Abstract
Sea buckthorn (Hippophae rhamnoides) is a valuable plant rich in biologically active compounds, mainly found in its berries and leaves. The harvesting process, which includes pruning, freezing, and shaking, leaves behind large amounts of biomass and juice-pressing residues, typically composted. The aim of this study is to expand knowledge of the valorization of sea buckthorn secondary raw materials by applying an innovative pressure cyclic solid–liquid (PCSL) extraction method and to develop value-added functional food products. Extraction was performed in 20 and 60 cycles, each lasting from 2 to 10 min. The highest concentrations of proanthocyanidins (5.51 gCE/L) and total phenolics (12.42 gGAE/L) were obtained under prolonged conditions, but the L-4 extract (20 cycles × 2 min) was selected for kombucha production due to its favorable balance between efficiency and sustainability. Microbial safety evaluation showed that kombucha with sea buckthorn leaf extract exhibited significantly stronger antimicrobial activity against tested pathogens compared to green tea kombucha. Additionally, sensory analysis revealed higher consumer acceptability of beverages enriched with sea buckthorn extracts. Shotgun metagenomic analysis identified high microbial diversity in the M. gisevii MI-2 starter culture and fermented kombucha products (227 bacteria and 44 eukaryotes), most of which (92.5% bacteria, 77.8% eukaryotes) remain viable and contribute to fermentation dynamics. New biotechnological strategies and genetic modifications raise concerns about the safe use of microorganisms in food production. To address these issues, these findings provide a foundation for future strategies aimed at the safe application of beneficial microorganisms in food biotechnology and support the long-term goals of the European Green Deal by promoting sustainable biomass valorization and circular economy advancement in the food sector.
1. Introduction
Sustainable use of agricultural by-products is a key objective of the European Green Deal, which emphasizes resource efficiency and circular economy principles. One significant challenge in this context is the underutilization of sea buckthorn (Hippophae rhamnoides) biomass. While the industry primarily focuses on berries, large quantities of biomass waste—particularly leaves and branches—are generated during harvesting and pruning yet remain largely unexploited. The sea buckthorn industry is dependent on its berries, but there are almost no applications for its secondary biomass use, which alone reaches about 12–15%. Valorizing this sea buckthorn biomass waste aligns with sustainability goals and can provide additional income for growers and rural communities [1].
In recent years, the interest and use of herbal infusions (extracts) from a wide range of edible plants have increased due to their potential health benefits and attractive flavor and taste. There is also growing interest in using sea buckthorn leaves and turning them into tea-type beverage products. However, most studies have focused on fresh sea buckthorn leaves, focusing on organic solvent extraction or sonication or microwave exposure to identify new compounds or maximize phenolic yield [2,3,4]. Less is known about the phenolic profiles and antioxidant activity of aqueous extracts of sea buckthorn leaves as tea product infusions and as beverages. It is known that biologically active compounds are sensitive to temperature changes, so it is important to apply innovative extraction methods. Pressure cyclic solid–liquid (PCSL) extraction using environmentally friendly solvents is suitable for this. However, this method has mostly been applied to sea buckthorn berries or pomace [5]. PCSL extraction is usually carried out by diffusion and osmosis, but it is difficult to find innovative methods based on the creation of a pressure gradient between solid and liquid matrices applied to sea buckthorn leaves in the literature. PCSL extraction preserves extracts containing biologically active compounds, thereby increasing the possibilities of using such extracts in the development of new value-added food products [6]. Compared to conventional methods such as maceration and liquid-liquid extraction, PCSL extraction has significant advantages, including significantly shorter extraction times, typically lasting from 2 to 24 h, lower temperature ranges, and the elimination of filtration steps due to the high quality of the extract produced [6]. This makes this extraction particularly suitable for the extraction of thermolabile substances, as it effectively avoids the decomposition that can occur during heating processes [7].
In parallel with extraction innovations, there is increasing consumer interest in fermented beverages such as kombucha, traditionally made from green and black teas. Kombucha is produced using a symbiotic culture of bacteria and yeast known as Medusomyces gisevii (SCOBY) [8], which ferments sweetened tea. This raises the potential to incorporate novel infusions—such as sea buckthorn leaf extracts—into the kombucha fermentation process. However, no scientific publications to date have assessed the efficacy of kombucha products derived from sea buckthorn leaf extracts. Moreover, ensuring the microbiological safety of fermentation processes is a growing regulatory concern. In 2021, the European Food Safety Authority (EFSA) published requirements for the whole-genome sequencing of microorganisms used in food chains [9], aiming to ensure traceability and safety. Shotgun sequencing, a high-throughput DNA sequencing technique, enables the analysis of entire microbial communities and their functional potential [10]. This is especially important in kombucha cultures, which vary in microbial composition and include complex interactions between yeast and acetic acid bacteria [11]. Therefore, evaluating the genetic profile of the SCOBY used in kombucha fermentation with sea buckthorn leaves is critical for meeting current food safety standards.
Previous studies have not applied PCSL extraction from sea buckthorn leaves for kombucha production. Therefore, this method can be considered innovative in terms of both extraction technique and potential for value-added product development and makes a significant contribution to the research field. By evaluating the bioactive compounds contained in the extracts and applying them to food matrices, it is possible to develop value-added products. This study may open new directions for the development of functional beverages using EFSA [9] compliant and microbiologically safe fermentation methods, using a low-energy, solvent-saving, and scalable method. In addition, this study will directly address the requirements by incorporating whole-genome shotgun sequencing to characterize the microbial starter culture used in the fermentation. This may ensure the microbiological safety and traceability of the production process, in line with modern standards for the development of novel foods.
The aim of this study is to evaluate the extraction of bioactive compounds from sea buckthorn secondary raw materials using the innovative PCSL extraction method and to evaluate their application in the production of kombucha-based functional beverages.
2. Materials and Methods
2.1. Materials
Sea buckthorn (Hippophae rhamnoides) leaves remaining after separating the sea buckthorn berries used in the production of kombucha were retrieved from a local production farm (“Amberry” farm identification code: 1365579). Before extraction, the leaves were grind (particle diameter 100–500 µm) and dried at room temperature (d.m. 94%). Green tea leaves were purchased from a local supermarket, and Chinese green tea “Gurman’s special gunpowder” was crushed (particle diameter 200–800 µm) and dried at room temperature (d.m. 97%). All reagents were of analytical grade. Hydrochloric acid (HCl), Folin–Ciocalteu’s reagent, sodium carbonate (Na2CO3), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and sodium hydroxide (NaOH), gallic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents were of analytical grade and were used as received without further purification.
2.2. Proximate Composition Analysis of Sea Buckthorn Leaves and Their Aqueous Extracts
Moisture content was determined according to the Association of Official Analytical Collaboration (AOAC) Method 925.10-1925 [12]. Crude protein content was determined by the Kjeldahl method (N × 6.25), according to the AOAC 978.04 [12]. Total ash content was determined according to the AOAC method 930.05 [12]. Total fat and fatty acids content was determined according to ISO:12966-2:2011 [13]. Dietary fiber content was determined according to the American Association of Cereal Chemists (AACC) method 32-07.01 and the AOAC method 991.43 using a Total Dietary Fiber assay kit (K-TDFR-200A Megazyme Int., Wicklow, Ireland) [14]. Total carbohydrate content and sugar (glucose, fructose, and saccharose) content were performed using Sucrose/D-Fructose/D-Glucose Assay Kit Megazyme (K-SUFRG Megazyme Int., Wicklow, Ireland) according to the manufacturer’s instructions. Microelements (calcium, potassium, and magnesium) content was determined according to European Standard: EN 15621:2017 [15].
2.3. Preparation of Sea Buckthorn Leaves Extracts
Sea buckthorn leaves (200 g) were put in a porous bag and then it was introduced in the extraction chamber of the Naviglio Extractor (Mod. 1 liter capacity, Nuova Estrazione S.a.s., Naples, Italy) [16] and 1 L of distillate water was added (leaves/water: 1:5 (g/mL)). The aqueous extract of sea buckthorn leaves was obtained by running the aqueous extraction for 20 to 60 cycles of 2 to 10 min at room temperature, under a pressure of 8.5 bar. The extraction conditions were selected based on literature data to optimize yield and quality [17,18]. In total, the extractions took between 2 and 12 h (2 min in the static phase and 2 min in the dynamic phase) and were performed at the end, obtaining the aquae extract to be used for the preparation of the kombucha.
