Abstract
Most previous studies on orofacial muscle strength have focused on older adults with conditions associated with sensorimotor deficits, such as stroke. However, the modifiable oral health factors that directly impact orofacial muscle strength and swallowing ability in healthy older adults remain unexplored. This pilot study explored the potential factors associated with orofacial muscle strength, particularly oral health conditions, in 70 healthy adults aged ≥65 years living independently without any diseases that cause dysphagia or sensorimotor deficits. The Iowa Oral Performance Instrument (IOPI) was used to assess orofacial muscle strength (tongue elevation, and cheek and lip compression). Statistical analyses were conducted using an independent t-test, one-way ANOVA, and multivariate linear regression. In the final regression models after adjustment, older age and fewer remaining teeth were significantly associated with reduced tongue and cheek strengths (p < 0.05). Socio-demographic factors, such as age, and oral health conditions, such as discomfort in pronunciation or mastication due to oral problems, poor self-rated oral health, and reduced salivary flow, were associated with tongue, cheek, and lip muscle strengths (p < 0.05). Early active oral health interventions can help prevent a decline in orofacial muscle strength in healthy older adults.
1. Introduction
According to the World Health Organization, one in six people worldwide will be aged 60 years or older by 2030, and the number of people aged 80 years and over will increase approximately threefold (426 million) from 2020 to 2050 [1]. Notably, South Korea has the fastest aging population globally, and it is expected to become a super-aged society, where individuals aged 65 years or older exceed 20% of the total population, by 2025 [2]. Consequently, interest in promoting healthy aging without any functional disability and improving the quality of life, rather than merely extending lifespan, is increasing.
Although older age is often associated with an improvement in emotional well-being, especially lower negative effects [3], cognitive and physical functions tend to decline with age [4,5]. Regarding oral health, the likelihood of developing oral diseases or systemic conditions such as diabetes and Parkinson’s disease, which directly or indirectly affect oral function, increases with age [6]. Furthermore, the incidence of periodontitis and dental caries, the leading causes of tooth loss, increases with age [7]. In addition, increasing numbers of older adults are experiencing xerostomia due to medications, such as cardiovascular medicine, that induce salivary gland dysfunction [8].
Moreover, the weakening of the orofacial muscles becomes more evident with age [9]. These muscles are located in the cheeks, lips, and tongue in the lower face and perform several functions that are crucial for the maintenance and quality of life, including mastication, deglutition, respiration, and articulation [9,10]. The orofacial muscles thus play a vital role in manipulating food and creating intraoral pressure during the swallowing process, particularly in the oral stage [9,11,12]. Several studies have shown that the thickness of the masticatory masseter muscle and the strength of the tongue muscle tend to decrease with age [10,13]. A reduction in the strength of orofacial muscles leads to poor chewing abilities [13] and dysphagia, a condition where the swallowing of food or liquid becomes difficult due to problems in the swallowing process [14]. Although geriatric diseases such as cerebrovascular diseases are the main cause of dysphagia [15], recent studies have suggested that older age itself is an independent risk factor for the disorder, as a growing number of older adults living in the community have dysphagia symptoms [16,17]. In addition, the weakening of the buccinators or tongue muscles, among other orofacial muscles, is a clinical indicator that can predict the development of dysphagia [18,19]. In particular, Byeon [12] reported that the diadochokinetic rate, which represents the tongue’s motor skill, is related to swallowing ability, and that the tongue’s role is more important in the oral phase of swallowing. Prolonged dysphagia in older adults can lead to changes in meal patterns, increasing the risk of malnutrition [20], and can cause life-threatening complications such as aspiration pneumonia and suffocation [21]. Moreover, the fear of eating and drinking or prolonged mealtimes due to dysphagia can reduce an individual’s quality of life [21,22].
Despite the critical role of orofacial muscles in mastication and swallowing, the function or status of these muscles in healthy older adults living independently has not received much attention. Most previous studies have focused on older adults staying in nursing homes or hospitals due to stroke or Parkinson’s disease for long periods of time [19,23,24], primarily because cerebrovascular diseases and neurogenic disorders negatively impact the sensorimotor system of orofacial muscles [25,26]. However, significant muscle strength loss is observed from the age of 40 years [27], and reduced muscle mass and strength also affect the orofacial muscles, including the tongue [28]. Fortunately, various interventions, such as myofunctional exercises, have been shown to improve the strength and response rate of orofacial muscles, such as the tongue and cheek, or enhance orofacial functions by increasing the muscles’ quantity and quality [12,29]. Although a few studies have reported an association between tongue strength and dental factors, such as tooth loss [30], scant research exists on the influence of oral health conditions, which are modifiable factors, on maintaining the strength of orofacial muscles in healthy older adults living independently. Accordingly, the potential factors associated with orofacial muscle strength should be identified in healthy older adults living independently in the community, to help develop effective interventions and improve the functional strength of these muscles and facilitate the early detection of dysphagia. Therefore, this study aimed to evaluate orofacial muscle strength (anterior tongue elevation, cheek compression, and lip compression strength) in healthy adults aged 65 years or older living independently in the community without any diseases that can cause dysphagia or sensorimotor deficits. In particular, we sought to assess the strength of any association between orofacial muscle strength and oral health conditions. Although these issues were addressed in previous studies, most of them focused on older adults living in institutions or on those with conditions affecting the sensorimotor system.
