Male Layer-Type Birds (Lohmann Brown Classic Hybrid) as a Meat Source for Chicken Pâtés
Abstract
1. Introduction
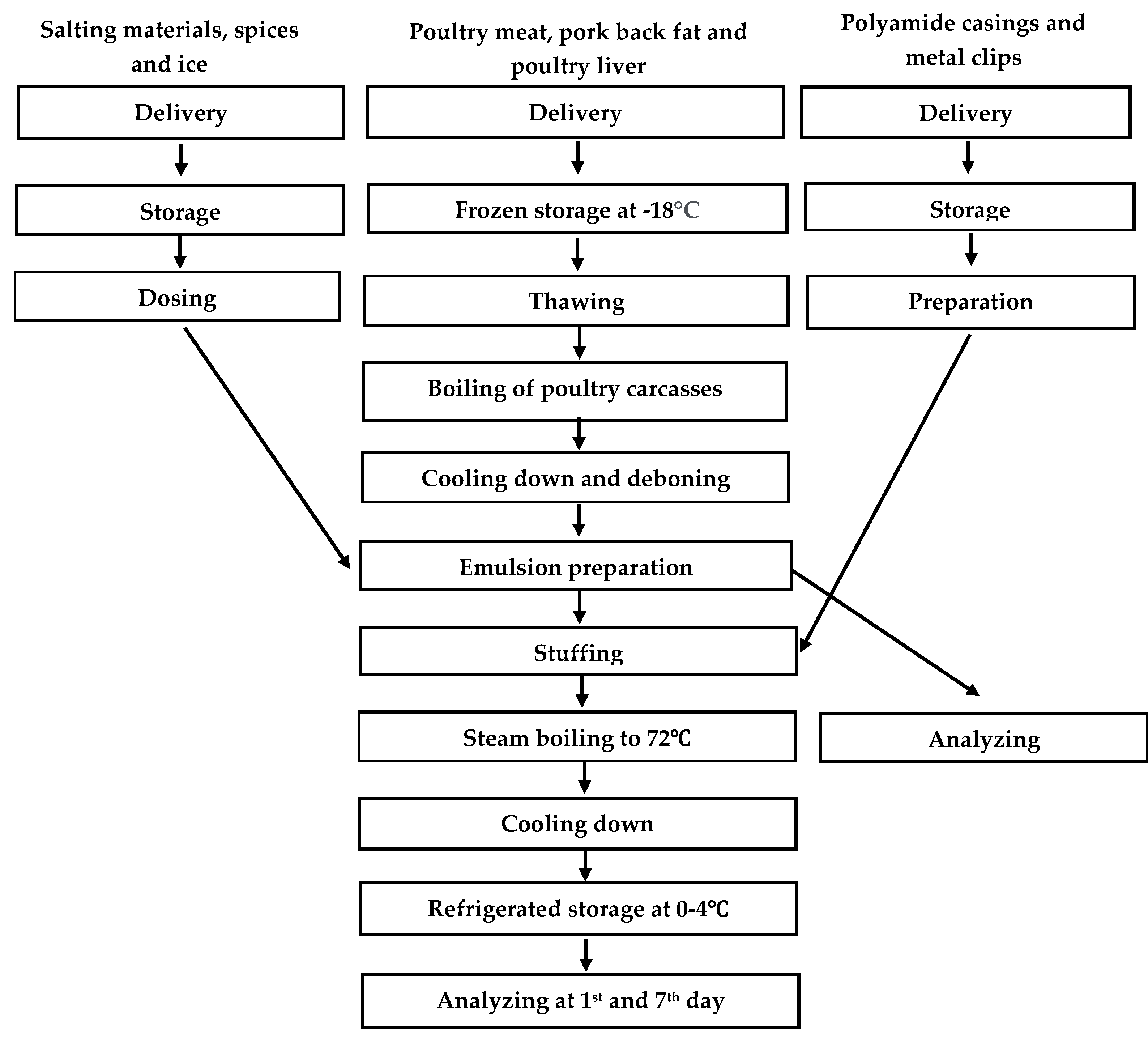
2. Materials and Methods
2.1. Raw Materials
2.2. Recipe and Formulation
2.3. Sampling
2.4. Emulsion Stability
2.5. Proximate Composition Analyses of Pâtés
2.6. pH and Intrumental Colour Determination of Pâtés
2.7. Texture Profile Analysis (TPA) of Pâtés
2.8. Hydrolytic and Oxidative Changes in Lipid Fraction of Pâtés
2.9. Data Analyses
3. Results
3.1. Emulsion Stability
3.2. Proximate Composition of Pâtés
3.3. pH and Instrumental Colour Characteristics of Pâtés
3.4. Texture Profile Analysis (TPA) of Pâtés
3.5. Oxidatative Changes in Pâtés During Storage
3.6. Fatty Acid Profile of Pâtés
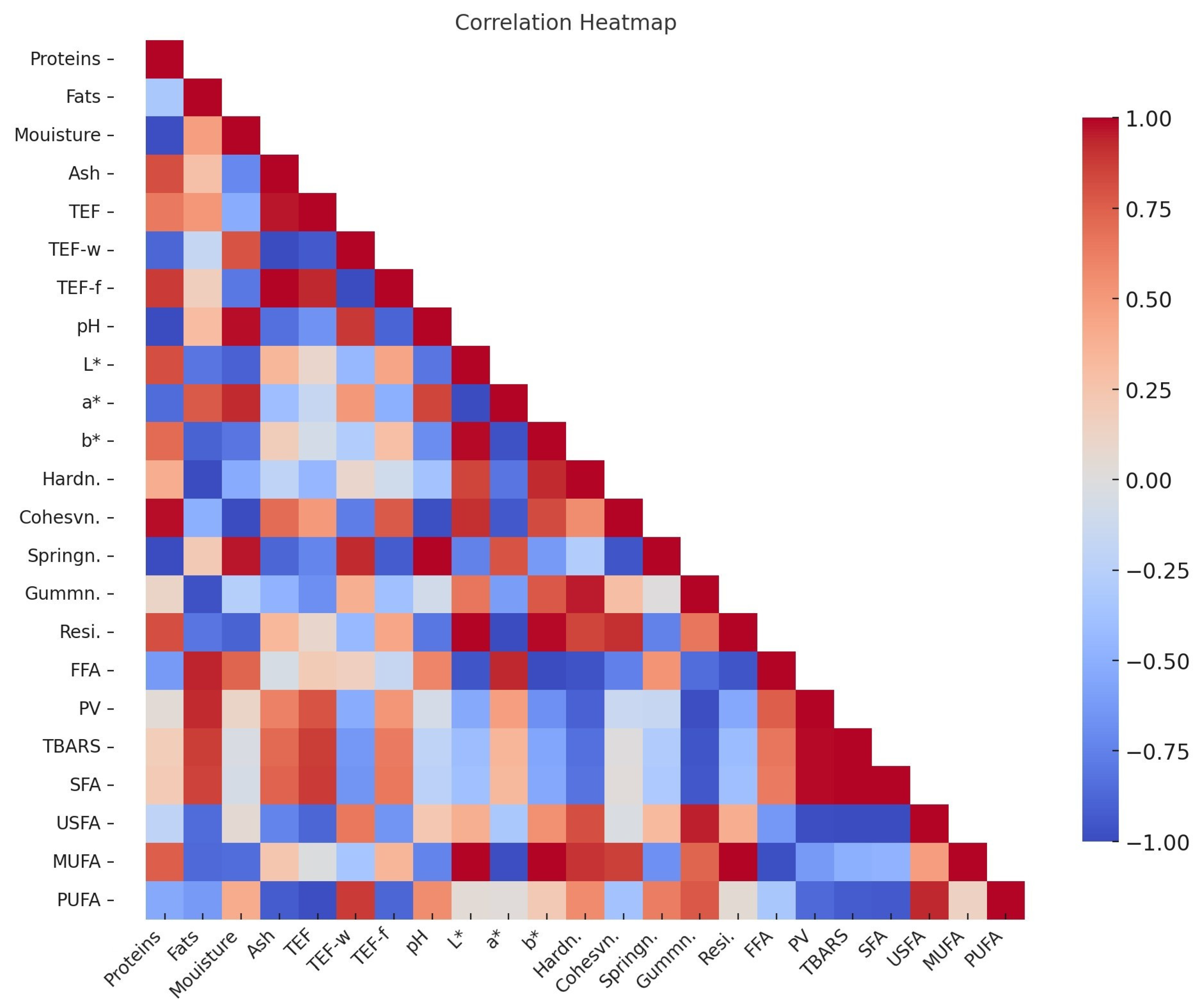
3.7. Correlation Analysis
4. Discussion
4.1. Emulsion Stability
4.2. Proximate Composition
4.3. pH and Instrumental Colour Characteristics
4.4. Texture Profile Analysis (TPA)
4.5. Oxidatative Changes Pâtés During Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TEF | Total expressible fluid |
| FFA | Free fatty acids |
| PV | Primary lipid oxidation products |
| SFA | Saturated fatty acid |
| USFA | Unsaturated fatty acid |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
