1. Introduction
In real-world workgroups, decision-making conversations can either foster agreement and group cohesion or deepen conflicts and amplify discord. A crucial determinant in steering these conversations toward constructive outcomes is the promotion of negotiation and compromise. Encouraging these strategies has been shown to enhance exploration and the exchange of diverse perspectives, facilitating a more integrative and adaptive approach to decision-making. Notably, dyads that adopt a compromise-oriented mindset tend to achieve more harmonious and mutually satisfactory outcomes than those engaging in competition [
1].
Previous research on decision-making conversations highlights two primary factors that shape their trajectory: intentions (as starting points) and communication strategies (which are adopted over the course of the conversation) [
2,
3]. First, the success of a decision-making conversation often hinges on individuals’ underlying intentions—their opinions, goals, and needs. These intentions, in turn, shape the communication strategies adopted to bridge differences and reach agreements. Effective negotiation relies on strategies that align thoughts and emotions, fostering shared understanding and consensus. The interplay between intention and communication strategies ultimately determines the effectiveness of the negotiation process and the quality of the final agreement.
A first key factor of conversational intentions is an individual’s decision-making orientation, which can be individual- or group-focused. While individual-oriented people may prioritize personal gains or fixed perspectives, group-oriented individuals are more inclined to integrate multiple viewpoints, making them less susceptible to biases, overconfidence, and cognitive errors [
4]. This openness to diverse perspectives not only improves decision accuracy but also fosters the creation of innovative, high-quality solutions [
5,
6]. In negotiation-driven conversations, group-oriented individuals tend to perceive the goal of acting as a cohesive unit as both socially and personally meaningful, reinforcing their commitment to cooperative dialogue.
The second key factor influencing decision-making conversations is the explicit goal of the interaction, which can be competitive or cooperative. These goals significantly shape the communication strategies employed. A cooperative mindset, strongly associated with group orientation, is characterized by prosocial intentions that prioritize joint success rather than individual gain. Dyads with cooperative goals actively engage with each other’s ideas, fostering a dynamic exchange that enhances learning, adaptation, and innovation [
7,
8]. Research has consistently linked cooperation to greater creativity and cognitive flexibility, which are essential for effective problem-solving and decision-making [
9,
10,
11,
12,
13].
Critically, cooperation is deeply intertwined with negotiation behaviors [
14,
15]. Negotiation involves the exchange of information about interests and perspectives, followed by strategic trade-offs that maximize collective benefits [
16]. Given its reliance on perspective-taking and shared goal-setting, negotiation thrives in environments where group orientation is strong.
To the best of our knowledge, no previous study has investigated the neurophysiological mechanisms underlying negotiation within group-oriented individuals during real-time, naturalistic decision-making interactions.
In neuroscience, negotiation and cooperative behaviors have been investigated using various tasks, including games, problem-solving activities, creativity exercises, mathematical challenges, and behavioral synchronization [
17]. These studies have employed both single-brain and brain-to-brain experimental paradigms, utilizing different neuroscientific tools. While communication between participants is a key element in this research, no study has specifically examined negotiation through a communicative ecologically valid scenario that involves synchrony and turn-taking [
18].
Although the importance of studying brain dynamics during real-time social interactions is widely acknowledged, most research has measured the electrophysiological (EEG) brain activity in single subjects rather than in interacting pairs or groups [
19]. Regarding the functional significance of EEG frequency bands, delta waves are thought to signal novelty in emotional contexts, responding more to a stimulus’s attentional salience than its emotional content [
20]. Theta activity has been linked to motivation, emotional processing, task relevance [
20], and adaptive behavior in social negotiation [
21]. Alpha and beta bands indicate cognitive effort, attention, and engagement in decision-making processes [
22], with alpha suppression playing a key role in decision-making by reflecting cognitive resource allocation [
23]. Beta waves are associated with emotional significance, attentional control, and cognitive regulation [
24,
25]. Gamma oscillations integrate sensory and emotional processing and are markers of top-down attention and conscious awareness [
26,
27]. Both beta and gamma bands also play a role in memory and decision-making processes [
28].
Also, the specific localization of EEG frequency bands correlate with different neural functions: frontal delta activity is linked to emotional and motivational processing [
20,
29], while theta waves in frontal regions reflect attentional engagement and strategic control [
21,
30]. Reduced parietal alpha activity is associated with enhanced cognitive performance [
30], and parietal–occipital beta waves indicate focused attention [
31]. Moreover, alpha and beta bands in parieto-occipital areas are associated with neural correlates of sense of agency, defined as the subjective experience of initiating and controlling actions to affect external events [
32] as well as index of working-memory performance [
33]. Gamma modulations in parieto-occipital regions, instead, may be interpreted as top-down activation of mental representations and the maintenance of auditory and visual information in working memory. This could reflect the increasing cognitive resources required for more effective planning, as previously evidenced [
34,
35].
