Abstract
Zirconia ceramics are widely used in dentistry, but maintaining long-term color stability remains challenging. This study investigated the combined effects of specimen thickness, immersion duration, and aging of coloring solutions on the color stability of two multilayer commercial zirconia materials: TT ONE Multilayer (TT) and DD cubeX2 ML (DD). Discs (1.0–2.5 mm thick) were immersed in A2-shade coloring liquids for 30 s, 1 min, 3 min, and 5 min and evaluated after three months of solution aging. Color parameters (L*, a*, b*, C*, ΔE) were assessed, along with pH variation and Fe/Er ion concentrations using ICP-MS. Thinner specimens showed higher ΔE values and greater chromatic shifts than thicker ones. Aging of the coloring solutions increased L* values and discoloration, particularly in TT. ICP-MS revealed rising Fe and declining Er levels, correlating with observed optical changes. DD showed greater chemical and optical stability under identical conditions. These findings highlight the need to control zirconia thickness and coloring solution aging to preserve long-term esthetics.
1. Introduction
Zirconia has emerged as one of the most promising materials for dental restorations, largely due to its outstanding mechanical strength [1,2], improved translucency [3,4], and versatile application [5]. Advancements in material formulations have increased zirconia’s popularity in modern dentistry. However, despite its robust mechanical properties and corrosion resistance, maintaining its color stability in the oral environment remains challenging [6]. The material’s exposure to staining agents, surface roughness, and mechanical wear can compromise its aesthetic appearance over time, which is critical to the clinical success of dental restorations [7,8,9]. Achieving optimal color stability is essential, as it directly influences restorative materials’ longevity, functionality, and visual appeal in clinical settings. Research has demonstrated that color stability is affected by various factors, including material composition [10,11] and environmental conditions [12,13].
One of the most critical aspects impacting the color stability of zirconia is the immersion in coloring liquids during manufacturing [14]. This immersion process not only alters the chemical composition of the material but also significantly affects its optical properties, resulting in changes in translucency and color stability [15,16]. Studies have identified that ion release from zirconia during immersion in these liquids is pivotal in causing color alterations [9,17].
Nam and Park (2017) demonstrated that the mechanical performance of zirconia, particularly its hardness, can be significantly affected by the chosen coloring [18], suggesting that the manufacturing protocol has implications beyond esthetics alone. In a subsequent study, Nam et al. (2019) reported that treatment with aged coloring solutions resulted in perceptible increases in L* and a* values, indicating surface brightening and reddish shifts [19]. While both studies underscore the sensitivity of zirconia to coloring processes, neither addressed the influence of immersion duration, specimen thickness, or ion release dynamics—factors crucial to understanding long-term optical stability.
As ion release is closely linked to the chemical environment, particular attention must be given to the pH of coloring solutions, which directly affects ion solubility and color outcomes [20,21]. In particular, monitoring the pH of coloring solutions has emerged as a crucial factor, as pH directly affects the solubility and retention of metal ions, thereby impacting the final color of zirconia [22,23] and its characteristics after the sintering process [24]. However, a notable gap exists in the literature regarding the correlation between ion release, immersion duration, and the material’s long-term color stability. Current studies lack comprehensive insights into how specimen thickness and soaking time impact ion release and subsequent color shifts, particularly in pH fluctuations over time.
This study aims to address the existing knowledge gap by evaluating the long-term color stability of two multilayer zirconia materials, specifically focusing on the effects of ion release, specimen thickness, and immersion duration on optical outcomes. In addition, the study examines the aging behavior of coloring solutions by analyzing pH variations and ion concentration changes over time, to better understand their contribution to zirconia discoloration. By integrating optical and chemical analyses, this work provides mechanistic insights into the factors influencing color changes and contributes to improving the esthetic durability of zirconia-based restorations.
This study aims to bridge this gap by investigating and comparing the long-term color stability of two zirconia materials, focusing specifically on how ion release, thickness variation, and immersion time affect optical outcomes. Furthermore, the study explores the dynamic changes in the coloring liquids over an extended usage period, analyzing pH fluctuations and ion concentration alterations to better understand their contribution to color shifts in zirconia. The remainder of this article is organized as follows: Section 2 describes the materials and methods, Section 3 presents the results, Section 4 discusses the findings and study limitations, and Section 5 provides the conclusions.
2. Materials and Methods
2.1. Sample Preparation
Two commercially available zirconia systems—TT ONE® Multilayer (hereafter referred to as TT) and DD cubeX2® ML (hereafter referred to as DD)—were selected for evaluation in this study. The material specifications summarized in Table 1 were derived from the official technical data sheets and product literature issued by the respective manufacturers.

Table 1.
Tested materials and their identifications.
This in vitro study involved a total of 96 disc-shaped zirconia specimens (10 mm in diameter), fabricated using a dental CAD/CAM system (Cameo 250i; Aidite Technology, Shenzhen, China). Zirconia, following the protocol-outlined study [24], was evaluated; TT (n = 48) and DD (n = 48). Each group was further stratified into four subgroups based on thickness (1.0 mm, 1.5 mm, 2.0 mm, and 2.5 mm; n = 12 per subgroup). Specimens were stained using brand-specific A2 shade coloring liquids via an immersion technique—ZirColor ST for TT and DD Shade Liquid (A2 shade) for DD. The sample size was determined with consideration of α = 0.05, β = 0.1, and from the results of previous studies [23,25,26,27].