References
- Govoni, C.; Chiarelli, D.D.; Luciano, A.; Ottoboni, M.; Perpelek, S.N.; Pinotti, L.; Rulli, M.C. Global assessment of natural resources for chicken production. Adv. Water Resour. 2021, 154, 103987. [Google Scholar] [CrossRef]
- Yue, K.; Cao, Q.Q.; Shaukat, A.; Zhang, C.; Huang, S.C. Insights into the evaluation, influential factors, and improvement strategies for poultry meat quality: A review. npj Sci. Food 2024, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Qi, L.; Fu, R.; Nie, Q.; Zhang, X.; Luo, W. A large-scale comparison of the meat quality characteristics of different chicken breeds in South China. Poult. Sci. 2024, 103, 103740. [Google Scholar] [CrossRef] [PubMed]
- Katamela, S.; Molee, A.; Thumanu, K.; Yongsawatdigul, J. Meat quality and Raman spectroscopic characterization of Korat hybrid chicken obtained from various rearing periods. Poult. Sci. 2021, 100, 1248–1261. [Google Scholar] [CrossRef]
- Eusemann, B.K.; Patt, A.; Schrader, L.; Weigend, S.; Thöne-Reineke, C.; Petow, S. The role of egg production in the etiology of keel bone damage in laying hens. Front. Vet. Sci. 2020, 7, 81. [Google Scholar] [CrossRef]
- Mishra, R.; Mishra, B.; Kim, Y.S.; Jha, R. Practices and issues of moulting programs for laying hens: A review. Br. Poult. Sci. 2022, 63, 720–729. [Google Scholar] [CrossRef]
