Anaerobic Digestion of Duckweed Used to Remediate Water Contaminated with Zinc and Ammonium
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Duckweed Cultivation
2.3. Zn and NH4+-N Removal by Duckweed
2.4. Duckweed Pretreatment
2.5. Biochemical Methane Production Potential Measurements
2.6. Analytical Methods
2.6.1. Analyses of Duckweed Samples
2.6.2. Analyses of Liquid Samples
2.6.3. Analysis of Biogas
2.7. Calculations
2.7.1. Growth Parameters
2.7.2. Kinetic Models
2.7.3. Statistical Analysis
3. Results
3.1. Growth of Duckweed in the Presence of Zn and NH4+-N
3.2. Zn Uptake and Zn/NH4+-N Removal by Duckweed
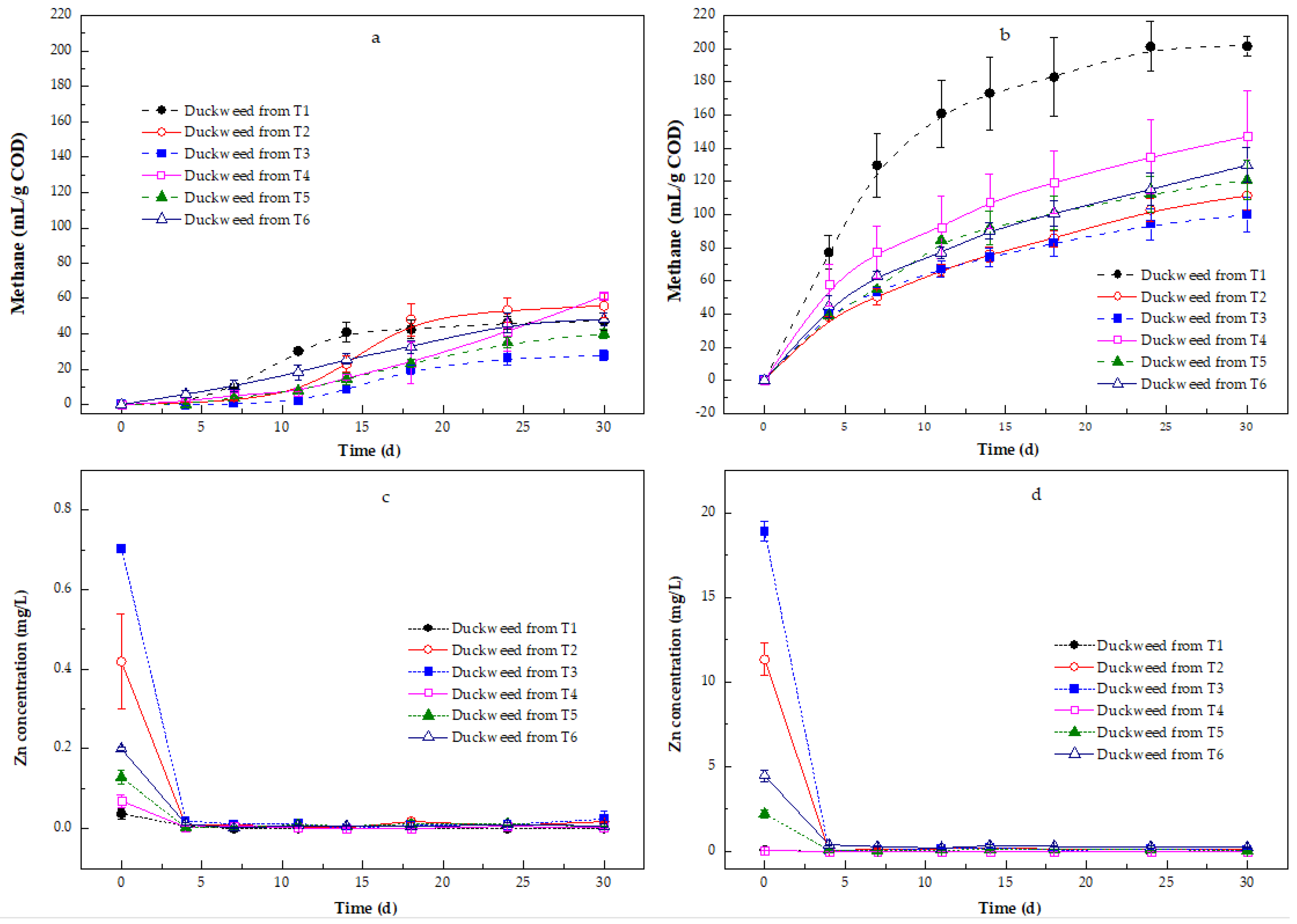
3.3. Methane Production Using the Solid and Liquid Fractions of the Duckweed
3.3.1. Methane Production
Kinetic Parameters of Methane Production
3.3.2. Variation in Process Parameters
Zn Removal in the Methane Production Process
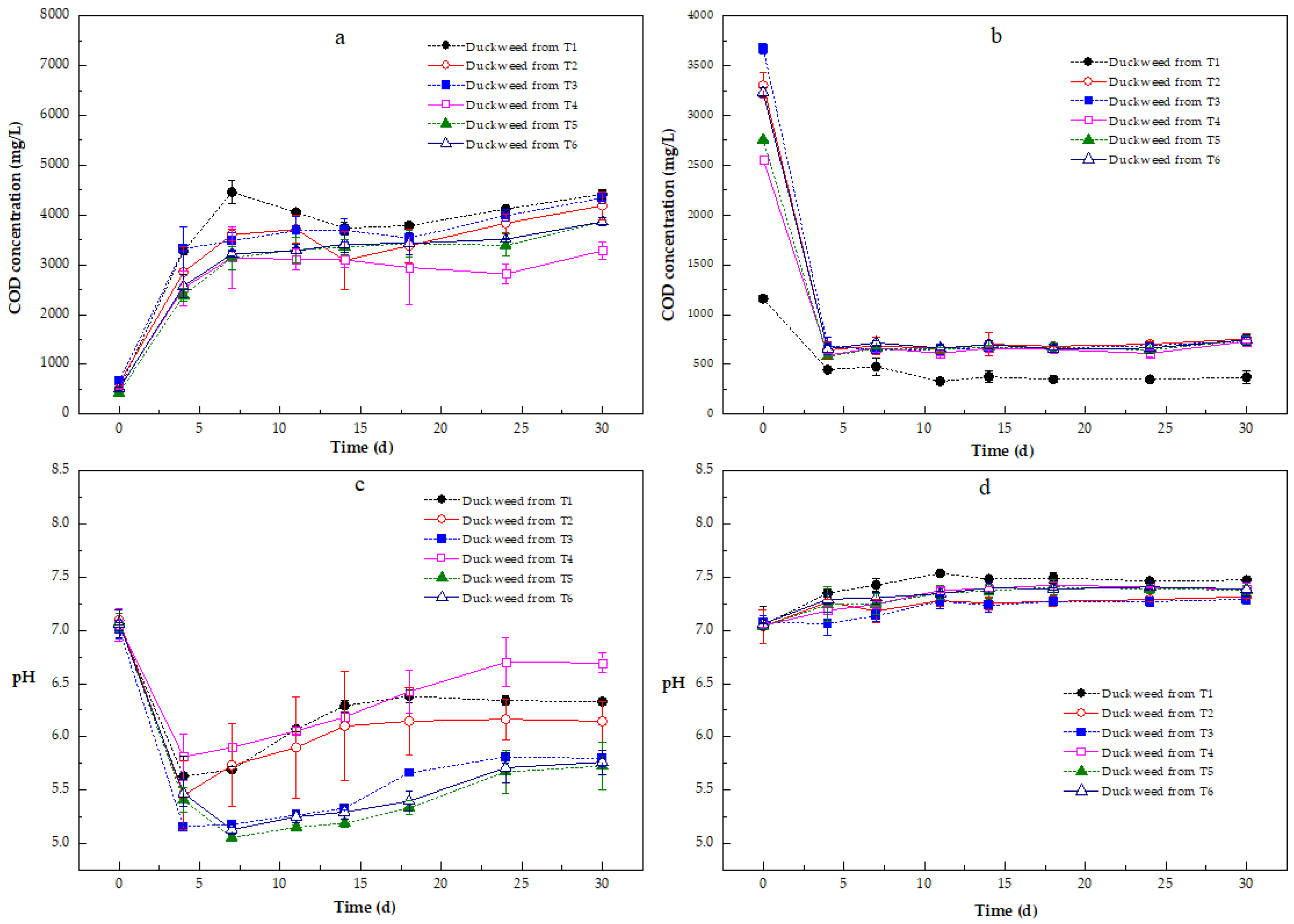
COD Variation in the Methane Production Processes
pH Variation During Anaerobic Digestion
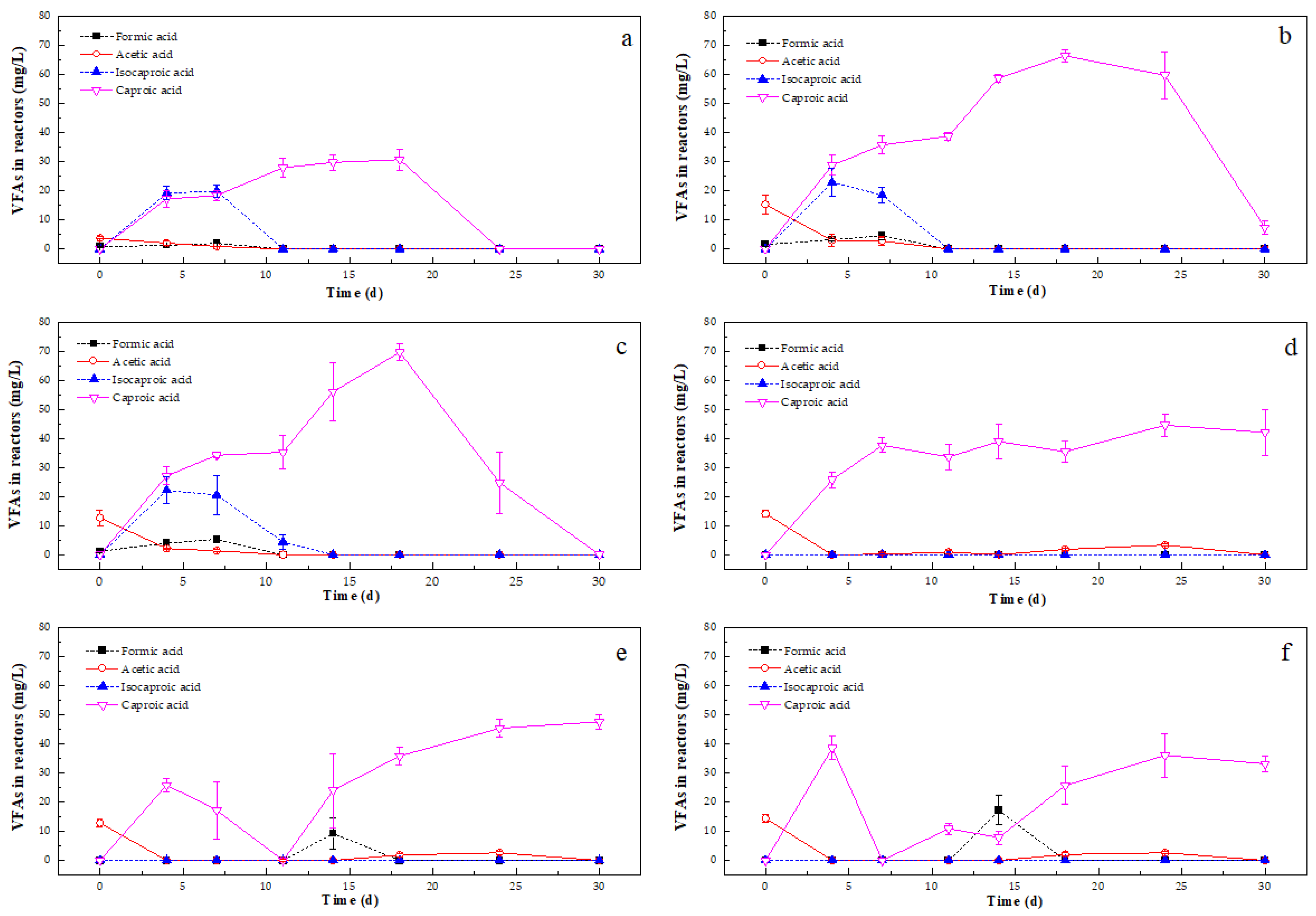
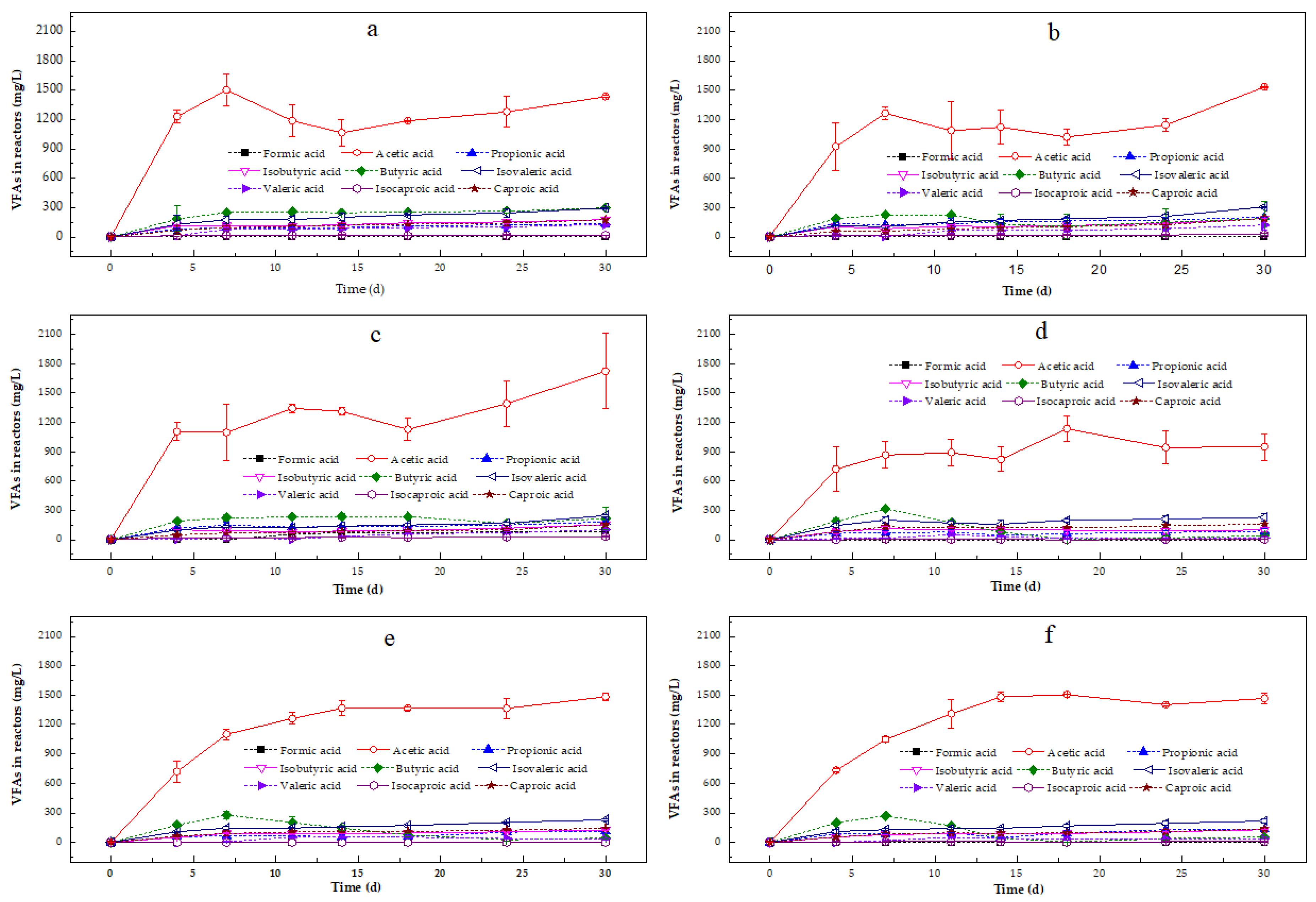
VFA Variation During Anaerobic Digestion
4. Discussion
4.1. Phytoremediation of Zn/NH4+-Polluted Water by Duckweed
4.1.1. Influence of Zn on Duckweed Growth and Zn Removal Efficiency
4.1.2. Effect of NH4+ on Zn Phytoremediation and Duckweed Growth
4.2. Anaerobic Digestion of Duckweed Used for Zn Phytoremediation
4.2.1. Effect of Zn on Methane Production Step
4.2.2. Effect of Zn on Acidification Step
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic Acid |
| AD | Anaerobic Digestion |
| BA | Butyric Acid |
| BCF | Bioconcentration Factor |
| BMP | Biomethane potential |
| CA | Caproic Acid |
| COD | Chemical Oxygen Demand |
| FA | Formic Acid |
| PA | Propionic Acid |
| IBA | Isobutyric Acid |
| ICA | Isocaproic Acid |
| IVA | Isovaleric Acid |
| RGR | Relative Growth Rate |
| TI | Tolerance Index |
| VA | Valeric Acid |
| VFAs | Volatile Fatty Acids |
| VS | Volatile Solids |
References