2. Materials and Methods
2.1. Participants
This pilot cross-sectional study was approved by the Institutional Review Board of Gachon University (No. 1044396-202403-HR-051-01) and conducted in accordance with the World Medical Association’s Declaration of Helsinki. To recruit participants, among the senior welfare facilities located in Incheon, South Korea, 10 facilities with similar geographical and social environments were selected through convenience sampling. The purpose and methods of the study were thoroughly explained to adults aged 65 years or older who visited the selected facilities. Based on the findings of previous studies [25,26], older adults with one or more of the following diseases or disorders associated with decreased sensorimotor function in the orofacial muscles or the development of dysphagia were excluded from the study: dysphagia, cerebrovascular diseases (e.g., stroke), neurasthenia, neurological diseases (e.g., Parkinson’s disease), chronic obstructive pulmonary diseases (e.g., chronic bronchitis), gastrointestinal disorders, amyotrophic lateral sclerosis, esophageal inflammation (e.g., reflux esophagitis), cancer, and depression. Furthermore, two preliminary tests were conducted to assess the eligibility of 90 older adults who were initially willing to participate. First, cognitive status was assessed using the Mini-Mental State Examination—Korean version (MMSE-K), and those with cognitive impairment (score < 24) (n = 9) were excluded [31,32]. The MMSE-K, standardized in Korean by Park and Kwon [32], is one of the most widely used scales in Korea, as it can be easily administered in a short time and its reliability and validity have been proven for screening moderate-to-severe dementia [31,33,34]. Next, the Korean Activities of Daily Living scale developed by Won et al. [35], a valid and reliable instrument for the functional assessment of older adults, was employed to assess the independence of the participants in daily living. All participants responded that they could perfectly perform the basic activities of daily living in all seven items without any assistance. After informed consent was obtained from the 81 participants who fully understood the research process and voluntarily agreed to participate, a survey and several clinical assessments were carried out. Data were collected from 30 April 2024 to 31 May 2024. After collecting the survey data from all participants, questionnaires with at least one missing value (n = 11) were excluded, and a total of 70 participants were finally analyzed in the study. Using G* Power 3.1.9.4 software (Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany) with a significance level of 0.05, power of 80%, effect size of 0.20, and five predictors [36], the minimum required sample size was calculated to be 70. The flowchart of the participant recruitment process is presented in Figure 1.
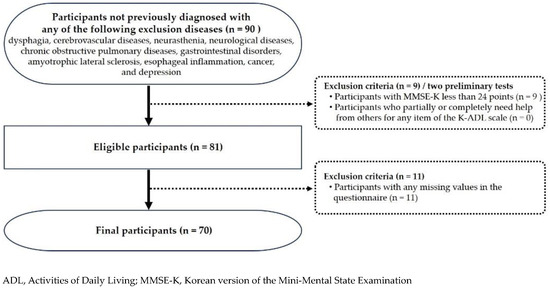
Figure 1.
Flowchart of participant recruitment and selection.
2.2. Measurements
Data were collected on the sociodemographic characteristics (age and four others), health-related characteristics (smoking status and two others), oral health conditions (number of remaining teeth and eight others), and orofacial muscle strength (anterior tongue elevation, cheek compression, and lip compression strengths).
For assessing oral health conditions, unstimulated whole saliva was collected in an upright position for 5 min using the spitting method to diagnose xerostomia [37], with the amount of collected saliva converted to the salivary flow rate (mL/min). Participants were instructed not to eat or brush their teeth at least 2 h before saliva collection. Using a cut-off value of <0.1 mL/min, participants were divided into two groups: xerostomia and normal [38]. To evaluate chewing ability, the number of remaining teeth and number of functional tooth units (FTUs) were identified [39]. FTUs are defined as pairs of upper and lower natural or artificial teeth that occlude during chewing [40]. Two molars were counted as 2 FTUs and two premolars were counted as 1 FTU; therefore, a participant with complete dentition was recorded as having 12 FTUs (excluding the third molars) [39,41]. To assess orofacial muscle strength, the anterior tongue elevation strength, cheek compression strength, and lip compression strength were measured using the Iowa Oral Performance Instrument (IOPI) (IOPI Medical, Redmond, WA, USA), following the manufacturer’s guidelines [10,42]. The IOPI consists of a pressure bulb, connecting tube, and main body. The bulb, made of soft rubber, is approximately 3.5 cm long, 1.5 cm wide, filled with approximately 2.8 mL of air, and connected to the pressure port of the main body. Each muscle strength was measured three times at 1 min intervals, with the highest value (kPa) recorded. The position of the pressure bulb changed depending on which muscle was being measured. To measure the anterior tongue elevation strength, the bulb was positioned longitudinally on the participant’s hard palate just posterior to the alveolar ridge [43]. Then, the participant was instructed to lightly close their lips and press against the bulb as hard as possible with the anterior part of their tongue for 2 s. The cheek compression strength was measured by positioning the bulb laterally between the cheek of the habitual chewing side and the buccal surface of the molar. Participants were asked to squeeze the bulb as hard as possible with their cheek muscle for 2 s, with the upper and lower teeth lightly occluded. When measuring the lip compression strength, two disposable wooden tongue depressors (150 × 19 × 1.6 mm) were used to evenly distribute the pressure exerted on the bulb to obtain an accurate pressure reading [10]. The bulb, inserted between two tongue depressors, was positioned between the upper and lower lips at the dental midline. Then, participants were instructed to slightly close their lips, protrude them slightly to prevent the use of the jaw muscles, and squeeze the bulb with their lips as hard as possible for 2 s.
