Abstract
Probiotics play a pivotal role in functional food development owing to their distinct health-promoting properties. This review comprehensively examines probiotics’ classifications and functional mechanisms and their roles in modulating intestinal microbiota, enhancing immunity, and intervening in metabolic diseases. The diverse applications of probiotics in dairy and meat products are examined alongside technological innovations, including microencapsulation, biofilm systems, and personalized strain screening that have been employed to enhance probiotic stability and efficacy in functional foods. We analyze safety concerns and regulatory challenges, emphasizing the need for rigorous pre-market evaluation and international regulatory harmonization. This study aims to review the existing scientific evidence on the application of probiotics in functional foods, providing a theoretical reference for the development of the next generation of high-quality functional foods.
1. Introduction
With continuous improvements in quality of life and increasing attention being paid to healthy living and nutrition, probiotics have gained popularity owing to their unique prebiotic properties and health benefits [1]. By the joint expert committees of the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), probiotics are defined as “live microorganisms that benefit the health of their hosts when taken in moderation” [2]. Probiotics, alternatively termed live bacterial preparations, microecological regulators, ecological products, or biotin promoters [3]. They colonize the intestinal microecological system and, through multiple interactions, collectively maintain homeostasis in the body.
Functional foods are foods that have potential health benefits beyond their basic nutritional value [4]. The health benefits of functional foods stem from their bioactive compound composition, which may be naturally occurring, formed during industrial processing, or extracted and added from other sources [5]. Probiotics, as essential functional ingredients, play a pivotal role in functional food manufacturing [6]. In recent years, the development and application of probiotics in functional foods have emerged as a significant focus in functional food research, while the global probiotic functional food market has undergone rapid expansion. According to Blue Weave data, the global market size was USD 60.23 billion in 2022, while China, as the second-largest market, had a market size of CNY 109.38 billion in the same year [7]. In the post-pandemic era, consumer demand for immune health has surged, further driving market expansion. For example, global sales of respiratory immune probiotics experienced a 45% surge in 2024 [8]. Furthermore, the global probiotic functional food market is projected to exceed USD 80 billion by 2025, exhibiting a compound annual growth rate of 8.5% during the forecast period [9]. With the increasing global burden of metabolic disorders and immune-related diseases [10], probiotics, as a scientifically validated natural intervention, have demonstrated their potential as an adjunctive strategy. Probiotics have demonstrated a positive impact on type 2 diabetes mellitus (T2DM) through mechanisms such as improving insulin sensitivity and modulating glucose metabolism [11]. The application scope of probiotics in functional foods is gradually expanding, extending from traditional fermented dairy products to diverse sectors, including fruit and vegetable products and meat products. Concurrently, the development of innovative technologies such as microencapsulation [12] and biofilm delivery systems [13] has effectively addressed probiotic stability challenges in practical applications, thereby expanding the potential applications of probiotics in functional foods.
This review comprehensively summarizes the latest developments in probiotic mechanisms and technological innovations in strain stabilization and provides a detailed introduction to the widespread application of probiotics in the field of functional foods. Furthermore, it comprehensively analyzes the safety assessment framework and regulatory discrepancies to achieve standardization in probiotic development for functional foods. With the continuous advancement of science and technology and the increasing market demand, the potential applications of probiotics in the field of functional foods are expected to expand, thereby bringing more benefits to human health.
2. Probiotic Strains, Sources, and Isolation Methods
The human body hosts over 400 types of probiotics, which can mostly be categorized into five major genera: Lactobacillus, Bifidobacterium, Bacillus, Streptococcus, and others (Table 1). Systematic taxonomy reveals their taxonomic distribution: Lactobacillus, Streptococcus, and Bacillus belong to the phylum Firmicutes; Bifidobacterium belongs to the phylum Actinobacteria; and Saccharomyces belongs to the phylum Ascomycota in the kingdom Fungi [14]. Currently, 56 species of Lactobacillus (of which more than 10 are in everyday use), more than 32 species of Bifidobacterium (14 of which are closely related to humans), more than 150 species of Bacillus (with more than a dozen in common use), and more than 60 species of Streptococcus, including various strains and subspecies, have been reported and classified.
Familiar sources of probiotics include the following: first, the human gut is the primary habitat for many probiotics, such as Bifidobacterium and Lactobacillus [15]. These symbiotic microbial communities play a crucial role in maintaining host health. Fermented foods are an important dietary source of probiotics. During the fermentation process involving specific microorganisms, changes occur in the flavor and texture of the food matrix, and a large number of active probiotics are enriched. Typical fermented foods include yogurt and fermented dairy products. Consuming these foods is an effective way to obtain active probiotics. Certain probiotic strains can also be isolated from natural environments, such as soil and water bodies. These environmentally sourced probiotics play an indispensable role in maintaining biochemical cycles and balance in the microbial community in their native ecosystems [16]. However, environmental isolates must undergo rigorous assessment, screening, and safety validation to confirm their probiotic properties before they can be used in human or animal health applications.
The development of probiotic isolation technology has progressed from traditional methods to modern multi-technology integration. The most basic and widely used method is the traditional plate isolation method, which involves diluting samples in a gradient and spreading them on selective culture media, followed by screening based on colony morphological characteristics. However, its significant limitations include low efficiency in isolating slow-growing strains and an inability to accurately reflect the true diversity of microbial communities. To address these shortcomings, optimized dilution plating and gradient dilution methods improved screening efficiency, but they still could not overcome the fundamental limitations of traditional culture techniques. To break through this bottleneck, molecular biology techniques have brought about a methodological revolution. Specifically, 16S rRNA gene sequencing directly analyzes the microbial composition of a sample [17], while metagenomics technology analyzes functional gene information to guide the targeted isolation of strains with specific functions [18]. Meanwhile, innovations in physical separation technologies have significantly enhanced single-cell manipulation capabilities. Microfluidic chips simulate the intestinal microenvironment to capture active bacterial cells [19], and flow cytometry combined with fluorescently labeled probes enables high-precision sorting at the single-cell level [20]. In recent years, the development of function-driven platforms has further driven technological innovation. High-throughput phenotypic screening systems can simultaneously assess the probiotic characteristics of thousands of strains, including acid tolerance and adhesion ability [21]. Notably, culturomics has successfully increased the cultivability rate of human gut microbiota to over 70% by optimizing combinations of cultivation conditions [22]. Looking ahead, probiotic isolation technology will continue to evolve toward multi-dimensional intelligent integration systems, ultimately establishing an efficient and targeted probiotic resource development framework.

Table 1.
Common probiotics and their representative genera.
Table 1.
Common probiotics and their representative genera.
| Category | Representative Bacteria | Reference |
|---|---|---|
| Lactobacillus | Lactobacillus gasseri, Lactobacillus fermentum, Lactobacillus delbrueckii, Lactobacillus plantarum, Lactobacillus salivarius and Lactobacillus reuteri, etc. | [23] |
| Bifidobacterium | Bifidobacterium bifidum, Bifidobacterium thermophilum, Bifidobacterium adolescentis, Bifidobacterium infantis and Bifidobacterium breve, etc. | [24] |
| Bacillus | Bacillus amyloliquefaciens, Bacillus cereus, Bacillus circulans, Bacillus licheniformis, Bacillus sphaericus, Bacillus subtilis and Bacillus thuringiensis, etc. | [25] |
| Streptococcus | Streptococcus thermophilus and Streptococcus salivarius, etc. | [26] |
| Other | Pediococcus pentosaceus, Lactococcus lactis, Saccharomyces cerevisiae and Saccharomyces boulardii, etc. | [27] |
3. The Function of Probiotics
Probiotics exert multifaceted health benefits through interactions with the human gastrointestinal tract and the regulation of systemic physiological processes. Their core functions include regulating the intestinal microbiota balance, enhancing intestinal epithelial barrier function, modulating local and systemic immune responses, and mediating metabolic pathways [28]. These functions are not isolated but are closely intertwined and mutually reinforcing through the metabolic activities of probiotics. Collectively, they maintain intestinal homeostasis and promote overall health, as shown in Figure 1. When probiotics are used in combination with prebiotics, their core mechanisms synergistically enhance each other, forming a comprehensive strategy for disease prevention and health promotion with broad application prospects. The specific roles of probiotics in the aforementioned functional areas, as well as their potential molecular pathways, are systematically discussed in subsequent discussions.
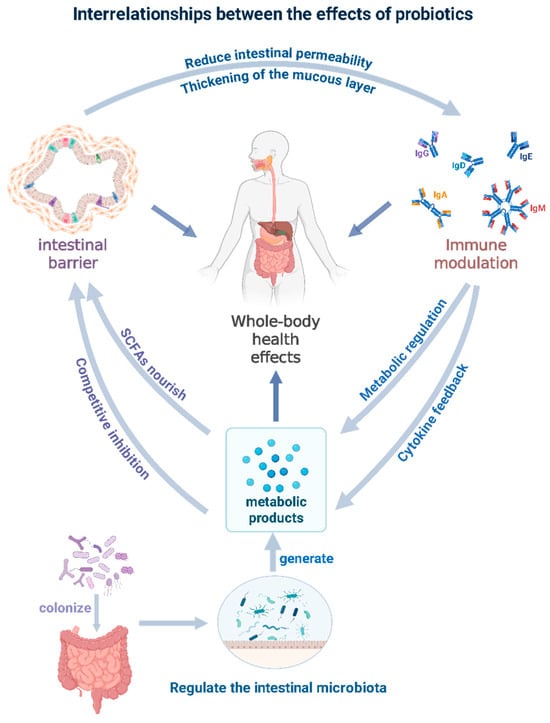
Figure 1.
Interrelationships between the effects of probiotics.
3.1. Regulating the Balance of Intestinal Flora
Intestinal flora balance is critical for intestinal homeostasis. The primary mechanism by which probiotics regulate intestinal balance is through competitive exclusion, a natural phenomenon involving competition for nutrients and adhesion sites [29]. On the one hand, probiotics express adhesins on their surface, which specifically bind to glycoprotein receptors on intestinal epithelial cells, thereby inhibiting the colonization of pathogenic bacteria [30]. On the other hand, probiotics efficiently absorb limited nutrients in the intestine, thereby depriving pathogens of essential resources, competitively inhibiting the growth of harmful bacteria, reducing the production of harmful substances, and maintaining intestinal microbiota balance [31]. Wang et al. [32] evaluated the inhibitory effects of chicken-derived lactic acid bacteria (LAB) on intestinal pathogens, such as Escherichia coli and Salmonella spp. The results showed that P. pentosaceus 2–5 and L. reuteri L-3 significantly reduced the adherence of pathogenic bacteria to Caco-2 cells in competition, exclusion, and replacement assays, suggesting that they can reduce the colonization of harmful bacteria through competitive inhibition mechanisms.
