Comparison of Selected Quality Parameters of Olive Oils Derived from Conventional and Organic Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
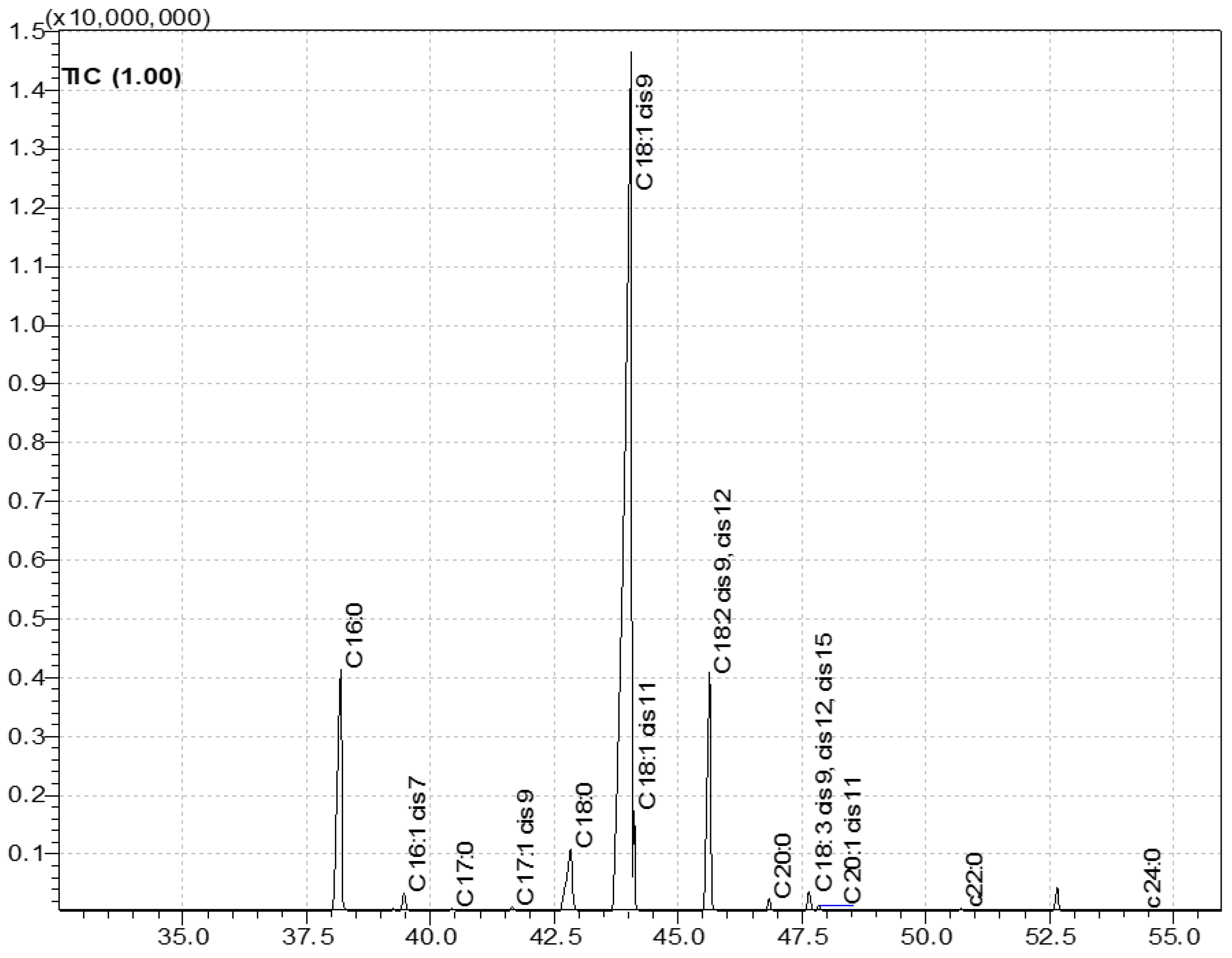
2.2.1. Fatty Acid Profile in Olive Oils [9]
2.2.2. Sterol Content Determination in Olive Oils [9]
2.2.3. Determination of Acid Value [1]
- V = volume of titrant (mL);
- c = concentration of KOH solution (mol/L);
- M = molar mass of oleic acid (282 g/mol);
- m = mass of sample (g).
2.2.4. Determination of Peroxide Value [1]
- V = volume of sodium thiosulfate solution used (mL);
- T = molarity of thiosulfate (mol/L);
- m = mass of sample (g).