- Ulusoy, A.; Atılgan, A.; Rolbiecki, R.; Jagosz, B.; Rolbiecki, S. Innovative Approaches for Sustainable Wastewater Resource Management. Agriculture 2024, 14, 2111. [Google Scholar] [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative approaches to sustainable wastewater treatment: A comprehensive exploration of conventional and emerging technologies. Environ. Sci. Adv. 2025, 4, 189–222. [Google Scholar] [CrossRef]
- Hejna, M.; Alessandra, M.; Elisabetta, O.; Antonella, B.; Salvatore, P.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, J.; Zou, P.; Zhou, Y.; Wang, B.; Yu, D.; Li, W.; Zhan, X. Effects of Dietary Supplementation of Humic Acid Sodium and Zinc Oxide on Growth Performance, Immune Status and Antioxidant Capacity of Weaned Piglets. Animals 2020, 10, 2104. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Alagawany, M.; Hashem, N.M.; Farag, M.R.; Alghamdi, E.S.; Hassan, F.U.; Bilal, R.M.; Elnesr, S.S.; Dawood, M.A.O.; Nagadi, S.A.; et al. Nanominerals: Fabrication Methods, Benefits and Hazards, and Their Applications in Ruminants with Special Reference to Selenium and Zinc Nanoparticles. Animals 2021, 11, 1916. [Google Scholar] [CrossRef]
- Daverey, A.; Chen, Y.-C.; Sung, S.; Lin, J.-G. Effect of zinc on anammox activity and performance of simultaneous partial nitrification, anammox and denitrification (SNAD) process. Bioresour. Technol. 2014, 165, 105–110. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Rouff, A.A.; Juarez, K.M. Zinc Interaction with Struvite During and After Mineral Formation. Environ. Sci. Technol. 2014, 48, 6342–6349. [Google Scholar] [CrossRef]
- Jain, D.; Kour, R.; Bhojiya, A.A.; Meena, R.H.; Singh, A.; Mohanty, S.R.; Rajpurohit, D.; Ameta, K.D. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci. Rep. 2020, 10, 13865. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Tsonev, T.; Lidon, F. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Zhou, Q.; Lin, Y.; Li, X.; Yang, C.; Han, Z.; Zeng, G.; Lu, L.; He, S. Effect of zinc ions on nutrient removal and growth of Lemna aequinoctialis from anaerobically digested swine wastewater. Bioresour. Technol. 2018, 249, 457–463. [Google Scholar] [CrossRef]
