Peri-Implantitis Causal Therapy with and Without Doxycycline: Retrospective Cohort Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
- Control Group (CG): This cohort included patients who received non-surgical peri-implant therapy between January 2021 and December 2022 before the introduction of Ligosan® in clinical practice. These individuals underwent a standardized non-surgical treatment protocol consisting of professional mechanical debridement without local or systemic antibiotic drugs.
- Test Group (TG): This cohort comprised patients who underwent the same non-surgical peri-implant therapy between January 2022 and March 2024, after the introduction of Ligosan®. In addition to mechanical debridement, these patients were treated with 14% topical doxycycline gel to assess its adjunctive efficacy in peri-implant disease management.
2.3. Participants
2.4. Inclusion Criteria
- Age ≥ 18 years;
- Presence of at least one osseointegrated implant diagnosed with peri-implantitis based on radiographic and clinical criteria, according to the 2018 Classification of Periodontal and Peri-Implant Diseases and Conditions [15];
- Subjected to exclusively screw-retained full-ceramic implant–prosthetic rehabilitation, regardless of implant design;
- Availability of complete clinical records, including baseline (T0) and follow-up (T1) evaluations;
- Patients with compensated systemic conditions (e.g., controlled diabetes or cardiovascular disease);
- Smokers were not excluded to reflect real-world clinical conditions and assess the impact of smoking on treatment outcomes.
- Exclusion criteria
- Patients were excluded if they met any of the following conditions:
- Lack of complete clinical documentation, including missing baseline or follow-up data;
- Previous history of surgical peri-implant therapy within 12 months prior to study inclusion;
- Use of local or systemic antibiotics for peri-implant treatment within the 3 months preceding non-surgical therapy;
- Presence of autoimmune diseases or uncompensated systemic conditions (e.g., uncontrolled diabetes, immunosuppression) that could interfere with tissue healing and disease progression after treatment;