- Krautwald-Junghanns, M.E.; Cramer, K.; Fischer, B.; Förster, A.; Galli, R.; Kremer, F.; Bartels, T. Current approaches to avoid the culling of day-old male chicks in the layer industry, with special reference to spectroscopic methods. Poult. Sci. 2018, 97, 749–757. [Google Scholar] [CrossRef]
- de Haas, E.N.; Oliemans, E.; van Gerwen, M.A. The need for an alternative to culling day-old male layer chicks: A survey on awareness, alternatives, and the willingness to pay for alternatives in a selected population of Dutch citizens. Front. Vet. Sci. 2021, 8, 662197. [Google Scholar] [CrossRef]
- Coppola, F.; Paci, G.; Profeti, M.; Mancini, S. Stop culling male layer-type chicks: An overview of the alternatives and public perspective. World’s Poult. Sci. J. 2024, 80, 611–631. [Google Scholar] [CrossRef]
- Popova, T.; Petkov, E.; Ignatova, M.; Vlahova-Vangelova, D.; Balev, D.; Dragoev, S.; Kolev, N. Male layer-type chickens—An alternative source for high-quality poultry meat: A review on the carcass composition, sensory characteristics, and nutritional profile. Braz. J. Poult. Sci. 2022, 24, eRBCA-2021. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj/eng (accessed on 7 May 2025).
