In Vitro Assays to Evaluate the Effects of Mango By-Product Polyphenolic Extracts Against Bacterial Species Associated with Food Spoilage and Human Diseases and the Relationship with Their Genotypes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
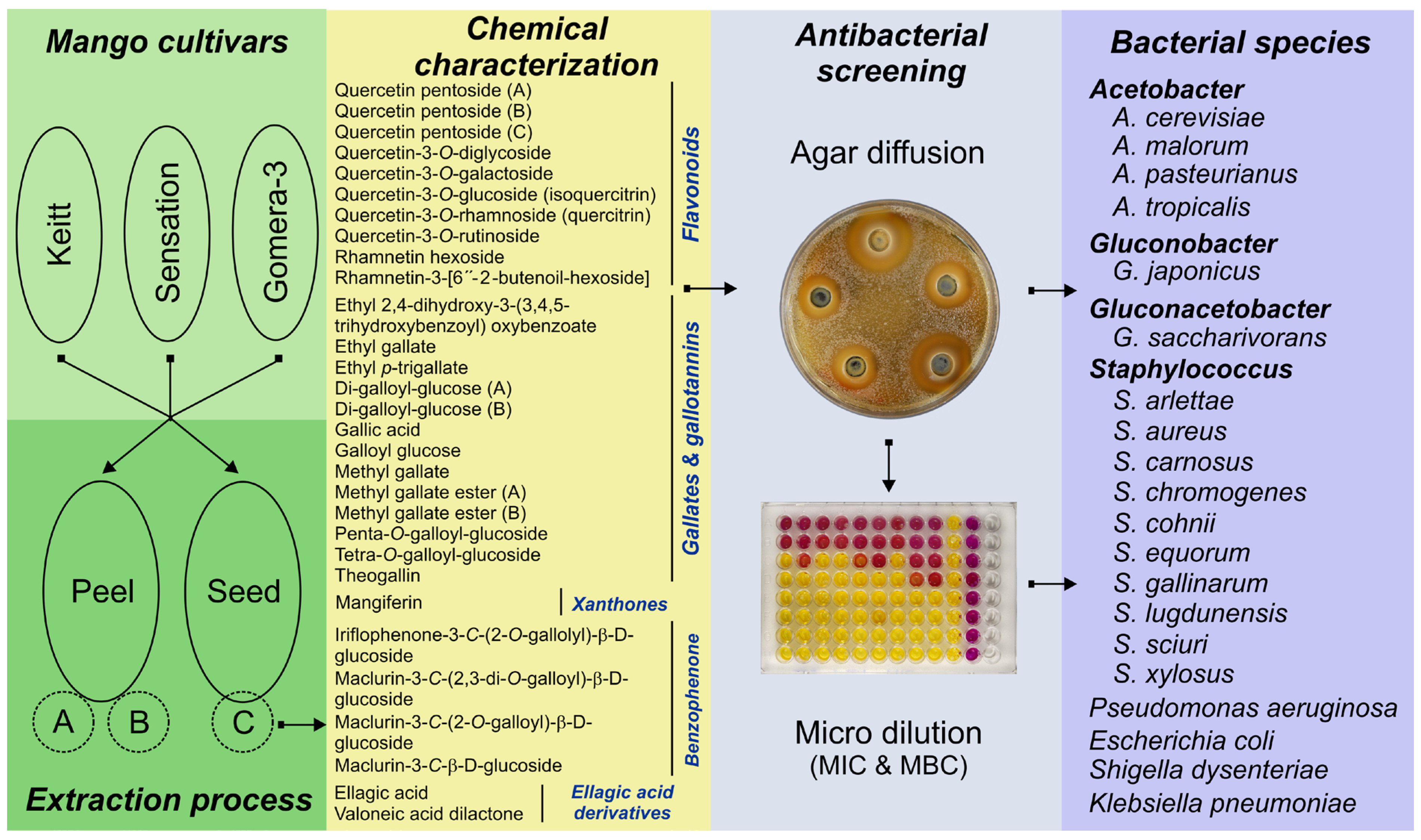
2.1. Plant Collection and Preparation of Mango By-Product Extracts
2.2. Bacterial Species and Culture Conditions
2.3. Antibacterial Screening
2.3.1. Agar Diffusion Assay
2.3.2. Microdilution Antibacterial Assay
2.4. Statistical Analysis
3. Results
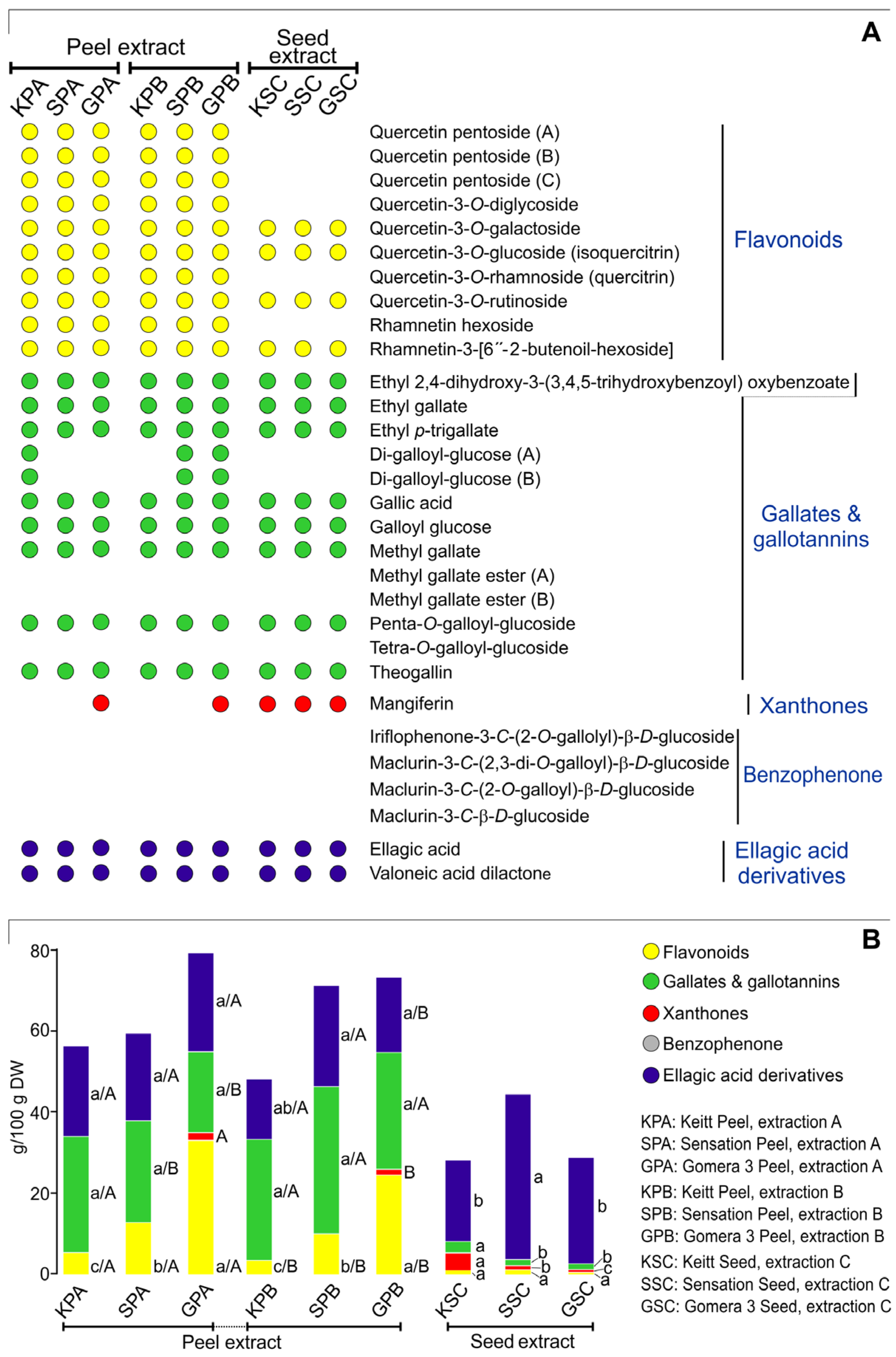
3.1. Bioactive Compound in Mango By-Product Extracts
3.2. Antibacterial Activity of Mango By-Products
3.2.1. Acetic Acid Bacteria
3.2.2. Staphylococcus Species
3.2.3. Human Pathogenic Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAB | Acetic Acid Bacteria |
| FAE | Fermentation-Assisted Extraction |
| GAE | mg gallic acid equivalents per mL |
| GPA | Gomera Peel, extraction A |
| GPB | Gomera Peel, extraction B |
| GSC | Gomera Seed, extraction C |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| KPA | Keitt Peel, extraction A |
| KPB | Keitt Peel, extraction B |
| KSC | Keitt Seed, extraction C |
| SAE | Solvent-Assisted Extraction |
| SPA | Sensation Peel, extraction A |
| SPB | Sensation Peel, extraction B |
| SSC | Sensation Seed, extraction C |
References
- Barata, A.; Caldeira, J.; Botelheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef][Green Version]