In addition, probiotics can secrete various metabolic substances with regulatory effects, thereby directly inhibiting the growth of pathogenic bacteria. First, probiotics can secrete bacteriocins, which effectively inhibit the growth of pathogenic bacteria. For example, Lactococcus lactis produces nisin, whose N-terminal domain binds to the pyrophosphate group of the bacterial cell wall precursor (Lipid II), thereby blocking the glycosylation reaction of the peptidoglycan chain and interrupting cell wall synthesis. Simultaneously, Lipid II serves as an anchor to fix nisin to the membrane, allowing its C-terminal amphiphilic helix to insert into the membrane phospholipid layer and flip over. This ultimately leads to the formation of transmembrane pores through the coordinated assembly of multiple molecules, thereby disrupting the integrity of the cell membrane and killing pathogenic bacteria [33]. Probiotics can also directly disrupt the collective cooperation and surface colonization capabilities of pathogenic bacteria by secreting hydrogen peroxide (H2O2). H2O2 oxidizes quorum sensing (QS) signaling molecules, thereby inactivating them and blocking interbacterial communication, which in turn inhibits the expression of virulence factors. Furthermore, H2O2 oxidizes the conserved cysteine residues in the GGDEF domain of the active center of di-guanosine cyclase, thereby inactivating it and upregulating phosphodiesterase expression, which leads to a sharp decrease in c-di-GMP levels. This both inhibits the expression of extracellular polysaccharide synthesis genes and blocks the mechanism of biofilm formation, thereby effectively inhibiting the biofilm formation ability of pathogenic bacteria [34]. Second, probiotics produce organic acids, which directly lower the intestinal pH, thereby inhibiting the growth and reproduction of pathogenic bacteria. Short-chain fatty acids (SCFAs) play a major role in this process. SCFAs are key metabolic products produced by probiotics through the fermentation of dietary fiber, primarily including acetate, propionate, and butyrate [35]. On one hand, the production of SCFAs significantly lowers the pH of the intestinal contents. On the other hand, acetate and lactate can be directly utilized by beneficial bacteria, such as butyrate-producing bacteria, promoting their growth and metabolic activities. As the population of beneficial bacteria expands, it inhibits the excessive proliferation of pathogens, thereby effectively maintaining microbial balance. Butyrate is a potent histone deacetylase (HDAC) inhibitor. It competitively binds to the zinc ion active site in the HDAC catalytic domain, thereby blocking its ability to remove acetyl groups from histone lysine residues and leading to increased histone acetylation within cells. This modification triggers chromatin structural remodeling into an open conformation, enhancing DNA accessibility, thereby promoting the binding of transcription factors to regulatory elements and activating the expression of key genes previously silenced by epigenetic mechanisms. These genes include tumor suppressor genes, anti-inflammatory genes, intestinal barrier function genes, and immune regulatory genes, which collectively coordinate and strengthen the body’s physiological defense functions [36]. Notably, unlike some common probiotics, Saccharomyces boulardii does not produce SCFAs or lactic acid in its metabolic pathways. Instead, it directly hydrolyzes and inactivates toxins A and B through the secretion of a 54 kDa serine protease, thereby protecting intestinal epithelial cells from damage [37].
3.2. Enhancement of Intestinal Barrier Function
The intestinal barrier is composed of mucosal epithelial cells, tight junction proteins, the mucus layer, and intestinal flora [38]. The gastrointestinal barrier is a key defense mechanism for maintaining epithelial integrity and preventing pathogen infection and excessive inflammation [39]. On the one hand, by increasing the expression of tight junction proteins, the intercellular gaps are reduced to 0.3–0.8 nm, thereby reducing intestinal permeability [40]. On the other hand, it directly stimulates goblet cells to secrete mucin (MUC2), which promotes the thickening of the mucus layer [41] and the proliferation and repair of epithelial cells [42], thereby enhancing the integrity of the mechanical barrier. Experimental evidence indicates that probiotic supplementation can increase intestinal mucus thickness by 30–50% [43]. Some strains reduce intestinal barrier damage by neutralizing pathogen toxins and harmful metabolites [44]. The lipopolysaccharide (LPS) fragment expressed by Escherichia coli Nissle 1917 (EcN) mimics the structure of the Shiga toxin receptor Gb3, causing the B subunit of Shiga toxin to be misrecognized and to specifically bind to EcN’s LPS, thereby firmly fixing the toxin to the EcN surface. The toxin–EcN complex is then expelled from the body via intestinal peristalsis, thereby protecting the host from toxin-mediated damage [45].
The surface molecules of probiotics significantly modulate intestinal barrier function through interactions with host intestinal epithelial cells. For example, surface-layer proteins of probiotics competitively inhibit pathogen adhesion and enhance intestinal epithelial integrity by regulating tight-junction proteins and cytoskeletal distribution [46]. Flagellin is recognized by toll-like receptor 5 (TLR5) on the intestinal epithelial cell membrane and activates the downstream PI3K/Akt signaling pathway through the adapter protein myeloid differentiation primary response 88 (MyD88). The TLR5–MyD88 complex recruits phosphoinositide 3-kinase (PI3K), which catalyzes the production of the second messenger phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 binds to and recruits protein kinase B (Akt) to the cell membrane, where it is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase 1 and mTOR complex 2. Activated phosphorylated Akt phosphorylates and inhibits the transcription factor FOXO1 while activating the transcription factors cAMP response element-binding protein and activator protein 1 (AP-1), thereby significantly promoting the transcription of antimicrobial peptides and exerting anti-inflammatory and antimicrobial effects [47]. In addition, flagellar proteins activate the pattern recognition receptor TLR4/MD2 complex by binding to glycoproteins on the surface of intestinal epithelial cells. This interaction triggers intracellular MyD88- and TRIF-dependent signaling pathways and induces the activation of transcription factors (nuclear factor kappa-B (NF-κB), AP-1, and interferon regulatory factor 3), thereby promoting intestinal barrier function and mucosal immunity [48]. Podocarp polysaccharides interact with host receptors to regulate cytokine secretion and reduce intestinal inflammation [49]. These surface molecules bind specifically to host receptors, activate downstream signaling pathways, and promote intestinal health by enhancing the tight junctions of intestinal epithelial cells and promoting mucus secretion while inhibiting pathogen adhesion and invasion, ultimately maintaining the integrity of the intestinal barrier [50].
3.3. Immunomodulatory Function
The intestinal tract is the largest immune organ in the human body, where approximately 70–80% of immune cells are concentrated [51]. Probiotics not only modulate the immune system locally in the gut but also influence the systemic immune response through various mechanisms. Benjamin et al. [52] described two ways in which probiotics activate the immune system. The first involves probiotics passing through the intestinal wall as living cells or undergoing limited proliferation within the intestinal tract; the second involves dead probiotics releasing antigens absorbed by the intestinal tract and directly stimulating the host’s immune system. Probiotics enhance host immunity by activating immune cells, including dendritic cells, macrophages, and lymphocytes, while modulating the production of anti-inflammatory and pro-inflammatory cytokines, thereby influencing both innate and adaptive immune responses [53]. On the one hand, LPS on the surface of probiotics activates toll-like receptor 2 (TLR2) negative regulatory proteins, thereby blocking the recruitment of MyD88 signal transduction proteins and inhibiting the activation of pro-inflammatory signaling pathways [54]. On the other hand, the metabolic products SCFAs stabilize inhibitors of κB alpha (IκBα) via HDAC, thereby blocking the phosphorylation of IκBα by iκB kinase and effectively preventing the nuclear translocation and activation of NF-κB. Additionally, SCFAs induce inhibitory histone methylation in the promoter regions of pro-inflammatory genes and upregulate peroxisome proliferator-activated receptor gamma expression, which competitively binds to NF-κB, thereby comprehensively reducing the secretion of pro-inflammatory factors and maintaining immune homeostasis [55]. Additionally, probiotics inhibit tumor necrosis factor-alpha-mediated (TNF-α) apoptosis by activating anti-apoptotic factors and Akt [56]. Chae et al. [57] induced colitis in mice using dextran sodium sulfate (DSS), resulting in significantly elevated TNF-α levels in colon tissue. The subsequent administration of Bifidobacterium bifidum BB12 effectively alleviated colitis symptoms. This intervention alleviated TNF-α-mediated intestinal epithelial cell apoptosis, enhanced the survival rate of intestinal epithelial cells, and promoted the balance of the intestinal immune environment. The metabolic products of probiotics, specifically SCFAs, promote the differentiation of regulatory T cells via multiple mechanisms. They inhibit Th2 overreactions, such as allergen sensitization, and enhance Th1 proinflammatory capabilities, thereby reducing intestinal inflammation and promoting immune homeostasis [58].
3.4. Metabolic Disease Intervention
Metabolic diseases, including obesity, T2DM, non-alcoholic fatty liver disease (NAFLD), and hypertension, have emerged as major public health challenges globally [59]. To systematically assess the effects of oral probiotics on these diseases, Koutnikova et al. [60] reviewed 105 randomized controlled trials involving 6826 subjects sourced from the Medline, EMBASE, and Cochrane databases. The study results demonstrated that probiotics significantly reduced body weight, BMI, waist circumference, body fat mass, and visceral adipose tissue mass in overweight but non-obese individuals. Additionally, in patients with T2DM, probiotics significantly reduced fasting blood glucose, glycosylated hemoglobin, and insulin levels. In patients with NAFLD, probiotics significantly lowered alanine aminotransferase and aspartate aminotransferase levels. These ameliorative effects were particularly prominent in mixtures containing Bifidobacterium (e.g., B. breve, B. longum), S. Thermophilus, and Lactobacillus (e.g., L. acidophilus, L. casei, L. delbrueckii).
Probiotics can intervene in metabolic diseases through multiple mechanisms. First, they can activate the Adenosine 5′-monophosphate (AMP)-activated protein kinase pathway via SCFAs to promote fat oxidation and stimulate the secretion of the satiety hormones glucagon-like peptide-1 and peptide YY, thereby reducing appetite [61]. Additionally, probiotics stimulate Glucagon-like peptide-1 secretion via SCFA-mediated signaling from intestinal L cells, which promotes insulin secretion and inhibits glucagon release, thereby improving glycemic control, as shown in Figure 2 [62]. Simultaneously, probiotics can inhibit α-glucosidase activity, delay carbohydrate absorption, reduce postprandial blood glucose peaks, and further enhance glycemic control in patients with T2DM [63]. To improve NAFLD, probiotics enhance hepatic lipid metabolism by regulating bile acid metabolism, increasing bile acid synthesis and secretion, and promoting cholesterol excretion. Meanwhile, probiotics protect liver cells from damage by reducing oxidative stress and inflammatory responses through their antioxidant and anti-inflammatory effects [64]. Moreover, probiotics exert antihypertensive and cardiovascular protective effects through various mechanisms. Probiotics can regulate the metabolic activity of the intestinal microbiota, promote the production of SCFAs, activate G-protein-coupled receptor 41/43, and subsequently promote the release of nitric oxide, which effectively dilates blood vessels and thereby lowers blood pressure [65]. Concurrently, probiotics can inhibit the activity of angiotensin-converting enzyme and reduce the generation of angiotensin II, further reducing blood pressure [66]. Additionally, by regulating cholesterol metabolism, probiotics reduce lipid deposition on blood vessel walls and lower serum total cholesterol and low-density lipoprotein cholesterol levels, thereby alleviating atherosclerosis and reducing the risk of cardiovascular disease [67].
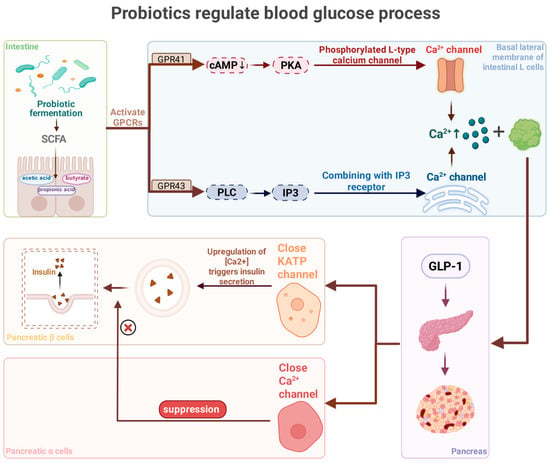
Figure 2.
Probiotic regulation of the blood glucose process.