2.2. Coloring Protocol and Color Measurement
For the immersion protocol, specimens in each subgroup were immersed for one of four durations: 30, 60, 180, or 300 s [16,28]. After immersion, all specimens were air-dried and placed under infrared light at 80 °C for 30 min to evaporate surface moisture. Sintering was conducted using a high-temperature zirconia furnace (inFire HTC; Dentsply Sirona, Manheimm, Germany) following manufacturer guidelines, with final temperatures ranging from 1450 °C to 1550 °C, depending on the material.
Two widely accepted colorimetric systems—CIELAB and CIEDE2000—were utilized to quantify color differences, consistent with established protocols in dental color research [29,30]. The CIEDE2000 model refines color difference calculations by incorporating factors such as chroma and hue, drawing from Munsell’s color theory. In contrast, the CIELAB system represents color in a three-dimensional space: L* (lightness, ranging from 0 = black to 100 = white), a* (green to red axis), and b* (blue to yellow axis), aligning with human visual perception [29,31]. This framework allows for a comprehensive and perceptually relevant evaluation of color characteristics. The color difference (ΔE*ab) for each group was calculated using the standard formula described in the previous literature [29,32].
ΔEab = [(ΔL*)2 + (Δa*)2 + (Δb*)2]½.
For CIEDE2000, color difference was calculated based on adjustments for lightness (ΔL′), chroma (ΔC′), and hue (ΔH′) according to parametric weighting factors (KL, KC, KH) using the formula:
Measurements using Shadepilot spectrophotometer (Degudent, Hanau, Germany) were taken twice: once after 24 h post-sintering and again after 90 days of water storage at 37 °C.
2.3. Assessment of pH Variation and Ion Release Dynamics
To assess the effect of coloring solution aging, additional specimens were immersed in solutions that had been aged for three months under laboratory conditions. Surface brightness (L*) and color change (ΔE) were compared between fresh and aged solution groups. pH measurements of the coloring liquids were conducted at eight time points over the 90-day period (days 1, 14, 28, 42, 56, 70, 84, and 90) using a calibrated digital pH meter (a-AB41PH ZH, OHAUS, Changzhou, China). Specimens were stored in individual containers at 37 °C in a shaking incubator, and extraction media were replaced every 14 days.
For ion release analysis, specimens were immersed in 10 mL of A2 coloring liquid at 37 °C, and leachate samples were collected at baseline, 30 days, and 90 days. Ion concentrations of iron (Fe) and erbium (Er) were measured using inductively coupled plasma mass spectrometry (ICP-MS; iCAP Qc, ThermoFisher Scientific, Waltham, MA, USA). At each interval, 5 mL of immersion liquid was withdrawn for analysis and replaced with fresh solution to simulate dynamic clinical exposure. Ion release values were recorded in parts per billion (ppb).
2.4. Statistical Analysis
Statistical analysis was performed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA). Two-way ANOVA was used to assess the influence of thickness and immersion time on ΔE values, followed by Tukey’s HSD test for pairwise comparisons. A post hoc power analysis was also conducted using G*Power 3.1.
3. Results
3.1. Colorimetric Results of Zirconia Test Pieces with Different Thicknesses at Different Immersion Times
The analysis of color difference (ΔE) based on L*a*b* and LCH systems across different thicknesses and immersion times demonstrated distinct material-dependent variations, as shown in Figure 1. In DD specimens, ΔE values remained relatively low and stable across all thickness groups (1.0 mm to 2.5 mm), with only modest increases observed at longer immersion times (3 and 5 min). Notably, the 2.5 mm DD specimens consistently exhibited the smallest color differences.
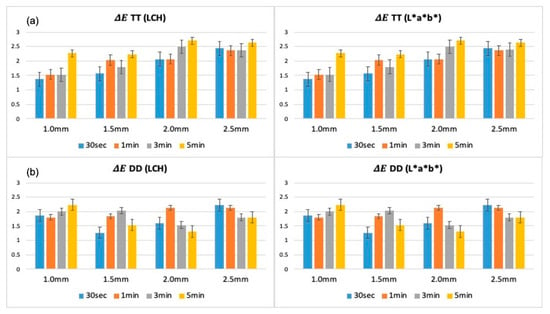
Figure 1.
Colorimetric results (ΔE) of TT (a) and DD (b) zirconia specimens assessed across different thicknesses and immersion durations, using L*a*b* and LCH systems. TT: TT ONE Multilayer; DD: cubeX2 ML.
In contrast, TT specimens exhibited higher ΔE values across all immersion times, particularly in thinner specimens (1.0 mm and 1.5 mm). Prolonged immersion (5 min) amplified color deviations in TT specimens, whereas increased thickness (2.0 mm and 2.5 mm) led to a general reduction in ΔE values. Consistent trends were observed when comparing Lab* and LCH calculations: thicker specimens showed better color stability in both zirconia brands, with DD exhibiting overall superior stability compared to TT.
3.2. Colorimetric Results of Different Immersion Times at Different Thicknesses
Analysis of colorimetric changes across different immersion durations at each thickness level revealed distinct trends between the two zirconia brands, as presented in Figure 2. For TT specimens, ΔE values increased with longer immersion times at all thickness levels. Thinner specimens (1.0 mm and 1.5 mm) exhibited the greatest color deviations, particularly at 5 min, whereas thicker specimens (2.0 mm and 2.5 mm) showed smaller but still measurable increases. In contrast, DD specimens maintained relatively stable ΔE values across all immersion times and thicknesses.