- Branco, P.; Coutinho, R.; Malfeito-Ferreira, M.; Prista, C.; Albergaria, H. Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide. Microorganisms 2021, 9, 2528. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V. Wine Features Related to Safety and Consumer Health: An Integrated Perspective. Crit. Rev. Food Sci. Nutr. 2012, 52, 31–54. [Google Scholar] [CrossRef]
- Alaiya, M.A.; Odeniyi, M.A. Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: A review. Future J. Pharm. Sci. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Hogg, T. Utilisation of natural and by-products to improve wine safety. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, M.V., BartoloméSualdea, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–49. [Google Scholar]
- Abdullah, A.; Saeed Mirghani, M.; Jamal, P. Antibacterial activity of malaysian mango kernel. Afr. J. Biotechnol. 2011, 10, 18739–18748. [Google Scholar]
- Widsten, P.; Cruz, C.D.; Fletcher, G.C.; Pajak, M.A.; McGhie, T.K. Tannins and Extracts of Fruit by products: Antibacterial Activity against Foodborne Bacteria and Antioxidant Capacity. J. Agric. Food. Chem. 2014, 62, 11146–11156. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Laich, F. Antifungal activity of mango peel and seed extracts against clinically pathogenic and food spoilage yeast. Nat. Prod. Res. 2015, 26, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.H.A.; Jahurul, I.S.M.; Ghafoor, Z.K.; Al-Juhaimi, F.Y. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gurjar, P.S.; Beer, K.; Pongener, A.; Ravia, S.C.; Singh, S.; Verma, A.; Singh, A.; Thakure, M.; Tripathyf, S.; et al. A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food Biosci. 