- Petkov, E.; Dimov, K.; Popova, T.; Ignatova, M. Attitude of consumers towards the possibility of avoiding the culling of male layer-type chickens: A survey on the acceptability of the derived meat products. Bulg. J. Agric. Sci. 2024, 30, 101–106. [Google Scholar]
- Popova, T.; Petkov, E.; Ignatova, M.; Vlahova-Vangelova, D.; Balev, D.; Dragoev, S.; Dimov, K. Meat quality of male layer-type chickens slaughtered at different ages. Agriculture 2023, 13, 624. [Google Scholar] [CrossRef]
- Popova, T.; Petkov, E.; Ignatova, M.; Dragoev, S.; Vlahova-Vangelova, D.; Balev, D.; Kolev, N. Growth performance, carcass composition, and tenderness of meat in male layer-type chickens slaughtered at different ages. Proc. Bulg. Acad. Sci. 2023, 76, 156–164. [Google Scholar]
- Dragoev, S.; Balev, D.; Vlahova-Vangelova, D.; Kolev, N.; Popova, T.; Ignatova, M.; Petkov, E. A comparative analysis of cooked smoked cockerel products derived from male layer-type chickens (Lohmann Brown Classic hybrid) and dual-purpose cocks (based on Bresse Gauloise). BIO Web Conf. 2024, 102, 01001. [Google Scholar] [CrossRef]
- Ibrahim, D.; Goshu, G.; Esatu, W.; Bino, G.; Abebe, T. Comparative study of production and reproductive performance of various strains of chicken parent layers raised in floor pens. Ethiop. J. Agric. Sci. 2018, 28, 79–93. [Google Scholar]
- Nguyen Van, D.; Moula, N.; Moyse, E.; Do Duc, L.; Vu Dinh, T.; Farnir, F. Productive performance and egg and meat quality of two indigenous poultry breeds in Vietnam, Ho and Dong Tao, fed on commercial feed. Animals 2020, 10, 408. [Google Scholar] [CrossRef]
- Gremmen, B.; Bruijnis, M.R.N.; Blok, V.; Stassen, E.N. A public survey on handling male chicks in the Dutch egg sector. J. Agric. Environ. Ethics 2018, 31, 93–107. [Google Scholar] [CrossRef]
- Kaufmann, F.; Andersson, R. Eignung männlicher legehybriden zur mast. 2012. Available online: https://www.researchgate.net/publication/280493097_Eignung_mannlicher_Legehybriden_zur_Mast_Suitability_of_egg-type_cockerels_for_fattening_purposes (accessed on 7 May 2025).
- Popova, T.; Petkov, E.; Dimov, K.; Vlahova-Vangelova, D.; Kolev, N.; Balev, D.; Ignatova, M. Performance, carcass composition, and meat quality during frozen storage in male layer-type chickens. Agriculture 2024, 14, 185. [Google Scholar] [CrossRef]
- Hamzeh, A.; Azizieh, A.; Yazagy, S. The effect of the fat percentage and liver type in the stability and pH value of locally prepared liver pate. Int. Food Res. J. 2016, 23, 1131–1135. [Google Scholar]
- Santos, M.M.; Lima, D.A.; Madruga, M.S.; Silva, F.A. Lipid and protein oxidation of emulsified chicken patties prepared using abdominal fat and skin. Poult. Sci. 2020, 99, 1777–1787. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I. Handbook of Poultry Science and Technology, Volume 2: Secondary Processing; John Wiley & Sons, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Kabdylzhar, B.K.; Kakimov, A.K.; Gurinovich, G.V.; Suychinov, A.K. Research of compositions of amino acids, fatty acids, and minerals in meat pate with addition of meat-and-bone paste. Teor. I Prakt. Pererab. Myasa 2022, 7, 66–72. [Google Scholar] [CrossRef]
- Tonchev, M.; Atanasov, T.; Todorova, A.; Atanasova, T.; Shtrankova, N.; Momchilova, M.; Zsivanovits, G. Sensory and instrumental texture analysis of Bulgarian commercial pates. Agric. Sci. Technol. 2017, 9, 251–256. [Google Scholar] [CrossRef]
- Kambarova, A.; Nurgazezova, A.; Nurymkhan, G.; Atambayeva, Z.; Smolnikova, F.; Rebezov, M.; Moldabaeva, Z. Improvement of quality characteristics of turkey pâté through optimization of a protein-rich ingredient: Physicochemical analysis and sensory evaluation. Food Sci. Technol. 2020, 41, 203–209. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Tiensa, B.E.; Barbut, S.; Marangoni, A.G. Influence of fat structure on the mechanical properties of commercial pate products. Food Res. Int. 2017, 100, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Esbensen, K.H.; Wagner, C. Theory of sampling (TOS) versus measurement uncertainty (MU)–A call for integration. TrAC Trends Anal. Chem. 2014, 57, 93–106. [Google Scholar] [CrossRef]
- Silva-Vazquez, R.; Flores-Giron, E.; Quintero-Ramos, A.; Hume, M.E.; Mendez-Zamora, G. Effect of inulin and pectin on physicochemical characteristics and emulsion stability of meat batters. CyTA J. Food 2018, 16, 306–310. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, AOAC 984.13–1994 Protein (Crude) in Animal Feed and Pet Food; AOAC: Rockville, MD, USA, 1996. [Google Scholar]
- ISO 1444:1996; Meat and Meat Products—Determination of Free Fat Content. ISO: Geneva, Switzerland, 1996.
