Abstract
Information about the trace elements content of goose carcass parts with or without skin can be important for consumers when making dietary choices. This study aimed to (1) determine the effects of popular heat processing techniques on the content of chromium (Cr), iodine (I), manganese (Mn), and bromine (Br) in goose breast muscle, and (2) estimate the extent to which 100 g of goose meat—both with and without skin—cover the Nutrient Reference Values (NRV) for Cr, I, Mn, and the Tolerable Daily Intake (TDI) for Br in adults. The heat processing techniques used in the study were water bath cooking (WBC), Oven Convection Roasting (OCR), grilling (G), and pan frying (PF). Grilled goose breast without skin had the highest Br retention (97.4%) and TDI (2.41%). Cooked goose breast (WBC) with skin exhibited the highest retention of Cr (73.8%) and I (73.6%). The highest Mn content was found in meat without skin after OCR processing and grilled meat with skin (0.170 and 0.191 mg/100 g, respectively). The iodine content in the meat decreased (from 0.020 raw to 0.003 mg/100 g after PF) with each heat treatment. The results of our study may provide helpful information for consumers when making dietary choices and using heat treatment techniques. Goose breast muscles, depending on heat treatment and the presence of skin, provide trace elements in the range of 2.21% of NRV (Nutrient Reference Value) for Br without skin to 740.7% of NRV for Cr with skin and may be a valuable component of a varied diet (apart from iodine). The Br content in the meat decreases after WBC treatment (1.29 without skin or 1.43 with skin mg/100 g). For this reason, it seems to be the most beneficial for the consumer’s health because it minimizes the risk of exceeding the TDI value. Total hazard quotients (THQ) in meat (regardless of the treatment and skin presence) for Cr, Mn, and Br contents were <1, indicating a low risk to Polish consumer health.
1. Introduction
In general, minerals constitute only 5% of a typical human diet, but they are essential for ensuring homeostasis in the body and, thus, health. Trace elements are minerals whose requirement for adults ranges from 1 to 100 mg daily or account for less than 0.01% of total body weight. According to the World Health Organization classification, chromium (Cr) and iodine (I) are classified as “essential elements,” and manganese (Mn) is a “probably essential element” for the proper development of living organisms and maintaining their vital functions [1,2]. These elements are responsible for the appropriate course of some tissue processes and are building blocks of cell organelles, enzymes, and hormones. Disturbances in trace element homeostasis may result in the development of pathological states and diseases. Both deficiency and excess trace elements can harm living organisms when their efficient use and excretion mechanisms fail. An example of such an element is bromine (Br), which has a physiological effect on the body but can also negatively affect the health of humans and animals.
Chromium (velogenic form III) is an element that takes part in the metabolism of carbohydrates (as Glucose Tolerance Factor GTF), proteins, and lipids (by activating enzymes involved in the metabolic pathways) in humans and animals. Its deficiency in the body contributes to increased blood glucose and cholesterol levels [3,4,5]. However, the velogenic form VI of chromium is a known toxic compound (especially hepatotoxic), which, due to its oxidizing potential, easily penetrates biological membranes and causes kidney damage, central nervous system diseases, and cancer in humans.
In poultry nutrition, chromium is used under temperature stress conditions (which induce proteolysis, lipolysis, and glycogenolysis) due to its strong antioxidant effect, preventing lipid peroxidation [6]. Additionally, chromium enhances the action of insulin in the metabolism of carbohydrates and proteins [7]. The response of poultry organisms to feed supplementation with chromium depends on its chemical form. It has been shown that organic forms—chelates, e.g., yeast, picolinate, nicotinate, propionate, methionine, and histidinate—are less toxic and show greater bioavailability from feed [6,8]. However, it should be noted that if heavy metals, such as chromium, are added to feed in amounts exceeding the required level [9], they may accumulate in the body tissues of poultry and humans consuming raw materials from poultry.
Iodine is an essential human trace element for proper growth, brain development, immune system function, and thyroid hormone synthesis [10]. Therefore, insufficient or excessive iodine intake causes thyroid dysfunction and thus affects the body’s metabolic functions [11]. Low iodine intake causes the development of iodine deficiency disorders (IDDs). Therefore, information on the actual content of iodine in food is the basis for creating recommendations regarding its consumption. It is also for implementing nutritional policy programs regarding food fortification with this element (mainly salt iodization), and is recommended by various international institutions [12,13].
Manganese, its physiological role in the body, and its occurrence in goose meat have recently been the subject of an extensive systematic review [14]; therefore, we have referred to the data in the Results and Discussion section to avoid repeating this content.
Bromine occurs naturally and is widely distributed in the environment as the inorganic anion, Br-. Bromide can be found in food and feed due to natural occurrences, environmental contamination from anthropogenic activity, the use of certain biocidal products, and the administration of veterinary medicinal products in food-producing animals [15].
In the body, bromine is necessary for the enzymatic activity of peroxidasin, which catalyzes the formation of sulfilimine cross-links in collagen IV, essential for the structural integrity of basement membranes in tissues [15]. However, in subsequent years, studies conducted in animal models and humans [15], showed that bromine causes a decrease in the concentration of total thyroxine (tT4) and triiodothyronine (tT3), thyrotropin (TSH) in serum, an increase in absolute/relative thyroid mass with morphological and histological changes and reduced iodide accumulation in the thyroid gland. Unfortunately, thyroid hormone disorders in critical developmental periods imply disorders in neurological development. Moreover, bromide has a direct neurotoxic effect, probably due to mimicking the neurotransmitter γ-aminobutyric acid (GABA gamma-aminobutyric-acid) [15,16]. Bromide can also cause kidney and liver damage and respiratory diseases [17,18].
Considering public health and the possible negative impact of bromine on the hormonal system and neurotoxicity [19], research interest in this element has increased in recent years. EFSA reviewed the opinions and scientific evidence on bromine in 2024. The EFSA Scientific Committee determined the TDI and Acute Reference Dose (ARfD) at 0.4 mg Br−/kg body mass/day for the general human population, including children, pregnant and breastfeeding women, but with sufficient iodine in the body. Furthermore, EFSA recommends that, to fully assess the risk, an assessment of exposure to bromide delivered to the human body via food should be carried out [15].
The bromine content in poultry meat depends on its concentration in feed and drinking water, as demonstrated by the study of Orobchenko et al. [20], In certain regions of Ukraine, bromine levels in these sources (between 2006 and 2020) exceeded safety thresholds. However, to date, there are no published studies assessing bromine content in goose meat—either raw or cooked—with consideration of skin presence. In recent years, consumer interest in waterfowl meat (particularly goose and duck) has grown across various regions of the world. This trend may be attributed to both the scale and profitability of production, as well as to demographic factors, dietary patterns, and cultural preferences specific to individual countries [21,22]. As of 2023, China is the leading producer and consumer of waterfowl meat, with an annual output of 5.5 million tons, followed by France (203 thousand tons) and Myanmar (174 thousand tons). In per capita terms, the highest consumption of goose and duck meat was observed in Taiwan (6.12 kg/person), China (3.77 kg/person), and Myanmar (3.23 kg/person) [23].
China, Taiwan, and Egypt are the leading producers of goose meat, whereas Poland, Hungary, and Belgium are among the main exporting countries. The current study projects that global goose meat production will increase by 246.32% between 2023 and 2027, compared to the average production over the previous 62 years [23]. In Poland, approximately 90% of goose meat is produced from the White Koluda® goose, and most of it is exported to European Union countries. Despite this, goose and duck meat combined represent only 3.2% of Poland’s poultry meat production, compared to chicken (81.6%) and turkey (15.3%). Between 2020 and 2022, the value of Polish goose meat exports increased significantly, from EUR 51 million to EUR 116 million—an almost 2.3-fold rise. According to data from the National Support Centre for Agriculture, Poland produced 1.11 million geese in 2023, and exported approximately 13.9 thousand tons, primarily to EU markets. The largest importer of Polish goose meat was Germany, which in 2023 purchased 9.4 thousand tons, valued at EUR 68 million. Smaller quantities were exported to Hong Kong, France, Italy, the Czech Republic, Austria, and the United Kingdom [24,25]. Information on the trace element content in goose carcass parts, both with and without skin, may be relevant to consumers when making informed dietary choices.
Few reports address the effect of cooking on the mineral content of goose meat. They mainly concern P, Na, K, Ca, Mg, Fe, Cu, Mn, B, Se, but a few concern trace elements. For example, Belinsky and Kuhnlein [26] found that Ca content was higher in oven-roasted carcasses than in cooked carcasses. Geldenhuys et al. [27] found higher Fe, P, Na, Ca, K, and Mg content in the breast muscles of Egyptian geese that were oven-roasted compared to raw muscles, but the boron content did not change significantly. Oz and Celik [28] showed that cooking methods only had a significant effect on the potassium content of breast meat and the iron content of breast and leg meat of Turkish geese. Goluch et al. [29] showed that cooking in water without skin, White Koluda® breast meat preserves the most minerals (Ca, Mg, Fe, Cu, Mn), compared to grilling, roasting, and frying methods without fat. On the other hand, the selectin content [30] was significantly higher in White Koluda® goose breast meat after roasting, grilling, frying without fat, and boiling than in raw meat. Therefore, in our opinion, the appropriate choice of cooking method is crucial to optimize the nutritional quality of poultry meat, also in terms of mineral content.
Given the limited number of scientific studies addressing the content of selected trace elements in goose meat, the present study aimed to (1) evaluate the impact of commonly used heat processing methods on the concentration of these elements in goose breast muscle, and (2) estimate the extent to which a 100 g portion of goose meat—with or without skin—can contribute to meeting the Nutrient Reference Values (NRV) for chromium, iodine, and manganese, as well as the Tolerable Daily Intake (TDI) for bromine in adults.
