Evaluating the Role of Canada Goose Populations in Transmission Dynamics During Peak HPAI Incidence in Iowa, February 2022–December 2023
Abstract
Featured Application
Abstract
1. Introduction
1.1. HPAI Epidemiology
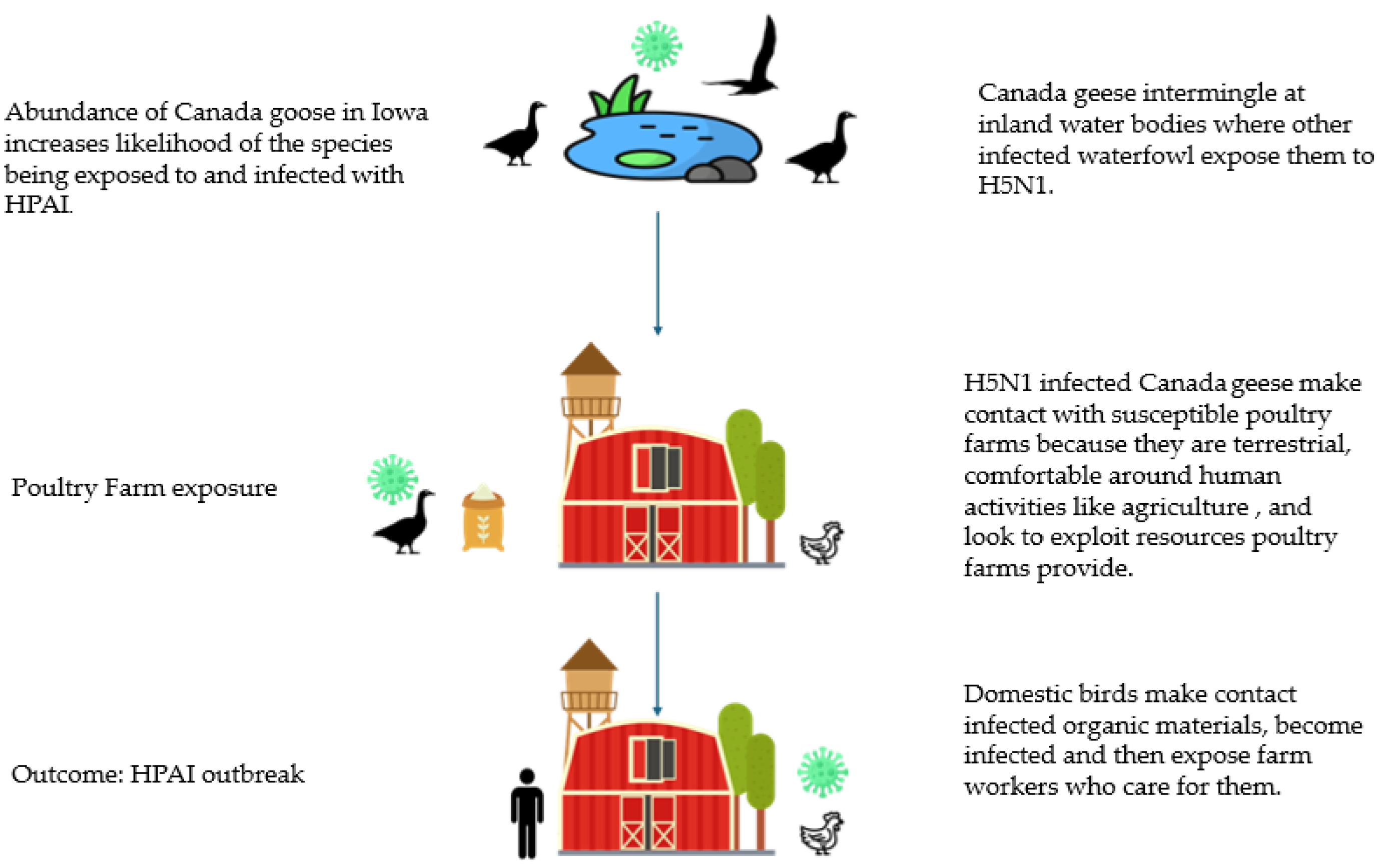
1.2. Canada Goose Ecology and Behavior
1.3. Possible Role of Canada Goose in HPAI Transmission
1.4. Infection of Multiple Mammal Species
1.5. HPAI Risk to Public Health: Human-Adapted Mutation
2. Materials and Methods
2.1. Period of Evaluation for Iowa: 6 March 2022–20 December 2023
2.2. Canada Goose Abundance Data
2.3. Outcome of Interest: HPAI Positive Counties
2.4. Statistical Methods: Bivariate Analysis
2.5. Multivariable Model Selection
2.6. Covariates
3. Results
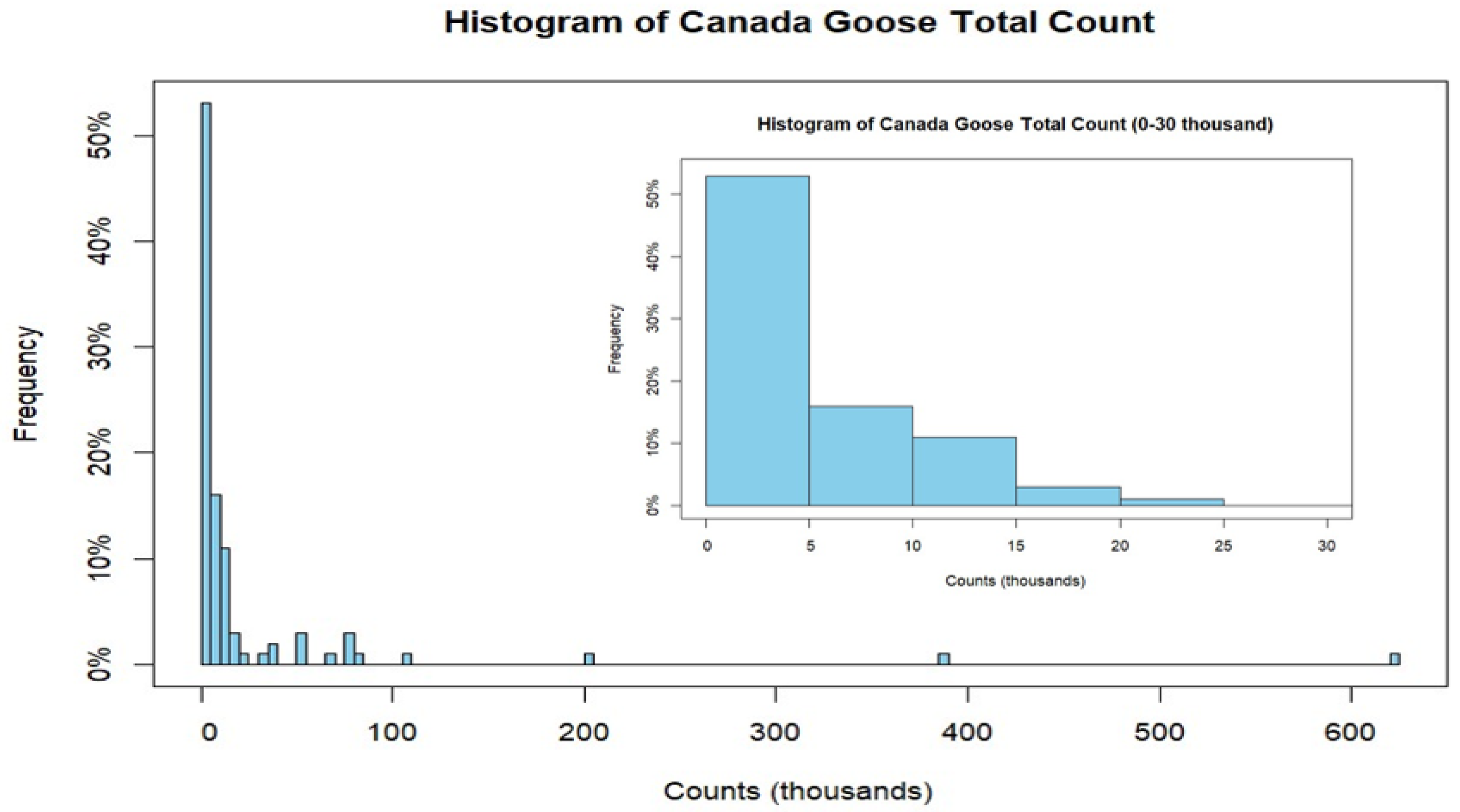
3.1. Descriptive Statistics and Bivariate Results
3.2. Model Diagnostic Results
3.3. Multivariate Zero-Inflated Negative Binomial Results
4. Discussion
4.1. Canada Goose Abundance Hypothesis
4.2. Short-Term Strategy to Reduce H5N1 Spread: Reinforcing Biosecurity Guidelines
4.2.1. Maintaining Biosecurity on All Farms
4.2.2. Rodent Control
4.3. Study Limitations
4.3.1. Poultry Farm Data Limitations
4.3.2. Canada Geese and Covariate Species Data Limitations
4.3.3. Study Design Limitations
4.3.4. Reason for Lack of Poultry Farm Location Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| AI | Artificial Intelligence |
| APHIS | Animal and Plant Health Inspection Service |
| BIC | Bayesian Information Criterion |
| HPAI | Highly Pathogenic Avian Influenza |