2.4. Determination of Proanthocyanidins by the Bate-Smith Assay
The proanthocyanidin concentration was determined using the methodology proposed by Bate-Smith [19,20]. Each aqueous sea buckthorn leaf extract sample was diluted at a ratio of 1:50 (v/v) with deionized water. In two separate test tubes, 4 mL of the diluted sample, 2 mL of deionized water, and 6 mL of hydrochloric acid were added. One test tube (reaction tube) was placed in a water bath at 100 °C for 30 min, and the other test tube (blank tube) was left to stand in the dark for the same time. After 30 min, 1 mL of ethanol was added to each tube, and the tubes were left in the dark until the heated reaction tube was cooled. The absorbance of each test tube was measured in a spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA, JAV) at 550 nm using deionized water as the blank. The absorbance difference was multiplied by the factor 19.33, and the concentration of proanthocyanidins was expressed in g (+)-catechin equivalent (CE) per liter.
2.5. Total Phenolic Content (TPC) by Folin−Ciocalteu’s Assay
The TPC was determined by Folin−Ciocalteu’s Assay as previously described by Singleton et al. [21] with the same modifications. Sea buckthorn leaf extract solution was mixed with 5 mL Folin−Ciocalteu’s reagent (1:9 v/v), and after 5 min, 4 mL 7.5% (w/v) sodium carbonate solution was added. The absorption was measured at 765 nm after 2 h using spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA, JAV). A gallic acid calibration curve was prepared (R2 = 0.999), and TPC was expressed as milligrams of gallic acid equivalents (GAE)/g per liter.
2.6. Preparation of Kombucha
Kombucha starter culture M. gisevii MI-2 was collected from a local family (Kaunas, Lithuania) and then stored in “starter fluid” (fermented acidic tea) at room temperature in the Kaunas University of Technology Food Institute (Kaunas, Lithuania). Prior to fermentation, M. gisevii MI-2 was activated for 7 days at room temperature (approximately 25 °C). Kombucha was prepared according to Gülhan [22] with slight modifications.
Different concentrations of sea buckthorn leaf extract and green tea (sourced from China; supplied and packaged by Gurman’s, Vilnius, Lithuania) were used for kombucha preparation. Sea buckthorn leaf extract, obtained as described in Section 2.3, was diluted 20 times with sterile distilled water to obtain a concentration similar to traditional tea. Green tea leaves were extracted in similar conditions as sea buckthorn leaves and diluted 20 times with sterile distilled water. The concentrations of sea buckthorn and green tea diluted extracts used for kombucha preparation were: 100% sea buckthorn leaves (L4-100); 75% sea buckthorn leaves +25% green tea (L4-75); 50% sea buckthorn leaves +50% green tea (L4-50); 25% sea buckthorn leaves + 75 green tea (L4-25); 100% green tea (G-100). Ten percent of saccharose (Panevėžio plius, Panevėžys, Lithuania) was added to all preparations and stirred until completely dissolved. The previous fermented kombucha tea (5%) and M. gisevii MI-2 (2%) were added, and fermentation was carried out at room temperature for 10 days. The samples were taken at 0, 1, 3, 4, 7 and 10 days. All appliances and flasks were sterilized with 70% ethanol before experiments. Fermentation assays were carried out in triplicates.
2.7. pH, Titratable Acidity, and Total Soluble Solids
The pH of kombucha was measured by directly immersing the electrode into 25 mL of sample immediately after sampling, using a pH meter (PP-15, Sartorius, Goettingen, Germany). Total acidity was determined by acid–base titration of a kombucha sample with 0.1 N NaOH using phenolphthalein as an indicator. The endpoint was determined by the appearance of a stable pale pink color persisting for at least 30 s. The results were expressed as a percentage (%) of acetic acid per mL of sample. Total soluble solids (TSS) were measured using a refractometer (Atago, RX 5000C, Tokyo, Japan) based on the principle of light refraction through the sample by applying 1 mL of the sample. The results were expressed in °Brix.
2.8. Shotgun Metagenomic Analysis of M. gisevi MI-2 and Fermented Product
Sample Collection and DNA extraction. Samples of M. gisevii MI-2 and fermented product (FP)—kombucha containing a mix of microorganisms—were collected for DNA extraction. DNA was extracted from 20 mg of washed “SCOBY” before the fermentation process and from 20 mg obtained after 10 min centrifugation at 6000 rpm of FP. DNA extraction with commercial Quick-DNA Fungal/Bacterial KIT according to manufacturer’s protocol (ZYMO Research, Irvine, CA, USA). DNA concentration of M. gisevii MI-2 and FP was 75.50 ng/µL and 25.17 ng/µL, respectively; concentrations were confirmed using Qubit® dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA).
Library construction and sequencing. Shotgun metagenome sequencing of samples was performed at the Novogene laboratory in the UK. DNA was fragmented into short fragments, which were polished at the ends, received A-tails, and ligated with full-size adapters for sequencing on the Illumina platform. Then, PCR amplification was performed. Purification was carried out using the AMPure XP system (Beverly, Singapore). The resulting library was evaluated using the Agilent fragment analyzer system (Agilent, Santa Clara, CA, USA) and quantified up to 1.5 nM using Qubit® (Thermo Fisher Scientific, San Diego, CA, USA) and quantitative PCR. Corresponding libraries were pooled and sequenced with 300 cycles via the Illumina NovaSeq 6000 system. Clean reads in size 7.07 Gb of M. gisevii MI-2 and 13.11 Gb of FP were processed with Fastp (GitHub, Inc., San Francisco, CA, USA, 2024). Free access to scaftigs data in BioProject PRJNA1163270 in NCBI database.
Assembly and data analysis. MEGAHIT v1.2.9 software was used for assembly analysis of clean data, with assembly parameter settings [23]. The shot reads 49,584,388 bp in the length of “SCOBY”, and 40,198,023 bp of FP were assembled in 8244 and 17,892 caftigs without N [24]. MetaGeneMark was used to perform ORF prediction for scaftigs (≥500 bp) of each sample [25], and the information with a length less than 100 nt in the prediction results was filtered out [23]. DIAMOND v2.1.6 software [26] was used for the alignment of unigenes sequences with the Micro_NR database to identify the organisms (bacteria, fungi, archaea, and viruses) in both samples. Since each sequence may have multiple alignment results, the LCA algorithm (applied to the systematic taxonomy of MEGAN (Meta Genome Analyzer) v.4) was adopted to determine the species annotation information of the sequence [27]. Prediction of the functional genes performed with DIAMOND software to align unigenes with those in the common functional database: KEGG [28] and CAZy [29]. For Venn diagram construction, we used (bioinformatics.psb.ugent.be/webtools/Venn/); SankeyMATIC.com was used for Sankey diagram construction.
2.9. Assessment of Antioxidant Activity
The assessment of kombucha antioxidant activity was evaluated by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay according to Brand-Williams et al. [30]. Briefly, 0.5 mL of kombucha sample was mixed with 1.5 mL of 0.004% of DPPH solution (dissolved in ethanol) and incubated for 30 min at room (approximately 25 °C) temperature in darkness. The DPPH radical scavenging activity was estimated using Equation.
DPPH radical scavenging activity (%) = (OD_control − OD_sample)/(OD_control) × 100
2.10. Ethanol Analysis
The ethanol analysis was performed using a gas chromatograph equipped with a flame ionization detector and an integrator for peak area measurement following the method described by Ebersole et al. [31]. A 30 m capillary column with a 0.25 mm internal diameter and a polyethylene glycol-based stationary phase (Stabilwax-DA) was used. The injection volume was 1 µL, with a carrier gas linear flow rate of 18 cm/s. The temperature program started at 38 °C (held for 3 min) and increased to 240 °C at 50 °C/min, with a 1-min hold. Samples before the analysis were degassed in an ultrasonic bath filtered through a membrane filter.
2.11. Assessment of Antimicrobial Activity
Antimicrobial activities of kombucha were evaluated using the agar diffusion assay method as previously described by Sreeramulu et al. [32] with slight modifications. The six strains of microorganisms were used (Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 19433, Salmonella enteritidis ATCC 13076, Pseudomonas aeruginosa ATCC 27853, Staphylococcus saprophyticus ATCC 15305). The microorganisms were propagated overnight at 37 °C temperature on plate count agar (Liofilchem, S.r.l., Roseto degli Abruzzi, Italy) slants. After cultivation, bacteria cells were collected from slants, and cell suspension was adjusted to McFarland standard No. 0.5 (Liofilchem, Waltham, MA, USA). Bacteria suspension was mixed with plate count agar medium (45 °C) to obtain 106 CFU/mL, and 10 mL was poured into Petri dishes. The kombucha (50 μL) or sterile distilled water (negative control) was added to each well (diameter of 8 mm) previously cut into agar plates. Plates were incubated for 24 h at 37 °C. The antimicrobial activities against microorganisms were recorded as clear zones around the wells (mm).