- ISO 1442:2023; Meat and Meat Products—Determination of Moisture Content (Reference Method). ISO: Geneva, Switzerland, 2023.
- ISO 936:1998; Meat and Meat Products—Determination of Total Ash. ISO: Geneva, Switzerland, 1998.
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Weber, M. American Meat Science Association Guidelines for Meat Color Measurement. Meat Muscle Biol. 2023, 6, 12473, 1–81. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Protein oxidation in frankfurters with increasing levels of added rosemary essential oil: Effect on color and texture deterioration. J. Food Sci. 2005, 70, c427–c432. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- Prasad, N.B.S.; Sivamani, S. Isolation of oleic acid from virgin and extra virgin olive oil and study their physicochemical properties. Int. J. Res. Eng. Sci. 2021, 9, 29–34. [Google Scholar]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 7, 421–424. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. A rapid, sensitive, iron-based spectrophotometric method for determination of peroxide values of food lipids. J. Agric. Food Chem. 1994, 42, 709–711. [Google Scholar]
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters. Part 4: Determination by Capillary Gas Chromatography. ISO: Geneva, Switzerland, 2015.
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.A.; Castro, A.; Rolo, C.; Torres, A.; Dorta-Guerra, R.; Acosta, N.G.; Rodríguez, C. Fatty acid profiles and omega-3 long-chain polyunsaturated fatty acids (LC-PUFA) biosynthesis capacity of three dual purpose chicken breeds. J. Food Compos. Anal. 2021, 102, 104005. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I.; Hui, Y.H. Processed poultry products: A primer. In Handbook of Poultry Science and Technology, Volume 2: Secondary Processing; John Wiley & Sons: New York, NY, USA, 2010; pp. 1–11. [Google Scholar]
- Hui, Y.H. (Ed.) Handbook of Meat and Meat Processing; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Phongpa-Ngan, P.; Grider, A.; Mulligan, J.H.; Aggrey, S.E.; Wicker, L. Proteomic analysis and differential expression in protein extracted from chicken with a varying growth rate and water-holding capacity. J. Agric. Food Chem. 2011, 59, 13181–13187. [Google Scholar] [CrossRef]
- Doherty, M.K.; McLean, L.; Hayter, J.R.; Pratt, J.M.; Robertson, D.H.; El-Shafei, A.; Beynon, R.J. The proteome of chicken skeletal muscle: Changes in soluble protein expression during growth in a layer strain. Proteomics 2004, 4, 2082–2093. [Google Scholar] [CrossRef]
- Lee, A.; Suh, Y.; Wick, M.P.; Lee, K. Temporal myosin heavy chain isoform expression transitions faster in broiler chickens compared with Single Comb White Leghorns. Poult. Sci. 2012, 91, 2872–2876. [Google Scholar] [CrossRef]