2.2.5. Spectrophotometric Measurement of UV Absorbance [10]
2.2.6. DPPH Radical Scavenging Activity [10]
Sample Preparation—Phenolic Compound Extraction
Preparation of Standard Curve Solutions
Spectrophotometric Measurement Using a Microplate Reader
2.2.7. FRAP Antioxidant Activity Assay [10]
Spectrophotometric Measurement Using Microplate Reader
Data Analysis
2.2.8. HPLC-DAD Analysis of Phenolic Compounds [10]
2.3. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
3.2. Sterol Content in Olive Oils
3.3. Acid Value (%), Peroxide Value (mEq O2/kg), and Extinction Coefficients K268, K232, and ∆K of Olive Olives
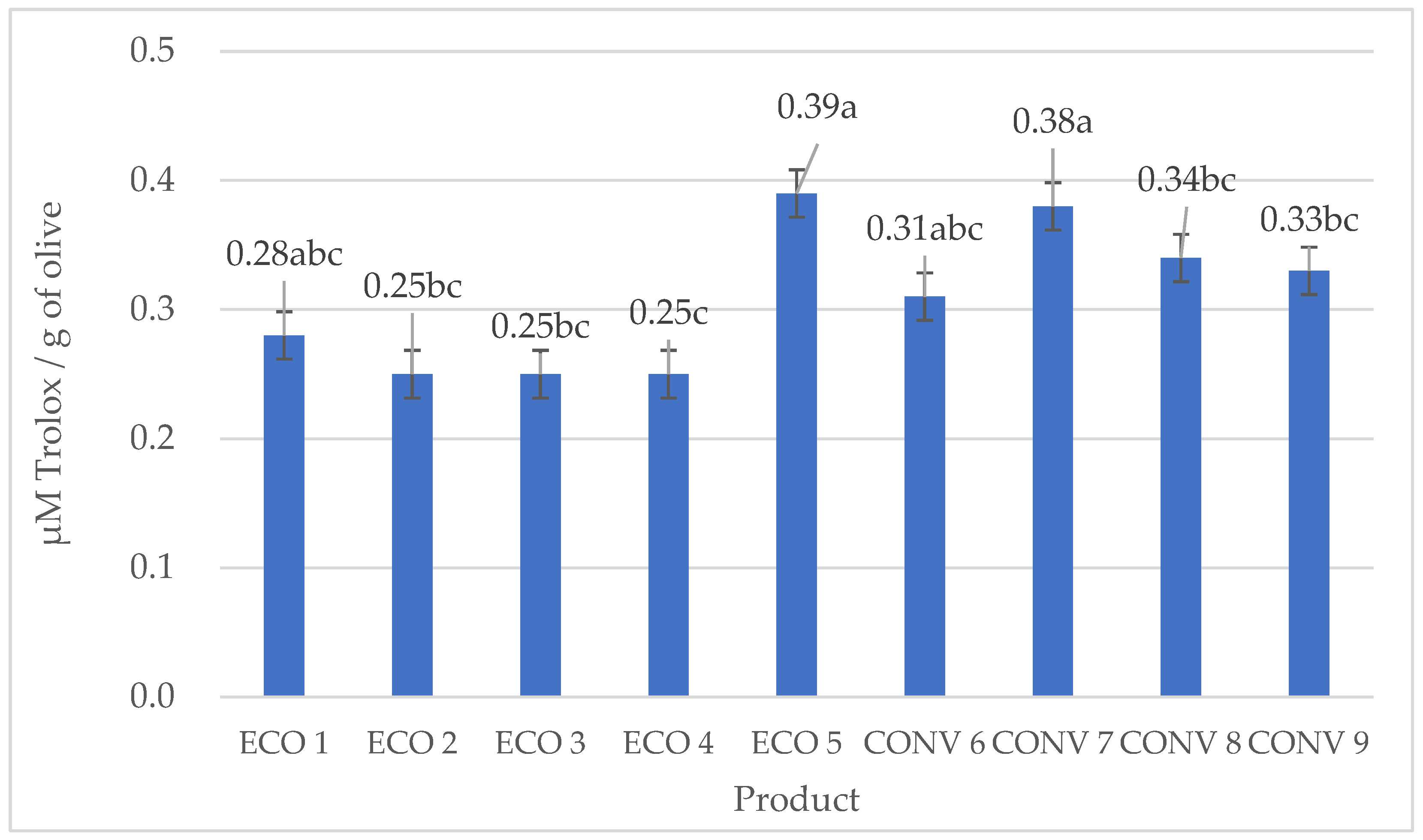
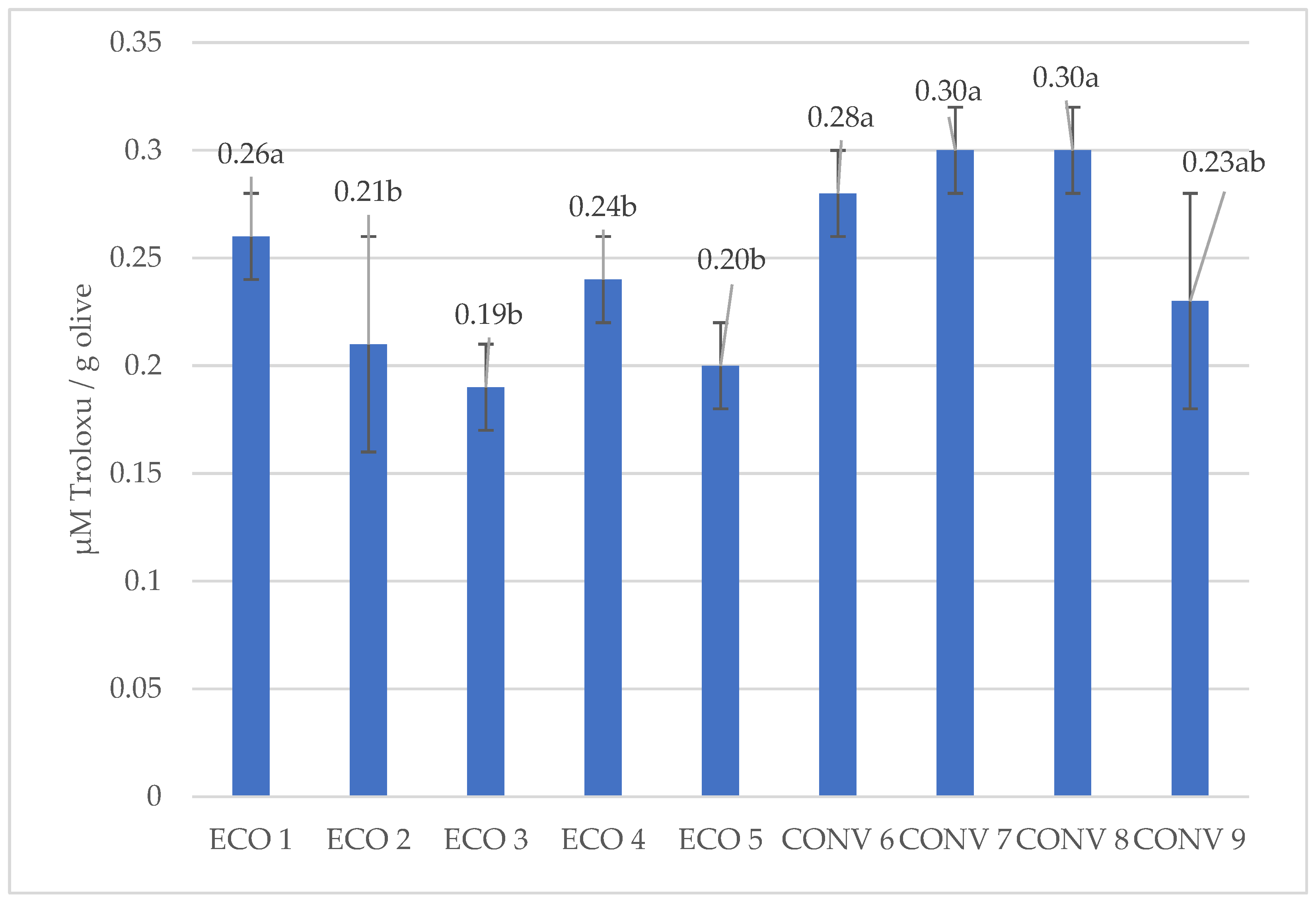
3.4. Analysis of the DPPH Antiradical Capacity of Olive Oils, FRAP Iron Ion Reduction, and Determination of Selected Phenolic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Olive Oil Council (IOOC). Trade Standard Applying to Olive Oils and Pomace-Oils; COI/T.15/NC No.3/Rev.19/2022; International Olive Oil Council (IOOC): Madrid, Spain, 2022. [Google Scholar]
- Skowron, M.; Zalejska-Fiolka, J.; Błaszczyk, U.; Birkner, E. Właściwości prozdrowotne oleju rzepakowego i oliwy. Postępy Hig. Med. Dośw. 2018, 72, 1104–1113. [Google Scholar] [CrossRef]
- Wroniak, M.; Maszewska, M. Oliwa z oliwek w diecie śródziemnomorskiej. Żywność Nauka Technol. Jakość 2011, 5, 26–36. [Google Scholar]
- Volakakis, N.; Kabourakis, E.; Kiritsakis, A.; Rempelos, L.; Leifert, C. Effect of Production System (Organic versus Conventional) on Olive Fruit and Oil Yields and Oil Quality Parameters in the Messara Valley, Crete, Greece; Results from a 3-Year Farm Survey. Agronomy 2022, 12, 1484. [Google Scholar] [CrossRef]
- Berg, H.; Maneas, G.; Engstrom, A.S. A Comparison between Organic and Conventional Olive Farming in Messenia, Greece. Horticulturae 2018, 4, 15. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Esposto, S.; Servili, M.; Rosati, A.; Montedoro, G. A 3-Year Study on Quality, Nutritional and Organoleptic Evaluation of Organic and Conventional Extra-Virgin Olive Oils. J. Am. Oil Chem. Soc. 2008, 85, 151–158. [Google Scholar] [CrossRef]
- Jimenez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Effect of Agronomical Practices on the Nutritional Quality of Virgin Olive Oil at Different Ripening Stages. J. Am. Oil Chem. Soc. 2015, 92, 1491–1501. [Google Scholar] [CrossRef]
- Jimenez, B.; Sánchez-Ortiz, A.; Lorenzo, M.L.; Rivas, A. Effect of Organic Cultivation of Picual and Hojiblanca Olive Varieties on the Quality of Virgin Olive Oil at Four Ripening Stages. Eur. J. Lipid Sci. Technol. 2014, 116, 1634–1646. [Google Scholar] [CrossRef]