2022, 48, 101783. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, T.; Liu, J.; Liu, T.; Gong, W. Biodegradable Antimicrobial Films Prepared in a Continuous Way by Melt Extrusion Using Plant Extracts as Effective Components. Food Chem. 2025, 464, 141643. [Google Scholar] [CrossRef]
- Sarker, M.S.; Alam, M.M.; Chen, J.; Wu, S.; Li, X.; Ali, N.; Mallasiy, L.O.; Alshehri, A.A. Maximizing Polyphenol Yield: Ultrasound-Assisted Extraction and Antimicrobial Potential of Mango Peel. Prep. Biochem. Biotechnol. 2025, 55, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kučuk, N.; Primožič, M.; Kotnik, P.; Knez, Ž.; Leitgeb, M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods 2024, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Lobo, M.G.; González, M. Improving the efficiency of antioxidant extraction from mango peel by using microwave-assisted extraction. Plant Food Hum. Nutr. 2013, 68, 190–199. [Google Scholar]
- Dorta, E.; Lobo, M.G.; González, M. Optimization of factors affecting extraction of antioxidants from mango seed. Food Bioprocess Technol. 2013, 6, 1067–1081. [Google Scholar]
- Dorta, E.; Gonzalez, M.; Lobo, M.G.; Sanchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Valera, M.J.; Laich, F.; González, S.S.; Torija, M.J.; Mateo, E.; Mas, A. Diversity of acetic acid bacteria present in healthy grapes from the Canary Islands. Int. J. Food Microbiol. 2011, 151, 105–112. [Google Scholar]
- WHO, World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 12 December 2024).
