Evaluation of the Antimicrobial Capacity of a White Grape Marc Extract Through Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Albariño Marc Extract
2.3. INFOGEST Static In Vitro Simulations
2.4. Target Polyphenols’ LC-MS/MS Characterization
2.5. Bacterial Strains and Culture
2.6. Antimicrobial Activity
2.7. Statistical Analysis and Visualization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses-A Narrative Review. Antioxidants 2025, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K.; Najda, A.; Kaźmierski, S.; Głowniak, P.; Saso, L. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials, and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Panagiotakos, D.B.; Pitsavos, C.; Antonopoulou, S.; Nomikos, T.; Argyropoulou, A.; Stefanadis, C.; Antonopoulos, A. Association of Mean Daily Polyphenols Intake with Mediterranean Diet Adherence and Anthropometric Indices in Healthy Greek Adults: A Retrospective Study. Appl. Sci. 2021, 11, 4664. [Google Scholar]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Vaz, A.A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Physicochemical Properties and Bioaccessibility of Phenolic Compounds of Dietary Fibre Concentrates from Vegetable By-Products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, P.; Rueda-Robles, A.; Borrás-Linares, I.; Quirantes-Piné, R.M.; Emanuelli, T.; Segura-Carretero, A.; Lozano-Sánchez, J. Grape and Grape-Based Product Polyphenols: A Systematic Review of Health Properties, Bioavailability, and Gut Microbiota Interactions. Horticulturae 2022, 8, 583. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Chaiwangyen, W.; Chumphukam, O.; Kangwan, N.; Pintha, K.; Suttajit, M. Anti-aging effect of polyphenols: Possibilities and challenges. Plant Bioact. as Nat. Panacea Against Age-Induc. Dis. 2023, 147–179. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; Rama, J.L.R.; Villarino, R.-A.; Calvo, L.G.; Castillo, A.; Celeiro, M.; de Miguel, T. Antibacterial Activity against Clinical Strains of a Natural Polyphenolic Extract from Albariño White Grape Marc. Pharmaceuticals 2023, 16, 950. [Google Scholar] [CrossRef]
- Rama, J.L.R.; Mallo, N.; Biddau, M.; Fernandes, F.; de Miguel, T.; Sheiner, L.; Choupina, A.; Lores, M. Exploring the powerful phytoarsenal of white grape marc against bacteria and parasites causing significant diseases. Environ. Sci. Pollut. Res. 2021, 28, 24270–24278. [Google Scholar] [CrossRef]
- Castillo, A.; Celeiro, M.; Rubio, L.; Bañobre, A.; Otero-Otero, M.; Garcia-Jares, C.; Lores, M. Optimization of bioactives extraction from grape marc via a medium scale ambient temperature system and stability study. Front. Nutr. 2022, 28, 9. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Mulero, M.; Cuevas-Rodríguez, E.O.; Mondor, M.; Arcand, Y.; Hernández-Álvarez, A.J. In vitro gastrointestinal digestion impact on stability, bioaccessibility and antioxidant activity of polyphenols from wild and commercial blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef]
- Lores, M.; García-Jares, C.; Álvarez-Casas, M.; Llompart, M. Polyphenol Extract from White-Grape Residue. European Patent EP2875822A1, 27 May 2015. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Celeiro, M.; Lamas, J.P.; Arcas, R.; Lores, M. Antioxidants profiling of by-products from eucalyptus greenboards manufacture. Antioxidants 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.G.; Castillo, A.; Villarino, R.-A.; Rama, J.L.R.; Abril, A.G.; de Miguel, T. Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens. Antibiotics 2023, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.G.; Villarino, R.A.; Rama, J.L.R.; Abril, A.G.; de Miguel, T. A modification of the resazurin cell viability assay, suitable for the quantification of lactic acid producing bacteria. LWT 2025, 215, 117259. [Google Scholar] [CrossRef]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro-A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef]
- Chiappero, J.; Monti, G.A.; Acevedo, D.F.; Paulucci, N.S.; Yslas, E.I. Harnessing Silver Nanoclusters to Combat Staphylococcus aureus in the Era of Antibiotic Resistance. Pharmaceutics 2025, 17, 393. [Google Scholar] [CrossRef]
- Teh, C.H.; Nazni, W.A.; Nurulhusna, A.H.; Norazah, A.; Lee, H.L. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017, 17, 36. [Google Scholar] [CrossRef]
- Fai, P.B.; Grant, A. A Rapid Resazurin Bioassay for Assessing the Toxicity of Fungicides. Chemosphere 2009, 74, 1165–1170. [Google Scholar] [CrossRef]
- Tye, K.-Y.; Gan, S.-Y.; Lim, S.-H.E.; Tan, S.-E.; Chen, C.-A.; Phang, S.-M. Comparison of Visual Observation and Emission Intensity of Resazurin for Antimicrobial Properties of Hexane, Dichloromethane, Methanol and Water Extracts from a Brown Alga, Turbinaria Ornata. Cogent Biol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Mendes, R.J.; Sario, S.; Luz, J.P.; Tassi, N.; Teixeira, C.; Gomes, P.; Tavares, F.; Santos, C. Evaluation of Three Antimicrobial Peptides Mixtures to Control the Phytopathogen Responsible for Fire Blight Disease. Plants 2021, 10, 2637. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 12, 950–988. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 12774. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 26, 362. [Google Scholar] [CrossRef]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.