- Derewiaka, D.; Rupert, M.; Wołosiak, R.; Bzducha-Wróbel, A.; Ścibisz, I.; Matuszewska-Janica, A. Volatiles as Markers of Bioactive Components Found in Croatian Extra Virgin Olive Oils. LWT—Food Sci. Technol. 2021, 139, 110589. [Google Scholar] [CrossRef]
- Fanali, C.; Posta, S.; Vilmercati, A.; Dugo, L.; Russo, M.; Petitti, T.; Mondello, L.; Gara, L. Extraction, Analysis, and Antioxidant Activity Evaluation of Phenolic Compounds in Different Italian Extra-Virgin Olive Oils. Molecules 2018, 23, 3249. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Gośliński, M.; Popławski, C. Antioxidant Properties and Fatty Acid Profile of Cretan Extra Virgin Bioolive Oils: A Pilot Study. Int. J. Food Sci. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union 2022, L282, 1–36. [Google Scholar]
- Bouymajane, A.; Majdoub, Y.; Cacciola, F.; Russo, M.; Salafia, F.; Trozzi, A.; Filali, F.R.; Dugo, P.; Mondello, L. Characterization of Phenolic Compounds, Vitamin E and Fatty Acids from Monovarietal Virgin Olive Oils of “Picholine Marocaine” Cultivar. Molecules 2020, 25, 5428. [Google Scholar] [CrossRef]
- Anastasopoulos, E.; Kalogeropoulos, N.; Kaliora, A.C.; Kountouri, A.; Andrikopoulos, N.K. The Influence of Ripening and Crop Year on Quality Indices, Polyphenols, Terpenic Acids, Squalene, Fatty Acid Profile and Sterols in Virgin Olive Oil (Koroneiki cv.) Produced by Organic versus Non-Organic Cultivation Method. Int. J. Food Sci. Technol. 2010, 46, 170–178. [Google Scholar] [CrossRef]
- Bilušić, T.; Melliou, E.; Giacometti, J.; Čaušević, A.; Čorbo, S.; Landeka, M.; Magiatis, P. Phenolic, Fatty Acids and Biological Potential of Selected Croatian EVOOs. Eur. J. Lipid Sci. Technol. 2017, 119, 1600461. [Google Scholar] [CrossRef]
- Gargouri, B.; Ammar, S.; Zribi, A.; Mansour, A.B.; Bouaziz, M. Effect of Growing Region on Quality Characteristics and Phenolic Compounds of Chemlali Extra Virgin Olive Oils. Acta Physiol. Plant. 2013, 35, 2801–2812. [Google Scholar] [CrossRef]
- Cano, M.M.; Gordillo, C.M.; Mendoza, M.F.; Vertedor, D.M.; Casasa, J.S. The Sterol and Erythrodiol + Uvaol Content of Virgin Olive Oils Produced in Five Olive-Growing Zones of Extremadura (Spain). J. Am. Oil Chem. Soc. 2016, 93, 227–235. [Google Scholar] [CrossRef]
- Sönmez, A.; Özdikicierler, O.; Gümüşkesen, A.S. Evaluation of Olive Oil Quality during the Ripening of the Organic Cultivated Olives and Multivariate Discrimination of the Variety with a Chemometric Approach. Riv. Ital. Delle Sostanze Grasse 2018, 95, 173–181. [Google Scholar]