2. Materials and Methods
2.1. Meat Samples
The study material consisted of breast muscles (Pectoralis major) from 17-week-old White Koluda® geese, which are marketed across Europe under the name “Polish oat geese.” This genotype was developed through decades of targeted breeding work conducted at the National Research Institute of Animal Production, Experimental Station in Kołuda Wielka [24]. The feeding program included a starter concentrate (weeks 1–4) containing 19% protein and 11.9 MJ of energy, a grower mixture (weeks 5–8) with 17% protein and 11.7 MJ, and a finisher mixture (weeks 9–13) containing 14% protein and 11.7 MJ. The commercial feed mixtures were composed of ground wheat, barley, triticale, oats, grass meal, and protein concentrate in varying proportions. From week 14 to 17, the geese were fattened freely on grass meal and oats [21,31,32]. Birds were reared on the same commercial farm and fed the same diet, including a premix containing 80.0 mg of manganese, 1.20 mg iodine per 1 kg of feed [29]. EFSA [15] has not established a maximum safe concentration of bromine in complete feed for geese, but only for broiler chickens and turkeys (analogously: 4.5 and 6.0 mg/kg feed). Before slaughter, the geese underwent a 12 h fasting period. They were then processed at a poultry facility in compliance with Polish industry standards [33]. After evisceration, the carcasses were chilled at 2–4 °C for 24 h. Following this, breast muscles were removed, refrigerated, and transported to the Wroclaw University of Economics and Business laboratory. Upon arrival, the muscle samples designated for heat treatment were sorted into groups of similar weight and subjected to specific cooking methods.
Muscles with skin weighed, on average, 384.7 g ± 26.1, and without skin and subcutaneous fat, 286.4 g ± 54.6 (N = 36, 12 raw breast muscles = 6 raw with skin and 6 raw without skin, and 24 cooked breast muscles = 6 for every heat treatment: 3 with skin and 3 without skin).
2.2. Heat Processing
The methodology has already been described in a previous publication [30]. Briefly—goose breasts without salt, spices, and other additives were boiled in water, grilled, pan-fried, and roasted in a convection oven. Heat treatment was carried out until the temperature reached 75 °C inside the muscle. After cooling to room temperature, the muscles were minced in an electric meat grinder (model MM/1000/887 Zelmer, Rzeszów, Poland) and sent for analysis.
Water Bath Cooking (WBC). The muscles, packed tightly in plastic bags, were cooked in a water bath (model SW 22, Julabo GmbH, Seelbach, Germany) at 90 °C for 30 min (to reach the above-mentioned final temperature).
Grilling (G). Entire muscle samples were grilled using an electric grill (PD 2020R, Red Fox, Warsaw, Poland) with two preheated plates set to 200 °C. The heat treatment lasted approximately 25 min.
Oven Convection Roasting (OCR). Breast muscles wrapped in aluminum foil were baked in a convection oven (EB7551B Fusion, Amica Ltd., Wronki, Poland) preheated to 200 °C. The roasting continued for 25 min until the target internal temperature was achieved.
Pan Frying (PF). Pan frying was carried out using an electric skillet (model 48155, Unold AG, Hockenheim, Germany). The meat was placed on the preheated surface at 160 °C and flipped once the internal temperature reached 40 °C. Frying continued until the core temperature reached 75 °C, which took approximately 15 min.
2.3. Chemical Analysis
The methods for determining moisture, ash content, and cooking loss have been described in [30]. The results of these analyses are presented in the Supplementary Materials.
Metal content was determined using inductively coupled plasma mass spectrometry (ICP-MS). Sample preparation for the analysis involved wet digestion in a microwave system, Mars Xpress (CEM Corporation, Matthews, NC, USA). Each sample (0.5 g) was digested with 4 cm3 of concentrated nitric acid (CAS: 7697-37-2, Suprapur, Supelco, Bellefonte, PA, USA). The digestion was conducted in Teflon vessels under the following conditions: the temperature was raised to 210 °C over 25 min, followed by a 15 min hold at 210 °C, using a magnetron power of 800 W.
In our study, each experimental group (i.e., each combination of heat processing method and the presence or absence of skin) consisted of three biological replicates, corresponding to independent goose breast muscle (Pectoralis major) samples collected from different birds. The total of n = 6 per heat treatment resulted from combining 3 samples with skin and 3 without skin, as described in Section 2.1. Each biological sample was analyzed twice for trace element content using ICP-MS.
After digestion, the solutions were quantitatively transferred to 50 mL volumetric flasks and diluted to the mark with demineralized water with a conductivity of 0.055 µS/cm (Direct-Q®, Millipore, Darmstadt, Germany).
The clear digests were analyzed using an inductively coupled plasma mass spectrometer (ICP-MS, Varian MS-820, Palo Alto, CA, USA). Plasma was generated using argon gas with a purity of 99.999%. A collision/reaction cell (CRI) was not employed during the analysis. The operational parameters of the ICP-MS system were as follows: plasma gas flow rate—16 L/min, nebulizer gas flow rate—0.98 L/min, RF power—1.38 kW, and sampling depth—6.5 mm.
Quantification was performed using the external calibration method with multielement standards from Ultra Scientific (purity 99.999%, Billerica, MA, USA).
The reliability of the measurements was assured by implementing quality control procedures, including the analysis of a blank sample, a duplicate sample, and a certified reference material (NIST-1577c Bovine Liver, National Institute of Standards and Technology, Gaithersburg, MD, USA).
The validation parameters used in the analysis are summarized in Table 1.

Table 1.
Validation parameters used in the analysis.
2.4. Determination of Retention Factors
The percentage of trace elements retention after heat processing (HP) was calculated by using the following equation [34]:
2.5. Statistical Analysis
Statistical analysis of the results performed using Statistica® 13.1 included the following:
- (1)
- Examination for normality of distribution with the Shapiro–Wilk Test and variation in homogeneity with Levene’s test;
- (2)
- Calculation of arithmetic means and standard errors of a mean (SEM),
- (3)
- Estimation of the significance of differences between the mean values of trace elements contents for individual heat processing (Raw, WBC, G, OCR, PF) and types of meat (with and without skin) and between treatments within particular types of meat using Tukey’s multiple comparison test (at the significance level of p ≤ 0.05 and p ≤ 0.01);
- (4)
- Two-way analysis of variance (ANOVA), according to the linear model Yij = μ + Ai + Bj + (AB)ij + eij, where Yij—feature value; μ—arithmetic mean; Ai—effect of thermal treatment; Bj—effect of meat type (without skin and with skin); (AB)ij—interaction; eij—residual error;
- (5)
- One-way analysis of variance (ANOVA) according to the linear model Yij = μ + Aj + eij, where Yij—feature value; μ—arithmetic mean; Aj—effect of heat treatment within one type of meat (without skin or with skin); eij—residual error.
All data are reported as means (±SEM) of 2 chemical determinations.
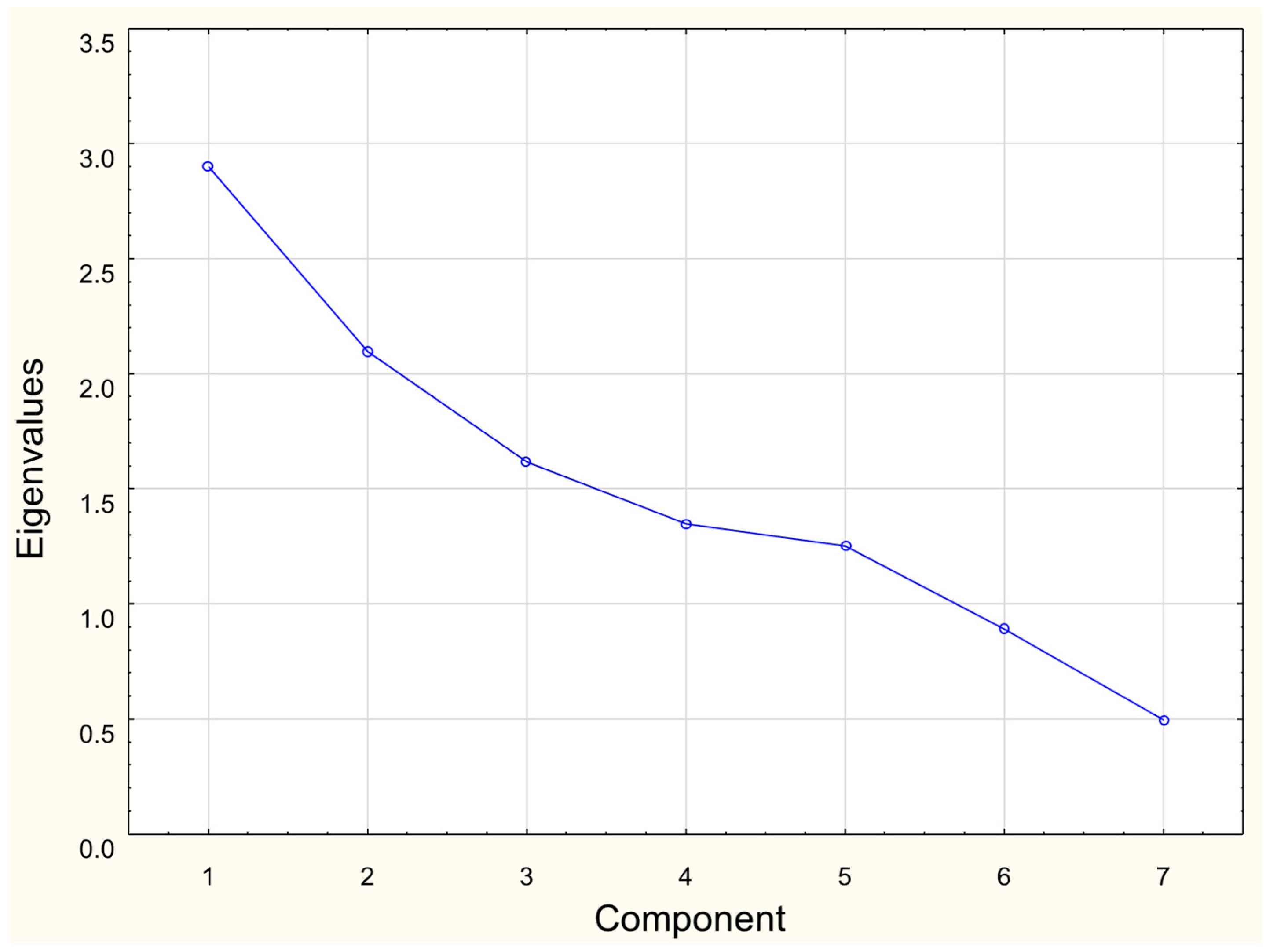
The Principal Component Analysis (PCA) was also conducted to visualize the interrelationships between the Cr, J, Mn, and Br contents, meat type, and kind of heat processing. Based on the scree plot (Figure 1), three Principal Components were included in the PCA model. The PCA was performed using Statistica® 13.1 software.
Figure 1.
