Featured Application
The work’s results can be used to apply oil-rich yeast biomass in food technology. In addition, a method of culturing cells in media based on untreated water containing a waste carbon source was presented, thereby minimizing the cost of culture.
Abstract
This study investigated the potential of Yarrowia lipolytica, an oleaginous yeast, for producing lipid-rich biomass and its application in food technology. According to EFSA guidelines, lipid-rich biomass is recognized as a novel food with potential nutritional and technological value. However, cost-effective and scalable production of such biomass remains a challenge. The yeast was cultured in a nitrogen-limited medium using a cost-containment strategy based on the use of waste carbon sources, such as post-frying oil and untreated tap water. The composed batch culture approach studied in the experiments presented an example that reduces the cost of yeast biomass biosynthesis. This research aimed to characterize the biomass to assess its nutritional quality and suitability for food applications. Cultures were conducted in a laboratory bioreactor with a working volume of 4 litres. Key kinetic parameters were determined, including biomass yield (X), maximum lipid concentration (Lmax), lipid yield, protein yield relative to substrate and the specific rate of lipid synthesis or protein content and other cellular components. The biomass of Y. lipolytica demonstrated a high lipid content (39.43–50.53%), with significant levels of protein (24.16–27.03%) and unsaturated fatty acids, including oleic acid (62.73–66.44%) and linoleic acid (19.40–21.40%). Lipid-rich biomass produced in cultures with shorter times (20 h), which ended in the logarithmic growth phase, exhibited lower oxidative stability than longer cultures (65 h), which ended in the stationary growth phase. The results of this study highlighted that waste carbon sources and untreated tap water did not significantly impact the biomass yield or the nutritional profile, but did affect the stability of the produced oil. The biomass of Y. lipolytica, containing over 20% lipids, could serve as a promising raw material for food technology, providing a sustainable alternative to traditional vegetable oils. This work makes an important contribution to the development of alternative lipid sources by integrating waste processing in bioreactor-scale culture and kinetic modelling.
1. Introduction
Yarrowia lipolytica, identified by David Yarrow in 1980, is an unconventional yeast species that is important in biotechnology due to its unique metabolic characteristics. Van der Walt and von Arx introduced the name Yarrowia, while the species lipolytica reflects its ability to hydrolyse lipids [1]. It is often found in food products such as cheeses (Camembert, Livarot, and Rokpol), yoghurts, and sausages [2].
Y. lipolytica is a non-pathogenic microorganism classified as GRAS (Generally Recognized as Safe) by the FDA [2]. It can, however, cause sporadic infections in critically ill and immunocompromised or patients who suffer prolonged hospitalisation [3]. The EFSA Panel evaluated Y. lipolytica yeast biomass as a novel food under Regulation (EU) 2015/2283. The registered dried biomass contains 45–55 g/100 g protein, 25 g/100 g dietary fibre, and 7–10 g/100 g fat, primarily as beneficial monounsaturated and polyunsaturated fatty acids [4]. Still, these yeasts can accumulate lipids equivalent to 40% of their dry biomass. Under nutrient-limited conditions, lipid accumulation can reach values exceeding 70% of their biomass [5]. Its protein content is comparable to S. cerevisiae, and proteins from Y. lipolytica yeast cells are also a source of micronutrients, including chromium and selenium in organic forms, which promotes their better absorption. Y. lipolytica yeast has a high content of vitamins and minerals [6,7,8]. The yeast Y. lipolytica efficiently metabolizes hydrophobic substrates, making it valuable for producing single-cell protein (SCP), single-cell oil (SCO), and citric acid. This yeast is an object of interest in research focusing on producing raw materials for biofuel synthesis and other industrial applications [9]. The current Commission Regulation (EU) 2024/2044 extends the possibility to use Y. lipolytica yeast biomass as a novel food and sets maximum levels for its content in various food products. The biomass can be used among others, in food supplements (up to 6 g/day for adults), meal replacements, foods for special medical purpose and a wide range of products such as beverages, dairy products, bread, sweets, snacks, and meat products, with specific limits ranging from a few grams to a few tens of grams per kilogram or litre [10].
Furthermore, only Y. lipolytica biomass has been approved by EFSA as a novel food, while the microbial oil obtained from this biomass still remains largely unexplored and unregulated, with many studies highlighting its valuable properties.
The microbial oil production process involves two main steps: biomass growth and lipid accumulation, followed by lipid extraction using solvents [11]. The yeast Y. lipolytica stores fatty acids (FAs) in lipid bodies, primarily as triacylglycerols (TAGs) and sterol esters (SEs). Fatty acids are synthesized via two pathways: the de novo pathway, requiring hydrophilic substrates like sugars and organic acids to produce FAs from acetyl-CoA, and the ex novo pathway, which involves the hydrolysis of hydrophobic substrates such as alkanes and TAGs into FAs and glycerol for TAG synthesis [12]. Single-cell oil (SCO) can be generated through de novo pathways using glucose or other hydrophilic sources. When nitrogen is limited, it accumulates lipids early in the growth cycle, increasing its potential for industrial applications. Its rapid cell growth and high biomass density make it one of the most studied microbial bioproduct cell factories [13]. This oleaginous yeast can rapidly convert low-cost substrates into SCOs, making large-scale production possible despite high culture costs. Unlike plant and animal sources, microbial oil production is unaffected by seasonal or geographical factors [14]. SCOs are a sustainable source of polyunsaturated fatty acids (PUFAs) and can serve as an alternative to fish oils, which face issues like declining fish populations and contamination. Microbial production is resilient to climate change and offers high-purity oils [15]. These oils can serve as sustainable alternatives to palm oil. Modifying oleaginous yeast to increase its production of saturated fatty acids can make its lipid profile more similar to palm oil [16]. Microbial oils could help preserve forests and reduce greenhouse gas emissions linked to palm oil production [17]. An economic analysis indicated that microbial oil production is more expensive than vegetable and animal lipids. This is primarily due to the high cost of glucose, a basic carbon source in microbial culture, which accounts for 80% of the raw material cost. Nevertheless, using agricultural and industrial waste in culture media can potentially reduce costs and enhance the economic viability of bioproduction [18]. Oleaginous yeast show potential in biodiesel and oleochemicals production, with better results than microalgae or vegetable oils. The rapid growth rate of yeast, replicating in less than an hour, gives it a competitive advantage over microalgae. It has been estimated that producing 1000 tonnes of refined oil and 1800 tonnes of dry whey yeast and protein blend by continuously fermenting the whey for 250 days a year would cost $2.8 million and provide a 14% internal rate of return. Other evidence suggests that producing microbial oil from yeast or fungi would cost more than US$3/kg in 2008. However, no detailed studies in the open literature estimate the cost of producing microbial oil [19].
Despite the many advantages of microbial oil production in yeast cells, work is still needed to increase the economic viability of this process on a commercial scale. The high cost of SCO biosynthesis lies in the price of culture and intracellular lipid extraction. The first cost can be reduced by using waste culture media, and the second by developing a way to use microbial oil without extracting it. This paper addresses both of these aspects. The utilisation of Y. lipolytica yeast in the production of lipids from low-quality waste material, with a concomitant examination of the impact of the type of water employed in culture (distilled versus tap water), signifies the innovation of the research presented. The waste, although often treated as a problem, is at the same time a rich source of energy and carbon that can be used in biotechnological processes to produce biomass, lipids, or other high-value-added products. This study aimed to obtain oleaginous yeast Y. lipolytica biomass rich in storage lipids in a nitrogen-limited culture medium with a waste carbon source and untreated tap water. The resulting lipid-rich biomass was characterized to evaluate its nutritive quality, which is important for its application in food technology, including microbial lipids’ oxygen stability and composition.
To the best of the authors’ knowledge, the biomass of unconventional yeast has been studied as a source of protein in the human diet and animal nutrition. Preparations of cell walls, valuable glucans, and mannans are also being studied. The current research is being conducted on storage lipids as an alternative to vegetable oils. The biomass of oleaginous yeast containing more than 20% fat in the cell’s dry weight was discussed as a possible raw material in food technology. Previous work evaluated the safety of the oil in terms of polycyclic aromatic hydrocarbons (PAHs), the residual content of the solvent used to extract fat from yeast cells and the presence of heavy metals [20]. Because only the protein-rich biomass of Y. lipolytica has been allowed for human nutrition by EFSA, the research presented in this paper might give new insights into the nutritive properties of the high lipid-content yeast biomass. This research highlights the importance of microbial oil as a food additive. Since only biomass of Y. lipolytica is currently approved for human nutrition, our study provides new insights into the nutritive properties of the high lipid-content yeast biomass and offers data that may support future regulatory approval for using microbial oil as a food additive.