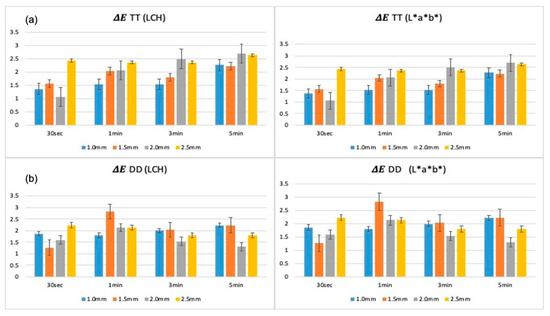
Figure 2.
Color differences (ΔE) of TT (a) and DD (b) zirconia specimens evaluated at multiple immersion times across a range of thicknesses, analyzed using L*a*b* and LCH methods. TT: TT ONE Multilayer; DD: cubeX2 ML.
3.3. Colorimetric Changes and Lightness Ratio (L*) of Zirconia Specimens After 3 Months of Coloring Solution Aging
After 3 months of continuous use, significant alterations in the optical properties of the coloring solutions were observed, impacting the colorimetric outcomes of zirconia specimens. Color differences (ΔE) increased notably, particularly in specimens immersed in aged liquids (Figure 3), and these changes were further supported by variations in the surface brightness (L*) of the zirconia (Figure 4).
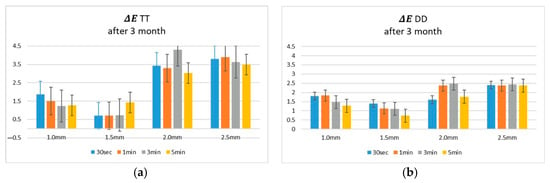
Figure 3.
Colorimetric results (ΔE L*, a*, b*) of coloring solutions after 3 months of use, measured following immersion of (a) TT and (b) DD zirconia specimens. TT: TT ONE Multilayer; DD: cubeX2 ML.
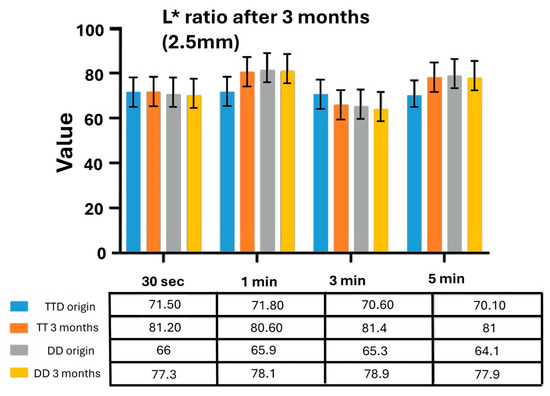
Figure 4.
Lightness ratio (L*) of TT and DD zirconia specimens with different thicknesses after immersion in coloring solutions aged for 3 months. TT: TT ONE Multilayer; DD: cubeX2 ML.
In TT specimens, ΔE values increased markedly after immersion in aged solutions, especially in thicker specimens (2.0 mm and 2.5 mm). Complementary analysis of the L* ratio at 2.5 mm thickness revealed a substantial increase in lightness after 3 months across all immersion times. In contrast, DD specimens demonstrated superior optical stability. ΔE values remained low across thicknesses and immersion times, even after 3 months of solution use. The L* ratio analysis further confirmed that the surface brightness of DD specimens remained relatively unchanged after immersion in aged solutions, indicating minimal optical alteration.
3.4. Subsection pH Fluctuations and Ion Release Dynamics During 3-Month Aging
The pH analysis of the extraction media over a 90-day period (Figure 5) revealed material-specific variations in chemical stability between TT and DD zirconia systems immersed in A2 shade coloring liquids. TT zirconia, treated with ZirColor ST, exhibited a dynamic pH trajectory, peaking at 1.30 on day 28, followed by a gradual decline to 1.03 by day 70, and a slight rebound to 1.05 at day 90. The overall mean pH value for TT was 1.14, indicating greater fluctuation over time. Conversely, DD zirconia, immersed in DD Shade Liquid, maintained a more consistent and lower pH profile, ranging from 0.90 on day 14 to a minimum of 0.82 on day 56, with a moderate increase to 1.01 by the end of the observation period. The mean pH value for DD remained at 0.88, underscoring its relatively stable chemical environment.
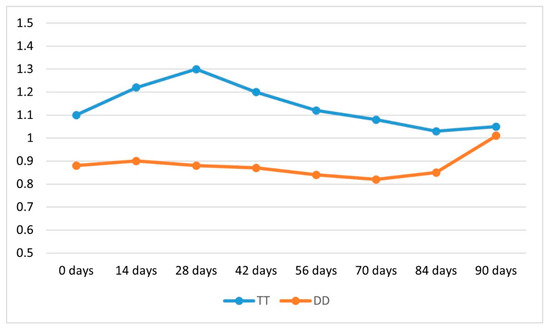
Figure 5.
pH variation of coloring solutions used for TT and DD zirconia over a 90-day period of continuous use. TT: TT ONE Multilayer; DD: cubeX2 ML.