Scree plot form based on the results of mineral composition analysis of meat.
3. Results and Discussion
Conventional heat treatment techniques used by consumers can be classified as either moist or dry processing techniques. Moist heat treatment typically involves cooking in a water bath, where the heat is transferred via a hot liquid medium such as water. In contrast, dry heat treatments include convection baking in an oven (where heat is transferred through hot air), pan frying, and grilling on hot surfaces. The primary objectives of meat heat treatment are to inactivate microorganisms and endogenous enzymes, ensure microbiological safety, enhance sensory attributes, and facilitate mastication and digestion [35,36].
In general, meat undergoes moisture loss during thermal processing, which may lead to an increased concentration of certain minerals. Additionally, structural modifications such as protein and collagen denaturation and fat melting influence the meat’s capacity to retain or release minerals [37,38].
3.1. Proximate Composition and Cooking Loss
Moisture and ash content in goose breast, along with ash retention and cooking loss (CL), are presented in the Supplementary Material (Table S1). The results indicate that the method of heat treatment significantly affects these meat quality parameters. Goose breast meat with skin exhibited significantly higher cooking loss compared to skinless samples. Heat processing also had a notable impact on cooking loss, with the highest values observed following grilling (G), oven roasting (OCR), and pan frying (PF), and the lowest after water bath cooking (WBC) (G, OCR, PF > WBC). Conversely, skinless goose breast meat retained significantly more moisture than meat with skin. Additionally, both raw and WBC-treated goose meat showed significantly higher moisture content compared to samples subjected to other thermal treatments. No statistically significant differences in ash content or retention were found between meat with and without skin. However, heat treatment itself did influence ash content, with higher levels observed after OCR and grilling compared to raw meat, PF, and WBC. In terms of retention, WBC resulted in the highest ash retention, followed by PF. A detailed discussion of these findings is available in [30]. This work, however, focuses on the content of selected trace elements.
3.2. Trace Elements Content in Goose Meat After Heat Processing
The content of trace elements in meat is influenced by various factors, including animal species, breed, production system, diet, meat type, and carcass processing techniques. According to Menezes et al. [37], higher temperatures and prolonged exposure to heat during meat processing may reduce protein bioavailability, which in turn affects mineral retention and bioaccessibility. A reduction in mineral content leads to a decline in the nutritional value of meat, which is increasingly important to consumers who prioritize not only sensory attributes but also a balanced, nutrient-rich diet [39].
Meat and meat products are considered significant dietary sources of chromium. In our study (Table 2), the Cr content in goose breast meat was found to be significantly higher (p ≤ 0.001) in samples with skin compared to skinless meat (0.172 vs. 0.120 mg/100 g fresh mass) (0.172 vs. 0.120 mg/100 g Fresh Mass). Heat treatment also significantly affected Cr levels.

Table 2.
Microelements composition (mg/100g FM) of raw and heat-processed White Kołuda® geese breast meat (mean, SEM).
The highest chromium content (p ≤ 0.001) was observed in samples subjected to oven roasting (OCR), when compared with raw, water bath cooked (WBC), and grilled (G) meat. In addition, pan-fried (PF) meat contained significantly more Cr (p ≤ 0.001) than grilled meat. A statistically significant interaction (p ≤ 0.001) was found between meat type (with or without skin) and heat treatment method.
Among skinless samples, the lowest Cr content was recorded in raw and WBC-treated meat, while significantly higher values were found in G, PF, and especially OCR-treated meat (p ≤ 0.001). The lower Cr content in WBC samples may be attributed to leaking from the meat.
In contrast, for skin-on samples, Cr levels were significantly highest (p ≤ 0.001) in OCR-treated meat, compared to all other treatments (Raw, WBC, PF, G). The lowest Cr content (p ≤ 0.001) was recorded in grilled meat relative to the remaining variants. Unfortunately, there is no specific literature data on chromium levels in goose breast meat, which limits direct comparisons. Available data indicate that the average chromium content in unspecified meat in the United Kingdom is approximately 230 μg/kg, while in the United States, meat and fish contain 110–230 μg/kg [40]. In broiler chicken, average Cr concentrations have been reported at 0.01–3.43 (±0.20) mg/kg [41]. According to Nawrocka and Szkoda [42], the Cr content in poultry muscles was 0.16 μg/g FM. Greek studies showed chromium levels in chicken breast and leg muscles ranging from 0.11 to 0.21 and 0.13–0.21 μg/g, respectively [43].
The stability of iodine in food is influenced by the type of food matrix (e.g., seafood, dairy products, eggs, vegetables, cereals) and by processing and storage conditions. In the case of fortified products, iodine stability further depends on the chemical form used—such as potassium iodate (KIO3) or potassium iodide (KI)—and on the type of carrier, including wheat fiber, soy protein, salt, or collagen [44,45,46]. Iodine derived from iodized salt or preparations like Yodozin has been shown to remain stable and thermotolerant in certain animal-based foods (e.g., semi-finished chicken and turkey meat, emulsion-type sausage type “Lyoner”, cooked sausage, boiled ham, salami) even after various technological processes such as heating, fermentation, freezing, smoking, and ripening [46,47]. However, since no iodized salt was used in our study, the applied heat treatments led to a reduction in iodine content in goose breast muscles.
There are relatively few studies reporting iodine concentrations in goose meat. The iodine content in poultry meat is primarily determined by its level in feed and by the physiological and individual ability of animals to absorb and utilize this element. Generally, around 80% of iodine is stored in the thyroid gland, with only a small proportion deposited in other tissues, including muscle [48]. According to Souci et al. [49], the iodine content in raw goose carcasses is 40.7 µg/100 g, whereas food composition databases from several European countries report values ranging from 1.20 to 6.0 µg/100 g [50,51]. In Poland, iodine content in raw goose carcasses has been reported at levels between 0.7 and 4 µg/100 g [52,53]. In our study, goose breast meat with skin had a significantly higher iodine content than skinless samples (p ≤ 0.001) (Table 2). All heat treatments applied led to statistically significant reductions in iodine levels compared to raw meat (Raw > WBC, G, ORC, PF; p ≤ 0.001). A significant interaction was also observed between the meat type (without skin or with skin) and the heat treatment method (p ≤ 0.001). The lowest iodine content was recorded in PF meat without skin and in OCR and PF meat with skin. In both meat types, a consistent decline in iodine content was observed following each heat treatment relative to raw samples. Iodine is a volatile element, and its loss during cooking is mainly attributed to evaporation at elevated temperatures. Additionally, iodine in its organic form is bound to proteins and lipids, meaning that fat and moisture losses during cooking, grilling, baking, and frying also contribute to overall iodine reduction. During dry-heat processing (e.g., baking), protein degradation leads to the oxidation and volatilization of iodine. The extent of iodine loss depends on both temperature and duration of heat exposure. In our study, iodine losses from heat-treated goose breast ranged from 15 to 20%, which aligns with WHO estimates [12]. Similar findings were reported in studies on minced pork, where heat treatment reduced total and inorganic iodine levels by 18–30%, regardless of the method used [44].
In general, meat—including goose meat—is not considered a major dietary source of iodine. Therefore, strategies to enhance iodine content in animal-derived foods are essential to help combat iodine deficiency in the human population. Our findings are consistent with those of Röttger et al. [54], who investigated iodine enrichment in Ross 308 broiler chickens by supplementing feed with KI and Ca(IO3)2 at doses up to 5 mg I/kg. Their results showed that even high-dose supplementation did not significantly increase iodine concentrations in breast or leg muscles—only in blood serum, liver, and thyroid—confirming that poultry meat is unlikely to contribute meaningfully to iodine intake. Current enrichment approaches include supplementing animal feed with inorganic iodine compounds or iodine-rich materials (e.g., seaweed) and plant biofortification through fertigation, foliar or soil application, or hydroponic cultivation using iodine compounds [55,56,57].
According to literature data [14,51,58], the manganese content in raw goose carcasses without skin may range from 0.012 to 0.050 mg/100 g, and in carcasses with skin, approximately 0.020 mg/100 g. In our study (Table 2), the Mn concentration in raw goose breast meat without skin was 0.149 mg/100 g, which is higher than the values reported for Turkish goose by Oz and Celik [28], Canadian goose by Horak et al. [59] and Egyptian goose by et al. [60] in (0.02; 0.03; 0.06 mg/100 g, respectively). However, it was slightly lower than the 0.170 mg/100 g reported in White Kołuda® by Goluch et al. [29]. For meat with skin, the Mn content in our study was 0.139 mg/100 g, which is also slightly lower than the value reported by Goluch et al. [29] (0.150 mg/100 g). Notably, the presence of skin did not significantly affect Mn levels in our findings. The available literature on manganese levels in goose meat after heat treatment generally refers to whole carcasses with or without skin, without distinguishing between muscle types (e.g., breast vs. leg). Reported Mn concentrations range from 0.027 to 0.270 mg/100 g in skinless carcasses subjected to water bath cooking (WBC). In carcasses processed by oven roasting (OCR), Mn content ranges from 0.024 mg/100 g (skinless meat) to 2.6 mg/100 g (meat with skin) [14,58,61].
In our study, heat treatment had a statistically significant effect (p ≤ 0.05) on Mn content in goose breast meat. The highest Mn concentrations were observed in OCR- and G-treated samples (0.159 and 0.149 mg/100g FM, respectively), while the lowest was found in WBC-treated meat (0.110 mg/100 g FM). In contrast, Goluch et al. [29] reported significantly higher Mn content (p ≤ 0.01) in grilled goose breast meat compared to Raw, OCR, and PF samples (0.29 vs. 0.16; 0.12; 0.12 mg/100 g DM, respectively). Taking into account interactions between meat type and heat treatment, a significantly higher Mn content (p ≤ 0.05) was observed in skinless meat after OCR compared to grilling (0.170 vs. 0.107 mg/100 g FM). However, contrary to Goluch et al. [29], we found that WBC-treated meat without skin had higher Mn content than PF-treated meat (0.25 vs. 0.11 mg/ 100 g).
On the other hand, Oz and Celik [28] reported no significant differences in Mn content in skinless breast muscles following various heat treatments, which may be attributed to differences in processing conditions.
In our study, the lowest Mn content in skin-on goose breast meat was observed after WBC treatment (p ≤ 0.001)compared to G, OCR, PF, and Raw samples (p ≤ 0.05). In contrast, Goluch et al. [29] found that grilled meat with skin had significantly higher Mn concentration (p ≤ 0.01) than Raw, WBC, PF, and OCR meat (0.38 vs. 0.15; 0.14; 0.13; 0.11 mg/100 g, respectively).
