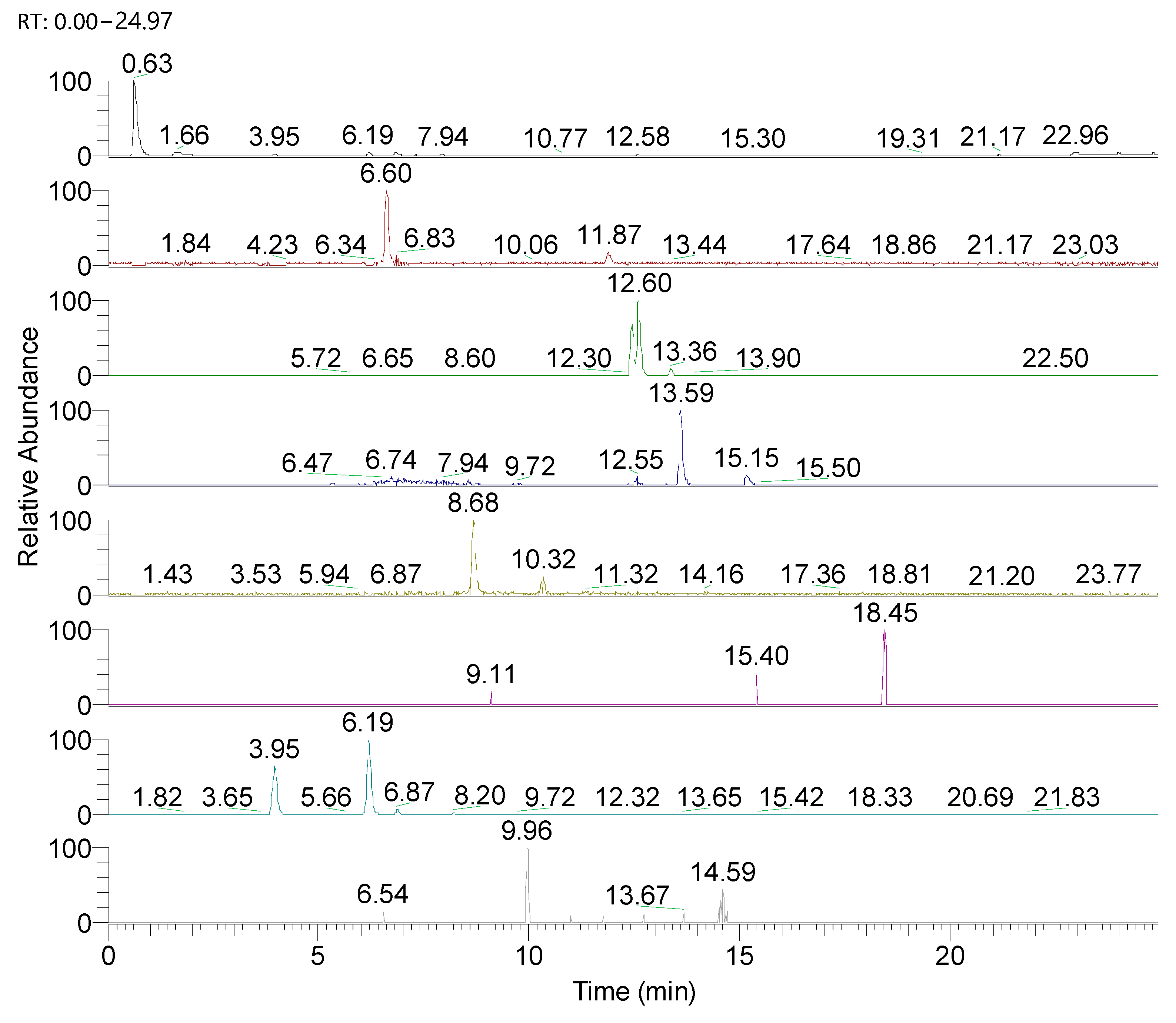
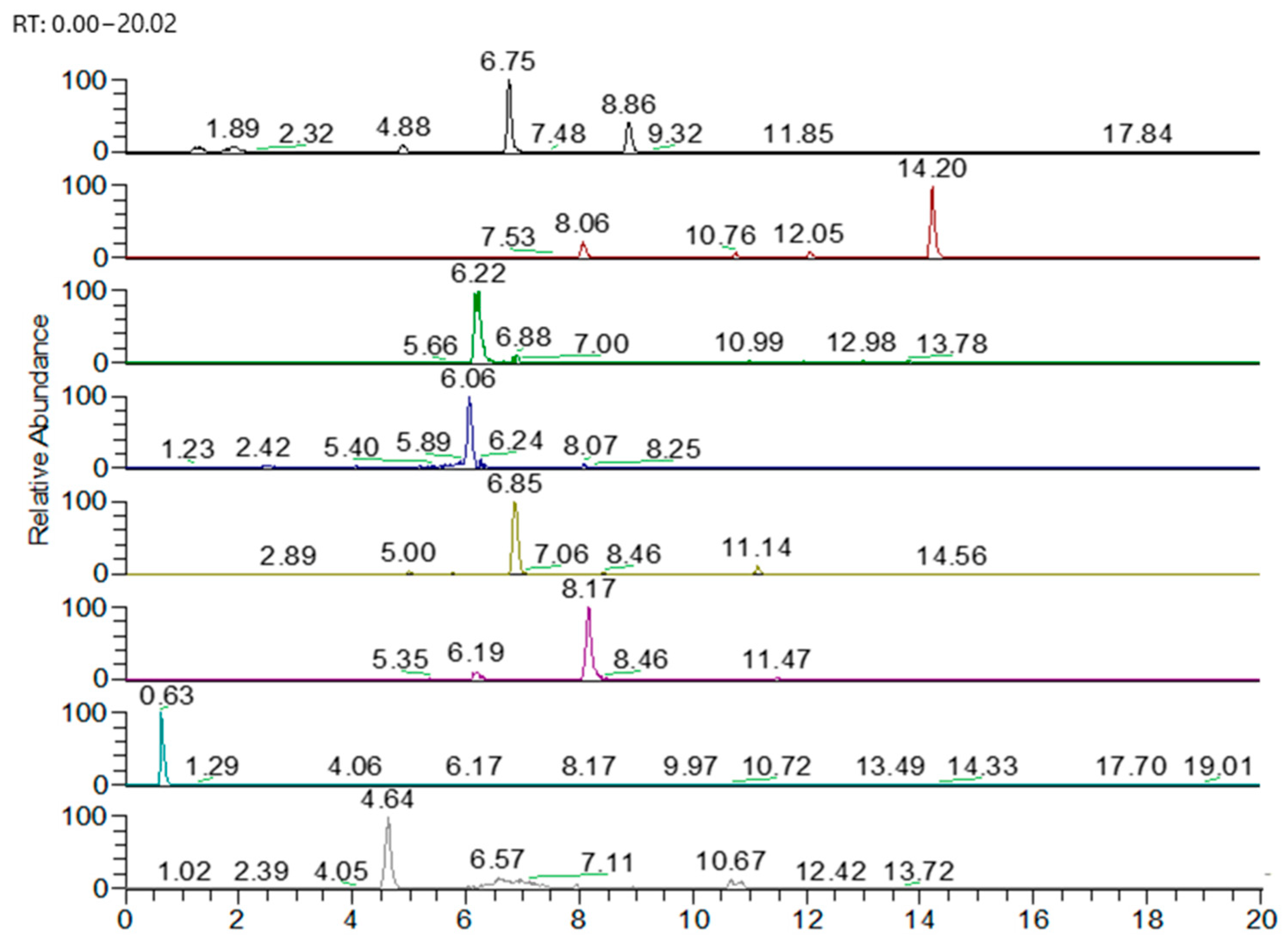
3.3. UHPLC-HRMS-MS Characterization
3.4. Antimicrobial Activity
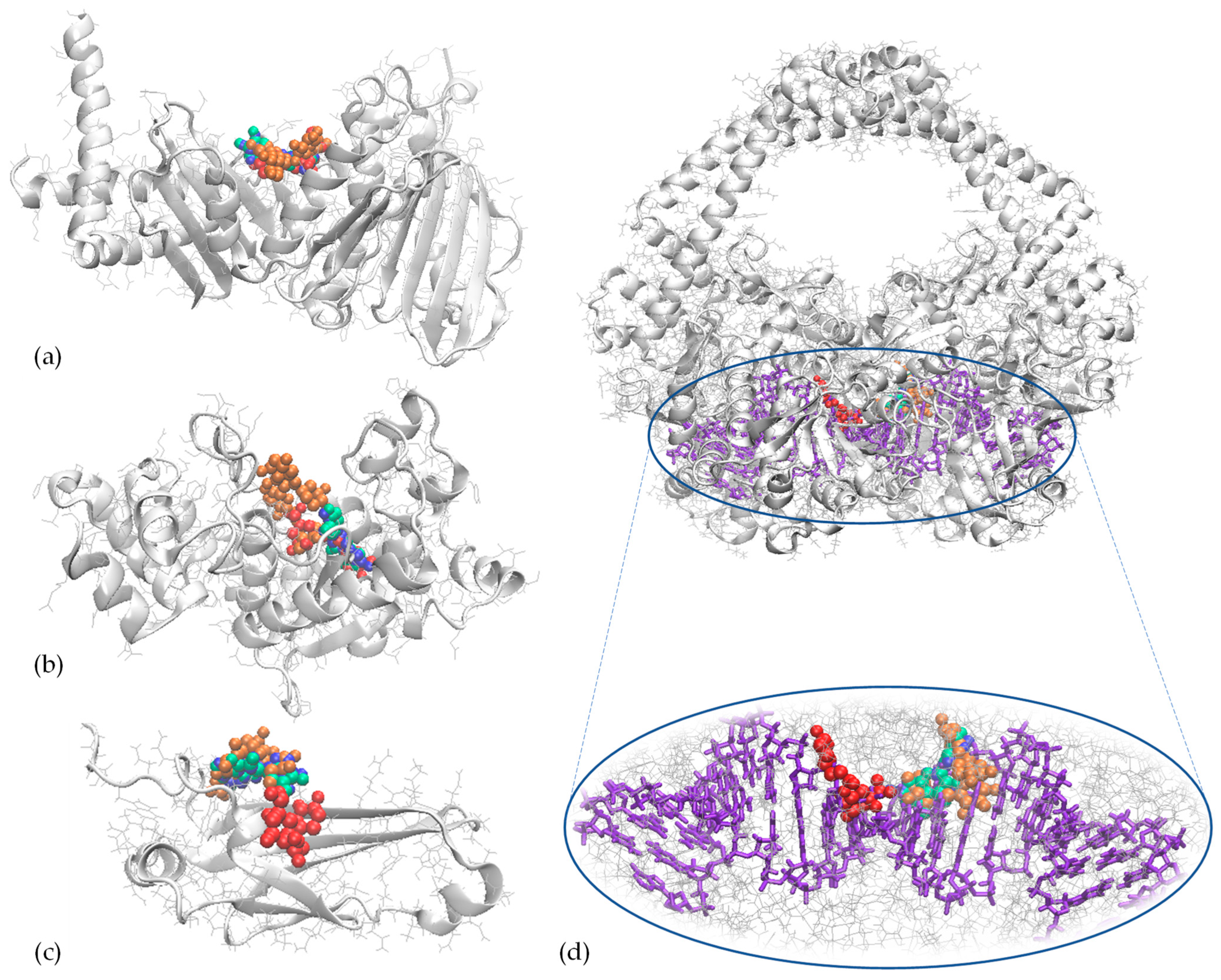
3.5. Molecular Docking Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devirgiliis, C.; Guberti, E.; Mistura, L.; Raffo, A. Effect of fruit and vegetable consumption on human health: An update of the literature. Foods 2024, 13, 3149. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, X.; Chen, X.; Ding, C.; Dong, L.; Zhang, J.; Sun, S.; Ding, Q.; Khatoom, S.; et al. Chokeberry (Aronia melanocarpa) as a new functional food relationship with health: An Overview. J. Future Foods 2021, 1–2, 168–178. [Google Scholar] [CrossRef]
- Lee, K.L.; Kim, S.; Lee, J.; Son, H.; Lee, J.; Park, C.; Yoo, H. Improved recovery of antioxidants from aronia juice processing residue via optimization of extraction variables based on multi-prediction models. Sus. Chem. Pharm. 2024, 39, 101546. [Google Scholar] [CrossRef]
- Available online: https://www.futuremarketinsights.com/reports/aronia-berries-market (accessed on 27 May 2025).
- Jakobek, L.; Šeruga, M.; Medvidovíc-Kosanovic, M.; Novak Jovanovic, I. Antioxidant Activity and Polyphenols of Aronia in Comparison to other Berry Species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Andrade, T.A.; Hamerski, F.; Lopez Fetzer, D.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Rohn, S.; Mocan, A. Fermented Black Chokeberry (Aronia melanocarpa (Michx.) Elliott) products—A systematic review on the composition and current scientific evidence of possible health benefits. Food Res. Int. 2024, 196, 115094. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Dro, A.; Gramza-Micha, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors—An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Ghanbari, S.A.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. A review of the anti-bacterial effects exerted by Aronia melanocarpa. Eur. J. Microbiol. Immunol. 2025, 15, 13–20. [Google Scholar] [CrossRef]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Rodríguez-Werner, M.; Winterhalter, P.; Esatbeyoglu, T. Phenolic Composition, radical scavenging activity and an approach for authentication of Aronia melanocarpa berries, juice, and pomace. J. Food Sci. 2019, 84, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. mechanisms, techniques, combinations, protocols and applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Sethi, S.; Rathod, V. Recent advances in ultrasound-assisted extraction of natural products using novel solvents: A mini-review. Chem. Eng. 2025, 48, 101132. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Qiu, Y.; Ren, H.; Ju, D.H.; Jia, H.L. Optimization of ultrasound-assisted aqueous two-phase system extraction of polyphenolic compounds from Aronia melanocarpa pomace by response surface methodology. Prep. Biochem. Biotechnol. 2017, 47, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Galvan D’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Enachi, E.; Stănciuc, N.; Aprodu, I. Optimization of ultrasound assisted extraction of phenolic compounds from cornelian cherry fruits using response surface methodology. CyTA-J. Food 2019, 17, 814–823. [Google Scholar] [CrossRef]
- Mihalcea, L.; Aprodu, I.; Dumitrașcu, L.; Cucolea, E.I.; Dănilă, G.-M.; Enachi, E.; Barbu, V.; Constantin, O.E.; Grigore-Gurgu, L.; Stănciuc, N. Whey proteins isolate-based biopolymeric combinations to microencapsulate supercritical fluid extracted oleoresins from sea buckthorn pomace. Pharmaceuticals 2021, 14, 1217. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, H.; Guo, X.; Guo, D.U.; Yang, Y.; Wang, X.; Zhang, C.; Shan, Z.; Xia, X.; et al. Antibiofilm activity of shonin against Listeria monocytogenes and inhibition of key virulence factors. Food Control 2021, 120, 107558. [Google Scholar] [CrossRef]
- Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and antibiofilm activities of essential oils against Escherichia coli o157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 730. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nuc. Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Stanger, F.V.; Dehio, C.; Schirmer, T. Structure of the N-terminal Gyrase B fragment in complex with ADP⋅ Pi reveals rigid-body motion induced by ATP hydrolysis. PLoS ONE 2014, 9, 107289. [Google Scholar] [CrossRef] [PubMed]
- Velarde, J.J.; Varney, K.M.; Inman, K.G.; Farfan, M.; Dudley, E.; Fletcher, J.; Weber, D.J.; Nataro, J.P. Solution structure of the novel dispersin protein of enteroaggregative Escherichia coli. Mol. Microbiol. 2007, 66, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Janson, C.A.; Smith, W.W.; Green, S.M.; McDevitt, P.; Johanson, K.; Carter, P.; Hibbs, M.; Lewis, C.; Chalker, A.; et al. Crystal structure of Staphylococcus aureus tyrosyl-tRNA synthetase in complex with a class of potent and specific inhibitors. Prot. Sci. 2001, 10, 2008–2016. [Google Scholar] [CrossRef]