While single-brain approaches help localize neural activations related to social processes, they fail to grasp the real-time brain-to-brain dynamics. In this field, the hyperscanning paradigm, which enables the simultaneous recording of brain activity from multiple individuals, may reveal functional connections and interbrain synchronization. This approach shifts the focus from isolated brain activity to dynamic interactions, where behaviors and neural patterns continuously adapt to each other.
Hyperscanning research has demonstrated the critical role of interbrain synchrony in joint attention, communication, coordination [
36,
37,
38], cooperation [
37], prosocial exchange [
38] and decision-making tasks. In particular, hyperscanning studies have shown that the dorsolateral prefrontal cortex, specifically in the left side, is a key area of cooperation processes [
37] as well as the prefrontal cortex, which is implicated in joint action performance [
39]. Moreover, during joint actions in cooperative behavior, a higher synchronization was reported in the delta and theta low frequency bands, as an index of social competencies, empathy, and emotional engagement [
39]. Additionally, the temporoparietal junction (TPJ) is involved in social cognition, including perspective-taking, Theory of Mind, and communication [
40]. TPJ also plays a role in managing social dynamics, including negotiation processes [
41,
42]. However, for a comprehensive understanding of cooperative processes, it is essential to consider real, dynamic interactions where partners are actively engaged, as social exchanges naturally occur in face-to-face settings [
39].
Building on these premises, this study aims to: (i) examine the single-brain neural correlates of group-oriented individuals during decision-making processes that explicitly involve negotiation; (ii) investigate inter-brain neural synchronization within dyads working toward a shared decision. The participants, paired in dyads, discussed a hypothetical situation where a workgroup member did not align with the team’s ideals. Before the interaction, their group decision-making orientation was assessed using the Likert scale. During the negotiation interaction, they were asked to negotiate on the best solution for the hypothetical situation previously presented, while EEG data were continuously recorded to analyze their neural activity.
Given the previous evidence, first at the single-brain level, we hypothesized that group-oriented individuals (for which a group decision-making solution would be a relevant option) will show a significantly increase in low-frequency bands (delta and theta) connected to emotional processing in the frontal brain region compared to parieto-occipital regions [
20,
29]. This is because frontal delta and theta activity has been associated with emotional engagement, motivation, and goal-directed control, all crucial for effective negotiation and group alignment, especially in group-oriented individuals.
Secondly, a greater high frequency band power (beta and gamma bands [
34,
35]) is expected in parieto-occipital regions, deputed to cognitive processing and awareness during the negotiation process. Beta and gamma oscillations in these areas are linked to cognitive control, memory, and decision-making, supporting the integration of different perspectives and the monitoring of the negotiation process. These bands have been associated with a greater ability to integrate multiple pieces of information, a key element for those who are group-oriented.
Furthermore, since previous hyperscanning studies showed an increase in delta and theta EEG coherence in the frontal area during cooperation and prosocial exchange [
38], we expect to observe a lower dissimilarity in the frontal area compared to other brain regions in this sample of group-oriented dyads during the negotiation process. While frontal low-frequency synchrony is typically observed in joint action and imitation paradigms, cooperative negotiation may involve functionally divergent but complementary regulatory processes, whereby each individual evaluates and modulates their own cognitive and affective strategies in the pursuit of a shared decision [
43,
44]. Frontal EEG coherence has been linked to interpersonal alignment, empathy, and joint decision-making, suggesting that a greater synchronization in this region reflects a shared mental framework essential for negotiation.
2. Materials and Methods
2.1. Sample
A total of 26 university students (6 males and 20 females; mean age = 25.4 years, standard deviation ± 6.4 years) participated in the study. They were recruited through a non-probabilistic convenience sampling method from [BLIND]. An a priori power analysis was conducted using G*Power (version 3.1.9.7 [
45]) to determine the required sample size for the planned repeated-measures analysis of variance (ANOVA), which included three Regions of Interest (ROIs), two groups, and two measurement points. Based on a medium effect size (
f = 0.40) derived from prior hyperscanning EEG studies reporting regional differences in spectral activity during social interaction (e.g., [
46]), the analysis indicated that a minimum of 24 participants would be required to achieve a statistical power of 0.95 at an alpha level of 0.05. This supports the adequacy of the recruited sample for detecting effects of interest in the present study. This analysis indicated that, for a medium effect size (
f = 0.40), an alpha level of 0.05, and a statistical power of 0.95, a minimum of 24 participants was necessary to achieve reliable results across two groups and two measurement points, confirming the sufficiency of the recruited sample.
The participants were randomly assigned to one of two experimental roles, labelled Member 1 and Member 2. This attribution was conducted to form 13 dyads, with each dyad comprising two individuals of the same gender. To control for potential biases and confounding variables, participants were explicitly selected to ensure that no prior familiarity or acquaintance existed between dyad members before the experimental session.