- Abadi, V.A.J.M.; Sepehri, M. Effect of Piriformospora indica and Azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.). Symbiosis 2016, 69, 9–19. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Ahern, R.; Burnell, G.; O’Mahoney, R.; Kuehnhold, H.; Jansen, M.A.K. Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants 2022, 11, 2170. [Google Scholar] [CrossRef]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef]
- Paolacci, S.; Stejskal, V.; Toner, D.; Jansen, M.A.K. Wastewater valorisation in an integrated multitrophic aquaculture system; assessing nutrient removal and biomass production by duckweed species. Environ. Pollut. 2022, 302, 119059. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Guo, B.; Liu, C.; Liu, J.; Qiu, G.; Fu, Q.; Li, H. Alleviation of aqueous nitrogen loss from paddy fields by growth and decomposition of duckweed (Lemna minor L.) after fertilization. Chemosphere 2023, 311, 137073. [Google Scholar] [CrossRef]
- Amulya, K.; Morris, S.; Lens, P.N.L. Aquatic biomass as sustainable feedstock for biorefineries. Biofuels Bioprod. Biorefining 2023, 17, 1012–1029. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khan, A.A.; Suthar, S. Effect of thermal pre-treatment on co-digestion of duckweed (Lemna gibba) and waste activated sludge on biogas production. Chemosphere 2017, 174, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Liu, H.; Lin, W.; Shukla, P.; Luo, J. Simultaneous biohydrogen production from dark fermentation of duckweed and waste utilization for microalgal lipid production. Bioresour. Technol. 2020, 302, 122879. [Google Scholar] [CrossRef] [PubMed]
- Arefin, M.A.; Rashid, F.; Islam, A. A review of biofuel production from floating aquatic plants: An emerging source of bio-renewable energy. Biofuels Bioprod. Biorefining 2021, 15, 574–591. [Google Scholar] [CrossRef]
- Sharma, S.S.; Gaur, J.P. Potential of Lemna polyrrhiza for removal of heavy metals. Ecol. Eng. 1995, 4, 37–43. [Google Scholar] [CrossRef]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef]