2.12. Sensory Evaluation
Sensory analysis of kombucha was carried out by 20 assessors (6 males and 14 females aged between 22 and 60), selected and trained according to ISO 8586:2012 [33] guidelines. All panelists were affiliated with Kaunas University of Technology (Kaunas, Lithuania) and had prior experience in participating in sensory evaluations. The sensory evaluation procedure followed ISO 6658:2017 [34]. Kombucha samples were served at room temperature (22 °C) in transparent glasses (50 mL) that were coded with three-digit random numbers, and water was provided for rinsing between samples. Kombucha sensory properties (color, odor, taste) were evaluated using a 9-point intensity scale, where 1 corresponded to the lowest intensity, and 9 corresponded to the highest intensity of the attribute. Kombucha was also evaluated for overall acceptability using a 9-point hedonic scale where point 9 was “extremely liked”, and point 1 was “extremely disliked”.
2.13. Statistical Analyses
All experiments were performed at least in triplicate. Average values and standard deviations were calculated using MS Excel 2019 (Microsoft Corp, Albuquerque, NM, USA), while statistical analysis was performed using the Statgraphics Centurion 19 statistical package. One-way analysis of variation (ANOVA), followed by Tukey’s honest significant difference (HSD) test, was performed to determine significant differences (p ≤ 0.05).
3. Results and Discussion
3.1. Sea Buckthorn Leaves Characteristic
The sea buckthorn, especially its fruits, is well-known for its nutritional and therapeutic value and has been extensively studied in scientific research. While the leaves are less valued, they also possess high nutritional content. The chemical composition of sea buckthorn leaves used in this study is presented in Table 1. In this study, dietary fiber, carbohydrates, and crude protein were predominant in sea buckthorn leaves (38.73, 27.85, 15.40 g/100 g, respectively), which confirms their potential as a valuable nutritional resource. The obtained results of sea buckthorn leaf proteins and dietary fiber content align with Bośko et al. [35], who reported that these values range between 15.66–20.74 g/100 g and 9.59–12.14 g/100 g, respectively, depending on the specific plant cultivar and the year of harvest. While Saracila et al. [36] described a lower content of protein (14.48%).

Table 1.
Nutritional, compositional, quantitative, and qualitative indicators of raw materials for sea buckthorn leaves.
Sea buckthorn leaves could also be considered to be a source of fat, and in this study, the fat fraction was found to be predominantly composed of polyunsaturated fatty acids (60.96% of total fats), including omega-3 and omega-6 fatty acids, which are essential for nutrition and have important physiological functions [37]. Bośko et al. [35] and Saracila et al. [36] reported lower content of fat in sea buckthorn leaves.
The sea buckthorn leaves also have high mineral content, which was in accordance with Bośko et al. [35], who described that mineral range between 4.06 and 5.79 g/100 g. The mineral profile of sea buckthorn leaves showed high concentrations of calcium (6.5% of all minerals), which is essential for nutrition [38]. Tkacz et al. [39] also reported that calcium is the predominant macroelement in sea buckthorn leaves.
Sea buckthorn leaves exhibit a notably high nutritional value and are distinguished by an elevated concentration of polyphenolic compounds, which are present in greater quantities in the leaves compared to the berries [40]. As a result, increasing attention is being directed toward the extraction and utilization of these bioactive compounds in the development of functional food products.
3.2. Effect of Extraction Conditions on Sea Buckthorn Leaves
Sea buckthorn berries and leaves have been used as medicine and health food for thousands of years due to their bioactive compounds with strong antioxidant properties and significant therapeutic benefits. Therefore, it is important to develop an efficient and environmentally friendly extraction method for these compounds [41,42]. Currently, there is a scarcity of scientific studies employing PCSL extraction of sea buckthorn leaves using water as a solvent. Although ultrasonic extraction is commonly used, there are few studies where water is used as a solvent [43,44]. More often, ethanol or methanol is used, which are not environmentally friendly [41,43]. Furthermore, additional procedures are required to remove these solvents before using the extracts in the food industry. In this study, six different extracts of sea buckthorn leaves collected post-berry harvest were prepared using water as the extraction solvent. The extracts were obtained through PCSL extraction with varying numbers of extraction cycles and durations (see Table 2). The effects of these extraction conditions on the yield and composition of biologically active compounds, such as phenolic compounds and proanthocyanidins, were assessed.

Table 2.
Extraction conditions and content of biologically active compounds in sea buckthorn leaf aqueous extracts.
Extraction conditions notably influenced the concentration of bioactive compounds in the sea buckthorn leaf extracts. The extraction was performed in 20 and 60 cycles, with each cycle lasting from 2 to 10 min. PCSL extraction of sea buckthorn leaves under these conditions resulted in extracts with proanthocyanidin content ranging from 2.47 to 5.51 gCE/L and TPC ranging from 8.39 to 12.42 gGAE/L. The highest amount of biologically active compounds was observed in extracts obtained using 60 cycles, each lasting 10 min. However, compared to scientific publications, prolonged aqueous extraction is not environmentally friendly, similar to ultrasonic extraction methods that use ethanol and have a shorter extraction time of only 2 h. In such extracts, Bosko et al. [41] identified proanthocyanidins ranging from 7.86 to 14.57 gCE/L, depending on the sea buckthorn variety and harvest year. Čulina et al. [2], using ethanol as a solvent in microwave-assisted extraction and accelerated solvent extraction, found about 6–11 gGAE/100 g dry extract of TPC in extracts dependent on extraction conditions. The higher concentrations of biologically active compounds are described in these studies; however, the solvents used are not environmentally sustainable. Aqueous extracts may offer greater potential for application in food product development, such as in kombucha production. It also avoids removing the solvent before further use in the food industry. Currently, it is difficult to find analogous studies to compare the efficiency of water extraction of sea buckthorn leaves remaining after berry harvesting using PCSL extraction.
As shown in Table 2, although the L-2 extract yielded the highest concentration of bioactive compounds during the long extraction, the L-4 and L-5 extracts had similar amounts of proanthocyanidins and TPC. Therefore, the L-4 extract, obtained by PCSL extraction with 20 cycles of 2 min each, was selected for further studies. Although the shorter extraction does not result in the maximum concentration of bioactive compounds, the shortened extraction time and the use of an environmentally friendly solvent make it superior to extracts reported in the literature using non-sustainable solvents. This demonstrates a trade-off between extraction efficiency and process sustainability.
3.3. Preparation and Characterization of Sea Buckthorn Leaves Kombucha
Although the sea buckthorn industry is primarily focused on the use of berries, the secondary utilization of biomass is limited. However, before composting, valuable bioactive compounds can be extracted from biomass and used to develop new value-added food products. Kombucha production is one such approach. While black and green tea are the most popular substrates, new variations of kombucha are emerging, where part of the traditional tea is replaced with extracts from secondary raw materials [22,45,46]. Five different kombucha samples were prepared using different ratios of sea buckthorn leaf extract and green tea: 100% sea buckthorn leaves (L4-100); 75% sea buckthorn leaves +25% green tea (L4-75); 50% sea buckthorn leaves +50% green tea (L4-50); 25% sea buckthorn leaves + 75 green tea (L4-25); 100% green tea (G-100). Fermentation was carried out for 10 days, and at different time points, the concentrations of biologically active compounds and the antioxidant effectiveness of the tea were evaluated (see Table 3).

Table 3.
Proanthocyanidins, TPC concentrations, and antioxidant efficacy parameters in kombuchas during fermentation.
Biological active compounds such as phenolic compounds or proanthocyanidins are secondary metabolites found abundantly in plant-based products. They possess notable antioxidant properties that contribute positively to human health. Additionally, these compounds influence the aroma, flavor, and color characteristics of food products [45,47]. They are essential in kombucha beverages due to their health-promoting characteristics. Hence, alternative substrates obtained from various plants may have the potential for biologically active compounds [48].