- Lu, Y.; Vibhute, S.; Li, L.; Okumu, A.; Ratigan, S.C.; Nolan, S.; Papa, J.L.; Mann, C.A.; English, A.; Chen, A.; et al. Optimization of TopoIV potency, ADMET properties, and hERG inhibition of 5-amino-1, 3-dioxane-linked novel bacterial topoisomerase inhibitors: Identification of a lead with in vivo efficacy against MRSA. J. Med. Chem. 2021, 64, 15214–15249. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Prot. Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Krissinel, E. Crystal contacts as nature’s docking solutions. J. Comput. Chem. 2010, 31, 133–143. [Google Scholar] [CrossRef]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef] [PubMed]
- Sady, S.; Matuszak, L.; Błaszczyk, A. Optimisation of ultrasonic-assisted extraction of bioactive compounds from Chokeberry Pomace using response surface methodology. Acta Sci. Pol. Technol. Aliment. 2019, 18, 249–256. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of bioactive components and antioxidant activity of Aronia melanocarpa fruits at various stages of their growth, using chemometric Methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Sagandyk, A.T.; Liberal, Â.; da Silveira, T.F.; Alves, M.J.; Ferreira, I.C.; Zhakupova, G.N.; Makangali, K.; Tultabayeva, T.C.; Barros, L. Nutritional, phytochemical, and bioactive prospects of black chokeberry (Aronia melanocarpa) and saskatoon berry (Amelanchier ovalis) grown in the Republic of Kazakhstan. App. Food Res. 2024, 4, 100564. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (Aronia melanocarpa L.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Higbee, J.; Solverson, P.; Zhu, M.; Carbonero, F. The emerging role of dark berry polyphenols in human health and nutrition. Food Front. 2022, 3, 3–27. [Google Scholar] [CrossRef]
- Rugină, D.; Diaconeasa, Z.; Coman, C.; Bunea, A.; Socaciu, C.; Pintea, A. Chokeberry anthocyanin extract as pancreatic β-cell protectors in two models of induced oxidative stress. Oxidative Med. Cell. Longev. 2015, 2015, 429075. [Google Scholar] [CrossRef]
- Meilan, L.; Beads, G.; Hongmei, L.; Chengzhe, J. Preliminary study on the antioxidant activity of sorbus nigrum on liver and kidney of mice. Livest. Feed. Sci. 2018, 39, 1388. [Google Scholar]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The effect of chlorogenic acid on Bacillus subtilis based on metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of catechins in the treatment of bacterial infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of Anti-Staphylococcus aureus Activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simões, M. Combinatorial activity of flavonoids with antibiotics against drug-resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef]