- Luna-delRisco, M.; Orupõld, K.; Dubourguier, H.-C. Particle-size effect of CuO and ZnO on biogas and methane production during anaerobic digestion. J. Hazard. Mater. 2011, 189, 603–608. [Google Scholar] [CrossRef]
- Baek, G.; Saeed, M.; Choi, H.-K. Duckweeds: Their utilization, metabolites and cultivation. Appl. Biol. Chem. 2021, 64, 73. [Google Scholar] [CrossRef]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat. Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A state-of-the-art of phytoremediation approach for sustainable management of heavy metals recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Wang, X.; Xia, M.; Ba, S.; Lim, B.L.; Hou, H. Biomonitoring of heavy metals and their phytoremediation by duckweeds: Advances and prospects. Environ. Res. 2024, 245, 118015. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Kanwar, N. Duckweed: A model for phytoremediation technology. Holist. Approach Environ. 2022, 12, 39–58. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Xie, Y.; Li, J.; Li, J.; Ran, C. Optimal cultivation concentration of duckweed for pollutant removal from biogas slurry. Sci. Rep. 2025, 15, 5193. [Google Scholar] [CrossRef]
- Murillo, A.M.; Kotamraju, A.; Mulkeen, C.J.; Healy, M.G.; Sulpice, R.; Lens, P.N. Selenite (IV) and selenate (VI) uptake and accumulation capacity of Lemna minor L. from an aquatic medium. Environ. Technol. 2024, 45, 5630–5640. [Google Scholar] [CrossRef]
- Kotamraju, A.; Logan, M.; Lens, P.N.L. Integrated bioprocess for Se(VI) remediation using duckweed: Coupling selenate removal to biogas production. J. Hazard. Mater. 2023, 459, 132134. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production From Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, J.; Ji, Z.; Zhang, X.; Ramaswamy, S.; Xu, F.; Sun, R.-c. Dilute acid pretreatment differentially affects the compositional and architectural features of Pinus bungeana Zucc. compression and opposite wood tracheid walls. Ind. Crops Prod. 2014, 62, 196–203. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mohan, M.; Veeranki, V.D.; Goud, V.V.; Pinnamaneni, S.R.; Benarjee, T. Dilute acid pretreatment of sorghum biomass to maximize the hemicellulose hydrolysis with minimized levels of fermentative inhibitors for bioethanol production. 3 Biotech 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, M.J.; Mushtaq, M.; Tian, L.; Jiménez-Rodríguez, A.; Rincón, B.; Gilroyed, B.H.; Borja, R. Evaluation and modelling of methane production from corn stover pretreated with various physicochemical techniques. Waste Manag. Res. 2022, 40, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Morais, N.W.S.; Coelho, M.M.H.; de Oliveira, M.G.; Mourão, J.M.M.; Pereira, E.L.; dos Santos, A.B. Kinetic Study of Methanization Process Through Mathematical Modeling in Biochemical Methane Potential Assays from Four Different Inoculants. Water Air Soil Pollut. 2021, 232, 423. [Google Scholar] [CrossRef]
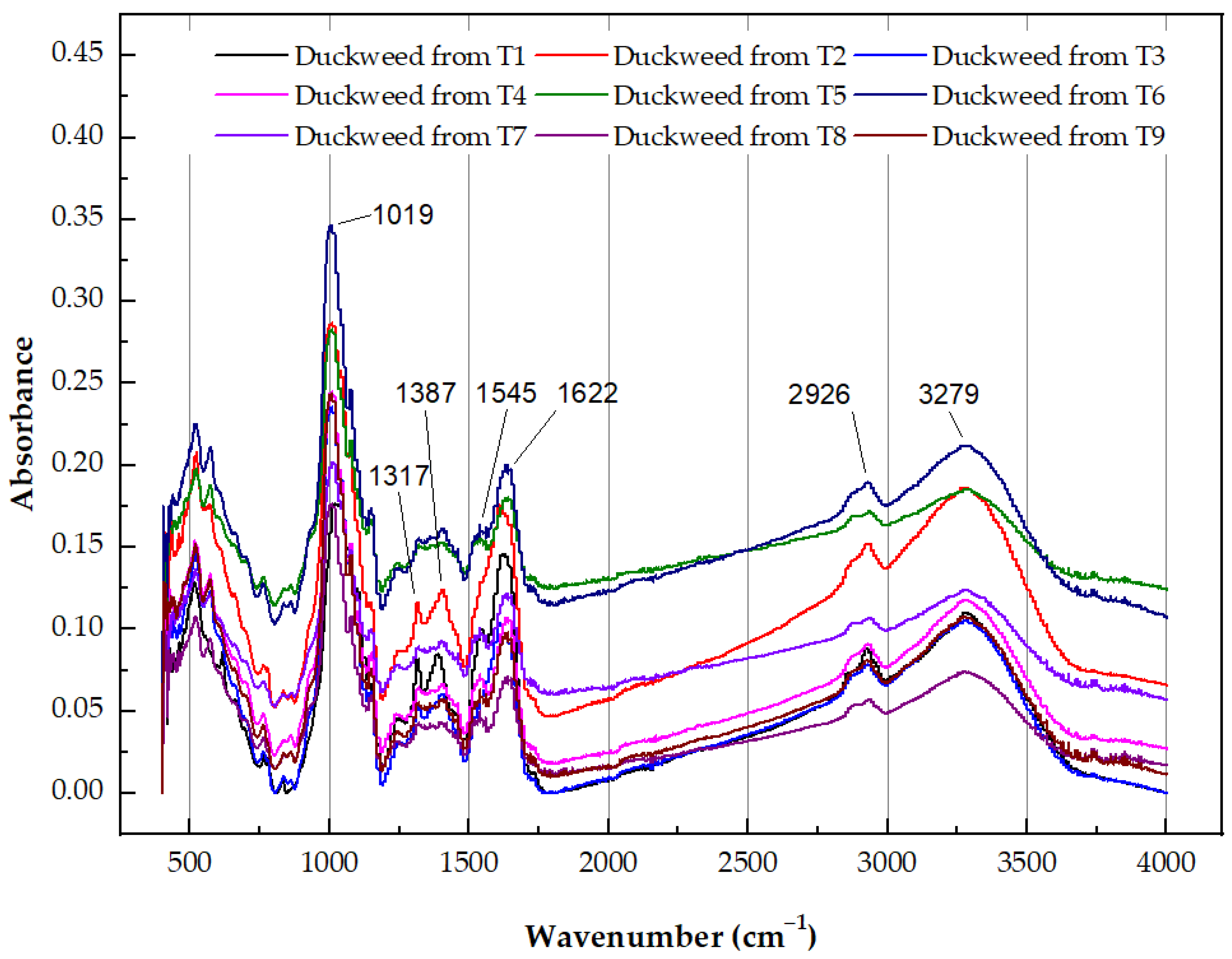
- Hu, L.X.; Ying, G.G.; Chen, X.W.; Huang, G.Y.; Liu, Y.S.; Jiang, Y.X.; Pan, C.G.; Tian, F.; Martin, F.L. Fourier-transform infrared spectroscopy as a novel approach to providing effect-based endpoints in duckweed toxicity testing. Environ. Toxicol. Chem. 2016, 36, 346–353. [Google Scholar] [CrossRef]
- Logan, M.; Tan, L.C.; Lens, P.N.L. Anaerobic co-digestion of dissolved air floatation slurry and selenium rich wastewater for simultaneous methane production and selenium bioremediation. Int. Biodeterior. Biodegrad. 2022, 172, 105425. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Logan, M.; Tan, L.C.; Nzeteu, C.O.; Lens, P.N.L. Effect of selenate on treatment of glycerol containing wastewater in UASB reactors. Renew. Energy 2023, 206, 97–110. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Wu, D.; Huang, T.; Zhang, B.; Cai, G.; Gao, G.; Liu, Z.; Huang, X.; Zhong, Z. Large-scale screening and characterization of Cd accumulation and ultrastructural deformation in duckweed. Sci. Total Environ. 2022, 832, 154948. [Google Scholar] [CrossRef]
- FTIR Functional Group Database Table with Search—InstaNANO. Available online: https://instanano.com/all/characterization/ftir/ftir-functional-group-search/ (accessed on 18 May 2025).