2.3. Statistical Analysis
The data collected were analyzed using SPSS (v23; IBM Corp., Armonk, NY, USA), with the significance level set at 0.05. The independent t-test and one-way ANOVA, followed by Scheffé’s post hoc analysis, were used to analyze the potential factors associated with orofacial muscle strength. In addition, after adjusting for several factors, stepwise multivariate linear regression was performed to identify the potential factors associated with orofacial muscle strength and the intensity of the associations. In the three regression models, the dependent variables were anterior tongue elevation strength, cheek compression strength, and lip compression strength, respectively. The independent variables comprised factors that showed a p-value of less than 0.05 in the univariate analysis.
3. Results
3.1. Distribution of Sociodemographic Characteristics and Orofacial Muscle Strength Levels of Participants
Of the participants, 75.7% (n = 53) were women. Participants aged 69 years or younger or aged 80 years or older accounted for more than half of the participants (each 28.6%), and the age range was 66–94 years, with an average age of 75.64 years (±7.38). Almost half (45.7%) of the participants had a final education level of elementary school or lower, 67.1% (n = 47) were engaged in no economic activity, and 45.7% (n = 32) of the participants were living with their spouses. In addition, the tongue elevation strength was 44.50 ± 12.00 kPa, the cheek compression strength was 23.53 ± 7.03 kPa, and the lip compression strength was 15.63 ± 3.91 kPa (Table 1).

Table 1.
Distribution of sociodemographic characteristics and orofacial muscle strength levels of participants.
3.2. Orofacial Muscle Strength by Sociodemographic Characteristics
Anterior tongue elevation strength was the lowest in women (42.87 ± 12.11 kPa), those aged 75 years or older (38.46 ± 11.82 kPa), those with an education level of elementary school or lower (38.63 ± 12.04 kPa), those who were not economically active (42.26 ± 12.28 kPa), and those living with their children (39.86 ± 12.05 kPa) (p < 0.05). Cheek compression strength was the lowest in females (22.58 ± 6.98 kPa), those aged 75 years or older (20.86 ± 7.42 kPa), those with an education level of elementary school or lower (21.03 ± 7.58 kPa), and those who were not economically active (22.02 ± 7.05 kPa) (p < 0.05). Lip compression strength was the lowest in females (14.89 ± 3.32 kPa), those aged 75 years or older (14.23 ± 4.31 kPa), those with an education level of elementary school or lower (13.75 ± 3.27 kPa), and those living with their children (14.18 ± 3.11 kPa) (p < 0.05) (Table 2).

Table 2.
Orofacial muscle strength by sociodemographic characteristics (units: kPa).
3.3. Orofacial Muscle Strength by General Health-Related Characteristics
Anterior tongue elevation strength was the lowest in non-drinkers (42.25 ± 12.30 kPa) (p < 0.05). Although it was lower in participants with cardiovascular disease (39.60 ± 11.79 kPa) compared to those without, the difference was not statistically significant (p = 0.074). Lip compression strength was the lowest in participants with three or more systemic diseases (14.22 ± 3.40 kPa) (p < 0.05). It was also lower in those with diabetes (13.76 ± 3.68 kPa) and cardiovascular disease (13.60 ± 3.83 kPa) than in those without these conditions (p < 0.05). No statistically significant differences were observed in cheek compression strength across general health-related characteristics (Table 3).

Table 3.
Orofacial muscle strength by general health-related characteristics (units: kPa).
3.4. Orofacial Muscle Strength by Oral Health Status
The anterior tongue elevation, cheek compression, and lip compression strengths were the lowest in those experiencing discomfort during chewing due to oral problems, those with a history of toothache in the past 30 days, and those with 0–9 remaining teeth (p < 0.05). Additionally, all orofacial muscle strengths were the lowest in participants using both upper and lower dentures and those who perceived their dentures as uncomfortable (p < 0.05). Tongue elevation strength and lip compression strength were also the lowest in participants with pronunciation difficulties due to oral problems or xerostomia (p < 0.05). The tongue elevation and cheek compression strengths were the lowest among those with six or fewer FTUs (p < 0.05). Lip compression strength was the lowest in participants who perceived their own oral health as poor (p < 0.05) (Table 4).

Table 4.
Orofacial muscle strength by oral health status (units: kPa).
3.5. Potential Factors Associated with Orofacial Muscle Strength
The results of the multiple linear regression analysis of the intensity of association of various potential factors with the three muscle strengths are presented in Table 5. The anterior tongue elevation strength was significantly lower in participants aged 75 years or older (B = −9.177), those with 0–9 remaining teeth (B = −11.011), and those with pronunciation difficulties due to oral problems (B = −9.931) (p < 0.05). The cheek compression strength was lower in participants aged 75 years or older (B = −4.186), those with 0–9 remaining teeth (B = −4.798), and those with mastication difficulties due to oral problems (B = −3.445) (p < 0.05). The lip compression strength was lower in women (B = −2.272) and patients with diabetes (B = −2.228), and it was higher in those with better self-perceived oral health (B = 1.374) and higher unstimulated salivary flow rates (B = 11.514) (p < 0.05). The cumulative variance explained by the independent variables significantly associated with each muscle strength was 44.0%, 24.0%, and 43.2%, respectively (adj. R2 = 0.440, 0.240, and 0.432, respectively). All regression models confirmed the absence of multicollinearity between the independent variables (Variance Inflation Factor <10, tolerance >0.10).

Table 5.
Potential factors associated with orofacial muscle strength.