- Kannan, S.; Balakrishnan, J.; Govindasamy, A.; Arunagiri, R. New insights into the antibacterial mode of action of quercetin against uropathogen Serratia marcescens in-vivo and in-vitro. Sci. Rep. 2022, 12, 21912. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2005, 48, 11–17. [Google Scholar]
- Salamon, I.; Şimşek Sezer, E.N.; Kryvtsova, M.; Labun, P. Antiproliferative and antimicrobial activity of anthocyanins from berry fruits after their isolation and freeze-drying. App. Sci. 2021, 11, 2096. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial Characteristics and Mechanisms of Action of Aronia melanocarpa Anthocyanins against Escherichia coli. LWT Food Sci. Technol. 2021, 150, 112018. [Google Scholar] [CrossRef]
- Apostolos, A.J.; Pires, M.M. Impact of crossbridge structure on peptidoglycan crosslinking: A synthetic stem peptide approach. In Methods in Enzymology; Carlson, E., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 665, pp. 259–279. [Google Scholar]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; Araújo, A.C.J.D.; Freitas, P.R.; Almeida, R.S.D.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Barbosa, C.R.; Scherf, J.R.; De Freitas, T.S.; De Menezes, I.R.A.; Pereira, R.L.S.; Dos Santos, J.F.S.; De Jesus, S.S.P.; Lopes, T.P.; De Sousa Silveira, Z.; De Morais Oliveira-Tintino, C.D.; et al. Effect of carvacrol and thymol on NorA efflux pump inhibition in multidrug-resistant (MDR) Staphylococcus aureus strains. J. Bioenerg. Biomembr. 2021, 53, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Giacomini, D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.B.; Kumar, A.; Medda, R.; Gatto, G.; Pal, R.; Fais, A.; Matos, M.J. Antibacterial activity and molecular docking studies of a selected series of hydroxy-3-arylcoumarins. Molecules 2019, 24, 2815. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, Y.; Zang, X.; Wu, T.; Qi, X.; Pan, S.; Xu, X. 3D-QSAR and docking studies of flavonoids as potent Escherichia coli inhibitors. Sci. Rep. 2016, 6, 23634. [Google Scholar] [CrossRef]
- Abishad, P.; Niveditha, P.; Unni, V.; Vergis, J.; Kurkure, N.V.; Chaudhari, S.; Rawool, D.B.; Barbuddhe, S.B. In silico molecular docking and in vitro antimicrobial efficacy of phytochemicals against multi-drug-resistant enteroaggregative Escherichia coli and non-typhoidal Salmonella spp. Gut Pathog. 2021, 13, 46. [Google Scholar] [CrossRef]
| Component | Conventional 24 h, 25 °C | Ultrasound Amplitude, % | |||
|---|---|---|---|---|---|
| 20 | 30 | 40 | 60 | ||
| Solvent: Water | |||||
| TPC mg GAE/g d.w. | 33.50 ± 1.32 A | 27.38 ± 1.46 Aa | 26.35 ± 3.08 Aa | 33.03 ± 3.15 Aa | 30.39 ± 3.66 Aa |