| HA | Hemagglutinin |
| NA | Neuraminidase |
| NaN | Not a Number |
| OR | Odds Ratio |
| VIF | Variance Inflation Factor |
| RR | Relative Risk |
| USDA | United States Department of Agriculture |
| US | United States |
| ZINB | Zero inflated negative binomial |
References
- United States Department of Agriculture-Animal Health and Plant Inspection Service. Confirmations of Highly Pathogenic Avian Influenza in Wild Birds. 2025. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/wild-birds (accessed on 20 March 2025).
- United States Department of Agriculture-Animal Health and Plant Inspection Service. Confirmations of Highly Pathogenic Avian Influenza in Commercial and Backyard Flocks. 2024. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections (accessed on 4 April 2024).
- Animal and Plant Health Inspection Service-Veterinary Services Surveillance, P. and Response Services. Epidemiologic and Other Analyses of HPAI-Affected Poultry Flocks 1 June 2023 Interim Report. 2023. Available online: https://www.aphis.usda.gov/sites/default/files/epi-analyses-avian-flu-poultry-2nd-interim-rpt.pdf (accessed on 24 January 2024).
- Center for Disease Control and Prevention. Avian Influenza in Birds: Causes and How It Spreads. 2022. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/bird-flu/virus-transmission/avian-in-birds.html (accessed on 20 May 2023).