During the study, the proanthocyanidin content of the original tea samples ranged from 7.19 gCE/L to 7.81 gCE/L, depending on the ratio of sea buckthorn leaves to green tea (Table 3). The results show that fermentation led to a decrease in proanthocyanidin content, with the lowest concentration observed in the sea buckthorn leaf kombucha sample L4-100. Proanthocyanidins are complex polyphenolic compounds composed of flavanol monomers such as catechin and epicatechin linked together. Therefore, during fermentation, microorganisms, especially yeasts, and bacteria, can secrete enzymes such as tannases or glycosidases that degrade proanthocyanidin polymers into smaller phenolic compounds or flavonoids [49,50]. For this reason, it can be observed that the TPC increases in proportion to the decreasing concentration of proanthocyanidins.
In this study, the TPC content of the tea samples ranged from 0.89 gGAE/L to 1.21 gGAE/L, depending on the ratio of sea buckthorn leaves to green tea (Table 3). Scientific publications have found that the introduction of new substrates into kombucha fermentation generally increases the content of bioactive molecules such as phenolic compounds [48,51]. Silva et al. [52], the TPC of unfermented beverages made from green tea and wax mallow (Malvaviscus arboreus Cav.) were 568.4 μGAE/mL and 50.9 μgGAE/mL, respectively. After fermentation into kombucha beverages at 24 °C for 14 days, the phenolic content of the wax mallow kombucha increased to 124.6 μgGAE/mL, whereas the phenolic content of the green tea kombucha did not differ significantly from that of the unfermented green tea beverage. In another work [46], the TPC of unfermented beverages made from hawthorn leaves, nettle leaves, and black tea were 163.33 mgGAE/L, 663.83 mgGAE/L, and 1896.83 mgGAE/L, respectively. After fermentation into kombucha beverages at 25 °C for 15 days, the phenolic content of the kombucha from hawthorn leaves, nettle leaves, and black tea were 995.33 mgGAE/L, 1479.83 mgGAE/L, and 2452.67 mgGAE/L, respectively. The biggest change was observed in hawthorn leaves and nettle leaves, while in black tea, the change was insignificant. Microbial metabolism converts certain compounds into more active isomers or smaller molecules with enhanced biological activity, thereby enhancing or diversifying kombucha’s physiological functions, properties, and sensory attributes [45].
He et al. [44] presented that the DPPH values of the different sea buckthorn leaf tea beverages showed significant variation, ranging from 20.69 ± 0.61 to 50.21 ± 2.71 mmol TE/g. The findings indicated that sea buckthorn leaf tea exhibited a greater capacity to scavenge DPPH free radicals compared to the other two analysis types. In the present study, assays such as DPPH (which measures the removal of DPPH free radicals) were used to evaluate the antioxidant activities of the five sea buckthorn leaf kombucha samples (Table 3). The lowest antioxidant changes during fermentation were observed in sea buckthorn leaf kombucha L4-100. It was also observed that the antioxidant potency of the samples decreased with increasing fermentation time. Similar results have been found in the work of other authors. For example, the free radical-binding capacity of black tea kombucha decreased from 36.4 to 35.4 during the fermentation of 15 days [53]. Fermentation can also produce other compounds, such as organic acids, which can reduce the overall antioxidant potential of the beverage, even if the amount of phenolic compounds increases. It was also noted that antioxidant efficiency decreased with longer fermentation duration. This observation is supported by changes in the pH values of the kombucha and the measured titratable acidity (TA). It was observed that as the fermentation time increased, the pH values decreased, and the total acetic acid content increased in all five samples (see Figure S1 in the Supplementary Material S1).
TA and pH are important parameters that help not only to evaluate fermentation and determine the end of the process but also have an impact on product sensory properties and safety. During fermentation pH value decreased from 5.0–5.1 to 2.97–3.33, while TA increased from 0.05–0.07 to 0.34–0.65%. Other authors also described reduced pH value after 10 days of kombucha fermentation, which reached pH 3 [54] in black tea kombucha and between 2.72 and 2.79 with fermentation with black, green, and oolong teas [55]. It is recommended that kombucha pH should be over 2.5, and with optimal pH range from 2.5 to 4.2 [56]. In this study, the highest decrease of pH (2.03) after 10 days of fermentation was observed in L4-100 kombucha, this sample also has the lowest content of saccharose (Table 4) after fermentation. The highest pH after fermentation was observed in G-100 kombucha, which also had the highest saccharose content. The saccharose content significantly decreased in all samples after 10 days, accompanied by a significant increase in glucose and fructose content. During fermentation, saccharose is hydrolyzed into glucose and fructose by the yeast-produced invertase (β-fructofuranosidase) [57]. This enzymatic hydrolysis provides fermentable sugars that serve as substrates for both yeasts and bacteria in the M. gisevii MI-2 consortium. Yeasts primarily metabolize glucose and fructose to produce ethanol and carbon dioxide, while acetic acid bacteria subsequently oxidize ethanol to organic acids, such as acetic acid and gluconic acid [57], which leads to increased acid production and a decrease in pH levels.

Table 4.
Changes in the TSS values and saccharides contain kombucha at different fermentation times.
In the preparation of kombucha, qualitative parameters such as TSS are important. In fermented beverages like kombucha, TSS measurements help monitor the fermentation process. Initial and final TSS measurements allow for determining the amount of sugar that has been converted into alcohol. In this study, alcohol content was measured in all samples before and after fermentation. No alcohol was detected in the initial samples before fermentation, while after fermentation, up to 0.32% alcohol (L4-25) was found in kombucha. In the kombucha medium, yeasts convert glucose and fructose into ethanol. On the other hand, acetic acid bacteria utilize ethanol to produce gluconic acid [22,58]. As yeasts and bacteria metabolize sucrose during fermentation, a decreasing trend in TSS values was observed across all infusion groups over time, although the differences were not statistically significant (Table 4). In this study, TSS was monitored throughout the entire fermentation period, ranging from 9.57 ± 0.42 to 8.79 ± 0.39 °Brix. The lowest TSS values in all tea samples were observed on the 10th day (Table 4). Additionally, the total saccharide content decreased by approximately 1.2%, while the glucose and fructose content increased (Table 4).
3.4. Antimicrobial Activity of Kombucha Evaluation
Sea buckthorn leaf extract, which is rich in polyphenols and has antioxidant properties, may enhance the therapeutic effects of kombucha. It is, therefore, very important to investigate the safety and efficacy of this drink against pathogenic bacteria to better understand its potential applications in the food industry and the health sector [59]. For this reason, it is essential to ensure its safety. In this study, the antimicrobial activity of kombucha after 10 days of fermentation was evaluated and is shown in Table 5. All tested bacteria were sensitive to fermented kombucha containing sea buckthorn leaf extract, and L4-100 showed a statistically significant (p < 0.05) increase in antimicrobial potential with inhibition zones ranging from 16.0 (P. aeruginosa ATCC 27853) to 19.80 (S. saprophyticus ATCC 15305) mm.

Table 5.
Inhibition zones of kombucha.
Green tea kombucha G-100, which does not contain sea buckthorn leaf extract, did not show antimicrobial activity against E. coli ATCC8739, S. aureus ATCC 25923, or E. faecalis ATCC 19433, indicating that during sea buckthorn leaf extract fermentation formed higher content of antimicrobial substances than during green tea fermentation. The antimicrobial activity could be attributed to the synthesis of organic acids and plant-derived phenolic compounds, as well as the action of enzymes, proteins, and bacteriocins [60,61]. In this study, the highest pH, lowest TA, and TPC were identified in green tea kombucha, which could explain its lower antimicrobial potential. Several studies described traditional green and black tea kombucha antimicrobial activity against E. coli, S. epidermidis, S. aureus, and P. aeruginosa [60,61], while Battikh et al. [60] reported that non-traditional kombucha prepared from different herbals showed stronger antimicrobial effect compared to traditional black and green tea kombucha. Sea buckthorn leaf extract (L4-100 at day 0) also showed antimicrobial activity against S. aureus ATCC 25923 and S. saprophyticus ATCC 15305. Janceva et al. [62] described sea buckthorn extract antibacterial activity against several bacteria, among them S. aureus, which is mostly related to polyphenolic compounds composition and quantity.