The bromine content in goose breast meat did not differ significantly between samples with and without skin. However, the applied heat treatments had a statistically significant effect on bromine levels (p ≤ 0.001). Meat subjected to grilling (G), oven roasting (OCR), and pan frying contained significantly more Br (p ≤ 0.001) compared to Raw and water bath cooked (WBC) meat.
A significant interaction (p ≤ 0.001) was also observed between meat type (with or without skin) and heat treatment method. Among skinless samples, grilled meat contained more Br than OCR- and raw samples, while PF-treated meat showed higher Br content than raw, WBC, and OCR. In skin-on meat, the highest Br content was observed in OCR-treated samples, which contained significantly more Br than raw and WBC-treated samples. Both grilled and raw meat had significantly higher Br levels than WBC-treated meat. Bromine is primarily located in the extracellular fluid and has been used in clinical settings to assess extracellular volume and nutritional status [62]. Our study suggests that bromine losses occurred via thermal leakage of extracellular fluid during processing [62]. Given the potential health risks associated with excessive bromine intake and the possibility of exceeding the Tolerable Daily Intake (TDI), WBC processing appears to be the most favorable method for minimizing Br retention in goose meat. Currently, there are no published studies on bromine content in goose meat. However, some data are available for broiler and turkey meat. Essary [63] reported Br concentrations of 0.98–1.21 in mechanically deboned poultry meat and 0.55–0.60 mg/kg in turkey meat. Rose et al. [64] found an average Br concentration of 2.2 mg/kg FM in poultry meat. In a more recent study [65], dietary supplementation of bromine-rich seaweed Ulva lactuca at a dose of 150 g/kg feed for 14 days in 22-day-old Ross-308 broilers resulted in an increase in Br content from 0.12 to 0.30 mg/100 g FM.
3.3. Principal Components Analysis
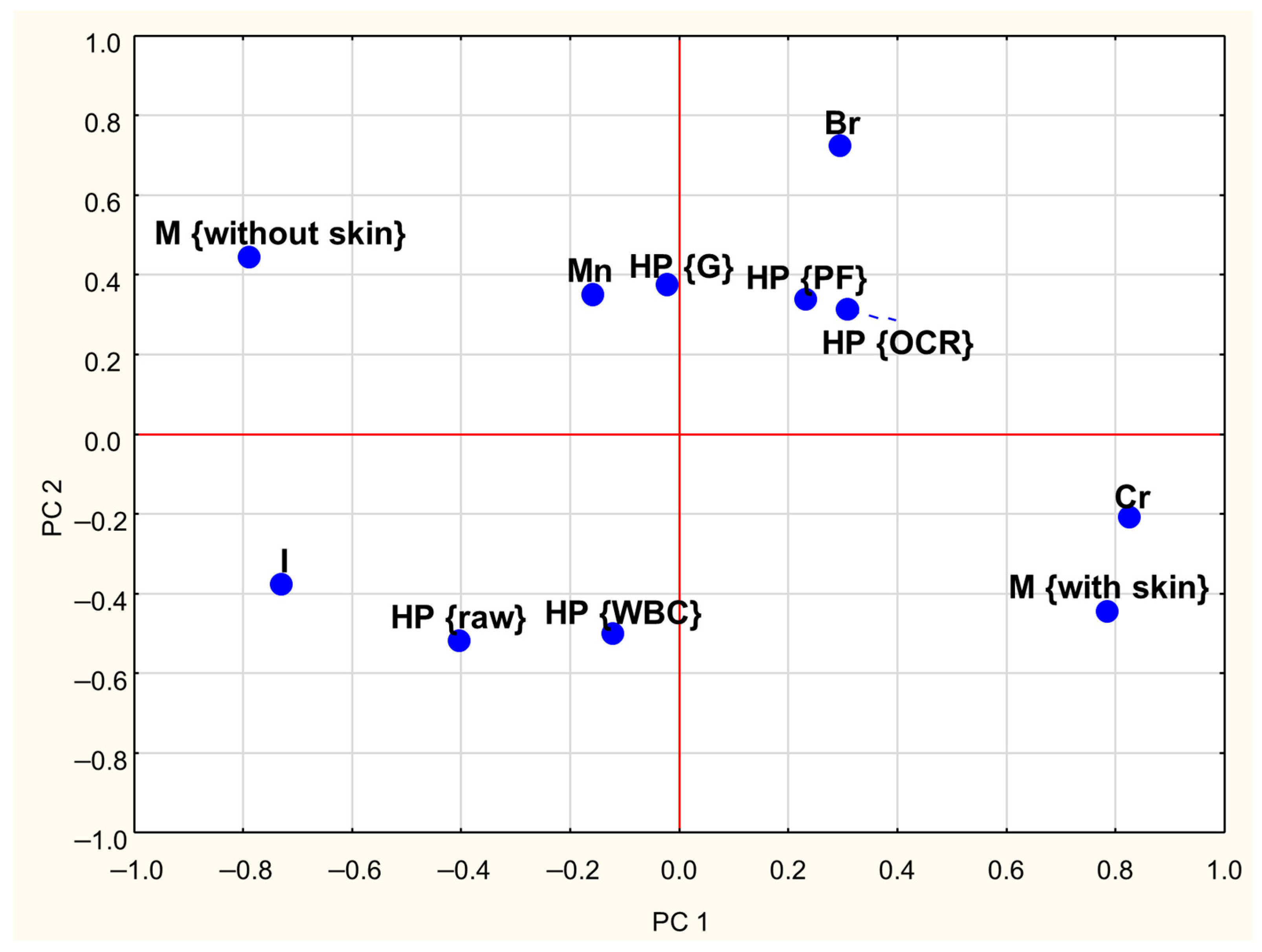
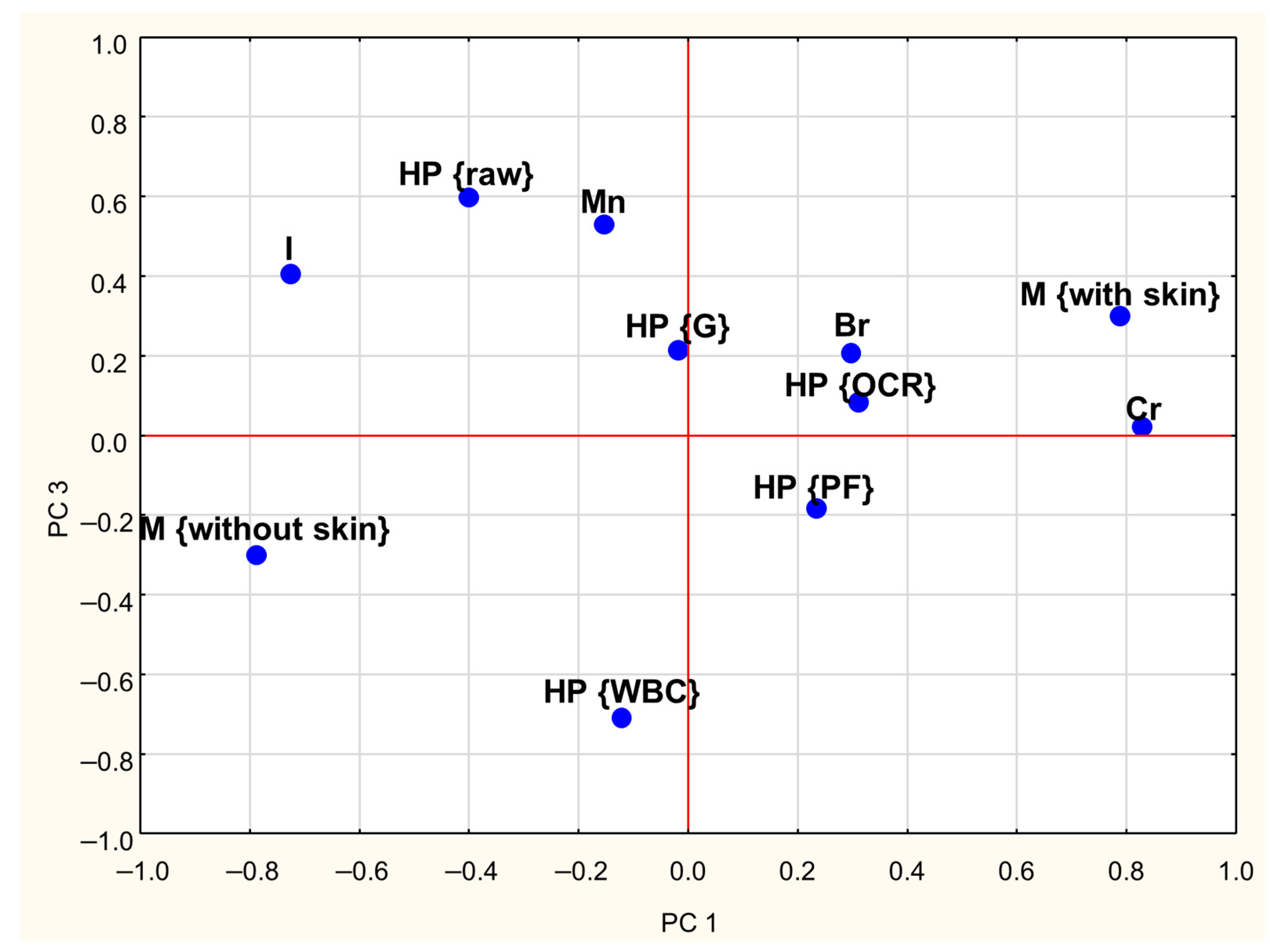
A PCA was conducted to investigate the relationships between the concentrations of Cr, Br, I, and Mn in goose meat and the effects of meat type (with and without skin) and heat processing methods. The first three Principal Components (PCs) explained approximately 60% of the total variance in the dataset. The first Principal Component (PC1) explained 26% of the total variance and showed strong positive loadings (absolute values ≥ 0.6) for Cr content while demonstrating a negative correlation with iodine content (Table 3). The second Principal Component (PC2) accounts for 19% of the variance and was positively associated with Br content. Moreover, Table 3 indicates that PC1 has a strong negative correlation with skinless meat and a strong positive correlation with skin-on meat. The third component (PC3) explained 15% of the total variance and showed high positive loadings for raw meat and negative loadings for WBC-treated meat.