The participants were enrolled in the study on a voluntary basis, with no monetary incentives or alternative compensation offered. Inclusion criteria were rigorously defined to ensure homogeneity in relevant variables: all participants were required to be right-handed, possess normal or corrected-to-normal visual acuity, and meet standardized thresholds for general health and cognitive functioning. Exclusion criteria were systematically applied to eliminate potential confounding factors. These included a self-reported history of psychiatric or neurological disorders, clinically significant depressive symptoms, diminished global cognitive functioning, and documented impairments in short- or long-term memory. Additionally, individuals currently using psychoactive medications with the potential to alter cognitive processes were excluded to maintain the integrity of the experimental outcomes. All participants signed a written informed consent before participating in the study.
Ethical approval for the study was granted by [BLIND]. The study was carried out in full accordance with the ethical standards delineated in the Declaration of Helsinki (2013). Additionally, the study adhered to the General Data Protection Regulation (GDPR, EU Reg. 2016/679).
2.2. Experimental Procedure
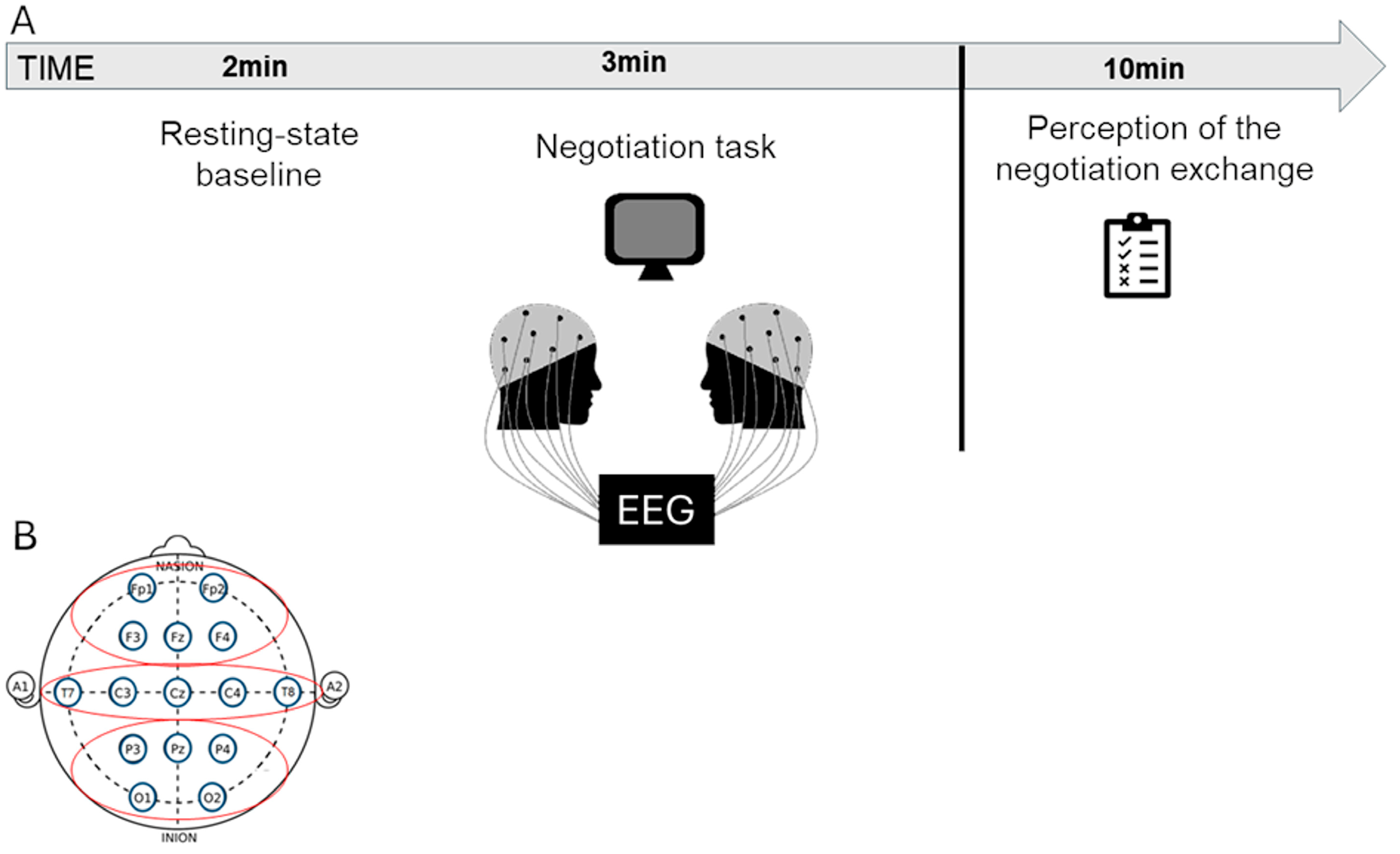
The experiment was conducted in a quiet darkened room, where participants, after providing informed consent forms, were seated comfortably next to one another to promote direct interaction for all the duration of the experimental procedure, which took approximately 30 min. Before starting the negotiation task, an EEG eyes-open resting-state baseline of 120 s was detected by the participants using the EEG hyperscanning paradigm. EEG activity was also recorded simultaneously by the participants during the negotiation task, which lasted approximately 3 min (
Figure 1A). Specifically, the experimental procedure followed three main steps: (i) the decision-making orientation assessment (step 1), (ii) the negotiation task (step 2), and (iii) the perception of negotiation exchange (step 3).