Complementary analysis of ion concentrations using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) revealed distinct temporal patterns in the release of Fe and Er ions during the 3-month immersion period (Figure 6). In both TT and DD groups, Fe concentrations increased over time, with TT showing a steady rise and DD exhibiting a more pronounced surge at the 3-month mark, ultimately exceeding TT’s Fe levels. In contrast, Er ion concentrations in both zirconia systems markedly declined by the third month (Figure 6b).
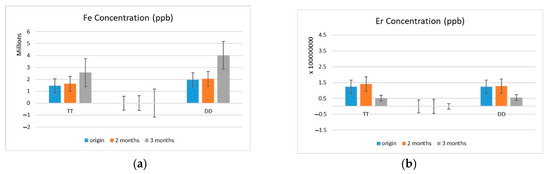
Figure 6.
(a) Fe and Er (b) ion concentrations in coloring solutions after 1, 2, and 3 months of use, measured by ICP-MS. TT: TT ONE Multilayer; DD: cubeX2 ML.
4. Discussion
This study investigated the intricate relationship between ion release, immersion conditions, and the resultant color stability of zirconia materials, focusing on two prominent commercial brands, TT and DD. By analyzing variations in specimen thickness and immersion durations, the research provides critical insights into the physicochemical mechanisms that govern the optical performance of zirconia [28,33]. The findings not only underscore the dynamic interaction between material properties and environmental factors but also align with prior studies highlighting the importance of ion leaching, particularly highlighting the influence of Fe and Er ion leaching on long-term color accuracy.
Despite extensive research on the optical properties of zirconia—particularly the influence of crystal structure, material purity, and processing techniques [34,35,36]—few studies have comprehensively examined the combined effects of ion release and immersion conditions on color stability across different commercial brands. Most of the existing literature has primarily focused on surface treatments, sintering protocols, and aging processes [37,38,39,40], with limited attention given to ion migration under prolonged immersion environments. This study addresses this gap by demonstrating how variations in material thickness and exposure duration influence ion dissolution and subsequent discoloration, while illustrating the interdependent role of the physicochemical environment.
A significant correlation was observed between specimen thickness and color parameters, particularly lightness (L*), chromatic coordinates (a*, b*), and chroma (C*). Thickness substantially impacted color stability more than immersion time across all experimental conditions. In this study, zirconia specimens with thicknesses ranging from 1.0 mm to 2.5 mm were evaluated, revealing that thicker specimens (2.0 mm and 2.5 mm) generally exhibited higher L* and C* values and demonstrated greater resistance to color change (lower ΔE values), even after prolonged immersion and aging. This behavior may be explained by the reduced penetration depth of coloring liquids and limited diffusion pathways for ionic migration in thicker zirconia specimens, in accordance with Fick’s second law of diffusion [41] and the Beer–Lambert law [42], which stabilizes the optical performance of zirconia.
Conversely, thinner specimens (1.0 mm and 1.5 mm) exhibited greater color deviations and more pronounced reddish (a*) and yellowish (b*) shifts, indicating higher susceptibility to environmental influences and ion absorption. These findings are consistent with the studies by Aydogdu et al. [43] and Tabatabaian et al. [44], which demonstrated that zirconia thickness significantly modulates translucency and color perception. Thicker zirconia layers exhibit reduced translucency due to increased light scattering, while simultaneously enhancing color masking capability, thus improving the ability to conceal underlying tooth structures [45,46,47,48]. However, it is also recognized that excessive thickness, while improving color masking, may limit light transmission, affecting the desired esthetic outcomes [49,50]. Additionally, variations in surface finishing and polishing procedures further influence translucency and color stability depending on the material thickness [51].
In the present study, ΔE values increased notably, particularly in specimens immersed in aged solutions, with TT specimens exhibiting more significant optical alterations than DD. In TT, thicker specimens (2.0 mm and 2.5 mm) showed marked increases in ΔE following aging, accompanied by substantial elevations in L* values across all immersion times, suggesting that surface brightening and instability contributed to overall color degradation. These results are consistent with the findings of Nam et al. [19], who reported that specimens processed using aged coloring solutions demonstrated increased brightness (L*) and chromatic shifts toward the red (a*) and yellow (b*) axes, indicating that prolonged use of such liquids alters their chemical composition and negatively impacts the final esthetic outcome. Conversely, DD specimens maintained low ΔE values and stable L* ratios even after three months of continuous solution use, demonstrating superior resistance to chemical degradation. These findings reinforce the clinical importance of controlling not only the physical parameters, such as thickness, but also the chemical conditions associated with the aging of coloring solutions, to ensure the long-term optical performance of zirconia restorations [52,53].
ICP-MS analysis confirmed substantial changes in ion concentrations in the coloring solutions over the three-month aging period. A notable increase in Fe ion levels and a concurrent decline in Er ion concentrations were observed. This shift in ion composition was accompanied by greater pH fluctuations and more pronounced color changes in the zirconia specimens. The interaction between pH variation, ion release, and optical degradation in zirconia is a multifactorial phenomenon rooted in the material’s physicochemical properties. Acidic conditions can accelerate the dissolution of metallic oxides and rare-earth dopants from the zirconia matrix, destabilizing its crystalline structure [7,54].