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Parvez, G.M. Pharmacological activities of mango (Mangifera indica): A review. J. Pharmacogn. Phytochem. 2016, 5, 1–7. [Google Scholar]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A review on ethnopharmacological applications, pharmacological activities and bioactive compounds of Mangifera indica (mango). Evid.-Based Complement. Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef]
- Maharaj, A.; Naidoo, Y.; Dewir, Y.H.; Rihan, H. Phytochemical screening and antibacterial and antioxidant activities of Mangifera indica L. Leaves. Horticulturae 2022, 8, 909. [Google Scholar] [CrossRef]
- Manzur, A.G.; Junior, S.M.; Morais-Costa, F.; Mariano, E.G.; Careli, R.T.; da Silva, L.M.; Duarte, E.R. Extract of Mangifera indica L. leaves may reduce biofilms of Staphylococcus spp. in stainless steel and teat cup rubbers. Food Sci. Technol. Int. 2019, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Gänzle, M.G.; Schieber, A. Fast LC–MS analysis of gallotannins from mango (Mangifera indica L.) kernels and effects of methanolysis on their antibacterial activity and iron binding capacity. Food Res. Int. 2012, 45, 422–426. [Google Scholar] [CrossRef]
- Kabuki, T.; Nakajima, H.; Arai, M.; Ueda, S.; Kuwabara, Y.; Dosoko, S. Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem. 2000, 71, 61–66. [Google Scholar] [CrossRef]
- Adams, M.R.; Moss, M.O. Food Microbiology, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2000; pp. 101–109. [Google Scholar]
- Gunnison, A.F.; Jacobsen, D.W. Sulfite hypersensitivity. A critical review. Crit. Rev. Toxicol. 1987, 17, 185–214. [Google Scholar] [CrossRef]
- Bomhard, E.M.; Brendler-Schwaab, S.Y.; Freyberger, A.; Herbold, B.A.; Leser, K.H.; Richter, M. Ophenylphenol and its sodium and potassium salts: A toxicological assessment. Crit. Rev. Toxicol. 2002, 32, 551–626. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Munuera, I.P.; Fiszman, S.; Martín-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Posth. Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, A.R.; Han, J. Antioxidant and antimicrobial activities of leafy green vegetable extracts and their application to meat product preservation. Food Control 2013, 29, 112–120. [Google Scholar] [CrossRef]
- Dillon, V.M.; Board, R.G. Future prospects for natural antimicrobial food preservation systems. In Natural Antimicrobial Systems and Food Preservation; Dillon, V.M., Board, R.G., Eds.; CAB International: Wallingford, UK, 1994; pp. 297–303. [Google Scholar]
- Parke, D.V.; Lewis, D.F.V. Safety aspects of food preservatives. Food Addit. Contam. 1992, 9, 561–577. [Google Scholar] [CrossRef]
- Bañón, S.; Díaz, P.; Rodríguez, M.; Garrido, M.D.; Price, A. Ascorbate, green tea and grape seed extracts increase the shelf-life of low sulphite beef patties. Meat Sci. 2007, 77, 626–633. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. Acetic acid bacteria spoilage of bottled red wine—A review. Int. J. Food Microbiol. 2008, 125, 60–70. [Google Scholar] [CrossRef]
- Alañón, M.E.; García-Ruíz, A.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Moreno-Arribas, M. Antimicrobial and antioxidant activity of pressurized liquid extracts from oenological woods. Food Control 2015, 50, 581–588. [Google Scholar] [CrossRef]
- Reguant, C.; Bordons, A.; Arola, L.; Rozès, N. Influence of phenolic compounds on the physiology of Oenococcusoeni from wine. J. Appl. Microbiol. 2000, 88, 1065–1071. [Google Scholar] [CrossRef]