2. Materials and Methods
2.1. Materials
The material for the study was the yeast strain Y. lipolytica KKP 379 purchased from the Collection of Industrial Microbial Cultures from Prof. Wacław Dąbrowski Institute of Agricultural and Food Biotechnology—National Research Institute in Warsaw. Yeast cultures were stored in glycerol stocks at −20 °C and on agar slants at 4 °C for the current use.
Post-frying rapeseed oil was kindly gifted from meat processing plants in the Mazowieckie Voivodeship in Poland. Poultry carcasses were fried in the oil.
2.2. Yeast Cultures and Biomass Treatments
The inoculum cultures in flasks were provided in yeast extract, peptone, and glucose medium (YPG). Other shaken cultures were performed in a rich YPO medium. YPG medium consisted of yeast extract (10 g/dm3), peptone (20 g/dm3), and glucose (20 g/dm3) as carbon sources. Instead of glucose as a carbon source, the YPO medium was characterized by including waste rapeseed oil in the composition. The shaken culture media’s pH was 5.0, adjusted with concentrated hydrochloric acid.
The nitrogen source-limited medium was used for shaken cultures and yeast culture in the BIOFLO 3000 laboratory bioreactor from New Brunswick Scientific (Edison, NK, USA) with a working volume of 4 dm3. Depending on the variant, distilled or untreated tap water was used to prepare the medium. All media, independent of the type of water used, were sterilized in an autoclave at 121 °C for 15 min using standard sterilization conditions. The culture medium contained rapeseed oil from meat processing plants at a concentration of 50 g/dm3, along with yeast extract (BTL sp. z o. o., Warsaw, Poland) at 2 g/dm3 and peptone (BTL sp. z o. o., Warsaw, Poland) at 1 g/dm3, ammonium sulfate ((NH4)2SO4) at 2.5 g/dm3, potassium dihydrogen phosphate (KH2PO4) at 7.0 g/dm3, sodium hydrogen phosphate (Na2HPO4) at 2.5 g/dm3, magnesium sulfate (MgSO4) at 1.5 g/dm3, calcium chloride (CaCl2) at 0.15 g/dm3, ferrous sulfate heptahydrate (FeSO4·7H2O) at 0.16 g/dm3, zinc sulfate (ZnSO4) at 0.02 g/dm3, and manganese chloride tetrahydrate (MnCl2·4H2O) at 0.08 g/dm3. The mineral salts listed were sourced from the following companies: Avantor (Gliwice, Poland) and Chempur (Piekary Śląskie, Poland). The final solution was adjusted to a total volume of 4 dm3. During the 65 h bioreactor culture, a waste carbon source of 100 g was added at 16, 50, and 62 h for the distilled water-based culture and at 16 h for the tap water-based culture to maintain further yeast cell growth.
The culture was carried out under conditions of 28 °C and 200–600 rpm with an adjustable relative oxygenation level of the medium, the concentration of which was maintained at 30% of its initial concentration as measured by an oxygen electrode. During the culture, samples were taken for analysis. In cultures lasting 65 h, biomass was taken at the 43rd hour of culture and at the end of the culture. In the one-day bioreactor, the biomass was taken at the end of the culture in the 20th hour. The biomass was collected by centrifugation in an MPW Centrifuge-352 (MPW Med. Instruments, Warsaw, Poland) at 8000 rpm for 10 min. After centrifugation, three layers were formed in the thimble: the first layer at the very top was the residual lipid carbon source, the second was the post-culture fluid, and at the very bottom, as a precipitate, was the yeast cell biomass. The supernatant and the residual lipid carbon source were poured into a separate thimble. The wet biomass was weighed and dried at 105 °C in a laboratory dryer, then weighed again after drying. The yeast biomass’s dry matter (DM) content was calculated from the difference between the wet and dry weights.
Part of the biomass was lyophilized. Before lyophilization, the yeast biomass was subjected to a quick freezing process at −40 °C. Freeze-drying was carried out for 24 h in a Christ Gamma 1–16 apparatus (Osterode am Harz, Germany) on a 10 °C shelf under a pressure of 63 Pa, corresponding to an ice temperature of −25 °C at thermodynamic equilibrium. The safety pressure was 103 Pa. The material was stored in sealed aluminium foil bags [9].
2.3. Water Composition Analysis
A physicochemical analysis of four different water samples was provided: water C—redistilled water, serving as a control sample; water P—tap water taken from left-bank Warsaw (Praga-Południe District); water B—tap water taken from right-bank Warsaw (Bielany District); water U—tap water taken from right-bank Warsaw (Ursynów District). The selection of these samples made it possible to compare the quality of water supplied in different areas of Warsaw and assess the possible influence of its parameters on the study. Physicochemical analysis of the water was carried out following current standards and testing procedures. Parameters such as pH (using the potentiometric method) and electrical conductivity (using the conductometric method PN-EN ISO 10523:2012 [21]) were assessed. The content of ions such as nitrates (PN-EN 26777:1999 [22]), nitrites (PN-82/C-04576.08 [23]), and ammonium ions (PN-ISO 7150-1:2002 [24]) was determined using spectrophotometry. Chlorides (PN-ISO 9297:1994 [25]), water hardness (PN-ISO 6059:1999 [26]), and permanganate index were determined using titration. The ICP–OES (inductively coupled plasma with optical emission separation atomic spectrometry) method was used to assess the water’s iron and manganese (PN-EN ISO 11885:2009 [27]) content. Sulfates were determined according to the ISO 15923-1:2013 [28] procedure, defining methods for automated spectrophotometric and turbidimetric analyses using a discrete analytical system.
2.4. Determination of Lipid Content in Yeast Cells and Content of Residual Oil in Medium
Microbial oil was extracted from the yeast biomass in a Soxhlet apparatus, and residual oil from the supernatant was extracted by simple double extraction using a separator. For the microbial oil, the dried biomass was ground. Fats from the previously dried biomass cells were extracted with n-hexane, and the extraction time was approximately 1.5 h. The resulting extract was dried for 10 min with anhydrous magnesium sulfate. The solvent was distilled under reduced pressure in a Buchi Rotavapor R-200 evaporator (Uster, Switzerland). The oil remaining in the flask was weighed, and its mass was related to the weight of the yeast biomass.
A twofold simple extraction method was used with 10 cm3 portions of hexane to separate the oil from the residual culture medium. Anhydrous magnesium sulfate was added to conical flasks with the organic phase to remove water. After 10 min, the whole was filtered into a dry and pre-weighed round-bottomed flask to remove the drying agent. The solvent was evaporated from the organic phase using a Buchi Rotavapor R-200 reduced-pressure evaporator (Uster, Switzerland). The remaining oil in the flask was weighed and related to the volume of the culture medium.
2.5. Protein Analysis by the Kjeldahl Method
The Kjeldahl method for determining protein content involves mineralizing the sample with concentrated sulphuric acid (VI). Approximately 0.5 g of yeast biomass was placed in a heat-resistant glass tube to which 10 cm3 of concentrated acid and a catalyst were added. The mixture was heated until the organic matter was completely burned off, as indicated by the formation of a clear solution. Once cooled, the contents were diluted with distilled water, followed by ammonia distillation. The distillation system added 10 cm3 of stock solution and phenolphthalein and 10–15 cm3 of 20% NaOH to the flask. Distillation continued until the solution flowing out of the condenser became neutral, as confirmed by the indicator paper. Finally, the solution was titrated with 0.1 M HCl until the colour changed from green to pink. The protein content per 100 g of sample was calculated from the average HCl used, using a titre of HCL and a conversion factor of 6.25 for yeast biomass.