3.5. The Synergistic Effect of Probiotics and Prebiotics
Prebiotics are food components that are either not digested or are challenging to digest. They selectively stimulate the growth or activity of one or more beneficial gut microbiota, positively impacting host health [68]. The main types include inulin, fructans, fructooligosaccharides, galactooligosaccharides, and resistant starch. When prebiotics are combined with probiotics, this synergistic effect is referred to as “synbiotics” [69]. Prebiotics provide probiotics with fermentation substrates, promoting their proliferation and fermentation metabolism. Probiotics, in turn, regulate intestinal health and positively affect the immune system and metabolic functions of the host [70]. Furthermore, several studies have demonstrated that synbiotics can enhance cognitive function by modulating gut microbiota. Chunchai et al. [71] administered a synbiotic formulation containing xylooligosaccharides and Lactobacillus paracasei HII01 to obese insulin-resistant rats. The results indicated that rats receiving the synbiotic intervention exhibited significant improvements in the time taken to locate the platform and the time spent in the target quadrant during the Morris water maze test, suggesting restored cognitive function. The gut microbiota can produce or influence the precursors of neurotransmitters, such as tryptophan metabolites, which can be converted into neurotransmitters such as gamma-aminobutyric acid, serotonin, and dopamine. These neurotransmitters can subsequently influence brain function through the circulatory system or vagus nerve signaling. Additionally, a synbiotic formulation can indirectly protect the brain from cognitive decline by reducing both intestinal and systemic inflammation [72]. SCFAs, particularly butyrate, exhibit neuroprotective effects by promoting the expression of brain-derived neurotrophic factor, inhibiting the overactivation of microglia; in this way, they reduce neuroinflammation and modulate the hypothalamic–pituitary–adrenal axis to regulate stress responses [73]. In a clinical trial involving Alzheimer’s disease patients, researchers found that a synbiotic formulation (containing probiotics and selenium) significantly improved patients’ cognitive abilities compared to selenium supplementation alone, as evidenced by higher Mini-Mental State Examination (MMSE) scores [74]. These findings suggest that synbiotics have significant potential in promoting overall health.
4. Application of Probiotics in Functional Foods
Due to the incorporation of various probiotics that regulate the balance of intestinal microorganisms and enhance immune function, functional foods can not only promote health but also stimulate metabolism and improve overall health [75]. The application of probiotics in functional foods has evolved from traditional fermented foods to modern scientific research and development, as shown in Figure 3. Currently, probiotics are widely used worldwide, not only in dairy products, meat products, fermented fruits, and vegetables, but also in tablets [76], capsules [77], granular probiotics [78], lyophilized probiotic powders [79], and the dried and lyophilized powders of fruits and vegetables. This has gradually formed a well-developed market, with sales increasing year by year, and the application areas are also expanding.
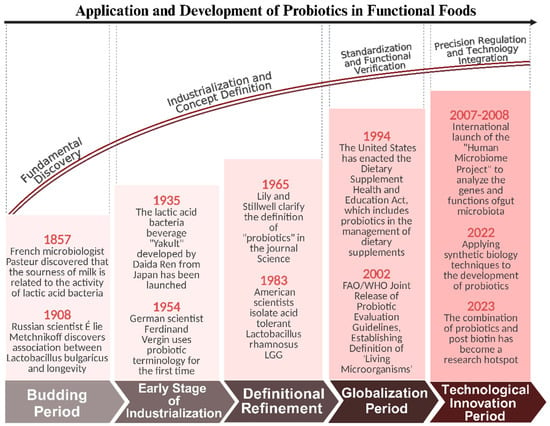
Figure 3.
Application and development of probiotics in functional foods.
4.1. Dairy Products
Probiotics have been widely applied in functional foods, particularly in dairy products. Currently, probiotics are widely used in various dairy products, including liquid milk products such as yogurt, pure milk, and milk drinks, as well as solid milk products such as milk powder and cheese (Table 2). These products meet the dietary needs of people across different age groups.
4.1.1. Probiotic Fermented Milk
As research into the fermentation characteristics of probiotic bacteria has deepened, a variety of probiotics have been added to fermented milk, including B. bifidum, L. bulgaricus, L. casei and also L. acidophilus, L. fermentum, and L. plantarum [80]. Fermented milk not only possesses excellent nutritional composition but also effectively regulates the balance of intestinal microorganisms and enhances immune and digestive functions [81]. Additionally, such products promote more effective absorption of various minerals and vitamins and help reduce body fat content [82]. Dairy products enriched with probiotics not only retain the nutritional value of raw milk but also introduce a variety of micronutrients during the fermentation process [83], thereby enhancing the body’s absorption and utilization of proteins. Notably, probiotics can effectively reduce aflatoxin levels in dairy products [84]. Sanaldi et al. [85] formed various experimental groups by adding L. acidophilus DSMZ 20079, L. rhamnosus GG, and B. bifidum DSMZ 20456 to Aflatoxin M1 (AFM1)-enriched contaminated milk and analyzed the AFM1 levels after one day of storage. The results showed that all three groups exhibited binding capacity for AFM1, with the highest binding capacity observed in the Bifidobacterium group (40.14 ± 0.38%). Regular consumption of probiotic-containing dairy products may reduce AFM1 exposure and improve overall health.
4.1.2. Probiotic Fermented Yogurt
Common microbial species found in probiotic-fermented yogurt include L. bulgaricus, S. thermophilus, and other probiotics, such as P. sapphimus [86] and B. bifidum [87]. Probiotics provide additional health benefits while improving yogurt’s flavor and consistency and regulating intestinal flora. Evidence suggests that increased intake of probiotic-fermented yogurt may protect individuals from bone loss. For instance, an Irish study involving over 4000 individuals aged 60 years and older found that higher yogurt intake was associated with a lower risk of osteoporosis [88]. Additionally, probiotics are believed to reduce inflammation and enhance insulin effectiveness. Daily consumption of probiotic yogurt may reduce the incidence of cardiovascular disease and T2DM [89]. For example, Gijsbers et al. [90] found that daily yogurt intake reduced the risk of T2DM by 18%. Studies have shown that different probiotic strains exhibit unique metabolic behaviors, such as lactic acid production and amino acid synthesis, in specific environments [91], thereby affecting the nutritional composition and sensory quality of yogurt. Researchers have recently combined probiotics with beneficial ingredients, such as sea buckthorn [92] and ginger juice [93], to develop novel probiotic yogurt products with antioxidant, anti-inflammatory, and intestinal health benefits. For example, Terpou et al. [94] used a commercial yogurt fermenter and L. casei ATCC 393 as an auxiliary fermenter, adding sea buckthorn berries as an immobilization carrier to prepare frozen yogurt. The results showed that frozen yogurt containing immobilized probiotics maintained a high survival rate during 90 days of frozen storage and exhibited excellent survival, superior organoleptic properties, and high commercial potential under gastrointestinal conditions.

Table 2.
Probiotic fermented milk products and their strains.
Table 2.
Probiotic fermented milk products and their strains.
| Probiotic Fermented Milk Products | Tested Strains | Reference |
|---|---|---|
| High-protein drink, pure probiotic fermented milk | Lactobacillus acidophilus, Bifidobacterium lactis, Lactobacillus paracasei, Streptococcus thermophilus | [95] |
| Compound lactobacillus beverage | Lactobacillus bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacterium | [96] |
| Probiotic fermented milk with compound fruit and vegetable juice | Lactobacillus bulgaricus, Streptococcus thermophilus | [97] |
| Probiotic fermented goat milk | Acidophilus, Lactobacillus casei, Lactobacillus paracasei 01, Lactobacillus paracasei 431 | [98] |
| Double-protein probiotic yogurt | Lactobacillus bulgaricus, Streptococcus thermophilus, Bifidobacterium | [99] |
| Probiotic yogurt ice cream | Streptococcus thermophilus DMST-H2, Lactobacillus delbrueckii DMLD-H1 | [100] |
| Compound probiotic fermented milk | Lactobacillus paracasei PC-01, Bifidobacterium Lactobacillus Probio-M8 | [101] |
4.2. Plant-Based and Fruit and Vegetable Products
4.2.1. Probiotic Fermentation of Fruit and Vegetable Juice
Fruits and vegetables, which are rich in vitamins, minerals, dietary fiber, and other nutrients, provide an ideal environment for probiotic growth. After processing, fruit and vegetable juices with a roughly neutral pH exhibit a relatively short shelf life and limited storage time, leading to suboptimal flavors and textures [102]. By acidifying the raw juice [103], the pH value was reduced from 6.5 to 4.2, extending the shelf life by 14 days and effectively prolonging the storage time, thereby enhancing the flavor when consumed directly. During the production of fermented fruit and vegetable products, adding LAB and yeast probiotics to the juice results in a gradual increase in lactic acid content [104]. Simultaneously, the concentrations of amino acids, polyphenols, organic acids, extracellular polysaccharides, vitamins, bacteriocins, minerals, and various biologically active enzymes in fruit juices increase [105]. These nutrients and beneficial components not only enhance the flavor of fruit and vegetable juices but also provide various health benefits to consumers, including antioxidant, antimicrobial, and hepatoprotective effects [106], the optimization of gastrointestinal function, a reduction in blood glucose levels [107], and enhanced immune function [108]. Nowadays, a wide variety of functional fruit and vegetable juices are emerging and gaining public attention due to their unique formulations and flavors. Quan et al. [109] inoculated L. plantarum, L. fermentum, L. acidophilus, L. rhamnosus, L. casei paracasei, and B. longum into orange juice and fermented the mixtures for 48 h. The results showed that all Lactobacillus strains grew well, and the fermented orange juice exhibited enhanced bioactive constituents, aroma compounds, total antioxidant activity, and sensory characteristics, with L. casei paracasei fermentation producing the best aroma and flavor. These findings provide a reference for the further processing of other fruit and vegetable juices. These innovative products not only enhance the health benefits of yogurt but also meet consumer expectations for product variety and personalization.
4.2.2. Probiotic Fermented Soy Products
Soy milk is rich in oligosaccharides, which serve as prebiotics and provide a high-quality growth environment for LAB, particularly bifidobacteria [93]. Importantly, probiotics can convert soy isoflavone glycosides, a functional component, into biologically active isoflavones [110]. Wei et al. [111] determined the isoflavone glycoside content in soy milk fermented with a single strain. The results showed that soy milk not only maintained high probiotic activity (107–109 CFU/mL) but also significantly increased the content of soy isoflavone glycosides. Isoflavone glycosides act as phytoestrogens to regulate endocrine secretion bi-directionally [112]. In cases of estrogen deficiency, isoflavones exhibit mild estrogenic activity, alleviating menopausal symptoms such as hot flushes, night sweats, and osteoporosis. Conversely, in cases of estrogen excess, they competitively bind to receptors to reduce the risk of breast cancer. In a study where rats with 2-Amino-1-Methyl-6-Phenylimidazo [4,5-B] Pyridine chemically induced mammary tumors were fed soymilk alone, L. casei alone, and a combination of soymilk and L. casei, the results showed that tumor incidence was reduced in the soymilk group, and tumor volume was decreased in the L. casei group. Moreover, the combination group exhibited significantly reduced tumor incidence and volume. L. casei exerts its anticancer effect by enhancing natural killer cells’ activity, which is significantly enhanced when combined with soymilk [113]. Therefore, the use of probiotics can complement the cancer prevention function that cannot be achieved when using soy milk alone.
Natto, a traditional Japanese fermented food, is made from soybeans fermented by Bacillus subtilis [114]. Natto kinase (NK), a key functional component, effectively dissolves fibrin. Furthermore, natto contains other bioactive compounds that collectively contribute to various health benefits, including improved blood circulation and reduced cardiovascular disease risk [115]. However, the odor and taste of natto may not be acceptable to most people. Thus, adjustments are made to improve its sensory properties and acceptability, such as modifying the fermentation strain formula and fermentation conditions. Additionally, natto contains biogenic amines, which may adversely affect human health when ingested in excess [116]. Lan et al. [117] conducted mono-, bi-, and tri-bacterial fermentations using B. subtilis GUTU09 (B9) in combination with Lactobacillus, Bifidobacterium, and Trichoderma. The results showed that bio-fermentation (especially the combination of B9 and Trichoderma) exhibited higher NK and protease activities under co-fermentation conditions; additionally, it significantly reduced biogenic amine content (total biogenic amine decreased to 16.16 mg/kg) and improved the flavor and texture of natto. In contrast, tri-bacterial fermentation did not show significant advantages. Ma et al. [118] used B. subtilis and L. bulgaricus in a mixed fermentation and found that, under the conditions of 4% inoculum, a 1:1 strain ratio, 20 hours of fermentation, and a fermentation temperature of 35 °C, the content of active ingredients in natto was elevated. The biogenic amine content was 16.30 mg/kg in mixed fermented natto and 9.46 mg/kg in single-fermented natto. This comparison showed that mixed fermentation significantly reduced the accumulation of biogenic amines compared to single fermentation. Meanwhile, mixed fermented natto contained higher levels of volatile compounds, including acids and esters, resulting in a unique fruity and lactic flavor.