-E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.-A.; Varvara, R.-A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato- Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, H.; Mc Clements, D.J. Application of Static In Vitro Digestion Models for Assessing the Bio-Accessibility of Hydrophobic Bioactives: A Review. Trends Food Sci. Technol. 2022, 122, 314–327. [Google Scholar] [CrossRef]
- Calvo, L.G.; Celeiro, M.; Lores, M.; Abril, A.G.; de Miguel, T. Assessing the Effect of Gastrointestinal Conditions and Solubility on the Bioaccessibility of Polyphenolic Compounds from a White Grape Marc Extract. Food Chem. 2025, 480, 143810. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Nagar, E.E.; Okun, Z.; Shpigelman, A. In Vitro Bioaccessibility of Polyphenolic Compounds: The Effect of Dissolved Oxygen and Bile. Food Chem. 2023, 404, 134490. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Xue, X.; Al Naggar, Y.; Wu, L.; Wang, K. Understanding the Gastrointestinal Protective Effects of Polyphenols using Foodomics-Based Approaches. Front. Immunol. 2021, 2, 671150. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Sakarya, F.B.; Tas, D.; Yurt, B.; Ercisli, S.; Capanoglu, E. In Vitro Bioaccessibility of Polyphenols in Different Fruit Matrices. ACS Omega 2023, 8, 12730–12738. [Google Scholar] [CrossRef] [PubMed]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Akritidou, T.; Akkermans, S.; Gaspari, S.; Azraini, N.D.; Smet, C.; Van de Wiele, T.; Van Impe, J.F.M. Effect of Gastric pH and Bile Acids on the Survival of Listeria monocytogenes and Salmonella Typhimurium during Simulated Gastrointestinal Digestion. Innov. Food Sci. Emerg. Technol. 2022, 82, 103161. [Google Scholar] [CrossRef]