- Giacalone, R.; Giuliano, S.; Gulotta, E.; Monfreda, M.; Presti, G. Origin Assessment of Extra Virgin Olive Oils by Esterified Sterols Analysis. Food Chem. 2015, 188, 279–285. [Google Scholar] [CrossRef]
- Sakouhi, F.; Absalon, C.; Flamini, G.; Cioni, P.L.; Kalel, H.; Boukhchina, S. Lipid Components of Olive Oil from Tunisian Cv. Sayali: Characterization and Authenticity. Comptes Rendus Biol. 2010, 333, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Noorali, M.; Barzegar, M.; Sahari, M.A. Sterol and Fatty Acid Compositions of Olive Oil as an Indicator of Cultivar and Growing Area. J. Am. Oil Chem. Soc. 2014, 91, 1571–1581. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Mungan, B. Effects of Variety, Maturation and Growing Region on Chemical Properties, Fatty Acid and Sterol Compositions of Virgin Olive Oils. J. Am. Oil Chem. Soc. 2016, 93, 1499–1508. [Google Scholar] [CrossRef]
- Dag, C.; Demirtas, I.; Ozdemir, I.; Bekiroglu, S.; Ertas, E. Biochemical Characterization of Turkish Extra Virgin Olive Oils from Six Different Olive Varieties of Identical Growing Conditions. J. Am. Oil Chem. Soc. 2015, 92, 1349–1356. [Google Scholar] [CrossRef]
- Borges, T.H.; Pereira, J.A.; Cabrera-Vique, C.; Lara, L.; Oliveira, A.F.; Seiquer, I. Characterization of Arbequina Virgin Olive Oils Produced in Different Regions of Brazil and Spain: Physicochemical Properties, Oxidative Stability and Fatty Acid Profile. Food Chem. 2017, 215, 454–462. [Google Scholar] [CrossRef]
- Arfaoui, M.; Boulares, M.; Bezzezi, A.; Ayachi, S.; Ghrab, M.; Jouini, N.E.; Hassouna, M.; Boudiche, S. Effect of the Enrichment with Natural Antioxidants Obtained by Maceration or Ultrasound-Assisted Extraction from Olive Leaves on Organic Extra Virgin Olive Oil. Riv. Ital. Delle Sostanze Grasse 2022, 99, 121–129. [Google Scholar]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Amir, Y.; Youyou, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Influence of Olive Ripeness on Chemical Properties and Phenolic Composition of Chemlal Extra-Virgin Olive Oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- Giacometti, J.; Milin, Č.; Giacometti, F.; Ciganj, Z. Characterization of Monovarietal Olive Oils Obtained from Croatian Cvs. Drobnica and Buza during the Ripening Period. Foods 2018, 7, 188. [Google Scholar] [CrossRef]