4.3. Meat Products
In developing new functional meat products, the primary application strategy for probiotics is their integration into fermented sausage production. The developed probiotic meat products are not only nutrient-rich and conducive to intestinal digestion and absorption but also possess a unique fermentation flavor and the distinct advantages of probiotics, allowing for extended storage. Wang Qian [119] used a compound starter of L. acidophilus and Staphylococcus xylosus in a 1:1 volume ratio, inoculating it into sausage at a concentration of 107 CFU/mL. The fermented sausage produced using this method has an excellent taste, is neither greasy nor sweet, has moderate saltiness, a slight wine aroma, and a bright red color. Gong et al. [120] used L. plantarum, P. pentosaceus, and Staphylococcus as fermentation agents to inoculate Sichuan sausage, effectively overcoming the issues of long fermentation times and susceptibility to contamination by other bacteria in traditional Sichuan sausage. The prepared sausage has an appropriate balance of sour and spicy flavors, successfully retaining the unique flavor characteristics of traditional Sichuan sausage. Rubio et al. [121] successfully inoculated sausage with three types of bacteria isolated from infant feces, substantially inhibiting the growth of harmful microbes and significantly increasing product safety. Bis-Souza et al. [122] added the screened strains SJRP66 and SJRP169 of L. casei to low-fat fermented sausage. The research indicates that the addition of probiotics affects the oxidation process of oil and the color value of the final product, demonstrating significant potential for application in low-fat fermented sausage. In the production of fermented sausage, its matrix structure effectively protects the survival and continuity of probiotics. However, this requires probiotics to have excellent tolerance to withstand adverse conditions such as high salinity and low pH during processing. Flores et al. [123] selected Debaryomyces Hansenii yeast as an aroma agent and inoculated it into a low-salt, low-fat sausage. This technology effectively increases the aromatic components in sausages, making their flavor similar to that of traditional sausages.
4.4. Other Functional Foods
4.4.1. Application of Probiotics in Health Products
With the continuous improvement in quality of life, the demand for health products in the market has also increased. Some medications for gastrointestinal health primarily take the form of drugs, and probiotics are incorporated into the production process to create a variety of probiotic health products, including oral liquids, hard capsules, soft capsules, granules, and microcapsules [124]. Probiotics have poor acid resistance, leading to their rapid degradation under the acidic conditions of gastric acid and bile [125], thus limiting their effectiveness in the small intestine or colon. Therefore, it is necessary to isolate probiotics from the external environment and use microencapsulation technology to produce probiotic capsules. Probiotic capsules are characterized by excellent stability and high tolerance to gastric acid and bile salts, effectively resisting phage invasion while increasing the number of viable bacteria in the gut, thereby offering significant potential for development in the health food sector [126]. However, traditional microencapsulation technologies, such as spray drying and emulsification, have limitations, prompting the exploration of new nano-packaging technologies to enhance probiotic survival rates and targeted delivery efficiency. Xu et al. [127] employed various nano-encapsulation systems, including nanofibers, nanoparticles, biofilms, and nano-coatings, to form a protective layer on probiotics, enhancing their tolerance in simulated gastrointestinal fluid and intestinal colonization ability. This significantly enhanced probiotic survival and targeted release efficiency in the digestive tract, offering a new direction for developing personalized and precise probiotic nanomaterials.
4.4.2. Application of Probiotics in Food Additives
Common probiotics that produce flavors include L. bulgaricus, S. thermophilus, L. lactis, Acetobacter aceti, Leuconostoc mesenteroides, and S. casei, among which L. bulgaricus is the most important. It produces a variety of aromatic compounds through fermentation, thereby influencing human taste perception [128]. Lactobacillus and S. thermophilus primarily produce acetaldehyde as a significant flavor compound [129], while L. lactis, A. aceti, L. mesenteroides, and S. casei produce various flavor components, including glyoxal, lactic acid, acetic acid, and butanedione [130]. Probiotics produce a range of unique odor components through metabolic processes, endowing products with distinct taste characteristics and reducing the need for artificial flavors and spices, thereby making products more natural and environmentally friendly.
5. Technological Innovation of Probiotics in Functional Foods
5.1. Microencapsulation Technology
Microencapsulation technology, a core method in probiotic delivery systems, effectively protects probiotics from gastric acid and bile through encapsulation within microcapsules, enabling targeted release under specific gastrointestinal conditions [131]. Different types of microencapsulation materials exhibit distinct physical and chemical properties, providing varying levels of protection for probiotics. Common microencapsulation wall materials and their respective advantages and disadvantages are listed in Table 3. Combining multiple types of wall materials can significantly enhance the protection of probiotics and improve their survival rates under adverse conditions [132]. Zaeim [133] demonstrated that sodium alginate–chitosan bilayered microcapsules, featuring a dense network structure formed through electrostatic complexation, exhibited 82.4% protection efficiency in simulated gastric fluid after two hours, representing a 4.3-fold improvement over the unencapsulated control group and significantly enhancing probiotic survival rates in the gastrointestinal environment. Advances in materials science continue to yield innovative material combinations. Patarroyo et al. [134] reported that gelatin-graphene oxide composite hydrogels maintained a >90% survival rate of L. plantarum LS14 at pH 3.0, with a compression modulus of 12.5 kPa, demonstrating superior mechanical stability. This formulation not only protects probiotics from gastric acid damage but also maintains structural stability in the gastrointestinal tract, ensuring slow release and sustained probiotic activity. These innovative microencapsulation approaches provide new strategic insights for probiotic delivery system development and help enhance the performance of probiotic products.

Table 3.
Commonly used probiotic microcapsule wall materials and their advantages and disadvantages.
5.2. Biofilm Delivery Systems
Biofilm delivery systems represent an innovative probiotic delivery method that overcomes traditional dosage form limitations by leveraging microbial self-assembly properties to construct protective carriers, significantly enhancing application efficacy and safety [146]. The formation of probiotic biofilm delivery systems involves multiple key stages. The initial stage involves the colonization of probiotics on specific surfaces, mediated by surface proteins and polysaccharides, which facilitate preliminary adsorption and attachment. Subsequently, bacteria synthesize and secrete extracellular polymers (EPS), which intertwine to form a complex extracellular matrix. This matrix provides structural support for the biofilm and forms a physical barrier to protect internal bacterial cells. As the biofilm matures, cells communicate via QS mechanisms to synergistically regulate the synthesis of EPS and other key components, thereby optimizing biofilm stability and functionality. During biofilm development, an internal network of water channels often forms to facilitate nutrient penetration and metabolic waste excretion, thereby maintaining internal homeostasis [147]. In the food industry, Zhu et al. [148] successfully applied biofilm-state Lactobacillus from dynamic cultures to ice cream production, resulting in 35% improved product hardness, a 28% reduced melting rate, and the maintenance of >10⁷ CFU/g viability after 28 days of storage at 4 °C compared to traditional free-state strains, thereby quantifying biofilm’s freezing tolerance. EPS in biofilms regulates the immune response of macrophages by interacting with TLR2 and TLR4 on the macrophage surface. This interaction suppresses the production of pro-inflammatory cytokines, such as TNF-α, thereby alleviating inflammatory responses, regulating the intestinal microenvironment, enhancing the barrier function of the intestinal mucosa, improving intestinal permeability, and promoting overall intestinal health [149]. With the aim of achieving lasting NEC protection, offering a new paradigm for functional food development and the clinical treatment of necrotizing enterocolitis in preterm infants, Olson et al. [150] enhanced biofilm formation in L. reuteri using dextran microspheres loaded with maltose, achieving a reduction in morbidity from 45% to 18% in a single-administration experimental model and a 62% decrease in the intestinal permeability index, while preserving intestinal flora alpha diversity. This study demonstrates that single biofilm-state probiotic administration can provide long-term therapeutics through the biofilm’s physical barrier effects, metabolite slow-release properties, and environmental response mechanisms.
5.3. Multidimensional Environmental Adaptation Techniques
Technological innovations in probiotic applications focus on enhancing strain environmental adaptability and optimizing stress tolerance through multidimensional strategies [151]. Studies demonstrate that probiotics can induce cross-protective effects through pretreatment. For example, L. plantarum KLDS 1.0628, heat-acclimatized at 45 °C for one hour, showed a 31.38-fold increased tolerance to subsequent high-temperature challenges. The proportion of unsaturated fatty acids in its cell membranes significantly increased from 32.7% (control group) to 47.5%, suggesting enhanced thermal stability through membrane fluidity regulation [152]. At the genetic level, membrane reorganization constitutes a key acid tolerance mechanism. Liu et al. [153] subjected B. coagulans to atmospheric pressure room-temperature plasma (ARTP) mutagenesis combined with 40 days of adaptive laboratory evolution, obtaining the artp-aleBC15 mutant strain. This strain exhibited a 22.4% survival rate after three hours of simulated gastric treatment at pH 2.5, 4.8-fold higher than the wild-type strain, with significantly improved cell membrane permeability and hydrophobicity. Research indicates that strain-specific pretreatment strategies enhance environmental adaptation. Bisson et al. [154] demonstrated that L. plantarum pre-acclimated to 2% NaCl achieved a maximum growth rate of 0.52 OD/h, a 40% increase over untreated controls, with the cell length extending from 2.8 μm to 4.2 μm to cope with osmotic stress. These techniques significantly increase probiotic functional stability in food processing, gastrointestinal, and medical applications, providing theoretical foundations and technical pathways for the development of precise probiotic formulations.
5.4. Genetic Engineering
To address the demands of precision nutrition, personalized medicine, and disease treatment, probiotic engineering has garnered increasing attention. Utilizing gene editing technologies, artificial intelligence, and other advanced methods, researchers perform precise genetic modifications and optimizations of probiotics to confer specific functional characteristics [155]. In gene editing applications, recombinant DNA technology has enabled the development of engineered probiotics that are capable of secreting targeted bioactive molecules. For instance, Carter et al. [156] constructed Lactobacillus paracasei LP-A through genetic modification, enabling angiotensin (1–7) secretion. In an aged rat model, this strain increased circulating Ang (1–7) levels by 25% during the acute phase and 32% during the chronic phase while significantly reducing AngII levels (p < 0.01), thereby effectively modulating renin–angiotensin system function. Fang et al. [157] demonstrated that E. coli Nissle 1917 modified with the manganese superoxide dismutase gene via clustered regularly interspaced short palindromic repeats/Cas9 technology exhibited a 50% increase in survival rate in a DSS-induced colitis model, accompanied by a 62% reduction in inflammatory factor IL-6 levels. In terms of process optimization, Abou Ayana et al. [158] developed an artificial neural network model that successfully increased total solids’ recovery of water-based sesame milk to 95.18%, achieved a live LAB count of 10⁸ CFU/mL, and improved sensory scores by 27% in plant-based dairy products through whey permeate integration. These technological innovations expand probiotic application scenarios while advancing their role in precision medicine and functional foods.