- Inability to comply with the scheduled follow-up protocol, due to either non-compliance or logistical constraints;
- Participation in concurrent clinical trials evaluating peri-implant disease management during the study period.
2.5. Variables
2.6. Non-Surgical Treatment Protocol (CG)
2.7. Non-Surgical Treatment Protocol Applying Doxycycline (TG)
2.8. Data Sources
2.9. Bias
2.10. Study Size Rationale
2.11. Statistical Methods
3. Results
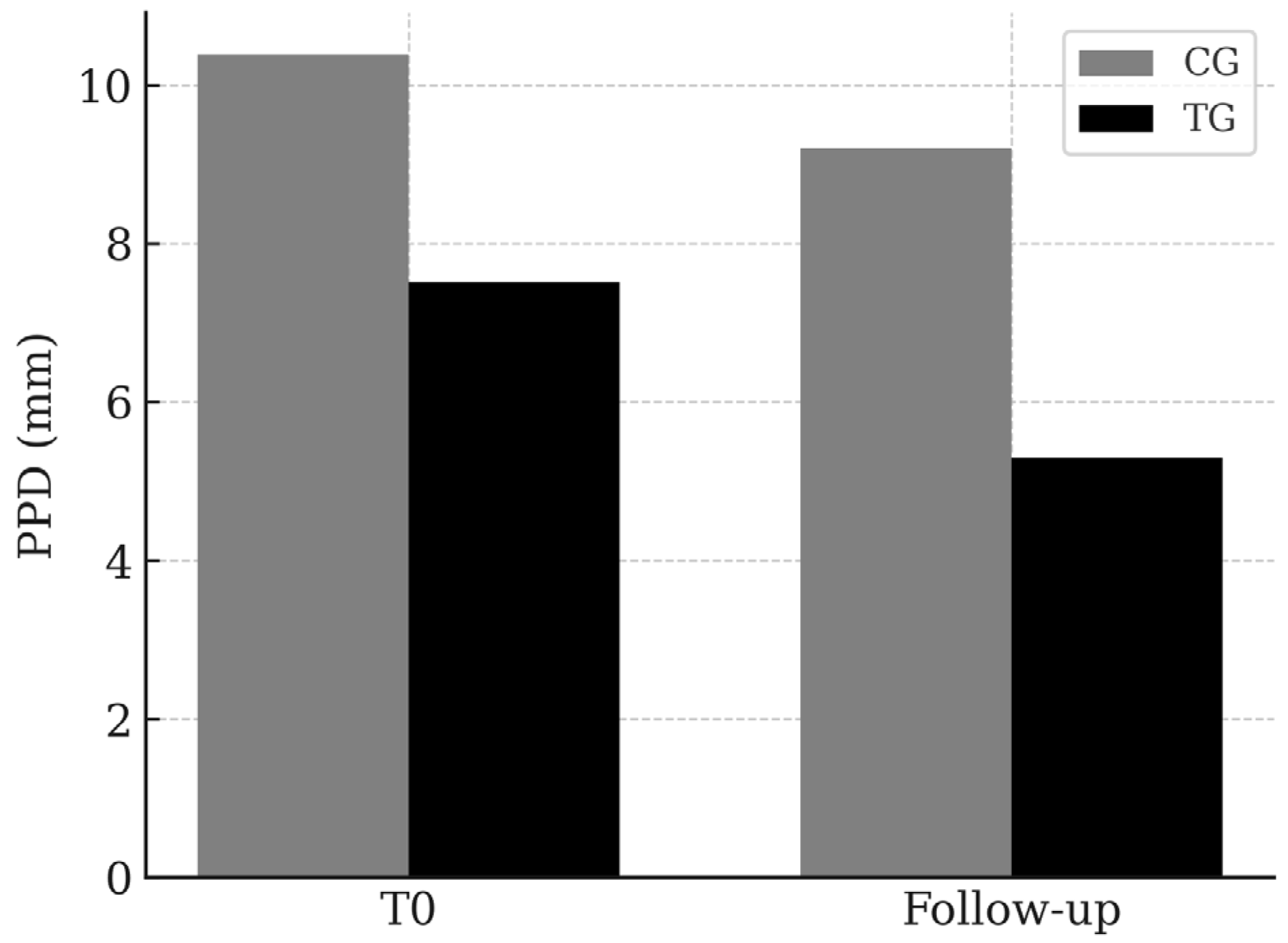
3.1. PPD
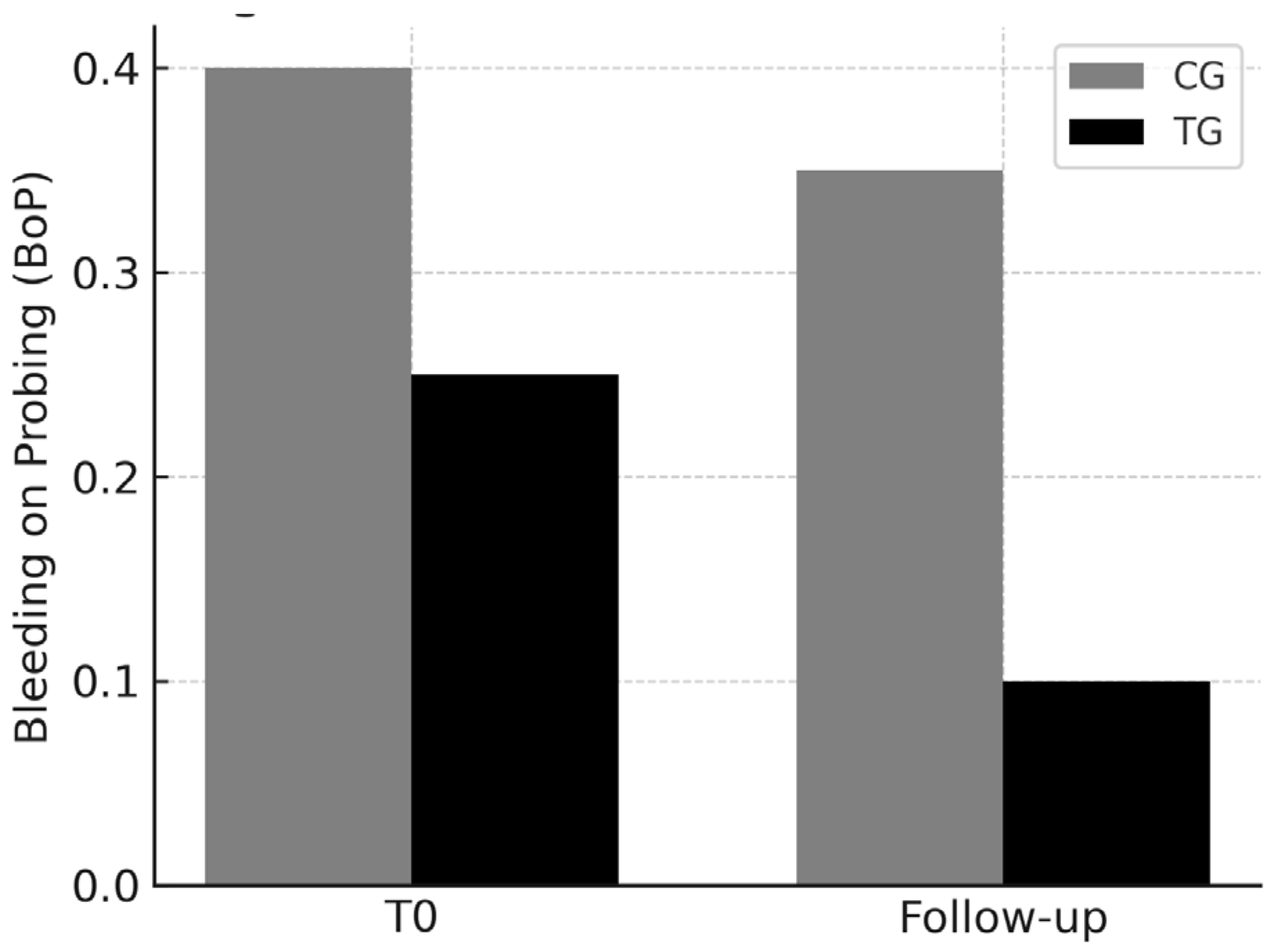
3.2. BoP
3.3. PI
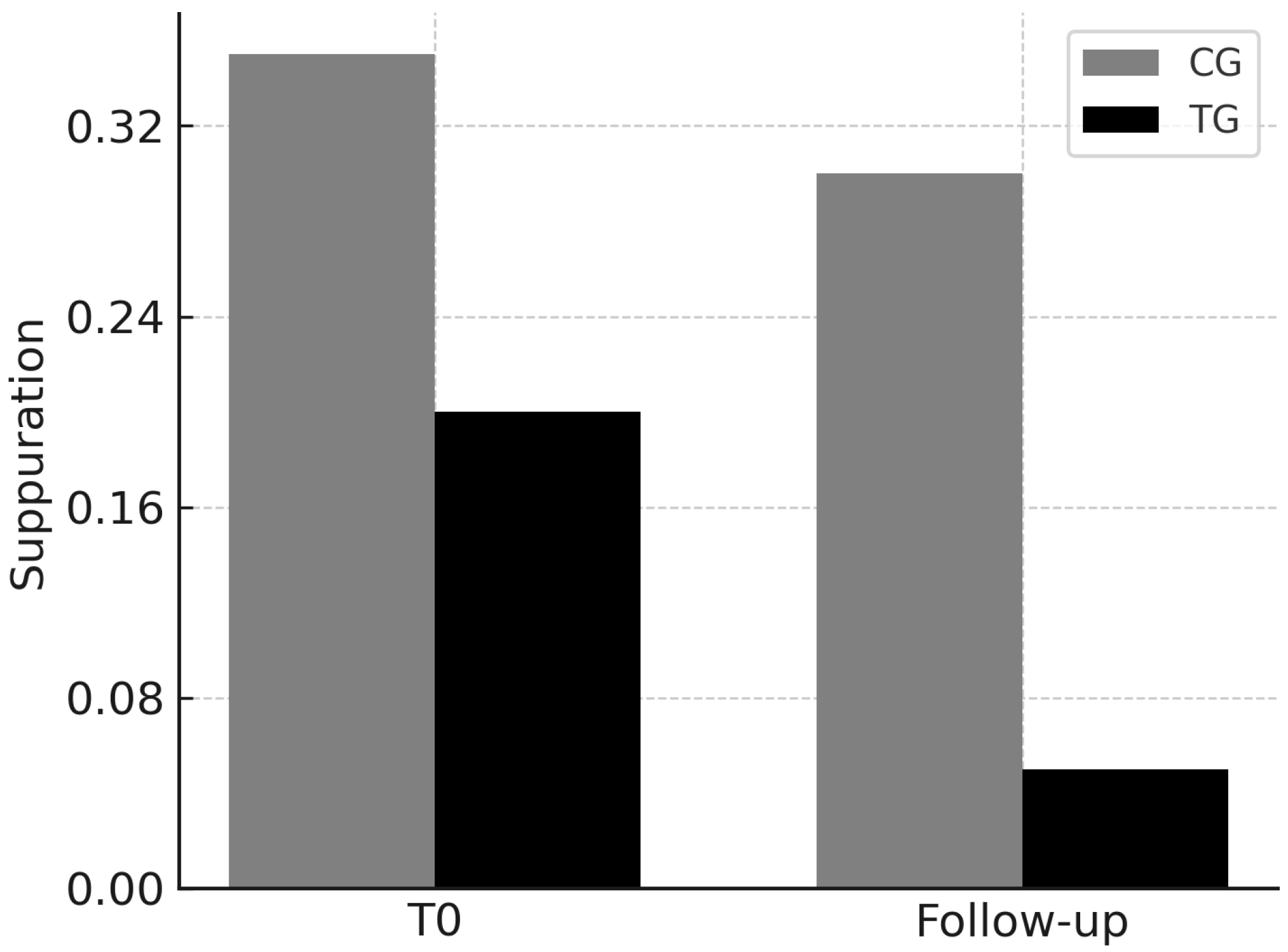
3.4. Suppuration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CG | Control Group |
| TG | Test Group |
| PPD | Peri-implant probing depth |
| BoP | Bleeding on Probing |
| PI | Placque Index |
References
- Howe, M.-S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nagni, M.; D’Orto, B.; Franceschi, L.; Zizza, A.; Cotticelli, C.; Ferrini, F. Five-Year Follow-Up Study on Full-Arch Implant-Prosthetic Rehabilitations: Evaluation of Immediate-Load Procedures with Digital Protocols. Eur. J. Musculoskelet. Dis. 2024, 13, S177–S190. [Google Scholar]
- Capparè, P.; Tetè, G.; D’Orto, B.; Nagni, M.; Gherlone, E.F. Immediate Loaded Full-Arch Mandibular Rehabilitations in Younger vs. Elderly Patients: A Comparative Retrospective Study with 7-Year Follow-Up. J. Clin. Med. 2023, 12, 4524. [Google Scholar] [CrossRef] [PubMed]
- Nagni, M.; Pirani, F.; D’Orto, B.; Ferrini, F.; Cappare, P. Clinical and Radiographic Follow-Up of Full-Arch Implant Prosthetic Rehabilitations: Retrospective Clinical Study at 6-Year Follow-Up. Appl. Sci. 2023, 13, 11143. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, Y.-W.; Zhu, L.; Weltman, R. Prevalence of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef]