3.5. Shotgun Metagenomic Analysis of M. gisevii MI-2 and Fermented Kombucha
Comparative metagenomic analysis of M. gisevii MI-2 and its fermented product—kombucha—provides insights into the evolution of microbial communities, metabolic shifts, and functional pathways that occur during fermentation. The composition of species in M. gisevii MI-2 and kombucha L4-100 is shown in Table 6. The list of microorganisms is revealed in Supplementary Material S3 (Excel file). Three Kingdoms were identified in the M. gisevii MI-2 sample and 4 in the kombucha L4-100 precipitate. More Bacteria species (91) were obtained in kombucha, which shows the introduction of microorganisms into the product from plant raw materials and during the technological process. However, in terms of percentage, the bacteria content remained very similar in both samples at 81.07 and 82.60%. Eukaryotes identified more in the symbiotic system of M. gisevii MI-2 than in kombucha (44 and 38 species, respectively). Nine viruses were found in M. gisevii MI-2 (5 plant-specific and 4 bacterial phages). A total of 28 viruses were found in the kombucha; 5 plant-specific viruses remained during fermentation, and others were introduced during the fermentation process by various hostplants and bacteria. Having entered the M. gisevii MI-2 microorganism complex from plant raw materials through a series of fermentations, plant viruses such as Cauliflower mosaic virus, Dahlia mosaic virus, Figwort mosaic virus, Grapevine-associated calicivirus, and Rudbeckia flower distortion virus were able to establish themselves and survive (Figure 1C). One member from the archaea species Candidatus Woesearchaeota archaeon was found in kombucha L4-100. It is widespread and abundant in soils, freshwater, sediments, and others [63].

Table 6.
Number of species in M. gisevii MI-2 and fermented—kombucha L4-100.
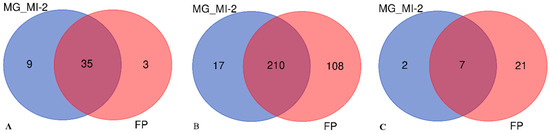
Figure 1.
Core-pan genome in Venn diagram of M. gisevii MI-2 (MG_MI-2) and fermented Kombucha (FP). (A)—eucaryote; (B)—bacteria; (C)—virus.
Based on the abundance list of species (Supplementary Material S2 (Excel file) in each sample, Venn diagrams were constructed, and information was acquired about the Core-pan genome for eucaryotes, bacteria, and virus taxons (Figure 1; Supplementary Material S3 (Excel file)). After comparing the microbiota, 79.5% of eukaryotes, 92.5% of bacteria, and 77.8% of viruses identified in M. gisevii MI-2 were also present in kombucha sediments. Specific to M. gisevii MI-2 were 9 species of eucaryotes, and 3 Dentiscutata erythropus, Puccinia coronata, and Grosmannia clavigera detected only after fermentation in kombucha (Figure 1A). These fungi, D. erythropus and P. coronata, are not typically associated with fermentation processes. Instead, each of them has a distinct ecological or pathological role. D. erythropus is widespread in the soil and plays an essential ecological role as a detritivore, as first published in 2013 [64]. P. coronata spores infect the alternate host buckthorn [65], which was the main biomass of fermented beverages. G. clavigera is also a fungal pathogen of plants DiGuistini et al. [66]. However, G. clavigera produces lignocellulolytic enzymes and could grow using monoterpenes like limonene as the sole carbon source [67]. Consequently, G. clavigera can degrade plant cellulose, so in theory, it could break down bacterial cellulose produced by K. xylinus, and using monoterpene limonene from buckhorn leaves. Unfortunately, until this described case, the interaction possibilities of these microorganisms were not studied because they inhabit very different ecosystems—forests and fermented tea, respectively.
Results showed (Figure 1B) that 210 bacterial species from the M. gisevii MI-2 metagenome were also detected in the metagenome of the fermented kombucha. Seventeen species were still in M. gisevii MI-2 and did not migrate to the kombucha microflora. A wide range of bacteria (108) and viruses (21) species were introduced into kombucha with plant material or other food supplements during the fermentation process (Figure 1B,C).
Using the classic microbiology method (cultivation on selective mediums in vitro) or 16S rRNA gene sequencing, it was possible to identify only a part of microorganisms, usually at the genus level. Coton et al. [68] identified 3 LAB (lactic acid bacteria), 13 AAB (acetic acid bacteria), and 11 species of yeasts from black or green tea kombucha according to 16S rRNA gene sequencing data and 26S metabarcoding analysis and other microoganisms in fermented product remained unidentified. This study revealed the full composition of the symbionts and showed that the biodiversity of M. gisevii can be much wider, and the amounts of substances produced can be modeled by creating the conditions for the action and dominance of more than 250 species of microorganisms. A wide range of bacteria of the genus Sphingobium in M. gisevii MI-2 is worth mentioning. It is known that they are notable for their ability to degrade a variety of complex and toxic organic compounds. Sphingobium bacteria is important in bioremediation efforts to clean up contaminated environments, such as soil and water [69]. Other microorganisms such as heterotrophic Geminicoccus harenae [70], obligate intracellular Rickettsiales bacterium [71], Zymomonas mobilis with the ability to ferment sugars into bioethanol [72] or Burkholderia spp. as source of novel antibiotics and bioactive secondary metabolites [73], halophilic black yeast Hortaea werneckii [74] or symbionts of Coccocarpia genus [75] are present in low abundance in M. gisevii MI-2, but must play an important role in the M. gisevii symbiotic system, which will need to be detailed in the future.
Comparison of top species (taxonomy abundance > 0.5% in Kingdom) of bacteria and eucaryotes obtained in metagenomes of M. gisevii MI-2 (MG) and fermented kombucha L4-100 are shown in the Sankey diagram (Figure 2). All the top acetic acid bacteria belong to the family Acetobacteraceae. Eukaryote—all yeasts dominated in both metagenomes and belonged to the families Pichiaceae and Saccharomycetaceae. In the metagenome of M. gisevii, MI-2 in the first place was K. xylinus (60.4%), and in the second place N. hansenii (16.4%). Meanwhile, the metagenome of the kombucha was dominated by N. hansenii (54.1%) and K. saccharivorans (25.6%). The most common yeast in both cases was the same species, B. bruxellensis—75.9% in M. gisevii MI-2 and 94.3% in FP. Results showed that P. membranifaciens was not dominant in kombucha. Other microorganisms that have a rather active influence on the fermented product were the bacteria K. oboediens, K. europeus, and K. intermedius and the yeasts—Pichia californica, Z. bailii, and Z. parabailii.
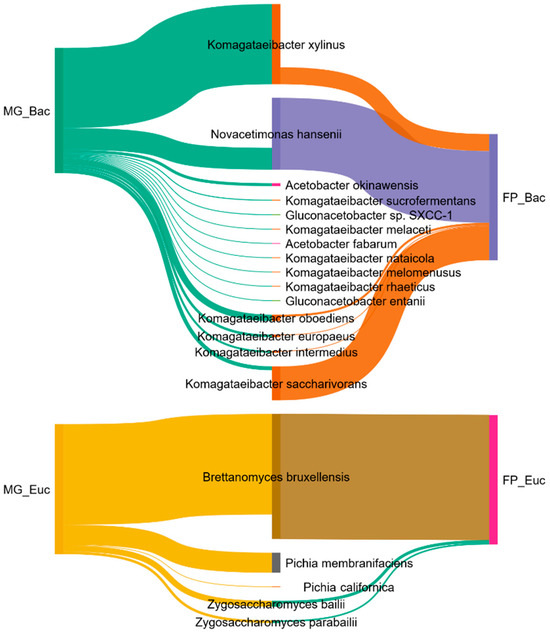
Figure 2.
Sankey Diagram of top species (taxonomy abundance > 0.5% in Kingdom) of bacteria and eucaryote obtained in metagenomes of M. gisevii MI-2 (MG) and fermented product (FP)—kombucha.
According to data from Içen et al. [76], the three main groups of microorganisms in M. gisevii are AAB (Komagataeibacter, Gluconobacter, Acetobacter spp.), LAB (Lactobacillaceae, Lactococcus spp.) and yeasts (Schizosaccharomyces pombe, Saccharomycodes ludwigii, Kloeckera apiculata, Saccharomyces cerevisiae, Zygosaccharomyces bailii, Torulaspora delbrueckii, Brettanomyces bruxellensis). However, after starting to apply this symbiont not only for food at home for functional beverages but also in industrial biotechnology in biocellulose production, there was a great opportunity for diversity between different M. gisevii [77]. In this study, the main bacterial composition of the M. gisevii MI-2 was AAB–Komagataeibacter 62%, Novacetimonas 9%, Acetobacter 4%, Gluconobacter 1% and Gluconacetobacter 0.8%. LAB belonging to the Lactobacillales order in the studied symbiont were identified, but taxon abundance was <0.05%. The main yeast identified in M. gisevii MI-2 belonged to three genera: Saccharomyces 60%, Zygosaccharomyces 18%, and Pichia 13%. In the fermentation process, this consortium converted sugars into various metabolites, including ethanol, acetic acid, and other organic compounds, which gave rise to a distinctive fermented product. More families (Supplementary Material S3) with less taxon abundance in the symbiosis process contribute to the metabolic diversity of AAB, LAB, and yeast found in the studied metagenome.