Table 3.
Loadings for the first three PCs.
Loadings plots (Figure 2 and Figure 3) graphically illustrate the relationships among individual treatments and meat types. Similar objects are arranged close to each other on a plane. Analysis of both plots reveals clear distinctions between skinless and skin-on meat, as they are located on opposite sides of the coordinate system. A similar pattern was observed concerning heat treatments: the position of WBC-treated meat on the graph suggests that this method exerts a different influence on mineral retention compared to grilling (G), pan-frying (PF), and oven roasting (OCR). Additionally, the proximity of Cr content and meat with skin on the PCA plots suggests that, regardless of the heat treatment method, the presence of skin is associated with higher Cr levels in meat.
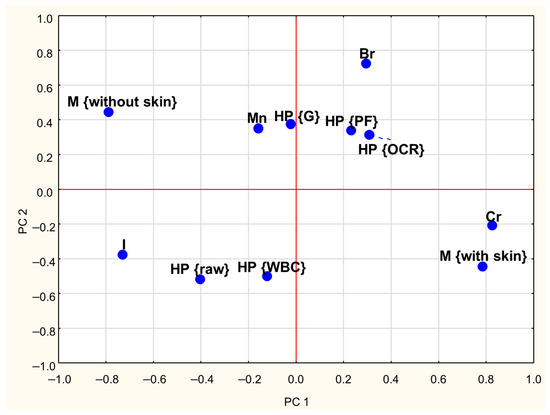
Figure 2.
Loadings plot of the two first PCs (PC1, PC2—first and second Principal Components; HP—heat processing; M—type of meat; WBC—water bath cooking; G—grilling; OCR—Oven Convection Roasting; PF—pan frying; Cr, Br, I, Mn—contents of particular trace elements).
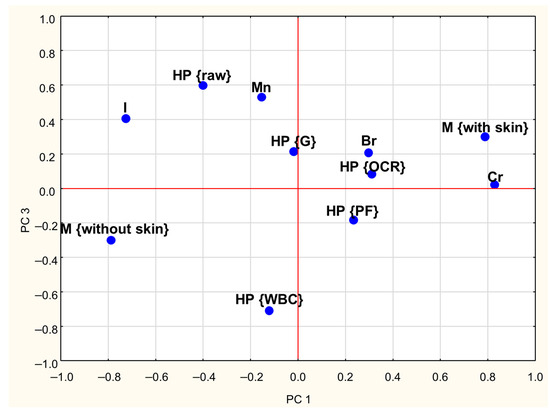
Figure 3.
Loadings plot of the first and third PCs (PC1, PC3—first and third Principal Components; HP—heat processing; M—type of meat; WBC—water bath cooking; G—grilling; OCR—Oven Convection Roasting; PF—pan frying; Cr, Br, I, Mn—contents of particular trace elements).
3.4. Trace Elements Retention in Goose Meat After Thermal Treatment
Heat transfer, temperature, duration of heat treatment, and the specific heat processing method all play critical roles in the physical and chemical changes that can affect the nutritional quality of meat, including mineral retention. When direct heat is applied—such as through hot air during roasting or surface contact during pan frying—thermal energy penetrates the meat gradually from the exterior to the interior. This process forms an outer layer or crust that may help reduce protein degradation and, consequently, the loss of associated minerals [37].
In our study, chromium retention in skinless goose breast meat was significantly higher (p ≤ 0.001) than in meat with skin (80.0% vs. 54.5%; Table 4). This may be attributed to greater cooking losses (CL) observed in meat with skin (36.3 vs. 45.4%, respectively; Table S1). Another contributing factor could be the higher fat content of meat with skin [29], as chromium may be lost along with the fat during heat processing.

Table 4.
Retention coefficients of microelements in the White Kołuda® geese breast meat after heat processing (mean, SEM).
Chromium retention was also significantly higher (p ≤ 0.05) in breast muscles subjected to oven roasting (OCR) compared to grilling (G). This may be explained by the fact that hot-air roasting rapidly raises the meat surface temperature above 70 °C, causing surface drying and crust formation, which reduces internal fluid loss [66,67]. Although CL in OCR-treated meat was lower than in grilled meat, the difference was not statistically significant (42.1% vs. 47.1%). A significant interaction (p ≤ 0.001) was observed between meat type and heat treatment regarding Cr retention. In meat with skin, WBC-treated samples showed significantly higher Cr retention (with significantly lower CL—Table S1) than PF and G, while retention was significantly (p ≤ 0.05) lower after grilling compared to OCR. The better retention in WBC-treated meat may be due to the protective effect of the skin, which acted as a barrier against the penetration of chromium into the leak from meat, thereby reducing mineral losses.
Iodine retention was also significantly higher (p ≤ 0.001) in meat with skin compared to skinless meat (37.0 vs. 16.4%), despite greater CL in the former (Table S1). Regarding heat treatment methods, WBC and grilling (G) resulted in significantly higher iodine retention (p ≤ 0.001) than OCR and PF, likely due to the lower CL observed for WBC (Table S1).
A significant (p ≤ 0.001) interaction was also observed between the meat type (with or without skin) and the heat treatment. In skinless meat, significantly greater iodine retention was observed following WBC and grilling than after frying (PF) and OCR, which again corresponds with lower CL values (Table S1). A similar trend was found in meat with skin, as the lowest iodine retention occurred after OCR and PF, while significantly higher values were recorded after WBC and G (18.5% and 18.3% vs. 73.6% and 37.4%, respectively).
In the case of manganese, similarly to findings reported by Goluch et al. [29], no significant differences in retention were observed based on meat type or heat treatment method, despite statistically significant differences in CL (Table S1).
Bromine retention was significantly higher (p ≤ 0.001) in skinless meat compared to meat with skin (83.9% vs. 71.9%; Table 4), which may again be due to lower CL in skinless samples (Table S1). No statistically significant effect of heat treatment alone was observed for Br retention. However, the interaction between skinless meat and heat treatment was significant. In particular, PF and G resulted in significantly higher Br retention (p ≤ 0.001) than OCR and WBC. Considering the potentially toxic effects of bromine and the risk of exceeding the Tolerable Daily Intake, WBC appears to be the most favorable heat treatment from a health perspective. This is because cooking reduces Br levels by losing meat juices containing extracellular bromine.
In summary, the quality of meat consumed is influenced by both the cooking method and the final internal temperature achieved during thermal processing [68,69]. In general, heat application causes a series of physical and chemical transformations in meat, including muscle fiber contraction, aggregation and gelation of sarcoplasmic proteins, and collagen dissolution within connective tissue structures [69]. During water bath cooking (WBC), water loss—and with it, mineral loss—occurs due to structural and conformational changes in proteins. These changes involve disruption of myofibrillar proteins and transverse and longitudinal contraction of muscle fibers, which weakens the fiber structure and binding forces, ultimately expelling intracellular fluids. Additionally, protein gelation exacerbates these losses by further reducing the water-holding capacity of the tissue [70].
Dry-heat treatments such as grilling, roasting, and pan frying promote collagen breakdown, leading to the formation of microscopic channels through which moisture and melted fat escape but simultaneously contribute to increased concentrations of retained minerals in the remaining tissue mass [67,71]. It has been reported [72] that during frying, the meat surface temperature rapidly reaches 115–120 °C or even higher, forming a coagulated protein layer on the surface. This thermal barrier limits the leaching of soluble compounds, such as nitrogenous substances and inorganic salts. In line with this, our study found that the highest retention levels of chromium, manganese, and bromine—in both skinless and skin-on goose meat—were observed following oven roasting (OCR), grilling (G), and pan frying (PF).
3.5. Coverage of the Nutrient Reference Values by Goose Meat in Adults
The estimated daily requirement for chromium in adults ranges from 20 to 35 µg/day [3], while reported intake levels in Europe and the United States vary between 25 and 120 µg/day [40]. The German (DGE), Austrian (ÖGE), and Swiss (SGE) Nutrition Societies jointly recommend an Adequate Intake (AI) of 30–100 µg/day for adults [73]. Similarly, the U.S. Food and Nutrition Board has established age- and sex-specific AI values, recommending 35 µg/day for men and 25 µg/day for women aged 19–50 years [3]. Adverse effects related to excessive chromium intake have been observed primarily in the context of dietary supplementation, particularly when doses exceed 1 mg Cr/day. However, to date, the European Food Safety Authority (EFSA) has not established a Tolerable Upper Intake Level (UL) for trivalent chromium (Cr III) due to insufficient evidence on toxicity at dietary intake levels [74]. The Nutrient Reference Value (NRV) for chromium in adults (as shown in Table 5 is more fully covered by the consumption of 100 g of goose breast meat with skin than by skinless meat (740.7% vs. 299.5%). The heat treatment method also significantly affects NRV coverage (p ≤ 0.001). The highest chromium NRV percentage was observed in meat subjected to oven-cooked roasting (OCR) (436.5%), while the lowest was noted after water bath cooking (WBC) (228.0%).

Table 5.
Realization (%) Nutrient Reference Values (NRV) or Tolerable Daily Intake (TDI) of trace elements resulting from the consumption of 100 g of Biała Kołudzka® goose breast meat, raw or heat processed (MEAN, SEM).
In skinless meat, the highest NRV coverage was found after grilling (359.7%), and the lowest again after WBC (234.3%). Conversely, in meat with skin, the highest chromium contribution came from OCR-treated meat (542.5%) and the lowest from grilled samples (286.1%).
It is worth noting that chromium is classified as a heavy metal. However, the average chromium content in 100 g of raw and heat-treated goose breast meat ranges from 0.96 to 2.17 mg, which does not exceed the TDI recommended by the EFSA, −0.3 mg/kg BW/day [5]. For an average adult weighing 70 kg, this corresponds to a TDI of 21 mg/day.
Considering the low absorption rate of trivalent chromium (III) from food—estimated at 0.4% to 2.5% [5]—a 100 g portion of goose breast may provide a bioavailable chromium amount of only 0.0038 to 0.055 mg. This level does not pose any health risk to consumers. Furthermore, no adverse effects have been confirmed for dietary intake of chromium (III) [1].