Critically, the interaction between pH variation and ion release plays a pivotal mechanistic role in optical degradation. Lower pH levels enhance the solubility of metallic oxides and destabilize the zirconia lattice, leading to increased Fe ion dissolution, which is closely associated with reddish discoloration [19]. In contrast, the depletion of rare-earth elements such as Er, essential for maintaining translucency and hue stability, contributes to optical degradation [34,55]. These observations suggest a direct, pH-dependent ionic migration mechanism, where acidic environments exacerbate chromophore imbalance within the zirconia structure. The increase in Fe ion concentration correlates with elevated ΔE values and higher L* ratios, indicating that surface brightening and color shifts are not merely superficial but stem from deeper chemical degradation.
The ΔE values obtained in this study were interpreted based on established clinical thresholds, with 1.2 considered the perceptibility threshold and 2.7 as the acceptability threshold [56]. Values exceeding these thresholds suggest clinically perceptible or unacceptable color changes, providing a more practical context for evaluating the material’s long-term esthetic performance.
In addition to the observed effects of ion release and coloring solution aging, the intrinsic translucency of zirconia must also be considered in the clinical context [57]. As translucency increases—particularly in restorations with reduced thickness—the optical influence of the underlying tooth structure and the color of the luting cement becomes more significant [49,58]. This phenomenon can alter the perceived shade of the restoration despite material-level color stability. Accordingly, the cement shade should be cautiously selected in highly translucent zirconia systems, especially those applied in esthetically demanding regions, to prevent undesired shade mismatches [59,60,61].
Overall, the findings of this study highlight the importance of optimizing material selection and processing conditions to enhance the long-term performance of zirconia restorations. Ion release directly influences color stability and, consequently, clinical reliability. While DD showed superior performance in terms of color retention, TT zirconia may still offer value in specific clinical applications, if exposure durations and environmental conditions are carefully managed.
Based on the findings of this study, it is recommended that DD multilayer zirconia with a minimum thickness of 2.0 mm be selected to achieve optimal long-term color stability and resistance to chemical degradation. The superior color stability observed in DD specimens under prolonged aging conditions supports its use in clinical situations requiring high esthetic durability. Additionally, carefully considering luting cement shade remains critical, particularly in thin zirconia restorations, due to increased translucency and potential shade influence from underlying structures. These findings offer practical guidance for material and procedural selection to enhance the long-term esthetic and functional success of zirconia-based restorations.
While this study offers initial insights into how specimen thickness, immersion duration, and coloring solution aging affect zirconia color stability and ion release, several methodological limitations should be noted. As a preliminary in vitro investigation, advanced surface and structural analyses—such as spectroscopy, diffraction, and topographic profiling—were excluded due to scope and resource constraints. Additionally, the absence of a non-aged control group limits the ability to isolate the specific effects of solution aging. Other parameters critical to optical assessment, including translucency, surface roughness, and standardized imaging, were also not evaluated, thereby narrowing the interpretative depth of the findings.
Future studies should adopt a more comprehensive and multidisciplinary approach to address these gaps, integrating advanced characterization techniques to unravel the physicochemical processes driving zirconia discoloration and degradation. Expanding the analysis to a broader range of material formulations and implementing clinically relevant aging models—such as pH cycling, thermomechanical loading, and dynamic salivary exposure—will enhance translational value. In vivo validation, particularly with attention to patient-specific variability in salivary composition and dietary habits, will be critical in establishing the clinical reliability of zirconia restorations. Such directions are essential for developing chemically stable, esthetically durable ceramic systems for long-term use in prosthetic dentistry.
5. Conclusions
This study demonstrated that zirconia thickness exerts a more pronounced influence on color stability than immersion time, with thicker specimens (2.5 mm) showing greater resistance to color deviation. Aging of coloring solutions over three months further compromised optical performance, particularly in TT specimens, as evidenced by increased ΔE and L* values. ICP-MS analysis confirmed shifts in Fe and Er ion concentrations, correlating with observed color changes and suggesting a chemical contribution to esthetic degradation. DD exhibited superior optical and chemical stability under identical conditions, underscoring the role of material composition in long-term performance. These findings highlight the multifactorial nature of zirconia color stability and the importance of managing fabrication variables, such as thickness and solution aging, to ensure consistent esthetic outcomes in clinical practice.
Author Contributions
Conceptualization, A.M.T. and C.-M.L.; methodology, A.M.T., K.E.G., I. and C.-M.L.; software, A.M.T. and F.-Y.F.; validation, F.-Y.F. and C.-M.L., formal analysis, A.M.T. and F.-Y.F.; investigation, A.M.T. and C.-M.L.; resources, F.-Y.F.; data curation, S.-M.W. and L.-R.K.; writing—original draft preparation, A.M.T.; writing—review and editing, A.M.T., K.E.G., F.-Y.F. and C.-M.L.; visualization, A.M.T. and F.-Y.F.; supervision, C.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their sincere gratitude to the lecture and administrative staff of China Medical University, Taiwan, and Taipei Medical University, Taiwan, for their invaluable support and assistance during this study.
Conflicts of Interest
The authors declare no conflicts of interest and no affiliation with or representation of the products evaluated in this study.