- Yang, C.; Wang, J.; Lei, M.; Xie, G.; Zeng, G.; Luo, S. Biosorption of zinc(II) from aqueous solution by dried activated sludge. J. Environ. Sci. 2010, 22, 675–680. [Google Scholar] [CrossRef]
- Kumar, M.; Matassa, S.; Bianco, F.; Oliva, A.; Papirio, S.; Pirozzi, F.; De Paola, F.; Esposito, G. Effect of Varying Zinc Concentrations on the Biomethane Potential of Sewage Sludge. Water 2023, 15, 729. [Google Scholar] [CrossRef]
- McGeer, J.C.; Szebedinszky, C.; McDonald, D.G.; Wood, C.M. Effects of chronic sublethal exposure to waterborne Cu, Cd or Zn in rainbow trout. 1: Iono-regulatory disturbance and metabolic costs. Aquat. Toxicol. 2000, 50, 231–243. [Google Scholar] [CrossRef]
- Jayasri, M.A.; Suthindhiran, K. Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef]
- Lanthemann, L.; van Moorsel, S.J. Species interactions in three Lemnaceae species growing along a gradient of zinc pollution. Ecol. Evol. 2022, 12, e8646. [Google Scholar] [CrossRef]
- Bodnar, I.S.; Cheban, E.V. Joint effects of gamma radiation and zinc on duckweed Lemna minor L. Aquat. Toxicol. 2023, 257, 106438. [Google Scholar] [CrossRef]
- Chen, X.; O’Halloran, J.; Jansen, M.A.K. The toxicity of zinc oxide nanoparticles to Lemna minor (L.) is predominantly caused by dissolved Zn. Aquat. Toxicol. 2016, 174, 46–53. [Google Scholar] [CrossRef]
- Li, J.; Lens, P.N.L.; Otero-Gonzalez, L.; Du Laing, G. Production of selenium- and zinc-enriched Lemna and Azolla as potential micronutrient-enriched bioproducts. Water Res. 2020, 172, 115522. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Phytoaccumulation of zinc by the aquatic plant, Lemna gibba L. Bioresour. Technol. 2009, 100, 6137–6140. [Google Scholar] [CrossRef]
- Jafari, N.; Akhavan, M. Effect of pH and heavy metal concentration on phytoaccumulation of zinc by three Duckweeds species. J. Agric. Environ. Sci. 2011, 10, 34–41. [Google Scholar]
- Chaudhry, F.M.; Loneragan, J.F. Zinc Absorption by Wheat Seedlings: I. Inhibition by Macronutrient Ions in Short-Term Experiments and its Relevance to Long-Term Zinc Nutrition. Soil Sci. Soc. Am. J. 1972, 36, 323–327. [Google Scholar] [CrossRef]