- Vivas, N.; Lonvaud-Funel, A.; Glories, Y. Effect of phenolic acids and anthocyanins on growth, viability and malolactic activity of a lactic acid bacterium. Food Microbiol. 1997, 14, 291–300. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Cueva, C.; González-Rompinelli, E.M.; Yuste, M.; Torres Martín-Álvarez, J.; Bartolomé, B.; Moreno-Arribas, M.V. Antimicrobial phenolic extracts able to inhibit lactic acid bacteria growth and wine malolactic fermentation. Food Control 2012, 28, 212–219. [Google Scholar] [CrossRef]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska, L. Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine Phenolic Compounds: Antimicrobial Properties against Yeasts, Lactic Acid and Acetic Acid Bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Ascacio, L.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic acid: Biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2011, 10, 4518–4523. [Google Scholar]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Torres-León, C.; de Azevedo Ramos, B.; dos Santos Correi, M.T.; Carneiro-da-Cunha, M.G.; Ramirez-Guzman, N.; Alves, L.C.; Brayner, F.A.; Ascacio-Valdes, J.; Álvarez-Pérez, O.B.; Aguilar, C.N. Antioxidant and anti-staphylococcal activity of polyphenolic-rich extracts from Ataulfo mango seed. LWT Food Sci. Technol. 2021, 148, 111653. [Google Scholar] [CrossRef]
- EFSA. European Food Safety Authority. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2012. EFSA J. 2014, 12, 3547. [Google Scholar]
- Götz, F.; Bannerman, T.; Schleifer, K.H. The Genera Staphylococcus and Macrococcus. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 4, pp. 5–75. [Google Scholar]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-Negative Staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Bieber, L.; Kahlmeter, G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin. Microbiol. Infect. 2010, 16, 385–388. [Google Scholar] [CrossRef]
- Liu, C.M.; Price, L.B.; Hungate, B.A.; Abraham, A.G.; Larsen, L.A.; Christensen, K.; Stegger, M.; Skov, R.; Andersen, P.S. Staphylococcus aureus and the ecology of the nasal microbiome. Sci. Adv. 2015, 1, e1400216. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, H.; Raju, F.; Shaikh, A.Z.; Lin, A.N.; Lin, A.T.; Abboud, J.; Reddy, S. Staphylococcus lugdunensis endocarditis and cerebrovascular accident: A systemic review of risk factors and clinical outcome. Cureus 2018, 10, e2469. [Google Scholar] [CrossRef]
- Liu, P.Y.; Huang, Y.F.; Tang, C.W.; Chen, Y.Y.; Hsieh, K.S.; Ger, L.P.; Chen, Y.S.; Liu, Y.C. Staphylococcus lugdunensis infective endocarditis: A literature review and analysis of risk factors. J. Microbiol. Immunol. Infect. 2010, 43, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.L.; Del Pozo, J.L.; Patel, R. From clinical microbiology to infection pathogenesis: How daring to be different works for Staphylococcus lugdunensis. Clin. Microbiol. Rev. 2008, 21, 111–133. [Google Scholar] [CrossRef]
- Douiri, N.; Hansmann, Y.; Lefebvre, N.; Riegel, P.; Martin, M.; Baldeyrou, M.; Christmann, D.; Prevost, G.; Argemi, X. Staphylococcus lugdunensis: A virulent pathogen causing bone and joint infections. Clin. Microbiol. Infect. 2016, 22, 747–748. [Google Scholar] [CrossRef]
- Argemi, X.; Prevost, G.; Riegel, P.; Keller, D.; Meyer, N.; Baldeyrou, M.; Douiri, N.; Lefebvre, N.; Meghit, K.; Ronde Oustau, C.; et al. VISLISI trial, a prospective clinical study allowing identification of a new metalloprotease and putative virulence factor from Staphylococcus lugdunensis. Clin. Microbiol. Infect. 2017, 23, 334.e1–334.e8. [Google Scholar] [CrossRef] [PubMed]