Studies by Saraiva et al. [75], who analyzed oil-free fried beef, confirmed that trivalent chromium (III) is not oxidized to its toxic hexavalent form (Cr VI) during heat treatment, indicating that chromium remains stable in the meat matrix. After absorption in the intestine, chromium binds to plasma transferrin and enters cells via endocytosis, where it is released and sequestered by low-molecular-weight chromium-binding substances (LMWCr). Importantly, chromium does not accumulate in the human body; it is rapidly excreted in the urine (primarily as picolinate and chromodulin), with only small amounts eliminated in feces [76].
WHO and EFSA recommend a daily iodine intake of 150 µg for adults. The Tolerable Upper Intake Levels (ULs) are set at 1100 µg/day by WHO and 600 µg/day by EFSA. According to WHO data, an adequate iodine intake exceeding 150 µg/day among adults was observed in only three out of ten analyzed European countries—Finland, the Netherlands, and Denmark. Notably, iodine intake tends to be lower in women than in men [74]. Dietary iodine—typically in the form of iodide (I−)—is rapidly and almost completely absorbed in the small intestine via the sodium/iodide symporter (Na⁺/I−), regardless of the dietary source or chemical form. Approximately 5–25% of absorbed iodine is taken up by the thyroid gland and used for the synthesis of thyroid hormones, namely thyroxine (T4) and triiodothyronine (T3), which are released into the bloodstream. The biosynthesis of these hormones is regulated by the hypothalamic-pituitary-thyroid axis through a negative feedback mechanism involving thyroid-stimulating hormone (TSH).
Unabsorbed or excess iodine is eliminated from circulation within 24 h, with approximately 90% excreted in urine, assuming adequate intake and normal thyroid function. Minor amounts may also be excreted in feces, sweat, or breast milk during lactation [77]. Our calculations show that 100 g of goose breast meat can contribute to meeting the Nutrient Reference Value (NRV) for iodine in adults, depending on the presence of skin is present and the cooking method applied (Table 5). The iodine NRV contribution was found to be significantly higher (p ≤ 0.001) in skinless meat compared to meat with skin (7.34% vs. 4.56%). This difference can be explained by the distribution of iodine within the tissue—iodine is primarily deposited in muscle, whereas poultry skin is composed mainly of fat (30–40%) and structural proteins (8–12%), including collagen (approx. 3%), and contains only small amounts of minerals [78,79]. As a result, skinless meat has a higher nutrient density, making it a richer dietary source of iodine per 100 g portion. Moreover, heat treatment significantly affected iodine retention (p ≤ 0.001). Raw goose meat, whether with or without skin, provided the highest percentage of NRV for iodine. However, following cooking, the iodine content decreased substantially from 13.5% to 2.29% for raw skinless meat and from 7.32% to 2.36% for meat with skin. These findings confirm that thermal processing significantly reduces the iodine content of goose breast meat, lowering its contribution to the daily iodine requirement in adults.
For manganese (Table 5), the percentage of NRV coverage for an adult consuming 100 g of goose breast meat is not influenced by the presence of skin. However, the heat treatment applied significantly affects the NRV for manganese (p ≤ 0.001). The highest percentage of NRV for manganese is found in meat subjected to OCR at 79.3%, while the lowest is in meat prepared using WBC at 54.9%. When considering skinless meat, the OCR treatment results in the highest NRV coverage for manganese at 85.2%, whereas grilling yields the lowest coverage at 53.4%. Conversely, for meat with skin, grilling provides the highest NRV coverage at 95.5%, while the lowest is observed with WBC treatment at 37.7%. This may be because the skin on the meat forms a physical protective barrier [78]. Under the temperature of the heating surface of the grill (a dry process that does not require water), the loss of skin components occurs, rather than from the meat, in which denaturation occurs. It limits the loss of water-soluble ingredients.
There is currently no established NRV for bromine. Instead, its dietary safety is assessed by comparing intake levels to the TDI defined by the EFSA, which is set at 0.4 mg of bromine per kilogram of body weight per day for the general population (Table 5). For an average adult weighing 70 kg, this corresponds to a TDI of 28 mg/day.
Based on our data, the consumption of 100 g of goose breast meat results in bromine intake ranging from 1.43% to 2.23% of the TDI, with an average contribution of 1.91%. This intake level is considered safe and poses no health risks to consumers.
After ingestion, ions (Br−) are rapidly and almost completely absorbed, mainly in the small intestine, with an absorption rate of 96 ± 6% [15,17]. Peak plasma concentrations are typically reached within 90 min. Once absorbed, bromide is distributed almost exclusively in the extracellular fluid compartment (excluding erythrocytes), like chloride ions. It is capable of crossing both the blood–brain barrier and the placental barrier. Bromide is not metabolized and competes with chloride for renal tubular reabsorption. Its biological half-life in the human body is approximately 285.6 ± 33.6 h, and elimination occurs primarily via the kidneys at a rate of about 26 ± 1.7 cm3/day/kg body weight [15]. Minor bromide excretion also occurs through exhaled air, feces, sweat, saliva, breast milk, and tears [15].
Literature reports [17,80] highlight the competitive relationship between bromine and iodine in the human body, particularly regarding their uptake by specialized thyroid transporters. As a member of the halogen group, bromine functions as a competitive inhibitor at iodine binding sites in the thyroid gland. Due to its lower atomic mass, bromine can displace iodine, interfering with its availability and uptake by the thyroid and thyroid-stimulating hormone (TSH) receptors. This may lead to altered thyroid hormone synthesis and the potential development of antithyroid antibodies.
Additionally, bromine may replace iodine at the 5-position in the structure of thyroid hormones triiodothyronine (T3) and thyroxine (T4) without losing hormonal activity. Such substitution may contribute to thyroid enlargement (goiter), even when iodine intake is adequate. Therefore, in our opinion, the low bromine content (and thus low TDI) observed in goose breast meat is favorable, as it is unlikely to interfere with iodine utilization.
According to Regulation (EU) No 1169/2011 [81], a food may be labeled as a “source” of a nutrient if it provides at least 15% of the adult NRV per 100 g. The established NRVs for chromium, iodine, and manganese are 40 μg, 150 μg, and 2000 μg per day, respectively. Thus, to qualify as a source, a food must contain at least 6 μg of chromium, 22.5 μg of iodine, and 300 μg of manganese per 100 g to qualify as a source of these nutrients. To be classified as a “high source,” the food must provide 30% of the NRV, i.e., 12 μg of chromium, 45 μg of iodine, and 600 μg of manganese.
Based on our findings, 100 g of goose breast meat—regardless of skin presence and heat treatments—can be a “high source” of chromium (162.5–690% NRV) and a potential source of manganese (29.9–148% NRV) but does not qualify as a source of iodine (1.67–30.2% NRV).
In summary, chromium and bromine retention were the highest after grilling skin-on goose breast. The highest manganese retention was found in skinless meat after OCR treatment and in skin-on meat after grilling. These heat treatments induce rapid surface dehydration and crust formation, which may reduce the loss of trace elements. In contrast, iodine content decreased consistently in skinless and skin-on meat after all heat treatments. However, from a dietary perspective, frequent consumption of grilled meat is not recommended due to the potential risks associated with polycyclic aromatic hydrocarbons (PAHs) [82].
To prevent the potential goitrogenic effects of excessive bromine intake, we recommend monitoring the cumulative dietary exposure to bromine from goose meat and other foods.
3.6. Risk Assessment Calculation
The total concentrations of chromium, manganese, and bromine in goose breast meat (regardless of skin and treatment) were Cr—1.46 mg/kg, Mn—1.41 mg/kg, and Br—5.35 mg/kg. The Estimated Daily Intake (EDI) for each element was calculated using the following equation:
where C—concentration of target element in food (mg/kg), IR—intake rate (kg/day), BW—body weight (kg).
To calculate the EDI value, it was assumed that the consumption of goose meat in Poland is 0.3 kg/year/person (~0.00082 kg/day), and the average body weight of an adult is 70 kg. The calculated values are shown in Table 6.

Table 6.
Estimated Daily Intake (EDI), Reference Dose (RfD), Hazard Quotient (HQ), and Total Hazard Quotient (THQ) for chromium, manganese, and bromine from goose breast meat consumption (Polish adult, BW = 70 kg, IR = 0.00082 kg/day).
Next, the Hazard Quotient (HQ) for each element was calculated using [83]:
where EDI—Estimated Daily Intake, RfD—Reference Dose = 0.003 mg/kg BW/day for Cr, 0.1 mg/kg BW/day for Mn, Cr and Mn or ARfD for Br (Acute RfD = 0.4 mg/kg BW/day for Br) [15,84,85].
The calculated HQ values are shown in Table 6. All HQ values were well below one, indicating no health risk associated with the consumption of goose meat. The HQ for iodine was not calculated because the RfD was not established.
The Total Hazard Quotient (THQ), summarizing the combined exposure to all three elements, was
Since THQ < 1, the combined exposure to chromium, manganese, and bromine from goose breast meat does not pose a health risk for Polish consumers.
4. Conclusions
Different heat treatment techniques significantly altered the levels of chromium (Cr), iodine (I), manganese (Mn), and bromine (Br) in goose breast meat, both with and without skin, compared to raw meat. These changes affected the retention of these trace elements and their contribution to the Nutrient Reference Values (NRV) for adults. Grilled, skinless goose breast exhibited the highest bromine retention (97.4%), contributing 2.41% of the Tolerable Daily Intake (TDI). In contrast, skin-on goose meat showed the highest retention of chromium (73.8%) and iodine (73.6%) following water bath cooking (WBC). The highest manganese content was recorded in skinless meat after oven roasting (0.170 mg/100 g) and in grilled meat with skin (0.191 mg/100 g). However, limiting the consumption of grilled meat is advisable due to the potential risk of exposure to polycyclic aromatic hydrocarbons (PAHs). Iodine content decreased across all heat treatments—from 0.020 mg/100 g in raw meat to 0.003 mg/100 g after pan frying (PF). Depending on the cooking method and the presence of skin, goose breast meat can contribute between 2.21% (Br, skinless) and 740.7% (Cr, skin-on) of the adult NRV for selected elements, making it a nutritionally valuable component of a varied diet—except in the case of iodine, which remains limited. To help ensure that bromine intake remains below the TDI, water-based cooking methods such as WBC may be the most beneficial, as they significantly reduce the bromine content in goose meat.
The estimated Hazard Quotients (HQ) and Total Hazard Quotients (HTQ) in goose breast meat for total Cr, Mn, and Br contents (regardless of treatment and meat type) were <1, indicating a low health risk for the Polish consumer. However, the low values of these indicators may result from the average Pole’s low consumption of goose meat. However, the limitations of our research are the lack of speciation of trace elements and small numbers in the groups.