4. Discussion
The weakening of the tongue and buccinator muscle, among other orofacial muscles, has been recognized as a risk factor for the development of swallowing difficulties [14,19]. A scoping review highlighted that tongue strength is an important predictor of cognitive decline in dementia, which has often been overlooked in previous studies [44]. Since the primary risk factors for reduced orofacial muscle strength include diseases that negatively impact the sensorimotor system, previous studies have largely focused on long-term hospitalized older adults with cerebrovascular diseases or neurogenic disorders [19,23,24]. Limited research exists on the influence of oral health conditions, a modifiable factor, on maintaining orofacial muscle strength in healthy older adults living independently. Given the high prevalence of dysphagia even among older adults without causal conditions such as stroke [18], the functional status of orofacial muscles that are directly related to swallowing abilities, as well as the risk factors contributing to their weakening, must be considered in healthy older adults. This study, therefore, assessed the anterior tongue elevation, cheek compression, and lip compression strengths in 70 healthy older adults aged 65 years or older, living independently in the community without diseases contributing to dysphagia, and analyzed the potential factors related to each muscle strength, particularly oral health status.
The final regression models used to elucidate the intensity of the associations with orofacial muscle strength demonstrated that the orofacial muscle strength was associated with several oral health-related factors. However, significant factors differed between tongue elevation strength, cheek compression strength, and lip compression strength. A reduced tongue elevation strength was most strongly associated with older age, followed by having 0–9 remaining teeth and discomfort in pronunciation due to oral problems. A reduced cheek compression strength was most strongly associated with older age, a smaller number of remaining teeth, and discomfort in mastication due to oral problems. A weakened lip compression strength was most significantly associated with poor self-rated oral health, followed by diabetes, female sex, and a low unstimulated salivary flow rate. We did not find any common factors associated with all three orofacial muscle strengths except for age, number of remaining teeth, and discomfort due to oral problems for tongue elevation strength and cheek compression strength. We speculated that the associated factors differed because each muscle plays a different role. However, this study was not able to identify the exact cause. The role of each orofacial muscle has been investigated in previous studies. Among the orofacial muscles, the buccinator, also known as the accessory mastication muscle, is a square-shaped major facial muscle located in the cheek area between the maxilla and mandible, which maintains the position of the food bolus in the mouth by compressing the cheeks against the teeth during chewing to help mastication [45,46]. The strength of the buccinator muscle has been considered a valuable indicator for diagnosing orofacial weakness [14]. The tongue is a complex muscle primarily responsible for manipulating food and pushing it from the oral cavity toward the pharynx [11]. Additionally, the orbicularis oris, the principal muscle of the lips, is crucial for creating a lip seal to prevent the food bolus from leaking out of the oral cavity during chewing [45]. Therefore, considering the specific role played by each muscle, it is necessary to confirm the results of this study in future studies with a larger sample size.
In this study, the main potential factors associated with orofacial muscle strength were as follows. First, older age was the most significant factor associated with both tongue and cheek muscle weakness (p < 0.05). This aligns with the findings in the existing literature [9,10], confirming that aging is a factor associated with the deterioration of orofacial muscle strength. As people age, in general, muscle mass and strength in the entire body tend to decrease [27]; this is accompanied by the weakening of the orofacial muscles, including the tongue [28]. Furthermore, older adults exhibit higher levels of intramuscular fat, a factor associated with lower muscle strength, than younger adults, irrespective of body weight [47,48]. Specifically, the fat mass and fat percentage of the tongue increase with age, while its force generation capacity decreases [49,50]. The reduction in muscle mass and strength during the aging process is largely attributed to an increase in catabolic factors, such as inflammatory cytokines, and a decrease in anabolic factors, such as growth hormones and physical activity [27,51]. Given the rapid rise in the super-aged population globally, including in South Korea [52,53], and the prevalence of dysphagia among community-dwelling older adults [18], further research on the aging of orofacial muscles as a risk factor for dysphagia is warranted. In addition, we suggest that the functional status of orofacial muscle is monitored periodically in community-dwelling older adults to prevent and detect a decline in their chewing and swallowing abilities. Early intervention programs should be implemented for those identified as having strong predictors of poor muscle strength. Moreover, since a low muscle volume and high intramuscular fat in the tongue are factors that contribute to weakened tongue strength and an increased risk of malnutrition in older adults [54], healthcare providers should closely monitor the motor function and strength of the tongue in this population.
Second, our study also found that participants with fewer remaining teeth had lower tongue elevation and cheek compression strengths. Although the number of remaining teeth was associated with all three orofacial muscle strengths in the univariate analysis, it was not related to lip compression strength in the final regression model. Nonetheless, these findings are consistent with those of a study that reported a close relationship between the masseter, one of the masticatory muscles, and the number of teeth [55]. Our results also support the findings of Huang et al. [56], who suggested that masticatory abilities, which are influenced by the number of remaining teeth, can enhance the tongue’s motor function, and conversely, that an increased tongue strength can improve masticatory abilities. Typically, 28 teeth are present in the oral cavity, excluding the third molars, which assist in cutting and crushing food with the aid of saliva and other organs, including the masticatory muscles, during swallowing and digestion [45]. A dentition of 20 teeth, although fewer than the normal number, has been proposed as a measure of healthy oral aging in terms of function [7]. Periodontitis, whose prevalence increases with age, breaks down periodontal tissues, including the alveolar bone, ultimately leading to tooth loss and diminished oral function [57,58]. Tooth loss causes anatomical changes around the oral cavity, particularly in the mandibular morphology [59]. These anatomical changes may affect the structure and function of the masticatory and orofacial muscles [55]. One study reported that tooth loss was significantly associated with the thickness of the masticatory muscles because muscle atrophy can occur due to muscle disuse [60]. Another study suggested that as tooth loss showed a significant association with lower tongue pressure, fixed prosthesis treatment, rather than removable dentures, could prevent the decrease in tongue pressure [30]. Furthermore, the preservation of natural teeth has been found to be significantly associated with general health status, including oropharyngeal dysphagia, and psychosocial well-being in older adults [61,62]. Therefore, the early treatment of oral diseases causing tooth loss and the early restoration of missing teeth may prevent irreversible changes in the orofacial muscles, particularly the tongue and buccinator, in healthy older adults.