Step 1: Decision-making orientation assessment.
To verify the decision-making orientation (individual versus group orientation), a real-life decision-making script based on a common group problem was administered to the members through the Qualtrics XM platform (Qualtrics LLC, Provo, UT, USA) before the negotiation interaction. Specifically, this script was presented to take advantage of a hypothetical scenario with high ecological validity. After instructing the participants to identify with the situation that will be presented to them, the following realistic decision-making scenario and instructions were shown to participants:
“A member of a workgroup does not appear to be in line with the ideals and style of the group. What does the group do? Think about your real work group.
Now rate with a number from 1 to 5 (where 1 stands for “not at all” and 5 for “very much”) how close the following decisions are to how your real group would behave.”
Subsequently, eight items about different ways in which the real group would face the situation were presented. The eight items were presented in a random order, with four of them representing a behavior characterized by an individual decision-making orientation (1–4 item, e.g., “The decision follows the perspective of the most innovative member of the group”) and the other four a group decision-making orientation (5–8 item, e.g., The group adopts the decision endorsed by the majority).
The individual orientation score (Individualorientation) and the group orientation score (Grouporientation) were calculated by averaging the four individual and four group orientation items.
Step 2: Negotiation task.
After the decision-making orientation assessment, the participants were introduced to the negotiation task, in which they had to negotiate and decide together how to deal with the realistic situation previously proposed in the first step. Specifically, the following instructions were provided:
“This study aims to investigate how individuals negotiate their opinions. You will therefore be asked to discuss the previously proposed topic and share your perspectives. Specifically, as a first step, we ask you to individually choose from the previous sentences the one that is closest to your decision and the way your real group would behave.”
After receiving these instructions, the participants had the chance to read again the eight previous statements, and the experimenter recorded each participant’s chosen statement as Member 1’s initial choice and Member 2’s initial choice.
Following this decision, the two participants engaged in a three-minute timed negotiation exchange, during which they were required to collaboratively decide on a single statement that best reflected their shared perception of their real workgroup, following this instruction:
“Now, reflecting on the same scenario you have just read, you must work together to agree on a single statement that represents what your real group means to both of you. Therefore, the statement must be mutually accepted and should best reflect how to act within the group and for the group. You will have a maximum of 3 min to reach an agreement.”
Finally, at the end of the negotiation exchange, the experimenter recorded the final agreed-upon statement as the dyad’s final negotiated choice. All dyads involved in this study ended the negotiation process within the given time by reaching a common agreement.
Step 3: Perception of the negotiation exchange: manipulation check.
At the end of the negotiation task, the perception of the negotiation exchange inside each dyad was investigated by asking the members to fill in an item presented on a 5-point Likert scale, where 1 stands for “completely in disagreement” and 5 for “completely in agreement”.
Specifically, the following two items were created ad hoc to investigate how the negotiation exchange influenced each member’s idea: “
The other agent influenced my decision” and “
The real group idea I had before the discussion has changed”. Moreover, 10 items, which were derived, translated, and modified from the validated Group Questionnaire [
47], were administrated to check the negotiation exchange in terms of cohesion, alliance, conflict, climate, and empathic failure.
The behavioral data showed how participants reported that the other member of the dyad influenced his/her decision (M = 3.23, SD = 0.92) and that he/she does not change his/her idea due to the negotiation exchange (M = 2.80, SD = 1.08). Additionally, a sense of cohesion, alliance, positive climate, as well as an absence of conflict and emphatic failure were reported as being above the average (cohesion: M = 4.40, SD = 0.23; alliance: M = 4.18, SD = 0.12; climate: M = 4.30, SD = 0.17; conflict: M = 1.50, SD = 0.13; emphatic failure: M = 1.58, SD = 0.05).
2.3. EEG Data: Acquisition and Processing
EEG data were acquired using a 16-channel direct current (DC) amplifier (SYNAMPS system) integrated with the NEUROSCAN 4.2 software. Recordings were performed during both resting-state and negotiation tasks, with electrode placement adhering to the international 10/20 system [
48]. A total of 15 Ag/AgCl electrodes (Fp1, Fp2, F3, F4, Fz, Cz, C3, C4, T7, T8, Pz, P3, P4, O1, O2) were positioned on the scalp via an ElectroCap (Compumedics Germany GmbH, Singen, Germany), with the earlobes serving as reference points (
Figure 1B). Additionally, two electrooculographic (EOG) electrodes were positioned at the outer canthus of the left eye to monitor ocular artifacts.
Figure 1.
(A) The picture displays the timeline of the experimental procedure encompassing the EEG recording during the resting state baseline and the negotiation task. After the negotiation task, participants filled in a scale on the perception of negotiation exchange. (B) Following the 10/20 international system, each subject’s EEG montage included 15 selected electrodes. For statistical analysis, three anatomically defined regions of interest (ROIs) were considered: the frontal region (Fp1, Fp2, F3, F4), the temporo-central region (T7, T8, C3, C4), and the parieto-occipital region (P3, P4, O1, O2).