- Barbieri, S.; Bendini, A.; Valli, E.; Toschi, T.G. Do Consumers Recognize the Positive Sensorial Attributes of Extra Virgin Olive Oils Related with Their Composition? A Case Study on Conventional and Organic Products. J. Food Compos. Anal. 2015, 44, 186–195. [Google Scholar] [CrossRef]
- Bouarroudj, K.; Tamendjari, A.; Larbat, R. Quality, Composition and Antioxidant Activity of Algerian Wild Olive (Olea europaea L. subsp. oleaster) Oil. Ind. Crops Prod. 2016, 83, 484–491. [Google Scholar] [CrossRef]
- Willenberg, I.; Matthäus, B.; Gertz, C. A New Statistical Approach to Describe the Quality of Extra Virgin Olive Oils Using Near Infrared Spectroscopy and Traditional Analytical Parameters. Eur. J. Lipid Sci. Technol. 2019, 121, 1800484. [Google Scholar] [CrossRef]
- Ceci, L.N.; Mattar, S.B.; Carelli, A.A. Chemical Quality and Oxidative Stability of Extra Virgin Olive Oils from San Juan Province (Argentina). Food Res. Int. 2017, 100, 764–770. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.L.; Aparicio-Ruiz, R.; Morales, M.T. Chemical Characterization of Organic and Non-Organic Virgin Olive Oils. OCL Oilseeds Fats Crops Lipids 2014, 21, D506. [Google Scholar] [CrossRef]
- Ballus, C.A.; Meinhart, A.D.; Souza Campos, F.A.; Teixeira-Godoy, H. Total Phenolics of Virgin Olive Oils Highly Correlate with the Hydrogen Atom Transfer Mechanism of Antioxidant Capacity. J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Borges, T.H.; Cabrera-Vique, C.; Seiquer, I. Antioxidant Properties of Chemical Extracts and Bioaccessible Fractions Obtained from Six Spanish Monovarietal Extra Virgin Olive Oils: Assays in Caco-2 Cells. Food Funct. 2016, 7, 209–218. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Q.; Wang, Y.; Ying, R.; Fan, G.; Huang, M.; Agyemang, M. Physicochemical Characterization and Antioxidant Activities of Chongqing Virgin Olive Oil: Effects of Variety and Ripening Stage. J. Food Meas. Charact. 2020, 14, 2010–2020. [Google Scholar] [CrossRef]
- Perez-Córdoba, R.; Quesada-Granados, J.J.; Ramirez-Anaya, J.P.; Peña-Díaz, J.; Blanca-Herrera, R.; Samaniego-Sánchez, C. Bioactive Compounds in Spanish Extra Virgin Olive Oils: Migration and Stability According to the Culinary Technique Used. Food Chem. 2023, 172, 1–11. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Morales, M.T.; Asuero, A.G. Characterization of Bioactive Compounds from Monovarietal Virgin Olive Oils: Relationship Between Phenolic Compounds and Antioxidant Capacities. Int. J. Food Prop. 2015, 18, 348–358. [Google Scholar] [CrossRef]