- Trindade, P.C.O.; Santos, B.A.D.; Hollweg, G.; Correa, L.P.; Pinton, M.B.; Padilha, M.; Payeras, R.H.Z.; Rosa, S.C.; Campagnol, A.J.; Cichoski, P.C.B. Pea protein isolate as a meat substitute in canned pork pâté: Nutritional, technological, oxidative, and sensory properties. Foods 2023, 12, 3486. [Google Scholar] [CrossRef]
- Wideman, N.; O’bryan, C.A.; Crandall, P.G. Factors affecting poultry meat color and consumer preferences—A review. World’s Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Qin, Z.; Ye, B.; Wu, Y.; Liu, L. Effect of pH on lipid oxidation mediated by hemoglobin in washed chicken muscle. Food Chem. 2022, 372, 131253. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Ventanas, S.; Cava, R. Effect of natural and synthetic antioxidants on protein oxidation and color and texture changes in refrigerated stored porcine liver pâté. Meat Sci. 2006, 74, 396–403. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A. Quality characteristics of ostrich liver pâté. J. Food Sci. 2004, 69, snq85–snq91. [Google Scholar] [CrossRef]
- Latoch, A.; Glibowski, P.; Libera, J. The effect of replacing pork fat with inulin on the physicochemical and sensory quality of guinea fowl pate. Acta Sci. Pol. Technol. Aliment. 2016, 15, 311–320. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.M.; Baek, I.K.; Auh, J.H. Carnosine and anserine in chicken: Distribution, age-dependency and their anti-glycation activity. Food Sci. Anim. Res. 2012, 32, 45–48. [Google Scholar] [CrossRef]
| Parameters | CP | 5wP | 9wP | RMSE * | p-Value |
|---|---|---|---|---|---|
| TEF, % | 14.35 b | 13.16 c | 15.58 a | 0.365139 | 0.0007 |
| TEFWater, % | 83.48 b | 85.56 a | 83.02 c | 0.175214 | <0.0001 |
| TEFFat, % | 16.52 b | 14.44 c | 16.98 a | 0.175214 | <0.0001 |
| Parameters | CP | 5wP | 9wP | RMSE * | p-Value |
|---|---|---|---|---|---|
| Moisture, % | 57.74 a | 58.89 a | 58.28 a | 2.913406 | 0.8916 |
| Ash, % | 0.76 a | 0.51 a | 0.86 a | 0.126183 | 0.0549 |
| Proteins, % | 16.70 a | 16.40 a | 16.60 a | 0.828358 | 0.9028 |
| Fats, % | 20.10 a | 20.50 a | 20.90 a | 1.023502 | 0.6507 |
| Carbohydrates, % | 4.70 a | 3.70 a | 3.26 a | 0.612545 | 0.0772 |
| Parameters | CP | 5wP | 9wP | RMSE * | p-Value | ||
|---|---|---|---|---|---|---|---|
| M | S | M × S | |||||
| pH, 1st day | 6.52 e | 6.61 c | 6.57 d | 0.009428 | <0.0001 | <0.0001 | 0.0008 |
| pH, 7th day | 6.65 b | 6.72 a | 6.65 b | ||||
| L*, 1st day | 69.32 ax | 65.16 bx | 65.71 bx | 0.406537 | <0.0001 | 0.8782 | 0.9387 |
| L*, 7th day | 69.34 ax | 65.18 bx | 65.58 bx | ||||
| a*, 1st day | 10.15 cx | 11.60 ax | 11.34 bx | 0.161727 | <0.0001 | 0.0184 | 0.9443 |
| a*, 7th day | 9.91 by | 11.42 ax | 11.13 ax | ||||
| b*, 1st day | 11.43 ab | 10.97 b | 11.25 ab | 0.218632 | 0.0038 | 0.1544 | 0.0425 |
| b*, 7th day | 11.79 a | 11.35 ab | 10.99 b | ||||
| ΔE | 0.43 | 0.42 | 0.36 | ||||
| Parameters | CP | 5wP | 9wP | RMSE * | p-Values | ||
|---|---|---|---|---|---|---|---|
| M | S | M × S | |||||
| Hardness, 1st day (N) | 5.38 ay | 4.62 by | 3.99 cy | 0.310416 | <0.0001 | <0.0001 | 0.4491 |