5.5. Personalized Strain Screening
In the realm of personalized applications, probiotic technology continues to innovate, achieving enhanced efficacy through precise strain screening and the optimization of host suitability. Personalized strain screening for probiotics involves identifying the optimal probiotic strain for an individual from a diverse pool of candidates. This process considers the individual’s specific requirements, physiological profile, current health condition, and genetic makeup to determine the most appropriate probiotic intervention [159]. Celiberto et al. [159] isolated native probiotic strains from healthy host feces using selective media (MRS and BIM-25). They identified three host-adapted bacterial strains through simulated gastrointestinal fluid tolerance screening, antibiotic susceptibility testing, and RAPD-PCR genotyping. These strains were reintroduced into healthy mice and evaluated for protective effects in a DSS-induced colitis model. The results demonstrated that this personalized probiotic group significantly reduced the disease activity index by 42% compared to commercial LGG, while being more effective in alleviating colitis symptoms, ameliorating histopathological damage, and modulating immune responses. Rudyka et al. [160] further revealed gender dimorphism effects, where a multi-strain formulation (containing 14 Lactobacillus/Bifidobacterium strains) exhibited superior metabolic modulation in female MSG-obese rats. This formulation effectively prevented changes, including blocking weight gain, reducing inflammatory cell activation, and improving gut microbial community composition. The screening and application of personalized probiotics represent a current research hotspot in probiotic science, with the possibility of the development of more targeted and efficient probiotic products that address diverse individual needs.
6. Safety and Evaluation of Probiotics
6.1. Safety Risks of Probiotics
Currently, there is no unified and clear consensus on issues including systemic infection, harmful metabolic processes, excessive immune activation for sensitive people, and gene delivery, as shown in Figure 4, where the severity or risk level of side effects is positively correlated with the pie chart area [161]. Regarding systemic infection risks, specific probiotic strains can release harmful metabolites. For example, L. rhamnosus and L. paracasei have been identified as pathogens that may cause a variety of diseases, such as infective endocarditis, meningitis, and dental caries [162]. In addition, some strains can also cause gastrointestinal inflammatory responses [163]. Research has shown that Clostridium innocuum can regulate epithelial and mucosal immune responses through interactions with the host, thereby influencing the pathogenicity of Salmonella. This interaction may promote the development of drug resistance in Salmonella, potentially triggering new diseases [164]. In addition, some microbial strains can also act as hosts to induce drug resistance of other pathogenic bacteria, thus causing new diseases. Experiments showed that some probiotics can cause a series of pathophysiological processes, such as abnormal immune response and inflammation in the human body, and eventually lead to the death of the body, posing significant health risks to patients with impaired immune system function or serious diseases [165]. Besselink et al. [166] explored the possible effects of probiotics on patients with severe acute pancreatitis. Studies have found that, in those patients who are expected to suffer from severe acute pancreatitis, the use of probiotics as a means of prevention does not significantly reduce the risk of infectious complications but may increase the risk of death [167]. In addition, probiotics may also lead to the spread of drug resistance and gene migration. In the current use environment, Enterococcus has become the most controversial group of LAB [168]. Enterococcus is a Gram-negative bacterium, a group that is found widely in nature, including in the human body, animals, plants, and other organisms; most are conditional pathogenic bacteria or pathogenic bacteria with weak conditional pathogenicity. Enterococcus is considered a microorganism that easily becomes infected, and it easily develops resistance to some antibiotics. Vancomycin-Resistant Enterococcus (VRE) is considered a super bacterium, showing obvious multi-drug resistance [169]. These strains can resist vancomycin by secreting a variety of proteins associated with the outer membrane. VRE has the ability to transmit this vancomycin-resistant gene to other multidrug-resistant pathogens, such as S. aureus [170].
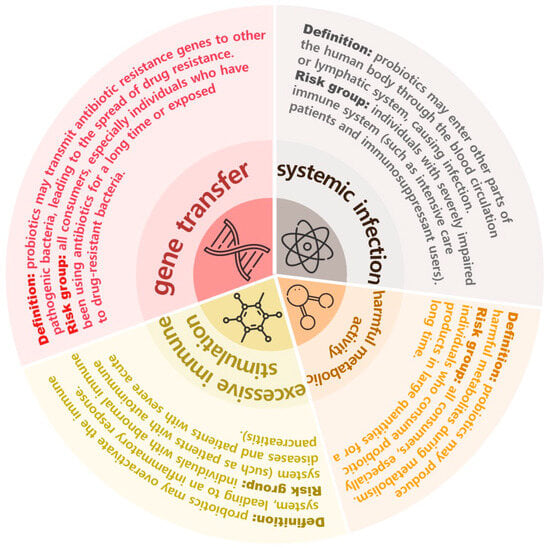
Figure 4.
Four possible side effects of probiotics.
6.2. Safety Evaluation System of Probiotics
Establishing a strong and scientifically sound safety evaluation methodology ensures that customers can make educated decisions when buying probiotic products. The FAO/WHO “Guidelines for the Evaluation of Probiotics in Food” clearly state that, for strains that are Generally Recognized As Safe, in-depth experimental evaluations are required in several areas, including antibiotic resistance, metabolic activity, potential adverse effects on human health, epidemiological monitoring, toxin production, and hemolytic activity [171]. The International Scientific Association for Probiotics and Prebiotics (ISAPP) has discussed the potential short-term and long-term risks associated with probiotic use, the threats to vulnerable populations, the importance of ensuring that probiotic product quality meets the needs of these populations, and the necessity of promptly reporting adverse events related to probiotic use. Based on these discussions, ISAPP has put forward evidence-based recommendations [172]: for specific patient groups, the use of probiotics should undergo rigorous quality testing, ideally conducted by an independent third party; for probiotics that have been used for extended periods or exhibit serious adverse effects but have not been licensed for use, immediate cessation or complete discontinuation is recommended; for probiotics that have not been safely used, their safety must be evaluated based on specific circumstances, including the safety of live microorganisms, the potential for antibiotic resistance gene transfer, changes in microbial tissue caused by probiotics, interactions with drugs, and the potential impact of probiotic colonization. ISAPP particularly emphasizes the crucial role of whole-genome sequencing technology, as it can accurately identify the toxicity, toxin production, and antibiotic resistance genes of strains, thereby determining the species characteristics of these strains [173].
7. Final Considerations and Future Perspectives
The functional food market has witnessed rapid growth in recent years, primarily driven by heightened consumer health and nutrition awareness. As a core functional ingredient, probiotics have emerged as a significant driver of innovation and health-conscious consumer trends in the global food industry by leveraging their unique health benefits, including gut microbiota modulation, immune enhancement, and metabolic disease intervention. The objective of this review was to promote the development of functional foods, investigate probiotic applications, and establish a theoretical foundation for developing the next generation of high-quality functional foods. Currently, probiotics are extensively utilized in dairy products, meat products, and dietary supplements, yet they encounter several technical and market challenges, including insufficient efficacy validation, product homogenization, and regulatory barriers. Moreover, probiotics encounter systemic challenges in translating scientific findings into commercial applications. Laboratory-scale encapsulation technologies face numerous challenges during industrialization, including strain survival rate reductions caused by shear forces and temperature fluctuations, material costs exceeding food industry thresholds, insufficient processing tolerance, shelf-life degradation, and other persistent issues. Future development necessitates the establishment of a multi-dimensional evidence system, including the construction of organ microarrays and organoid models, to overcome limitations in probiotic efficacy validation. Research and development efforts should prioritize strain innovation while fostering interdisciplinary collaboration across medicine, food science, and data science to develop probiotic strains with unique functional characteristics. The regulatory framework must be strengthened through coordinated communication and scientifically rationalized approaches. The involvement of governments and industry associations is critical for developing and harmonizing international regulations and standards, promoting global regulatory harmonization of probiotic products, and reducing trade barriers. Continuous technological innovation, the optimization of microencapsulation technologies, the integration of advanced methods such as gene editing, and the refinement of continuous flow production processes are essential to enhance strain stability. Additionally, developing low-cost alternative materials is crucial for producing commercially viable, scalable, probiotic-rich functional foods, bridging the gap between research and industry. In conclusion, probiotics hold significant potential in functional food development. Through continuous technological innovation and interdisciplinary collaboration, the current challenges can be addressed, the application scope of probiotics can be expanded, and safer, more effective, and palatable functional foods can be developed, thereby making substantial contributions to human health.
Author Contributions
Y.H.: investigation, methodology, and writing—original draft. Y.Z.: investigation and methodology. H.X.: funding acquisition, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Research Project of State Key Laboratory of Food Science and Resources, Nanchang University, China (Project No. SKLF-ZZB-202328).