Our findings can assist consumers in choosing the most effective cooking methods for goose breast meat to preserve or enhance trace element content. The retention values of these trace elements may also serve as valuable data for dietitians when estimating their levels in cooked meat based on raw meat content. It is important to note that the values presented refer to the absolute mineral content in the meat without accounting for their absorption by the body, which depends on various factors, including the relationship between bromine and iodine. Therefore, further research is needed to investigate the digestibility of goose meat using different heat treatment methods and the bioavailability of its minerals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126795/s1, Table S1: Cooking loss, moisture, ash content, and retention of raw and heat-processed White Kołuda goose muscles (Mean, SEM).
Author Contributions
Conceptualization, Z.G.; methodology, Z.G. and T.C.; formal analysis, Z.G. and G.H.; investigation, Z.G., T.C., G.H., A.O. and M.W.; data curation, Z.G. and T.C.; writing—original draft preparation, Z.G. and G.H.; writing—review and editing, Z.G., T.C., G.H., A.O. and M.W.; visualization, G.H.; supervision, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization, International Atomic Energy Agency & Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; ISBN N92-4-156173-4.
- Institute of Medicine (US). Panel on Micronutrients Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001; ISBN 0-309-07279-4. [Google Scholar]
- Mikulewicz, M.; Chojnacka, K.; Kawala, B.; Gredes, T. Trace Elements in Living Systems: From Beneficial to Toxic Effects. BioMed Res. Int. 2017, 2017, 2–3. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for Chromium. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef]
- Sahin, N.; Hayirli, A.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Komorowski, J.R.; Sahin, K. Effects of the Supplemental Chromium Form on Performance and Oxidative Stress in Broilers Exposed to Heat Stress. Poult. Sci. 2017, 96, 4317–4324. [Google Scholar] [CrossRef]
- Untea, A.E.; Panaite, T.D.; Dragomir, C.; Ropota, M.; Olteanu, M.; Varzaru, I. Effect of Dietary Chromium Supplementation on Meat Nutritional Quality and Antioxidant Status from Broilers Fed with Camelina-Meal-Supplemented Diets. Animal 2019, 13, 2939–2947. [Google Scholar] [CrossRef]
- Ali, R.; Ali, S.; Azeem, T.; Umar, W.; Irfan, M.; Ali, A.; Ali, M. Effects of Chromium on Meat and Egg Production in Poultry—A Review. Sci. Lett. 2014, 2, 1–4. [Google Scholar]
- Ahmed, S.; Fatema-Tuj-Zohra; Khan, M.S.H.; Hashem, M.A. Chromium from Tannery Waste in Poultry Feed: A Potential Cradle to Transport Human Food Chain. Cogent Environ. Sci. 2017, 3, 1312767. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Nedić, O. Iodine: Physiological Importance and Food Sources. eFood 2023, 4, e63. [Google Scholar] [CrossRef]
- World Hearlth Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination, 3rd ed.; World Hearlth Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-159582-7.
- Fan, L.; Meng, F.; Sun, Q.; Zhai, Y.; Liu, P. Assessment of Sustainable Elimination Criteria for Iodine Deficiency Disorders Recommended by International Organizations. Front. Nutr. 2022, 9, 852398. [Google Scholar] [CrossRef]
- Goluch, Z.; Haraf, G. Goose Meat as a Source of Dietary Manganese—A Systematic Review. Animals 2023, 13, 840. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Risks to Human and Animal Health from the Presence of Bromide in Food and Feed. EFSA J. 2025, 23, e9121. [Google Scholar] [CrossRef]
- Sangster, B.; Krajnc, E.I.; Loeber, J.G.; Rauws, A.G.; Van Logten, M.J. Study of Sodium Bromide in Human Volunteers, with Special Emphasis on the Endocrine System. Hum. Exp. Toxicol. 1982, 1, 393–402. [Google Scholar] [CrossRef]
- Pavelka, S. Metabolism of Bromide and Its Interference with the Metabolism of Iodine. Physiol. Res. 2004, 53, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, X.; Wang, T.; Ji, X.; Wu, X. Advances in Organic Fluorescent Probes for Bromide Ions, Hypobromous Acid and Related Eosinophil Peroxidase-A Review. Anal. Chim. Acta 2023, 1244, 340626. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Okonkwo, J.O.; Olukunle, O.I.; Botha, B.M. Brominated Flame Retardants: Sources, Distribution, Exposure Pathways, and Toxicity. Environ. Rev. 2011, 19, 238–253. [Google Scholar] [CrossRef]
- Orobchenko, O.; Koreneva, Y.; Paliy, A.; Rodionova, K.; Korenev, M.; Kravchenko, N.; Pavlichenko, O.; Tkachuk, S.; Nechyporenko, O.; Nazarenko, S. Bromine in Chicken Eggs, Feed, and Water from Different Regions of Ukraine. Potravin. Slovak J. Food Sci. 2022, 16, 42–54. [Google Scholar] [CrossRef]
- Buzała, M.; Adamski, M.; Janicki, B. Characteristics of Performance Traits and the Quality of Meat and Fat in Polish Oat Geese. Worlds Poult. Sci. J. 2014, 70, 531–542. [Google Scholar] [CrossRef]
- Çimen, H.; Kızılkaya, P.; Sayın, B.; Alp Baltakesmez, D.; Kırmacı, F. Goose Meat: Salting/Drying Effect on Nutritional Value, Physicochemical and Sensory Properties. Gıda Yem Bilim. Teknol. Derg. 2025, 33, 40–50. [Google Scholar] [CrossRef]
- Dumlu, B. The Global Goose Meat Production Quantity Forecast for the 2023–2027 Years. Selcuk J. Agric. Food Sci. 2024, 38, 326–341. [Google Scholar] [CrossRef]
- Gąsior, R.; Wojtycza, K.; Majcher, M.A.; Bielińska, H.; Odrzywolska, A.; Bączkowicz, M.; Migdał, W. Key Aroma Compounds in Roasted White Kołuda Goose. J. Agric. Food Chem. 2021, 69, 5986–5996. [Google Scholar] [CrossRef] [PubMed]
- National Support Centre for Agriculture. Polish Goose Market; National Support Centre for Agriculture: Warsaw, Poland, 2023.
- Belinsky, D.L.; Kuhnlein, H.V. Macronutrient, Mineral, and Fatty Acid Composition of Canada Goose (Branta Canadensis): An Important Traditional Food Resource of the Eastern James Bay Cree of Quebec. J. Food Compos. Anal. 2000, 13, 101–115. [Google Scholar] [CrossRef]
- Geldenhuys, G.; Hoffman, L.C.; Muller, N. Aspects of the Nutritional Value of Cooked Egyptian Goose (Alopochen Aegyptiacus) Meat Compared with Other Well-Known Fowl Species. Poult. Sci. 2013, 92, 3050–3059. [Google Scholar] [CrossRef]
- Oz, F.; Celik, T. Proximate Composition, Color and Nutritional Profile of Raw and Cooked Goose Meat with Different Methods. J. Food Process. Preserv. 2015, 39, 2442–2454. [Google Scholar] [CrossRef]
- Goluch, Z.; Król, B.; Haraf, G.; Wołoszyn, J.; Okruszek, A.; Wereńska, M. Impact of Various Types of Heat Processing on the Energy and Nutritional Values of Goose Breast Meat. Poult. Sci. 2021, 100, 101473. [Google Scholar] [CrossRef]
- Goluch, Z.; Bąkowska, M.; Haraf, G.; Pilarczyk, B. Selenium Content of Goose Breast Meat Depending on the Type of Heat Processing. Appl. Sci. 2024, 14, 4693. [Google Scholar] [CrossRef]
- Nowicka, K.; Przybylski, W. The Genetic Background of Slaughter Value and Quality of Goose Meat—A Review. Anim. Sci. Pap. Rep. 2018, 36, 245–262. [Google Scholar]
- Wojciechowski, J. Hodowla i Chów Gęsi Białych Kołudzkich w Realiach XXI Wieku. In National Research Institute of Animal Production; Koluda Wielka & KPODR: Minikowo, Poland, 2016; pp. 1–20. [Google Scholar]
- Minister of Agriculture and Rural Development. Regulation of the Minister of Agriculture and Rural Development of 15 July 2004 on Veterinary Requirements for the Production of Poultry Meat. J. Laws 2004, item 1636. [Google Scholar]
- Bognár, A.; Piekarski, J. Guidelines for Recipe Information and Calculation of Nutrient Composition of Prepared Foods (Dishes). J. Food Compos. Anal. 2000, 13, 391–410. [Google Scholar] [CrossRef]
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food Bioprocess Technol. 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Thippareddi, H.; Sanchez, M. Thermal Processing of Meat Products. In Thermal Food Processing. New Technologies and Quality Issues; Sun, D.W., Ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006; pp. 155–196. ISBN 1-57444-628-2. [Google Scholar]
- Menezes, E.A.; Oliveira, A.F.; França, C.J.; Souza, G.B.; Nogueira, A.R.A. Bioaccessibility of Ca, Cu, Fe, Mg, Zn, and Crude Protein in Beef, Pork and Chicken after Thermal Processing. Food Chem. 2018, 240, 75–83. [Google Scholar] [CrossRef]
- Bognár, A. Comparative Study of Frying to Other Cooking Techniques Influence on the Nutritive Value. Grasas Aceites 1998, 49, 250–260. [Google Scholar] [CrossRef]
- Teixeira, A.; Rodrigues, S. Consumer Perceptions towards Healthier Meat Products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Committee on Food. Scientific Panel on Dietetic Products Nutrition and Allergies. In Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Parma, Italy, 2006; ISBN 9291990140. [Google Scholar]
- Iwegbue, C.M.A.; Nwajei, G.E.; Iyoha, E.H. Heavy Metal Residues of Chicken Meat and Gizzard and Turkey Meat Consumed in Southern Nigeria. Bulg. J. Vet. Med. 2008, 11, 275–280. [Google Scholar]
- Nawrocka, A.; Szkoda, J. Determination of Chromium in Biological Material by Electrothermal Atomic Absorption Spectrometry Method. Bull. Vet. Inst. Pulawy 2012, 56, 585–589. [Google Scholar] [CrossRef]
- Bratakos, M.S.; Lazos, E.S.; Bratakos, S.M. Chromium Content of Selected Greek Foods. Sci. Total Environ. 2002, 290, 47–58. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Waszkowiak, K. Iodine Retention in Ground Pork Burgers Fried in Fat Free Conditions. Acta Sci. Pol. Technol. Aliment. 2004, 3, 157–162. [Google Scholar]
- Winger, R.J.; König, J.; House, D.A. Technological Issues Associated with Iodine Fortification of Foods. Trends Food Sci. Technol. 2008, 19, 94–101. [Google Scholar] [CrossRef]
- Meinhardt, A.K.; Müller, A.; Lohmayer, R.; Dederer, I.; Manthey-Karl, M.; Münch, S.; Brüggemann, D.; Fritsche, J.; Greiner, R. Influence of Processing and Storage on the Iodine Content of Meat and Fish Products Using Iodized Salt. Food Chem. 2022, 389, 133092. [Google Scholar] [CrossRef]
- Khramova, V.N.; Korolyov, A.V.; Khramova, Y.I.; Korotkova, A.A.; Kartushina, Y.N. Development of Natural Semi-Finished Poultry Meat Products Enriched with Iodine. IOP Conf. Ser. Earth Environ. Sci. 2021, 848, 012032. [Google Scholar] [CrossRef]
- Herzig, I.; Trávniček, J.; Kursa, V.; Kroupová, J.; Řezníček, I. Content of Iodine in Broiler Meat. Acta Vet. Brno 2007, 76, 137–141. [Google Scholar] [CrossRef][Green Version]
- Souci, S.W.; Scherz, H.; Fachmann, W.; Kraut, H.; Senser, F. Food Composition and Nutrition Tables, 6th ed.; Medpharm Scientific Publications: Centurion, Gauteng, 2000; ISBN 9783887630768. [Google Scholar]
- Food Composition Database for Epidemiological Studies in Italy. Available online: https://bda.ieo.it/?page_id=690&lang=en (accessed on 22 April 2025).