| DPPH scavenging activity mM Trolox/g d.w. | 103.84 ± 2.65 A | 73.10 ± 0.11 b | 82.95 ± 0.55 a | 80.84 ± 1.76 a | 78.03 ± 1.76 ab |
| ABTS+ scavenging activity mM Trolox/g d.w. | 163.03 ± 0.43 A | 150.26 ± 17.63 Ab | 228.41 ± 10.32 a | 226.58 ± 0.0 a | 228.41 ± 12.9 a |
| Solvent: 70% Ethanol | |||||
| TPC mg GAE/g d.w. | 78.89 ± 3.00 A | 72.15 ± 1.39 Ac | 86.16 ± 0.66 a | 73.60 ± 1.39 Abc | 78.95 ± 2.34 Ab |
| DPPH scavenging activity mM Trolox/g d.w. | 149.12 ± 0.33 A | 149.59 ± 0.77 Aa | 151.16 ± 0.55 Aa | 149.91 ± 0.77 Aa | 153.19 ± 0.99 Aa |
| ABTS+ scavenging activity mM Trolox/g d.w. | 178.81 ± 1.50 A | 348.81 ± 8.6 a | 360.67 ± 4.73 a | 360.67 ± 3.87 a | 360.37 ± 5.16 a |
| Component | Conventional 24 h, 25 °C | Ultrasound Exposure, min | |||
|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | ||
| Solvent: Water | |||||
| TPC mgGAE/g d.w. | 33.50 ± 1.32 A | 25.09 ± 0.0 b | 26.65 ± 3.08 b | 31.79 ± 0.95 abA | 37.29 ± 1.68 aA |
| DPPH scavenging activity mM Trolox/g d.w. | 103.84 ± 2.65 A | 64.10 ± 4.64 c | 82.95 ± 0.55 b | 79.51 ± 2.54 b | 98.75 ± 2.98 aA |
| ABTS+ scavenging activity mM Trolox/g d.w. | 163.03 ± 0.43 A | 163.34 ± 7.74 Ab | 201.34 ± 20.21 Ab | 183.10 ± 15.05 Ab | 269.15 ± 17.2 a |
| Solvent: 70% Ethanol | |||||
| TPC mg GAE/g d.w. | 78.89 ± 3.00 A | 67.74 ± 2.34 a | 67.90 ± 3.44 a | 73.08 ± 0.51 Aa | 73.19 ± 1.39 Aa |
| DPPH scavenging activity mM Trolox/g d.w. | 149.12 ± 0.33 A | 149.12 ± 3.65 Aa | 154.99 ± 1.10 Aa | 154.05 ± 1.99 Aa | 155.22 ± 1.21 Aa |
| ABTS+ scavenging activity mM Trolox/g d.w. | 178.81 ± 1.50 A | 356.42 ± 0.43 a | 363.41 ± 4.3 a | 359.76 ± 5.16 a | 364.93 ± 1.29 a |
| Compound | Chemical Formula | Monitored Ion [M-H]− | Retention Time (min) | Amount (mg/100 g d.w.) |
|---|---|---|---|---|
| Chlorogenic acid | C16H18O9 | 353.08780 | 6.17 | 9045.88 |
| Caffeic acid | C9H8O4 | 179.03501 | 6.6 | 0.265 |
| Epicatechin | C15H14O6 | 289.07180 | 5.48 | 791.44 |
| Catechin | C15H14O6 | 289.07180 | 3.29 | 65.55 |
| Syringic acid | C9H10O5 | 197.04560 | 4.13 | 11.41 |
| p-Coumaric acid | C9H8O3 | 163.03950 | 6.76 | 45.10 |
| Ferulic acid | C10H10O4 | 193.05070 | 12.63 | 27.60 |
| Hyperoside (quercetin-3-O-galactoside) | C21H20O12 | 463.08768 | 10.82 | 757.85 |
| Rutin | C27H30O16 | 609.14610 | 12.6 | 1098.05 |
| Naringin | C27H32O14 | 579.17185 | 9.96 | 11.96 |
| Abscisic acid | C15H20O4 | 263.12890 | 14.82 | 13.49 |
| Gallic acid | C7H6O5 | 169.01430 | 11.41 | 27.62 |
| Quercetin | C15H10O7 | 301.03540 | 13.59 | 564.80 |
| Chrysin | C15H10O4 | 253.05066 | 14.69 | 22.61 |