- Monje, A.; Aranda, L.; Diaz, K.T.; Alarcón, M.A.; Bagramian, R.A.; Wang, H.L.; Catena, A. Impact of Maintenance Therapy for the Prevention of Peri-implant Diseases: A Systematic Review and Meta-analysis. J. Dent. Res. 2016, 95, 372–379. [Google Scholar] [CrossRef]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J. Periodontol. 2010, 81, 231–238. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Helbling, C.; Weber, H.P.; Matuliene, G.; Salvi, G.E.; Brägger, U.; Zwahlen, M.; Lang, N.P. Peri-implantitis susceptibility as it relates to periodontal status: A systematic review of the literature. Clin. Oral. Implant. Res. 2012, 23, 67–81. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Silva, G.L.M.; Cortelli, J.R.; Costa, J.E.; Costa, F.O. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J. Clin. Periodontol. 2006, 33, 929–935. [Google Scholar] [CrossRef]
- Tomasi, C.; Derks, J. Clinical research of peri-implant diseases—Quality assessment and future perspectives. Periodontol. 2000 2022, 88, 213–225. [Google Scholar] [CrossRef]
- Diaz, P.; Gonzalo, E.; Gil Villagra, L.J.; Miegimolle, B.; Suarez, M.J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral. Health 2022, 22, 449. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Orlandi, M.; Suvan, J.; Harden, S.; Smith, J.; D’Aiuto, F. Association between peri-implantitis and systemic inflammation: A systematic review. Front Immunol. 2023, 14, 1235155. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Alcoforado, G.; Guerrero, A.; Jönsson, D.; Klinge, B.; Lang, N.; Mattheos, N.; Mertens, B.; Pitta, J.; Ramanauskaite, A.; et al. Peri-implantitis: Summary and consensus statements of group 3. The 6th EAO Consensus Conference 2021. Clin. Oral Implant. Res. 2021, 32 (Suppl. S21), 245–253. [Google Scholar] [CrossRef]
- Liu, G.; Sun, H.; Shi, B.; Xia, H.; Wu, T. Rat Peri-implantitis Models: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2024, 39, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Giok, K.C.; Veettil, S.K.; Menon, R.K. Risk factors for Peri-implantitis: An umbrella review of meta-analyses of observational studies and assessment of biases. J. Dent. 2024, 146, 105065. [Google Scholar] [CrossRef]