- The Danish Food Composition Database Version 5.3. Available online: https://frida.fooddata.dk/data?lang=en (accessed on 22 April 2025).
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tables of Composition and Nutritional Value of Food; PZWL: Warszawa, Poland, 2020; ISBN 9788320062588. [Google Scholar]
- Łoś-Kuczera, M.; Piekarska, J. Composition and Nutritional Value of Food Products. Part II-VII; Państwowy Zakład Wydawnictw Lekarskich: Warsaw, Poland, 1988; ISBN 83-200-1110-8. [Google Scholar]
- Röttger, A.S.; Halle, I.; Wagner, H.; Breves, G.; Flachowsky, G. The Effect of Various Iodine Supplementations and Two Different Iodine Sources on Performance and Iodine Concentrations in Different Tissues of Broilers. Br. Poult. Sci. 2011, 52, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Behroozlak, M.; Daneshyar, M.; Farhomand, P. The Effects of Dietary Iodine and Its Consumption Duration on Performance, Carcass Characteristics, Meat Iodine, Thyroid Hormones and Some Blood Indices in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2020, 104, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Krzepiłko, A.; Prazak, R.; Skwaryło-Bednarz, B.; Molas, J. Agronomic Biofortification as a Means of Enriching Plant Foodstuffs with Iodine. Acta Agrobot. 2019, 72, 1–9. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of Edible Plants with Selenium and Iodine—A Systematic Literature Review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef]
- ANSES-CIQUAL French Food Composition Table Version 2020. Available online: https://ciqual.anses.fr/ (accessed on 22 April 2025).
- Horak, K.; Chipman, R.; Murphy, L.; Johnston, J. Environmental Contaminant Concentrations in Canada Goose (Branta canadensis) Muscle: Probabilistic Risk Assessment for Human Consumers. J. Food Prot. 2014, 77, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, G.; Hoffman, L.C.; Muller, N. The Fatty Acid, Amino Acid, and Mineral Composition of Egyptian Goose Meat as Affected by Season, Gender, and Portion. Poult. Sci. 2015, 94, 1075–1087. [Google Scholar] [CrossRef]
- McCance, W. McCance Widdowsons Composition of Foods Integrated Dataset 2021. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 22 April 2025).
- Silva, A.M.; Heymsfield, S.B.; Gallagher, D.; Albu, J.; Pi-Sunyer, X.F.; Pierson, R.N.; Wang, J.; Heshka, S.; Sardinha, L.B.; Wang, Z.M. Evaluation of Between-Methods Agreement of Extracellular Water Measurements in Adults and Children. Am. J. Clin. Nutr. 2008, 88, 315–323. [Google Scholar] [CrossRef][Green Version]
- Essary, E. Moisture, Fat, Protein and Mineral Content of Mechanically Deboned Poultry Meat. J. Food Sci. 1979, 44, 1070–1073. [Google Scholar] [CrossRef]
- Rose, M.; Miller, P.; Baxter, M.; Appleton, G.; Crews, H.; Croasdale, M. Bromine and Iodine in 1997 UK Total Diet Study Samples. J. Environ. Monit. 2001, 3, 361–365. [Google Scholar] [CrossRef]
- Costa, M.M.; Pestana, J.M.; Carvalho, P.; Alfaia, C.M.; Martins, C.F.; Carvalho, D.; Mourato, M.; Gueifão, S.; Delgado, I.; Coelho, I.; et al. Effect on Broiler Production Performance and Meat Quality of Feeding Ulva Lactuca Supplemented with Carbohydrases. Animals 2022, 12, 1720. [Google Scholar] [CrossRef]
- Alfaifi, B.M.; Al-Ghamdi, S.; Othman, M.B.; Hobani, A.I.; Suliman, G.M. Advanced Red Meat Cooking Technologies and Their Effect on Engineering and Quality Properties: A Review. Foods 2023, 12, 2564. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Marais, J.; Strydom, P.E.; Hoffman, L.C. Effects of Increasing Internal End-Point Temperatures on Physicochemical and Sensory Properties of Meat: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2843–2872. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Roskilly, A.P. Quality and Energy Evaluation in Meat Cooking. Food Eng. Rev. 2016, 8, 435–447. [Google Scholar] [CrossRef]
- Ángel-Rendón, S.V.; Filomena-Ambrosio, A.; Hernández-Carrión, M.; Llorca, E.; Hernando, I.; Quiles, A.; Sotelo-Díaz, I. Pork Meat Prepared by Different Cooking Methods. A Microstructural, Sensorial and Physicochemical Approach. Meat Sci. 2020, 163, 108089. [Google Scholar] [CrossRef]
- Macharáčková, B.; Bogdanovičová, K.; Ježek, F.; Bednář, J.; Haruštiaková, D.; Kameník, J. Cooking Loss in Retail Beef Cuts: The Effect of Muscle Type, Sex, Ageing, PH, Salt and Cooking Method. Meat Sci. 2021, 171, 108270. [Google Scholar] [CrossRef]
- Purchas, R.W.; Wilkinson, B.H.P.; Carruthers, F.; Jackson, F. A Comparison of the Nutrient Content of Uncooked and Cooked Lean from New Zealand Beef and Lamb. J. Food Compos. Anal. 2014, 35, 75–82. [Google Scholar] [CrossRef]
- Yong, W.; Amin, L.; Dongpo, C. Status and Prospects of Nutritional Cooking. Food Qual. Saf. 2019, 3, 137–143. [Google Scholar] [CrossRef]
- Referenzwerte Für Die Nährstoffzufuhr. Neustadt an Der Weinstraße, Duitsland. Available online: https://www.dge.de/wissenschaft/referenzwerte/kupfer-mangan-chrom-molybdaen/ (accessed on 22 April 2025).
- European Food Safety Authority (EFSA). Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Eur. Food Saf. Auth. 2024, 10, 1–8. [Google Scholar]
- Saraiva, M.; Chekri, R.; Guérin, T.; Sloth, J.J.; Jitaru, P. Chromium Speciation Analysis in Raw and Cooked Milk and Meat Samples by Species-Specific Isotope Dilution and HPLC-ICP-MS. Food Addit. Contam. Part A 2021, 38, 304–314. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe; Iodine Global Network. Prevention and Control of Iodine Deficiency in the WHO European Region: Adapting to Changes in Diet and Lifestyle; World Health Organization, Ed.; WHO Regional Office for Europe: Copenhagen, Denmark, 2024; ISBN 9789289061193.
- Ockerman, H.W.; Basu, L. Hides and Skins, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, ISBN 9780323851251. [Google Scholar]
- da Silva Araújo, Í.B.; Pereira Da Silva, F.A.; Moreira Fernandes Santos, M.; do Nascimento Alves, R. Chapter 25—Recovery and Application of Bioactive Proteins from Poultry by-Products. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Oxford, UK, 2021; pp. 497–514. ISBN 978-0-12-824044-1. [Google Scholar]
- Zaichick, V. Determination the Content of Bromine, Calcium, Chlorine, Iodine, Potassium, Magnesium, Manganese, and Sodium in the Nodular Goiter of Human Thyroid Gland Using Neutron Activation Analysis. Aditum J. Clin. Biomed. Res. 2021, 3, 1–8. [Google Scholar]
- European Parliament Regulation (EU). No 1169/2011 of the European Parliament and of the Counsil of 25 October 2011 on the Provision of Food Information to Consumers. Off. J. Eur. Union 2011, 304, 18–41. [Google Scholar]
- Mariussen, E.; Alexander, J.; Bukhvalova, B.A.; Dahl, L.; Olsen, A.-K.H.; Kvalem, H.E.; Schlabach, M.; Amlund, H.; Hannisdal, R.; Ruus, A.; et al. Risk Assessment of Grilled and Barbecued Food Scientific Opinion of the Panel on Contaminants of the Norwegian Scientific Committee for Food and Environment. Food Risk Assess Eur. 2024, 2, 0024E. [Google Scholar]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population Health Risk Due to Dietary Intake of Heavy Metals in the Industrial Area of Huludao City, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef]
- CASRN 16065-83-1; US EPA-IRIS Chromium (III), Insoluble Salts. Integrated Risk Information System (IRIS), U.S. Chemical Assessment Summary National Center for Environmental Assessment: Washington, DC, USA, 1998; pp. 1–16.
- CASRN 7439-96-5; US EPA-IRIS Manganese. Integrated Risk Information System (IRIS): Washington, DC, USA, 1995; pp. 1–46.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).