| Pinocembrin | C15H12O4 | 255.06630 | 18.45 | 25.19 |
| Naringenin | C15H12O5 | 271.06120 | 15.64 | 5.93 |
| Daidzin | C21H20O9 | 415.10348 | 16.26 | 0.49 |
| Daidzein | C15H10O4 | 253.05066 | 19.08 | 0.23 |
| Vanillic acid | C8H8O4 | 167.03500 | 8.68 | 47.13 |
| Compound | Chemical Formula | Monitored Ion [M-H]− | Retention Time (min) | Fragments |
|---|---|---|---|---|
| Liquiritigenin | C15H12O4 | 255.06631 | 18.44 | 211.0764; 135.00761; 119.04889; 117.03323 |
| Kaempferol 3-O-rutosid | C27H30O15 | 593.15122 | 9.56 | 299.05615; 255.02997; 227.0341 |
| Azelaic acid | C9H16O4 | 187.09761 | 14.12 | 169.08600; 143.10655; 123.08015 |
| Cynarine | C25H24O12 | 515.11950 | 12.4 | 515; 353; 191 |
| Rosmarinic acid | C18H16O8 | 359.07727 | 5.29 | 161.0237; 135.0455; 133.0283 |
| Carnasol | C20H26O4 | 329.17586 | 18.86 | 285.1856; 201.0897 |
| Carnosic acid | C20H28O4 | 331.19151 | 20.05 | 287.2015 |
| Neochlorogenic acid | C16H18O9 | 353.08780 | 3.93 | 192.05876; 191.05544; 173.04474 |
| Oleanoic acid | C30H48O3 | 455.35309 | 25.16 | 455.3507; 456.3572 |
| Gallocatechin | C15H14O7 | 305.06668 | 6.06 | 137.0247; 125.0247; 109.0298 |
| Aesculetin | C9H6O4 | 177.01933 | 10.51 | 133.038; 105.0354 |
| Naringenin-7-O-glucoside | C21H22O10 | 433.11402 | 16.45 | 271.0656; 151.0058 |
| Chrysoeriol 7-O-glucoside | C22H22O11 | 461.10896 | 15.62 | 446.0844; 283.024; 255.0296 |
| Apigenin-7-O-glucosid | C21H20O10 | 431.09839 | 17.45 | 269.0428; 268.037 |
| Procyanidin B1 | C30H26O12 | 577.13515 | 6.85 | 125.0240; 289.0698; 407.0802 |
| Procyanidin C1 | C45H38O18 | 865.19854 | 8.17 | 866.2419; 407.0965; 289.0844 |
| Apigenin-O-glucuronide | C21H18O11 | 445.07763 | 6.52 | 269.0455; 151.0012 |
| Caraphenol B/C | C28H22O7 | 469.12928 | 6.86 | 541.1187; 281.0819; 227.0714 |
| Petunidin caffeoyl diglucoside | C37H39O20 | 802.19622 | 6.22 | 640.1502; 478.0974 |
| Dihydroquercetin 3-O-rhamnoside | C21H22O11 | 449.10896 | 11.02 | 285.0407; 151.0026; 123.0233 |
| Dihydroquercetin-3-O-glucoside | C21H22O12 | 465.10387 | 7.99 | 303.2508; 257.0452 |
| Quercetin 3-vicianoside | C26H28O16 | 595.1305 | 4.26 | 300.0262; 271.1450; 255.0276 |
| Myricetin 3-O-galactoside | C21H20O13 | 479.08314 | 4.64 | 317.0284; 271.0223; 151.0022 |
| Hydroxyferulic acid | C16H20O10 | 371.09839 | 6.76 | 209.0434; 165.0544 |
| Quinic acid | C7H12O6 | 191.05611 | 0.63 | 171.02702; 127.03691 |
| Quercetin 3-O-glucuronide | C21H18O13 | 477.06740 | 8.17 | 301.03558; 178.9986; 151.0013 |
| Quercetin (quercetin 3-rhamnoside) | C24H22O15 | 549.0886 | 8.35 | 300.0042; 505.0595 |
| Eriodictyol | C15H12O6 | 287.0561 | 14.20 | 135.0452; 117.0347; 89.0397 |