Figure 1.
(A) The picture displays the timeline of the experimental procedure encompassing the EEG recording during the resting state baseline and the negotiation task. After the negotiation task, participants filled in a scale on the perception of negotiation exchange. (B) Following the 10/20 international system, each subject’s EEG montage included 15 selected electrodes. For statistical analysis, three anatomically defined regions of interest (ROIs) were considered: the frontal region (Fp1, Fp2, F3, F4), the temporo-central region (T7, T8, C3, C4), and the parieto-occipital region (P3, P4, O1, O2).
Prior to data collection, the impedance levels of all electrodes were carefully measured and maintained below 5 kΩ to ensure optimal signal integrity. The EEG signal was digitized at a 1000 Hz sampling rate, with a 50 Hz notch filter applied to mitigate electrical interference from power lines. For offline processing, a 0.01–50 Hz infinite impulse response (IIR) bandpass filter with a slope of 48 dB/octave was applied to the data. Continuous EEG recordings were then segmented into two-second epochs, with visual inspection to identify and exclude any artifacts originating from ocular, muscular, or movement-related sources. Artifact-free segments were subjected to spectral analysis using fast Fourier transform (FFT) with a Hamming window, achieving a spectral resolution of 0.5 Hz. This procedure facilitated the computation of average power spectral density (PSD) for both resting-state and negotiation conditions. In addition, a rigorous multistep preprocessing pipeline was applied to enhance signal quality. Raw EEG data were re-referenced to linked earlobes and filtered with a 0.01–50 Hz band-pass filter and a 50 Hz notch filter. An Independent Component Analysis (ICA) was employed to identify and remove components linked to ocular and muscular artifacts. Data were then segmented into 2-s epochs, and epochs contaminated by residual artifacts were excluded following combined automated and manual inspection. These procedures ensured high-fidelity signal integrity across participants and dyads, enabling reliable spectral analyses in all frequency bands of interest.
Power spectral densities (PSDs) were calculated for the standard EEG frequency bands: delta (0.5–3.5 Hz), theta (4–7.5 Hz), alpha (8–12.5 Hz), beta (13–30 Hz), and gamma (30.5–50 Hz). Signal processing and analysis were conducted using Brain Vision Analyzer 2.0 (Brain Products GmbH, Gilching, Germany). To assess task-induced EEG modulation, the PSD values recorded during the negotiation phase were normalized against the baseline resting-state PSD values. The normalization procedure was performed using the following formula: [Normalized PSD = (PSDtask − PSDBL)/PSDBL], where PSDtask represents the power measured during the task and PSDBL corresponds to the baseline power. This normalization was applied separately to each frequency band and electrode location for both the negotiator and receiver within each dyad, allowing for the individualized assessment of task-related changes in EEG activity.
During the negotiation interaction, the participants were instructed to remain relaxed and maintain a steady posture, minimize motor movements, articulate their speech clearly, and avoid whispering and overlapping with the other participant’s speech, adhering to turn-taking conventions. Furthermore, the negotiation exchange was video-recorded, confirming the absence of noticeable changes in facial expressions or posture throughout the interaction. Thanks to these guidelines and to the video recordings, it was possible to consider and analyze only negotiation-speaking turns, with conversational pauses and non-negotiation utterances excluded. Accordingly, the data for each individual were defined as the average duration of all speech segments in which they actively participated in negotiation. Any utterances unrelated to the negotiation process, as well as conversational pauses, were omitted from the analysis.
For statistical analysis, three anatomically defined Regions of Interest (ROIs) were identified: frontal (Fp1, Fp2, F3, F4), temporo-central (T7, T8, C3, C4), and parieto-occipital (P3, P4, O1, O2). Within each ROI, power spectral density (PSD) values were calculated as the mean spectral power across the corresponding electrodes. This method allowed for a precise assessment of regional EEG activity across frequency bands, facilitating the comparison of task-related neural dynamics during the negotiation phase relative to the resting-state baseline.
2.4. Statistical Data Analyses
Prior to conducting statistical analyses, it was verified that all 13 dyads had reached an agreement within the three-minute negotiation task.
The statistical data analyses conducted in this study encompassed both behavioral and EEG data evaluations. For the behavioral analysis, a repeated measures ANOVA was performed to examine the group decision-making orientation based on Likert scale scores.
EEG data were analyzed at both the individual and dyadic levels (single-brain and dyads analyses), as outlined below.
2.4.1. Behavioral Data Analyses
As the preliminary analysis, one repeated-measures ANOVA with Orientation (2: Grouporientation, Individualorientation) as within-subject factor was computed on the score of the Likert scale to verify the group decision-making orientation of the members.
2.4.2. EEG Data Analysis
For EEG data, two main types of statistical analysis were included: a single-brain brain and an inter-brain analysis.