2.6. Determination of the Microbial Kinetics
Kinetic parameters in Y. lipolytica cultures were calculated according to the methodology described by Papanikolaou and Aggelis [29]. The following parameters were determined: initial concentration of carbon source [S—g/dm3], time [t—h], duration of lag phase [tlag—h], biomass yield [X gd.m./dm3], maximum concentration of lipids produced [Lmax—g/dm3], conversion yield of biomass per carbon substrate [YX/S—gd.m./g], conversion yield of storage lipids per biomass formed [YL/X—g/gd.m.], conversion yield of storage lipids per carbon substrate [YL/S—g/g], volumetric rate of storage lipids production [qLv—g/dm3/h], and specific rate of storage lipid production [qL—g/gd.m./h]. Analogous calculations were performed for protein content and other cell constituents. All values were calculated manually using the following formulas. The carbon source concentration (S) was determined based on the total amount of rapeseed oil added to the culture medium per 1 dm3, including supplementation during the culture, depending on the biomass sampling time. Culture time (t) corresponded to the time at which biomass was sampled for analysis (in the case of the 65 h culture, this was at 43 h and at the end of the culture, and in the 20 h culture at the end of the culture). Biomass yield [X] was expressed in grams of dry mass per 1 dm3. The maximum concentration of lipids, protein and other cell components was calculated as the product of their content in the dry biomass (%w/w) and the biomass yield (X). For the conversion yield of biomass to carbon substrate, the biomass yield was divided by the amount of carbon source [X/S], while the yield of storage lipids per biomass formed was calculated by dividing by Lmax/S, and for per carbon substrate, the maximum concentration was divided by the amount of supplemented oil—the carbon source. The calculation formula for the volumetric rate of storage lipids production was calculated using the following formula: qLy = Lmax/t, and the specific rate of production was calculated as qL = Lmax/t/X.
2.7. Determination of Fatty Acid Composition
A gas chromatography method using a YL6100 GC flame ionization detector (GC-FID) (Young Lin Bldg., Anyang, Hogye-dong, Republic of Korea) with a Zebron ZB-FFAP GC capillary column (30 m × 0.25 mm × 0.25 µm) was used to determine the fatty acid composition of the oil extracted from the yeast cells and the frying oil used in this study. Oil samples were dissolved in n-hexane and then derivatized to fatty acid esters with 1 M KOH solution in methanol for 30 min at 40 °C. Nitrogen was used as carrier gas at a 35 cm3/min flow rate. The analysis program was planned as follows: 80 °C (2 min) to 200 °C (10 min) (heating rate 5 °C/min). The injector temperature was 250 °C, the detector temperature was 350 °C, and the injection volume was 1 µL. The retention times of the fatty acid standard were used for identification, and the area of the corresponding peaks was used to determine the relative fatty acid content. Each sample was analysed twice [30,31].
2.8. Oxidative Stability Analysis of Microbial Oil
A Q20 TA Instrument differential scanning calorimeter (TA Instruments, New Castle, DE, USA) with a high-pressure DSC cell (Q Series DSC Pressure Cell, PDSC—TA Instruments, New Castle, DE, USA) was used for the study. Oil samples of 3–4 mg were placed in an aluminium vessel and heated to 120 °C. The analysis was carried out under isothermal conditions with an oxygen pressure of 1400 kPa and a gas flow rate (oxygen flow rate) of 100 cm3/min; from the oxidation isotherms obtained, the oxidation induction time and the maximum oxidation time were determined by interpreting the PDSC curves via TA Universal Analysis 2000 software.
2.9. Determination of the Number of Bacteria and Yeast
The general number of bacteria and yeast was evaluated in heat-inactivated biomass and microbial oil, utilising solid media: nutrient agar (Oxoid, Hampshire, UK) for bacteria and DRBC agar (Dichloran-Rose Bengal Chloramphenicol agar, Graso Biotech, Jabłowo, Poland) for yeast was used to solidify the media at a concentration of 20 g/dm3. After 48 h of incubation at 30 °C, the grown bacterial colonies were counted and converted to log CFU/g, and after 72 h of incubation at 28 °C, the grown yeast colonies were counted and converted to log CFU/g.
2.10. Statistical Analysis
Statistical analysis was performed using Statistica software (STATISTICA 13.3, Statsoft, Poland). The standard deviation was used to measure the central tendency. Confidence in the results obtained was 95%. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were performed to separate homogeneous groups. The normality of the distribution was assessed using the Shapiro–Wilk test.
3. Results
3.1. Impact of Tap Water Quality on Yeast Biomass Yield and Lipid Content
The analysis compared the physicochemical parameters of redistilled water (Water C, control) and tap water samples from three districts in Warsaw: Praga-Południe (Water P), Bielany (Water B), and Ursynów (Water U) (Table 1). Significant differences were observed in conductivity, hardness, and the concentrations of key ions. Water C exhibited the lowest conductivity (3.0 µS/cm) and negligible concentrations of dissolved substances, confirming its purity. In contrast, tap water samples displayed significantly higher conductivity, with the highest value recorded in Water P (1021 µS/cm). This suggests greater mineral content. The hardness of the tap water varied considerably, with the highest value in Water U (400 mg/L) and the lowest in Water B (230 mg/L). This variation is linked to the geological composition of the water sources, with Water U likely containing a higher proportion of groundwater, rich in calcium and magnesium. Chloride concentrations followed a similar trend, with Water P showing the highest value (186 mg/L), indicating the influence of water disinfection and distribution system characteristics. Iron and manganese levels remained low in most samples, except for Water U, which exhibited increased concentrations (18 µg/L Fe, 10 µg/L Mn). This suggests possible leaching from older pipes or a higher contribution of deep groundwater. Sulfates were also highest in Water U (162 mg/L), further supporting the hypothesis of groundwater influence. Nitrate levels were relatively low, with the highest concentrations detected in Water P (5.41 mg/L) and Water B (5.31 mg/L), reflecting possible surface water contamination. In contrast, Water U contained significantly lower nitrate concentrations (0.71 mg/L). The observed variations are attributed to differences in water sources, treatment processes, and local distribution system conditions [32,33,34]. Tap water from the left bank of the Vistula (Water P) showed higher mineralization, likely due to its origin from river infiltration. In contrast, water from the right bank (Water B and Water U) exhibited distinct compositions, with Water U’s characteristics resembling water from bad pipe conditions. These results highlight the impact of geological factors, treatment methods, and infrastructure on the final composition of tap water used to prepare culture media for yeast.

Table 1.
Water analysis was used to prepare yeast culture media. Water C—control redistilled water, Water P—tap water collected at the left bank of the Vistula River in Warsaw (Praga-Południe District), Water B—tap water collected at the right bank of the Vistula River in Warsaw (Bielany District) and Water U—tap water collected at the right bank of the Vistula River in Warsaw (Ursynów District).
To confirm the safety of the water used in this study, the values obtained were compared with the parameters specified in the Regulation of the Minister of Health of 7 December 2017 on the quality of water intended for human consumption [Dz.U. 2017 poz. 2294]. All the values obtained in the laboratory tests were within the permissible limits specified in this regulation, which confirms that the water used in the study was safe [35].
The results presented in Figure 1 show the biomass yield of yeast grown in a shaken flask culture in a nitrogen-limited medium with a waste carbon source prepared with tap water from different water supply sources (Water P, B, and U) and control redistilled water (C). Statistical analysis did not reveal any significant differences among the various water sources, suggesting that the different water supplies did not significantly impact yeast biomass growth in flask-shaken cultures. It was also confirmed that the Y. lipolytica yeast could be cultured in untreated tap water, which is beneficial from the point of view of commercial large-scale production.
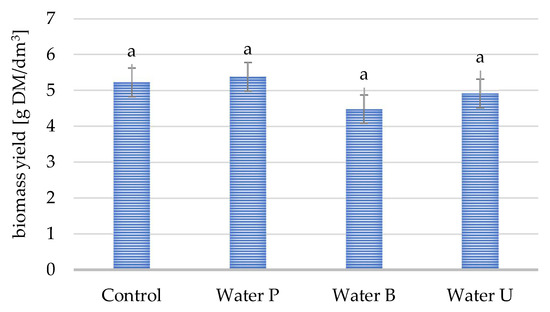
Figure 1.
Biomass yield after 96 h yeast culture in medium with waste carbon source, where medium ingredients were dissolved in water: Water C—control redistilled water; Water P, B and U—tap water collected at different water intakes (p < 0.05).