| Eriodictyol-7-O-glucoside | C21H22O11 | 449.1089 | 8.34 | 345.08292; 135.0453; 139.03893 |
| Eriodictyol-7-glucuronide | C21H20O12 | 463.0882 | 10.82 | 287.0655; 151.0038 |
| Bacterial Strain | |||
|---|---|---|---|
| E. coli | S. aureus | L. monocytogenes | |
| Inhibition zone diameter, mm | 25.50 ± 0.70 | 17.00 ± 1.41 | nd |
| MIC, mg/mL | 0.5 | 1.0 | - |
| MBC, mg/mL | >1.0 | >1.0 | - |
| Complexes formed between GyrB-N and | ||||
| CAT | CLA | QUE | RUT | |
| ΔGint, kcal/mol | −7.22 | −7.04 | −7.26 | −9.01 |
| ΔGdiss, kcal/mol | 0.0 | 10.2 | 9.7 | 0.0 |
| Interaction surface, Å2 | 68.6 | 68.8 | 97.3 | 48.9 |
| Interfacing residues | Phe41, Glu193, Tyr267, Cys268, Phe269, Pro274, Gln275, Arg276 | Phe41, Ile186, Lys189, Arg190, Glu193, Pro274, Thr336 | His38, Phe41, Arg190, Ile266, Pro274, Gln275, Arg276 | His38, Phe41, Ile186, Lys189, Arg190, Pro274, Arg276, Thr336 |
| Amino acids involved in different types of interactions with the ligands | Hydrophobic contacts: Phe41, Pro274, Arg276 | Ionic bonds: His38 Hydrophobic contacts: Tyr267, Phe269, Pro274 | H bonds: Ile266 Hydrophobic contacts: Phe41, Pro274, Arg276 | H bonds: His38 Hydrophobic contacts: Lys189, Tyr267, Arg276, Pro274 |
| Complexes formed between dispersin and | ||||
| CAT | CLA | QUE | RUT | |
| ΔGint, kcal/mol | −5.72 | −5.36 | −6.08 | −5.97 |
| ΔGdiss, kcal/mol | 0.0 | 0.0 | 9.2 | 9.8 |
| Interaction surface, Å2 | 49.7 | 69.9 | 91.4 | 65.5 |
| Interfacing residues | Asp11, Pro12, Leu90, Thr91, Glu92, Trp93 | Ser13, Gln14, Ile16, Lys17, Gln18, Tyr23, Thr91 | Ala7, Asp8, Val10, Pro12, Leu90, Thr91, Glu92, Trp93, Ser98 | Ala7, Asp8, Val10, Asp11, Pro12, Glu92, Trp93, Ser96, Ser98 |
| Amino acids involved in H bonds (no. of H bonds) | Thr91, Trp93 | Lys17, Gln18, Thr91 | Thr91 (2), Trp93 | Val10, Ser96, Ser98 |
| Complexes formed between TyrRS and | ||||
| CAT | CLA | QUE | RUT | |
| ΔGint, kcal/mol | −8.16 | −7.95 | −8.82 | −8.82 |
| ΔGdiss, kcal/mol | 0.0 | 0.0 | 8.1 | 0.0 |
| Interaction surface, Å2 | 86.4 | 80.1 | 135.7 | 82.0 |
| Interfacing residues | Gly38, Ala39, Asp40, His50, Asp80, Thr75, Tyr170, Gln174, Asp195, Gln196 | Cys37, Gly38, Ala39, Asp40, His50, Pro53, Thr75, Asp80, Gly192, Gly193, Asp195, Gln196 | Tyr36, Gly38, Ala39, Asp40, Thr42, His50, Leu70, Thr75, Asp80, Asn124, Tyr170, Gln174, Asp177, Gln190, Asp195, Gln196 | Gly38, His47, Gly49, His50, Pro53, Gly193, Asp195, Gln196, Leu223, Val224, Phe232, Gly233, Lys234 |