References
- Akhlaghi, O.; Camposilvan, E.; Garnier, V.; Goharibajestani, Z.; Khabbaz, S.; Ow-Yang, C.; Jorand, Y.; Gremillard, L.; Chevalier, J. Conventional sintering of nano-crystalline Yttria-Stabilized Zirconia enables high-strength, highly translucent and opalescent dental ceramics. Dent. Mater. 2024, 40, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cokic, S.; Van Meerbeek, B.; Vleugels, J.; Zhang, F. Novel zirconia ceramics for dental implant materials. J. Mater. Sci. Technol. 2025, 210, 97–108. [Google Scholar] [CrossRef]
- Lin, W.S.; Chen, L.; Alfaraj, A. 3D-printed Zirconia and Lithium Disilicate in Dentistry and Their Clinical Applications. Int. J Prosthodont. 2025, 38, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zhang, Y.; Jin, C.; Zhang, Q.; Lu, J.; Liu, Z.; Wang, Q.; Zhang, X.; Ma, J. 3D printed zirconia used as dental materials: A critical review. J. Biol. Eng. 2023, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Kui, A.; Manziuc, M.; Petruțiu, A.; Buduru, S.; Labuneț, A.; Negucioiu, M.; Chisnoiu, A. Translucent Zirconia in Fixed Prosthodontics-An Integrative Overview. Biomedicines 2023, 11, 3116. [Google Scholar] [CrossRef]
- Huang, B.; Chen, M.; Wang, J.; Zhang, X. Advances in zirconia-based dental materials: Properties, classification, applications, and future prospects. J. Dent. 2024, 147, 105111. [Google Scholar] [CrossRef]
- Lakhloufi, S.; Labjar, N.; Labjar, H.; Serghini- Idrissi, M.; El Hajjaji, S. Electrochemical behavior and surface stability of dental zirconia ceramics in acidic environments. J. Mech. Behav. Biomed. Mater. 2024, 150, 106288. [Google Scholar] [CrossRef]
- Fadavi, F.; Mohammadi-Bassir, M.; Sarabi, N.; Rezvani, M.B.; Jafari-Semnani, S.; Rastegar Moghaddam, M.; Labbaf, H. Effect of Low-Temperature Degradation, Ph-Cycling and Simulated Tooth Brushing on Surface Roughness, Topography, and Polish Retention of Yttrium-Stabilized Tetragonal Zirconia. J. Dent. 2023, 24, 293–304. [Google Scholar] [CrossRef]
- Alnassar, T.M. Color Stability of Monolithic Zirconia in Various Staining Liquids: An In Vitro Study. Appl. Sci. 2022, 12, 9752. [Google Scholar] [CrossRef]
- Mahrous, A.I.; Salama, A.A.; Shabaan, A.A.; Abdou, A.; Radwan, M.M. Color stability of two different resin matrix ceramics: Randomized clinical trial. BMC Oral Health 2023, 23, 665. [Google Scholar] [CrossRef]
- Falkensammer, F.; Arnetzl, G.V.; Wildburger, A.; Freudenthaler, J. Color stability of different composite resin materials. J. Prosthet. Dent. 2013, 109, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Rodgers, B.; Suliman, A.A.; Johnston, W.M. Color and translucency stability of contemporary resin-based restorative materials. J. Esthet. Restor. Dent. 2021, 33, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Jaâfoura, S.; Kikly, A.; Fejjeri, M.; Nasri, S.; Brini, M.; Kammoun, D. Color Stability of Microhybrid Composite Resins Depending on the Immersion Medium. Eur. J. Dent. 2025, 19, 500–512. [Google Scholar] [CrossRef]
- Souza, L.F.B.; Soares, P.M.; Ribeiro, V.F.; Scotti, N.; Kleverlaan, C.J.; Bacchi, A.; Pereira, G.K.R. Influence of coloring techniques on the surface characteristics and color stability of a monolithic zirconia ceramic. J. Prosthet. Dent. 2023, 130, 392.e391–392.e399. [Google Scholar] [CrossRef]
- İnal, C.B.; Bankoğlu Güngör, M.; Karakoca Nemli, S. Effects of Coloring Liquid Dipping Time and Surface Finishing Procedures on the Optical Properties of Monolithic Zirconia. Niger. J. Clin. Pract. 2024, 27, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, M.; Zhang, R.; Hao, P. Effect of the coloring liquid shade and dipping time on the color, transparency, and flexural strength of monolithic zirconia. J. Prosthet. Dent. 2024, 132, 229.e221–229.e228. [Google Scholar] [CrossRef]
- Alzahrani, K.M.; Alhaqbani, M.M.; Basalem, S.A.; Alsubaie, F.; AlRabiah, M.; Alzahrani, A.; AlAali, K.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Effect of Staining Drinks on the Color Stability of Grit Blasted and Non-Grit Blasted Monolithic Zirconia: An In Vitro Study. Crystals 2022, 12, 1331. [Google Scholar] [CrossRef]
- Nam, J.Y.; Park, M.G. Effects of aqueous and acid-based coloring liquids on the hardness of zirconia restorations. J. Prosthet. Dent. 2017, 117, 662–668. [Google Scholar] [CrossRef]
- Nam, J.Y.; Park, M.G. Effects of treatment with aqueous and acid-based coloring liquid on the color of zirconia. J. Prosthet. Dent. 2019, 121, 363.e361–363.e365. [Google Scholar] [CrossRef]
- Sala, D.; Ciambellotti, S.; Giachetti, A.; Turano, P.; Rosato, A. Investigation of the Iron(II) Release Mechanism of Human H-Ferritin as a Function of pH. J. Chem. Inf. Model 2017, 57, 2112–2118. [Google Scholar] [CrossRef]