In a study by Dobrowolski et al. (2019) [36] on the effect of seawater on biomass and microbial oil production, it was observed that in a medium containing seawater, the Y. lipolytica strain produced significantly more biomass than in a freshwater medium (36.4 and 48 g/L, respectively), which could be related to the fact that higher osmotic stress redirected the carbon flux from glycerol to biomass instead of to lipids. The carbon flux was evenly distributed between biomass and lipid synthesis in freshwater conditions. It is worth noting that the type of water used did not significantly affect the fatty acid profile [36]. The above results do not directly show the quality of tap water, but they confirm the adaptability of Y. lipolytica yeast to various conditions.
In the second step, three batch or fed-batch cultures were conducted. This study evaluated the effects of water quality and culture time on yeast biomass yield, lipid biosynthesis, and biomass nutritional value. In addition, how the yeast growth phase affected the yield of microbial oil was investigated. Cultures were conducted for 20 or 65 h, with variants using untreated tap water or distilled water. Key parameters such as stirrer speed and medium oxygenation were monitored throughout the process (Figure 2a–c).
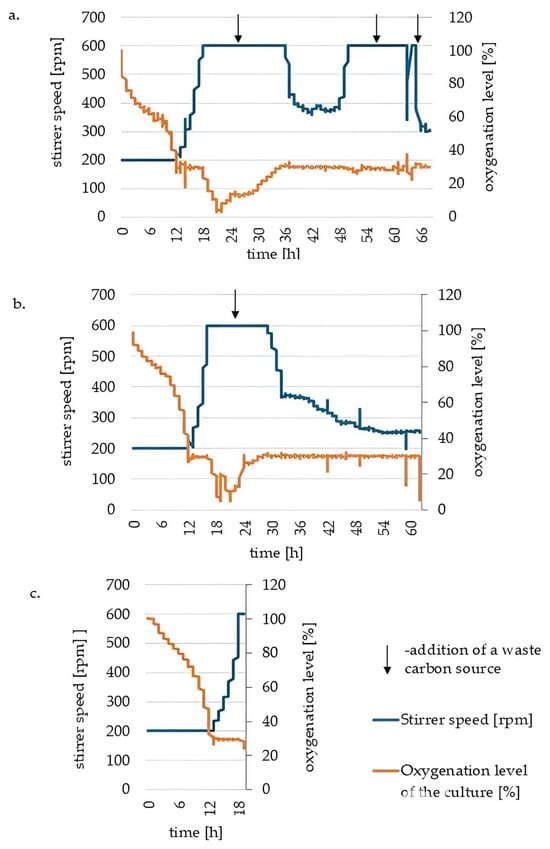
Figure 2.
Changes in stirrer speed and oxygenation level of the culture medium during yeast culture in the bioreactor carried out in the medium prepared based on distilled water (a), in a tap water-based medium (b) and during a 20 h yeast culture in a bioreactor conducted in a tap water-based medium (c).
In a 65 h culture with distilled water (Figure 2a), the cells entered a logarithmic growth phase after about 10 h, causing the oxygenation of the medium to drop to 50% of the initial value. As a result of intensive cell proliferation, there was a significant decrease in the oxygenation of the medium. Supplementation of the carbon source at 16, 50 and 62 h promoted lipid storage accumulation. Most of the waste oil depletion in medium occurred during the logarithmic growth phase, and each carbon source supplementation increased the stirrer speed, reflecting the higher oxygen demand as cell growth intensified. According to the knowledge of the biochemical pathways of microbial oil accumulation, a high carbon source-to-nitrogen ratio promotes lipid synthesis in oleaginous microbial cells [30].
The culture conducted in the tap water-based medium proceeded similarly to the medium prepared with distilled water for 65 h (Figure 2b). Waste oil was added at the 16th hour of culture, during the logarithmic phase of yeast growth. At a similar time to the culture in the distilled water-based medium, the oxygenation level of the medium dropped sharply, signalling the entry of the cells into the logarithmic phase of growth. After 30 h, a reduced oxygen demand was observed by a decrease in stirrer speed. It was not supplemented additionally, as an accumulation of unused substrate, impaired biomass separation, and problems with excessive foaming of the culture were observed in the first culture.
Due to the short duration of the 20 h culture (Figure 2c), a batch mode was used without introducing carbon sources. The degree of oxygenation of the medium decreased rapidly, as in the previous variants. Still, it remained at the appropriate assumed level of 30% until the end of the culture, when the stirrer speed reached the maximum set value of 600 rpm. The cells did not enter the stationary growth phase as in the previous two variants.
The observed oxygenation changes were due to the yeast cells utilizing the oxygen present in the medium. In more extensive cultures, due to the intensive multiplication of yeast cells, it was necessary to add oil during the culture, i.e., an additional portion of the carbon source. For each culture cycle, changes in the biomass content of the culture medium were observed. In a study by Snopek et al. (2021) [37], yeast cultures of Y. lipolytica in bioreactors showed a significant decrease in dissolved oxygen during the early logarithmic phase of growth, leading to an increase in stirrer speed until reaching 600 rpm after 21 h, with oxygenation remaining around 5%. Similarly, the experiments showed a sharp decrease in oxygenation with increased agitator speed, but substrate oxygenation remained at around 30% [37]. Fabiszewska et al. (2022) [38] observed a similar trend, noting a sharp decrease in substrate oxygenation after 12 h, which varied depending on the carbon sources used (glucose and olive oil), highlighting the significant oxygen requirement of yeast for growth. The cited experiments presented a comparable trend to our study, indicating that, irrespective of the carbon source and medium, Y. lipolytica yeast cells require significant amounts of oxygen for cell growth [38]. In a study by Magdouli et al. (2018), it was also shown that the level of oxygenation affects lipid synthesis, cell morphology and physical properties, and the maximum lipid content was recorded at 30–35% saturation (44.8%), while higher aeration seemed to reduce the lipid content and promote biodegradation of the accumulated SCO [39].
As demonstrated in Figure 3, the pH of the medium during the cultivation of Y. lipolytica yeast remained consistent across all experiments. Initially, the pH level remained stable for approximately 14 to 16 h, followed by a rapid decline, reaching its minimum around 22 h in cultures lasting 65 h. After this point, the pH level remained at a similar level for the remainder of the culture. In the experiment conducted in a distilled water-based medium, the pH level was higher at the beginning of the experiment; however, after 20 h, it had decreased to a level that was lower than that of the variant with tap water. The initial pH of the medium for each of the three cultures was set at 5, and the final pH in the 65 h cultures in the experiment with medium prepared with distilled water was within 2, while in the tap water it was within 3. Following the completion of the 20 h culture, the culture was concluded when the pH level reached approximately 4.
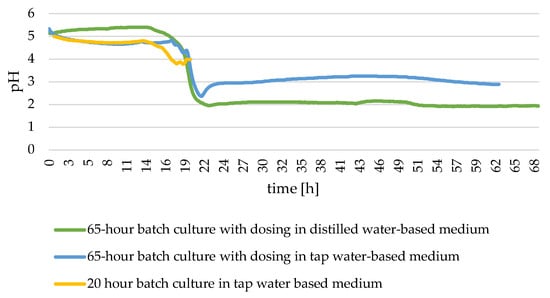
Figure 3.
Changes in substrate pH during batch culture of Y. lipolytica yeast.
In a study by Fabiszewska et al. (2019) [9], in cultures in medium with a limited nitrogen source to stimulate the synthesis of spare lipids, a sharp drop in pH in the logarithmic phase was also characteristic and resulted in a low level around 2.0 [40]. Such results indicate that the drop in pH may be the result of organic acid production by the yeast under nitrogen-limited conditions, which is characteristic of this type of culture [41]. The reason why the pH decreases during culture may be due to the synthesis of by-products, i.e., organic acids, obtained both during the synthesis of spare lipids by oleaginous microorganisms in glucose-containing medium (de novo pathway) and lipids (ex novo pathway), when part of the lipids are metabolised and then, in the form of acetyl-CoA, directed to the Krebs cycle. These compounds (most notably citric acid) were mainly responsible for significantly lowering the pH of the medium in cultures by other authors [40].
In the next step, the yeast biomass yield obtained in cultures in media with distilled and tap water was compared, and the cell lipid content and the concentration of the lipid carbon source in the medium were compared at different stages of the batch yeast culture (Table 2).

Table 2.
Comparison of yeast biomass yield, cell lipid content, and residual oil content of the culture medium as a function of the type of water used in the medium and the time of biomass sampling (p < 0.05).