Third, we found an association between diabetes and reduced lip compression strength, although no such association with tongue elevation strength or cheek compression strength was observed. A study by Wang et al. [63] on community-dwelling older adults similarly reported that diabetes can lead to a decline in or loss of muscle mass and function. The primary mechanisms underlying this decline in older adults with diabetes are reportedly the loss of anabolism due to increased insulin resistance, an increase in inflammatory mediators such as tumor necrosis factor-α and interleukin-6, and an increase in oxidative stress [64]. Conversely, another study suggested that diabetes increased the prevalence of sarcopenia by two to three times [65]. Unfortunately, this study did not measure the participants’ blood glucose levels, and this hypothesis requires further research.
Finally, in our univariate analysis, all orofacial muscle strengths tended to be lower in participants who used dentures or who reported that their dentures were uncomfortable compared to those who did not use dentures, although the final regression model did not confirm this association. This may be due to the fact that only a small number of participants (approximately 30%) used dentures; therefore, in future studies, the sample size should be expanded to accurately evaluate various characteristics related to dental prostheses. Contrary to our hypothesis, unstimulated salivary flow rates were associated with lip compression strength in the final regression model but not with tongue or cheek compression strengths. One possible explanation is that since over half of the participants (54.2%) had normal salivary flow rates, a reduced saliva flow may not have had significant adverse effects on tongue or cheek muscle strengths in most of the older adults. We also speculated that the function of lip muscles may be more sensitive to the amount of saliva, which acts as a lubricant, because the orbicularis oris, the main muscle of the lips, is not firmly attached directly to the bone but rather to other oral muscles [66]. A normal individual secretes about 1.5 L of saliva per day [45], and the decrease in salivary flow rates with age is largely due to polypharmacy [67]. Saliva protects teeth and mucosa because of its antibacterial properties and contains, in addition to approximately 99% water, mucins produced in various salivary glands that function as lubricants during mastication, swallowing, and speaking [68]. Therefore, future studies should further explore the relationship between various properties of saliva, including its quantity and composition, with orofacial muscle strength.
This study is significant as it demonstrates that orofacial muscle strength (tongue elevation, cheek compression, and lip compression) can be negatively impacted by a poor oral health status, such as a reduced number of remaining teeth, leading to weakened orofacial strength in healthy older adults living independently in the community without a history of dysphagia. These findings may contribute to the early detection of the functional decline of orofacial muscles, a risk factor for dysphagia, among healthy older adults living in the community, and could support the development of effective intervention strategies to improve orofacial muscle strength.
However, this study has several limitations. Firstly, although the number of participants in this study exceeded the minimum sample size required for multiple linear regression, the generalizability of the findings may be limited due to the small number of final participants and the sampling method (convenience sampling). Secondly, as the study was a cross-sectional survey, establishing causal relationships among the variables, particularly between orofacial muscle strength and oral health conditions, was not possible. Additionally, although multiple methods exist for evaluating the functional status of orofacial muscles, this study chose to measure muscle strength, one of the most commonly used methods [10,45]. We measured the pressure generated by compressing the IOPI bulb, positioned at each designated location, to assess the strength of the participant’s tongue, cheek, and lip muscles. Considering a study that suggested that the motor skill of the tongue tip or lips is related to the lip-seal strength [69], we judged that the method used in this study may reflect the function or condition of the orofacial muscles. Finally, even though individuals with any disease related to dysphagia were excluded, the health status of the participants, including craniofacial morphology, dental arch morphology, and tooth position [70], which may affect orofacial muscle strength, was not comprehensively evaluated. Therefore, our suggestions for improving orofacial muscle strength may be somewhat limited, and the above potential factors should be considered in future studies to accurately evaluate orofacial muscle strength. Based on our findings, a longitudinal study design combined with a comprehensive health status assessment is necessary in future research to accurately identify the potential risk factors for reduced tongue, cheek, and lip strengths in a larger population of community-dwelling healthy older adults.
5. Conclusions
In this pilot cross-sectional study, an older age and fewer remaining teeth were significantly associated with a reduced tongue and cheek compression strength in healthy older adults aged 65 years or older, living independently in the community and without any diseases contributing to dysphagia or sensorimotor deficits. Additionally, oral health conditions, including discomfort in pronunciation or mastication due to oral problems, poor self-rated oral health, and low salivary flow rates, as well as socio-demographic factors, such as sex, were associated with tongue, cheek, and lip strengths. We suggest that orofacial muscle strength should be regularly screened in community-dwelling older adults for the early detection of reduced orofacial muscle strength, which can adversely impact mastication and swallowing abilities. Furthermore, early oral health interventions, including the prevention and treatment of oral diseases leading to tooth loss, dental restoration to replace missing teeth, the address of self-perceived oral problems, and the management of hyposalivation, may contribute to maintaining orofacial muscle strength. However, the generalizability of these findings may be limited due to the small number of participants and the convenience sampling approach used in this study.