- Santostasi, N.; Gerardi, D.; Rinaldi, F.; Bernardi, S.; Bianchi, I.; Pinchi, V.; Piattelli, M.; Varvara, G. Relationship between interleukin 1 (IL-1) genetic polymorphism and periimplantitis: Systematic literature review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3566–3582. [Google Scholar] [CrossRef]
- Giok, K.C.; Veettil, S.K.; Menon, R.K. Comparative effectiveness of interventions for the treatment of peri-implantitis: A systematic review with network meta-analysis. J. Prosthet. Dent. 2024, in press. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, S.; Liu, Y.; Cui, S. Adjunctive Use of Active Compounds such as Chlorhexidine in the Nonsurgical Treatment of Peri-Implant Mucositis for Oral Health: A Systematic Review and Meta-Analysis. Oxidative Med. Cell Longev. 2022, 2022, 2312784. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Treatment of peri-implantitis: What interventions are effective? A Cochrane systematic review. Eur. J. Oral Implant. 2012, 5, S21–S41. [Google Scholar]
- Polymeri, A.; van der Horst, J.; Moin, D.A.; Wismeijer, D.; Loos, B.G.; Laine, M.L. Non-surgical peri-implantitis treatment with or without systemic antibiotics: A randomized controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 548–557. [Google Scholar] [CrossRef]
- Liñares, A.; Sanz-Sánchez, I.; Dopico, J.; Molina, A.; Blanco, J.; Montero, E. Efficacy of adjunctive measures in the non-surgical treatment of peri-implantitis: A systematic review. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 224–243. [Google Scholar] [CrossRef] [PubMed]
- Baus-Domínguez, M.; Bakkali, S.; Hermida-Cabrera, P.; Serrera-Figallo, M.-A.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. A Systematic Review and Meta-Analysis of Systemic and Local Antibiotic Therapy in the Surgical Treatment of Peri-Implantitis. Antibiotics 2023, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of local antibiotic therapy in the treatment of peri-implantitis: A systematic review and meta-analysis. J. Dent. 2021, 113, 103790. [Google Scholar] [CrossRef] [PubMed]
- Grusovin, M.G.; Pispero, A.; Del Fabbro, M.; Sangiorgi, M.; Simion, M.; Stefanini, M.; Varoni, E.M. Antibiotics as Adjunctive Therapy in the Non-Surgical Treatment of Peri-Implantitis: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1766. [Google Scholar] [CrossRef]