For the single-brain analysis, five repeated-measures ANOVAs considering ROIs (3: frontal, temporo-central, and parieto-occipital) as the within-subject variable were applied to the five different frequency bands (delta, theta, alpha, beta, and gamma) as the distinct dependent variable.
For the inter-brain analysis, EEG activity was analyzed at the dyadic level to quantify the extent to which Member 1 and Member 2 differed in their EEG activation. This was achieved by calculating the Euclidean Distance (PSD) [
49,
50,
51] between the power spectral density (PSD) values of each frequency band for corresponding ROIs within each dyad. Then, five repeated mixed-measures ANOVAs considering
ROIs (3: frontal, temporo-central, and parieto-occipital) as within-subject variables were performed considering the Euclidean Distance (PSD) for the five frequency bands (delta, theta, alpha, beta, and gamma) as the distinct dependent variables.
For all repeated-measures ANOVAs, degrees of freedom were adjusted using the Greenhouse-Geisser epsilon to correct for violations of sphericity, as assessed by Mauchly’s test of sphericity. When significant effects or interactions were identified, post hoc pairwise comparisons were performed with Bonferroni correction to control Type I error. Effect sizes were reported using eta-squared (η2), and the significance threshold was set at α = 0.05. Prior to conducting the primary analyses, the assumption of normality was checked by assessing skewness, kurtosis, and Q-Q plots. Levene’s test was applied to verify the homogeneity of variances.
Descriptive statistics, including means and standard errors, were computed for each condition to facilitate the interpretation of results. All statistical analyses were performed using Jamovi (version 2.6.22; The Jamovi Project, 2022).
4. Discussion
This study investigated the single-brain EEG correlates of group-oriented individuals during a decision-making process with an explicit negotiation goal, as well as the inter-brain neural activity of dyads converging on the decision. The findings contribute to the growing body of literature on social neuroscience by elucidating how negotiation processes in dyads shape individual and inter-brain neural dynamics. Specifically, negotiation was studied through a communicative ecologically valid scenario that involves synchrony and turn-taking. The modulation of different EEG frequency bands and inter-brain synchronization during negotiation allowed to explore the extent of shared cognitive and emotional states between interacting partners.
The behavioral findings confirmed that participants demonstrated a clear preference for group-oriented decision-making over individualistic approaches. This behavioral predisposition is mirrored by the neurophysiological patterns observed during the negotiation task. The increase in frontal delta and theta power likely reflects the affective–motivational engagement and strategic regulation characteristic of cooperative social cognition, while the enhanced parieto-occipital beta and gamma activity suggests a reliance on integrative and monitoring processes necessary for converging toward a shared solution. Together, these findings support the notion that group-oriented decision-making is instantiated through a specific configuration of distributed cortical activity during negotiation. This aligns with prior research suggesting that individuals with a cooperative orientation tend to prioritize collective interests and shared perspectives in decision-making contexts [
1]. Interestingly, participants reported that their partners influenced their decisions to a moderate extent, but their initial perceptions of group dynamics remained relatively stable. This suggests that, while negotiation fosters an exchange of ideas, individuals may integrate new perspectives without completely altering their initial viewpoints. Additionally, high ratings for cohesion and alliance, coupled with low scores for conflict and empathic failure, indicate that the negotiation process was largely cooperative and prosocial in nature. This interpersonal pattern may also highlight the observed inter-brain dissociation in delta power, which we interpret as reflecting individual regulatory engagement within a non-adversarial, co-constructed exchange.
The EEG findings reveal distinct neural patterns associated with negotiation, providing insights into the cognitive and emotional mechanisms underlying group-oriented decision-making.