- Kurmi, B.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Dubey, S.C.; Kumar, M. Survivability of Highly Pathogenic Avian Influenza H5N1 Virus in Poultry Faeces at Different Temperatures. Indian J. Virol. 2013, 24, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nakamura, K.; Mase, M. Survival of Highly Pathogenic Avian Influenza H5N1 Virus in Tissues Derived from Experimentally Infected Chickens. Appl. Environ. Microbiol. 2017, 83, e00604-17. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. H5 Bird Flu: Current Situation. 2025. Available online: https://www.cdc.gov/bird-flu/situation-summary/index.html (accessed on 1 April 2025).
- Center for Disease Control and Prevention. CDC Confirms Human Cases of H5 Bird Flu Among Colorado Poultry Workers. 2024. Available online: https://www.cdc.gov/media/releases/2024/p-0715-confirm-h5.html (accessed on 16 August 2024).
- Luukkonen, B.Z.; Jones, O.E., III; Klaver, R.W. Canada Goose Survival and Recovery Rates in Urban and Rural Areas of Iowa, USA. J. Wildl. Manag. 2021, 85, 283–292. [Google Scholar] [CrossRef]
- United States Department of Agriculture-Animal Health and Plant Inspection Service. Avian Influenza. 2025. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza (accessed on 3 April 2025).
- Runstadler, J.A.; Puryear, W.B. The virus is out of the barn: The emergence of HPAI as a pathogen of avian and mammalian wildlife around the globe. Am. J. Vet. Res. 2024, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cornell Lab of Ornithology. All About Birds-Canada Goose. 2022. Available online: https://www.allaboutbirds.org/guide/Canada_Goose (accessed on 19 April 2024).