- Casaes Nunes, R.S.; Pires de Souza, C.; Pereira, K.S.; Del Aguila, E.M.; Flosi Paschoalin, V.M. Identification and molecular phylogeny of coagulase-negative staphylococci isolates from Minas Frescal cheese in southeastern Brazil: Superantigenic toxin production and antibiotic resistance. J. Dairy Sci. 2016, 99, 2641–2653. [Google Scholar] [CrossRef]
- Gupta, P.; Deshmukh, P.; Ravishankerv. Antimicrobial and phytochemical screening of Mangifera indica against skin aliments. J. Pure Appl. Microbiol. 2010, 4, 387–392. [Google Scholar]
- Mirghani, M.E.S.; Yosuf, F.; Kabbashi, N.A.; Vejayan, J.; Yosuf, Z.B.M. Antibacterial activity of mango Kernel extracts. J. Appl. Sci. 2009, 9, 3013–3019. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Chomnawang, M.T. The inhibitory potential of Thai mango seed kernel extract against methicillin-resistant Staphylococcus aureus. Molecules 2011, 16, 6255–6270. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Q.; Hu, T.-G.; Xu, Y.-J.; Wu, J.-J.; Song, X.-L.; Yu, Y.-S. Interaction Mechanism of Carotenoids and Polyphenols in Mango Peels. Food Res. Int. 2023, 173, 113303. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Pasquel-Realtegui, J.L.; Dorronsoro-Guerrero, O.H.; Martínez-Correa, H.A. Phenolics and Carotenoids Recovery from Agroindustrial Mango Waste Using Microwave-Assisted Extraction: Extraction and Modeling. J. Food Process Eng. 2021, 44, e13774. [Google Scholar] [CrossRef]
- Sinha, S.; Das, S.; Saha, B.; Paul, D.; Basu, B. Anti-Microbial, Anti-Oxidant, and Anti-Breast Cancer Properties Unraveled in Yeast Carotenoids Produced via Cost-Effective Fermentation Technique Utilizing Waste Hydrolysate. Front. Microbiol. 2023, 13, 1088477. [Google Scholar] [CrossRef]
- Luo, F.; Lv, Q.; Zhao, Y.; Hu, G.; Huang, G.; Zhang, J.; Sun, C.; Li, X.; Chen, K. Quantification and purification of mangiferin from chinese mango (Mangifera indica L.) cultivars and its protective effect on human umbilical vein endothelial cells under H2O2 induced stress. Int. J. Mol. Sci. 2012, 13, 11260–11274. [Google Scholar] [CrossRef]
- Salomon, S.; Sevilla, I.; Betancourt, R.; Romero, A.; Nuevas-Paz, L.; Acosta-Esquijarosa, J. Extraction of mangiferin from Mangifera indica L. leaves using microwave assisted technique. Emir. J. Food Agric. 2014, 26, 616–622. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, Y.; Kumar, S.S.; Sharma, V.K.; Dua, K.; Samad, A. Antimicrobial evaluation of mangiferin analogues. Indian J. Pharm. Sci. 2009, 71, 328–331. [Google Scholar] [CrossRef]
| Acetic Acid Bacteria | Peel Extracts (mg GAE/mL) | Seed Extracts (mg GAE/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPA | SPA | KPA | GPB | SPB | KPB | GSC | SSC | KSC | |||
| Group I | Acetobacter A. cerevisiae (Ac3-6A2) | MIC | 8.3 | 8.3 | 8.3 | 1.7 | 8.3 | 1.7 | 8.3 | 17 | 17 |
| MBC | 25 | 25 | 25 | 17 | 25 | 17 | 50 | 50 | 50 | ||
| A. cerevisiae (Ac5-T5) | MIC | 8.3 | 8.3 | 8.3 | 1.7 | 8.3 | 8.3 | 17 | 17 | 17 | |
| MBC | 25 | 25 | 25 | 17 | 25 | 25 | 50 | 50 | 50 | ||
| A. cerevisiae (Ac2-6A1) | MIC | 8.3 | 8.3 | 8.3 | 1.7 | 1.7 | 1.7 | 8.3 | 25 | 17 | |
| MBC | 25 | 25 | 25 | 17 | 25 | 17 | 25 | 50 | 50 | ||
| A. pasteurianus (Ap16-Lz75) | MIC | 50 | 50 | 25 | 8.3 | 50 | 25 | 17 | 8.3 | 8.3 | |
| MBC | 100 | 100 | 100 | 50 | 100 | 100 | 50 | 50 | 50 | ||
| A. malorum (Am17-T33) | MIC | 50 | 50 | 50 | 25 | 50 | 50 | 25 | 25 | 100 | |
| MBC | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 200 | ||
| A. malorum (Am25-P21) | MIC | 50 | 50 | 50 | 25 | 50 | 50 | 25 | 25 | 100 | |
| MBC | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 200 | ||