Due to its ability to achieve high biomass concentrations with the rapid cell growth necessary for efficient SCO production, the yeast species Y. lipolytica is considered the most promising microbial producer of these bioproducts on an industrial scale [14]. Biomass yields in both experiment variants (Table 2) were determined after 43 and 65 h of culture (in late log phase and stationary phase). It can be seen that the biomass yield in both cultures increased with time. The type of water used for substrate preparation had no significant effect on the biomass yield obtained. The biomass yield could have been influenced by adding waste oil to the culture to supplement the carbon source. Hence, a non-significantly higher average biomass yield was obtained in the fed-batch culture in the variant with distilled water when this addition was made three times during the experiment, and the biomass yield was 24.89 g/dm3. In contrast, in the culture with tap water, it was 22.33 g/dm3. A 20 h culture was conducted to test whether the shorter culture time was as productive as cultures of several days (Table 2). In this culture, the biomass yield was non-significantly lower at 16.44 g/dm3. The lipid content in the 20 h culture was higher at 39%, whereas in the 65 h cultures, it was 30% for the distilled water-based medium and 33% for the tap water-based culture. No addition of a carbon source was needed in this case for the cells to proliferate; this was sufficient time as the yeast cells were in the logarithmic growth phase, and the end of the culture was around that time, whereas in the 65 h culture, waste oil was added to the culture.
In the study by Fabiszewska et al. (2021) [40], where waste oil from fish processing plants was analysed as the substrate used in batch culture, the biomass concentration was 20 g/dm3 in the variant with agitator speed control (identical to our work). Meanwhile, in the variant without regulation, the biomass yield was only 15 g/dm3 [40]. Therefore, it is worth noting that using oxygenation control in culture is an effective way to increase the biomass yield. The above results and literature data indicate that the biomass concentration of oleaginous yeast obtained in the experiments of the present study and other authors is similar. Factors that may have influenced some differences in the values were using different carbon sources and interfering with regulating the selected culture parameters in the bioreactor.
In the culture conducted with distilled water-based medium, the concentration of the lipid carbon source remaining in the culture medium was lower at the end of the culture. In contrast, in the second variant, the residual oil content in the culture medium was higher in the last hours of culture. The amount of carbon source dosed in the distilled water-based culture was three times higher than in the second culture (Table 2). The total was 300 g of waste oil supplementation (the lipid substrate concentration in the medium was 4.44 after 43 h and 2.89 g/dm3 after 65 h). In the tap water-based culture, where the carbon source was a single addition, these contents were lower (0.44 after 43 h and 1.44 g/dm3 after 65 h). It should be noted that although the added waste substrate was significantly consumed in both cultures, the amount of substrate added did not significantly affect the biomass yield and intracellular lipid content. In the 20 h culture, the residual oil content of the culture medium was high (36.22%) due to the rapidly interrupted culture and the high C/N ratio, as the carbon source was not consumed in the logarithmic phase.
Lipid accumulation is influenced by the substrate used in the medium. Pereira et al. (2022) [42] studied lipids produced in media with three carbon sources: linoleic and oleic acids, glucose, and volatile fatty acids. In the linoleic and oleic acid medium, the lipid content decreased by half after 72 h. Conversely, the glucose medium showed intensive lipid production, with a fivefold increase in content, reaching 37% after 72 h. No significant changes were noted in the volatile fatty acid medium. The authors concluded that yeast readily assimilates glucose, promoting growth and lipogenesis, particularly when there is a high carbon-to-nitrogen ratio [42].
3.2. Biomass Characteristics of the Oleaginous Yeast Y. lipolytica
Y. lipolytica yeast cells are excellent producers of single-cell protein (SCP). Y. lipolytica biomass contains high protein concentrations ranging from 30.5% to 56.4% of cell dry weight, with an average protein value of 43%. Beneficial protein concentrations are thought to be just above 40%. EFSA (European Food Safety Authority, Parma, Italy) and FAO (Food and Agriculture Organisation of the United Nations, Rome, Italy) recommend an intake of 50 g of protein in the daily human diet for adults [7].
It can be seen that the protein content of the yeast biomass with high lipid content was around 25–27% DM in all three cultures carried out (Table 3). The lipid content of the dry yeast biomass was lowest when cultured in a distilled water-based medium. The lipid content in all media ranged from 40–50%.

Table 3.
The contents of individual components in the dry biomass of Y. lipolytica yeast cultured in batch and fed-batch cultures. The standard deviation is given in brackets (p < 0.05).
Louhasakul et al. (2019) [43] studied lipid production in Y. lipolytica yeast and optimized their accumulation efficiency. The biomass obtained in shaken cultures consisted mainly of lipids, 52%, and the protein content was 20% [43]. In the study by Juszczyk et al. (2019) [44], Y. lipolytica strains were cultured in a medium with pure and crude glycerol and with waste from linseed oil production. Laboratory experiments conducted in flasks resulted in a biomass with a protein content between 19.4 and 48.2% m/m [44]. A study by Fabiszewska et al. (2019) [9] assessed the potential for valorising waste fish oil through its use as a carbon source for Y. lipolytica yeast. They also investigated the lipid content of cells cultured in media with vegetable, fish, and chicken frying oils, which ranged from 40 to 60%, with the highest value for post-frying waste oil, possibly due to the medium’s different nitrogen sources [40].
The following table (Table 4) summarizes the kinetic parameters of Y. lipolytica batch cultures, including storage lipid, protein, and other cell constituents synthesis. The research observed discrepancies in productivity and yields contingent on culture time and initial carbon source concentration. Increased culture time resulted in elevated levels of lipids, protein, and other cell constituents (L). The highest values for the volumetric production rate of all components (qLy) and the average volumetric specific production rate of storage lipids (qL) were recorded in the 20 h culture. They were more than three times higher than in the 65 h culture. No important differences were noticed between the 65 h cultures in media prepared on the basis of distilled and tap water, suggesting that time was a critical factor for high kinetic parameters of the culture.

Table 4.
Kinetic parameters of storage lipid biosynthesis, cell lipid, protein, and other cell constituents content in a batch bioreactor culture of Y. lipolytica, depending on the type of water used in the culture medium and the biomass sampling time (p < 0.05).
In addition to the composition and efficiency of biomass, the economic viability of its production on an industrial scale is also of significant importance. Techno-economic studies estimate that the cost of single-cell protein (SCP) from Y. lipolytica can range from 1.0 to 4.2 USD/kg, depending on scale, substrate, and process configuration [45,46]. Still, volumetric productivity of yeast lipids remains low (<100 g/L), requiring large-scale processing for industrial viability [47]. Although engineered Y. lipolytica strains have achieved up to 1.2 g/L/h in glucose-based media [48], the economic feasibility of heterotrophic processes is still uncertain [45]. Techno-economic analyses estimate microbial oil production costs at $1.7–$5.9/kg, which remain significantly higher than those of plant oils ($0.5–$1.9/kg). The short generation time of yeast cultures, the long history of their use, and the valuable composition of the bioproducts extracted from these microorganisms are motivating searches for methods to reduce costs. The concept of coupled fermentation, integrating the co-production of multiple bioproducts in a single process, improves the economic feasibility of microbial oil or protein production. Engineered strains of Y. lipolytica have been developed to simultaneously synthesize lipids and valuable compounds from waste substrates, supporting circular bioeconomy goals [49,50]. An approach to simultaneously synthesize SCO and SCP was presented in this paper. Strategies to reduce SCO and SCP costs include continuous processing, whole-cell utilization, co-production of bulk chemicals [45,51], and genetic modifications to enhance bioproducts yield and quality [15].
3.3. Characteristics of Microbial Oil Extracted from Y. lipolytica Yeast Cells
It can be seen (Figure 4) that the intracellular lipids of Y. lipolytica yeast are rich in monounsaturated fatty acids—they comprised close to 70% of the total of all fatty acids. Such a result is similar to the composition of fats found in cells, multiplied in a culture shaken in rich YPO medium as a control variant. The medium used in the batch and fed-batch cultures was characterised by a high carbon to nitrogen source ratio, while no nitrogen source limitation was applied in the YPO medium. The saturated fatty acid content of the microbial oils was low (6.61% to 7.44% on average), which was considered a positive characteristic from a nutritional point of view.