| Amino acids involved in different types of interactions with the ligands | H bonds: Thr75, Asp177, Gln190 Hydrophobic contacts: Gln174, Gln190 | H bonds: Thr75 Hydrophobic contacts: Tyr36, Pro53, Gln196 | H bonds: Gly38, Thr75 (2), Asp177, Gln190 Hydrophobic contacts: Gln174 | H bonds: Gly49, Gly193, Val224, Phe232 Hydrophobic contacts: Ala39, Phe54, His50 |
| Complexes formed between DNA gyrase and | ||||
| CAT | CLA | QUE | RUT | |
| ΔGint, kcal/mol | −7.39 | −7.45 | −7.56 | −9.37 |
| ΔGdiss, kcal/mol | 13.5 | 13.6 | 13.4 | 13.6 |
| Interaction surface with chains A/B, Å2 | 45.6/41.8 | 71.7/30.4 | 72.0/43.3 | 0/70.4 |
| Interfacing residues | Chain A: Arg323, Phe324 Chain B: Glu28, Gly29, Asp30, Ser31, Gly52, Asp101 | Chain A: Glu28, Gly29, Asp30, Ser31, Ala32, Gly52, Asp101, Pro281, His282, Gly283 Chain B: Arg323, Phe324 | Chain A: Arg323, Phe324 Chain B: Glu28, Gly29, Asp30, Ser31, Arg51, Gly52, Asp101, Lys140 | Chain B: Glu28, Ser31, Gly52, Lys53, Asp105, Ile109, Lys140, Arg234, Ser285, Ser286 |
| Amino acids (chain) involved in different types of interactions with the ligands | H bonds: Arg323(A), Asp30(B), 2 Hb Ser31(B) Hydrophobic contacts: Phe324(A) π-stacking interactions: Phe324(A) | H bonds: Asp30(A), Ser31(A), Pro281(A), 2 Hb Arg323(B) Ionic bonds: Arg382(B) Hydrophobic contacts: Phe324(B) | H bonds: Asp30(B), 3 Hb Ser31(B), Lys140(B), Asp323(A) π-stacking interactions: Phe324(A) | H bonds: 2 Hb Glu28(B), Ser31(B), Lys53(B), Lys140(B), 2 Hb Ser285(B), 2 Hb Ser286(B) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aprodu, I.; Chitescu, C.L.; Grigore-Gurgu, L.; Dumitrașcu, L. Investigation of the Antioxidant and Antimicrobial Properties of Ultrasound-Assisted Extracted Phenolics from Aronia melanocarpa Pomace. Appl. Sci. 2025, 15, 7070. https://doi.org/10.3390/app15137070
Aprodu I, Chitescu CL, Grigore-Gurgu L, Dumitrașcu L. Investigation of the Antioxidant and Antimicrobial Properties of Ultrasound-Assisted Extracted Phenolics from Aronia melanocarpa Pomace. Applied Sciences. 2025; 15(13):7070. https://doi.org/10.3390/app15137070
Chicago/Turabian StyleAprodu, Iuliana, Carmen Lidia Chitescu, Leontina Grigore-Gurgu, and Loredana Dumitrașcu. 2025. "Investigation of the Antioxidant and Antimicrobial Properties of Ultrasound-Assisted Extracted Phenolics from Aronia melanocarpa Pomace" Applied Sciences 15, no. 13: 7070. https://doi.org/10.3390/app15137070
APA StyleAprodu, I., Chitescu, C. L., Grigore-Gurgu, L., & Dumitrașcu, L. (2025). Investigation of the Antioxidant and Antimicrobial Properties of Ultrasound-Assisted Extracted Phenolics from Aronia melanocarpa Pomace. Applied Sciences, 15(13), 7070. https://doi.org/10.3390/app15137070