- Chairidchai, P.; Ritchie, G.S.P. The Effect of Citrate and pH on Zinc Uptake by Wheat. Agron. J. 1993, 85, 322–328. [Google Scholar] [CrossRef]
- Yu, L.; Kim, D.-G.; Ai, P.; Yuan, H.; Ma, J.; Zhao, Q.; Chen, S. Effects of Metal and Metal Ion on Biomethane Productivity during Anaerobic Digestion of Dairy Manure. Fermentation 2023, 9, 262. [Google Scholar] [CrossRef]
- Myszograj, S.; Stadnik, A.; Płuciennik-Koropczuk, E. The Influence of Trace Elements on Anaerobic Digestion Process. Civ. Environ. Eng. Rep. 2018, 28, 105–115. [Google Scholar] [CrossRef]
- Raja Ram, N.; Nikhil, G.N. A critical review on sustainable biogas production with focus on microbial-substrate interactions: Bottlenecks and breakthroughs. Bioresour. Technol. Rep. 2022, 19, 101170. [Google Scholar] [CrossRef]
- Wu, L.-J.; Kobayashi, T.; Kuramochi, H.; Li, Y.-Y.; Xu, K.-Q. Effects of Potassium, Magnesium, Zinc, and Manganese Addition on the Anaerobic Digestion of De-oiled Grease Trap Waste. Arab. J. Sci. Eng. 2016, 41, 2417–2427. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.b.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Huang, Z.; Niu, Q.; Nie, W.; Li, X.; Yang, C. Effects of heavy metals and antibiotics on performances and mechanisms of anaerobic digestion. Bioresour. Technol. 2022, 361, 127683. [Google Scholar] [CrossRef]
- Yu, H.Q.; Fang, H.H.P. Inhibition on Acidogenesis of Dairy Wastewater by Zinc and Copper. Environ. Technol. 2001, 22, 1459–1465. [Google Scholar] [CrossRef]


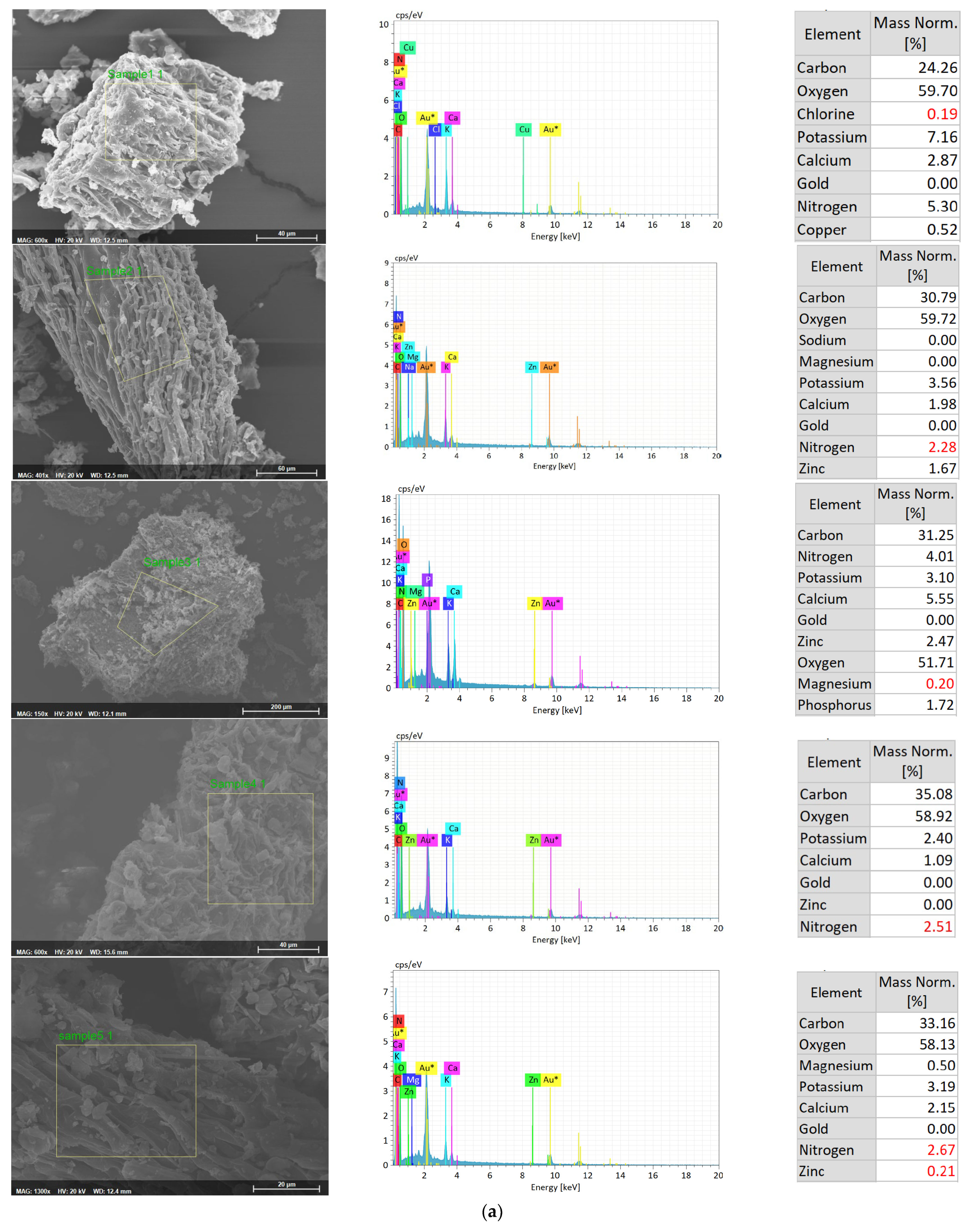
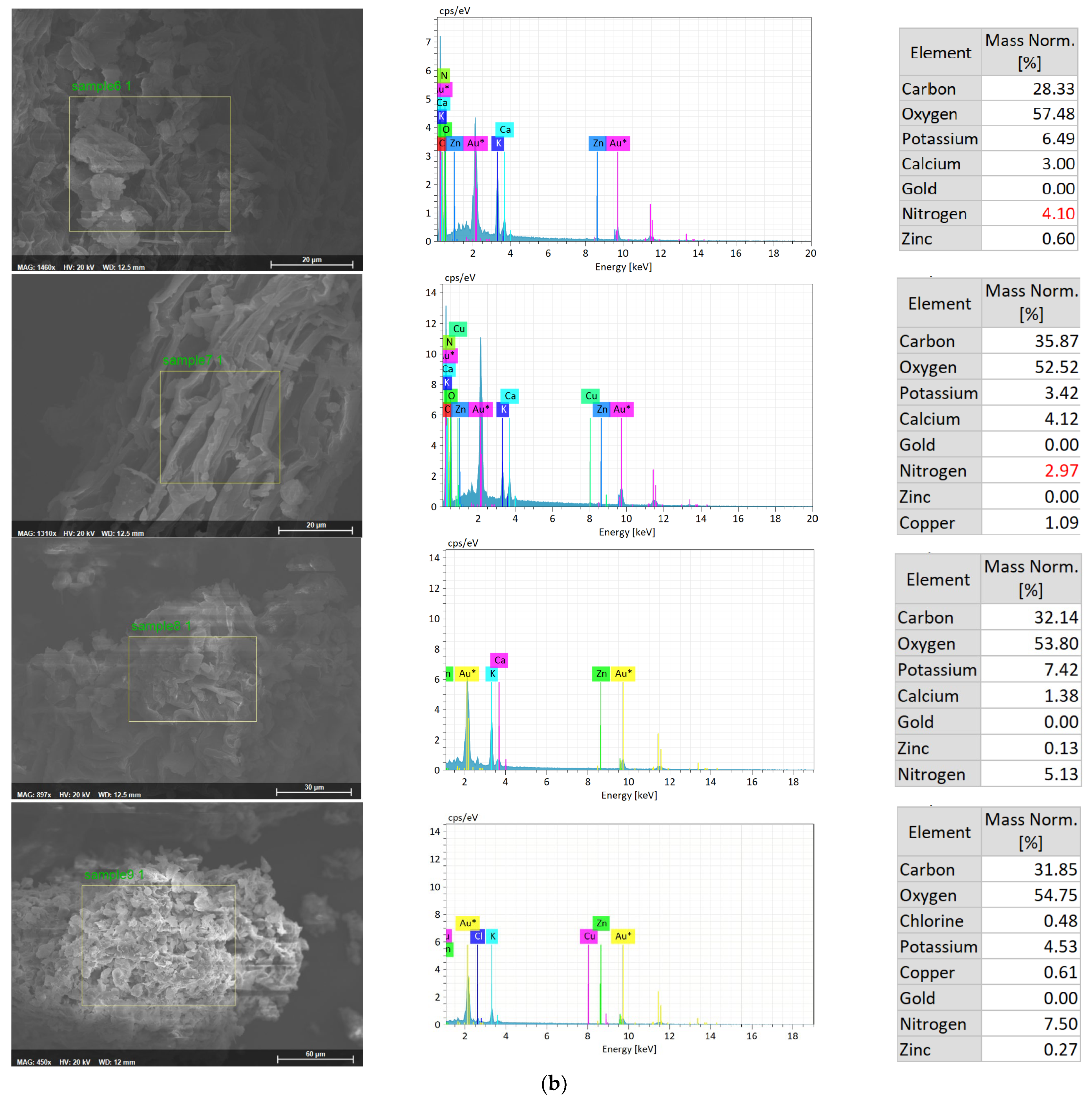
| Treatment | Content (mg/L) | |
|---|---|---|
| Zn | NH4+-N | |
| T1 (Control) | 0 | 0 |
| T2 | 2.5 | 0 |
| T3 | 5.0 | 0 |
| T4 | 0 | 20 |
| T5 | 2.5 | 20 |
| T6 | 5.0 | 20 |
| T7 | 0 | 40 |
| T8 | 2.5 | 40 |
| T9 | 5.0 | 40 |
| Duckweed Fraction | Treatment Condition | Mmax | Rmax | λ | Tmax | R2 |
|---|---|---|---|---|---|---|
| Duckweed solid fraction | T1 | 46.196 | 5.602 | 5.261 | 8.295 | 0.997 |
| T2 | 56.066 | 7.138 | 10.500 | 13.400 | 0.993 | |