| Hardness, 7th day (N) | 6.30 ax | 5.30 bx | 4.56 cx | ||||
| Cohesiveness, 1st day | 0.47 a | 0.38 b | 0.44 a | 0.023452 | <0.0001 | 0.2866 | 0.0279 |
| Cohesiveness, 7th day | 0.46 a | 0.42 ab | 0.43 a | ||||
| Springiness, 1st day | 0.50 b | 0.57 b | 0.60 b | 0.234325 | 0.0001 | <0.0001 | 0.0002 |
| Springiness, 7th day | 0.67 b | 1.63 a | 0.80 b | ||||
| Gumminess, 1st day (N) | 2.40 ay | 1.84 aby | 1.78 by | 0.604078 | 0.0357 | 0.0165 | 0.2485 |
| Gumminess, 7th day (N) | 2.81 ax | 2.93 ax | 1.98 bx | ||||
| Resilience, 1st day | 0.09 ax | 0.05 bx | 0.06 bx | 0.004655 | <0.0001 | 0.0006 | 0.1066 |
| Resilience, 7th day | 0.08 ay | 0.05 bx | 0.05 by | ||||
| Parameters | CP | 5wP | 9wP | RMSE * | p-Values | ||
|---|---|---|---|---|---|---|---|
| M | S | M × S | |||||
| FFA, 1st day (% Oleic acid) | 0.16 a | 0.17 a | 0.17 a | 0.007716 | 0.0013 | 0.1731 | 0.0106 |
| FFA, 7th day (% Oleic acid) | 0.14 b | 0.17 a | 0.18 a | ||||
| PV, 1st day (meq O2/kg fat) | 3.23 ax | 3.44 ax | 3.42 ax | 0.170569 | 0.8133 | <0.0001 | 0.2268 |
| PV, 7th day (meq O2/kg fat) | 1.80 ay | 1.72 ay | 1.66 ay | ||||
| TBARS, 1st day (mg MDA/kg) | 2.05 cd | 1.75 d | 3.21 b | 0.174032 | <0.0001 | <0.0001 | <0.0001 |
| TBARS, 7th day (mg MDA/kg) | 1.34 e | 2.44 c | 5.08 a | ||||
| Fatty Acid, % | CP 1st day | 5wP 1st day | 9wP 1st day | CP 7th day | 5wP 7th day | 9wP 7th day | RMSE * | p-Values | ||
|---|---|---|---|---|---|---|---|---|---|---|
| M | S | M × S | ||||||||
| C 6:0 | 0.1 c | 0.1 c | 0.1 c | 0.4 a | 0.3 b | 0.3 b | 0.216667 | <0.0001 | <0.0001 | <0.0001 |
| C 8:0 | N.F. | 0.1 b | N.F. | 0.2 a | 0.1 b | 0.1 b | 0.002415 | <0.0001 | <0.0001 | <0.0001 |
| C 10:0 | N.F. | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.003000 | <0.0001 | <0.0001 | <0.0001 |
| C 12:0 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.003606 | 1.0000 | 1.0000 | 1.0000 |
| C 14:0 | 1.2 bc | 1.3 b | 1.3 b | 1.1 c | 1.8 a | 1.3 b | 0.048484 | <0.0001 | <0.0001 | <0.0001 |
| C 15:0 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.003786 | 1.0000 | 1.0000 | 1.0000 |
| C 15:1 | N.F. | N.F. | N.F. | 0.1 a | 0.1 a | 0.1 a | 0.002345 | 1.0000 | <0.0001 | 1.0000 |
| C 16:0 | 21.7 bx | 23.0 ax | 23.5 ax | 21.6 bx | 23.1 ax | 23.0 ax | 0.696649 | 0.0035 | 0.6210 | 0.7545 |
| C 16:1 | 2.2 bx | 2.5 ax | 2.5 ax | 2.2 bx | 2.4 ay | 2.4 ay | 0.042695 | <0.0001 | 0.0062 | 0.1045 |
| C 17:0 | 0.3 b | 0.4 a | 0.3 b | 0.4 a | 0.4 a | 0.4 a | 0.009678 | <0.0001 | <0.0001 | <0.0001 |
| C 17:1 | 0.3 b | 0.4 a | 0.3 b | 0.4 a | 0.4 a | 0.4 a | 0.011540 | <0.0001 | <0.0001 | <0.0001 |
| C 18:0 | 10.2 bc | 10.5 ab | 9.7 c | 10.2 bc | 11.1 a | 10.9 ab | 0.280923 | 0.0066 | 0.0007 | 0.0104 |
| C 18:1 | 46.7 a | 47.2 a | 46.1 a | 46.3 a | 46.3 a | 45.9 a | 1.336086 | 0.6243 | 0.4427 | 0.8974 |
| C 18:2 Trans | 0.1 a | N.F. | 0.1 a | N.F. | N.F. | N.F. | 0.002380 | <0.0001 | <0.0001 | <0.0001 |