Author Contributions
Conceptualization, D.-S.L. and J.-S.C.; methodology, D.-S.L., J.-Y.K. and J.-S.C.; software, D.-S.L.; formal analysis, D.-S.L. and J.-S.C.; investigation, D.-S.L., J.-Y.K. and J.-S.C.; data curation, D.-S.L. and J.-S.C.; writing-original draft preparation, D.-S.L. and J.-S.C.; writing-review and editing, D.-S.L., J.-Y.K. and J.-S.C.; visualization, D.-S.L. and J.-S.C.; supervision, J.-Y.K. and J.-S.C.; project administration, J.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any financial support.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Gachon University (approval no: 1044396-202403-HR-051-01, approval date: 29 April 2024) and all involved procedures were performed in accordance with the World Medical Association Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful to all the subjects who participated in this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- World Health Organization. Aging and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 May 2024).
- South Korea Ranks Worst in OECD for Poverty in Old Age. Available online: https://english.hani.co.kr/arti/english_edition/e_national/1013419.html (accessed on 30 April 2024).
- Fields, E.C.; Kensinger, E.A.; Garcia, S.M.; Ford, J.H.; Cunningham, T.J. With age comes well-being: Older age associated with lower stress, negative affect, and depression throughout the COVID-19 pandemic. Aging Ment. Health 2022, 26, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L. The impact of age on cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, A.J.; Ford, B.Q.; Floerke, V.A.; Mauss, I.B. Getting better with age: The relationship between age, acceptance, and negative affect. J. Pers. Soc. Psychol. 2013, 104, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Korean Academy of Orofacial Pain & Oral Medicine. Dental Management of the Medically Compromised, the Elderly, and the Disabled Patients, 2nd ed.; YENANG Inc.: Seoul, Republic of Korea, 2019; pp. 380–381. [Google Scholar]
- Lamster, I.B.; Asadourian, L.; Del Carmen, T.; Friedman, P.K. The aging mouth: Differentiating normal aging from disease. Periodontology 2000 2016, 72, 96–107. [Google Scholar] [CrossRef]
- Wolff, A.; Joshi, R.K.; Ekström, J.; Aframian, D.; Pedersen, A.M.L.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: A systematic review sponsored by the World Workshop on Oral Medicine VI. Drugs R&D 2017, 17, 1–28. [Google Scholar]
- Park, J.S.; You, S.J.; Kim, J.Y.; Yeo, S.G.; Lee, J.H. Differences in orofacial muscle strength according to age and sex in East Asian healthy adults. Am. J. Phys. Med. Rehabil. 2015, 94, 677–686. [Google Scholar] [CrossRef]
- Clark, H.M.; Solomon, N.P. Age and sex differences in orofacial strength. Dysphagia 2012, 27, 2–9. [Google Scholar] [CrossRef]
- Felton, S.M.; Gaige, T.A.; Reese, T.G.; Wedeen, V.J.; Gilbert, R.J. Mechanical basis for lingual deformation during the propulsive phase of swallowing as determined by phase-contrast magnetic resonance imaging. J. Appl. Physiol. 2007, 103, 255–265. [Google Scholar] [CrossRef]
- Byeon, H.W. Effect of orofacial myofunctional exercise on the improvement of dysphagia patients’ orofacial muscle strength and diadochokinetic rate. J. Phys. Ther. Sci. 2016, 28, 2611–2614. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Feng, Q. Dynamic change in the thickness of the masseter muscle between contraction and relaxation is associated with the masticatory function in older adults: A cross-sectional study. Ann. Palliat. Med. 2022, 11, 3755–3763. [Google Scholar] [CrossRef]
- Mul, K.; Berggren, K.N.; Sills, M.Y.; McCalley, A.; van Engelen, B.G.M.; Johnson, N.E.; Statland, J.M. Effects of weakness of orofacial muscles on swallowing and communication in FSHD. Neurology 2019, 92, e957–e963. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.; Ottenstein, L.; Atanelov, L.; Christian, A.B. Dysphagia after stroke: An overview. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.N.; Ho, W.C.; Wang, L.H.; Chang, F.C.; Nhu, N.T.; Chou, L.W. Prevalence and methods for assessment of oropharyngeal dysphagia in older adults: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 2605. [Google Scholar] [CrossRef] [PubMed]
- Umay, E.; Eyigor, S.; Bahat, G.; Halil, M.; Giray, E.; Unsal, P.; Unlu, Z.; Tikiz, C.; Vural, M.; Cincin, A.T.; et al. Best practice recommendations for geriatric dysphagia management with 5 Ws and 1H. Ann. Geriatr. Med. Res. 2022, 26, 94–124. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, H.E.; Choi, J.S. Associated with dysphagia risk among older, healthy, community dwelling Korean adults: A pilot study. Healthcare 2024, 12, 267. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.-S.; Yun, D.H.; Chon, J.; Han, Y.J.; Yoo, S.D.; Kim, D.H.; Lee, S.A.; Joo, H.I.; Park, J.-S.; et al. The relationship between tongue pressure and oral dysphagia in stroke patients. Ann. Rehabil. Med. 2016, 40, 620–628. [Google Scholar] [CrossRef]
- Saleedaeng, P.; Korwanich, N.; Muangpaisan, W.; Korwanich, K. Effect of dysphagia on the older adults’ nutritional status and meal pattern. J. Prim. Care Commun. Health 2023, 14, 21501319231158280. [Google Scholar] [CrossRef]