According to the relative abundance in the KEGG database (Supplementary Material S4 (Excel file)), a cartogram of the functional abundance of the Top 10 modules in both samples was drawn (Figure 3A). Highlighted are the pentose phosphate pathway (M0004) is a crucial metabolic pathway in cells; Glycolysis (M0001, M0002), Gluconeogenesis (M0003), and Citrate cycle (M0009, M00011) pathways; De novo purine biosynthesis (M00048) for cell division; Shikimate (M00022) and Lysine biosynthesis (M000165) pathways are crucial for the synthesis of amino acids; Histidine biosynthesis (M00026) for protein synthesis and enzyme function regulation. All pathways were with higher abundance in M. gisevii MI-2 than in kombucha. A higher abundance of KEGG modules in the M. gisevii metagenome indicated that the symbionts have more active specialized functions and metabolic capabilities for adaptation.
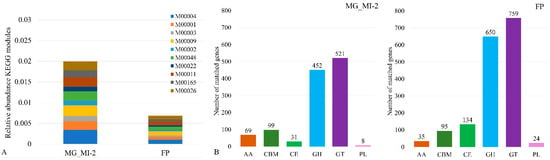
Figure 3.
Relative abundance of KEGGs (Kyoto Encyclopedia of Genes and Genomes) modules (A) and functional abundance of CAZy (B) in metagenomes of M. gisevii (MG) and fermented product (FP)—kombucha.
CAZy (Carbohydrate-Active Enzymes) are enzymes involved in the degradation, modification, and carbohydrate synthesis. Analysis of the functional abundance of CAZy enzymes in metagenomes shows the metabolic capabilities of microbial communities, particularly in their ability to process carbohydrates. The same six functional classes of CAZy were defined in both samples (Figure 3B). In kombucha, there is a clear enzymatic advantage in carbohydrate esterases, glycoside hydrolases (GH), glycosyl transferases (GT), and polysaccharide lyases (PL). The result shows more active work of carbohydrate esterases in synergy with GH and PL enzymes to degrade complex polysaccharides in kombucha. Enzymes GH are crucial for microbial carbohydrate metabolism and a well-established set of microorganisms in the kombucha L4-100 (FP), breaking down complex sugars into simpler forms (650 genes).
The M. gisevii MI-2 was richer (65 genes) in Auxiliary Activity Enzymes (AA) as FP (35 genes) (Figure 3B). These enzymes can oxidize cellulose, making it more accessible for GH to degrade into simpler sugars, which the microbial community could utilize for fermentation. Carbohydrate-binding modules (CBM) enzymes were almost equal; there is an equal opportunity to improve enzyme access to substrates, particularly for insoluble polysaccharides in both investigated microorganism complexes. In nature, communities adapt to the required source of nutrients and differ in the quantity, quality, and activity of enzymes. CAZy analysis in metagenomes provides insights into how microbial communities process carbohydrates. The composition and ratio of enzymes are essential for understanding ecosystem function, host-microbe interactions, and potential industrial applications [78]. Different groups and abundance of CAZys are required to achieve different biotechnological goals. A high abundance of GH and AA is often observed in soil and plant-associated metagenomes, reflecting the community’s ability to degrade complex polysaccharides like cellulose, hemicellulose, and pectin [79]. Metagenomes from environments such as compost heaps, rumen, or biofuel production sites show a high functional abundance of GH and GT, reflecting microbial communities’ capability to degrade lignocellulosic biomass [80].
3.6. Sensory Evaluation
During kombucha fermentation, organic acids, including acetic acid, are produced, significantly influencing the flavor profile of the beverage. When assessing the characteristics of kombucha, it is crucial to evaluate both its sensory attributes and overall acceptability to gain a comprehensive understanding of its quality. The sensory attributes of color, odor, taste, and overall acceptability for kombucha beverages after 10 days of fermentation are shown in Figure 4. Sea buckthorn leaf extract increased the darkness of the kombucha beverage, and according to accessors, the darkest sample was L4-100. The different combinations of green tea and sea buckthorn leaf extract did not have a significant influence on the alcohol, fruit odors, and transparency attributes. L4-100 kombucha showed the most acceptable aroma. Panelists evaluated this sample with the strongest fruit and tea aromas. Panelists noticed that L4-75 kombucha had the highest sour intensity (score of 6.7) compared with other kombucha, while L4-25 kombucha had the most acceptable sourness, with intensity the lowest (score of 4.8). The highest sweetness intensity (score of 6.1) was noted in L4-100 and G-100; however, L4-100 had the most acceptable sweetness (score of 6.6). This could be related to L4-100 kombucha having the highest glucose content after fermentation, while G-100 exhibited the highest pH and total saccharide content.
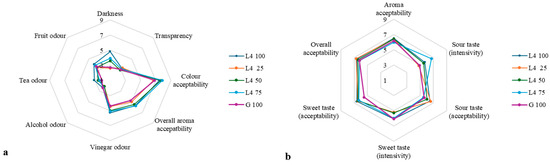
Figure 4.
Sensory properties of kombucha: color, aroma (a), taste, and acceptability (b).
In this study, the most acceptable was L4-25 and L4-100 kombucha, while traditional green tea kombucha was less acceptable for panelists. G. Cohen [81] reported that sweetness intensity had a strong relationship with the overall acceptability of kombucha, while sourness, perception of acetic acid, and yeast flavor have a negative influence on overall acceptability.
These results indicate that sea buckhorn leaves could be used as an alternative material for kombucha preparation.
4. Conclusions
This study introduces a novel application of PCSL extraction for isolating biologically active compounds from sea buckthorn leaves. Six different extracts of sea buckthorn leaves were obtained with varying numbers of extraction cycles and durations. The highest amount of biologically active compounds (5.51 gCE/L of proanthocyanidins and 12.42 gGAE/ L phenolic compounds) was observed in extracts obtained using 60 cycles, each lasting 10 min. However, shorter extraction resulted in lower concentrations of biologically active compounds. The reduced extraction time is more environmentally friendly. Therefore, the L-4 extract, obtained through PCSL extraction with 20 cycles of 2 min each, was selected for kombucha preparation. The highest concentration of phenolic compounds and lowest antioxidant changes during fermentation were observed in sea buckthorn leaf kombucha L4-100, while the lowest phenolic compound content and highest antioxidant changes during fermentation were found in green tea kombucha G-100. Microbial analysis revealed that M. gisevii MI-2 supports diverse and active microbiota beneficial for fermentation. The culture of M. gisevii MI-2 has a broad microbial diversity (227 bacteria and 44 eucaryotes) suitable for fermented kombucha beverages. Most of them (77.8% eukaryote and 92.5% bacteria) have a microbiological impact and can migrate and stay in the fermented drink. Evaluation of microbial safety demonstrated that kombucha with sea buckthorn leaf extract had stronger antimicrobial properties against tested pathogens compared to traditional green tea kombucha. Furthermore, sensory analysis revealed greater consumer acceptability for kombucha beverages enriched with sea buckthorn leaf extracts.
Considering the obtained results, secondary raw materials such as sea buckthorn leaves are not only nutritional materials but also could be used as bioactive compound sources. This approach highlights the potential for utilizing underappreciated biomass in creating functional foods that contribute to a more sustainable and circular economy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126608/s1.