- Brala, C.J.; Brnčić, D.; Šindrak, Z.; Barbarić, M.; Uršić, S. Labeled Extra Virgin Olive Oil as Food Supplement; Phenolic Compounds in Oils from Some Autochthonous Croatian Olives. Grasas Aceites 2015, 66, e099. [Google Scholar] [CrossRef]
| Organic Farming | Conventional Farming | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| SFA | 15.80 cd ± 0.86 | 14.60 d ± 0.60 | 16.02 cd ± 0.45 | 16.67 c ± 0.22 | 18.43 b ± 0.19 | 15.86 cd ± 0.12 | 16.99 bc ± 0.74 | 16.58 c ± 0.50 | 20.49 a ± 0.02 |
| C 16:0 palmitic acid | 11.27 ef ± 0.77 | 10.56 f ± 0.47 | 11.93 de ± 0.40 | 12.70 cd ± 0.15 | 14.65 b ± 0.16 | 11.74 def ± 0.09 | 13.93 bc ± 0.68 | 12.95 cd ± 0.43 | 16.45 a ± 0.19 |
| C 17:0 heptadecanoic acid | 0.11 ab ± 0.01 | 0.05 f ± 0.01 | 0.10 bc ± 0.01 | 0.07 de ± 0.01 | 0.06 ef ± 0.01 | 0.05 ef ± 0.01 | 0.13 a ± 0.01 | 0.08 cd ± 0.01 | 0.11 ab ± 0.01 |
| C 18:0 stearic acid | 3.79 a ± 0.49 | 3.22 b ± 0.13 | 3.30 ab ± 0.0 | 3.15 b ± 0.07 | 2.98 b ± 0.03 | 3.25 b ± 0.04 | 2.15 c ± 0.06 | 2.84 b ± 0.06 | 3.13 b ± 0.18 |
| C 20:0 arachidic acid | 0.45 f ± 0.03 | 0.53 abc ± 0.02 | 0.47 ef ± 0.01 | 0.51 cde ± 0.01 | 0.52 bcd ± 0.01 | 0.57 a ± 0.01 | 0.49 de ± 0.01 | 0.48 ef ± 0.01 | 0.55 ab ± 0.01 |
| C 22:0 behenic acid | 0.12 e ± 0.02 | 0.18 abc ± 0.01 | 0.14 de ± 0.01 | 0.16 cd ± 0.01 | 0.15 de ± 0.00 | 0.19 ab ± 0.01 | 0.21 a ± 0.00 | 0.16 cd ± 0.01 | 0.17 bcd ± 0.01 |
| C 24:0 lignoceric acid | 0.05 a ± 0.02 | 0.07 a ± 0.01 | 0.08 a ± 0.01 | 0.08 a ± 0.01 | 0.08 a ± 0.01 | 0.06 a ± 0.03 | 0.08 a ± 0.01 | 0.07 a ± 0.01 | 0.08 a ± 0.00 |
| MUFA | 74.50 cde ± 1.26 | 78.50 a ± 0.84 | 75.20 bc ± 0.66 | 72.50 d ± 0.38 | 66.50 e ± 0.31 | 76.35 b ± 0.19 | 73.40 cd ± 1.04 | 75.50 bc ± 0.67 | 65.60 e ± 0.41 |
| C 16:1. (cis 9) palmitoleic acid | 0.80 e ± 0.23 | 0.69 e ± 0.03 | 1.07 cd ± 0.02 | 1.18 c ± 0.03 | 1.67 ab ± 0.01 | 0.84 de ± 0.01 | 1.64 b ± 0.07 | 1.16 c ± 0.02 | 1.88 a ± 0.02 |
| C 17:1 (cis 10) heptadecenoic acid | 0.17 b ± 0.01 | 0.07 c ± 0.01 | 0.18 b ± 0.00 | 0.09 c ± 0.01 | 0.09 c ± 0.01 | 0.08 c ± 0.02 | 0.31 a ± 0.02 | 0.16 b ± 0.02 | 0.18 b ± 0.01 |
| C 18:1 (cis 9) oleic acid | 71.12 b ±1.01 | 75.36 a ± 0.99 | 71.03 b ± 0.86 | 68.24 cd ± 0.52 | 61.44 e ± 0.41 | 72.57 b ± 0.25 | 67.33 d ±1.47 | 70.61 bc ± 0.84 | 59.54 e ± 0.47 |
| C 18:1 (cis isomers) | 2.14 ef ± 0.40 | 2.10 f ± 0.13 | 2.67 cdef ± 0.18 | 2.68 cde ± 0.11 | 3.07 cd ± 0.09 | 2.51 def ± 0.04 | 3.80 a ± 0.33 | 3.18 bc ± 0.14 | 3.68 ab ± 0.04 |
| C 20:1 (cis 11) eicosenoic acid | 0.25 d ± 0.03 | 0.30 b ± 0.01 | 0.28 de ± 0.01 | 0.30 c ± 0.01 | 0.26 d ± 0.00 | 0.35 a ± 0.02 | 0.36 a ± 0.02 | 0.34 ab ± 0.01 | 0.31 bc ± 0.01 |