- Ney, D.M.; Weiss, J.M.; Kind, A.J.H.; Robbins, J.A. Senescent swallowing: Impact, strategies and interventions. Nutr. Clin. Pract. 2009, 24, 395–413. [Google Scholar] [CrossRef]
- Roy, N.; Stemple, J.; Merrill, R.M.; Thomas, L. Dysphagia in the elderly: Preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann. Otol. Rhinol. Laryngol. 2007, 116, 858–865. [Google Scholar] [CrossRef]
- Nakamori, M.; Ishikawa, K.; Imamura, E.; Yamamoto, H.; Kimura, K.; Ayukawa, T.; Mizoue, T.; Wakabayashi, S. Relationship between tongue pressure and dysphagia diet in patients with acute stroke. PLoS ONE 2021, 16, e0252837. [Google Scholar] [CrossRef]
- Minagi, Y.; Ono, T.; Hori, K.; Fujiwara, S.; Tokuda, Y.; Murakami, K.; Maeda, Y.; Sakoda, S.; Yokoe, M.; Mihara, M.; et al. Relationships between dysphagia and tongue pressure during swallowing in Parkinson’s disease patients. J. Oral Rehabil. 2018, 45, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Carmona, R.; Traube, M. Dysphagia in the elderly. Clin. Geriatr. Med. 2014, 30, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Prosiegel, M.; Riecker, A.; Weinert, M.; Dziewas, R.; Lindner-Pfleghar, B.; Stanschus, S.; Warnecke, T. Management of dysphagic patients with acute stroke. Nervenarzt 2012, 83, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013, 3, 346–350. [Google Scholar] [CrossRef]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Taniguchi, Y.; Seino, S.; Abe, T.; et al. Association between sarcopenia and oral functions in community-dwelling older adults: A cross-sectional study. J. Cachexia Sarcopenia Muscle 2023, 14, 429–438. [Google Scholar] [CrossRef]
- Takano, S.; Yamaguchi, K.; Nakagawa, K.; Yoshimi, K.; Nakane, A.; Okumura, T.; Tohara, H. Effect of isometric exercises on the masseter muscle in older adults with missing dentition: A randomized controlled trial. Sci. Rep. 2021, 11, 7285. [Google Scholar]
- Tashiro, K.; Soutome, S.; Funahara, M.; Kawashita, Y.; Kitamura, M.; Fukuda, H.; Furugen, R.; Iwasaki, T.; Hayashida, H.; Kawasaki, K.; et al. The relationship between dental findings and tongue pressure: A survey of 745 community-dwelling adults and elderly persons in Japan. Gerontology 2021, 67, 517–524. [Google Scholar] [CrossRef]
- Kang, I.W.; Beom, I.G.; Cho, J.Y.; Son, H.R. Accuracy of Korean-Mini-Mental Status Examination Based on Seoul Neuro-Psychological Screening Battery II results. Korean J. Fam. Med. 2016, 37, 177–181. [Google Scholar] [CrossRef]
- Park, J.; Kwon, Y. Standardization of Korean version of Mini-Mental State Examination (MMSE-K) for use in the elderly. Part II. diagnosis validity. J. Korean Neuropsychiatr. Assoc. 1989, 28, 508–513. [Google Scholar]
- Shin, J.H. Diagnosis of dementia: Neuropsychological test. Korean J. Fam. Med. 2010, 31, 253–266. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, Y.N.; Jeong, E.H.; Chang, S.O. Developing a Korean version of the scale for the observation of agitation in persons with dementia of alzheimer-type. J. Korean Gerontol. Nurs. 2020, 22, 316–325. [Google Scholar] [CrossRef]
- Won, C.W.; Rho, Y.G.; Kim, S.Y.; Cho, B.R.; Lee, Y.S. The validity and reliability of Korean Activities of Daily Living (K-ADL) Scale. J. Korean Geriatr. Soc. 2002, 6, 98–106. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G* power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Wiener, R.C.; Wu, B.; Crout, R.; Wiener, M.; Plassman, B.; Kao, E.; McNeil, D. Hyposalivation and xerostomia in dentate older adults. J. Am. Dent. Assoc. 2020, 141, 279–284. [Google Scholar] [CrossRef]
- Bergdahl, M.; Bergdahl, J. Low unstimulated salivary flow and subjective oral dryness: Association with medication, anxiety, depression, and stress. J. Dent. Res. 2000, 79, 1652–1658. [Google Scholar] [CrossRef]
- Samnieng, P.; Ueno, M.; Shinada, K.; Zaitsu, T.; Wright, F.A.; Kawaguchi, Y. Oral health status and chewing ability is related to mini-nutritional assessment results in an older adult population in Thailand. J. Nutr. Gerontol. Geriatr. 2011, 30, 291–304. [Google Scholar] [CrossRef]
- Ueno, M.; Yanagisawa, T.; Shinada, K.; Ohara, S.; Kawaguchi, Y. Category of functional tooth units in relation to the number of teeth and masticatory ability in Japanese adults. Clin. Oral Investig. 2010, 14, 113–119. [Google Scholar] [CrossRef]
- Jung, H.J.; Min, Y.G.; Kim, H.J.; Lee, J.Y.; Lee, E.S.; Kim, B.I.; Ahn, H.J. Factors affecting objective and subjective masticatory ability assessment of Korean elderly people. J. Korean Acad. Oral Health 2018, 42, 216–223. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, D.H.; Chang, M.Y. Effect of expiratory muscle strength training on swallowing-related muscle strength in community-dwelling elderly individuals: A randomized controlled trial. Gerodontology 2017, 34, 121–128. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ryu, J.H.; Baek, S.H.; Lim, W.H.; Yang, I.H.; Kim, T.W.; Jung, S.K. Comparative analysis of the differences in dentofacial morphology according to the tongue and lip pressure. Diagnostics 2021, 11, 503. [Google Scholar] [CrossRef]