- Giannella, R.A.; Broitman, S.A.; Zamcheck, N. Gastric acid barrier to ingested microorganisms in man: Studies in vivo and in vitro. Gut 1972, 13, 251–256. [Google Scholar] [CrossRef]
- Tennant, S.M.; Hartland, E.L.; Phumoonna, T.; Lyras, D.; Rood, J.I.; Robins-Browne, R.M.; van Driel, I.R. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect. Immun. 2008, 76, 639–645. [Google Scholar] [CrossRef]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of In Vitro Digestion on Bioactive Compounds, Antioxidant and Antimicrobial Activities of Coffee (Coffea arabica L.) Pulp Aqueous Extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef]
- González-Montiel, L.; Figueira, A.C.; Medina-Pérez, G.; Fernández-Luqueño, F.; Aguirre-Álvarez, G.; Pérez-Soto, E.; Pérez-Ríos, S.; Campos-Montiel, R.G. Bioactive Compounds, Antioxidant and Antimicrobial Activity of Propolis Extracts during In Vitro Digestion. Appl. Sci. 2022, 12, 7892. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to Antimicrobial Susceptibility Testing of Plant-Derived Polyphenolic Compounds. Arh. Hig. Rada Toksikol. 2020, 71, 300–311. [Google Scholar] [CrossRef]
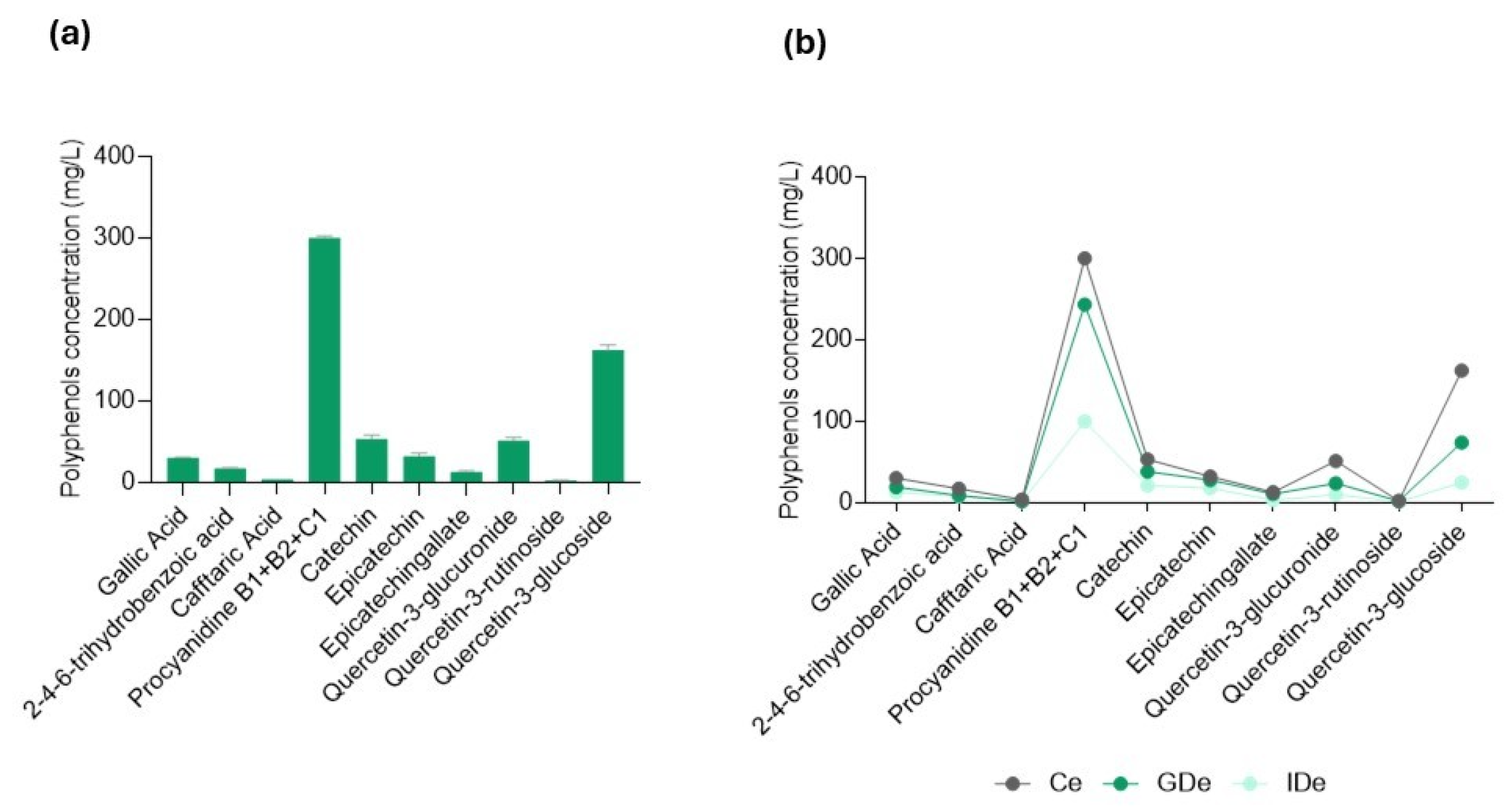
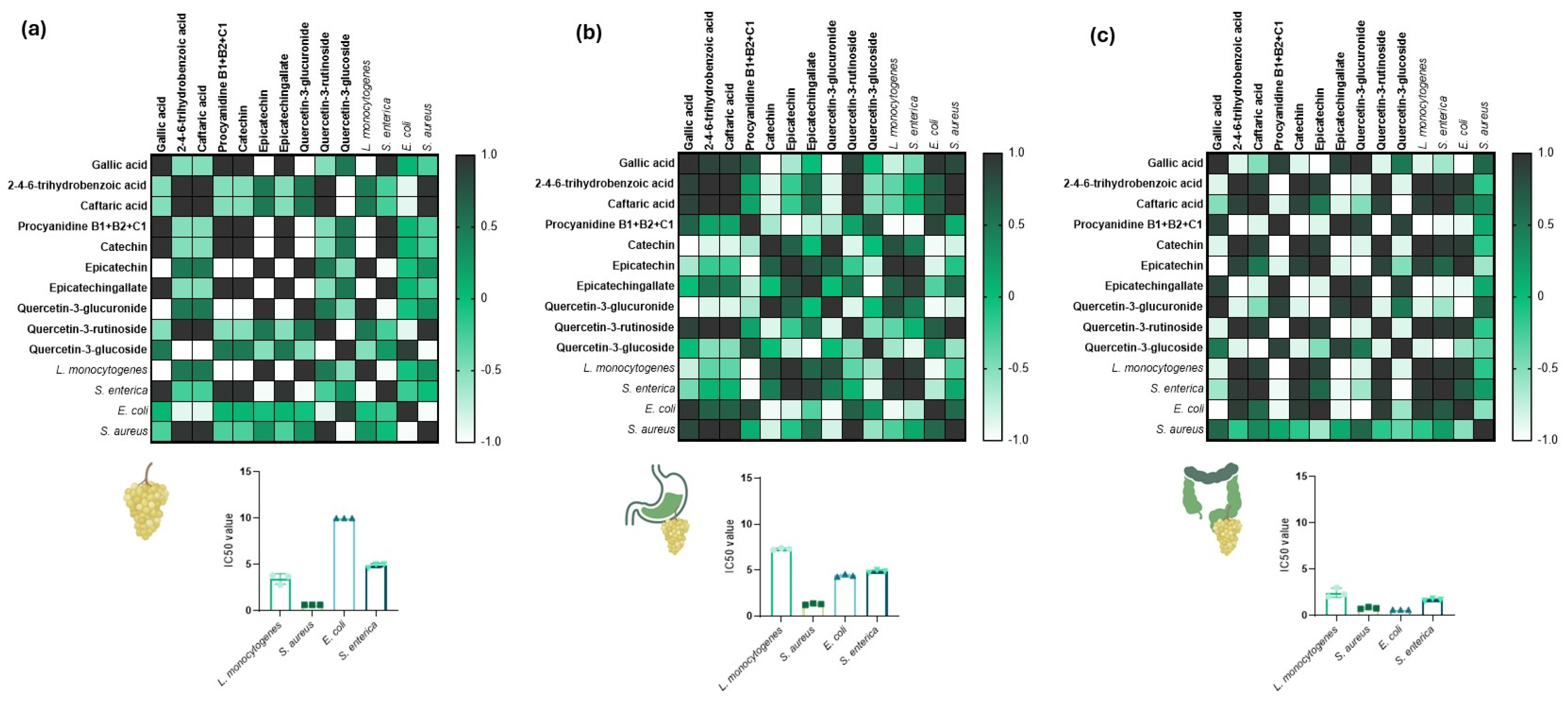
| Polyphenol | Ce | GDe | IDe |
| Gallic acid | 31 ± 1.8 a | 19 ± 1.4 b | 13 ± 0.25 b,c |
| 2-4-6-trihydrobenzoic acid | 18 ± 4 a | 10 ± 1.5 b | 7 ± 0.91 b,c |
| Caftaric acid | 4 ± 0.7 a | 2 ± 0.2 a | 1 ± 0.06 a |
| Procyanidine B1 + B2 + C1 | 302 ± 8.1 a | 241 ± 18.5 b | 104 ± 2.52 c |
| Catechin | 50 ± 1 a | 37 ± 1.2 b | 21 ± 0.48 c |
| Epicatechin | 30 ± 0.2 a | 27 ± 0.8 a | 17 ± 0.38 b |
| Epicatechingallate | 14 ± 0.2 a | 12 ± 0.9 a | 4 ± 0.22 b |
| Quercetin-3-glucuronide | 54 ± 1.3 a | 23 ± 1.2 b | 11 ± 0.24 c |