| PUFA | 9.70 d ± 0.90 | 6.90 g ± 0.23 | 8.70 ef ± 0.20 | 10.90 c ± 0.16 | 15.04 a ± 0.11 | 7.80 fg ± 0.07 | 9.60 de ± 0.30 | 7.90 f ± 0.16 | 13.90 b ± 0.02 |
| C 18:2 cis 9, cis 12 linoleic acid | 8.99 d ± 0.85 | 6.24 f ± 0.22 | 8.05 e ± 0.20 | 10.17 c ± 0.15 | 14.34 a ± 0.10 | 7.05 f ± 0.06 | 8.83 de ± 0.28 | 7.09 f ± 0.15 | 13.16 b ± 0.02 |
| C 18:3 cis 9, cis 12, cis 15 α-linolenic acid | 0.74 bc ± 0.04 | 0.66 e ± 0.02 | 0.69 de ± 0.0 | 0.68 de ± 0.01 | 0.70 cde ± 0.01 | 0.73 bcd ± 0.01 | 0.75 bc ± 0.02 | 0.87 a ± 0.01 | 0.77 b ± 0.01 |
| Organic Farming | Conventional Farming | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Campesterol | 18.26 e ± 0.72 | 22.50 cd ± 1.23 | 27.93 b ± 1.30 | 25.05 bc ± 0.51 | 28.34 b ± 1.69 | 25.63 bc ± 2.04 | 35.37 a ± 2.02 | 20.50 de ± 0.58 | 35.37 a ± 1.39 |
| Stigmasterol | 6.23 de ± 0.09 | 4.20 f ± 0.07 | 8.08 bcd ± 0.52 | 6.65 cde ± 0.09 | 6.10 e ± 0.16 | 5.53 ef ± 0.09 | 14.05 a ± 1.34 | 8.21 bc ± 0.28 | 9.34 b ± 1.27 |
| Clerosterol | 5.94 bc ± 1.30 | 7.24 bc ± 0.40 | 8.07 b ± 1.74 | 6.68 ab ± 0.57 | 4.90 c ± 0.86 | 6.94 bc ± 0.49 | 7.99 b ± 1.25 | 5.89 bc ± 0.72 | 8.27 b ± 0.49 |
| β-sitosterol | 609.08 b ± 56.29 | 476.08 d ± 14.76 | 611.86 d ± 10.39 | 578.31 d ± 5.57 | 635.95 c ± 32.49 | 519.28 cd ± 31.59 | 774.79 a ± 9.56 | 510.42 d ± 1.53 | 711.56 a ± 24.37 |
| ∆5-avenasterol | 97.04 b ± 10.23 | 140.47 a ± 4.50 | 76.20 c ± 1.41 | 93.96 b ± 1.17 | 88.59 bc ± 5.95 | 94.87 b ± 6.40 | 134.73 a ± 4.15 | 45.22 d ± 0.78 | 94.28 b ± 3.25 |
| Cycloartenol | 34.24 f ± 5.25 | 42.29 e ± 2.99 | 58.98 bc ± 3.38 | 82.61 a ± 2.17 | 52.51 cd ± 1.84 | 51.17 d ± 2.00 | 23.75 g ± 0.66 | 28.30 fg ± 1.54 | 60.67 b ± 1.50 |
| 24-methylenearthenol | 176.69 ab ± 22.06 | 117.91 c ± 7.07 | 185.93 a ± 5.51 | 157.62 b ± 3.84 | 173.73 ab ± 9.42 | 102.67 c ± 6.89 | 63.27 d ± 2.80 | 118.47 c ± 0.28 | 115.91 c ± 4.73 |
| Citrostadienol | 34.69 cd ± 5.81 | 82.75 a ± 6.11 | 32.63 cd ± 1.81 | 42.34 c ± 1.22 | 29.62 d ± 1.05 | 59.83 b ± 5.02 | 61.76 b ± 2.94 | 38.22 cd ± 0.89 | 38.18 cd ± 1.31 |
| Total | 982.15 bcd ± 101.74 | 893.43 cde ± 37.14 | 1009.69 abc ± 26.06 | 993.22 abcd ± 15.14 | 1019.74 abc ± 53.46 | 865.94 de ± 54.52 | 1115.70 a ± 24.73 | 775.23 e ± 6.60 | 1073.58 ab ± 38.31 |
| Farming Type | No. | Acid Value (%) | Peroxide Value (mEq O2/kg) | K268 | K232 | ∆K |
|---|---|---|---|---|---|---|
| Organic | 1 | 0.28 f ± 0.00 | 7.25 f ± 0.35 | 0.09 a ± 0.00 | 0.1 a ± 0.01 | −0.011 a ± 0.003 |
| 2 | 0.41 c ± 0.02 | 13.39 cd ± 0.24 | 0.08 a ± 0.01 | 0.12 a ± 0.01 | −0.011 a ± 0.004 | |