| C 18:2 | 13.8 ax | 10.8 cx | 12.3 bx | 13.7 ay | 10.3 cy | 11.6 by | 0.388433 | <0.0001 | 0.0356 | 0.4220 |
| C 18:3 n-6 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.002309 | 1.0000 | 1.0000 | 1.0000 |
| C 18:3 n-3 | 0.5 c | 0.5 c | 0.7 a | 0.5 c | 0.6 b | 0.7 a | 0.015449 | <0.0001 | 0.0006 | 0.0001 |
| C 20:0 | 0.2 ax | 0.2 ax | 0.1 bx | 0.2 ax | 0.2 ax | 0.1 bx | 0.005686 | <0.0001 | 1.0000 | 1.0000 |
| C 20:1 | 1.2 b | 1.3 a | 1.3 a | 1.1 c | 1.2 b | 1.1 c | 0.033643 | 0.0009 | <0.0001 | 0.0365 |
| C 20:2 | 0.5 a | 0.5 a | 0.5 a | 0.5 a | 0.5 a | 0.5 a | 0.016753 | 1.0000 | 1.0000 | 1.0000 |
| C 20:3 n-6 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.003000 | 1.0000 | 1.0000 | 1.0000 |
| C 20:4 | 0.3 b | 0.4 a | 0.4 a | 0.3 b | 0.3 b | 0.3 b | 0.011518 | <0.0001 | <0.0001 | <0.0001 |
| C 20:3 n-3 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.003028 | 1.0000 | 1.0000 | 1.0000 |
| C 20:5 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | N.F. | 0.002739 | <0.0001 | <0.0001 | <0.0001 |
| C 24:0 | N.F. | N.F. | N.F. | N.F. | 0.1 b | 0.2 a | 0.004163 | <0.0001 | <0.0001 | <0.0001 |
| C 24:1 | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.1 a | 0.003440 | 1.0000 | 1.0000 | 1.0000 |
| SFA | 33.9 by | 35.9 ay | 35.3 ay | 34.4 bx | 37.4 ax | 36.6 ax | 0.562670 | <0.0001 | 0.0014 | 0.3016 |
| USFA | 66.1 ax | 64.1 bx | 64.7 abx | 65.6 ax | 62.6 bx | 63.4 abx | 1.358902 | 0.0212 | 0.1116 | 0.7999 |
| MUFA | 50.5 a | 51.5 a | 50.3 a | 50.2 a | 50.5 a | 50.0 a | 1.068031 | 0.3836 | 0.3103 | 0.8098 |
| PUFA | 15.6 a | 12.6 d | 14.4 b | 15.4 a | 12.1 d | 13.4 c | 0.230146 | <0.0001 | 0.0002 | 0.0324 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolev, N.; Balev, D.; Dragoev, S.; Popova, T.; Petkov, E.; Dimov, K.; Suman, S.; Salim, A.P.; Vlahova-Vangelova, D. Male Layer-Type Birds (Lohmann Brown Classic Hybrid) as a Meat Source for Chicken Pâtés. Appl. Sci. 2025, 15, 6702. https://doi.org/10.3390/app15126702
Kolev N, Balev D, Dragoev S, Popova T, Petkov E, Dimov K, Suman S, Salim AP, Vlahova-Vangelova D. Male Layer-Type Birds (Lohmann Brown Classic Hybrid) as a Meat Source for Chicken Pâtés. Applied Sciences. 2025; 15(12):6702. https://doi.org/10.3390/app15126702
Chicago/Turabian StyleKolev, Nikolay, Desislav Balev, Stefan Dragoev, Teodora Popova, Evgeni Petkov, Krasimir Dimov, Surendranath Suman, Ana Paula Salim, and Desislava Vlahova-Vangelova. 2025. "Male Layer-Type Birds (Lohmann Brown Classic Hybrid) as a Meat Source for Chicken Pâtés" Applied Sciences 15, no. 12: 6702. https://doi.org/10.3390/app15126702
APA StyleKolev, N., Balev, D., Dragoev, S., Popova, T., Petkov, E., Dimov, K., Suman, S., Salim, A. P., & Vlahova-Vangelova, D. (2025). Male Layer-Type Birds (Lohmann Brown Classic Hybrid) as a Meat Source for Chicken Pâtés. Applied Sciences, 15(12), 6702. https://doi.org/10.3390/app15126702