- Ress, J.; Martin, U.; Bosch, J.; Bastidas, D.M. pH-Triggered Release of NaNO(2) Corrosion Inhibitors from Novel Colophony Microcapsules in Simulated Concrete Pore Solution. ACS Appl. Mater. Interfaces 2020, 12, 46686–46700. [Google Scholar] [CrossRef] [PubMed]
- Al-Haj Husain, N.; Özcan, M.; Dydyk, N.; Joda, T. Conventional, Speed Sintering and High-Speed Sintering of Zirconia: A Systematic Review of the Current Status of Applications in Dentistry with a Focus on Precision, Mechanical and Optical Parameters. J. Clin. Med. 2022, 11, 4892. [Google Scholar] [CrossRef]
- Tabatabaian, F. Color in Zirconia-Based Restorations and Related Factors: A Literature Review. J. Prosthodont. 2018, 27, 201–211. [Google Scholar] [CrossRef]
- Celik, S.; Ucar, Y.; Ekren, O. Effect of coloring liquids on color of zirconia frameworks and bond strength of zirconia/veneering ceramic. J. Prosthet Dent. 2020, 124, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Takahashi, H.; Iwasaki, N. Translucency of dental ceramics with different thicknesses. J. Prosthet. Dent. 2013, 110, 14–20. [Google Scholar] [CrossRef]
- Choi, Y.J.; Razzoog, M.E. Masking ability of zirconia with and without veneering porcelain. J. Prosthodont. 2013, 22, 98–104. [Google Scholar] [CrossRef]
- Kumagai, N.; Hirayama, H.; Finkelman, M.D.; Ishikawa-Nagai, S. The effect of translucency of Y-TZP based all-ceramic crowns fabricated with different substructure designs. J. Dent. 2013, 41, e87–e92. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Savaş, T.; Akın, C. Effects of sintering protocol and dipping time on the optical properties of monolithic zirconia. J. Prosthet. Dent. 2022, 127, e801. [Google Scholar] [CrossRef]
- Ghinea, R.; Pérez, M.M.; Herrera, L.J.; Rivas, M.J.; Yebra, A.; Paravina, R.D. Color difference thresholds in dental ceramics. J. Dent. 2010, 38, e57–e64. [Google Scholar] [CrossRef]
- Pérez Mdel, M.; Saleh, A.; Yebra, A.; Pulgar, R. Study of the variation between CIELAB delta E* and CIEDE2000 color-differences of resin composites. Dent. Mater. J. 2007, 26, 21–28. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Muñoz, M.P.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martín Casado, A.M. Comparison of the CIELab and CIEDE2000 color difference formulas. J. Prosthet. Dent. 2016, 115, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Schanda, J. CIE Colorimetry; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 25–78. [Google Scholar]
- Sen, N.; Isler, S. Microstructural, physical, and optical characterization of high-translucency zirconia ceramics. J. Prosthet. Dent. 2020, 123, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Čokić, S.M.; Li, M.; Huang, S.; Vleugels, J.; Van Meerbeek, B.; Zhang, F. Coloring Multilayer Zirconia May Affect Its Optical and Mechanical Properties. J. Dent. Res. 2024, 103, 1091–1099. [Google Scholar] [CrossRef]
- Chopra, D.; Guo, T.; Gulati, K.; Ivanovski, S. Load, unload and repeat: Understanding the mechanical characteristics of zirconia in dentistry. Dent. Mater. 2024, 40, e1–e17. [Google Scholar] [CrossRef]
- Yılmaz Savaş, T.; Aykent, F. Effect of fabrication techniques on the optical properties of zirconia-based systems. J. Prosthet. Dent. 2021, 125, e521–e528. [Google Scholar] [CrossRef]
- Dokuzlu, S.N.; Subaşı, M.G. Effect of sintering programs and surface treatments on monolithic zirconia. J. Adv. Prosthodont. 2024, 16, 25–37. [Google Scholar] [CrossRef]
- Hafezeqoran, A.; Sabanik, P.; Koodaryan, R.; Ghalili, K.M. Effect of sintering speed, aging processes, and different surface treatments on the optical and surface properties of monolithic zirconia restorations. J. Prosthet. Dent. 2023, 130, 917–926. [Google Scholar] [CrossRef]
- Wei, C.; Gremillard, L. Surface treatment methods for mitigation of hydrothermal ageing of zirconia. J. Eur. Ceram. Soc. 2019, 39, 4322–4329. [Google Scholar] [CrossRef]
- Mayinger, F.; Buser, R.; Laier, M.; Schönhoff, L.M.; Kelch, M.; Hampe, R.; Stawarczyk, B. Impact of the material and sintering protocol, layer thickness, and thermomechanical aging on the two-body wear and fracture load of 4Y-TZP crowns. Clin. Oral. Investig. 2022, 26, 6617–6628. [Google Scholar] [CrossRef]
- DiDomizio, R.; Lupulescu, A.; Glicksman, M. Simulation of Fick′s Verification of the 2nd Law. Diffus. Fundam. 2006, 4, 1–14. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Rigos, A.E.; Ilia, C.; Istantsos, T. Monolithic Zirconia: An Update to Current Knowledge. Optical Properties, Wear, and Clinical Performance. Dent. J. 2019, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Aydoğdu, H.M.; Yıldız, P.; Ünlü, D.G. A comparative study of translucency and color perception in monolithic zirconia and lithium disilicate veneers. Heliyon 2024, 10, e23789. [Google Scholar] [CrossRef]