| 3 | 0.32 e ± 0.02 | 12.68 d ± 0.45 | 0.10 a ± 0.01 | 0.12 a ± 0.00 | −0.008 a ± 0.001 | |
| 4 | 0.42 c ± 0.03 | 14.80 bc ± 0.41 | 0.09 a ± 0.01 | 0.12 a ± 0.01 | −0.006 a ± 0.001 | |
| 5 | 0.36 d ± 0.02 | 17.40 a ± 0.39 | 0.10 a ± 0.01 | 0.12 a ± 0.00 | −0.005 a ± 0.002 | |
| Conventional | 6 | 0.54 b ± 0.00 | 15.33 bc ± 0.50 | 0.10 a ± 0.01 | 0.12 a ± 0.00 | −0.007 a ± 0.002 |
| 7 | 0.26 g ± 0.02 | 14.60 def ± 1.13 | 0.09 a ± 0.00 | 0.12 a ± 0.01 | −0.005 a ± 0.001 | |
| 8 | 0.56 a ± 0.00 | 9.46 e ± 0.33 | 0.09 a ± 0.00 | 0.12 a ± 0.00 | −0.011 a ± 0.005 | |
| 9 | 0.40 c ± 0.03 | 16.01 ab ± 0.01 | 0.10 a ± 0.00 | 0.12 a ± 0.01 | −0.009 a ± 0.004 |
| Farming Type | No. | Oleuropein | Hydroxytyrosol | Tyrosol | Total |
|---|---|---|---|---|---|
| Organic | 1 | 59.01 b ± 14.17 | 46.83 a ± 2.06 | 71.76 a ± 12.60 | 177.60 ab ± 19.67 |
| 2 | 62.63 ab ± 14.17 | 59.35 a ± 22.38 | 59.73 a ± 25.69 | 181.71 ab ± 33.90 | |
| 3 | 90.77 ab ± 6.18 | 42.99 a ± 9.53 | 44.97 a ± 8.19 | 178.73 ab ± 23.77 | |
| 4 | 78.47 ab ± 13.96 | 57.78 a ± 4.88 | 73.43 a ± 0.57 | 209.68 ab ± 19.40 | |
| 5 | 58.89 b ± 12.03 | 51.87 a ± 13.79 | 55.56 a ± 14.99 | 166.31 ab ± 39.45 | |
| Conventional | 6 | 88.13 ab ± 17.79 | 56.23 a ± 7.85 | 82.45 a ± 9.71 | 226.82 a ± 2.50 |
| 7 | 78.97 ab ± 6.72 | 3.56 ab ± 1.09 | 50.75 a ± 18.80 | 133.20 b ± 17.24 | |
| 8 | 75.92 ab ± 6.16 | 36.04 ab ± 2.96 | 67.39 a ± 7.87 | 179.35 ab ± 11.07 | |
| 9 | 100.05 a ± 12.58. | 40.84 a ± 16.46 | 51.24 a ± 7.46 | 192.14 ab ± 32.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derewiaka, D.; Majdak, K.; Pakosz, P.; Drużyńska, B. Comparison of Selected Quality Parameters of Olive Oils Derived from Conventional and Organic Farming. Appl. Sci. 2025, 15, 7158. https://doi.org/10.3390/app15137158
Derewiaka D, Majdak K, Pakosz P, Drużyńska B. Comparison of Selected Quality Parameters of Olive Oils Derived from Conventional and Organic Farming. Applied Sciences. 2025; 15(13):7158. https://doi.org/10.3390/app15137158
Chicago/Turabian StyleDerewiaka, Dorota, Karolina Majdak, Paulina Pakosz, and Beata Drużyńska. 2025. "Comparison of Selected Quality Parameters of Olive Oils Derived from Conventional and Organic Farming" Applied Sciences 15, no. 13: 7158. https://doi.org/10.3390/app15137158
APA StyleDerewiaka, D., Majdak, K., Pakosz, P., & Drużyńska, B. (2025). Comparison of Selected Quality Parameters of Olive Oils Derived from Conventional and Organic Farming. Applied Sciences, 15(13), 7158. https://doi.org/10.3390/app15137158