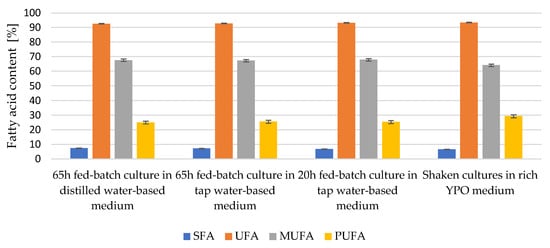
Figure 4.
Fatty acid content of microbial oils of Y. lipolytica yeast cultures in bioreactor cultures in nitrogen source-limited medium and rich YPO medium without nitrogen limitation in flasks (p < 0.05).
The yeast biomass of Y. lipolytica cultured in the study by Jach et al. (2022) [7] in a medium with agricultural waste (i.e., rye and oat straw or bran) or industrial waste (i.e., industrial or crude glycerol) was characterized by low concentrations of total cellular lipids (8.2–20.0%, respectively), with high contents of unsaturated fatty acids (65.0–90.0% of total lipids). Among unsaturated fatty acids, MUFA monounsaturated fatty acids (57.6%), represented by oleic acid (34.0–59.2%), predominated, and polyunsaturated fatty acids (PUFA) (34.0%), represented by linoleic acid (1.9–27.0%), were present in low concentrations. The high-protein yeast biomass contained a small amount of SFA-saturated fatty acids (8.3–34.9% of total lipids) [7]. Those results were in line with our results.
Based on the results (Table 5), it can be concluded that the yeast strain Y. lipolytica preferentially accumulated oleic acid, an unsaturated fatty acid, and linoleic acid, an unsaturated omega-6 fatty acid, which are essential dietary components.

Table 5.
The fatty acid content of the microbial oils of Y. lipolytica yeast multiplied in cultures in a bioreactor in a nitrogen source-limited medium, a rich YPO medium without nitrogen limitation, and in post-frying waste oil used as a carbon source (standard deviation is given in brackets) (p < 0.05).
Mazurczak et al. (2017) [52] analysed the fatty acid composition of triacylglycerols in lipid waste carbon sources such as residual oil after a fish smoking process, waste fat after smoking cured meats, rancid butter, and rendered fat after roasting duck. Analysis of the fatty acid composition showed that duck roast fat had the highest oleic and linoleic acid content, at around 42%. Waste oil from animal products is a rich source of fatty acids and was successfully used as a rich carbon source in the culture of Y. lipolytica yeast [52]. A study by Fabiszewska et al. (2021) [40] compared the fatty acid profile of carbon sources, waste oil from a fish smoking process, and olive oil and analysed the biomass of yeast cultured in YPG-rich medium (with glucose) and microbial oil extracted from yeast cells cultured in limited carbon source media: MF5 (medium with waste fish oil as the carbon source), MO5 (medium with olive oil as the carbon source) and MG7 (medium with glucose as the carbon source). The fatty acid content was studied as the total fatty acid concentration (%). It was noted that the content of fatty acids, mainly oleic, linoleic, and linolenic acid, differed from our study, probably due to the different carbon sources used in the culture. For example, the oleic acid content was the highest, reaching from 66.55% to 62.73% in each culture. In a study by Fabiszewska, specifically in a culture where waste fish oil was used as the carbon source, the content of oleic acid was significantly lower—around 30%, which means that this carbon source is not optimal. In the case of the medium with glucose, this value was the highest, around 56%, but still lower than the value that was obtained in the current study. That paper thus indicated that yeast accumulated components from the medium (free fatty acids), which were later found in the microbial oil [40].
Oxidation of fats changes the chemical properties of molecules, leading to the deterioration of taste, smell, and food spoilage. Radicals, as intermediate products of oxidation, can negatively affect human health. Factors favouring the oxidation of fats include light, oxygen, and high temperature. Key factors affecting the oxidation rate include the composition of fatty acids and the presence of prooxidants and antioxidants. Oils that contain polyunsaturated fatty acids are the most susceptible to oxidation [52]. The PDSC method was used to observe the stability and shelf life of the microbial oil obtained in this study by checking the oxidation induction time, i.e., the initial step of the fat oxidation process and the maximum oxidation time of the microbial oil. A longer oxidation induction time indicated greater oxidative stability of the oil (Figure 5). Microbial oil extracted from cells from a 20 h culture had the lowest oxidative stability values. Oil extracted from yeast cells grown in culture in a distilled water-based medium showed the highest stability and persistence. Similar conclusions could be made when analysing the maximum oxidation time (Figure 6). Microbial oil extracted from yeast cells characterized as cells derived from the stationary phase had significantly higher oxidation stability.
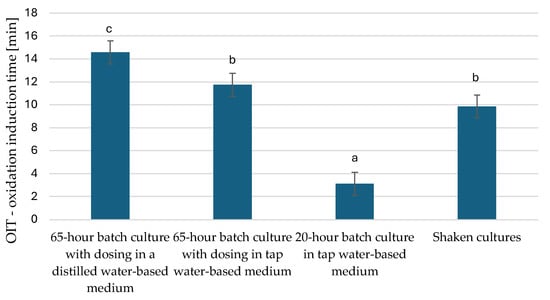
Figure 5.
Oxidation induction time of microbial oils of Y. lipolytica yeast multiplied in bioreactor cultures in nitrogen source-limited medium and in rich YPO medium without nitrogen limitation (p < 0.05). a,b,c—non-homogeneous groups.
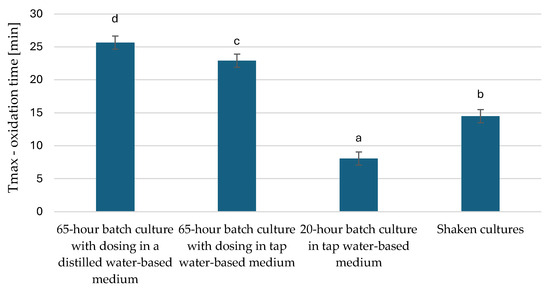
Figure 6.
Maximum oxidation time of microbial oils of Y. lipolytica yeast multiplied in bioreactor cultures in nitrogen source-limited medium and in rich YPO medium without nitrogen limitation (p < 0.05). a,b,c,d—non-homogeneous groups.
In the study by Wierzchowska et al. (2022), where post-frying rapeseed oil was used in culture, the value of the T max parameter was 26.23 min in a 62 h culture [53], which is very similar to our results obtained in a 65 h culture with distilled water. In a study by Bryś et al. (2019) [54], where oil derived from roasted hemp seeds was analysed, the oxidation induction time ranged from 13.6 to 28.9 min. Compared to other oils, hemp oil is highly susceptible to oxidation, which can be linked to the high content of polyunsaturated fatty acids in the oil [54]. The authors of this publication related their results to a study by Ciemniewska-Żytkiewicz et al. (2014) [55], where the oxidative stability of hazelnut oil, olive oil, and rapeseed oil was analysed. The results were characterized by values above 100 min (hazelnut oil and olive oil), showing that these oils are the most stable [55]. In our study, the fat oxidation times obtained are certainly related to the fatty acid composition and the high content of unsaturated fatty acids. The data obtained by these experimental methods can be successfully used to optimise processing and storage conditions to extend the oil’s shelf life [56].
3.4. Evaluation of Microbial Oil and Biomass Safety
The safety of products derived from microorganisms cultured in industrial oil-based media is an important issue, especially in the context of their use in food applications. Indeed, this is a critical point when considering the future potential of using oleaginous yeasts, such as Y. lipolytica, for oil production and inclusion in the human diet, and from a broader food safety perspective. The issue of safety has already been addressed in Wierzchowska et al. (2024), where the same yeast strain was used and a similar carbon source was used in the medium for obtaining the microbial oil [20]. Additionally, in the present study, microbial quality was evaluated.