- Luukkonen, B.Z.K.; Jones, O.E., III. Movement of Canada Geese in urban and rural areas of Iowa, USA. Avian Conserv. Ecol. 2022, 17, 27. [Google Scholar] [CrossRef]
- Askren, R.J.; Eichholz, M.; Sharp, C.M.; Washburn, B.E.; Beckerman, S.F.; Pullins, S.; Fournier, A.; VonBank, J.; Weegman, M.D.; Hagy, H.M.; et al. Behavioral responses of Canada geese to winter harassment in the context of human-wildlife conflicts. Wildl. Soc. Bull. 2022, 46, e1384. [Google Scholar] [CrossRef]
- Green, A.L.; Branan, M.; Fields, V.L.; Patyk, K.; Kolar, S.K.; Beam, A.; Marshall, K.; McGuigan, R.; Vuolo, M.; Freifeld, A.; et al. Investigation of risk factors for introduction of highly pathogenic avian influenza H5N1 virus onto table egg farms in the United States, 2022: A case–control study. Front. Vet. Sci. 2023, 10, 1229008. [Google Scholar] [CrossRef] [PubMed]
- Iowa Department of Natural Resoruces. Waterfowl Management, Seasons, and Harvests in Iowa. 2023. Available online: https://www.iowadnr.gov/media/1706/download?inline (accessed on 6 March 2024).
- Pasick, J.; Berhane, Y.; Embury-Hyatt, C.; Copps, J.; Kehler, H.; Handel, K.; Babiuk, S.; Hooper-McGrevy, K.; Li, Y.; Mai Le, Q.; et al. Susceptibility of Canada Geese (Branta canadensis) to highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2007, 13, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.B.; Trovão, N.S.; Ineson, K.M.; Schaefer, A.L.; Puryear, W.B.; Zhou, K.; Foss, A.D.; Clark, D.E.; MacKenzie, K.G.; MacKenzie, K.G.; et al. Ecological divergence of wild birds drives avian influenza spillover and global spread. PLoS Pathog. 2022, 8, e1010062. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, C.S.; Masto, N.M.; Sullivan, J.D.; Keever, A.; Poulson, R.; Bradshaw, B.; Highway, C.; Feddrsem, J.; Hagy, H.; Gerhold, R.; et al. North American wintering mallards infected with highly pathogenic avian influenza show few signs of altered local or migratory movements. Sci. Rep. 2023, 13, 14473. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Current Situation: Bird Flu in Dairy Cows. 2025. Available online: https://www.cdc.gov/bird-flu/situation-summary/mammals.html (accessed on 20 February 2025).
- Arizona Emergency Information Network. Avian Influenza Found in Dairy Cattle in Maricopa County. 2025. Available online: https://ein.az.gov/avian-influenza-found-dairy-cattle-maricopa-county (accessed on 17 February 2025).
- United States Department of Agriculture- Animal Health and Plant Inspection Service. Detections of Highly Pathogenic Avian Influenza in Mammals. 2025. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals (accessed on 20 March 2025).
- Shao, W.L.; Goraya, M.; Wang, S.; Chen, J.L. Evolution of Influenza A Virus by Mutation and Re-Assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Sun, L.; Xiong, J.; Wang, C.; Chen, L.; Yang, P.; Yu, H.; Cheng, Y.; Jiang, L.; Chen, Y.; et al. Semiaquatic mammals might be intermediate hosts to spread avian influenza viruses from avian to human. Sci. Rep. 2019, 9, 11641. [Google Scholar] [CrossRef] [PubMed]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- eBird. eBird Review Process. 2024. Available online: https://support.ebird.org/en/support/solutions/articles/48000795278?_gl=1*1b9zjoy*_gcl_au*MTE1NDA1ODQ1MS4xNzI5NDgzMzQx*_ga*MTY1MzU0ODUwOC4xNzE1MzUxNzQ1*_ga_QR4NVXZ8BM*MTczNDM4MjY4Ny4zMS4xLjE3MzQzODI2OTkuNDguMC4w&_ga=2.194236781.1517257474.1734382688-1653548508.1715351745 (accessed on 12 April 2024).
- eBird. eBird Status and Trends. 2024. Available online: https://science.ebird.org/en/status-and-trends (accessed on 13 May 2024).
- Iowa Department of Natural Resources. Canada Goose Control Guidelines; Iowa Department of Natural Resources: Des Moines, IA, USA, 2025; p. 7.
- United States Department of Agriculture- Animal Health and Plant Inspection Service. H5N1. 2025. Available online: https://www.aphis.usda.gov/h5n1-hpai#detections-hpai (accessed on 5 April 2025).