| Quercetin-3-rutinoside | 2 ± 0.1 a | 2 ± 0.4 a | 1 ± 0.37 a |
| Quercetin-3-glucoside | 167 ± 4.6 a | 71 ± 4.1 b | 24 ± 0.45 c |
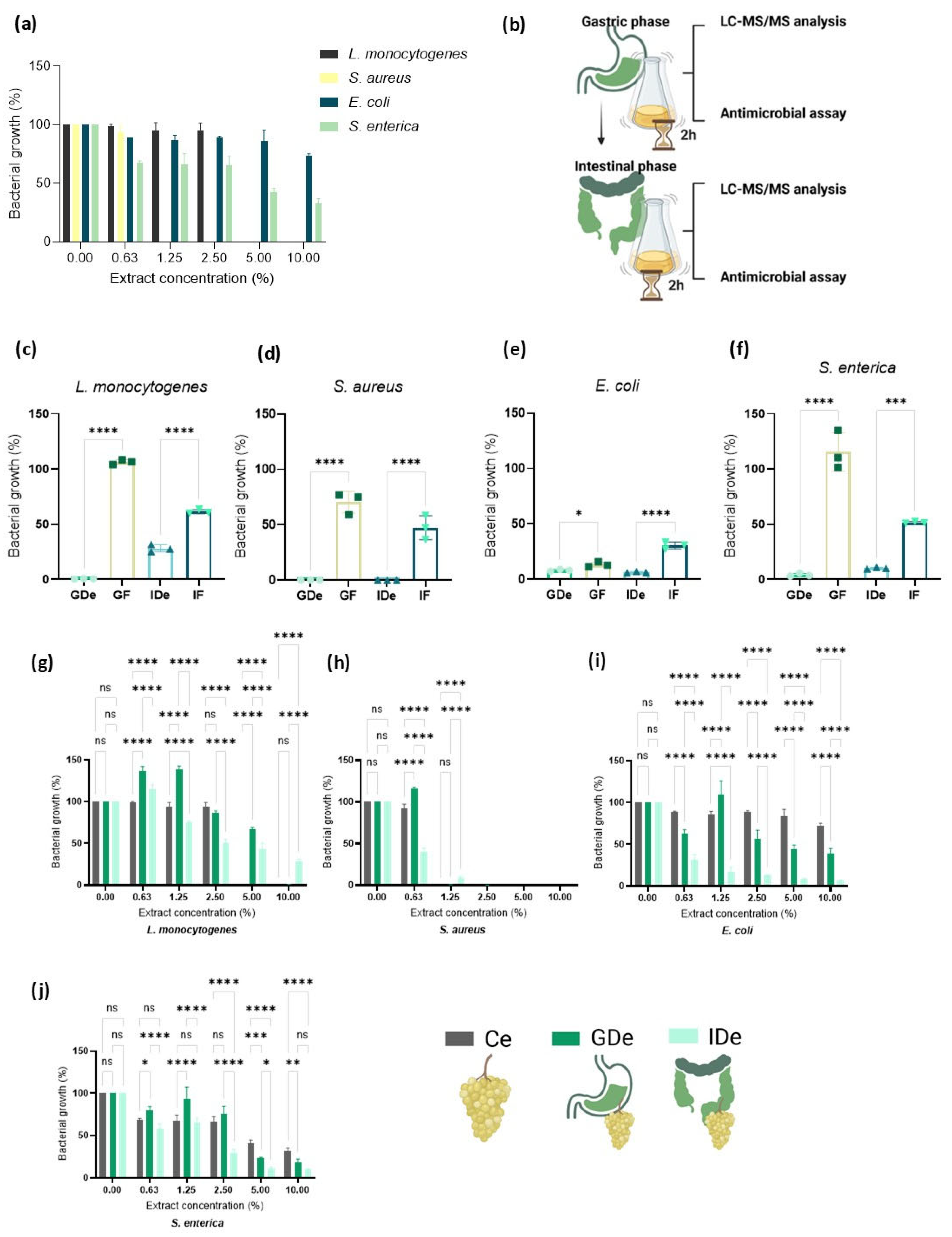
| Strains | Ce | GDe | IDe | |
|---|---|---|---|---|
| E. coli | IC50 | >10% | 4.58% | ≤0.625% |
| MBC | >10% | >10% | ≥10% | |
| S. aureus | IC50 | <0.625% | 1.22% | 0.72% |
| MBC | ≤0.625% | <2.5% | <2.5% | |
| S. enterica | IC50 | 4.98% | 4.84% | 1.67% |
| MBC | >10% | >10% | ≥10% | |
| L. monocytogenes | IC50 | 3.81% | 7.19% | 2.04% |
| MBC | ≤5% | ≤10% | ≥10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, L.G.; Celeiro, M.; Villarino, R.-A.; Abril, A.G.; Sánchez, S.; Rama, J.L.R.; de Miguel, T. Evaluation of the Antimicrobial Capacity of a White Grape Marc Extract Through Gastrointestinal Digestion. Appl. Sci. 2025, 15, 6390. https://doi.org/10.3390/app15126390
Calvo LG, Celeiro M, Villarino R-A, Abril AG, Sánchez S, Rama JLR, de Miguel T. Evaluation of the Antimicrobial Capacity of a White Grape Marc Extract Through Gastrointestinal Digestion. Applied Sciences. 2025; 15(12):6390. https://doi.org/10.3390/app15126390
Chicago/Turabian StyleCalvo, Lorena G., María Celeiro, Rosa-Antía Villarino, Ana G. Abril, Sandra Sánchez, José Luis R. Rama, and Trinidad de Miguel. 2025. "Evaluation of the Antimicrobial Capacity of a White Grape Marc Extract Through Gastrointestinal Digestion" Applied Sciences 15, no. 12: 6390. https://doi.org/10.3390/app15126390
APA StyleCalvo, L. G., Celeiro, M., Villarino, R.-A., Abril, A. G., Sánchez, S., Rama, J. L. R., & de Miguel, T. (2025). Evaluation of the Antimicrobial Capacity of a White Grape Marc Extract Through Gastrointestinal Digestion. Applied Sciences, 15(12), 6390. https://doi.org/10.3390/app15126390