Data Availability Statement
No data were used in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goldin, B.R. Health benefits of probiotics. Br. J. Nutr. 1998, 80, S203–S207. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Onubi, O.J.; Poobalan, A.S.; Dineen, B.; Marais, D.; McNeill, G. Effects of probiotics on child growth: A systematic review. J. Health Popul. Nutr. 2015, 34, 8. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Boggia, R.; Zunin, P.; Turrini, F. Functional foods and food supplements. Appl. Sci. 2020, 10, 8538. [Google Scholar] [CrossRef]
- Fentie, E.G.; Lim, K.; Jeong, M.; Shin, J.-H. A comprehensive review of the characterization, host interactions, and stabilization advancements on probiotics: Addressing the challenges in functional food diversification. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13424. [Google Scholar] [CrossRef]
- Chakravarty, K.; Gaur, S.; Kumar, R.; Jha, N.K.; Gupta, P.K. Exploring the multifaceted therapeutic potential of probiotics: A review of current insights and applications. Probiot. Antimicrob. Proteins 2025, 17, 341–363. [Google Scholar] [CrossRef]
- Ray, M.; Manjunath, A.; Halami, P.M. Effect of probiotics as an immune modulator for the management of COVID-19. Arch. Microbiol. 2023, 205, 182. [Google Scholar] [CrossRef]
- Penha Rodrigues Pereira, E.; Silva da Graça, J.; Manfrinato Ferreira, B.; Fasura Balthazar, C.; Xavier-Santos, D.; França Bezerril, F.; Magnani, M.; Sant’Ana, A.S. What are the main obstacles to turning foods healthier through probiotics incorporation? A review of functionalization of foods by probiotics and bioactive metabolites. Food Res. Int. 2024, 176, 113785. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Porras, D.; García-Mediavilla, M.V.; Román-Sagüillo, S.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Aging, gut microbiota and metabolic diseases: Management through physical exercise and nutritional interventions. Nutrients 2021, 13, 16. [Google Scholar] [CrossRef]
- Kocsis, T.; Molnár, B.; Németh, D.; Hegyi, P.; Szakács, Z.; Bálint, A.; Garami, A.; Soós, A.; Márta, K.; Solymár, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Romero, T.; Flores-Andrade, E.; Bonilla-Zavaleta, E.; González-Arnao, M.T.; Rascón-Díaz, M.P. Probiotic microencapsulation at low temperatures. Cryobiology 2019, 91, 173–174. [Google Scholar] [CrossRef]
- Han, J.; McClements, D.J.; Liu, X.; Liu, F. Oral delivery of probiotics using single-cell encapsulation. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13322. [Google Scholar] [CrossRef] [PubMed]
- Lorena, C.; Imen, N. Taxonomy and systematics of plant probiotic bacteria in the genomic era. AIMS Microbiol. 2017, 3, 383–412. [Google Scholar]
- Papizadeh, M.; Rohani, M.; Nahrevanian, H.; Javadi, A.; Pourshafie, M.R. Probiotic characters of Bifidobacterium and Lactobacillus are a result of the ongoing gene acquisition and genome minimization evolutionary trends. Microb. Pathog. 2017, 111, 118–131. [Google Scholar] [CrossRef]
- Thullner, M.; Regnier, P. Microbial Controls on the biogeochemical dynamics in the subsurface. Rev. Mineral. Geochem. 2019, 85, 265–302. [Google Scholar] [CrossRef]
- Matchado, M.S.; Rühlemann, M.; Reitmeier, S.; Kacprowski, T.; Frost, F.; Haller, D.; Baumbach, J.; List, M. On the limits of 16S rRNA gene-based metagenome prediction and functional profiling. Microb. Genom. 2024, 10, 001203. [Google Scholar] [CrossRef]
- Arikawa, K.; Ide, K.; Kogawa, M.; Saeki, T.; Yoda, T.; Endoh, T.; Matsuhashi, A.; Takeyama, H.; Hosokawa, M. Recovery of strain-resolved genomes from human microbiome through an integration framework of single-cell genomics and metagenomics. Microbiome 2021, 9, 202. [Google Scholar] [CrossRef]
- Cardona, S.; Mostafazadeh, N.; Luan, Q.; Zhou, J.; Peng, Z.; Papautsky, I. Numerical modeling of physical cell trapping in microfluidic chips. Micromachines 2023, 14, 1665. [Google Scholar] [CrossRef]
- Pereira, A.C.; Tenreiro, A.; Cunha, M.V. When FLOW-FISH met FACS: Combining multiparametric, dynamic approaches for microbial single-cell research in the total environment. Sci. Total Environ. 2022, 806, 150682. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Jiang, T.; Yan, Y. Biosensor-assisted titratable CRISPRi high-throughput (BATCH) screening for over-production phenotypes. Metab. Eng. 2023, 75, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Kim, Y.B.; Kim, J.Y.; Roh, S.W.; Whon, T.W. Advances in culturomics research on the human gut microbiome: Optimizing medium composition and culture techniques for enhanced microbial discovery. J. Microbiol. Biotechnol. 2024, 34, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Giemza, M.; Ponikiewska, W.; Strus, M. Importance of Lactobacilli for human health. Microorganisms 2024, 12, 2382. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.A.; Milani, C.; Duranti, S.; Mancabelli, L.; Mangifesta, M.; Turroni, F.; Viappiani, A.; Sinderen, D.v.; Ventura, M. Tracking the taxonomy of the genus Bifidobacterium based on a phylogenomic approach. Appl. Environ. Microbiol. 2018, 84, e02249-17. [Google Scholar] [CrossRef]
- Gopikrishna, T.; Suresh Kumar, H.K.; Perumal, K.; Elangovan, E. Impact of Bacillus in fermented soybean foods on human health. Ann. Microbiol. 2021, 71, 1–16. [Google Scholar] [CrossRef]
- Martinovic, A.; Cocuzzi, R.; Arioli, S.; Mora, D. Streptococcus thermophilus: To survive, or not to survive the gastrointestinal tract, that is the question! Nutrients 2020, 12, 2175. [Google Scholar] [CrossRef]
- Qin, H.; Wu, H.; Shen, K.; Liu, Y.; Li, M.; Wang, H.; Qiao, Z.; Mu, Z. Fermented minor grain foods: Classification, functional components, and probiotic potential. Foods 2022, 11, 3155. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Z. Health promoting activities of probiotics. J. Food Biochem. 2019, 43, e12944. [Google Scholar] [CrossRef]
- Knipe, H.; Temperton, B.; Lange, A.; Bass, D.; Tyler, C.R. Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Rev. Aquac. 2021, 13, 324–352. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Nie, X.; Chitrakar, B.; Gao, J.; Sang, Y. Mutual adhesion of Lactobacillus spp. to intestinal cells: A review of perspectives on surface layer proteins and cell surface receptors. Int. J. Biol. Macromol. 2024, 282, 137031. [Google Scholar] [CrossRef]
- Pan, C.; Jiang, X.; Wei, J.; Liu, C.; Zhang, M.; Gao, C.; Chen, R.; Yang, C.; Wang, B.; Yu, M.; et al. Ameba-inspired strategy enhances probiotic efficacy via prebound nutrient supply. Nat. Commun. 2025, 16, 1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, S.; Liao, C.; Jia, Y.; Li, J.; Shang, K.; Chen, J.; Cao, P.; Li, W.; Li, Y.; et al. Probiotic properties of chicken-derived highly adherent lactic acid bacteria and inhibition of enteropathogenic bacteria in Caco-2 Cells. Microorganisms 2022, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Çelen, T.; Anumudu, C.; Miri, T.; Onyeaka, H.; Fernandez-Trillo, P. Pathogen-responsive delivery of nisin. Food Hydrocoll. 2024, 154, 110076. [Google Scholar] [CrossRef]
- Yang, S.M.; Lee, D.W.; Park, H.J.; Kwak, M.H.; Park, J.M.; Choi, M.-G. Hydrogen peroxide enhances the antibacterial effect of methylene blue-based photodynamic therapy on biofilm-forming bacteria. Photochem. Photobiol. 2019, 95, 833–838. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-chain fatty acids and human health: From metabolic pathways to current therapeutic implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Nayebhashemi, M.; Enayati, S.; Zahmatkesh, M.; Madanchi, H.; Saberi, S.; Mostafavi, E.; Mirbzadeh Ardakani, E.; Azizi, M.; Khalaj, V. Surface display of pancreatic lipase inhibitor peptides by engineered Saccharomyces boulardii: Potential as an anti-obesity probiotic. J. Funct. Foods 2023, 102, 105458. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Qi, R.; Wang, J.; Qiu, X.; Liu, Z.; Huang, J. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1908–1918. [Google Scholar] [CrossRef]
- Pridmore, R.D.; Pittet, A.-C.; Praplan, F.; Cavadini, C. Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol. Lett. 2008, 283, 210–215. [Google Scholar] [CrossRef]
- Prado-Rebolledo, O.F.; Delgado-Machuca, J.d.J.; Macedo-Barragan, R.J.; Garcia-Marquez, L.J.; Morales-Barrera, J.E.; Latorre, J.D.; Hernandez-Velasco, X.; Tellez, G. Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens. Avian Pathol. 2017, 46, 90–94. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kakuta, S.; Aihara, Y.; Kamiya, T.; Watanabe, Y.; Osakabe, N.; Hazato, N.; Miyawaki, A.; Yoshikawa, S.; Usami, T.; et al. Visualization of probiotic-mediated Ca2+ signaling in intestinal epithelial cells in vivo. Front. Immunol. 2016, 7, 601. [Google Scholar] [CrossRef] [PubMed]
- Vangay, P.; Ward, T.; Lucas, S.; Beura, L.K.; Sabas, D.; Abramson, M.; Till, L.; Hoops, S.L.; Kashyap, P.; Hunter, R.C.; et al. Industrialized human gut microbiota increases CD8+T cells and mucus thickness in humanized mouse gut. Gut Microbes 2023, 15, 2266627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Song, J.; Lan, X.; Ma, F.; Jiang, M.; Jiang, C. Calcium-sensitive receptors alters intestinal microbiota metabolites especially SCFAs and ameliorates intestinal barrier damage in neonatal rat endotoxemia. Infect. Drug Resist. 2023, 16, 5707–5717. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Thaker, H.; Dong, M. Shiga toxins: An update on host factors and biomedical applications. Toxins 2021, 13, 222. [Google Scholar] [CrossRef]
- Fagan, R.P.; Fairweather, N.F. Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 2014, 12, 211–222. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Lasaro, M.A.; Salinger, N.; Zhang, J.; Wang, Y.; Zhong, Z.; Goulian, M.; Zhu, J. F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl. Environ. Microbiol. 2009, 75, 246–251. [Google Scholar] [CrossRef]
- Nzakizwanayo, J.; Kumar, S.; Ogilvie, L.A.; Patel, B.A.; Dedi, C.; Macfarlane, W.M.; Jones, B.V. Disruption of Escherichia coli Nissle 1917 K5 capsule biosynthesis, through loss of distinct kfi genes, modulates interaction with intestinal epithelial cells and impact on cell health. PLoS ONE 2015, 10, e0120430. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Benjamin-Neelon, S.E. Position of the academy of nutrition and dietetics: Benchmarks for nutrition in child care. J. Acad. Nutr. Diet. 2018, 118, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Perdigon, G.; Alvarez, S.; Rachid, M.; Aguero, G.; Gobbato, N. Immune system stimulation by probiotics. J. Dairy Sci. 1995, 78, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, M.; Liu, Y. TLR2 promotes the progression of diabetes mellitus with atherosclerosis via activating NLRP3 inflammasome and MyD88/NF-κB signaling pathway. Sci. Rep. 2025, 15, 16348. [Google Scholar] [CrossRef]
- Shoji, S.; Hanada, K.; Takahashi, M.; Watanabe, K.; Yonemochi, M.; Tomabechi, Y.; Shirouzu, M. The NF-κB regulator IκBβ exhibits different molecular interactivity and phosphorylation status from IκBα in an IKK2-catalysed reaction. FEBS Lett. 2020, 594, 1532–1549. [Google Scholar] [CrossRef]
- Vincenzi, A.; Goettert, M.I.; Volken de Souza, C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.-S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of orally-administered Bifidobacterium animalis subsp lactis strain BB12 on dextran sodium sulfate-induced colitis in mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef]
- Watanabe, N.; Kaminuma, O.; Kitamura, N.; Hiroi, T. Induced treg cells augment the Th17-mediated intestinal inflammatory response in a CTLA4-dependent manner. PLoS ONE 2016, 11, e0150244. [Google Scholar] [CrossRef]