| A. malorum (Am26-Lz67) | MIC | 50 | 50 | 50 | 25 | 50 | 50 | 25 | 25 | 100 | |
| MBC | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 50 | 200 | ||
| Group II | A. tropicalis (At1-T191) | MIC | 100 | 200 | 200 | 100 | 200 | 100 | 50 | 50 | 200 |
| MBC | 200 | 400 | 400 | 200 | 400 | 200 | 100 | 100 | 400 | ||
| A. tropicalis (At2-T59) | MIC | 100 | 200 | 200 | 100 | 200 | 100 | 50 | 50 | 200 | |
| MBC | 200 | 400 | 400 | 200 | 400 | 200 | 100 | 100 | 400 | ||
| Gluconobacter G. japonicas (Gj1-P37) | MIC | 100 | 50 | 200 | 100 | 50 | 50 | 50 | 50 | 50 | |
| MBC | 200 | 100 | 400 | 200 | 100 | 100 | 100 | 100 | 100 | ||
| G. japonicas (Gj3-Lz59) | MIC | 100 | 50 | 200 | 100 | 50 | 50 | 50 | 50 | 50 | |
| MBC | 200 | 100 | 400 | 200 | 100 | 100 | 100 | 100 | 100 | ||
| G. japonicas (Gj2-P92) | MIC | 100 | 100 | 200 | 50 | 50 | 50 | 50 | 50 | 50 | |
| MBC | 200 | 200 | 400 | 200 | 100 | 100 | 100 | 200 | 100 | ||
| Gluconacetobacter G. saccharivorans (Gs1-T80) | MIC | R * | R | R | R | R | R | R | R | R | |
| MBC | R * | R | R | R | R | R | R | R | R | ||
| Species | Isolate Code | Flavonoids | Xanthones | Gallates Gallotannins | Ellagic Acid Derivatives | Total Phenol Compounds |
|---|---|---|---|---|---|---|
| Acetobacter | ||||||
| A. cerevisiae | Ac3-6A2 | −0.4518 | 0.3708 | −0.5809 | 0.5809 | −0.5164 |
| A. cerevisiae | Ac5-T5 | −0.8385 * | 0.2725 | −0.7267 * | 0.5404 | −0.8385 * |
| A. cerevisiae | Ac2-6A1 | −0.4433 | 0.4445 | −0.7714 * | 0.4699 | −0.5497 |
| A. pasteurianus | Ap16-Lz75 | 0.5285 | −0.5339 | 0.4766 | 0.0693 | 0.5632 |
| A. malorum | Am17-T33 | 0.0559 | 0 | 0.3354 | −0.4099 | −0.0745 |
| A. malorum | Am25-P21 | 0.0559 | 0 | 0.3354 | −0.4099 | −0.0745 |
| A. malorum | Am26-Lz67 | 0.0559 | 0 | 0.3354 | −0.4099 | −0.0745 |
| A. tropicalis | At1-T191 | 0.2673 | −0.2745 | 0.5612 | −0.3742 | 0.1604 |
| A. tropicalis | At2-T59 | 0.2673 | −0.2745 | 0.5612 | −0.3742 | 0.1604 |
| Gluconobacter | ||||||
| G. japonicus | Gj1-P37 | 0.5578 | 0.13 | 0.2191 | −0.1594 | 0.5578 |
| G. japonicus | Gj3-Lz59 | 0.5578 | 0.13 | 0.2191 | −0.1594 | 0.5578 |
| G. japonicus | Gj2-P92 | 0.4781 | −0.2445 | 0.1394 | 0 | 0.3984 |
| Staphylococcus | ||||||
| S. arlettae | NRRL B-14764 | 0.414 | −0.1622 | −0.1035 | 0.6211 | 0.5175 |
| S. equorum subsp. equorum | NRRL B-14765 | 0.414 | −0.1622 | −0.1035 | 0.6211 | 0.5175 |
| S. carnosus subsp. carnosus | NRRL B-14760 | 0.1369 | 0.3575 | −0.4108 | 0.5477 | 0.1369 |
| S. xylosus | NRRL B-14776 | 0.1369 | 0.3575 | −0.4108 | 0.5477 | 0.1369 |
| S. sciuri subsp. sciuri | NRRL B-14767 | 0.1369 | 0.3575 | −0.4108 | 0.5477 | 0.1369 |
| S. gallinarum | NRRL B-14763 | 0.3651 | 0.0953 | −0.2739 | 0.5477 | 0.4564 |
| S. cohnii subsp. cohnii | NRRL B-14756 | 0.6739 | −0.4167 | 0.7625 * | −0.5763 | 0.6739 |
| S. lugdunensis | 99705-65 | −0.0791 | −0.2615 | 0.4480 | −0.4216 | −0.0264 |
| S. aureus | 11923-76 | 0.4099 | −0.0973 | 0.4845 | −0.5963 | 0.4099 |
| Pseudomonas aeruginosa | ATCC 27853 | −0.8216 * | 0.4767 | −0.8216 * | 0.4564 | −0.8216 * |
| Klebsiella pneumoniae | ATCC 13883 | −0.2739 | 0.286 | −0.7303 * | 0.4564 | −0.3651 |
| Staphylococcus Species | Peel Extracts (mg GAE/mL) | Seed Extracts (mg GAE/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPA | SPA | KPA | GPB | SPB | KPB | GSC | SSC | KSC | |||
| Group I | S. arlettae NRRL B-14764 | MIC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 1 |
| MBC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | ||
| S. equorum subsp. equorum NRRL B-14765 | MIC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 1 | |