Author Contributions
Conceptualization, K.A., J.J. and I.M.; methodology, K.A., J.J. and I.M.; validation, K.A., J.J. and I.M.; formal analysis, K.A.; investigation, K.A., J.J., J.G. and I.M.; resources, A.Š. and K.A.; data curation, K.A.; writing—original draft preparation, K.A., J.J. and I.M.; writing—review and editing, K.A.; visualization, K.A., J.J. and I.M.; supervision, K.A.; project administration, A.Š.; funding acquisition, A.Š., J.J., I.M. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the Lithuanian Rural Development Program 2014–2020 under the activity “Support for the creation and development of EIP activity groups” project “Circular manufacturing model for producing biologically active material” No. 35BV-KK-22-1-05005-PR001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analyzed within the frame of the present investigation are included in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Auxiliary Activity |
| AAB | acetic acid bacteria |
| AACC | American Association of Cereal Chemists |
| AOAC | Association of Official Analytical Collaboration |
| CAZy | Carbohydrate-Active Enzymes |
| CBM | Carbohydrate-binding modules |
| CE | (+)-catechin equivalent |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EFSA | European Food Safety Authority |
| FP | fermented product |
| GAE | gallic acid equivalents |
| GH | glycoside hydrolases |
| GT | glycosyl transferases |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LAB | lactic acid bacteria |
| TPC | Total phenolic content |
| MG | Medusomyces gisevii MI-2 |
| PCSL | Pressurized Cyclic Solid–Liquid |
| PL | polysaccharide lyases |
| Scoby | symbiotic culture of bacteria and yeast |
| TSS | Total soluble solids |
References
- Gâtlan, A.; Gutt, G. Sea buckthorn in plant based diets. An analytical approach of sea buckthorn fruits composition: Nutritional value, applications, and health benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef] [PubMed]
- Čulina, P.; Repajić, M.; Elez Garofulić, I.; Dragović-Uzelac, V.; Pedisić, S. Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Leaf and Berry Extracts Obtained via Optimized Microwave-Assisted and Accelerated Solvent Extraction. Processes 2024, 12, 126. [Google Scholar] [CrossRef]
- Sanwal, N.; Mishra, S.; Sharma, N.; Sahu, J.K.; Raut, P.K.; Naik, S.N. Evaluation of the phytoconstituents and bioactivity potentials of Sea buckthorn (Hippophae sp.) leaves using GC-MS, HPLC-PDA and ICP-MS: A gender-based comprehensive metabolic profiling. J. Food Meas. Charact. 2023, 17, 2767–2781. [Google Scholar] [CrossRef]
- Żuchowski, J. Phytochemistry and pharmacology of sea buckthorn (Elaeagnus rhamnoides; syn. Hippophae rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2022, 22, 3–33. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, J.; Zheng, L.; Xu, W.; Xie, Y. Mechanochemical-Assisted Extraction and Hepatoprotective Activity Research of Flavonoids from Sea Buckthorn (Hippophaë rhamnoides L.) Pomaces. Molecules 2021, 26, 7615. [Google Scholar] [CrossRef]
- Zarrelli, A.; Pollio, A.; Aceto, S.; Romanucci, V.; Carella, F.; Stefani, P.; De Natale, A.; De Vico, G. Optimisation of artemisinin and scopoletin extraction from Artemisia annua with a new modern pressurised cyclic solid–liquid (PCSL) extraction technique. Phytochem. Anal. 2019, 30, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Culurciello, R.; Bosso, A.; Di Fabio, G.; Zarrelli, A.; Arciello, A.; Carella, F.; Leonardi, L.; Pazzaglia, L.; De Vico, G.; Pizzo, E. Cytotoxicity of an innovative pressurised cyclic solid–liquid (pcsl) extract from Artemisia annua. Toxins 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.; Mutukumira, A.N. Kombucha: Production and Microbiological research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef]
- European Food Safety Authority. EFSA Statement on the Requirements for Whole Genome Sequence Analysis of Microorganisms Intentionally Used in the Food Chain. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6506 (accessed on 28 July 2024).
- La Reau, A.J.; Strom, N.B.; Filvaroff, E.; Mavrommatis, K.; Ward, T.L.; Knights, D. Shallow shotgun sequencing reduces technical variation in microbiome analysis. Sci. Rep. 2023, 13. [Google Scholar] [CrossRef]
- Jarrell, J.; Cal, T.; Bennett, J. The Kombucha consortia of yeasts and bacteria. Mycologist 2000, 14, 166–170. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- ISO 12966-2:2011; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl Esters. Preparation of Methyl esters of Fatty Acids. ISO: Geneva, Switzerland, 2011.
- Megazyme. Total Dietary Fiber Assay Procedure. 2017. Available online: https://d1kkimny8vk5e2.cloudfront.net/documents/Assay_Protocol/K-TDFR-200A_DATA.pdf (accessed on 15 February 2024).
- BS EN 15621:2017; Animal Feeding Stuffs: Methods of Sampling and Analysis. Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Sulphur, Iron, Zinc, Copper, Manganese and Cobalt After Pressure Digestion by ICP-AES. European Standards s.r.o.: Pilsen, Czech Republic, 2017. Available online: https://www.en-standard.eu/bs-en-15621-2017-animal-feeding-stuffs-methods-of-sampling-and-analysis-determination-of-calcium-sodium-phosphorus-magnesium-potassium-sulphur-iron-zinc-copper-manganese-and-cobalt-after-pressure-digestion-by-icp-aes/ (accessed on 15 February 2024).
- Naviglio, D. Naviglio’s principle and presentation of an innovative Solid–Liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Gallo, M.; Formato, A.; Giacco, R.; Riccardi, G.; Luongo, D.; Formato, G.; Amoresano, A.; Naviglio, D. Mathematical optimization of the green extraction of polyphenols from grape peels through a cyclic pressurization process. Heliyon 2019, 5, e01526. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Biosa, G.; Zayed, H.; Abou-Saleh, H.; Cossu, A.; Nasrallah, G.K.; Giordo, R.; Pagnozzi, D.; Porcu, M.C.; Pretti, L.; et al. Protective Effect of Cyclically Pressurized Solid–Liquid Extraction Polyphenols from Cagnulari Grape Pomace on Oxidative Endothelial Cell Death. Molecules 2018, 23, 2105. [Google Scholar] [CrossRef]
- Bate-Smith, E. Astringent tannins of the leaves of Geranium species. Phytochemistry 1981, 20, 211–216. [Google Scholar] [CrossRef]
- Gaižauskaitė, Ž; Klavins, L.; Almonaityte, K. Optimised extraction of bioactive compounds from spruce needles for sustainable applications. Waste Manag. 2025, 201, 114784. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gülhan, M.F. A New Substrate and Nitrogen Source for Traditional Kombucha Beverage: Stevia rebaudiana Leaves. Appl. Biochem. Biotechnol. 2023, 195, 4096–4115. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Almeida, M.; Juncker, A.S.; Rasmussen, S.; Li, J.; Sunagawa, S.; Plichta, D.R.; Gautier, L.; Pedersen, A.G.; Chatelier, E.L.; et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat. Biotechnol. 2014, 32, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Luo, R.; Sadakane, K.; Lam, T. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2014, 12, 59–60. [Google Scholar] [CrossRef]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2016, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ebersole, B.; Liu, Y.; Schmidt, R.; Eckert, M.; Brown, P.N. Determination of ethanol in kombucha products: Single-laboratory validation, first action 2016.12. J. AOAC Int. 2017, 100, 732–736. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Characterization of antimicrobial activity in Kombucha fermentation. Acta Biotechnol. 2001, 21, 49–56. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- ISO 6658:2017; Sensory Analysis—Methodology—General Guidance 2017. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Bośko, P.; Biel, W.; Witkowicz, R.; Piątkowska, E. Chemical composition and nutritive value of sea buckthorn leaves. Molecules 2024, 29, 3550. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Panaite, T.D.; Varzaru, I.; Oancea, A.; Turcu, R.P.; Vlaicu, P.A. Effects of Supplementing Sea Buckthorn Leaves (Hippophae rhamnoides L.) and Chromium (III) in Broiler Diet on the Nutritional Quality and Lipid Oxidative Stability of Meat. Antioxidants 2022, 11, 2220. [Google Scholar] [CrossRef]
- Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [CrossRef]
- Joint, F.A.O. Human Vitamin and Mineral Requirements. 2002. Available online: https://www.who.int/publications/i/item/9241546123 (accessed on 15 July 2024).