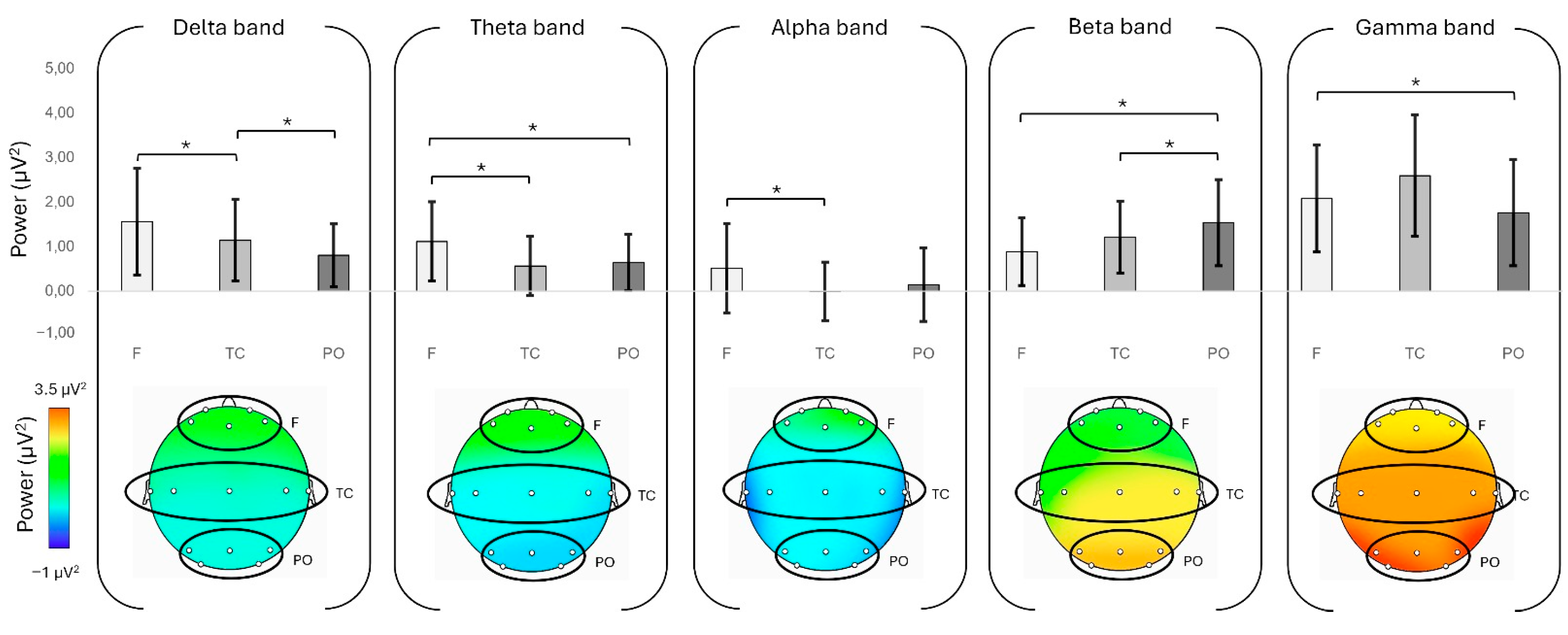
Firstly, the single-brain analysis demonstrated increased delta and theta activity in the frontal region compared to the parieto-occipital region. This is consistent with previous studies linking frontal delta and theta oscillations to emotional engagement, motivation, and goal-directed control [
20,
29]. Moreover, former hyperscanning works demonstrated the key role of frontal areas in cooperation process [
37] as well as the prefrontal cortex, implicated in joint action performance [
39]. These findings suggest that negotiation involves heightened emotional and cognitive effort, particularly in this sample of individuals committed to group-oriented goals.
Moreover, alpha activity was higher in the frontal region than in the temporo-central region during the negotiation task. The suppression of oscillations within the alpha frequency range is an index of cortical excitability [
52] and enhances the efficiency of cognitive processing [
53]. Moreover, previous work on social bargaining showed how alpha oscillations could serve as a mechanism by which different brain areas flexibly interact in order to adapt ongoing behavior in socially demanding contexts [
21].
Temporoparietal regions are also involved in social cognition and communication [
40], managing social dynamics, including negotiation processes [
41,
42]. Given that alpha suppression in posterior regions has been previously associated with enhanced cognitive performance [
30], a possible interpretation can be that this reduction in alpha power in the temporo-central regions, compared to the frontal ones, marks the cognitive effort derived from the negotiation process in our sample of participants.
The study also found that beta and gamma activity was more prominent in the parieto-occipital region compared to the frontal and temporo-central regions. Since beta and gamma oscillations are associated with cognitive control, memory integration, and decision-making [
34,
35], their increased presence in parieto-occipital areas suggests that negotiation requires the integration of multiple perspectives and the monitoring of shared goals. Elevated beta and gamma power in these regions may reflect working memory demands and the multimodal integration of verbal and social cues, alongside the ongoing evaluation of semantic relevance—key processes for managing complex, dialogic interactions in which information must be actively maintained and dynamically updated.
Secondly, at the dyadic level, the analysis revealed significant patterns of dissimilarity within the dyads. Specifically, and differently from what hypothesized, the Euclidean Distance analysis indicated greater dissimilarity in delta band activity in the frontal region compared to other brain regions. The increased dissimilarity in delta band activity likely reflects divergent cognitive and affective states between interactants rather than uniform synchronization. Delta oscillations, particularly in frontal regions, are implicated in large-scale neural coordination, executive control, and predictive coding in social contexts [
54]. The observed increase in frontal delta dissimilarity, contrary to classic models of inter-brain synchrony during cooperation, may reflect individualized engagement of top-down regulatory processes such as conflict monitoring, prediction updating, or fairness evaluation—each governed independently in real time. This dissociation aligns with emerging views that cooperation does not always entail neural alignment but may also involve parallel regulatory roles within a shared social frame. While previous studies have reported frontal delta synchrony during joint tasks, the negotiation paradigm used in this paper emphasizes strategic divergence and autonomous cognitive control. Thus, the inter-brain dissimilarity in delta activity may reflect asynchronous but complementary activation of conflict regulation and decision evaluation circuits, independently engaged by each partner under shared but non-identical cognitive loads [
55]. Unlike mid- and high-frequency bands, delta activity is critical for integrating top-down regulatory mechanisms essential for decision-making and social evaluation [
54,
56]. The higher dyadic dissimilarity in frontal delta activity, compared to the temporo-central and parieto-occipital regions, may reflect the distinct functional roles of these areas in social interactions. The temporo-central and parieto-occipital regions facilitate the processing of social cues, such as speech prosody and facial expressions, supporting higher synchronization through perceptual-motor mirroring [
57]. In contrast, the frontal cortex is engaged in executive functions, including conflict monitoring, strategic adaptation, and inhibition of prepotent responses, which are inherently more individualized and context dependent [
58]. As a result, lower coherence in frontal delta activity may indicate asymmetric cognitive control processes, where interactants independently regulate uncertainty, strategic shifts, and fairness perceptions [
59]. This neural dissociation is consistent with the behavioral profile reported by participants, characterized by low conflict and moderate influence. Rather than reflecting interpersonal discord, frontal inter-brain dissimilarity may index self-regulated cognitive engagement within a shared, non-adversarial task structure. Hyperscanning research suggests that inter-brain synchronization does not necessarily reflect functional alignment but may arise from cooperative or adversarial neural dynamics [
60,
61]. The observed lower delta synchronization in the frontal regions of the dyads may thus signal increased cognitive effort in conflict resolution, suppression of competing responses, or the negotiation of divergent interpretations.