| MBC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | ||
| S. carnosus subsp. carnosus NRRL B-14760 | MIC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | |
| MBC | 10 | 10 | 10 | 10 | 10 | 5 | 10 | 10 | 5 | ||
| S. xylosus NRRL B-14776 | MIC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | |
| MBC | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| S. sciuri subsp. sciuri NRRL B-14767 | MIC | 5 | 5 | 5 | 5 | 5 | 1 | 5 | 5 | 5 | |
| MBC | 30 | 15 | 15 | 30 | 15 | 15 | 120 | 120 | 120 | ||
| S. chromogenes NRRL B-14759 | MIC | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| MBC | 5 | 10 | 10 | 5 | 10 | 5 | 10 | 10 | 5 | ||
| Group II | S. gallinarum NRRL B-14763 | MIC | 10 | 10 | 5 | 10 | 10 | 5 | 10 | 10 | 5 |
| MBC | 30 | 15 | 10 | 15 | 15 | 10 | 240 | 240 | 240 | ||
| S. aureus subsp. aureus NRRL B-767 | MIC | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| MBC | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| S. aureus 11923-76 | MIC | 30 | 10 | 30 | 30 | 10 | 120 | 10 | 10 | 10 | |
| MBC | 60 | 15 | 60 | 60 | 15 | 240 | 30 | 15 | 15 | ||
| S. cohnii subsp. cohnii NRRL B-14756 | MIC | 15 | 15 | 10 | 15 | 15 | 60 | 5 | 5 | 5 | |
| MBC | nd | nd | nd | nd | nd | nd | nd | nd | nd | ||
| S. lugdunensis 99705-65 | MIC | 30 | 60 | 30 | 120 | 120 | 240 | 60 | 60 | 60 | |
| MBC | 120 | >240 | 120 | 240 | 240 | >240 | 120 | 120 | 120 | ||
| Species | Peel Extracts (mg GAE/mL) | Seed Extracts (mg GAE/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GPA | SPA | KPA | GPB | SPB | KPB | GSC | SSC | KSC | ||
| Pseudomonas aeruginosa ATCC 27853 | MIC | 10 | 10 | 10 | 10 | 10 | 10 | 30 | 30 | 30 |
| MBC | 30 | 30 | 30 | 30 | 30 | 30 | 60 | 60 | 30 | |
| Escherichia coli ATCC 25922 | MIC | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| MBC | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | |
| Shigella dysenteriae ATCC 13313 | MIC | 120 | 120 | 120 | 60 | 60 | 60 | 120 | 120 | 120 |
| MBC | >240 | >240 | >240 | >240 | >240 | >240 | >240 | >240 | >240 | |
| Klebsiella pneumoniae ATCC 13883 | MIC | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 |
| MBC | >240 | >240 | >240 | >240 | >240 | >240 | >240 | >240 | >240 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorta, E.; González, M.; Lobo, M.G.; Laich, F. In Vitro Assays to Evaluate the Effects of Mango By-Product Polyphenolic Extracts Against Bacterial Species Associated with Food Spoilage and Human Diseases and the Relationship with Their Genotypes. Appl. Sci. 2025, 15, 5845. https://doi.org/10.3390/app15115845
Dorta E, González M, Lobo MG, Laich F. In Vitro Assays to Evaluate the Effects of Mango By-Product Polyphenolic Extracts Against Bacterial Species Associated with Food Spoilage and Human Diseases and the Relationship with Their Genotypes. Applied Sciences. 2025; 15(11):5845. https://doi.org/10.3390/app15115845
Chicago/Turabian StyleDorta, Eva, Mónica González, María Gloria Lobo, and Federico Laich. 2025. "In Vitro Assays to Evaluate the Effects of Mango By-Product Polyphenolic Extracts Against Bacterial Species Associated with Food Spoilage and Human Diseases and the Relationship with Their Genotypes" Applied Sciences 15, no. 11: 5845. https://doi.org/10.3390/app15115845
APA StyleDorta, E., González, M., Lobo, M. G., & Laich, F. (2025). In Vitro Assays to Evaluate the Effects of Mango By-Product Polyphenolic Extracts Against Bacterial Species Associated with Food Spoilage and Human Diseases and the Relationship with Their Genotypes. Applied Sciences, 15(11), 5845. https://doi.org/10.3390/app15115845