In the study by Wierzchowska et al. 2024 [20], analysis of the PAH content (Table 6) showed that microbial oil obtained by culturing Y. lipolytica in post-frying rapeseed oil medium (as in our study) met the requirements for oils and fats marketed to end consumers or used as food ingredients in relation to maximum levels of process contaminants, such as benzo(a)pyrene and the sum of benzo(a)pyrene (B[a]P), benzo(a)anthracene (B[a]A), benzo(b)fluoranthene (B[k]F) and chrysene (Chry). As defined in Regulation (EU) 2023/915 (Commission Regulation (EU) 2023/915 of 25 April 2023), the maximum level for B[a]A is 2 μg/kg. The B[a]A content in the microbial oil analysed was 0.61 μg/kg. The guideline indicated in the same regulation for the sum of the four PAHs—B[a]P, Chry, B[b]F and B[a]A, which should not exceed 10 μg/kg oil, was also met. The sum of the four PAHs was 4.12 μg/kg for SCO obtained from post-frying rapeseed oil medium. In the case of hexane residue content, it was 0.0007 mg/kg, and according to European Union regulations, its permissible content in fats and oils should not exceed 1 mg/kg [4,20,57,58].

Table 6.
Assessment of the safety of biomass and microbial oil obtained from Y. lipolytica yeast.
To provide microbiological safety and to eliminate metabolic activity, the collected Y. lipolytica yeast biomass was heat-inactivated. Samples were autoclaved at 121 °C for 15 min following standard sterilisation conditions. In the context of a microbiological assessment of Y. lipolytica yeast biomass, total bacterial count and the counts of yeast were evaluated. The experiment did not maintain 100% sterility of the conditions as would be observed under industrial conditions, and still the bacterial count in the yeast biomass and microbial oil did not exceed 100 CFU/g and it did not contain viable yeast cells. In light of these findings and the available evidence, the resulting microbial product cannot be considered unsafe or unsuitable for food use.
4. Conclusions
This research was built upon a long-standing history of previous studies demonstrating this model yeast species’ safety and nutritional potential as a source of nutrients. This study assessed the suitability of oleaginous yeast of the Y. lipolytica species for the production of microbial oil and determined the specific qualitative characteristics of the biomass and oil, such as the protein content of the biomass, the unsaturated fatty acid content of the yeast lipids, and the oxidative stability of the oil. The effect of the culture water used and the duration of the culture on the yield of the obtained microbial oil was evaluated. Obtaining oleaginous microbial oil is gaining popularity, and more and more research is being undertaken to introduce alternative oil sources with similar properties to vegetable oils, such as rapeseed or palm oil, in food technology. Post-frying waste oil from the food industry was an easily assimilable carbon source for the yeast Y. lipolytica. Untreated tap water used for the preparation of the culture medium did not significantly affect the yeast biomass yield obtained, the microbial oil biosynthesis efficiency or its quality compared to the results obtained for biomass multiplied in a distilled water-based medium, except for a significant effect on the oxidative stability of the oil. The oleaginous yeast biomass of Y. lipolytica and the microbial oil were characterized by high nutritional value. The yeast biomass contained high amounts of lipids (39.43–50.53%), proteins (24.16–27.03%), and unsaturated fatty acids, essential dietary components. The lipids of oleaginous yeast contained predominantly unsaturated fatty acids (92.56–93.39%), mainly oleic acid (62.73–66.44%) and linoleic acid (19.40–21.40%). The time of batch culture of oleaginous yeast of the species Y. lipolytica influenced the yield and quality of the microbial oil (stock lipids) obtained from it. The yeast biomass from the 20 h culture was characterized by the lowest oxidative stability of the oil (more than twice as low as the 65 h culture).
Using untreated tap water to prepare the culture medium containing post-frying oil as a carbon source did not affect the yield or nutritional value of the biomass. The results from both distilled and tap water-based cultures were similar to each other. In terms of culture time, the oxidative stability of the oil was shorter after a shorter culture time, and a shorter culture time was associated with a shorter maximum oxidation time. In addition, the microbiological analysis of the tap water and the microbial oil obtained confirmed their safety for use, demonstrating that culturing Y. lipolytica in waste frying oil and untreated tap water can yield safe and nutritionally valuable biomass. This further highlights the novelty and potential of this approach for future food applications. The issue could be pursued further, as it is an interesting alternative for the future use of oleaginous microorganisms in oil production and its introduction into human nutrition.
Author Contributions
Conceptualization, A.U.F.; investigation, A.U.F., J.K., M.G., A.P., I.P. and D.N.; resources, A.U.F., M.G. and D.N.; data curation, A.U.F., J.K. and I.P.; writing—original draft preparation, A.U.F. and J.K.; writing—review and editing, A.U.F.; visualization, A.U.F. and J.K.; supervision, A.U.F.; project administration, A.U.F.; funding acquisition, A.U.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by sources of the Polish Ministry of Science and Higher Education grant no. SKN/SP/601167/2024 titled “Kapsułkowany olej mikrobiologiczny jako źródło cennych składników bioaktywnych/Encapsulated microbial oil as a source of valuable bioactive components”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety Assessment of an Oleaginous Yeast with a Great Industrial Potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A Model Yeast for Citric Acid Production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, J.F.-W.; Tsang, C.-C.; Wang, H.; Guo, D.; Pan, Y.; Xiao, Y.; Yue, N.; Chen, J.H.-K.; Lau, S.K.-P.; et al. Clinical Characteristics, Laboratory Identification, and in Vitro Antifungal Susceptibility of Yarrowia (Candida) lipolytica Isolates Causing Fungemia: A Multicenter, Prospective Surveillance Study. J. Clin. Microbiol. 2015, 53, 3639–3645. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Yarrowia lipolytica Yeast Biomass as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and Its Multiple Applications in the Biotechnological Industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef]
- Jach, M.E.; Masłyk, M.; Juda, M.; Sajnaga, E.; Malm, A. Vitamin B12-Enriched Yarrowia lipolytica Biomass Obtained from Biofuel Waste. Waste Biomass Valorization 2020, 11, 1711–1716. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Misiukiewicz-Stępień, P.; Paplińska-Goryca, M.; Zieniuk, B.; Białecka-Florjańczyk, E. An Insight into Storage Lipid Synthesis by Yarrowia lipolytica Yeast Relating to Lipid and Sugar Substrates Metabolism. Biomolecules 2019, 9, 685. [Google Scholar] [CrossRef]
- Implementing Regulation—EU—2024/2044—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_impl/2024/2044/oj/eng (accessed on 9 June 2025).
- Finco, A.M.d.O.; Mamani, L.D.G.; Carvalho, J.C.d.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Soccol, C.R. Technological Trends and Market Perspectives for Production of Microbial Oils Rich in Omega-3. Crit. Rev. Biotechnol. 2017, 37, 656–671. [Google Scholar] [CrossRef]
- El Kantar, S.; Khelfa, A.; Vorobiev, E.; Koubaa, M. Strategies for Increasing Lipid Accumulation and Recovery from Y. lipolytica: A Review. OCL 2021, 28, 51. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The History, State of the Art and Future Prospects for Oleaginous Yeast Research. Microb. Cell Fact. 2021, 20, 221. [Google Scholar] [CrossRef]
- Al-Obeidi, W.D.M.; Al-Rawi, D.F.; Ali, L.H. Production of Single-Cell Oil from a Local Isolate Bacillus subtilis Using Palm Fronds. Int. J. Biomater. 2023, 2023, 8882842. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.-E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial Oils as Food Additives: Recent Approaches for Improving Microbial Oil Production and Its Polyunsaturated Fatty Acid Content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous Yeasts for Sustainable Lipid Production-from Biodiesel to Surf Boards, a Wide Range of “Green” Applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, L.A.; Chuck, C.J.; Donnelly, J.; Bannister, C.D.; Scott, R.J. Optimizing the Lipid Profile, to Produce Either a Palm Oil or Biodiesel Substitute, by Manipulation of the Culture Conditions for Rhodotorula glutinis. Biofuels 2014, 5, 33–43. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of Volatile Fatty Acids into Lipids by the Oleaginous Yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and Techno-Economic Evaluation of Microbial Oil Production as a Renewable Resource for Biodiesel and Oleochemical Production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Roszko, M.; Derewiaka, D.; Szulc, K.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Yeast Lipids as a Sustainable Source of Nutrients in Dairy Products Analogs. Food Biosci. 2024, 62, 105321. [Google Scholar] [CrossRef]
- PN-EN ISO 10523:2012; Water Quality—Determination of pH. Polish Committee for Standardisation: Warsaw, Poland, 2012.
- PN-EN 26777:1999; Water Quality—Determination of Nitrites—Molecular Absorption Spectrometry Method. Polish Committee for Standardisation: Warsaw, Poland, 1999.