- Drisko, C.H. The use of locally delivered doxycycline in the treatment of periodontitis. Clinical results. J. Clin. Periodontol. 1998, 25 Pt 2, 947–952; discussion 978–979. [Google Scholar] [CrossRef]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, P.; Wang, X.; Kasugai, S. A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: A radiographic and histological study in mice. Clin. Implant. Dent. Relat. Res. 2019, 21, 154–159. [Google Scholar] [CrossRef]
- Nastri, L.; De Rosa, A.; De Gregorio, V.; Grassia, V.; Donnarumma, G. A New Controlled-Release Material Containing Metronidazole and Doxycycline for the Treatment of Periodontal and Peri-Implant Diseases: Formulation and In Vitro Testing. Int. J. Dent. 2019, 2019, 9374607. [Google Scholar] [CrossRef]
- Passarelli, P.C.; Netti, A.; Lopez, M.A.; Giaquinto, E.F.; De Rosa, G.; Aureli, G.; Bodnarenko, A.; Papi, P.; Starzyńska, A.; Pompa, G.; et al. Local/Topical Antibiotics for Peri-Implantitis Treatment: A Systematic Review. Antibiotics 2021, 10, 1298. [Google Scholar] [CrossRef]
- Büchter, A.; Meyer, U.; Kruse-Lösler, B.; Joos, U.; Kleinheinz, J. Sustained release of doxycycline for the treatment of peri-implantitis: Randomised controlled trial. Br. J. Oral Maxillofac. Surg. 2004, 42, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Mahgoli, H.; Akhoundinasab, F.; Sadrai, S.; Shirani, G.; Mahgoli, M.H.; Bassir, S.H. Effect of prophylactic application of doxycycline at the implant-abutment interface on the outcomes of implant therapy: A split-mouth randomized clinical trial. Quintessence Int. 2022, 53, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Vivares-Builes, A.M. Antibiotic Resistance in Patients with Peri-Implantitis: A Systematic Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 15609. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J. Antibiotics in the treatment of peri-implantitis. Eur. J. Oral Implantol. 2012, 5, S43–S50. [Google Scholar]