- Tang, C.; Kong, L.; Shan, M.; Lu, Z.; Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res. Int. 2021, 147, 110490. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.-M.; Rizkalla, S.; Schrezenmeir, J.; Clément, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, K.-l.; Chen, L.; Wu, C.; Hu, X.; Zeng, T.; Chen, X.-q.; Li, W.-j.; Deng, X.; Li, H.; et al. Insulin sensitizers improve the GLP-1 secretion and the amount of intestinal L cells on high-fat-diet–induced catch-up growth. Nutrition 2017, 39–40, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-R.; Singhania, R.R.; Patel, A.K.; Tsai, T.-R.; Tsai, M.-L.; Sharma, V.; Dong, C.-D. Novel food isolates with striking α-glucosidase inhibitory activity and probiotic potential for an antidiabetic role. J. Food Sci. Technol. 2024, 61, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Duseja, A.; Acharya, S.K.; Mehta, M.; Chhabra, S.; Shalimar; Rana, S.; Das, A.; Dattagupta, S.; Dhiman, R.K.; Chawla, Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019, 6, e000315. [Google Scholar] [CrossRef]
- Mishima, E.; Abe, T. Role of the microbiota in hypertension and antihypertensive drug metabolism. Hypertens. Res. 2022, 45, 246–253. [Google Scholar] [CrossRef]
- Saleh, M.A.; Norlander, A.E.; Madhur, M.S. Inhibition of interleukin-17a, but not interleukin-17f, signaling lowers blood pressure, and reduces end-organ inflammation in angiotensin ii–induced hypertension. JACC Basic. Transl. Sci. 2016, 1, 606–616. [Google Scholar] [CrossRef]
- Krege, J.H.; Moyer, J.S.; Langenbach, L.L.; Peng, L.; Zhang, S.H.; Maeda, N.; Reddick, R.L.; Smithies, O. Angiotensin-converting enzyme gene and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1245–1250. [Google Scholar] [CrossRef]
- Deehan, E.C.; Al Antwan, S.; Witwer, R.S.; Guerra, P.; John, T.; Monheit, L. Revisiting the concepts of prebiotic and prebiotic effect in light of scientific and regulatory progress—A consensus paper from the global prebiotic association. Adv. Nutr. 2024, 15, 100329. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: Current evidence, controversies, and perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Plant-polyphenols based second-generation synbiotics: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; O’Riordan, K.J.; Clarke, G.; Cryan, J.F. Feeding gut microbes to nourish the brain: Unravelling the diet–microbiota–gut–brain axis. Nat. Metab. 2024, 6, 1454–1478. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Catapano, A.; Trinchese, G.; Cimmino, F.; Penna, E.; Pizzella, A.; Cristiano, C.; Lama, A.; Crispino, M.; Mollica, M.P. Butyrate improves neuroinflammation and mitochondrial impairment in cerebral cortex and synaptic fraction in an animal model of diet-induced obesity. Antioxidants 2023, 12, 4. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Liu, H.; Guo, W.; Jiang, Z.; Han, Q.; Liu, Y. Proliferation of probiotics and antioxidant effects of functional oligosaccharides added in fermented dairy product. Int. J. Dairy Technol. 2024, 77, 893–904. [Google Scholar] [CrossRef]
- Azhar, M.A.; Munaim, M.S.A. Design and optimization of a probiotic tablet for gastrointestinal tolerance by a simplex-centroid mixture. Drug Dev. Ind. Pharm. 2020, 47, 1–8. [Google Scholar] [CrossRef]
- Balaghi, Z.; Azima, S.; Motamedifar, M.; Kaviani, M.; Poordast, T.; Zare, N. The effect of lactofem oral probiotic capsule on Lactobacilli colonization and some vaginal health parameters. Gynecol. Obstet. Investig. 2020, 85, 245–251. [Google Scholar] [CrossRef]
- Talwalkar, A.; Miller, C.W.; Kailasapathy, K.; Nguyen, M.H. Effect of packaging materials and dissolved oxygen on the survival of probiotic bacteria in yoghurt. Int. J. Food Sci. Technol. 2010, 39, 605–611. [Google Scholar] [CrossRef]
- Sornsenee, P.; Chimplee, S.; Saengsuwan, P.; Romyasamit, C. Characterization of probiotic properties and development of banana powder enriched with freeze-dried Lacticaseibacillus paracasei probiotics. Heliyon 2022, 8, e11063. [Google Scholar] [CrossRef]
- Zhou, J.S.; Gopal, P.K.; Gill, H.S. Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro. Int. J. Food Microbiol. 2001, 63, 81–90. [Google Scholar] [CrossRef]
- Alansari, M.M.; Sahlah, S.A.; Alhumaid, L.; Singh, A.J.R. Probiotic lactobacilli: Can be a remediating supplement for pandemic COVID-19. A review. J. King Saud Univ. Sci. 2020, 33, 101286. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Fonseca Faria, J.d.A.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Szajnar, K.; Znamirowska, A.; Kuzniar, P. Sensory and textural properties of fermented milk with viability of Lactobacillus rhamnosus and Bifidobacterium animalis ssp. lactis Bb-12 and increased calcium concentration. Int. J. Food Prop. 2020, 23, 582–598. [Google Scholar] [CrossRef]
- Martínez, M.P.; Magnoli, A.P.; González Pereyra, M.L.; Cavaglieri, L. Probiotic bacteria and yeasts adsorb aflatoxin M1 in milk and degrade it to less toxic AFM1-metabolites. Toxicon 2019, 172, 1–7. [Google Scholar] [CrossRef]
- Sanaldi, K.; Coban, A.Y. Detoxification of aflatoxin M1 in different milk types using probiotics. An. Acad. Bras. Cienc. 2023, 95, e20220794. [Google Scholar] [CrossRef]
- Gunenc, A.; Khoury, C.; Legault, C.; Mirrashed, H.; Rijke, J.; Hosseinian, F. Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt. LWT Food Sci. Technol. 2016, 66, 490–495. [Google Scholar] [CrossRef]
- Mani-Lopez, E.; Palou, E.; Lopez-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Laird, E.; Molloy, A.M.; McNulty, H.; Ward, M.; McCarroll, K.; Hoey, L.; Hughes, C.F.; Cunningham, C.; Strain, J.J.; Casey, M.C. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos. Int. 2017, 28, 2409–2419. [Google Scholar] [CrossRef]
- Saleem, G.N.; Gu, R.; Qu, H.; Bahar Khaskheli, G.; Rashid Rajput, I.; Qasim, M.; Chen, X. Therapeutic potential of popular fermented dairy products and its benefits on human health. Front. Nutr. 2024, 11, 1328620. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef]
- Farag, M.A.; Saleh, H.A.; El Ahmady, S.; Elmassry, M.M. Dissecting yogurt: The impact of milk types, probiotics, and selected additives on yogurt quality. Food Rev. Int. 2022, 38, 634–650. [Google Scholar] [CrossRef]
- Brodziak, A.; Król, J.; Matwijczuk, A.; Czernecki, T.; Glibowski, P.; Wlazło, Ł.; Litwińczuk, A. Effect of sea buckthorn (Hippophae rhamnoides L.) mousse on properties of probiotic yoghurt. Appl. Sci. 2021, 11, 545. [Google Scholar] [CrossRef]
- Ghafarloo, M.H.; Jouki, M.; Tabari, M. Production and characterization of synbiotic doogh, a yogurt-based Iranian drink by gum arabic, ginger extract and B. bifidum. J. Food Sci. Technol. 2020, 57, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT Food Sci. Technol. 2019, 105, 242–249. [Google Scholar] [CrossRef]
- da Cruz, M.F.; Rocha, R.S.; Silva, R.; Freitas, M.Q.; Pimentel, T.C.; Esmerino, E.A.; Cruz, A.G.; Fidalgo, T.K.d.S.; Maia, L.C. Probiotic fermented milks: Children’s emotional responses using a product-specific emoji list. Food Res. Int. 2021, 143, 110269. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; He, Q.; Hu, J.; Zheng, J. Changes in proteolysis in fermented milk produced by Streptococcus thermophilus in co-culture with Lactobacillus plantarum or Bifidobacterium animalis subsp. lactis during refrigerated storage. Molecules 2019, 24, 3699. [Google Scholar] [CrossRef]
- Barat, A.; Ozcan, T. Growth of probiotic bacteria and characteristics of fermented milk containing fruit matrices. Int. J. Dairy Technol. 2018, 71, 120–129. [Google Scholar] [CrossRef]
- Mituniewicz-Malek, A.; Zielinska, D.; Ziarno, M. Probiotic monocultures in fermented goat milk beverages—Sensory quality of final product. Int. J. Dairy Technol. 2019, 72, 240–247. [Google Scholar] [CrossRef]
- Soni, R.; Jain, N.K.; Shah, V.; Soni, J.; Suthar, D.; Gohel, P. Development of probiotic yogurt: Effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J. Food Sci. Technol. 2020, 57, 2038–2050. [Google Scholar] [CrossRef]
- Soukoulis, C.; Lyroni, E.; Tzia, C. Sensory profiling and hedonic judgement of probiotic ice cream as a function of hydrocolloids, yogurt and milk fat content. LWT Food Sci. Technol. 2010, 43, 1351–1358. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, T.; Guo, S.; Kwok, L.-Y.; Zhang, H.; Wang, J. Metabolic dynamics of fermented milk beverages co-fermented with Bifidobacterium animalis subsp. lactis Probio-M8 and Lacticaseibacillus paracasei PC-01 during storage. LWT Food Sci. Technol. 2023, 185, 115196. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Nederhoff, A.L.; Groot, M.N.N.; van Boekel, M.A.J.S.; Mastwijk, H.C. Effect of electrical field strength applied by PEF processing and storage temperature on the outgrowth of yeasts and moulds naturally present in a fresh fruit smoothie. Int. J. Food Microbiol. 2016, 230, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, D.; Bao, Y.; Liao, X.; Wu, J. Characterization of diversity and probiotic efficiency of the autochthonous lactic acid bacteria in the fermentation of selected raw fruit and vegetable juices. Front. Microbiol. 2018, 9, 2539. [Google Scholar] [CrossRef] [PubMed]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Szutowska, J.; Rybicka, I.; Pawlak-Lemanska, K.; Gwiazdowska, D. Spontaneously fermented curly kale juice: Microbiological quality, nutritional composition, antioxidant, and antimicrobial properties. J. Food Sci. 2020, 85, 1248–1255. [Google Scholar] [CrossRef]
- Mantzourani, I.; Nikolaou, A.; Kourkoutas, Y.; Alexopoulos, A.; Dasenaki, M.; Mastrotheodoraki, A.; Proestos, C.; Thomaidis, N.; Plessas, S. Chemical profile characterization of fruit and vegetable juices after fermentation with probiotic strains. Foods 2024, 13, 1136. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Yang, J.; Li, X.; Lin, D.; Wang, M. Fermentation broth from fruit and vegetable waste works: Reducing the risk of human bacterial pathogens in soil by inhibiting quorum sensing. Environ. Int. 2024, 188, 108753. [Google Scholar] [CrossRef]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in fruit juices and production of bioactive compounds during their fermentation. Foods 2024, 13, 3851. [Google Scholar] [CrossRef]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of six lactic acid bacteria strains on physicochemical characteristics, antioxidant activities and sensory properties of fermented orange juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef]
- Golestani, M.; Pourahmad, R. Comparison of three treatments (two fermented treatments and one nonfermented treatment) in production of synbiotic ice cream. J. Food Process. Preserv. 2017, 41, e12839. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mortazavian, A.M. Review article: Technological aspects of prebiotics in probiotic fermented milks. Food Rev. Int. 2011, 27, 192–212. [Google Scholar] [CrossRef]
- Jeminiwa, B.O.; Knight, R.M.; Braden, T.D.; Cruz-espindola, C.; Boothe, D.M.; Akingbemi, B.T. Regulation of the neuroendocrine axis in male rats by soy-based diets is independent of age and due specifically to isoflavone action. Biol. Reprod. 2020, 103, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Kano, M.; Kaga, C. Possibility of breast cancer prevention: Use of soy isoflavones and fermented soy beverage produced using probiotics. Int. J. Mol. Sci. 2015, 16, 10907–10920. [Google Scholar] [CrossRef]
- Lampe, B.J.; English, J.C. Toxicological assessment of nattokinase derived from Bacillus subtilis var. natto. Food Chem. Toxicol. 2016, 88, 87–99. [Google Scholar] [CrossRef]
- Maslarov, D.; Drenska, D.; Maslarova-Gelov, J.; Gelov, I. Understanding the concept of Nattokinase use: A few years after beginning. Biotechnol. Biotechnol. Equip. 2023, 37, 2249552. [Google Scholar] [CrossRef]
- Kim, B.; Byun, B.Y.; Mah, J.-H. Biogenic amine formation and bacterial contribution in Natto products. Food Chem. 2012, 135, 2005–2011. [Google Scholar] [CrossRef]
- Lan, G.; Li, C.; He, L.; Zeng, X.; Zhu, Q. Effects of different strains and fermentation method on nattokinase activity, biogenic amines, and sensory characteristics of natto. J. Food Sci. Technol. 2020, 57, 4414–4423. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; He, Y.; Zhou, Y. Process optimization of co-fermentation natto with Lactobacillus bulgaricus and characteristic analysis. J. Food Sci. Technol. 2024, 62, 716–726. [Google Scholar] [CrossRef]