- Tabatabaian, F.; Motamedi, E.; Sahabi, M.; Torabzadeh, H.; Namdari, M. Effect of thickness of monolithic zirconia ceramic on final color. J. Prosthet. Dent. 2018, 120, 257–262. [Google Scholar] [CrossRef]
- Supornpun, N.; Oster, M.; Phasuk, K.; Chu, T.G. Effects of shade and thickness on the translucency parameter of anatomic-contour zirconia, transmitted light intensity, and degree of conversion of the resin cement. J. Prosthet. Dent. 2023, 129, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaian, F.; Dalirani, S.; Namdari, M. Effect of Thickness of Zirconia Ceramic on Its Masking Ability: An In Vitro Study. J. Prosthodont. 2019, 28, 666–671. [Google Scholar] [CrossRef]
- Çakmak, G.; Donmez, M.B.; Kashkari, A.; Johnston, W.M.; Yilmaz, B. Effect of thickness, cement shade, and coffee thermocycling on the optical properties of zirconia reinforced lithium silicate ceramic. J. Esthet. Restor. Dent. 2021, 33, 1132–1138. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.H.; Lee, J.B.; Han, J.S.; Yeo, I.S.; Ha, S.R. Effect of the amount of thickness reduction on color and translucency of dental monolithic zirconia ceramics. J. Adv. Prosthodont. 2016, 8, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pala, K.; Reinshagen, E.M.; Attin, T.; Hüsler, J.; Jung, R.E.; Ioannidis, A. Masking capacity of minimally invasive lithium disilicate restorations on discolored teeth-The impact of ceramic thickness, the material′s translucency, and the cement color. J. Esthet. Restor. Dent. 2024, 36, 107–115. [Google Scholar] [CrossRef]
- Saláta, J.; Szabó, F.; Csuti, P.; Antal, M.; Márton, P.; Hermann, P.; Borbély, J.; Ábrám, E. Effect of thickness, translucency, and substrates on the masking ability of a polymer-infiltrated ceramic-network material. J. Esthet. Restor. Dent. 2023, 35, 886–895. [Google Scholar] [CrossRef]
- Saker, S.; Özcan, M. Effect of surface finishing and polishing procedures on color properties and translucency of monolithic zirconia restorations at varying thickness. J. Esthet. Restor. Dent. 2021, 33, 953–963. [Google Scholar] [CrossRef]
- Turgut, S. Optical properties of currently used zirconia-based esthetic restorations fabricated with different techniques. J. Esthet. Restor. Dent. 2020, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ângela Mazıero Volpato, C.; Francısco Cesar, P.; Antônıo Bottıno, M. Influence of Accelerated Aging on the Color Stability of Dental Zirconia. J. Esthet. Restor. Dent. 2016, 28, 304–312. [Google Scholar] [CrossRef] [PubMed]
- McGlinn, P.J.; McLeod, T.; Leturcq, G.; Aly, Z.; Blackford, M.G.; Zhang, Z.; Li, H.; Lumpkin, G.R. Aqueous Dissolution and Surface Alteration Studies of Nd-bearing Zirconolite in 0.001M Citric Acid at 90 °C. MRS Proc. 2003, 807, 219. [Google Scholar] [CrossRef]
- Nakamura, T.; Okamura, S.; Nishida, H.; Kawano, A.; Tamiya, S.; Wakabayashi, K.; Sekino, T. Fluorescence and physical properties of thulium and erbium co-doped dental zirconia. Dent. Mater. J. 2021, 40, 1080–1085. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Perez Mdel, M. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef]
- Pekkan, G.; Özcan, M.; Subaşı, M.G. Clinical factors affecting the translucency of monolithic Y-TZP ceramics. Odontology 2020, 108, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, M.; Vichi, A.; Tozzi, G.; Louca, C.; Ferrari, M. Cement opacity and color as influencing factors on the final shade of metal-free ceramic restorations. J. Esthet. Restor. Dent. 2022, 34, 423–429. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C.; Tang, M.; Chen, Y.; Yang, L.; He, F.; Chen, B.; Xie, H. Choice of resin cement shades for a high-translucency zirconia product to mask dark, discolored or metal substrates. J. Adv. Prosthodont. 2019, 11, 286–296. [Google Scholar] [CrossRef]
- Kang, C.M.; Huang, Y.W.; Wu, S.H.; Mine, Y.; Lee, I.T.; Peng, T.Y. Evaluation of shade correspondence between high-translucency pre-colored zirconia and shade tab by considering the influence of cement shade and substrate materials. Heliyon 2023, 9, e23046. [Google Scholar] [CrossRef]
- Alrabeah, G.; Alamro, N.; Alghamdi, A.; Almslam, A.; Azaaqi, M. Influences of luting cement shade on the color of various translucent monolithic zirconia and lithium disilicate ceramics for veneer restorations. J. Adv. Prosthodont. 2023, 15, 238–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).