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Triterpenoids, phenolic compounds, macro- and microelements in anatomical parts of sea buckthorn (Hippophaë rhamnoides L.) berries, branches and leaves. J. Food Compos. Anal. 2021, 103, 104107. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae rhamnoides L.) Varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef] [PubMed]
- Bośko, P.; Biel, W.; Smetanska, I.; Witkowicz, R.; Piątkowska, E. Sea buckthorn leaves as a potential source of antioxidant substances. Appl. Sci. 2024, 14, 5038. [Google Scholar] [CrossRef]
- Ma, P.; Li, Z.; Jin, Y.; Zuo, J.; Zhang, Y.; Dong, A.; Xiao, D.; Burenjargal, M. Green and efficient extraction process of flavonoids from sea buckthorn fruits by natural deep eutectic solvents aided with ultrasound. Microchem. J. 2024, 205, 111265. [Google Scholar] [CrossRef]
- Jung, H.; Cho, H.; Hwang, K.T.; Lee, B.; Yi, H.C.; Cho, E. Antioxidant activities of sea buckthorn leaf tea extracts compared with green tea extracts. Food Sci. Biotechnol. 2014, 23, 1295–1303. [Google Scholar] [CrossRef]
- He, Q.; Yang, K.; Wu, X.; Zhang, C.; He, C.; Xiao, P. Phenolic compounds, antioxidant activity and sensory evaluation of sea buckthorn (Hippophae rhamnoides L.) leaf tea. Food Sci. Nutr. 2022, 11, 1212–1222. [Google Scholar] [CrossRef]
- Kilic, G.; Sengun, I.Y. Bioactive properties of Kombucha beverages produced with Anatolian hawthorn (Crataegus orientalis) and nettle (Urtica dioica) leaves. Food Biosci. 2023, 53, 102631. [Google Scholar] [CrossRef]
- Xiong, R.; Wu, S.; Cheng, J.; Saimaiti, A.; Liu, Q.; Shang, A.; Zhou, D.; Huang, S.; Gan, R.; Li, H. Antioxidant Activities, Phenolic Compounds, and Sensory Acceptability of Kombucha-Fermented Beverages from Bamboo Leaf and Mulberry Leaf. Antioxidants 2023, 12, 1573. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef]
- Leonarski, E.; Guimarães, A.C.; Cesca, K.; Poletto, P. Production process and characteristics of kombucha fermented from alternative raw materials. Food Biosci. 2022, 49, 101841. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of fermentation on bioactivity and the composition of polyphenols contained in Polyphenol-Rich foods: A review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Yang, T.; Mac Regenstein, J.; Zhou, P. Functional properties and sensory characteristics of kombucha analogs prepared with alternative materials. Trends Food Sci. Technol. 2022, 129, 608–616. [Google Scholar] [CrossRef]
- Silva, K.A.; Uekane, T.M.; De Miranda, J.F.; Ruiz, L.F.; Da Motta, J.C.B.; Silva, C.B.; De Souza Pitangui, N.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha beverage from non-conventional edible plant infusion and green tea: Characterization, toxicity, antioxidant activities and antimicrobial properties. Biocatal. Agric. Biotechnol. 2021, 34, 102032. [Google Scholar] [CrossRef]
- Chu, S.; Chen, C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2005, 98, 502–507. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, Y.; Wang, S.; Zou, Y.; Sun, Z.; Yuan, L. Changes on physiochemical properties and volatile compounds of Chinese kombucha during fermentation. Food Biosci. 2023, 55, 103029. [Google Scholar] [CrossRef]
- Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Modelling pH dynamics, SCOBY biomass formation, and acetic acid production of kombucha fermentation using black, green, and oolong teas. Processes 2024, 12, 1301. [Google Scholar] [CrossRef]
- Nummer, B.A. Kombucha Brewing Under the Food and Drug Administration Model Food Code: Risk Analysis and Processing Guidance. J. Environ. Health 2013, 76, 8–11. Available online: https://www.jstor.org/stable/26329709 (accessed on 15 April 2024). [PubMed]
- Malbaša, R.; Lončar, E.; Djurić, M.; Došenović, I. Effect of sucrose concentration on the products of Kombucha fermentation on molasses. Food Chem. 2007, 108, 926–932. [Google Scholar] [CrossRef]
- Ayed, L.; Abid, S.B.; Hamdi, M. Development of a beverage from red grape juice fermented with the Kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Battikh, H.; Chaieb, K.; Bakhrouf, A.; Ammar, E. Antibacterial and antifungal activities of black and green kombucha teas. J. Food Biochem. 2012, 37, 231–236. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Bhattacharya, S.; Patra, M.M.; Chakravorty, S.; Sarkar, S.; Chakraborty, W.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef]
- Janceva, S.; Andersone, A.; Lauberte, L.; Bikovens, O.; Nikolajeva, V.; Jashina, L.; Zaharova, N.; Telysheva, G.; Senkovs, M.; Rieksts, G.; et al. Sea Buckthorn (Hippophae rhamnoides) Waste Biomass after Harvesting as a Source of Valuable Biologically Active Compounds with Nutraceutical and Antibacterial Potential. Plants 2022, 11, 642. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gu, J.D. Ecological distribution and potential roles of Woesearchaeota in anaerobic biogeochemical cycling unveiled by genomic analysis. Comput. Struct. Biotechnol. J. 2021, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Sowa, S.; Paczos-Grzęda, E. Virulence Structure of Puccinia coronata f. sp. avenae and Effectiveness of Pc Resistance Genes in Poland During 2017–2019. Phytopathology 2020, 111, 1158–1165. [Google Scholar] [CrossRef]
- DiGuistini, S.; Wang, Y.; Liao, N.Y.; Taylor, G.; Tanguay, P.; Feau, N.; Henrissat, B.; Chan, S.K.; Hesse-Orce, U.; Alamouti, S.M.; et al. Genome and transcriptome analyses of the mountain pine beetle-fungal symbiont Grosmannia clavigera, a lodgepole pine pathogen. Proc. Natl. Acad. Sci. USA 2011, 108, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Kligun, E.; Ostretsov, B.; Titievsky, A.; Farkov, M.; Alamouti, S.M.; Brodsky, L. Adaptation of the pine fungal pathogen Grosmannia clavigera to monoterpenes: Biochemical mechanisms revealed by RNA-seq analysis. For. Pathol. 2017, 47, e12372. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Song, D.; Chen, X.; Xu, M. Characteristics and functional analysis of the secondary chromosome and plasmids in sphingomonad. Int. Biodeterior. Biodegrad. 2022, 171, 105402. [Google Scholar] [CrossRef]
- Jiang, Z.; Deng, Y.; Han, X.; Su, J.; Wang, H.; Yu, L.; Zhang, Y. Corrigendum: Geminicoccus flavidas sp. nov. and Geminicoccus harenae sp. nov., two IAA-producing novel rare bacterial species inhabiting desert biological soil crusts. Front. Microbiol. 2023, 14, 1285950. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.M.; Rossmann, F.M.; Ferreira, J.L.; Matthews-Palmer, T.R.; Beeby, M.; Pallen, M.J. Bacterial flagellins: Does size matter? Trends Microbiol. 2017, 26, 575–581. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Lignocellulosic conversion into value-added products: A review. Process Biochem. 2019, 89, 110–133. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of metabolic activity, beneficial and Pathogenic aspects of Burkholderia spp. Metabolites 2021, 11, 321. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef]
- Gostinčar, C.; Zalar, P.; Gunde-Cimerman, N. No need for speed: Slow development of fungi in extreme environments. Fungal Biol. Rev. 2021, 39, 1–14. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, X.L.; Han, K.S.; Koh, Y.J.; Hur, J. Taxonomic study on the lichen Genus Coccocarpia (Lecanorales, ascomycota) in South Korea. Mycobiology 2007, 35, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Içen, H.; Corbo, M.R.; Sinigaglia, M.; Korkmaz, B.I.O.; Bevilacqua, A. Microbiology and antimicrobial effects of kombucha, a short overview. Food Biosci. 2023, 56, 103270. [Google Scholar] [CrossRef]
- Harrison, K.; Curtin, C. Microbial Composition of SCOBY Starter Cultures Used by Commercial Kombucha Brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2013, 42, D490–D495. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Z.; Zhong, Z.; Li, Q.; Bian, F.; Yang, C. Metagenomic insights into the characteristics of soil microbial communities in the decomposing biomass of Moso bamboo forests under different management practices. Front. Microbiol. 2022, 13, 1051721. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Sahoo, K.; Gaur, M.; Sahoo, R.K.; Dey, S.; Gupta, V.K.; Subudhi, E. A meta-omics approach to explore the biofuel-producing enzyme potential from extreme environmental conditions. Renew. Sustain. Energy Rev. 2023, 186, 113670. [Google Scholar] [CrossRef]
- Cohen, G.; Sela, D.A.; Nolden, A.A. Sucrose concentration and fermentation temperature impact the sensory characteristics and liking of kombucha. Foods 2023, 12, 3116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).