- Qian, W. Study on the Fermentation of Sausage with Composite Strains. Ph.D. Thesis, Jilin Agricultural University, Jilin, China, 2013. [Google Scholar]
- Gong, Y.; Sun, X.; Zhang, L.; Guo, Y.J.; Li, J.; Yang, Y.; Li, C.; Hu, B.; He, L. Processing technology of low-acidity Sichuan-flavor sausage produced by mixed-strain fermentation. Food Ind. Technol. 2015, 36, 227–232+239. [Google Scholar]
- Rubio, R.; Jofré, A.; Martín, B.; Aymerich, T.; Garriga, M. Characterization of lactic acid bacteria isolated from infant faeces as potential probiotic starter cultures for fermented sausages. Food Microbiol. 2014, 38, 303–311. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Barretto Penna, A.L.; da Silva Barretto, A.C. Applicability of potentially probiotic Lactobacillus casei in low-fat Italian type salami with added fructooligosaccharides: In vitro screening and technological evaluation. Meat Sci. 2020, 168. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Corral, S.; Cano-Garcia, L.; Salvador, A.; Belloch, C. Yeast strains as potential aroma enhancers in dry fermented sausages. Int. J. Food Microbiol. 2015, 212, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Marinova, V.Y.; Rasheva, I.K.; Kizheva, Y.K.; Dermenzhieva, Y.D.; Hristova, P.K. Microbiological quality of probiotic dietary supplements. Biotechnol. Biotechnol. Equip. 2019, 33, 834–841. [Google Scholar] [CrossRef]
- Saxelin, M.; Lassig, A.; Karjalainen, H.; Tynkkynen, S.; Surakka, A.; Vapaatalo, H.; Jarvenpaa, S.; Korpela, R.; Mutanen, M.; Hatakka, K. Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int. J. Food Microbiol. 2010, 144, 293–300. [Google Scholar] [CrossRef]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control. Release 2022, 349, 184–205. [Google Scholar] [CrossRef]
- Castro, A.; Aleman, R.S.; Tabora, M.; Kazemzadeh, S.; Pournaki, L.K.; Cedillos, R.; Marcia, J.; Aryana, K. Probiotic characteristics of Streptococcus thermophilus and Lactobacillus bulgaricus as influenced by new food sources. Microorganisms 2023, 11, 2291. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, Y.; Yu, X.; Liu, C.; Li, H.; Wang, X.; Yao, S. Fermentation characteristics of fermented milk with Streptococcus thermophilus CICC 6063 and Lactobacillus helveticus CICC 6064 and volatile compound dynamic profiles during fermentation and storage. Molecules 2024, 29, 1257. [Google Scholar] [CrossRef]
- Vandera, E.; Kakouri, A.; Koukkou, A.-I.; Samelis, J. Major ecological shifts within the dominant nonstarter lactic acid bacteria in mature Greek Graviera cheese as affected by the starter culture type. Int. J. Food Microbiol. 2019, 290, 15–26. [Google Scholar] [CrossRef]
- Sbehat, M.; Mauriello, G.; Altamimi, M. Microencapsulation of probiotics for food functionalization: An update on literature reviews. Microorganisms 2022, 10, 1948. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H. Electrospray assisted fabrication of hydrogel microcapsules by single- and double-stage procedures for encapsulation of probiotics. Food Bioprocess. Technol. 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Patarroyo, J.L.; Fonseca, E.; Cifuentes, J.; Salcedo, F.; Cruz, J.C.; Reyes, L.H. Gelatin-graphene oxide nanocomposite hydrogels for Kluyveromyces lactis encapsulation: Potential applications in probiotics and bioreactor packings. Biomolecules 2021, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Tian, H.; Yin, X.; Zhou, Y.; Zhu, L. Microencapsulation of 2-phenyl ethanol with methylcellulose/alginate/methylcellulose as the wall material and stability of the microcapsules. Polym. Bull. 2020, 77, 989–1001. [Google Scholar] [CrossRef]
- Al-Hamayda, A.; Abu-Jdayil, B.; Ayyash, M.; Tannous, J. Advances in microencapsulation techniques using Arabic gum: A comprehensive review. Ind. Crops Prod. 2023, 205, 117556. [Google Scholar] [CrossRef]
- Qi, F.; Zhu, J.; Li, M.; Ren, J.; Hu, Y.; Sun, Q. Preparation of W/O/W Lactiplantibacillus plantarum L3 microcapsules using modified low methoxy pectin as wall materials and their application in simulated yoghurt fermentation systems. Int. J. Dairy Technol. 2024, 77, 114–131. [Google Scholar] [CrossRef]
- Díaz Vergara, L.I.; Arata Badano, J.; Aminahuel, C.A.; Vanden Braber, N.L.; Rossi, Y.E.; Pereyra, C.M.; Cavaglieri, L.R.; Montenegro, M.A. Chitosan-glucose derivative as effective wall material for probiotic yeasts microencapsulation. Int. J. Biol. Macromol. 2023, 253, 127167. [Google Scholar] [CrossRef]
- Fauzi, M.A.R.D.; Pudjiastuti, P.; Wibowo, A.C.; Hendradi, E. Preparation, properties and potential of carrageenan-based hard capsules for replacing gelatine: A review. Polymers 2021, 13, 2666. [Google Scholar] [CrossRef]
- Shu, G.; He, Y.; Chen, L.; Song, Y.; Meng, J.; Chen, H. Microencapsulation of Lactobacillus acidophilus by xanthan-chitosan and its stability in yoghurt. Polymers 2017, 9, 733. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Li, X.; Michelon, M.; Leopercio, B.C.; Carvalho, M.S.; Frostad, J.M. Effects of aging on the shelf life and viscoelasticity of gellan gum microcapsules. Food Hydrocoll. 2021, 121, 106982. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Z.; Xia, J.; Kang, Y.; Sun, P.; Xiao, Z.; Niu, Y. Research progress of starch as microencapsulated wall material. Carbohydr. Polym. 2023, 318, 121118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, P.; Hu, Z.; Chen, Y.; Jin, X.; Deng, R.; Kirk, T.V.; Chen, X.D. Curcumin-loaded microcapsules with soy and whey protein as wall material: In vitro release, and ex vivo absorption based on the rat small intestine. J. Food Eng. 2024, 383, 112254. [Google Scholar] [CrossRef]
- Ye, S.; Fan, Y.; Cui, Y.; Chen, D.; Kong, L.; Liu, Y. Preparation of safflower oil microcapsules with gelatin and a novel exopolysaccharide as wall matrix. J. Food Process Eng. 2017, 40, e12573. [Google Scholar] [CrossRef]
- Boldoo, T.; Chinnasamy, V.; You, N.; Cho, H. Experimental analysis on thermal energy storage performance of micro-encapsulated stearic acid and stearyl alcohol PCM slurries; A comparative study. J. Energy Storage 2023, 73, 109218. [Google Scholar] [CrossRef]
- Yang, S.; Hu, X.; Cang, W.; Ji, S.; Wu, R.; Wu, J. Biofilm-based probiotic delivery system and its application in the food industry. Food Biosci. 2024, 62, 105172. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Zhang, Y.; Zhao, D.; Li, S.; Dou, H.; Wang, H.; Xia, X. The role of rcpA gene in regulating biofilm formation and virulence in Vibrio parahaemolyticus. Int. J. Food Microbiol. 2024, 418, 110714. [Google Scholar] [CrossRef]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef]
- Olson, J.K.; Navarro, J.B.; Allen, J.M.; McCulloh, C.J.; Mashburn-Warren, L.; Wang, Y.; Varaljay, V.A.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G408–G419. [Google Scholar] [CrossRef]
- Crook, N.; Ferreiro, A.; Gasparrini, A.J.; Pesesky, M.W.; Gibson, M.K.; Wang, B.; Sun, X.; Condiotte, Z.; Dobrowolski, S.; Peterson, D.; et al. Adaptive strategies of the candidate probiotic E. coli nissle in the mammalian gut. Cell Host Microbe 2019, 25, 499–512.e498. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Liu, F.; Hou, J.; Shao, H.; Yu, W. Stress adaptation and cross-protection of Lactobacillus plantarum KLDS 1.0628. CyTA-J. Food 2021, 19, 72–80. [Google Scholar] [CrossRef]
- Liu, K.; Fang, H.; Cui, F.; Nyabako, B.A.; Tao, T.; Zan, X.; Chen, H.; Sun, W. ARTP mutation and adaptive laboratory evolution improve probiotic performance of Bacillus coagulans. Appl. Microbiol. Biotechnol. 2020, 104, 6363–6373. [Google Scholar] [CrossRef] [PubMed]
- Bisson, G.; Marino, M.; Poletti, D.; Innocente, N.; Maifreni, M. Turbidimetric definition of growth limits in probiotic Lactobacillus strains from the perspective of an adaptation strategy. J. Dairy Sci. 2021, 104, 12236–12248. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, V.; Baweja, M.; Shukla, P. Gene editing and genetic engineering approaches for advanced probiotics: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1735–1746. [Google Scholar] [CrossRef]
- Carter, C.S.; Morgan, D.; Verma, A.; Lobaton, G.; Aquino, V.; Sumners, E.; Raizada, M.; Li, Q.; Buford, T.W. Therapeutic delivery of ang(1–7) via genetically modified probiotic: A dosing study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 75, 1299–1303. [Google Scholar] [CrossRef]
- Fang, T.; Liu, S. Metal-Phenolic Network Directed Coating of Single Probiotic Cell Followed by Photoinitiated Thiol-Ene Click Fortification to Enhance Oral Therapy. Small 2024, 20, 2308146. [Google Scholar] [CrossRef]
- Abou Ayana, I.A.A.; Elgarhy, M.R.; Al-Otibi, F.O.; Omar, M.M.; El-Abbassy, M.Z.; Khalifa, S.A.; Helmy, Y.A.; Saber, W.I.A. Artificial intelligence-powered optimization and milk permeate upcycling for innovative sesame milk with enhanced probiotic viability and sensory appeal. ACS Omega 2024, 9, 25189–25202. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Pinto, R.A.; Rossi, E.A.; Vallance, B.A.; Cavallini, D.C.U. Isolation and characterization of potentially probiotic bacterial strains from mice: Proof of concept for personalized probiotics. Nutrients 2018, 10, 1684. [Google Scholar] [CrossRef]
- Rudyk, M.; Hurmach, Y.; Serhiichuk, T.; Akulenko, I.; Skivka, L.; Berehova, T.; Ostapchenko, L. Multi-probiotic consumption sex-dependently interferes with MSG-induced obesity and concomitant phagocyte pro-inflammatory polarization in rats: Food for thought about personalized nutrition. Heliyon 2023, 9, e13381. [Google Scholar] [CrossRef]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L.K. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef]
- Tang, Q.; Hao, Y.; Wang, L.; Lu, C.; Lu, Z. Characterization of a bacterial strain Lactobacillus paracasei LP10266 recovered from an endocarditis patient in Shandong, China. BMC Microbiol. 2021, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.S.; Chen, C.W.; Kuo, Y.W.; Ho, H.H. Lactobacillus spp. reduces ethanol-induced liver oxidative stress and inflammation in a mouse model of alcoholic steatohepatitis. Exp. Ther. Med. 2021, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A. Antibiotic resistome of Salmonella typhi: Molecular determinants for the emergence of drug resistance. Front. Med. 2021, 15, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.R.; Achkar, J.P.; Brinich, M.A.; Farrell, R.M. Helping patients make informed choices about probiotics: A need for research. Am. J. Gastroenterol. 2009, 104, 809–813. [Google Scholar] [CrossRef]
- Besselink, M.G.H.; Santvoort, H.C.V.; Buskens, E.; Boermeester, M.A.; Gooszen, H.G. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Botes, M.; Reenen, C.A.V.; Dicks, L.M.T. Evaluation of Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 as probiotics by using a gastro-intestinal model with infant milk formulations as substrate. Int. J. Food Microbiol. 2008, 128, 362–370. [Google Scholar] [CrossRef]
- Moreno, M.R.F.; Sarantinopoulos, P.; Tsakalidou, E.; Vuyst, L.D. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef]
- Paudel, R.; Nepal, H.P. Linezolid resistance in vancomycin resistant Enterococci: A worrisome situation. Int. J. Basic. Clin. Pharmacol. 2021, 10, 464–465. [Google Scholar] [CrossRef]
- Nicas, T.I.; Wu, C.Y.; Hobbs, J.N.; Preston, D.A.; Allen, N.E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 1989, 33, 1121–1124. [Google Scholar] [CrossRef]
- Graham, K.; Stack, H.; Rea, R. Safety, beneficial and technological properties of enterococci for use in functional food applications—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3836–3861. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Ganzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Lim, M.Y.; Cho, Y.; Rho, M. Diverse distribution of resistomes in the human and environmental microbiomes. Curr. Genomics 2018, 19, 701–711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).