| T3 | 28.130 | 3.039 | 11.126 | 14.531 | 0.999 | |
| T4 | 153.409 | 3.364 | 11.763 | 28.539 | 0.997 | |
| T5 | 46.791 | 2.253 | 7.514 | 15.154 | 0.997 | |
| T6 | 56.395 | 2.316 | 2.922 | 11.880 | 0.992 | |
| Duckweed liquid fraction | T1 | 194.689 | 18.936 | 0.174 | 3.956 | 0.982 |
| T2 | 112.207 | 5.448 | <0.01 | 6.079 | 0.957 | |
| T3 | 95.569 | 6.097 | <0.01 | 4.422 | 0.945 | |
| T4 | 141.182 | 8.309 | <0.01 | 4.765 | 0.944 | |
| T5 | 117.230 | 7.749 | <0.01 | 5.329 | 0.978 | |
| T6 | 126.647 | 6.584 | <0.01 | 5.590 | 0.951 |
| Plant | Growth Medium | Temperature (°C) | Exposure Time (d) | Zn Concentration (mg/L) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Lemna minor | Synthetic media, i.e., Quarter Coic and Lessaint solution | 25 | 4 | 10 | ~70–80% | [53] |
| Lemna trisulca, Lemna minuta Lemna minor | Wastewater | 25 | 10 | 1, 5, 10, 15, and 20 | 97%, 89%, and 83% maximum | [59] |
| Lemna gibba | Quarter Coïc solution | Ambient temperature | 7 | 18, 14, 10 and 6 | 61–71% | [58] |
| Hybrid of Lemna minor and Lemna turionifera | Steinberg growth medium | 18 | 7 | 2.5 and 5.0 | >82% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhan, X.; Hatzikioseyian, A.; Lens, P.N.L. Anaerobic Digestion of Duckweed Used to Remediate Water Contaminated with Zinc and Ammonium. Appl. Sci. 2025, 15, 6212. https://doi.org/10.3390/app15116212
Zhang Y, Zhan X, Hatzikioseyian A, Lens PNL. Anaerobic Digestion of Duckweed Used to Remediate Water Contaminated with Zinc and Ammonium. Applied Sciences. 2025; 15(11):6212. https://doi.org/10.3390/app15116212
Chicago/Turabian StyleZhang, Yan, Xinmin Zhan, Artin Hatzikioseyian, and Piet Nicolaas Luc Lens. 2025. "Anaerobic Digestion of Duckweed Used to Remediate Water Contaminated with Zinc and Ammonium" Applied Sciences 15, no. 11: 6212. https://doi.org/10.3390/app15116212
APA StyleZhang, Y., Zhan, X., Hatzikioseyian, A., & Lens, P. N. L. (2025). Anaerobic Digestion of Duckweed Used to Remediate Water Contaminated with Zinc and Ammonium. Applied Sciences, 15(11), 6212. https://doi.org/10.3390/app15116212