- Velkers, F.C.; Blokhuis, S.J.; Veldhuis Kroeze, E.J.B.; Burt, S.A. The role of rodents in avian influenza outbreaks in poultry farms: A review. Vet. Q. 2017, 37, 182–194. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture-Animal Health and Plant Inspection Service. Prevent Avian Influenza at Your Farm-Improving Your Biosecurity with Simple Wildlife Management Practices. 2015. Available online: https://www.aphis.usda.gov/sites/default/files/hpaifactsheet_wildlife-biosecurity.pdf (accessed on 20 March 2025).
- Biothreats Emergence, Analysis and Communications Network (BEACON). 2025. Available online: https://www.bu.edu/ceid/projects-partnerships/beacon/ (accessed on 29 May 2025).
- Park, M.; Britton, D.; Daley, W.; McMurray, G.; Navaei, M.; Samoylov, A.; Usher, C.; Xu, J. Artificial intelligence, sensors, robots, and transportation systems drive an innovative future for poultry broiler and breeder management. Anim. Front. 2022, 12, 40–48. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. USDA Announces Grants and Technical Assistance Funding for Urban Agriculture and Innovative Production. 2025. Available online: https://www.usda.gov/about-usda/news/press-releases/2025/01/08/usda-announces-grants-and-technical-assistance-funding-urban-agriculture-and-innovative-production (accessed on 13 June 2025).
- Backhans, A.; Fellström, C. Rodents on pig and chicken farms—A potential threat to human and animal health. Infect Ecol. Epidemiol. 2012, 2, 17093. [Google Scholar] [CrossRef] [PubMed]
- Iowa State Assembly. Infectious and contagious diseases among animals: Foreign animal disease preparedness and response strategy. Available online: https://www.legis.iowa.gov/docs/ico/chapter/163.pdf (accessed on 15 February 2025).

| Dichotomous Variable | Variable Description | Variable Type |
|---|---|---|
| Combined Canada goose count greater than median value | Whether a county’s cumulative Canada goose count, for years 2022–2023, was above the median value created from count data for 99 Iowa counties. Primary predictor. | Categorical 1 = Yes, 0 = No |
| 2022 Canada goose count greater than median value | Whether a county’s cumulative Canada goose count, for 2022, was above the median value created from count data for 99 Iowa counties. Stratified variable. | Categorical 1 = Yes, 0 = No |
| 2023 Canada goose count greater than median value | Whether a county’s cumulative Canada goose count, for 2023, was above the median value created from count data for 99 Iowa counties. Stratified variable. | Categorical 1 = Yes, 0 = No |
| HPAI outbreak | Whether a county experienced an HPAI outbreak during the reference period (2022–2023). Outcome of interest. | Categorical 1 = Yes, 0 = No |
| Predictor Variable and Covariates | Variable Description | Variable Type |
|---|---|---|
| Canada goose species abundance by quartile. | Primary predictor: Canada geese abundance derived from species counts stored in eBird for all 99 Iowa counties. Counts represent the entire reference period (January 2022–December 2023). | Categorical 1 = counts within the first quartile (25%), 2 = counts falling between the second and third quartile (25–75%), 3 = counts above the third quartile (>75%). |
| Mallard duck species abundance by quartile. | Covariate: mallard duck abundance derived from species counts stored in eBird for all 99 Iowa counties. Counts represent the entire reference period (January 2022–December 2023). | Categorical 1 = counts within the first quartile (25%), 2 = counts falling between the second and third quartile (25–75%), 3 = counts above the third quartile (>75%). |
| Wood duck abundance by quartile. | Covariate: Wood duck abundance derived from species counts stored in eBird for all 99 Iowa counties. Counts represent the entire reference period (January 2022–December 2023). | Categorical 1 = counts within the first quartile (25%), 2 = counts falling between the second and third quartile (25–75%), 3 = counts above the third quartile (>75%). |