- PN-82/C-04576.08; Water and waste water—Tests for Nitrogen Compounds -Determination of Nitrate Nitrogen by the Colorimetric Method with Sodium Salicylate. Polish Committee for Standardisation: Warsaw, Poland, 2012.
- PN-ISO 7150-1:2002; Water Quality—Determination of Ammoniacal Nitrogen—Part 1: Manual Spectrometric Method. Polish Committee for Standardisation: Warsaw, Poland, 2002.
- PN-ISO 9297:1994; Water Quality—Determination of Chlorides—Method of Titration with Silver Nitrate in the Presence of Chromate as Indicator (Mohr Method). Polish Committee for Standardisation: Warsaw, Poland, 1994.
- PN-ISO 6059:1999; Water Quality—Determination of Total Calcium and Magnesium Content—Titration Method with EDTA. Polish Committee for Standardisation: Warsaw, Poland, 1999.
- PN-EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Polish Committee for Standardisation: Warsaw, Poland, 2009.
- ISO 15923-1:2013; Water quality—Determination of selected parameters by discrete analysis systems—Part 1: Ammonium, nitrate, nitrite, chloride, orthophosphate, sulfate and silicate with photometric detection. International Organization for Standardization: Geneva, Switzerland, 2013.
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part II: Technology and Potential Applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Taskin, M.; Saghafian, A.; Aydogan, M.N.; Arslan, N.P. Microbial Lipid Production by Cold-adapted Oleaginous Yeast Yarrowia lipolytica B9 in Non-sterile Whey Medium. Biofuels Bioprod. Biorefin. 2015, 9, 595–605. [Google Scholar] [CrossRef]
- Derewiaka, D.; Stepnowska, N.; Bryś, J.; Ziarno, M.; Ciecierska, M.; Kowalska, J. Chia Seed Oil as an Additive to Yogurt. Grasas Aceites 2019, 70, e302. [Google Scholar] [CrossRef]
- WisłaWarszawska.Pl. Available online: http://wislawarszawska.pl (accessed on 19 February 2025).
- Miejskie Przedsiębiorstwo Wodociągów i Kanalizacji. Available online: http://www.mpwik.com.pl (accessed on 19 February 2025).
- Official Portal of the City of Warsaw. Available online: http://um.warszawa.pl (accessed on 19 February 2025).
- Zdrowia, R.M. Warszawa, Dnia 11 Grudnia 2017 r. Poz. 2294. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20170002294/O/D20172294.pdf (accessed on 9 June 2025). (In Polish)
- Dobrowolski, A.; Drzymała, K.; Rzechonek, D.A.; Mituła, P.; Mirończuk, A.M. Lipid Production from Waste Materials in Seawater-Based Medium by the Yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 547. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and Stirring in Yarrowia lipolytica Lipase Biosynthesis during Batch Cultures with Waste Fish Oil as a Carbon Source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Paplińska-Goryca, M.; Misiukiewicz-Stępień, P.; Wołoszynowska, M.; Nowak, D.; Zieniuk, B. Expression Profile of Selected Genes Involved in Storage Lipid Synthesis in a Model Oleaginous Yeast Species Yarrowia lipolytica. Int. J. Mol. Sci. 2022, 23, 1041. [Google Scholar] [CrossRef] [PubMed]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Morphology and Rheological Behaviour of Yarrowia lipolytica: Impact of Dissolved Oxygen Level on Cell Growth and Lipid Composition. Process Biochem. 2018, 65, 1–10. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zieniuk, B.; Kozłowska, M.; Mazurczak-Zieniuk, P.M.; Wołoszynowska, M.; Misiukiewicz-Stępień, P.; Nowak, D. Studies on Upgradation of Waste Fish Oil to Lipid-Rich Yeast Biomass in Yarrowia lipolytica Batch Cultures. Foods 2021, 10, 436. [Google Scholar] [CrossRef]
- Giacomobono, R.; Albergo, R.; Valerio, V.; Caporusso, A.; De Bari, I. Modelling of the Citric Acid Production from Crude Glycerol by Wild-Type Yarrowia lipolytica DSM 8218 Using Response Surface Methodology (RSM). Life 2022, 12, 621. [Google Scholar] [CrossRef]
- Pereira, A.S.; Lopes, M.; Miranda, S.M.; Belo, I. Bio-Oil Production for Biodiesel Industry by Yarrowia lipolytica from Volatile Fatty Acids in Two-Stage Batch Culture. Appl. Microbiol. Biotechnol. 2022, 106, 2869–2881. [Google Scholar] [CrossRef]
- Louhasakul, Y.; Cheirsilp, B.; Maneerat, S.; Prasertsan, P. Potential Use of Flocculating Oleaginous Yeasts for Bioconversion of Industrial Wastes into Biodiesel Feedstocks. Renew. Energy 2019, 136, 1311–1319. [Google Scholar] [CrossRef]
- Juszczyk, P.; Rymowicz, W.; Kita, A.; Rywińska, A. Biomass Production by Yarrowia lipolytica Yeast Using Waste Derived from the Production of Ethyl Esters of Polyunsaturated Fatty Acids of Flaxseed Oil. Ind. Crops Prod. 2019, 138, 111590. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Parsons, S.; McManus, M.C.; Chuck, C.J. Using Techno-Economic Modelling to Determine the Minimum Cost Possible for a Microbial Palm Oil Substitute. Biotechnol. Biofuels 2021, 14, 57. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Zhang, B.; Chen, H.; He, Y.; Bai, Z.; He, Q. Techno-economic evaluation of large-scale SCP production by Pichia pastoris using methanol as sole carbon source. Bioresour. Technol. 2023, 368, 128331. [Google Scholar] [CrossRef]
- Khot, M.; Raut, G.; Ghosh, D.; Alarcón-Vivero, M.; Contreras, D.; Ravikumar, A. Lipid Recovery from Oleaginous Yeasts: Perspectives and Challenges for Industrial Applications. Fuel 2020, 259, 116292. [Google Scholar] [CrossRef]
- Qiao, K.; Wasylenko, T.M.; Zhou, K.; Xu, P.; Stephanopoulos, G. Lipid Production in Yarrowia lipolytica Is Maximized by Engineering Cytosolic Redox Metabolism. Nat. Biotechnol. 2017, 35, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Qiao, K.; Ahn, W.S.; Stephanopoulos, G. Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc. Natl. Acad. Sci. USA 2016, 113, 10848–10853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Nielsen, J. Production of advanced biofuels in yeast. Biotechnol. J. 2021, 16, 2000304. [Google Scholar]
- Uğur, Ş.; Zieniuk, B.; Fabiszewska, A. Nutritional and Medicinal Properties of Microbial Oil. Appl. Sci. 2024, 14, 4232. [Google Scholar] [CrossRef]
- Mazurczak, P.; Białecka-Florjańczyk, E.; Fabiszewska, A.; Nowak, D.; Wołoszynowska, M.; Zieniuk, B. Utylizacja odpadów pochodzących z zakładów przemysłu spożywczego i paliwowego z wykorzystaniem lipolitycznych drożdży Yarrowia lipolytica. Zesz. Probl. Postępów Nauk. Rol. 2017, nr 588, 15–24. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Pakulska, A.; Derewiaka, D.; Piasecka, I.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Concept of Batch and Fed-Batch Cultures of Yarrowia lipolytica as a Valuable Source of Sterols with Simultaneous Valorization of Molasses and Post-Frying Rapeseed Oil. Appl. Sci. 2022, 12, 12877. [Google Scholar] [CrossRef]
- Bryś, A.; Bryś, J.; Mellado, Á.F.; Głowacki, S.; Tulej, W.; Ostrowska-Ligęza, E.; Koczoń, P. Characterization of Oil from Roasted Hemp Seeds Using the PDSC and FTIR Techniques. J. Therm. Anal. Calorim. 2019, 138, 2781–2786. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the Oxidative Stability of Hazelnut Oils by PDSC and Rancimat Methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- Islam, M.; Kaczmarek, A.; Tomaszewska-Gras, J. Differential Scanning Calorimetry as a Tool to Assess the Oxidation State of Cold-Pressed Oils during Shelf-Life. J. Food Meas. Charact. 2023, 17, 6639–6651. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0915 (accessed on 19 May 2025).
- Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ%3AL%3A2009%3A141%3A0003%3A0011%3AEN%3APDF (accessed on 19 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).