Despite the study’s contributions to understanding the neural dynamics of negotiation, several methodological limitations should be acknowledged to guide future research.
Firstly, the interpretation of frontal delta dissimilarity as an index of cognitive conflict regulation is supported by research on low-frequency frontal oscillations and executive control. However, the current study did not include time-locked behavioral markers of conflict dynamics or parametric measures of decisional divergence, and the sample size limited the ability to test direct neuro-behavioral correlations. Future research should integrate continuous measures of negotiation dynamics, synchronized behavioral annotations, and temporally resolved EEG analyses to better understand the neural mechanisms of conflict regulation during negotiation.
Secondly, although the study was adequately powered for detecting medium within-subject effects, the relatively small sample limits broader generalization and the exploration of interaction-level variability.
Thirdly, although gender was not a variable of interest in the present study, the sample’s imbalance (20 females, 6 males) may limit generalizability, particularly given documented sex differences in negotiation styles, emotional regulation, and neural synchrony patterns. Future studies should implement stratified dyadic designs or include gender as a planned covariate to test its specific impact.
Finally, while designed to simulate a realistic group decision, the absence of real-world stakes may have limited affective arousal, potentially influencing the magnitude of neural activation during negotiation.
An interesting avenue for future research would be to examine how individual differences, such as personality traits, empathy levels, or prior negotiation experience, influence the observed neural patterns. It is possible that more experienced negotiators exhibit different frontal delta dynamics, reflecting more efficient strategic adaptation. Similarly, individuals with higher trait empathy may show reduced inter-brain dissimilarity, indicating a greater tendency to align with their partner’s cognitive and emotional states. Investigating these moderating factors could provide a more nuanced understanding of how neural mechanisms underpin real-world negotiation behavior. In addition, future studies could benefit from incorporating a time-resolved analysis of the negotiation process. Segmenting interactions into distinct phases—such as initial disagreement, deadlock, and resolution—could help uncover more detailed temporal dynamics of neural engagement and clarify how cognitive and affective processes evolve throughout the negotiation.
5. Conclusions
These findings provide first evidence for understanding the EEG neural basis of negotiation in group-oriented decision-making. The observed frontal delta and theta activity highlight the role of emotional engagement and motivation in shaping negotiation strategies, while parieto-occipital beta and gamma oscillations underscore the cognitive demands associated with integrating diverse perspectives. Furthermore, the presence of greater frontal dissimilarity in the delta band suggests that negotiation is not a process of complete alignment at the neural level, but rather it requires increased cognitive effort in conflict resolution, suppression of competing responses, or the negotiation of divergent interpretations. This has potential applications in various applied settings, such as the organizational one, where negotiation appears essential in group decision-making dynamics and knowing the neural bases of this process can promote empowering interventions in professionals. Indeed, beyond its theoretical contributions, this study has potential applications in domains such as professional negotiation training, conflict resolution, and teamwork optimization. The finding that negotiation involves distinct cognitive and affective regulation processes suggests that interventions aimed at enhancing strategic flexibility and emotional regulation could improve negotiation outcomes. Furthermore, the observed inter-brain dissimilarity in frontal delta activity highlights the importance of developing training programs that foster adaptive cognitive control rather than mere synchrony, particularly in high-stakes decision-making contexts.
In conclusion, this study provides first evidence that negotiation in group-oriented decision-making is supported by distinct neural mechanisms at both the individual and dyadic levels. These findings advance our understanding of the neurophysiological foundations of negotiation and offer new perspectives on how neural alignment contributes to effective group interactions.
Future research should explore additional factors that may influence neural synchronization during negotiation, such as personality traits, cultural differences, and the complexity of decision-making scenarios. Moreover, extending the investigation to larger and more diverse groups could provide deeper insights into how neural dynamics scale in multi-person interactions.