| Green-winged teal abundance by quartile. | Covariate: Green-winged teal abundance derived from species counts stored in eBird for all 99 Iowa counties. Counts represent the entire reference period (January 2022–December 2023). | Categorical 1 = counts within the first quartile (25%), 2 = counts falling between the second and third quartile (25–75%), 3 = counts above the third quartile (>75%). |
| Year | Canada Geese | Mallard Ducks | Wood Ducks | Blue-Winged Teals | Green-Winged Teals | Snow Geese |
|---|---|---|---|---|---|---|
| 2022 | 1,293,380 | 334,310 | 28,872 | 66,320 | 67,224 | 568,426 |
| 2023 | 1,076,316 | 297,374 | 28,649 | 57,007 | 68,878 | 433,246 |
| Total | 2,369,666 | 631,697 | 57,521 | 123,327 | 136,102 | 1,001,672 |
| Variable | Species Count | HPAI+ Counties Count | Median | HPAI+ Counties > Median | χ2 (p-Value) |
|---|---|---|---|---|---|
| Canada goose counts greater than or equal to median (full reference period) | 2,369,666 | 310,198 | 3947 | 9 (32%) | 4.29 (0.04) |
| Canada goose counts greater than or equal to median 2022 | 1,293,380 | 152,166 | 2363 | 11 (39%) | 1.39 (0.24) |
| Canada goose counts greater than or equal to median 2023 | 1,076,316 | 158,032 | 1600 | 11 (39%) | 1.39 (0.24) |
| Variables/Covariates | RR | 95 CI% | p-Value |
|---|---|---|---|
| Canada goose abundance between 25th and 75th percentiles | 0.21 ** | 0.04, 0.90 ** | 0.04 ** |
| Canada goose abundance above 75th percentile | 0.20 | 0.02, 1.11 | 0.13 |
| Mallard duck abundance between 25th and 75th percentile | >100 | <0.01, >100 | 0.95 |
| Mallard duck abundance above 75th percentile | >100 | <0.01, >100 | 0.98 |
| Snow goose abundance between 25th and 75th percentiles | 1.44 | 0.31, 6.63 | 0.64 |
| Snow goose abundance above 75th percentile | 0.81 | 0.20, 3.30 | 0.77 |
| Wood duck abundance between 25th and 75th percentiles | <0.01 | <0.01, >100 | 0.95 |
| Wood duck abundance above 75th percentile | <0.01 | <0.01, >100 | 0.96 |
| Zero-inflated component | |||
| Canada goose abundance between 25th and 75th percentiles | 0.18 | <0.01, 37.12 | 0.52 |
| Canada goose abundance above 75th percentile | >100 | <0.01, >100 | 0.95 |
| Mallard duck abundance between 25th and 75th percentiles | <0.01 | <0.01, >100 | 0.94 |
| Mallard duck abundance above 75th percentile | 1.73 | <0.01, >100 | 0.99 |
| Snow goose abundance between 25th and 75th percentiles | 8.51 | 0.29, 17.84 | 0.21 |
| Snow goose abundance above 75th percentile | 0.18 | <0.01, 3.85 | 0.28 |
| Wood duck abundance between 25th and 75th percentiles | >100 | <0.01, >100 | 0.92 |
| Wood duck abundance above 75th percentile | >100 | <0.01, >100 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, C.; Kolokotronis, S.-O.; Rosenbaum, J.E.; Hoepner, L.A. Evaluating the Role of Canada Goose Populations in Transmission Dynamics During Peak HPAI Incidence in Iowa, February 2022–December 2023. Appl. Sci. 2025, 15, 6900. https://doi.org/10.3390/app15126900
Jimenez C, Kolokotronis S-O, Rosenbaum JE, Hoepner LA. Evaluating the Role of Canada Goose Populations in Transmission Dynamics During Peak HPAI Incidence in Iowa, February 2022–December 2023. Applied Sciences. 2025; 15(12):6900. https://doi.org/10.3390/app15126900
Chicago/Turabian StyleJimenez, Christopher, Sergios-Orestis Kolokotronis, Janet E. Rosenbaum, and Lori A. Hoepner. 2025. "Evaluating the Role of Canada Goose Populations in Transmission Dynamics During Peak HPAI Incidence in Iowa, February 2022–December 2023" Applied Sciences 15, no. 12: 6900. https://doi.org/10.3390/app15126900
APA StyleJimenez, C., Kolokotronis, S.-O., Rosenbaum, J. E., & Hoepner, L. A. (2025). Evaluating the Role of Canada Goose Populations in Transmission Dynamics During Peak HPAI Incidence in Iowa, February 2022–December 2023. Applied Sciences, 15(12), 6900. https://doi.org/10.3390/app15126900