- dos Reis, I.N.R.; Amaral, G.C.L.S.D.; Hassan, M.A.; Villar, C.C.; Romito, G.A.; Spin-Neto, R.; Pannuti, C.M. The influence of smoking on the incidence of peri-implantitis: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 34, 543–554. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin. Oral Implant. Res. 2015, 26, e62–e67. [Google Scholar] [CrossRef]
- Cheng, L.L. Smoking May Be Strongly Associated with Peri-Implantitis. J. Evid. Based Dent. Pract. 2023, 23, 101913. [Google Scholar] [CrossRef]
- D’ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? Dent. J. 2023, 11, 146. [Google Scholar] [CrossRef]
- Chhina, M.S. Is there a correlation between peri-implantitis and systemic inflammation? Evid. Based Dent. 2024, 25, 69–70. [Google Scholar] [CrossRef]



| CG | TG | |
|---|---|---|
| Number of patients | 96 | 108 |
| Average age (Range) | 55 (38–72) | 54.5 (34–75) |
| Female | 37 | 45 |
| Males | 59 | 63 |
| Affected by systemic diseases and no smokers | 19 | 23 |
| Affected by systemic diseases and smokers | 7 | 21 |
| Healthy patients and no smokers | 55 | 46 |
| Healthy patients and smokers | 15 | 18 |
| Single implant | 43 | 48 |
| Bridge-supported implants | 16 | 19 |
| Full-arch implant–prosthetic rehabilitation | 37 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Orto, B.; Polizzi, E. Peri-Implantitis Causal Therapy with and Without Doxycycline: Retrospective Cohort Clinical Study. Appl. Sci. 2025, 15, 6367. https://doi.org/10.3390/app15116367
D’Orto B, Polizzi E. Peri-Implantitis Causal Therapy with and Without Doxycycline: Retrospective Cohort Clinical Study. Applied Sciences. 2025; 15(11):6367. https://doi.org/10.3390/app15116367
Chicago/Turabian StyleD’Orto, Bianca, and Elisabetta Polizzi. 2025. "Peri-Implantitis Causal Therapy with and Without Doxycycline: Retrospective Cohort Clinical Study" Applied Sciences 15, no. 11: 6367. https://doi.org/10.3390/app15116367
APA StyleD’Orto, B., & Polizzi, E. (2025). Peri-Implantitis Causal Therapy with and Without Doxycycline: Retrospective Cohort Clinical Study. Applied Sciences, 15(11), 6367. https://doi.org/10.3390/app15116367