- Yitbarek, G.Y.; Alty, J.; Lawler, K.; Goldberg, L.R. Current evidence on the association of tongue strength with cognitive decline in older adults and the known risk factors of frailty, sarcopenia and nutritional health: A scoping review protocol. BMJ Open 2023, 13, e076005. [Google Scholar] [CrossRef] [PubMed]
- The Korean Dysphagia Society. Swallowing Disorders, 1st ed.; Koonja Publishing: Paju-si, Republic of Korea, 2017; pp. 13–145. [Google Scholar]
- Dutra, E.H.; Caria, P.H.; Rafferty, K.L.; Herring, S.W. The buccinator during mastication: A functional and anatomical evaluation in minipigs. Arch. Oral Biol. 2010, 55, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, A.; Hioki, M.; Kanehira, N.; Shimaoka, K.; Koike, T.; Sakakibara, H.; Oshida, Y.; Akima, H. Three-dimensional comparison of intramuscular fat content between young and old adults. BMC Med. Imaging 2017, 17, 12. [Google Scholar] [CrossRef]
- Health AgingBody Composition Study; Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Park, J.; Oh, D.; Chang, M. Comparison of maximal tongue strength and tongue strength used during swallowing in relation to age in healthy adults. J. Phys. Ther. Sci. 2016, 28, 442–445. [Google Scholar] [CrossRef]
- Nakao, Y.; Yamashita, T.; Honda, K.; Katsuura, T.; Hama, Y.; Nakamura, Y.; Ando, K.; Ishikura, R.; Kodama, N.; Uchiyama, Y.; et al. Association among age-related tongue muscle abnormality, tongue pressure, and presbyphagia: A 3D MRI study. Dysphagia 2021, 36, 483–491. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef]
- Korea Institute for Health and Social Affairs. 2020 National Survey of Older Koreans; Korea Institute for Health and Social Affairs: Sejong, Republic of Korea, 2020; pp. 126–127. [Google Scholar]
- Park, J.S.; You, S.J.; Jeong, C.H. Age and sex differences in orofacial strength of healthy Korean adult. Korea J. Occup. Ther. 2013, 21, 103–116. [Google Scholar]
- Borda, M.G.; Hassan, E.B.; Weon, J.H.; Wakabayashi, H.; Tovar-Rios, D.A.; Oppedal, K.; Aarsland, D.; Duque, G. Muscle volume and intramuscular fat of the tongue evaluated with MRI predict malnutrition in people living with dementia: A 5-year follow-up study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 228–234. [Google Scholar] [CrossRef]
- Daboul, A.; Schwahn, C.; Bülow, R.; Kiliaridis, S.; Kocher, T.; Klinke, T.; Mundt, T.; Mourad, S.; Völzke, H.; Habes, M.; et al. Influence of age and tooth loss on masticatory muscles characteristics: A population based MR imaging study. J. Nutr. Health Aging 2018, 22, 829–836. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chang, W.H.; Liao, Y.F.; Chen, M.H.; Chang, C.T. Lip and tongue strength associated with chewing patterns in aging population. BMC Oral Health 2023, 23, 848. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.; Ower, P. Practical Periodontics, 1st ed.; Elsevier: St. Louis, MI, USA, 2015; pp. 3–12. [Google Scholar]
- Fouda, S.M.; Gad, M.M.; El Tantawi, M.; Virtanen, J.I.; Sipila, K.; Raustia, A. Influence of tooth loss on mandibular morphology: A cone-beam computed tomography study. J. Clin. Exp. Dent. 2019, 11, e814–e819. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67. [Google Scholar] [CrossRef]
- Rodakowska, E.; Jamiolkowski, J.; Baginska, J.; Kaminska, I.; Gabiec, K.; Stachurska, Z.; Kondraciuk, M.; Dubatowka, M.; Kaminski, K.A. Oral health–related quality of life and missing teeth in an adult population: A cross-sectional study from Poland. Int. J. Environ. Res. Public Health 2022, 19, 1626. [Google Scholar] [CrossRef]
- Mituuti, C.T.; Bianco, V.C.; Bentim, C.G.; de Andrade, E.C.; Rubo, J.H.; Berretin-Felix, G. Influence of oral health condition on swallowing and oral intake level for patients affected by chronic stroke. Clin. Interv. Aging 2015, 10, 29–35. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 8, 1057–1072. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Rogers, C.R.; Mooney, M.P.; Smith, T.D.; Weinberg, S.M.; Waller, B.M.; Parr, L.A.; Docherty, B.A.; Bonar, C.J.; Reinholt, L.E.; Deleyiannis, F.W.; et al. Comparative microanatomy of the orbicularis oris muscle between chimpanzees and humans: Evolutionary divergence of lip function. J. Anat. 2009, 214, 36–44. [Google Scholar] [CrossRef]
- Fortuna, G.; Whitmire, S.; Sullivan, K.; Alajbeg, I.; Andabak-Rogulj, A.; Pedersen, A.M.L.; Vissink, A.; di Fede, O.; Aria, M.; Jager, D.J.; et al. Impact of medications on salivary flow rate in patients with xerostomia: A retrospective study by the Xeromeds Consortium. Clin. Oral Investig. 2023, 27, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, Y.; Oki, T.; Ohta, M.; Ryu, M.; Kobayashi, K.; Sakurai, K.; Ueda, T. Distribution of lip-seal strength and its relation to oral motor functions. Clin. Exp. Dent. Res. 2021, 7, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Masuda, Y.; Kageyama, T.; Okazaki, R.; Murakami, M.; Yamada, K. The relationship between lip-closing force and dental arch morphology in patient with Angle Class I malocclusion. J. Oral Rehabil. 2017, 44, 205–212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).